Simple Summary
Yaks are one of the sources of greenhouse gas emissions from livestock farming on the Qinghai–Tibet Plateau region, and regulating greenhouse gas emissions from yaks has important ecological significance. In this study, we evaluated potential links between basal diet, rumen microbiota composition, and CH4 and CO2 emissions of yaks under different feeding regimes. We found the CO2 and CH4 emissions of yaks were lower in traditional grazing than in warm-grazing and cold-indoor feeding regimes. The rumen microbiota of the yaks changed because of differences in basal diet. The CO2 and CH4 emissions of yaks were related to complementarity among members of the rumen functional genera. We believe that shifts in feeding regimes are effective measures reducing greenhouse gas emissions from yaks and rumen microbiome characterization could be useful screening tools for selecting yaks with low gas emissions.
Abstract
Shifts in feeding regimes are important factors affecting greenhouse gas (GHG) emissions from livestock farming. However, the quantitative values and associated drivers of GHG emissions from yaks (Bos grunniens) following shifts in feeding regimes have yet to be fully described. In this study, we aimed to investigate CH4 and CO2 emissions differences of yaks under different feeding regimes and their potential microbial mechanisms. Using static breathing chamber and Picarro G2508 gas concentration analyzer, we measured the CO2 and CH4 emissions from yaks under traditional grazing (TG) and warm-grazing and cold-indoor feeding (WGCF) regimes. Microbial inventories from the ruminal fluid of the yaks were determined via Illumina 16S rRNA and ITS sequencing. Results showed that implementing the TG regime in yaks decreased their CO2 and CH4 emissions compared to the WGCF regime. The alpha diversity of ruminal archaeal community was higher in the TG regime than in the WGCF regime. The beta diversity showed that significant differences in the rumen microbial composition of the TG regime and the WGCF regime. Changes in the rumen microbiota of the yaks were driven by differences in dietary nutritional parameters. The relative abundances of the phyla Neocallimastigomycota and Euryarchaeota and the functional genera Prevotella, Ruminococcus, Orpinomyces, and Methanobrevibacter were significantly higher in the WGCF regime than in the TG regime. CO2 and CH4 emissions from yaks differed mainly because of the enrichment relationship of functional H2- and CO2-producing microorganisms, hydrogen-consuming microbiota, and hydrogenotrophic methanogenic microbiota. Our results provided a view that it is ecologically important to develop GHG emissions reduction strategies for yaks on the Qinghai–Tibet Plateau based on traditional grazing regime.
1. Introduction
The atmospheric concentration of greenhouse gases (GHGs) has increased steadily in the last few decades [1,2], at the same time, they have a significant impact on global warming. Global livestock farming contributes significantly to GHG emissions, generating approximately 14.5% of total global GHG emissions [3,4]. As a result of population growth, rising incomes, and changes in lifestyles and diets, the global demand and production of livestock products are increasing rapidly [5], especially in the developing world, which is possibly further accelerating global GHG emissions [6,7,8]. To meet the Paris Agreement goal of keeping global temperature rise to well below 1.5 °C above preindustrial levels [9], there is a need to balance the livestock farming keeping for nutrition, health, and well-being, with the urgent need to reduce GHG emissions to deal with the climate crisis [10,11].
The Qinghai–Tibet Plateau is the most important livestock areas in China. In recent decades, the expansion of livestock farming and relatively backward technological means of livestock production have exacerbated GHG emissions [12,13], which has caused widespread concern in China. The government is actively promoting the reduction of GHG emissions from the livestock farming on the Qinghai–Tibet Plateau [14,15]. The yak living on the Qinghai–Tibet Plateau is an important ruminant and supports the development of the livestock farming. However, yaks are also one of the largest contributors to GHG emissions from livestock farming on the Qinghai–Tibet Plateau [16,17]. Therefore, GHG emissions from yaks should be investigated.
Under most environmental conditions, feeding management is the most feasible strategy for reducing GHG emissions from livestock farming [18,19,20]. Currently, most of the effects of feeding management on GHG emissions from cattle production systems have been analyzed using models, an approach that has the advantage of allowing estimates at different scales [21,22,23,24]. These studies have shown consistent results that feeding management can affect GHG emissions by influencing the dietary conditions and habitat of cattle [25,26]. The basis of these model estimates requires empirical data to support them, and in previous studies, region specific emission factors are lacking, therefore having an enormous impact on model estimation results [27,28,29]. This knowledge gap may limit our understanding of the feedback of livestock development on the global climate, especially in the Qinghai–Tibet Plateau region, where livestock development of yaks is dominant. Therefore, accurate measurements of GHG emissions from yaks on the Qinghai–Tibet Plateau is necessary to inform the estimation of GHG emissions from livestock farming in the region.
Most of the GHGs are produced by their rumen fermentation in ruminants, which efficiently breaks down plant biomass and its complex dietary carbohydrates into soluble sugars, which are subsequently converted into nutrients and metabolites usable by host animals, as well as expelling CO2 and CH4 [30,31,32]. Bacteria, fungi, and archaea in the rumen play a key role in this process by interacting to form a complex mixture of microorganisms [33]. Studies have reported that molecular hydrogen (H2) and carbon dioxide (CO2) is produced during carbohydrate fermentation by bacteria and fungi and is primarily consumed by archaea in the rumen, thereby metabolizing the production of GHGs [34,35,36]. Changes in feeding management are usually accompanied by changes in dietary nutrition levels and environmental conditions, and these changes may individually or interactively affect the rumen microbiome in ruminants [37,38]. For instance, different diets have different rumen methanogenic community characteristics [39], Succinivibrionaceae with implicated in lower CH4 emissions from starch-containing diets [40], and fiber-based diet leads to increased relative abundance of Prevotellaceae, which was closely related to GHG emissions [41]. In summary, deterministic effects driven by diet shape the composition and function of the rumen microbiota, which in turn affects GHG emissions. Therefore, studies on changes in the rumen microbiota of yaks after changes in feeding management can help us understand the differences in their GHG emissions.
In this study, we aimed to explore (i) the emissions and differences in CH4 and CO2 emissions in yaks under different feeding regimes, (ii) differences in the rumen microbiota of yaks under different feeding regimes, and (iii) the potential mechanisms by which changes in rumen microbiota regulate CH4 and CO2 gas emissions of yaks. Overall, this study aimed to provide a reference for reducing GHG emissions in the livestock farming on the Qinghai–Tibet Plateau.
2. Materials and Methods
2.1. Experimental Animal Management
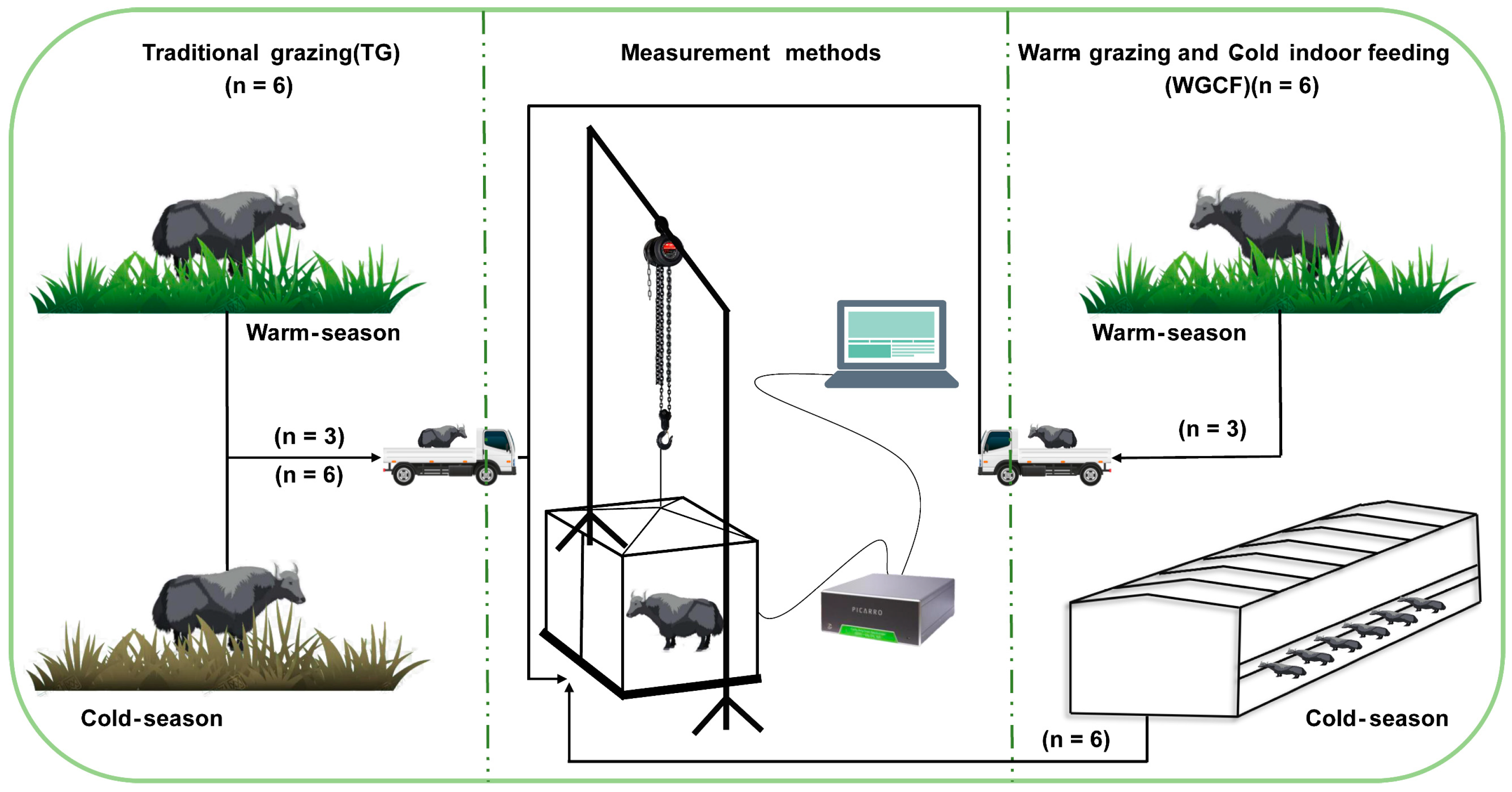
An experiment was performed in pastoral areas of Qinghai–Tibet Plateau (3004 m; 37°55′ N, 100°57′ E), Qinghai Province, China. The study period was from May 2021 to May 2022, in the warm season (May to October) and the cold season (November to April). In May 2021, twelve female yaks of similar weight and morphological characteristics at approximately 2 years of age were randomly divided into two feeding regimes: six yaks in the traditional grazing regime (warm-season grazing + cold-season grazing, TG) and six yaks in the warm-grazing and cold-indoor feeding regime (warm-season grazing + cold-season indoor feeding, WGCF). In the warm season, yaks in both TG (n = 6) and WGCF (n = 6) regimes were managed by grazing in natural pastures, and three yaks in each of the two feeding regimes were randomly selected to form a warm-season grazing group (YWG, n = 6), taking into account the experimental conditions and feasibility. In the cold season, yaks in the TG regime were managed in natural pasture for the cold-season grazing group (YCG, n = 6). yaks in the WGCF regime were managed indoors for feeding for the cold-season feeding group (YCF, n = 6). The diet with forage to concentrate ratios (60:40) were fed to yaks of the indoor feeding, which is the most common feeding strategy in the region. The ingredients and nutrient level of concentrated feed in Table 1. The yaks had access to water throughout the experimental period.

