Simple Summary
C-reactive protein is a major positive acute phase protein in dogs. It is commonly used as a marker of inflammation that, although nonspecific, is highly sensitive. The high clinical value lies in its rapid response and relatively short half-life time; these qualities make the C-reactive protein a good therapeutic guide; among others, it can be used to determine when an antimicrobial therapy could be ceased. Various tests are available on the market and the measurement is becoming a part of routine biochemistry blood panels in many countries. Although it is very useful, especially in conjunction with white blood cell count or other acute phase response proteins measurements, it does not allow a complete evaluation as a single parameter.
Abstract
Acute phase response is a nonspecific reaction to disturbances in homeostasis during which the production of some Acute Phase Proteins (APPs) is stimulated; they are sensitive but nonspecific markers of systemic inflammatory processes. The major positive APP in dogs is the C-reactive protein (CRP). The dynamic of its concentration changes fast, rising and decreasing rapidly with the onset and removal of the inflammatory stimulus. It increases within the first 4–24 h after the stimulus and reaches up to a 50–100-fold increase of the baseline level. It has been documented that this APP’s concentration is elevated during several diseases, such as pyometra, panniculitis, acute pancreatitis, polyarthritis, sepsis, immune-mediated hemolytic anemia, and neoplasia in dogs. In clinical practice, canine CRP is mostly measured to detect and monitor systemic inflammatory activity and the efficacy of treatments, because it is a more sensitive marker than shifts in leukocyte counts. Blood serum CRP concentration is becoming a part of routine biochemistry panels in many countries. In this article, changes in CRP concentration and its clinical application in healthy and diseased dogs are discussed.
1. Introduction
Biomarkers are widely discussed in veterinary medicine, mostly those that can be easily measured in blood. However, finding the most sensitive and specific one is often impossible, especially in inflammatory disorders. The acute phase response (APR) is the initial nonspecific systemic reaction induced by disturbances of homeostasis such as injuries, infection, neoplasia, and other pathologies (Figure 1). After such stimulation, the release of acute phase proteins (APPs, mostly from the liver to the bloodstream) occurs. Thus, the evaluation of their blood concentrations changes seems to be a good, real-time indicator of ongoing systemic inflammation.
Figure 1.
Factors inducing acute phase response, including CRP.
APPs are proteins whose concentration changes a minimum 25% [1,2] in response to inflammatory cytokines (e.g., IL-1, IL-6, TNFα); they are considered positive or negative, depending on whether the concentration rises or decreases, respectively. In addition, based on their magnitude, they are classified as major (APPs that increase 10–1000-fold, reaching a peak 24–48 h after the insult), moderate (5–10-fold, peaking around 48–72 h after insult), and minor (less than 2-fold increase). APPs share several anti-inflammatory and immunomodulatory functions such as the promotion of phagocytosis, induction of cytokine production, inhibition of chemotaxis, and modulation of neutrophil function, among others [1,2].
The changes in APPs are species-specific. C-reactive protein (CRP) is a major positive APP in dogs, and it is the most sensitive APP in this species [3]. Canine CRP has a molecular weight of approximately 100 kDa and consists of 5 subunits with an apparent molecular weight (MW) of approximately 20 kDa each [4]. It is mostly produced after proinflammatory stimulation by cytokines such as IL-1, IL-6, and tumor necrosis factor-α (TNF-α) [5]. The blood concentration of CRP changes dramatically within 4–6 h after the inflammatory stimulus and reaches its maximum concentration after about 24–48 h, which in most cases reflects the physical degree of tissue trauma. Some of the CRP’s biological functions are shown in Figure 2.
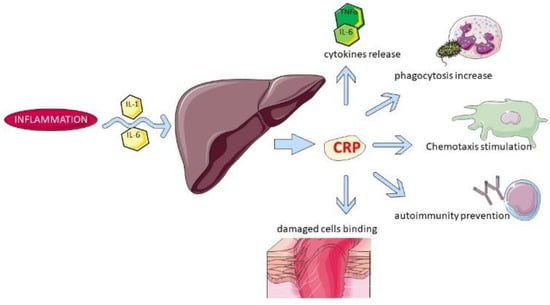
Figure 2.
Some of the biological functions of CRP.
An increase in CRP should not be considered a marker of etiologic diagnosis due to its low specificity. However, it is highly sensitive and has great diagnostic value in the presence of subclinical disease. CRP’s increase in several inflammatory or non-inflammatory conditions [6,7,8,9,10,11,12] is shown in Table 1. Thus, such an assay should be taken into consideration when deciding on early treatment and can guide the recovery.

Table 1.
CRP concentration values have been recorded in various studies.
2. Clinical Resources Available in Veterinary Medicine
There are various methods to measure CRP, including ELISA, immunoturbidometric assays, latex agglutination, and a time-resolved immunofluorometric assay [5]. Only validated assays should be used for the measurement of all APPs because of the cross-reactivity of the used antibodies and different limits of detection (LOD), especially when different body fluids are evaluated (ex. blood vs. synovial fluid) [26]. Assays available in veterinary medicine are summarized in Table 2.

Table 2.
A comparison of CRP assays validated in veterinary medicine. Abbreviations: POCT—Point of Care Testing.
The immunoturbidimetric assay is the most commonly used in clinical laboratories when rapid results are needed to measure the CRP blood level in dogs. Due to its higher precision compared to ELISA assays, it is also commonly used for research projects. The samples can be stored for later analysis, as CRP is resistant to −10 °C for up to 4 months of storage [39] and even longer in lower temperatures [34].
In most clinical veterinary laboratories, only blood serum is recommended for routine CRP analysis. Various anticoagulants can also be used, but they may distort the result of the measurement. In one study, values for CRP were significantly lower in samples with trisodium citrate than with serum (30%), whereas there were no differences when lithium heparin or tripotassium ethylenediamine tetraacetic acid (EDTA K3) were used [40]. EDTA and citrate both remove calcium to prevent coagulation while heparin acts instead by binding to antithrombin III, which suggests that these mechanisms are not responsible for the observed disturbances and so far, the reason is unknown. However, the levels of CRP in samples used for this anticoagulant study were very low; thus, it should be interpreted with caution. On the other hand, human studies showed no difference in CRP serum and plasma concentration) [41].
According to several studies various values are considered the upper point value of the reference interval (RI): <5 μg/mL [1,42], <10 μg/mL [43], 0.22–4.04 μg/mL [44], 0.8–16.4 μg/mL [45], 8.4 ± 4.6 μg/mL [37], 0.48 ± 0.17 μg/mL [46]. Therefore, RIs reported in the literature are variable, however, the reported ranges are not extremely wide (the URLs vary between approx. 5 and approx. 20 μg/mL). The possible sources of this variation among studies or laboratories are based on the method, protocol, reagent and anticoagulant, and instruments storage, as previously discussed [9]. The reading may also be affected by lipemia, hemolysis, and bilirubinemia when ELISA is used, but it is not observed in immunoturbidimetric assays [2,29]. Especially, the measurement may vary to the clinically critical extent when polyclonal human antibodies are used, and the machinery is calibrated with human CRP. Such practice requires batch-to-batch validation since different cross-reactivity can be expected in various batches [47].
Although highly accurate, species-specific validated assays are available worldwide, CRP should never be interpreted as a single parameter. Some laboratories include CRP as a part of biochemistry panels, but the Seven Points Plan, published in 2008 and updated in 2019 [48], recommends a broader APPs profile that includes major positive, major negative, and moderate acute phase proteins to be implemented because of the high diagnostic value of their divergence. As an example, the authors explain that a simultaneous increase in haptoglobin, accompanied by a normal level of CRP, could suggest a possible diagnosis of hyperadrenocorticism. Another major part of an evaluation of an inflammatory process is the white blood cell count (WBC); but APPs are more stable than WBC [48]. WBC shifts even when inflammation is not involved. Neutrophilia and lymphopenia can be a result of the inhibition of margination, and it is well known that stress and glucocorticoid therapy can induce both [49]. When in doubt, even the measurement of CRP alone can help determine the underlying cause of such shifts.
3. CRP Concentrations in Healthy Dogs
Compared with other markers, small changes in CRP over time may be relevant for diagnostic or prognostic purposes. It is best recommended to obtain baseline CRP concentration in a healthy individual to then compare with further results [50]; this is the most adequate approach due to the wide reference values and individual differences. However, it is rarely done in veterinary medicine. In clinical practice, baseline CRP is a part of routine biochemistry panels in many countries, but it is not the worldwide standard yet.
In countries where it is not included, it should be recommended to run it in addition; in cases where the value was not additionally requested to be recorded before the potential sickness, the most practical recommendation is that the patient’s health status can still be monitored through repeated measurements of CRP over time during the follow-ups, to assess possible changes compared with the initial value (which also should be compared to the population-based RI).
There may exist some breed-related differences. It was documented that in clinically healthy Miniature Schnauzers (MS) [51] (median 4.0 μg/mL, minimum–maximum 0–18.2 μg/mL), CRP was found to be higher than in non-MS of the study (median 0.1 μg/mL, minimum–maximum 0–10.7 μg/mL). It is unclear whether the baseline serum CRP concentration could predict or be correlated with hyperlipidemia and pancreatitis in MS dogs, to which they are prone, but an association between hypertriglyceridemia and pancreatitis is well documented in humans [52].
No changes in CRP concentration were observed over day and night circadian rhythms [44]. Shifts are noticeable in pregnant bitches, peaking at a 2–4-fold increase (70–90 μg/mL), 30–45 days after the ovulation [53]. Even though the concentration drops after the peak, it remains elevated until the parturition [54]. So far, no evidence suggests differences in CRP levels as dogs age. However, in one study, where dogs were inoculated with turpentine oil, dogs 1 month of age showed a maximum 15- fold CRP rise, while 3 months and older increased the value up to 26-fold [55]. Therefore, CRP might not be diagnostic in canines younger than 3 months, because the response to inflammation is significantly lower than in those below 3 months of age
4. Physical Exercise
Physical activity is associated with a change in APPs concentrations in different species [56] and is connected with duration and type of exercise. In a study focused on the influence of short-duration exercise in racing Greyhounds, a decrease in CRP concentration was found, whereas other APPs levels increased in 2 h after the exercise (e.g., serum amyloid A and haptoglobin) [57]. In German Shepherds, CRP was measured right before and 6 h after the exercise; showing a lower than 1-fold increase (shift from 40.69 ± 16.48 μg/mL to 45.00 ± 13.37 μg/mL) meaning the change was statistically insignificant [58]. Disturbances in glucose homeostasis, acid-base, and electrolyte balance were suggested as the underlying causes of the multi-level reaction of the organism.
Other authors measured CRP concentrations over a shorter period in hunting dogs. With the exercise’s duration of 3 h, the assay was performed immediately before (T0), immediately after (T1), and one hour after it was over (T2) [1]. A significant 4-fold rise was observed (from 1 μg/mL (T0) to 4.5 μg/mL (T1)) in comparison to no changes at T0, T1, and T2 in the control group. Notably, in this study, the CRP value 1 h after the workout has already decreased to the same value as T0. It was discussed by the authors that the overall APPs rise could have to do with subclinical arthritis in these animals, but the white blood cells (WBC) count performed before and after the exercise showed no variability, which likely excludes such a possibility. The CRP increase may be connected with the exercise APR-like response, which was documented in other species [56].
The fact that endurance exercise-induced changes in CRP might be indistinguishable from those caused by an actual inflammatory response was proven by another study [57,58,59]. Sled dogs are elite athletes and can endure days running. Candidates who showed elevated WBC count and/or rectal temperature above 39.4 °C were excluded from the study; 12 healthy, mixed-breed dogs ran 557 km over 4–5 days. Testing 48 h before the workout began and within 20 min of completing it, over a 10-time fold increase was observed (rising from a mean of 22.4 ± 16.3 µg/mL to 263.3 ± 103.8 µg/mL). CRP concentration was also measured in sled dogs completing a 1650-km race and significant changes were observed: CRP (median [range]) start: 18 μg/mL [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58]; midpoint: 119 76 μg/mL [12,13,14,15,16,17,18,19]; finish: 60 μg/mL [12,13,14,15,16,17,60]. In another study, performed on 65 sled dogs, it was documented that CRP concentration increased after a training session and a long-distance race [61]. There, it was confirmed that CRP level was elevated in both finishing and non-finishing dogs. Thus, this APP cannot indicate a dog’s readiness for race. On the other hand, in sled dogs with rhabdomyolysis, CRP level was 2-fold elevated in comparison to the healthy animals (308 vs. 164 μg/mL) [62].
Thus, changes in CRP concentration may be a useful marker for monitoring the physical adaptation in the competing dogs, especially in endurance training. More studies are necessary to establish the patterns of interpretation of the results, however, since the half-life time of CRP is 19 h [63], a clinician should not be surprised by a higher value in an athlete patient, despite the lack of inflammatory disease. Vice versa, whenever an athlete canine requires medical attention midst or shortly after exercise or competition, a dramatic elevation of CRP is to be expected regardless of the emergency.
In conclusion, serum CRP should not be used to determine which animal is capable of physical effort. It is additionally supported by the evidence that degenerative joint diseases do not increase this parameter in the bloodstream [26].
5. Bacterial and Viral Etiology Diseases
Bacterial infections can produce the most intense inflammatory processes, and, alongside anaphylaxis, can result in distributive shock resulting in the death of the affected animal [64]. In human medicine, there used to be an outdated generalization that high CRP blood concentration is a marker of somewhat specificity for bacterial infections. Similarly in veterinary medicine, septic pneumonia would be linked to levels above 100 μg/mL in dogs [65]; this approach has shifted in human medicine, and it has been shown that the parameter is rather used to reduce the number of antibiotic treatments prescribed in patients with acute cough [66].