Table 1.
The ingredients and nutrient level of concentrated feed (dry matter basis, %).
2.2. Measurement of GHG Emissions
The GHG emissions of the yaks were measured outside the feedlots of the YCF group; thus, the yaks had to be transported from the grazing site to the measurement site for the YWG and YCG groups, but for the YCF group, the yaks were not transported. The GHG emissions were measured during the warm season, July, August, and September, and the cold season, December, January, and February, using the static breath chamber method [42,43]. The GHG measurement system of the static breathing chamber method consisted of three parts: first, a lifting system consisting of a lifting frame and lifting guide chain for lifting the sealed outer cage cover of the static breathing chamber; second, a measurement system consisting of an iron base (2.50 m × 1.90 m), a sealing tank (welded to the iron base, 0.10 m wide), an iron metabolic cage (2.20 m × 1.50 m × 1.65 m) the sealing outer cage (2.40 m × 1.80 m × 1.80 m), four small fans (fixed on the top of the iron metabolic cage used to mix the gas inside the chamber), two thermometers (fixed on the side of the iron metabolic cage used to record the temperature inside the chamber), one food tank (to ensure diet), one water tank (to ensure drinking water) during the test period in the sealing tank filled with absorbent sponge or filled with water, ensuring the hermetic seal between the sealing tank and the sealing outer cage; and third, a gas analysis system consisting of the Picarro G2508 gas concentration analyzer, which is a real-time analytics system that measures the concentrations of gases along with CO2 and CH4. On the basis of real-time data analysis and yak condition observation, the GHG emissions of yaks were determined every 2 h during the day for 20 min for a total of 12 measurements. Measurements were taken at the same time of the month for each measurement group, and a 5-day acclimatization test, including transport and static breathing chamber acclimatization, was conducted prior to the measurements. The yak diet materials were kept constant during measurement. The YWG and YCG groups used mowing methods to obtain natural pasture, while the YCF group obtained ration directly from the feedlot and had free access to ration and water throughout the trial period. The overall measurement process is illustrated in Figure 1. The GHG emissions of yak were calculated using a linear least-squares fit to all points in the time series of the gas concentration:
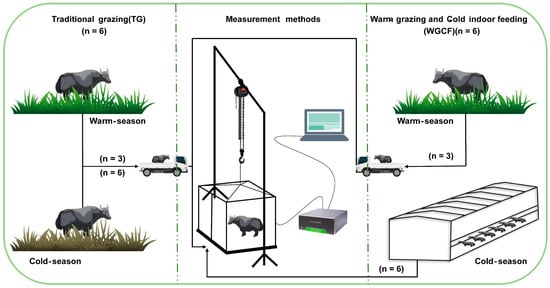
Figure 1.
Schematic illustration of the GHG measurement process in two different feeding regimes.
Yaks’ GHG fluxes (F, g head−1 h−1) were calculated using the following equation [42]:
where dc/dt is the rate of change in GHG concentration in the chamber over time (ppm s−1), gas samples that showed linear GHG concentration shift with time (dc/dt) with R2 ≥ 0.9 were used for further analysis; V is the volume of the chamber (in m3); N is the number of yaks; M is the molecular weight of GHGs (CO2: 44 and CH4: 16); 22.4 is the molar volume of gas at standard temperature and pressure (in 1 mol−1); P is the air pressure in the chamber (in Pa); P0 is the atmospheric pressure at standard conditions, 1.013 × 105 Pa; T is the temperature in the closed static chamber (in °C).
Annual, seasonal, and day cumulative yak GHG emissions were calculated using the following equation [44]:
where E is the annual or seasonal cumulative CO2, CH4 (kg head−1) emissions or the day cumulative CO2, CH4 (g head−1 d−1), f represents the emission of CO2 (g head−1 h−1), CH4 (g head−1 h−1), i is the measurements ith measurement, (ti+1 − ti) is the interval between measurements.
2.3. Dietary Collection and Nutritional Quality Determination
The diet was collected during each experimental period. The natural pasture was sampled using sample squares (0.5 m × 0.5 m) randomly thrown in the center of the grazing pasture, with 10 samples collected at a time in which the pasture was cut to approximately 2 cm above the ground and composed of a mixture of young, mature, and dry pastures to mimic the feeding behavior of cattle [45]. The indoor feed rations were collected from 10 random 50 g samples. All collected diets were oven dried at 60–70 °C for 48 h and individually ground with a grinder to pass a 1 mm sieve for nutritional quality determination. Natural pasture and indoor ration samples were mixed separately in warm and cold seasons to determine the nutritional composition of each test group.
Dry matter (DM) and total ash content were analyzed in these samples by drying at 105 °C and 550 °C, respectively; organic matter (OM) was calculated as dry matter minus total ash [46]; crude protein (CP) was quantified using the Kjeldahl method of nitrogen determination [47]; neutral detergent fiber (NDF) and acid detergent fiber (ADF) contents were determined using an automatic fiber analyzer [48].
2.4. Ruminal Fluid Collection and Sequencing Analysis
Ruminal fluid samples were collected once during the warm season (August) and once during the cold season (January). Ruminal fluid samples from each animal were collected using a gastric tube the morning before feeding. For sampling, approximately 50 ml of ruminal fluid was extracted by inserting a gastric tube through the mouth and manually suctioning. The fluid was filtered through four layers of muslin, and the filtered ruminal fluid was collected, immediately frozen on dry ice, and stored at −80°C for microbial sequencing.
Total genomic DNA samples were extracted using an OMEGA soil DNA kit (M5635-02) (Omega Bio-Tek, Norcross, GA, USA), in accordance with the manufacturer’s instructions, and stored at −20 °C before further analysis. The quantity and quality of extracted DNAs were examined using a NanoDrop NC2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and agarose gel electrophoresis, respectively.
PCR amplification (15–35 cycles) was carried out in quadruplicate 25 μL eactions using Q5® High-Fidelity DNA polymerase (New England Biolabs, Hitchin, UK). The primer sets 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTC-TAAT-3′) for bacteria, ITS5 (5′-GGAAGTAAAAGTCGTAACAAGG-3′) and ITS2(5′-GCT-GCGTTCTTCATCGATGC-3′) for fungi, and 1106F (5′-TTWAGTCAGGCAACGAGC-3′) and 1378R (5′-TGTGCAAGGAGCAGGGAC-3′) for archaea were used to amplify the target regions. Sample-specific 7 bp barcodes were incorporated into the primers for multiplex sequencing. Thermal cycling involved initial denaturation at 98 °C for 5 min, followed by 25 cycles including denaturation at 98 °C for 30 s, annealing at 53 °C for 30 s, and extension at 72 °C for 45 s, with a final extension of 5 min at 72 °C.
PCR products were cleaned and quantitated using the Vazyme VAHTSTM DNA Clean Beads (Vazyme, Nanjing, China). The band at the expected size containing the amplicons was cut and purified using the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA, USA). After the individual quantification step, amplicons were pooled in equal amounts, and 2 × 250 bp pair-end sequencing was performed using the Illlumina MiSeq platform with MiSeq Reagent Kit v3 at Shanghai Personal Biotechnology Co., Ltd. (Shanghai, China).
Raw sequence data were filtered and analyzed using QIIME2 (https://docs.qiime2.o-rg/2019.4/tutorials/, accessed on 30 June 2022) [49], and the Dada2 method was used for primer removal, quality filtering [50], denoising, splicing and chimer removal. Database sequence counts in each library were normalized by subsampling to the same sequencing depth per sample. Amplicon sequence variant (ASV) based approach was used for phylotyping [51,52].
2.5. Statistical Analysis
One-way ANOVA was performed to examine the data on dietary nutrients and GHG emissions, and Duncan’s test was conducted for multiple comparisons when treatments had differences. The 16S rRNA and ITS (internal transcribed spacer) microbiome sequencing data were statistically analyzed via Kruskal–Wallis, PERMANOVA, and Mantel test [53]. Redundancy analysis (RDA), introduced by Capblancq [54], was used to analyze the correlation between dietary nutrients and rumen microbial communities in this study. Data were statistically analyzed using SPSS24.0 or R software v3.6.1 [55,56], and data were visualized using Origin 2021, ImageGP (http://www.ehbio.com/Cloud_Platform/front/, accessed on 15 July 2022) [57] and Genescloud (https://www.genescloud.cn, accessed on 12 July 2022).
3. Results
3.1. Dietary Nutrition and CO2 and CH4 Emissions from Yaks
In the TG regime (YWG+YCG), DM, NDF, ADF, and OM were higher by 0.92%, 11.22%, 36.84% and 1.56% in the YCG group than in the YWG group, respectively, and CP was lower by 56.33%. In the WGCF regime (YWG+YCF), DM, ADF, and OM were higher by 0.79%, 10.24%, and 1.57%, respectively, in the YCF group than in the YWG group. Conversely, NDF was lower by 6.06% in the YCF group than in the YWG group. CP was similar in the two groups. Based on the overlap between the two feeding regimes, the difference in the YCG and YCF groups was equivalent to that between the TG and WGCF regimes. Between feeding regimes (YCG vs. YCF), NDF and ADF were higher by 18.40% and 24.13%, respectively, while CP was lower by 56.11%, and DM and OM showed no differences in the YCG than in the YCF groups (Table 2).

Table 2.
Nutrient level and feed intake of experimental groups dietary (dry matter basis, %).
In the TG regime (YWG+YCG), CO2 and CH4 emissions were 28.59% and 87.67% higher, respectively, in the YWG than in the YCG groups. In the WGCF regime (YWG+YCF), CO2 emissions were higher by 24.88%, while CH4 emissions were lower by 18.06% in the YCF group than the YWG group. Between feeding regimes, CO2 and CH4 emissions were higher by 26.50% and 18.69%, respectively, in the WGCF than in the TG regimes (Table 3).

Table 3.
The day, seasonal, and annual cumulative CO2 and CH4 emissions from yaks.
3.2. Rumen Microbiome Structure of Yaks
Sparse analysis showed that our population captured most of the rumen microbiota from each rumen digestive fluid sample (Figure S1a–c). By quality control and leveling (leveling depth set to 95% of the minimum sample sequence volume) [49], a total of 2,427,106 raw reads and 1,206,851 high-quality sequences were obtained for bacteria, 2,561,329 raw reads and 1,935,483 high-quality sequences for fungi, and 2,524,601 raw reads and 1,791,387 high-quality sequences for archaea in the 18 sample sequences. The statistical analysis of the ASV tables after flat sampling, revealed that the rumen microbial communities of yaks changed in the different experimental groups.