For reference, in a study including 147 dogs with CRP concentrations greater than 100 ug/mL, 86/147 dogs had an associated inflammation, but only 33/147 (22%) of them had a confirmed bacterial infection and 11 dogs had a viral infection. No statistically significant differences were found between infected dogs and any other group (animals with trauma, neoplasia, etc.) [65]. Thus, extremely high CRP concentrations do not allow a simple conclusion of the underlying etiology or identification of bacterial inflammation and cannot be used to excuse the administration of an antibiotic. The authors also concluded that CRP does not rise in an organ-specific manner, nor is it specific for infectious diseases.
In another recent study, such high values of CRP concentration have not been observed. However, a comparison between dogs with sepsis and trauma showed a significantly higher increase in the first group [67]. Additionally, the evaluation of CRP at point-of-admission was a reliable prognostic marker. Similarly in a different publication, although it concluded that sepsis cannot be differentiated from aseptic Systemic Inflammatory Response Syndrome (SIRS) based on CRP, in both cases, a rapid decrease of CRP, rather than initial concentration, was a good prognostic marker [68]. In addition, higher mean CRP concentrations were found in dogs with SIRS or sepsis (165 ± 82 μg/mL) than in those with localized inflammation (108 ± 70 μg/mL) [8].
CRP proves to be useful in guiding antibiotic therapy and allows one to determine when it should be discontinued. An in-depth analysis was run regarding, among other parameters, CRP levels accompanying various respiratory diseases, including the infectious [69]. In a total of 106 dogs, the 22 diagnosed with bacterial pneumonia experienced a significantly higher rise in CRP than dogs with other respiratory diseases (bacterial tracheobronchitis, idiopathic pulmonary fibrosis, chronic bronchitis, cardiogenic pulmonary edema, eosinophilic bronchopneumopathy). In dogs with bacterial pneumonia, the serum CRP was successfully used to guide the duration of antibiotic treatment and it was stopped 5–7 days after the CRP normalization [70]; this finding supports the idea that the APPs evaluation could be used in support of the therapeutic approach (i.e., the need to use the antibiotic or supportive treatment) in veterinary medicine as well to minimalize the risk of creating resistant bacterial strains.
In experimental conditions, where the exact inoculation time is set, more precise diagnostic criteria could be established. CRP concentrations as high as 720 μg/mL (mean 478 μg/mL) were noted 24 h after intratracheal inoculation with Bordetella bronchiseptica and later continuously dropped until day 20 [13]. In the dogs that were inoculated again on day 36, the initial peak was not as high. Clinical symptoms, such as coughing, started 1–3 days after the first inoculation, and until day 5, all the dogs showed CRP > 100 μg/mL. In contrast, a comparison of CRP concentrations in dogs with Bordetella bronchiseptica infection and aspiration bronchopneumonia was described by other researchers in a retrospective study [10]. In all of the dogs CRP was elevated. However, such dramatic CRP elevation was not recorded at all, in fact, in the aspiration bronchopneumonia group, the increase was significantly higher (20 vs. 118 μg/mL). The findings of both those studies show that patients cannot be classified as affected by bacterial pneumonia only based on the CRP values being higher than 100 μg/mL and respiratory symptoms; this would a severe conceptual mistake; as results from different studies and laboratories cannot be compared to each other, or because the criteria to name a disease are always based on the clinical presentation, diagnostic imaging, and in the case of septic conditions also on culture and of course also on laboratory results; these, however, with rare exceptions, may provide ancillary information and do not work alone as a diagnostic criterion. In addition, dogs with other airway diseases such as upper respiratory diseases may not experience a rise in CRP at all (e.g., rhinitis) [9].
In parvoviral enteritis, serum CRP concentration was associated with the prognosis in puppies [3]. During this study, CRP levels at admission and 12 and 24 h later were positively associated with the odds ratio and negatively associated with the survival time. Regarding differences between survivors and non-survivors, sensitivity and specificity of CRP concentration at 24 h after admission were 86.7% and 78.7%, respectively.
In conclusion, an increase in CRP concentration may only suggest the ongoing infection and encourage the clinician to order a culture and an antibiogram. Further diagnostic techniques such as susceptibility testing and targeting of pathogen(s) should be performed as often as possible because of the increasing global problem with antibiotic resistance.
6. Parasitic Etiology Diseases
The dynamics of CRP changes in many parasitic diseases have been extensively investigated. Especially Babesia invasions were explored, where it has been used to study the pathogenicity of the disease, to understand its diagnostic or prognostic role, or to assess responses to treatments and the severity of the disease. In 50 dogs with Babesia canis, the highest CRP concentration noted prior to imidocarb treatment exceeded 200 μg/mL [16].
In cases of leishmaniasis, no correlation seems to exist between CRP concentrations and antibody levels, but symptomatic dogs show higher CRP than the asymptomatic [14]. The finding that canine leishmaniasis doesn’t always result in changes in WBC is very important in a clinical setting, where CRP could be the only parameter suggesting an inflammation caused by the parasite [48]. However, when it comes to the treatment of this disorder, the CRP changes should be interpreted with caution [71]. Similarly, Neospora caninum invasion could influence APR leading to an increase in the concentration of APPs [12]. Some alteration was noted after Toxocara canis invasion [72], as well as in canine monocytic ehrlichiosis [73]. CRP is also observed to rise significantly in demodicosis [10]. Interestingly, in Trypanosoma brucei infections, CRP rose very slowly and did not peak until days 7–10 (>160 μg/mL) [15].
CRP has been researched in dogs with filariasis to differentiate those infected by different Filarioidea species, to differentiate dogs with latent infection from dogs with overt disease, dogs with complications (e.g., vena cava syndrome, thromboembolism, pulmonary hypertension) from dogs without complications. Several species of filarial worms such as Dirofilaria immitis may induce the 1–4-fold increase of CRP in dogs (69.9 μg/mL) whereas others do not, e.g., Brugia pahangi (12.9 μg/mL) [17].
In a dog suffering from Anaplasma phagocytophilum infection, the CRP rise was correlated with upcoming periods of immune-mediated polyarthritis (IMPA) symptoms. CRP values were also negatively correlated with corticosteroid treatment doses. CRP dropped 3-fold during the initial high dose 30-day prednisolone treatment (1–2 mg/kg/day). CRP rose again when the prednisolone dose was lowered to 0.5 mg/kg/day, and shortly the next symptomatic period occurred [74]; this leads to the conclusion that CRP could be used to predict the symptomatic periods in such diseases and also as a guide when establishing the lowest effective dose.
7. Surgery
Regardless of whether the injury of the tissue was caused by a surgical procedure or by accident, CRP can be expected to increase within 24 h as a result of the event alone [75,76]. CRP may be used for early detection of post-surgical complications, disregarding the type, length, or site of the surgery, before their clinical manifestation [77]. Regarding routine, less invasive procedures, the ovariohysterectomy (OH) in bitches did not influence the CRP level at all [78]. In another study, OH performed by laparoscopy induced a smaller CRP response than by laparotomy (2-fold) and the overall response was very mild. In both groups, CRP concentration started dropping after the 24 h. In the same study, dogs who underwent vasectomy showed barely any CRP response [79]. In beagles undergoing orchidectomy and treated with amoxicillin and carprofen, a 17-fold increase in the first 24 h post-surgery was noted (reaching over 100 μg/mL) [80]. The degree of severity of trauma does not always match the CRP level, but research proves that when the surgery is performed by an inexperienced surgeon, then the prolonged duration is the most likely reason for such inconsistency [81,82].
Various anesthetic protocols do not seem to affect post-surgical CRP levels [82,83]. In addition, single CRP measurements are of limited value regarding post-surgical inflammation and prognosis [84]. We believe that this claim can be extended beyond surgical procedures.
8. Orthopedic Diseases
In cases of lameness, CRP evaluation can facilitate differential diagnosis between immune-mediated or septic polyarthritis and other conditions that do not produce changes in CRP, such as intravertebral disc protrusion [9] as well paraplegia secondary to acute intervertebral disc extrusion [85]. Patellar luxation often requires surgery but does not cause severe trauma or inflammation by itself, as otherwise healthy dogs expecting surgery showed physiological CRP concentrations. Twenty-four hours after the surgery a 2–6-fold rise (median 92 μg/mL) was noted [86]. CRP may be a nonspecific biomarker for discospondylitis—but probably more sensitive than fever, leukocytosis, neutrophilia, and hyperglobulinemia. However, there is no association with the positive bacterial culture [87]. In 14/16 cases in dogs with bacterial discospondylitis the level of CRP was elevated (100.7 μg/mL), in contrast to 12 dogs with pyrexia and 6 experiencing leukocytosis [87]. Thus, it is still unclear if CRP can be used to differentiate between septic and non-septic articular diseases, or to guide clinical management (e.g., the necessity of surgery, after which it is also expected to rise).
9. Autoimmune Diseases
CRP increases in symptomatic (i.e., associated with SIRS) immune-mediated conditions, independent of the type of disease (e.g., immune-mediated hemolytic anemia—IMHA, immune-mediated thrombocytopenia—IMTP). Acute onset of IMHA is a life-threatening event. Evidence suggests it is accompanied by SIRS [88], which is supported by the high CRP findings in these dogs [9,20]. In most cases, dogs with IMHA display high values of CRP (143 ± 89 μg/mL) on the day of admission, which normalizes with treatment [11]. There is no significant difference in CRP levels in dogs with IMHA, IMTP, and immune-mediated polyarthritis (IMPA) [20]. In addition, CRP concentrations are almost 1-fold lower in dogs with IMHA receiving corticosteroids, but no correlation was found between initial CRP levels and survival until discharge.
CRP increases in Steroid Responsive Meningitis-Arteritis (SRMA) but not in meningoencephalitides of unknown origin, although the magnitude of increase in SRMA is similar to that of other inflammatory diseases. In a group of autoimmune diseases of the brain, meningoencephalitides of unknown origin, CRP often stays within reference values as systemic inflammation does not take place [89] in opposite to steroid-responsive meningitis arthritis (SRMA), where CRP rise is observed [21,90,91]. It is a valuable finding since the differential diagnosis between these two often requires a costly MRI scan, but SRMA-caused blood CRP shift is indistinguishable from one caused by systemic inflammatory disease [22].
CRP might be useful to differentiate between pemphigus foliaceous and superficial pyoderma, but only in more severe cases [92]. However, it is not a useful prognostic or treatment efficacy biomarker for canine atopic dermatitis [32]. Thus, the common conclusion of several studies is that CRP, in addition, alone or in addition to other diagnostic tests, may theoretically help differentiate between some potential diagnoses, regardless of disease types, the severity of clinical conditions, and the presence of complications (e.g., overwhelming bacterial dermatitis). CRP’s role in autoimmune diseases protection and prevention is also being investigated [93].
10. Neoplasia
The increase of CRP in patients with tumors (both in humans and in veterinary medicine) does not depend on the direct action of the tumor. Rather, CRP increases as a consequence of the secondary inflammatory/immune-mediated stimuli. In support of this hypothesis, it is widely demonstrated by dozens of studies (especially in humans) that increases in CRP are associated with increased staging, metastatization, or paraneoplastic complications (e.g., ulcers, immune depression, and so on). A study on a small population concluded that very often, there is no influence of the neoplastic condition on CRP concentration [65]. However, an extensive research paper published in 2020 shows that CRP concentrations in patients with immune-mediated and neoplastic diseases rise most frequently of all and to the greatest extent [9]. Hemangiosarcoma, nasal adenocarcinoma, cholangiocellular carcinoma, acute lymphoblastic leukemia, malignant histiocytosis, lymphoma, malignant mesothelioma, and intestinal adenocarcinoma were found to significantly increase CRP concentration. Of these cases, hematopoietic tumors might provoke the greatest elevation. In addition, disseminated tumors tend to cause higher spikes of CRP than localized tumors, which may not raise CRP at all (e.g., leiomyosarcoma, mammary gland tumors) [9,94]. In dogs with multicentric lymphoma, low, physiological CRP level was shown to be related to obtaining remission [33]. Furthermore, a correlation between high CRP and malignancy was suggested. In stage I, II, III, and IV of mammary tumors in bitches, a significant rise (approx. ten-fold) was observed only in stage IV. It was hypothesized that unless there is a metastasis or the tumor is greater than 5 cm in diameter, the lesions might be too poor of a stimulus for APPs production. It is unknown why stage IV showed higher concentrations than V [94].
However, the change in CRP concentration should only be an ancillary tool to predict the outcome, the response to treatment and the invasiveness of the tumor as well as to monitor over time the response to treatments and it cannot replace diagnostic imaging.
11. Other Diseases
Generally, in inflammatory bowel disease (IBD), CRP rises periodically along with symptoms and drops after successful prednisolone and metronidazole treatment, regardless of the organ affected [95]. No differences in CRP concentrations were found in dogs with IBD and other chronic gastrointestinal diseases among 51 dogs [23]. However, in a very recent study, the clinical value of CRP as indicative of duodenal histopathologic severity marker was evaluated and a positive correlation with canine IBD assessment index (CIBDAI) was confirmed [24].
Canine acute pancreatitis is a common disease in veterinary practice; however, the clinical presentations vary from subclinical to severe and life-threatening. Thus, there are differences between studies, and they may be due to the patient selection and inclusion criteria (e.g., different types/origins of pancreatitis, different phase, or duration of the disease and so on) and ultimately to the presence or absence (and severity) of SIRS. In 16 dogs with spontaneous acute pancreatitis, CRP measured on the day of admission was 2–5-fold higher and did not return to RI on day 5 [25]. Other authors report CRP within RI in dogs with pancreatitis, possibly due to a longer, subclinical period before the presentation [96]. In a recent study, it was suggested that serum canine pancreatic lipase immunoreactivity (cPLI) which is the most commonly used marker for pancreatitis confirmation, together with CRP could be used as objective biomarkers for clinical improvement in hospitalized dogs (8). In addition, a meta-analysis of 31 canine, rodent, and human studies provides evidence indicating the beneficial effect of corticosteroids on circulating CRP levels and hospitalization, whose administration during a pancreatitis episode is sometimes controversial [97].