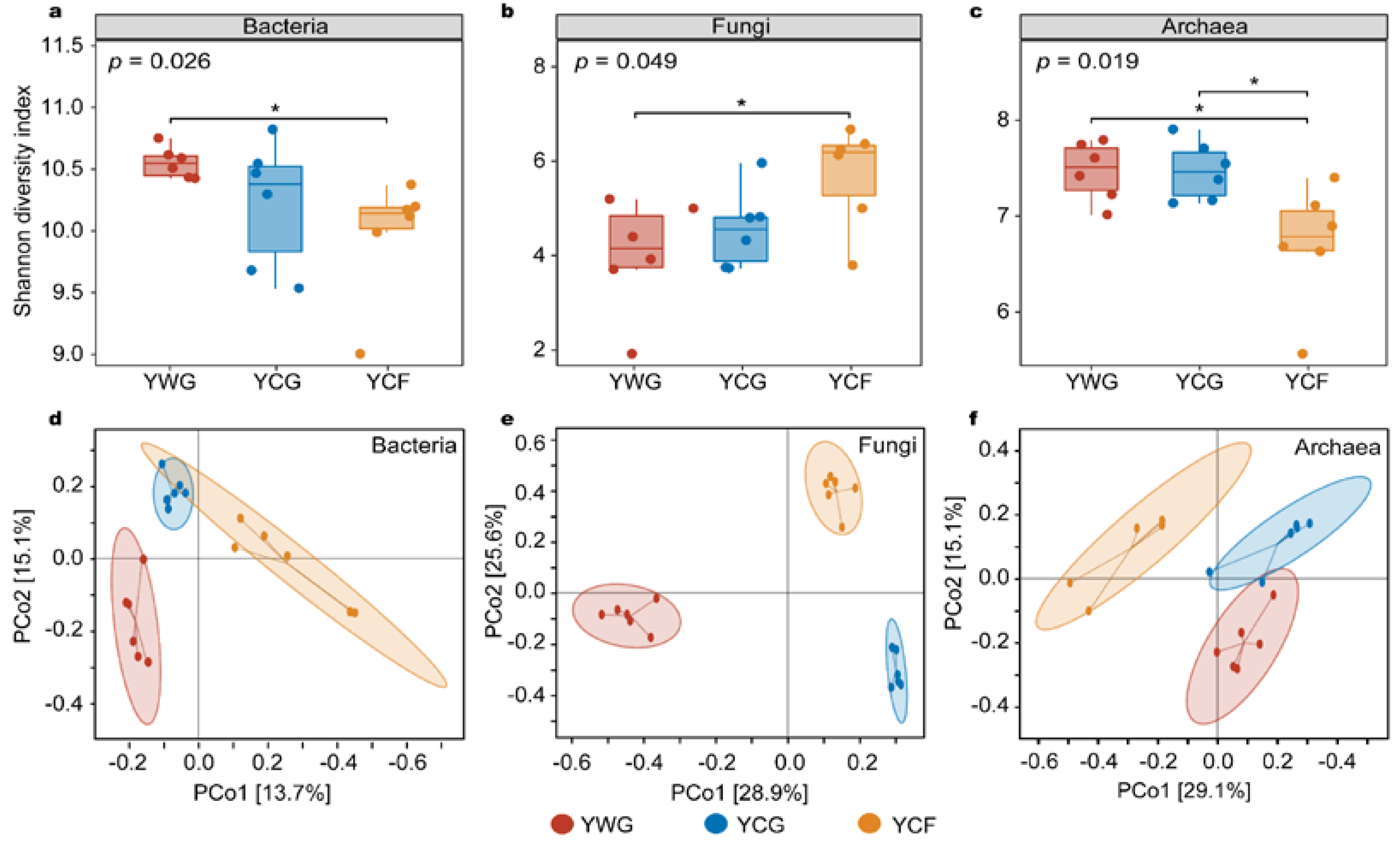
The variation in the alpha and beta diversity of the rumen microbiota was analyzed to compare the response of the rumen microbiota to changes in the two feeding regimes. In the TG regime (YWG+YCG), the alpha diversity between the YWG and YCG did not vary significantly in the bacterial, fungal, and archaeal communities (p > 0.05; Figure 2a–c). In the WGCF regime (YWG+YCF), the alpha diversity of the YWG was significantly higher than that of the YCF in the bacterial and archaeal communities (p < 0.05; Figure 2a,c), whereas the YWG was significantly lower than that of the YCF in fungal communities (p < 0.05; Figure 2b). Between feeding regimes (YCG vs. YCF), the alpha diversity of the YCG was significantly higher than that of the YCF in the archaeal communities (p < 0.05; Figure 2c), but the alpha diversity between the YCG and YCF did not vary significantly in the bacterial and fungal communities (p > 0.05; Figure 2a,b).
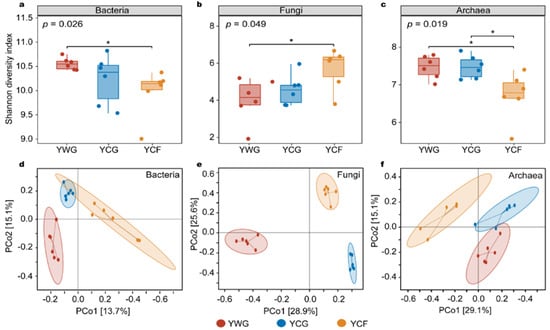
Figure 2.
Diversity analysis of rumen microbiota between different experimental groups of yaks. Alpha diversity of the rumen microbiota as determined using the Shannon index. (a–c) are alpha diversity of the rumen bacteria, fungi, and archaea in the three experimental groups, respectively. Principal coordinates analysis (PCoA) based on Bray–Curtis distances. PCoA analysis of rumen bacteria (d), fungi (e), and archaea (f) composition between different experimental groups. YWG: Warm-season grazing, YCG: Cold-season grazing, YCF: Cold-season indoor feeding. * p < 0.05 (Wilcoxon rank-sum test).
Unconstrained principal coordinates analysis (PCoA) based on Bray–Curtis distance showed that rumen bacterial, fungal, and archaeal communities were significantly clustered in all three experimental groups (p < 0.001) (Figure 2d–f). In other words, the rumen microbiota was significantly clustered within the TG (YWG vs. YCG) and WGCF (YWG vs. YCF) regimes, and between the TG and WGCF regimes (YWG vs. YCF).
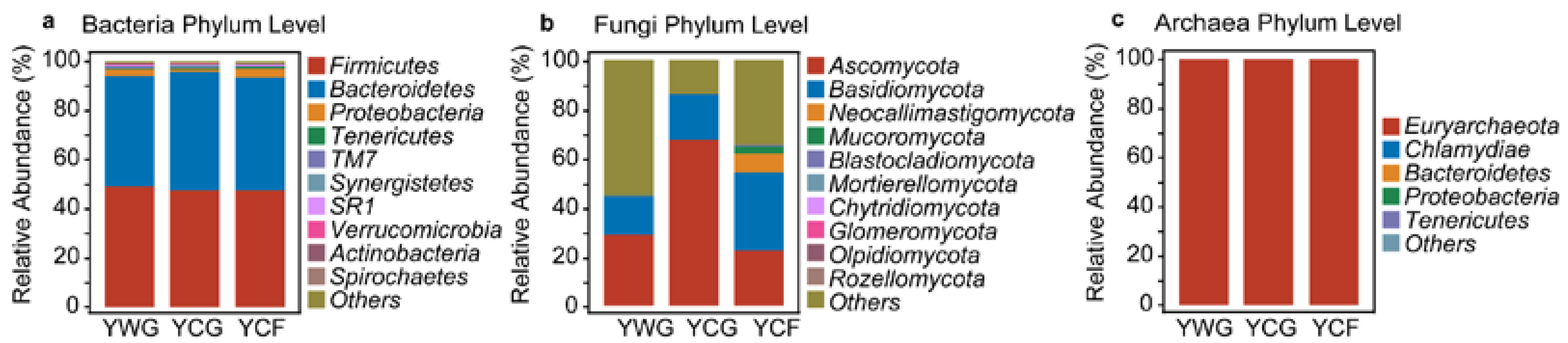
Overall, at the phylum level, bacterial communities were predominated by Firmicutes (47.39–49.14%), Bacteroidetes (44.88–48.26%), and Proteobacteria (1.16–3.87%); fungal communities were predominated by Ascomycota (22.70–67.74%), Basidiomycota (15.94–31.48%) and Neocallimastigomycota (0.21–7.79%); and archaeal communities were predominated by Euryarchaeota (99.98–100.00%) (Figure 3a–c). Under the TG regime (YWG+YCG) an increase in the relative abundance of Ascomycota (p < 0.05) was observed in the YCG group (Tables S1–S3). In the WGCF regime (YWG+YCF), an increase in the relative abundance of Neocallimastigomycota (p < 0.01) and Euryarchaeota (p < 0.05) was observed in the YCF group (Tables S1–S3). Between the feeding regimes (YCG vs. YCF), the relative abundance of Neocallimastigomycota (p < 0.01) and Euryarchaeota (p < 0.01) increased in the YCF group, and the relative abundance of Ascomycota (p < 0.05) decreased (Tables S1–S3).

Figure 3.
Phylum-level distribution of the rumen bacteria (a), fungi (b), and archaea (c) in three groups. Relative abundance of top 10 for rumen microbiota of yaks. YWG: Warm-season grazing, YCG: Cold-season grazing, YCF: Cold-season indoor feeding.