CRP was found to be higher in dogs displaying pyrexia, regardless of the underlying cause (median 140 μg/mL) in 825 dogs, but with no linear correlation. Similarly, higher WBC count (>17,500) was correlated with higher CRP (385 dogs, median 38 μg/mL) and with no linear correlation as well. CRP was higher in dogs with low plasma albumin concentrations (albumin < 26 μg/mL, 128 dogs with median 50 μg/mL) than those with the higher value (408 dogs, median 8 μg/mL) [9]. Thus, CRP correlates with positive (fever) and negative (albuminemia) indicators of inflammation.
CRP was also investigated as a potential marker that would allow differentiating pyometra from cystic endometrial hyperplasia (CEH) [6]. Higher CRP values were expected to be reached in those with pyometra, as the underlying cause is septic, opposite to CEH. CEH is also not accompanied by systemic inflammation that often develops in pyometra. Indeed, the mean values were 3–4-fold higher for pyometra (200.28 ± 93.51 μg/mL and 53.51 ± 66.24 μg/mL for CEH). However, the values overlap.
Acute abdomen syndrome (AAS) is a group of nonspecific symptoms that may be caused by many diseases. In a study consisting of 32 dogs admitted to a clinic with AAS [96], 21 showed elevated CRP upon presentation. No correlation was found between CRP level and the later made diagnosis (e.g., pyometra, acute pancreatitis, gastric ulceration, etc.). None of the dogs whose CRP was within RI died. One dog with splenic hemangiosarcoma died (CRP 16.6 μg/mL) and among 6 euthanized dogs CRP ranged from 25 μg/mL to 202.5 μg/mL. In all dogs who survived until the second assay in 48–72 h but were euthanized/died later, CRP dropped from initial values. CRP also dropped to the greatest extent in dogs who died, rather than in survivors. In dogs with gastric dilatation, gastric volvulus and urolithiasis CRP was within the normal range, but other authors report a significant increase in CRP concentration 24 and 48 h after the gastric dilatation-volvulus surgery [98]. Thus, it may be concluded that CRP increases with the severity of the condition, although the rapid decrease over a short time may carry a negative prognostic value and should be taken into consideration by clinicians as well as researchers.
12. Conclusions
One of the most important conclusions drawn from our review is that canine CRP has a short half-life time and rises very shortly after the initial inflammatory factor affects homeostasis, which is consistent with current knowledge [2,16]. Generally, high CRP concentrations do not allow a conclusion of the underlying etiology or identification of bacterial inflammation [65]. Changes in CRP concentration in most of the reviewed studies have been summarized in Table 1.
CRP is widely used in veterinary medicine and increases in dogs when the disease is severe and associated with SIRS, independent of the type (primary inflammation or inflammation associated with other diseases). However, retrospective studies, even when big populations are evaluated, might have a limited value as the patients were not necessarily in the same stage of disease at the time of presentation, but were presented when the owner found it necessary—especially when neoplasia was involved. Regarding infectious diseases, it can seldom be assumed that the animal was naïve to the pathogen prior to the ongoing infection.
To obtain the full picture of the CRP dynamics, it would probably be necessary to perform future research in a controlled environment, where the studied condition would be artificially induced, and CRP be measured in specified time intervals. An ethical question would have to be answered if the potential benefits of such research outweigh the animal suffering caused by the condition. Based on our review, the answer is probably negative, as CRP is never crucial to obtain the diagnosis.
Some of the research suggests that it is not the CRP’s absolute value, but the decrease in time that might be a good prognostic factor, especially in life-threatening events, such as sepsis [68]. However, an unusually fast decrease might be a negative prognostic marker, since in dogs with acute abdomen those who experienced it died [96]. Nonetheless, the dynamics of CRP values, based on multiple measurements until recovery, are probably of much higher value as a guiding factor rather than a single measurement, often performed at admission and not repeated, when it is treated as an additional and less valuable diagnostic marker. Additionally, monitoring the concentration of CRP over time during the follow-up may allow differentiating responsive from non-responsive dogs, with the aforementioned exception.
Furthermore, we suggest that CRP cannot be evaluated as an isolated parameter or be the only diagnostic assay performed, and it should be complementary to good diagnostic practice and not a cheaper alternative to such. More extensive APPs profiles and multiple tests through the treatment should be recommended for the detection of inflammatory diseases.
Author Contributions
K.M. and O.W.-P.—contributed to the conception and design of the work; data analysis and interpretation, and drafting the manuscript. O.W.-P. supervised the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The Figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Casella, S.; Fazio, F.; Russo, C.; Giudice, E.; Piccione, G. Acute phase proteins response in hunting dogs. J. Vet. Diagn. Investig. 2013, 25, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Ceron, J.J.; Eckersall, P.D.; Martinez-Subiela, S. Acute Phase Proteins in dogs and cats: Current knowledge and future perspectives. Vet. Clin. Pathol. 2005, 34, 85–99. [Google Scholar] [CrossRef] [PubMed]
- McClure, V.; van Schoor, M.; Thompson, P.N.; Kjelgaard-Hansen, M.; Goddard, A. Evaluation of the use of serum C-reactive protein concentration to predict outcome in puppies infected with canine parvovirus. J. Am. Vet. Med. Assoc. 2013, 243, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Jasensky, A.K.; Bondzio, A.; Murugaiyan, J.; Siebert, U.; Roesler, U.; Kohn, B.; Einspanier, R. Characterization of the native C-reactive protein (cCRP) and the corresponding liver mRNA in dogs. Biochem. Biophys. Res. Commun. 2014, 452, 462–467. [Google Scholar] [CrossRef]