3.3. Relationships between Dietary Nutrition and Rumen Microbial Communities
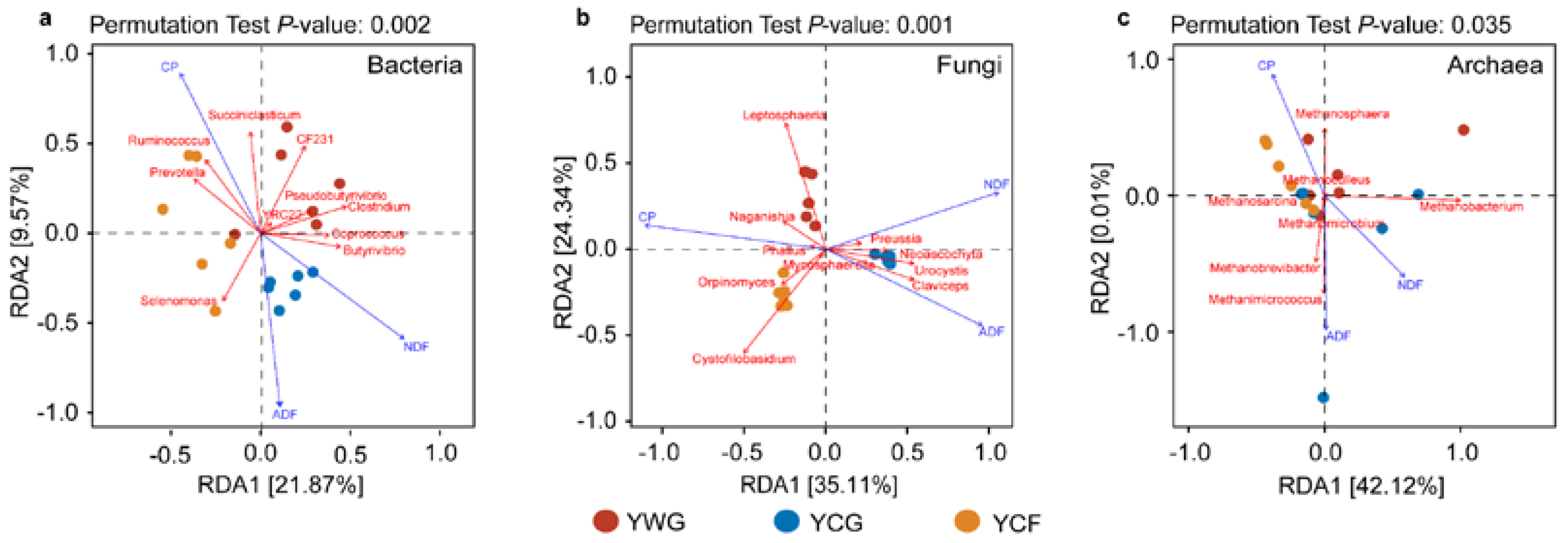
Changes in dietary nutrition parameters were key factors driving the succession of rumen bacterial, fungal, and archaeal communities (Mantel test R = 0.385 (p = 0.001), R = 0.640 (p = 0.001), and R = 0.250 (p = 0.010), respectively) (Figure S1d–f). The results from the best identified CP, NDF, and ADF contents were the prevailing factors that explain rumen microbial community succession. Redundancy analysis (RDA) between nutritional parameters and rumen microbial genus levels depicted the interdependence of nutrition parameters and rumen microbial communities. For bacterial communities, the first two canonical axes explained 21.87% and 9.57% of the changes, respectively. Succiniclasticum was positively correlated with CP, whereas Selenomonas and Butyrivibrio were positively correlated with ADF and NDF, respectively (Figure 4a). For fungal communities, the first two canonical axes explained 35.11% and 24.34% of the changes, respectively. Leptosphaeria and Cystofilobasidium were positively associated with CP, whereas Claviceps were positively associated with NDF and ADF (Figure 4b). For archaeal communities, the first two canonical axes explained 42.12% and 0.01% of the changes, respectively. Methanosphaera was positively associated with CP, while Methanobacterium and Methanobrevibacter were positively related to NDF and ADF (Figure 4c). In the bacterial, fungal, and archaeal communities, CP was negatively correlated with NDF and ADF.
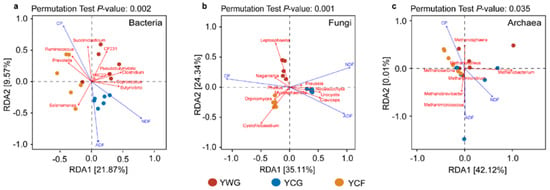
Figure 4.
Correlation analysis of the rumen microbial communities with dietary nutrition characteristics. Redundancy analysis (RDA) plot showing the correlations between fermentation characteristics and rumen bacteria (a), fungi (b), and archaea (c) community composition. The canonical axes are labelled with the percentage of total variance explained (%). Arrow lengths indicate the variance explained by dietary nutrition characteristics. Individual yaks from different experimental groups are presented as separate data points. YWG: Warm-season grazing, YCG: Cold-season grazing, YCF: Cold-season indoor feeding.
3.4. Relationships between GHG Emissions and Functional Microbial Genera
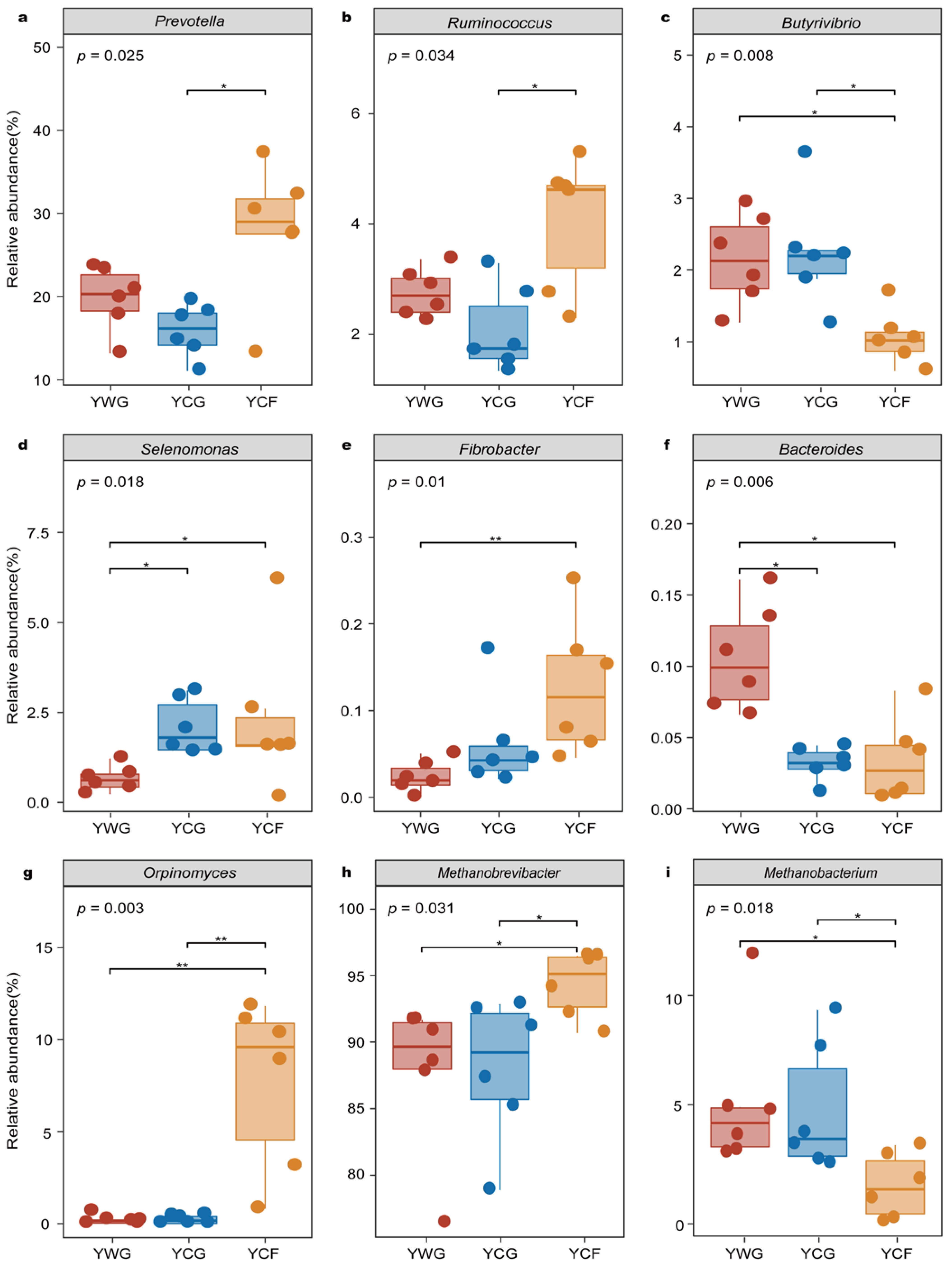
The microbial genera potentially involved in CO2 and CH4 gas emissions were investigated in greater detail because of their known significance in rumen ecosystem functioning (Table S4). Based on differential abundance analysis performed in microbial genera putatively capable of gas production, the studied system included members of the bacterial genera Prevotella, Ruminococcus, Butyrivibrio, Selenomona, Fibrobacter, and Bacteroides; the fungal genera Orpinomyces, and the archaeal genera Methanobrevibacter and Methanobacterium. In the TG regime (YWG+YCG), Bacteroides exhibited higher relative abundance in the YWG group (p < 0.05) (Figure 5f). Although Selenomonas was the predominant functional bacterial in the YCG group (p < 0.05) (Figure 5d), none of the other functional microbial genera showed significant enrichment (p > 0.05). In the WGCF regime (YWG+YCF), in the YCF group, the relative abundance of Fibrobacter (p < 0.01), Selenomonas (p < 0.05), Orpinomyces (p < 0.01), and Methanobrevibacter (p < 0.05) increased (Figure 5d,e,g,h). in the YWG group, the relative abundance of Butyrivibrio (p < 0.05), Bacteroides (p < 0.05), and Methanobacterium (p < 0.05) increased (Figure 5c,f,i). Between feeding regimes (YCG vs. YCF), the taxa were significantly enriched in the YCF group comprising functional microbial genera from Prevotella (p < 0.05), Ruminococcus (p < 0.05), Orpinomyces (p < 0.01), and Methanobrevibacter (p < 0.05) (Figure 5a,b,g,h). The taxa were significantly enriched in the YCG group included functional microbial genus from Butyrivibrio (p < 0.05) and Methanobacterium (p < 0.05) (Figure 5c,i).
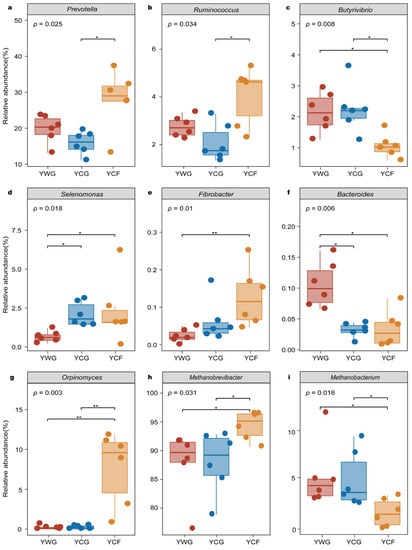
Figure 5.
Relative abundance of putative functional bacteria (a–f), fungi (g), and archaea (h,i) by rumen microbiota compartment and GHG emissions phenotype of yaks. YWG, Warm-season grazing, YWG: Warm-season grazing, YCG: Cold-season grazing, YCF: Cold-season indoor feeding. * p < 0.05, ** p < 0.01 (Wilcoxon rank-sum test).
4. Discussion
Typically, changes in feeding regimes are accompanied by differences in dietary conditions because feeding regimes are linked to seasonal and human factors [58,59]. These results were consistent with our observations, which showed that the nutritional parameters of the diet varied significantly within and between the feeding regimes. In ruminant feeding, changes in dietary conditions modulate rumen microsystems by regulating the development and colonization of rumen microbiota [38], influencing diversity, composition, and function of rumen microbiota, and interfering with the metabolism and energy absorption of hosts [60,61]. In addition, changes in the rumen microbiota allow ruminants to adapt to changes in dietary conditions [17,62,63]. Our study also found that rumen microbiota was significantly correlated with dietary conditions. The fiber and protein composition of the diet were the key factors influencing the rumen microbiota. The results of covariation in dietary conditions and rumen microbiota caused by shifts in feeding regimes enhanced our confidence in the potential mechanisms explaining the differences in GHG emissions from yaks within and between feeding regimes.
Previous studies reported conflicting results regarding the relationship between feeding regimes and GHG emissions [64,65,66,67]. We found that the CO2 and CH4 emissions of yaks were lower in the TG regime (YWG+YCG) than in the WGCF regime (YWG+YCF). This result was consistent with previous findings that grazing systems have lower GHG emissions than indoor feeding systems in cattle [65], sheep [66], and that the addition of a high-concentrate diet promotes GHG emissions from beef cattle [62]. In contrast to the results of Ding et al. [67], who found higher GHG emissions in grazing yaks than in indoor feeding yaks, our findings exhibited differences possibly because of methodological and experimental design limitations in a previous work. In our study, the method of GHG measurement was a combination of static breathing chamber and Picarro G2508 gas concentration analyzer, which can accurately measure the GHG emissions of yaks in real-time. During the measurement process, we supplied a wild natural diet for yaks, which can greatly preserve the native microbial community of the yak rumen and improve measurement accuracy [68]. In addition, CO2 and CH4 emissions from yaks in the cold and warm seasons within different feeding regimes have been rarely investigated. In our study, we found that CO2 and CH4 emissions were higher in the warm season than the cold season in the TG regime (YWG+YCG). In the WGCF regime (YWG+YCF), CH4 emissions were higher in the warm season than in the cold season, whereas CO2 emissions were lower than in the cold season. The reason why these results have appeared might be that rumen microbiome of yak cope with seasonal changes in diets, temperature, and environmental factor demands [62,69,70]. We did not detect any obvious evidence of these tradeoffs in yaks, but future work that seasonal temperature and environmental factor data is needed to help determine if such tradeoffs exist.
The rumen of yaks is a stable and extremely complex microecosystem with a complex microbial community that includes numerous bacteria, fungi, and archaea [70]. These microbiota ferment indigestible carbohydrates and derive energy from them to grow and continue to actively produce volatile fatty acids, CO2, H2, CH4, and others [71], the main gases produced by bacteria and fungi are CO2 and H2, and part of the dissolved H2 and CO2 is used by methanogenic bacteria to form CH4 [36]. Therefore, we will explain these differences in GHG emissions in the analysis of the yak rumen microbiota.
In the TG regime (YWG+YCG), alpha diversity had no differences, but the beta diversity of rumen bacterial, fungal, and archaeal communities significantly changed between the YWG and YCG groups in yaks. At the phylum level, the relative abundance of Ascomycota increased in the YCG group. These results were due to the stable structure and composition of rumen microbiota developed in yaks over a long period of evolution. Similar to our results, Guo et al. [63] found relatively stable changes in the gut microbiota composition in response to changes in diet composition across seasons. This implied that high-altitude mammals have evolved stable systems of gut microbiota composition across seasons. Thus, differences in the GHG emissions of yaks in this regime may be due to differences in the key functional microbiota. Surprisingly, we found that Bacteroides was significantly enriched in the YWG group. Bacteroides is a group of well-characterized carbohydrate fermenters that produce CO2 and H2 [72], which are major substrates supporting ruminal methanogenesis and usually observed to be enriched in ruminants with high gas emissions [73]. Selenomonas was enriched in the YCG group, a group of bacteria confirmed in most studies to be hydrogenophilic, which are mainly involved in fumarate and nitrate reduction metabolic pathways, which were important H2 sinks and can effectively compete with methanogenic bacteria for H2 [36]. Selenomonas is usually enriched in ruminants with low GHG emissions [58,71]. These results confirmed our findings, which showed that CO2 and CH4 emissions were higher in yaks in the YWG group than in the YCG group.
In the WGCF regime (YWG+YCF), the alpha diversities of rumen bacteria and archaea were lower in the YCF group than in the YWG group. The beta diversity of yak rumen microorganisms was also significantly different. This result was similar to those of previous studies [59,74]. The YCF group allowed yaks to live in a limited space and lost contact with a complex environment, which in turn hindered the maintenance of rumen microbial diversity [75]. This diversity also fits neutral diffusion limitation theory [76]. In addition, diet is an important factor in rumen microbial communities as the YWG group has more diverse dietary options and consumes more micronutrients than the YCF group condition; consequently, rumen microbial diversity was higher in the YWG group than in the YCF group [63]. At the phylum level, we found that the relative abundances of Neocallimastigomycota and Euryarchaeota increased in yaks in the YCF group. Neocallimastigomycota has a high fiber-degrading ability, which can secrete a series of fiber degrading enzymes and produce H2, CO2, and other substances, during fermentation [77,78]. Simultaneously, Euryarchaeota uses these metabolites to produce CH4 [79]. However, the role of Neocallimastigomycota may be greater than that of Euryarchaeota, which may also explain the higher CO2 emissions in the YCF group than in the YWG group in our study [80]. In terms of functional microbiota studies, our results showed that the relative abundances of the functional microbiota Fibrobacter and Orpinomyces were higher in the rumen of the YCF group with high CO2 emissions than in the rumen of the YWG group. Fibrobacter uses cellulose as a substrate to produce short-chain fatty acids, H2, and CO2 [35]. Orpinomyces, an anaerobic fungus with a longer life cycle and a more indeterminate (polycentric) growth regime, favors its proliferation in animals grazing fresh forage and can utilize various substrates to produce H2, CO2, acetate, lactate, and ethanol [81,82], which support our results. In the rumen microbiota of the YWG group with high CH4 emissions, the relative abundance of Methanobacterium, which is a hydrogenotrophic methanogen, significantly increased [83]; the increased relative abundance of hydrogen-producing Butyrivibrio [84] and Bacteroides [36] also provided fermentation substrates for Methanobacterium, leading to a high CH4 expression. These results indicated that the CH4-producing metabolic process in the rumen microbiota of the YWG group was superior to H2 and CO2 metabolic processes, whereas H2 and CO2 metabolic processes were superior to the CH4-producing metabolic process in the rumen microbiota of the YCF group.
Between the TG and WGCF regimes (YCG vs. YCF), the yaks in the YCF group had higher concentrate diets than in the YCG group, thereby reducing the diversity of their microbiota [85]. Dietary fiber intake was positively correlated with microbial diversity and pasture in the YCG group with a higher fiber content [86]. Consistent with our results, our findings indicated that the alpha diversity in the rumen archaeal microbiota of the YCG group was higher than that in the YCF group, whereas the beta diversity in rumen bacterial, fungal, and archaeal microbiota differed. It has been shown that higher CH4 emissions are usually associated with lower archaeal diversity, and that higher archaeal diversity facilitates efficient energy use and thus reduces GHG emissions [87]. In addition, the rumen microbiota in the YCF group altered the microbial community at the phylum level, including the abundances of Neocallimastigomycota and Euryarchaeota. They are involved in carbohydrate metabolic conversion and CH4 production through various carbohydrate active enzymes [78,79,81]. These results suggested that the YCF group had better-quality dietary conditions that favored the growth of gas production-related microbial taxa. In terms of functional microbiota studies, our results showed that the relative abundances of Prevotella, Ruminococcus, and Orpinomyces were higher in the rumen of the YCF group than in the rumen of the YCG group. Prevotella is an important protein and polysaccharide degrading genus, and Ruminococcus and Orpinomyces are involved in fiber degradation and biohydrogenation in the rumen [16,33,83]. Their enrichment is positively correlated with high GHG emissions in ruminants [35,88,89]. Similarly, Methanobrevibacter is the dominant archaebacterium and a typical hydrogenotrophic methanogenic bacterium in the rumen [90]. In our study, Methanobrevibacter was enriched in the YCF group. Previous studies demonstrated that Methanobrevibacter plays a significant role in rumen CH4 production and its relative abundance is positively correlated with CH4 emissions [91,92,93]. These changes in functional microbiota explained the phenomenon that CO2 and CH4 emissions were higher in the WGCF regime than in the TG regime.
5. Conclusions
We investigated the differences in CO2 and CH4 emissions from yaks within and between feeding regimes by examining the characteristics of the diversity, composition, and functional microbiota of the yak rumen microbiota to characterize the potential causes of differences in CO2 and CH4 emissions. We found that dietary differences in feeding regimes were crucial factors of variations in yak rumen microbiota. Differences in GHG emissions from yaks were attributed to the enrichment relationship of functional H2- and CO2-producing and hydrogen-consuming microbiota and hydrogenotrophic methanogenic bacteria. The functional microbiota within and between feeding regimes differed, but they belonged to gas-producing, hydrogen-consuming, and hydrogenotrophic methanogenic bacteria. Accordingly, we presumed that strong ecotopic complementarity among members of the rumen microbial community could cause differences in CO2 and CH4 emissions. Combined with GHG emission measurements, rumen microbiome characterization would be a useful screening tool for selecting yaks with low gas emission.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani12212991/s1, Figure S1: Rarefaction curves of the bacteria (a), fungi (b), archaea (c) gene reads based on ASVs. Mantel test revealed the correlation between rumen bacteria (d), fungi (e), archaea (f) and dietary nutrition (ASV level); Table S1: Relative abundance of bacterial phylum levels of experimental groups; Table S2: Relative abundance of fungal phylum levels of experimental groups; Table S3: Relative abundance of archaeal phylum levels of experimental groups; Table S4: Functional genera of the rumen microbiome. References [31,33,36,40,41,78,80,88,89,90,91,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, S.X. and T.X.; methodology, Q.Z. and X.W.; formal analysis, Q.Z.; investigation, Q.Z., T.G., X.W., X.Z. and Y.G.; data curation, H.L., L.H. and N.Z.; writing—original draft preparation, Q.Z.; writing—review and editing, Q.Z., T.G. and S.X.; visualization, Q.Z.; project administration, S.X.; funding acquisition, S.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2021YFD1600205), the Qinghai Province Natural Science Foundation (2019-ZJ-974Q), the Joint fund project of NSFC (U21A20250), the CAS “Light of West China” for Interdisciplinary Innovation Team (CASLWC-2021), the Strategic Priority Research Program of Chinese Academy of Sciences (XDA20050104, XDA23060603).
Institutional Review Board Statement
All animal procedures were performed in accordance with the Institutional Animal Care and Use Committee at the Northwest Institute of Plateau Biology, Chinese Academy of Sciences, Davis under protocol number NWIPB20160302. No endangered or protected animal species were involved in this study and no harm was inflicted on the experimental animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
All raw sequences for this study can be found in the NCBI Sequence Read Archive under BioProject PRJNA874346 with the accession number SUB11978917 (https://dataview.ncbi.nlm.nih.gov/object/PRJNA874346, accessed on 11 October 2022).