- Paul, C.; Hansson, L.-O.; Seierstad, S.L.; Kriz, K. Canine C-Reactive Protein—A Clinical Guide, 1st ed.; LifeAssays AB: Lund, Sweden, 2011; p. 3. [Google Scholar]
- Fransson, B.A.; Karlstam, E.; Bergstrom, A.; Lagerstedt, A.S.; Park, J.S.; Evans, M.A.; Ragle, C.A. C-reactive Protein in the Differentiation of Pyometra From Cystic Endometrial Hyperplasia/Mucometra in Dogs. J. Am. Anim. Hosp. Assoc. 2004, 40, 391–399. [Google Scholar] [CrossRef]
- Keany, K.M.; Fosgate, G.T.; Perry, S.M.; Stroup, S.T.; Steiner, J.M. Serum concentrations of canine pancreatic lipase immunoreactivity and C-reactive protein for monitoring disease progression in dogs with acute pancreatitis. J. Vet. Intern. Med. 2021, 35, 2187–2195. [Google Scholar] [CrossRef]
- Torrente, C.; Manzanilla, E.G.; Bosch, L.; Fresno, L.; Rivera Del Alamo, M.; Andaluz, A.; Saco, Y.; Ruiz de Gopegui, R. Plasma iron, C-reactive protein, albumin, and plasma fibrinogen concentrations in dogs with systemic inflammatory response syndrome. J. Vet. Emerg. Crit. Care 2015, 25, 611–619. [Google Scholar] [CrossRef]
- Nakamura, M.; Takahashi, M.; Ohno, K.; Koshino, A.; Nakashima, K.; Setoguchi, A.; Fujino, Y.; Tsujimoto, H. C-Reactive Protein Concentration in Dogs with Various Diseases. J. Vet. Med. Sci. 2008, 70, 127–131. [Google Scholar] [CrossRef]
- Canonne, A.M.; Menard, M.; Maurey, C.; Benchrekroun, G.; Fernandes Rodrigues, N.; Billen, F.; Clecx, C. Comparison of C-reactive protein concentrations in dogs with Bordetella bronchiseptica infection and aspiration bronchopneumonia. J. Vet. Intern. Med. 2021, 35, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Griebsch, C.; Arndt, G.; Raila, J.; Schweigert, F.J.; Kohn, B. C-reactive protein concentration in dogs with primary immune-mediated hemolytic anemia. Vet. Clin. Pathol. 2009, 38, 421–425. [Google Scholar] [CrossRef]
- Ferreira, R.F.; Dittrich, R.L.; Zimmermann, I.B.; Ljubic, B.B.; Mrljak, V.; Eckersall, P.D. Differential acute-phase protein responses in dogs seropositive or seronegative for Neospora caninum. Parasitol. Res. 2021, 120, 3529–3535. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Shida, T.; Honda, M.; Ashida, Y.; Rikihisa, Y.; Odakura, M.; Hayashi, S.; Nomura, M.; Isayama, Y. Serum C-reactive protein and immune responses in dogs inoculated with Bordetella bronchiseptica (phase I cells). Vet. Res. Commun. 1994, 18, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Subiela, S.; Tecles, F.; Eckersall, P.D.; Cerón, J.J. Serum concentrations of acute phase proteins in dogs with leishmaniasis. Vet. Rec. 2002, 150, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Ndung’u, J.M.; Eckersall, P.D.; Jennings, F.W. Elevation of the concentration of acute phase proteins in dogs infected with Trypanosoma brucei. Acta Trop. 1991, 49, 77–86. [Google Scholar] [CrossRef]
- Matijatko, V.; Mrljak, V.; Kis, I.; Kucer, N.; Forsek, J.; Zivicnjak, T.; Romić, Z.; Simec, Z.; Ceron, J.J. Evidence of an acute phase response in dogs naturally infected with Babesia canis. Vet. Parasitol. 2007, 144, 242–250. [Google Scholar] [CrossRef]
- Asawakarn, S.; Sirisawadi, S.; Kunnasut, N.; Kamkong, P.; Taweethavonsawat, P. Serum protein profiles and C-reactive protein in natural canine filariasis. Vet. World 2021, 14, 860–864. [Google Scholar] [CrossRef]
- Nye, G.; Liebel, F.X.; Harcourt-Brown, T. C-reactive protein in dogs with suspected bacterial diskospondylitis: 16 cases (2010-2019). Vet. Rec. Open 2020, 7, e000386. [Google Scholar] [CrossRef]
- Mitchell, K.D.; Kruth, S.A.; Wood, R.D.; Jefferson, B. Serum acute phase protein concentrations in dogs with autoimmune hemolytic anemia. J. Vet. Intern. Med. 2009, 23, 585–591. [Google Scholar] [CrossRef]
- Grobman, M.; Outi, H.; Rindt, H.; Reinero, C. Serum Thymidine Kinase 1, Canine-C-Reactive Protein, Haptoglobin, and Vitamin D Concentrations in Dogs with Immune-Mediated Hemolytic Anemia, Thrombocytopenia, and Polyarthropathy. J. Vet. Intern. Med. 2017, 31, 1430–1440. [Google Scholar] [CrossRef]
- Zilli, J.; Olszewska, A.; Farke, D.; Schmidt, M.J. Successful surgical and medical treatment of a severe, acute epidural bleed in a young dog due to steroid responsive meningitis-arteritis. Acta Vet. Scand. 2021, 63, 27. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, C.; Monreal, L.; Cerón, J.; Pastor, J.; Viu, J.; Añor, S. Fibrinolytic activity in cerebrospinal fluid of dogs with different neurological disorders. J. Vet. Intern. Med. 2012, 26, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- McCann, T.; Ridyard, A.E.; Simpson, J.W. Evaluation of the Utility of C-Reactive Protein in the Diagnosis of Chronic Gastrointestinal Disease in Dogs. In Proceedings of the British Small Animal Veterinary Congress, Birmingham, England, 3–6 April 2008. [Google Scholar]
- Lee, J.H.; Kim, H.S.; Lee, D.; Yun, T.; Koo, Y.; Chae, Y.; Kang, J.H.; Kang, B.T.; Yang, M.P.; Kim, H. Clinical signs, duodenal histopathological grades, and serum high-mobility group box 1 concentrations in dogs with inflammatory bowel disease. J. Vet. Intern. Med. 2021, 35, 2205–2214. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.L.; Rozanski, L.; Freeman, L.M.; Webster, C.R.L. C-reactive protein concentrations in canine acute pancreatitis. J. Vet. Emerg. Crit. Care 2004, 14, 183–186. [Google Scholar] [CrossRef]
- Boal, S.; Carreira, M.L. Serum and synovial fluid C-reactive protein level variations in dogs with degenerative joint disease and their relationships with physiological parameters. Vet. Res. Commun. 2015, 39, 163–169. [Google Scholar] [CrossRef]
- Kjelgaard-Hansen, M.; Kristensen, A.T.; Jensen, A.L. Evaluation of a commercially available enzyme-linked immunosorbent assay (ELISA) for the determination of C-reactive protein in canine serum. J. Vet. Med. Ser. A 2003, 50, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Covin, M.A.; Gomez, R.R.; Suchodolski, J.S.; Steiner, J.M.; Lidbury, J.A. Analytical validation of a point-of-care test and an automated immunoturbidimetric assay for the measurement of canine C-reactive protein in serum. Can. J. Vet. Res. 2021, 85, 285–292. [Google Scholar]