Acknowledgments
We would like to thank Quanmin Dong and Huakun Zhou for their active support during the field experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Crippa, M.; Guizzardi, D.; Solazzo, E.; Muntean, M.; Schaaf, E.; Monforti-Ferrario, F.; Banja, M.; Olivier, J.G.J.; Grassi, G.; Rossi, S. GHG Emissions of All World Countries; 2021 Report, EUR 30831 EN; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar]
- Minx, J.C.; Lamb, W.F.; Andrew, R.M.; Canadell, J.G.; Crippa, M.; Döbbeling, N.; Forster, P.M.; Guizzardi, D.; Olivier, J.; Peters, G.P. A comprehensive dataset for global, regional and national greenhouse gas emissions by sector 1970–2019. Earth Syst. Sci. Data 2021, 13, 5213–5252. [Google Scholar] [CrossRef]
- Caro, D.; Davis, S.J.; Bastianoni, S.; Caldeira, K. Global and regional trends in greenhouse gas emissions from livestock. Clim. Chang. 2014, 126, 203–216. [Google Scholar] [CrossRef]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock: A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Godfray, H.C.J.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Lorimer, J.; Pierrehumbert, R.T.; Scarborough, P.; Springmann, M.; Jebb, S.A. Meat consumption, health, and the environment. Science 2018, 361, eaam5324. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Thornton, P.K. Livestock and global change: Emerging issues for sustainable food systems. Proc. Natl. Acad. Sci. USA 2013, 110, 20878–20881. [Google Scholar] [CrossRef]
- Reisinger, A.; Clark, H. How much do direct livestock emissions actually contribute to global warming? Glob. Chang. Biol. 2018, 24, 1749–1761. [Google Scholar] [CrossRef]
- Shafiullah, M.; Khalid, U.; Shahbaz, M. Does meat consumption exacerbate greenhouse gas emissions? Evidence from US data. Environ. Sci. Pollut. Res. 2021, 28, 11415–11429. [Google Scholar] [CrossRef]
- Rogelj, J.; Popp, A.; Calvin, K.V.; Luderer, G.; Emmerling, J.; Gernaat, D.; Fujimori, S.; Strefler, J.; Hasegawa, T.; Marangoni, G. Scenarios towards limiting global mean temperature increase below 1.5 C. Nat. Clim. Chang. 2018, 8, 325–332. [Google Scholar] [CrossRef]
- Havlík, P.; Valin, H.; Herrero, M.; Obersteiner, M.; Schmid, E.; Rufino, M.C.; Mosnier, A.; Thornton, P.K.; Böttcher, H.; Conant, R.T. Climate change mitigation through livestock system transitions. Proc. Natl. Acad. Sci. USA 2014, 111, 3709–3714. [Google Scholar] [CrossRef]
- Herrero, M.; Henderson, B.; Havlík, P.; Thornton, P.K.; Conant, R.T.; Smith, P.; Wirsenius, S.; Hristov, A.N.; Gerber, P.; Gill, M. Greenhouse gas mitigation potentials in the livestock sector. Nat. Clim. Chang. 2016, 6, 452–461. [Google Scholar] [CrossRef]
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Harrigan, T.; Woznicki, S.A. Climate change and livestock: Impacts, adaptation, and mitigation. Clim. Risk Manag. 2017, 16, 145–163. [Google Scholar] [CrossRef]
- Bai, Y.; Guo, C.; Li, S.; Degen, A.A.; Ahmad, A.A.; Wang, W.; Zhang, T.; Huang, M.; Shang, Z. Instability of decoupling livestock greenhouse gas emissions from economic growth in livestock products in the Tibetan highland. J. Environ. Manag. 2021, 287, 112334. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Ge, Y.; Ren, Y.; Fan, X.; Pan, K.; Lin, L.; Wu, X.; Min, Y.; Meyerson, L.A.; Heino, M. A global strategy to mitigate the environmental impact of China’s ruminant consumption boom. Nat. Commun. 2018, 9, 4133. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Ma, W.; Ma, L.; Velthof, G.L.; Wei, Z.; Havlík, P.; Oenema, O.; Lee, M.R.F.; Zhang, F. China’s livestock transition: Driving forces, impacts, and consequences. Sci. Adv. 2018, 4, eaar8534. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Wang, L.; Wang, K.; Yang, Y.; Ma, T.; Wang, Z.; Zhang, X.; Ni, Z.; Hou, F.; Long, R. Yak whole-genome resequencing reveals domestication signatures and prehistoric population expansions. Nat. Commun. 2015, 6, 10283. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Ding, L.; Zhou, J.; Huang, X.; Degen, A.; Long, R. The adaptive strategies of yaks to live in the Asian highlands. Anim. Nutr. 2022, 9, 249–258. [Google Scholar] [CrossRef]
- de Souza Filho, W.; de Albuquerque Nunes, P.A.; Barro, R.S.; Kunrath, T.R.; de Almeida, G.M.; Genro, T.C.M.; Bayer, C.; de Faccio Carvalho, P.C. Mitigation of enteric methane emissions through pasture management in integrated crop-livestock systems: Trade-offs between animal performance and environmental impacts. J. Clean Prod. 2019, 213, 968–975. [Google Scholar] [CrossRef]
- Zubieta, Á.S.; Savian, J.V.; de Souza Filho, W.; Wallau, M.O.; Gómez, A.M.; Bindelle, J.; Bonnet, O.J.F.; de Faccio Carvalho, P.C. Does grazing management provide opportunities to mitigate methane emissions by ruminants in pastoral ecosystems? Sci. Total Environ. 2021, 754, 142029. [Google Scholar] [CrossRef]
- Ouatahar, L.; Bannink, A.; Lanigan, G.; Amon, B. Modelling the effect of feeding management on greenhouse gas and nitrogen emissions in cattle farming systems. Sci. Total Environ. 2021, 776, 145932. [Google Scholar] [CrossRef]
- Berton, M.; Agabriel, J.; Gallo, L.; Lherm, M.; Ramanzin, M.; Sturaro, E. Environmental footprint of the integrated France–Italy beef production system assessed through a multi-indicator approach. Agric. Syst. 2017, 155, 33–42. [Google Scholar] [CrossRef]
- Chen, Z.; An, C.; Fang, H.; Zhang, Y.; Zhou, Z.; Zhou, Y.; Zhao, S. Assessment of regional greenhouse gas emission from beef cattle production: A case study of Saskatchewan in Canada. J. Environ. Manag. 2020, 264, 110443. [Google Scholar] [CrossRef]
- Zhuang, M.; Li, W. Greenhouse gas emission of pastoralism is lower than combined extensive/intensive livestock husbandry: A case study on the Qinghai-Tibet Plateau of China. J. Clean Prod. 2017, 147, 514–522. [Google Scholar] [CrossRef]
- Angerer, V.; Sabia, E.; von Borstel, U.K.; Gauly, M. Environmental and biodiversity effects of different beef production systems. J. Environ. Manag. 2021, 289, 112523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, D.; Wang, L.I.; Hao, J.; Wang, J.; Zhou, X.; Wang, W.; Qiu, Q.; Huang, X.; Zhou, J. Convergent evolution of rumen microbiomes in high-altitude mammals. Curr. Biol. 2016, 26, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Romano, E.; Roma, R.; Tidona, F.; Giraffa, G.; Bragaglio, A. Dairy Farms and Life Cycle Assessment (LCA): The allocation criterion useful to estimate undesirable products. Sustainability 2021, 13, 4354. [Google Scholar] [CrossRef]
- Weiss, F.; Leip, A. Greenhouse gas emissions from the EU livestock sector: A life cycle assessment carried out with the CAPRI model. Agric. Ecosyst. Environ. 2012, 149, 124–134. [Google Scholar] [CrossRef]
- Sykes, A.J.; Topp, C.F.E.; Wilson, R.M.; Reid, G.; Rees, R.M. A comparison of farm-level greenhouse gas calculators in their application on beef production systems. J. Clean Prod. 2017, 164, 398–409. [Google Scholar] [CrossRef]
- Vibart, R.; de Klein, C.; Jonker, A.; Van der Weerden, T.; Bannink, A.; Bayat, A.R.; Crompton, L.; Durand, A.; Eugène, M.; Klumpp, K. Challenges and opportunities to capture dietary effects in on-farm greenhouse gas emissions models of ruminant systems. Sci. Total Environ. 2021, 769, 144989. [Google Scholar] [CrossRef]
- Janssen, P.H. Influence of hydrogen on rumen methane formation and fermentation balances through microbial growth kinetics and fermentation thermodynamics. Anim. Feed Sci. Technol. 2010, 160, 1–22. [Google Scholar] [CrossRef]
- Hess, M.; Sczyrba, A.; Egan, R.; Kim, T.-W.; Chokhawala, H.; Schroth, G.; Luo, S.; Clark, D.S.; Chen, F.; Zhang, T. Metagenomic discovery of biomass-degrading genes and genomes from cow rumen. Science 2011, 331, 463–467. [Google Scholar] [CrossRef]
- Morgavi, D.P.; Kelly, W.J.; Janssen, P.H.; Attwood, G.T. Rumen microbial (meta) genomics and its application to ruminant production. Animals 2013, 7, 184–201. [Google Scholar] [CrossRef]
- Seshadri, R.; Leahy, S.C.; Attwood, G.T.; Teh, K.H.; Lambie, S.C.; Cookson, A.L.; Eloe-Fadrosh, E.A.; Pavlopoulos, G.A.; Hadjithomas, M.; Varghese, N.J. Cultivation and sequencing of rumen microbiome members from the Hungate1000 Collection. Nat. Biotechnol. 2018, 36, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Shabat, S.K.B.; Sasson, G.; Doron-Faigenboim, A.; Durman, T.; Yaacoby, S.; Berg Miller, M.E.; White, B.A.; Shterzer, N.; Mizrahi, I. Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants. ISME J. 2016, 10, 2958–2972. [Google Scholar] [CrossRef] [PubMed]
- Tapio, I.; Snelling, T.J.; Strozzi, F.; Wallace, R.J. The ruminal microbiome associated with methane emissions from ruminant livestock. J. Anim. Sci. Biotechnol. 2017, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Greening, C.; Geier, R.; Wang, C.; Woods, L.C.; Morales, S.E.; McDonald, M.J.; Rushton-Green, R.; Morgan, X.C.; Koike, S.; Leahy, S.C. Diverse hydrogen production and consumption pathways influence methane production in ruminants. ISME J. 2019, 13, 2617–2632. [Google Scholar] [CrossRef]
- Henderson, G.; Cox, F.; Ganesh, S.; Jonker, A.; Young, W.; Janssen, P.H. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 2015, 5, 14567. [Google Scholar] [CrossRef]
- Wolff, S.M.; Ellison, M.J.; Hao, Y.; Cockrum, R.R.; Austin, K.J.; Baraboo, M.; Burch, K.; Lee, H.J.; Maurer, T.; Patil, R. Diet shifts provoke complex and variable changes in the metabolic networks of the ruminal microbiome. Microbiome 2017, 5, 60. [Google Scholar] [CrossRef]