- Hillström, A.; Hagman, R.; Tvedten, H.; Kjelgaard-Hansen, M. Validation of a commercially available automated canine-specific immunoturbidimetric method for measuring canine C-reactive protein. Vet. Clin. Pathol. 2014, 43, 235–243. [Google Scholar] [CrossRef]
- Hindenberg, S.; Klenner-Gastreich, S.; Kneier, N.; Zielinsky, S.; Gommeren, K.; Bauer, N.; Moritz, A. Evaluation of a species-specific C-reactive protein assay for the dog on the ABX Pentra 400 clinical chemistry analyzer. BMC Vet. Res. 2017, 13, 146. [Google Scholar] [CrossRef] [PubMed]
- Jasensky, A.K.; Klenner, S.; Einspanier, R.; Kohn, B. Evaluation of three different point-of-care tests for quantitative measurement of canine C-reactive protein. Vet. Clin. Pathol. 2015, 44, 205–214. [Google Scholar] [CrossRef]
- Favrot, C.; Fischer, N.; Rostaher, A.; Olivry, T. Evaluation of Plasma C-Reactive Protein as a Biomarker in Dogs with Atopic -Dermatitis Receiving Allergen-Specific Immunotherapy: A Pilot Study. Schweiz. Arch. Tierheilkd. 2021, 163, 67–72. [Google Scholar] [CrossRef]
- Nielsen, L.; Toft, N.; Eckersall, P.D.; Mellor, D.J.; Morris, J.S. Serum C-reactive protein concentration as an indicator of remission status in dogs with multicentric lymphoma. J. Vet. Intern. Med. 2007, 21, 1231–1236. [Google Scholar] [CrossRef]
- Kjelgaard-Hansen, M.; Jensen, A.L.; Kristensen, A.T. Evaluation of a commercially available human C-reactive protein (CRP) turbidometric immunoassay for determination of canine serum CRP concentration. Vet. Clin. Pathol. 2003, 32, 81–87. [Google Scholar] [CrossRef]
- Klenner, S.; Bauer, N.; Moritz, A. Evaluation of three automated human immunoturbidimetric assays for the detection of C-reactive protein in dogs. J. Vet. Diagn. Investig. 2010, 22, 544–552. [Google Scholar] [CrossRef]
- Hindenberg, S.; Keßler, M.; Zielinsky, S.; Langenstein, J.; Moritz, A.; Bauer, N. Evaluation of a novel quantitative canine species-specific point-of-care assay for C-reactive protein. BMC Vet. Res. 2018, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Shida, T.; Okimura, T.; Otabe, K.; Honda, M.; Ashida, Y.; Furukawa, E.; Sarikaputi, M.; Naiki, M. Determination of C-reactive protein in serum and plasma from healthy dogs and dogs with pneumonia by ELISA and slide reversed passive latex agglutination test. Vet. Q. 1994, 16, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Tagata, K.; Yokoyama, S.; Ginbo, T.; Honda, M.; Okimura, T.; Odakura, M.; Nomura, M.; Yamamoto, S. Quantitative capillary reversed passive latex agglutination test for C-reactive protein (CRP) in the dog. Vet. Res. Commun. 1996, 20, 21–30. [Google Scholar] [CrossRef]
- Riley, R.F.; Zontine, W. Further observations on the properties of dog C-reactive protein and the C-reactive protein response in the dog. J. Lab. Clin. Med. 1972, 80, 698–703. [Google Scholar]
- Martínez-Subiela, S.; Cerón, J.J. Effects of hemolysis, lipemia, hyperbilirrubinemia, and anticoagulants in canine C-reactive protein, serum amyloid A, and ceruloplasmin assays. Can. Vet. J. 2005, 46, 625–629. [Google Scholar]
- Dossus, L.; Becker, S.; Achaintre, D.; Kaaks, R.; Rinaldi, S. Validity of multiplex-based assays for cytokine measurements in serum and plasma from “non-diseased” subjects: Comparison with ELISA. J. Immunol. Methods 2009, 31, 125–132. [Google Scholar] [CrossRef]
- Yamashita, K.; Fujinaga, T.; Miyamoto, T. Canine acute phase response: Relationship between serum cytokine activity and acute phase protein in dogs. J. Vet. Med Sci. 1994, 56, 487–492. [Google Scholar] [CrossRef]
- Eckersall, P.D.; Conner, J.G.; Parton, H. An enzyme-linked immunosorbent assay for canine C-reactive protein. Vet. Rec. 1989, 124, 490–491. [Google Scholar] [CrossRef]
- Martínez-Subiela, S.; Ginel, P.; Ceron, J.J. Effects of different glucocorticoid treatments on serum acute phase proteins in dogs. Vet. Rec. 2004, 154, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Otabe, K.; Sugimoto, T.; Jinbo, T.; Honda, M.; Kitao, S.; Hayashi, S.; Shimizu, M.; Yamamoto, S. Physiological levels of C-reactive protein in normal canine sera. Vet. Rec. Commun. 1998, 22, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Tagata, K.; Nagahata, H.; Ishikawa, Y.; Morimatsu, M.; Naiki, M. Isolation of canine Creactive protein and characterization of its properties. Vet. Immunol. Immunopathol. 1992, 30, 329–339. [Google Scholar] [CrossRef]
- Kjelgaard-Hansen, M. Comments on measurement of C-reactive protein in dogs. Vet. Clin. Pathol. 2010, 39, 402–403. [Google Scholar] [CrossRef] [PubMed]
- Cerón, J.J. Acute phase proteins, saliva and education in laboratory science: An update and some reflections. BMC Vet. Res. 2019, 15, 197. [Google Scholar] [CrossRef]
- Hekman, J.P.; Karas, A.Z.; Sharp, C.R. Psychogenic Stress in Hospitalized Dogs: Cross Species Comparisons, Implications for Health Care, and the Challenges of Evaluation. Animals 2014, 4, 331–347. [Google Scholar] [CrossRef]
- Carney, P.C.; Ruaux, C.G.; Suchodolski, J.S.; Steiner, J.M. Biological variability of C-reactive protein and specific canine pancreatic lipase immunoreactivity in apparently healthy dogs. J. Vet. Intern. Med. 2011, 25, 825–830. [Google Scholar] [CrossRef]
- Wong, V.M.; Kidney, B.A.; Snead, E.C.; Myers, S.L.; Jackson, M.L. Serum C-reactive protein concentrations in healthy Miniature Schnautzer dogs. Vet. Clin. Pathol. 2011, 40, 380–383. [Google Scholar] [CrossRef]
- Gan, S.I.; Edwards, A.L.; Symonds, C.J.; Beck, P.L. Hypertriglyceridemia-induced pancreatitis: A case-based review. World J. Gastroenterol. 2006, 12, 7197–7202. [Google Scholar] [CrossRef]
- Kuribayashi, T.; Shimad, T.; Matsumoto, M.; Kawato, K.; Honjyo, T.; Fukuyama, M.; Yamamoto, Y.; Yamamoto, S. Determination of serum C-reactive protein (CRP) in healthy beagle dogs of various ages and pregnant beagle dogs. Exp. Anim. 2003, 52, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kotani, K. Perinatal veterinary medicine-related evaluation in hematological and serum biochemical profiles of experimental beagles throughout pregnancy and parturition. Anim. Model. Exp. Med. 2018, 1, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Jinbo, T.; Iguchi, K.; Shimizu, M.; Shimada, T.; Nomura, M.; Ishida, Y.; Yamamoto, S. A Comparison of the Concentrations of C-reactive Protein and a1-Acid Glycoprotein in the Serum of Young and Adult Dogs with Acute Inflammation. Vet. Res. Commun. 2001, 25, 117–126. [Google Scholar] [CrossRef]