- Zhou, M.; Hernandez-Sanabria, E.; Guan, L.L. Characterization of variation in rumen methanogenic communities under different dietary and host feed efficiency conditions, as determined by PCR-denaturing gradient gel electrophoresis analysis. Appl. Environ. Microbiol. 2010, 76, 3776–3786. [Google Scholar] [CrossRef]
- Pope, P.B.; Smith, W.; Denman, S.E.; Tringe, S.G.; Barry, K.; Hugenholtz, P.; McSweeney, C.S.; McHardy, A.C.; Morrison, M. Isolation of Succinivibrionaceae implicated in low methane emissions from Tammar wallabies. Science 2011, 333, 646–648. [Google Scholar] [CrossRef]
- Li, Q.S.; Wang, R.; Ma, Z.Y.; Zhang, X.M.; Jiao, J.Z.; Zhang, Z.G.; Ungerfeld, E.M.; Yi, K.L.; Zhang, B.Z.; Long, L. Dietary selection of metabolically distinct microorganisms drives hydrogen metabolism in ruminants. ISME J. 2022, 16, 2535–2546. [Google Scholar] [CrossRef]
- Xu, T.; Zhao, N.; Hu, L.; Xu, S.; Liu, H.; Ma, L.; Zhao, X. Characterizing CH4, CO2 and N2O emission from barn feeding Tibetan sheep in Tibetan alpine pastoral area in cold season. Atmos. Environ. 2017, 157, 84–90. [Google Scholar] [CrossRef]
- Ku-Vera, J.C.; Valencia-Salazar, S.S.; Piñeiro-Vázquez, A.T.; Molina-Botero, I.C.; Arroyave-Jaramillo, J.; Montoya-Flores, M.D.; Lazos-Balbuena, F.J.; Canul-Solís, J.R.; Arceo-Castillo, J.I.; Ramírez-Cancino, L. Determination of methane yield in cattle fed tropical grasses as measured in open-circuit respiration chambers. Agric. For. Meteorol. 2018, 258, 3–7. [Google Scholar] [CrossRef]
- Yuan, J.; Xiang, J.; Liu, D.; Kang, H.; He, T.; Kim, S.; Lin, Y.; Freeman, C.; Ding, W. Rapid growth in greenhouse gas emissions from the adoption of industrial-scale aquaculture. Nat. Clim. Chang. 2019, 9, 318–322. [Google Scholar] [CrossRef]
- Benvenutti, M.A.; Gordon, I.J.; Poppi, D.P.; Crowther, R.; Spinks, W.; Moreno, F.C. The horizontal barrier effect of stems on the foraging behaviour of cattle grazing five tropical grasses. Livest. Sci. 2009, 126, 229–238. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Kil, D.Y.; Kong, C.; Kim, B.G. Comparison of oven-drying methods for determination of moisture content in feed ingredients. Asian Australas. J. Anim. Sci. 2014, 27, 1615. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W. Official Methods of Analysis of AOAC International, Agricultural Chemicals, Contaminants, Drugs; Horwitz, W., Ed.; AOAC International: Gaithersburg, Maryland, 2010; Volume 1. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Parks, D.H.; Chuvochina, M.; Rinke, C.; Mussig, A.J.; Chaumeil, P.-A.; Hugenholtz, P. GTDB: An ongoing census of bacterial and archaeal diversity through a phylogenetically consistent, rank normalized and complete genome-based taxonomy. Nucleic Acids Res. 2022, 50, 785–794. [Google Scholar] [CrossRef]
- Ten-Doménech, I.; Moreno-Torres, M.; Castell, J.V.; Quintás, G.; Kuligowski, J. Extracting consistent biological information from functional results of metabolomic pathway analysis using the Mantel’s test. Anal. Chim. Acta 2021, 1187, 339173. [Google Scholar] [CrossRef]
- Capblancq, T.; Luu, K.; Blum, M.G.B.; Bazin, E. Evaluation of redundancy analysis to identify signatures of local adaptation. Mol. Ecol. Resour. 2018, 18, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Liu, Y.-X.; Qin, Y.; Chen, T.; Lu, M.; Qian, X.; Guo, X.; Bai, Y. A practical guide to amplicon and metagenomic analysis of microbiome data. Protein Cell 2021, 12, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, Y.X.; Huang, L. ImageGP: An easy-to-use data visualization web server for scientific researchers. iMeta 2022, 1, e5. [Google Scholar] [CrossRef]
- Fang, L.; Zhou, Z.; Ren, L.; Shi, F.; Can, M.; Chai, S.; Meng, Q. Ruminal bacterial diversity of Yaks (Bos grunniens) fed by grazing or indoor regime on the Tibetan Plateau by analysis of 16S rRNA gene libraries. Ital. J. Anim. Sci. 2015, 14, 3970. [Google Scholar] [CrossRef]
- Huang, X.; Mi, J.; Denman, S.E.; Zhang, Q.; Long, R.; McSweeney, C.S. Changes in rumen microbial community composition in yak in response to seasonal variations. J. Appl. Microbiol. 2022, 132, 1652–1665. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, H.; Liu, S.; Chai, S.; Meng, Q.; Zhou, Z. Dynamic alterations in yak rumen bacteria community and metabolome characteristics in response to feed type. Front. Microbiol. 2019, 10, 1116. [Google Scholar] [CrossRef] [PubMed]
- Furman, O.; Shenhav, L.; Sasson, G.; Kokou, F.; Honig, H.; Jacoby, S.; Hertz, T.; Cordero, O.X.; Halperin, E.; Mizrahi, I. Stochasticity constrained by deterministic effects of diet and age drive rumen microbiome assembly dynamics. Nat. Commun. 2020, 11, 1904. [Google Scholar] [CrossRef] [PubMed]
- Snelling, T.J.; Auffret, M.D.; Duthie, C.-A.; Stewart, R.D.; Watson, M.; Dewhurst, R.J.; Roehe, R.; Walker, A.W. Temporal stability of the rumen microbiota in beef cattle, and response to diet and supplements. Anim. Microbiome 2019, 1, 16. [Google Scholar] [CrossRef]
- Guo, N.; Wu, Q.; Shi, F.; Niu, J.; Zhang, T.; Degen, A.A.; Fang, Q.; Ding, L.; Shang, Z.; Zhang, Z. Seasonal dynamics of diet–gut microbiota interaction in adaptation of yaks to life at high altitude. NPJ Biofilms Microbomes 2021, 7, 38. [Google Scholar] [CrossRef]
- Bergier, I.; Silva, A.P.S.; de Abreu, U.G.P.; de Oliveira, L.O.F.; Tomazi, M.; Dias, F.R.T.; Urbanetz, C.; Nogueira, É.; Borges-Silva, J.C. Could bovine livestock intensification in Pantanal be neutral regarding enteric methane emissions? Sci. Total Environ. 2019, 655, 463–472. [Google Scholar] [CrossRef]
- Alemu, A.W.; Doce, R.R.; Dick, A.C.; Basarab, J.A.; Kröbel, R.; Haugen-Kozyra, K.; Baron, V.S. Effect of winter feeding systems on farm greenhouse gas emissions. Agric. Syst. 2016, 148, 28–37. [Google Scholar] [CrossRef]
- Zhuang, M.; Zhang, J.; Li, W. Community-based seasonal movement grazing maintains lower greenhouse gas emission intensity on Qinghai-Tibet Plateau of China. Land Use Pol. 2019, 85, 155–160. [Google Scholar] [CrossRef]
- Ding, X.Z.; Long, R.J.; Kreuzer, M.; Mi, J.D.; Yang, B. Methane emissions from yak (Bos grunniens) steers grazing or kept indoors and fed diets with varying forage: Concentrate ratio during the cold season on the Qinghai-Tibetan Plateau. Anim. Feed Sci. Technol. 2010, 162, 91–98. [Google Scholar] [CrossRef]
- Martínez-Mota, R.; Kohl, K.D.; Orr, T.J.; Denise Dearing, M. Natural diets promote retention of the native gut microbiota in captive rodents. ISME J. 2020, 14, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Baniel, A.; Amato, K.R.; Beehner, J.C.; Bergman, T.J.; Mercer, A.; Perlman, R.F.; Petrullo, L.; Reitsema, L.; Sams, S.; Lu, A.; et al. Seasonal shifts in the gut microbiome indicate plastic responses to di et in wild geladas. Microbiome 2021, 9, 26. [Google Scholar] [CrossRef]
- Xu, C.; Liu, W.; Sun, B.; Zhang, S.; Zhang, S.; Yang, Y.; Lei, Y.; Chang, L.; Xie, P.; Suo, H. Multi-Omics analysis reveals a dependent relationship between rumen bacteria and diet of grass-and grain-fed yaks. Front. Microbiol. 2021, 12, 642959. [Google Scholar] [CrossRef]
- Pereira, A.M.; de Lurdes Nunes Enes Dapkevicius, M.; Borba, A.E.S. Alternative pathways for hydrogen sink originated from the ruminal fermentation of carbohydrates: Which microorganisms are involved in lowering methane emission? Anim. Microbiome 2022, 4, 5. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Zhang, Q.; Difford, G.; Sahana, G.; Løvendahl, P.; Lassen, J.; Lund, M.S.; Guldbrandtsen, B.; Janss, L. Bayesian modeling reveals host genetics associated with rumen microbiota jointly influence methane emission in dairy cows. ISME J. 2020, 14, 2019–2033. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Q.; Song, J.; Xin, J.; Zhang, S.; Lei, Y.; Yang, Y.; Xie, P.; Suo, H. Comparison of gut microbiota of yaks from different geographical regions. Front. Microbiol. 2021, 12, 666940. [Google Scholar] [CrossRef]
- Hu, C.; Ding, L.; Jiang, C.; Ma, C.; Liu, B.; Li, D.; Degen, A.A. Effects of management, dietary intake, and genotype on rumen morphology, fermentation, and microbiota, and on meat quality in yaks and cattle. Front. Nutr. 2021, 8, 755255. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.R.; Stephens, W.Z.; Stagaman, K.; Wong, S.; Rawls, J.F.; Guillemin, K.; Bohannan, B.J.M. Contribution of neutral processes to the assembly of gut microbial communities in the zebrafish over host development. ISME J. 2016, 10, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Shi, Q.; Sun, R.; Liang, D.; Li, Y.; Li, Y.; Jin, W.; Zhu, W. The biotechnological potential of anaerobic fungi on fiber degradation and methane production. World J. Microbiol. Biotechnol. 2018, 34, 155. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meng, Z.; Xu, Y.; Shi, Q.; Ma, Y.; Aung, M.; Cheng, Y.; Zhu, W. Interactions between anaerobic fungi and methanogens in the rumen and their biotechnological potential in biogas production from lignocellulosic materials. Microorganisms 2021, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Choudhury, P.K.; Carro, M.D.; Griffith, G.W.; Dagar, S.S.; Puniya, M.; Calabro, S.; Ravella, S.R.; Dhewa, T.; Upadhyay, R.C. New aspects and strategies for methane mitigation from ruminants. Appl. Microbiol. Biotechnol. 2014, 98, 31–44. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Li, Y.; Cheng, Y.; Zhu, W. The enrichment of anaerobic fungi and methanogens showed higher lignocellulose degrading and methane producing ability than that of bacteria and methanogens. World J. Microbiol. Biotechnol. 2020, 36, 125. [Google Scholar] [CrossRef]
- Edwards, J.E.; Forster, R.J.; Callaghan, T.M.; Dollhofer, V.; Dagar, S.S.; Cheng, Y.; Chang, J.; Kittelmann, S.; Fliegerova, K.; Puniya, A.K.; et al. PCR and Omics based techniques to study the diversity, ecology and biology of anaerobic fungi: Insights, challenges and opportunities. Front. Microbiol. 2017, 8, 1657. [Google Scholar] [CrossRef]
- Belanche, A.; Kingston-Smith, A.H.; Griffith, G.W.; Newbold, C.J. A multi-kingdom study reveals the plasticity of the rumen microbiota in response to a shift from non-grazing to grazing diets in sheep. Front. Microbiol. 2019, 10, 122. [Google Scholar] [CrossRef]
- Mauerhofer, L.-M.; Reischl, B.; Schmider, T.; Schupp, B.; Nagy, K.; Pappenreiter, P.; Zwirtmayr, S.; Schuster, B.; Bernacchi, S.; Seifert, A.H.; et al. Physiology and methane productivity of Methanobacterium thermaggregans. Appl. Microbiol. Biotechnol. 2018, 102, 7643–7656. [Google Scholar] [CrossRef]
- Palevich, N.; Kelly William, J.; Leahy Sinead, C.; Denman, S.; Altermann, E.; Rakonjac, J.; Attwood Graeme, T. Comparative genomics of rumen Butyrivibriospp. uncovers a continuum of polysaccharide-degrading capabilities. Appl. Environ. Microbiol. 2019, 86, e01993-19. [Google Scholar] [CrossRef]
- Lin, B.; Henderson, G.; Zou, C.; Cox, F.; Liang, X.; Janssen, P.H.; Attwood, G.T. Characterization of the rumen microbial community composition of buffalo breeds consuming diets typical of dairy production systems in Southern China. Anim. Feed Sci. Technol. 2015, 207, 75–84. [Google Scholar] [CrossRef]
- Sheflin, A.M.; Melby, C.L.; Carbonero, F.; Weir, T.L. Linking dietary patterns with gut microbial composition and function. Gut Microbes 2017, 8, 113–129. [Google Scholar] [CrossRef]
- Huang, X.D.; Tan, H.Y.; Long, R.; Liang, J.B.; Wright, A.-D.G. Comparison of methanogen diversity of yak (Bos grunniens) and cattle (Bos taurus) from the Qinghai-Tibetan plateau, China. BMC Microbiol. 2012, 12, 237. [Google Scholar] [CrossRef] [PubMed]
- Wallace, R.J.; Rooke, J.A.; McKain, N.; Duthie, C.-A.; Hyslop, J.J.; Ross, D.W.; Waterhouse, A.; Watson, M.; Roehe, R. The rumen microbial metagenome associated with high methane production in cattle. BMC Genom. 2015, 16, 839. [Google Scholar] [CrossRef] [PubMed]
- Kamke, J.; Kittelmann, S.; Soni, P.; Li, Y.; Tavendale, M.; Ganesh, S.; Janssen, P.H.; Shi, W.; Froula, J.; Rubin, E.M.; et al. Rumen metagenome and metatranscriptome analyses of low methane yield sheep reveals a Sharpea-enriched microbiome characterised by lactic acid formation and utilisation. Microbiome 2016, 4, 56. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.Q.; Yang, H.J.; Luan, Y.; Long, R.J.; Wu, Y.J.; Wang, Z.Y. Isolation, identification and fibrolytic characteristics of rumen fungi grown with indigenous methanogen from yaks (Bos grunniens) grazing on the Qinghai-Tibetan Plateau. J. Appl. Microbiol. 2016, 120, 571–587. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, R.; Dicksved, J.; Sun, L.; Gonda, H.; Müller, B.; Schnürer, A.; Bertilsson, J. Methane production in dairy cows correlates with rumen methanogenic and bacterial community structure. Front. Microbiol. 2017, 8, 226. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, R.; Wang, M.; Zhang, X.; Mao, H.; Tan, Z. Short communication: Variability in fermentation end-products and methanogen communities in different rumen sites of dairy cows. J. Dairy Sci. 2018, 101, 5153–5158. [Google Scholar] [CrossRef]
- Wang, K.; Nan, X.; Chu, K.; Tong, J.; Yang, L.; Zheng, S.; Zhao, G.; Jiang, L.; Xiong, B. Shifts of hydrogen metabolism from methanogenesis to propionate production in response to replacement of forage fiber with non-forage fiber sources in diets in vitro. Front. Microbiol. 2018, 9, 2764. [Google Scholar] [CrossRef]
- Ndeh, D.; Rogowski, A.; Cartmell, A.; Luis, A.S.; Baslé, A.; Gray, J.; Venditto, I.; Briggs, J.; Zhang, X.; Labourel, A.; et al. Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature 2017, 544, 65–70. [Google Scholar] [CrossRef]
- Solden, L.M.; Naas, A.E.; Roux, S.; Daly, R.A.; Collins, W.B.; Nicora, C.D.; Purvine, S.O.; Hoyt, D.W.; Schückel, J.; Jørgensen, B.; et al. Interspecies cross-feeding orchestrates carbon degradation in the rumen ecosystem. Nat. Microbiol. 2018, 3, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.D.; Auffret, M.D.; Warr, A.; Walker, A.W.; Roehe, R.; Watson, M. Compendium of 4,941 rumen metagenome-assembled genomes for rumen microbiome biology and enzyme discovery. Nat. Biotechnol. 2019, 37, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.Y.; Wu, J.J.; Xie, Y.Y.; Zhu, S.L.; Zhong, Y.F.; Liu, J.X.; Sun, H.Z. Investigation of fiber utilization in the rumen of dairy cows based on metagenome-assembled genomes and single-cell RNA sequencing. Microbiome 2022, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Jin, W.; Si, H.Z.; Yuan, Y.; Tao, Y.; Liu, J.H.; Wang, X.X.; Yang, C.J.; Li, Q.S.; Yan, X.T.; et al. An integrated gene catalog and over 10,000 metagenome-assembled genome s from the gastrointestinal microbiome of ruminants. Microbiome 2021, 9, 137. [Google Scholar] [CrossRef]
- Weimer, P.J. Why don’t ruminal bacteria digest cellulose faster? J. Dairy Sci. 1996, 79, 1496–1502. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Jin, W.; Mao, S.Y.; Zhu, W.Y. Production of Citrate by Anaerobic fungi in the Presence of Co-culture Methanogens as Revealed by 1H NMR Spectrometry. Asian-Australas J. Anim. Sci. 2013, 26, 1416–1423. [Google Scholar] [CrossRef]
- Cunha, C.S.; Marcondes, M.I.; Veloso, C.M.; Mantovani, H.C.; Pereira, L.G.R.; Tomich, T.R.; Dill-McFarland, K.A.; Suen, G. Compositional and structural dynamics of the ruminal microbiota in dairy heifers and its relationship to methane production. J. Sci. Food Agric. 2019, 99, 210–218. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.H.; Chen, Y.X.; Cheng, Z.H.; Duan, Q.H.; Meng, Q.H.; Tao, X.P.; Shang, B.; Dong, H.M. Age-Related response of rumen microbiota to mineral salt and effects of their interactions on enteric methane emissions in cattle. Microb. Ecol. 2017, 73, 590–601. [Google Scholar] [CrossRef]
- Aguilar-Marin, S.B.; Betancur-Murillo, C.L.; Isaza, G.A.; Mesa, H.; Jovel, J. Lower methane emissions were associated with higher abundance of rumin al Prevotella in a cohort of Colombian buffalos. BMC Microbiol. 2020, 20, 364. [Google Scholar] [CrossRef]
- Wang, S.; Huang, H.; Kahnt, J.; Thauer, R.K. A reversible electron-bifurcating ferredoxin- and NAD-dependent [FeFe]-hydrogenase (HydABC) in Moorella thermoacetica. J. Bacteriol. 2013, 195, 1267–1275. [Google Scholar] [CrossRef]
- Bauchop, T.; Mountfort, D.O. Cellulose Fermentation by a rumen anaerobic fungus in both the absence and the presence of rumen methanogens. Appl. Environ. Microbiol. 1981, 42, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, W.; Cheng, Y.; Zhu, W. Effect of the associated methanogen methanobrevibacter thaueri on the dynamic profile of end and intermediate metabolites of anaerobic fungus piromyces sp. F1. Curr. Microbiol. 2016, 73, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Comtet-Marre, S.; Parisot, N.; Lepercq, P.; Chaucheyras-Durand, F.; Mosoni, P.; Peyretaillade, E.; Bayat, A.R.; Shingfield, K.J.; Peyret, P.; Forano, E. Metatranscriptomics reveals the active bacterial and eukaryotic fibrolytic communities in the rumen of dairy cow fed a mixed diet. Front. Microbiol. 2017, 8, 67. [Google Scholar] [CrossRef]
- Liang, J.; Zheng, W.; Zhang, H.; Zhang, P.; Cai, Y.; Wang, Q.; Zhou, Z.; Ding, Y. Transformation of bacterial community structure in rumen liquid anaerobic digestion of rice straw. Environ. Pollut. 2021, 269, 116130. [Google Scholar] [CrossRef] [PubMed]
- Ozbayram, E.G.; Kleinsteuber, S.; Nikolausz, M.; Ince, B.; Ince, O. Bioaugmentation of anaerobic digesters treating lignocellulosic feedstock by enriched microbial consortia. Eng. Life Sci. 2018, 18, 440–446. [Google Scholar] [CrossRef]
- Youssef, N.H.; Couger, M.B.; Struchtemeyer, C.G.; Liggenstoffer, A.S.; Prade, R.A.; Najar, F.Z.; Atiyeh, H.K.; Wilkins, M.R.; Elshahed, M.S. The genome of the anaerobic fungus Orpinomyces sp. strain C1A reveals the unique evolutionary history of a remarkable plant biomass degrader. Appl. Environ. Microbiol. 2013, 79, 4620–4634. [Google Scholar] [CrossRef]
- Hagen, L.H.; Brooke, C.G.; Shaw, C.A.; Norbeck, A.D.; Piao, H.; Arntzen, M.Ø.; Olson, H.M.; Copeland, A.; Isern, N.; Shukla, A.; et al. Proteome specialization of anaerobic fungi during ruminal degradation of recalcitrant plant fiber. ISME J. 2020, 5, 421–434. [Google Scholar] [CrossRef]
- Dai, X.; Tian, Y.; Li, J.T.; Su, X.Y.; Wang, X.W.; Zhao, S.G.; Liu, L.; Luo, Y.F.; Liu, D.; Zheng, H.J.; et al. Metatranscriptomic analyses of plant cell wall polysaccharide degradation by microorganisms in the cow rumen. Appl. Environ. Microbiol. 2014, 81, 1375–1386. [Google Scholar] [CrossRef]
- Hirakata, Y.; Hatamoto, M.; Oshiki, M.; Watari, T.; Araki, N.; Yamaguchi, T. Food selectivity of anaerobic protists and direct evidence for methane production using carbon from prey bacteria by endosymbiotic methanogen. ISME J. 2020, 14, 1873–1885. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).