- Witkowska-Piłaszewicz, O.; Bąska, P.; Czopowicz, M.; Żmigrodzka, M.; Szczepaniak, J.; Szarska, E.; Winnicka, A. Changes in Serum Amyloid A (SAA) Concentration in Arabian Endurance Horses During First Training Season. Animals 2019, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Tharwat, M.; Al-Sobayil, F.; Buczinski, S. Influence of racing on the serum concentrations of acute-phase proteins and bone metabolism biomarkers in racing greyhounds. Vet. J. 2014, 202, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Goldírová, K.; Fialkovičová, M.; Benková, M.; Tóthová, C.; Harčárová, M. The Influence of Short Duration Exercise on the Concentration of C-Reactive Protein and Selected Haematological and Biochemical Parameters in the Blood of German Shepherd Dogs. FoliaVet 2017, 61, 35–43. [Google Scholar] [CrossRef][Green Version]
- Wakshlag, J.J.; Stokol, T.; Geske, S.M.; Greger, C.E.; Angle, C.T.; Gillette, R.L. Evaluation of exercise-induced changes in concentrations of C-reactive protein and serum biochemical values in sled dogs completing a long-distance endurance race. Am. J. Vet. Res. 2010, 71, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Yazwinski, M.; Milizio, J.G.; Wakshlag, J.J. Assessment of serum myokines and markers of inflammation associated with exercise in endurance racing sled dogs. J. Vet. Intern. Med. 2013, 27, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Fergestad, M.E.; Jahr, T.H.; Krontveit, R.I.; Skancke, E. Serum concentration of gastrin, cortisol and C-reactive protein in a group of Norwegian sled dogs during training and after endurance racing: A prospective cohort study. Acta Vet. Scand. 2016, 58, 24. [Google Scholar] [CrossRef]
- Frye, C.W.; Mann, S.; Joseph, J.L.; Hansen, C.; Sass, B.; Wakshlag, J.J. Serum Biochemistry and Inflammatory Cytokines in Racing Endurance Sled Dogs With and Without Rhabdomyolysis. Front. Vet. Sci. 2018, 5, 145. [Google Scholar] [CrossRef]
- Coventry, B.J.; Ashdown, M.L.; Quinn, M.A.; Markovic, S.N.; Yatomi-Clarke, S.L.; Robinson, A.P. CRP identifies homeostatic immune oscillations in cancer patients: A potential treatment targeting tool? J. Transl. Med. 2009, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- The Many Types of Shock. Available online: https://www.cliniciansbrief.com/article/many-types-shock-hypovolemic-cardiogenic-distributiveobstructive-hypoxic-metabolic (accessed on 9 October 2022).
- Hindenberg, S.; Bauer, N.; Moritz, A. Extremely high canine C-reactive protein concentrations > 100 mg/L—Prevalence, etiology and prognostic significance. BMC Vet. Res. 2020, 16, 147. [Google Scholar] [CrossRef] [PubMed]
- Cals, J.W.; Ebell, M.H. C-reactive protein: Guiding antibiotic prescribing decisions at the point of care. Br. J. Gen. Pract. 2018, 68, 112–113. [Google Scholar] [CrossRef] [PubMed]
- Ruggerone, B.; Scavone, D.; Troìa, R.; Giunti, M.; Dondi, F.; Paltrinieri, S. Comparison of Protein Carbonyl (PCO), Paraoxonase-1 (PON1) and C-Reactive Protein (CRP) as Diagnostic and Prognostic Markers of Septic Inflammation in Dogs. Vet. Sci. 2021, 8, 93. [Google Scholar] [CrossRef]
- Gebhardt, C.; Hirschberger, J.; Rau, S.; Arndt, G.; Krainer, K.; Schweigert, F.J.; Brunnberg, L.; Kaspers, B.; Kohn, B. Use of C-reactive protein to predict outcome in dogs with systemic inflammatory response syndrome or sepsis. J. Vet. Emerg. Crit. Care 2009, 19, 450–458. [Google Scholar] [CrossRef]
- Viitanen, S.J.; Laurila, H.P.; Lilja-Maula, L.I.; Melamies, M.A.; Rantala, M.; Rajamäki, M.M. Serum C-Reactive Protein as a Diagnostic Biomarker in Dogs with Bacterial Respiratory Diseases. J. Vet. Intern. Med. 2014, 28, 84–91. [Google Scholar] [CrossRef]
- Viitanen, S.J.; Lappalainen, A.K.; Christensen, M.B.; Sankari, S.; Rajamäki, M.M. The Utility of Acute-Phase Proteins in the Assessment of Treatment Response in Dogs With Bacterial Pneumonia. J. Vet. Intern. Med. 2017, 31, 124–133. [Google Scholar] [CrossRef]
- Paltrinieri, S.; Ibba, F.; Barbè, F.; Rossi, G. Influence of domperidone supplementation on short-term changes in C-reactive protein and paraoxonase-1 in dogs with leishmaniasis undergoing meglumine antimoniate and allopurinol therapy. Vet. Clin. Pathol. 2020, 49, 618–623. [Google Scholar] [CrossRef]
- Zheng, W.B.; Zou, Y.; He, J.J.; Liu, G.H.; Hu, M.H.; Zhu, X.Q. Proteomic alterations in the plasma of Beagle dogs induced by Toxocara canis infection. J. Proteom. 2021, 232, 104049. [Google Scholar] [CrossRef]
- Mylonakis, M.E.; Ceron, J.J.; Leontides, L.; Siarkou, V.I.; Martinez, S.; Tvarijonaviciute, A.; Koutinas, A.F.; Harrus, S. Serum acute phase proteins as clinical phase indicators and outcome predictors in naturally occurring canine monocytic ehrlichiosis. J. Vet. Intern. Med. 2011, 25, 811–817. [Google Scholar] [CrossRef]
- Kjelgaard-Hansen, M.; Jensen, A.L.; Houser, G.A.; Jessen, L.R.; Kristensen, A.T. Use of serum C-reactive protein as an early marker of inflammatory activity in canine type II immune-mediated polyarthritis: Case report. Acta Vet. Scand. 2006, 48, 9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freeman, L.J.; Rahmani, E.Y.; Sherman, S.; Chiorean, M.; Selzer, D.J.; Constable, P.D.; Snyder, P.W. Oophorectomy by natural orifice transluminal endoscopic surgery: Feasibility study in dogs. Gastrointest. Endosc. 2009, 69, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Nevill, B.; Leisewitz, A.; Goddard, A.; Thompson, P. An evaluation of changes over time in serum creatinine kinase activity and C-reactive protein concentration in dogs undergoing hemilaminectomy or ovariohysterectomy. J. South Afr. Vet. Assoc. 2010, 81, 22–26. [Google Scholar] [CrossRef][Green Version]
- Löfqvist, K.; Kjelgaard-Hansen, M.; Nielsen, M.B.M. Usefulness of C-reactive protein and serum amyloid A in early detection of postoperative infectious complications to tibial plateau leveling osteotomy in dogs. Acta Vet. Scand. 2018, 60, 30. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Bae, H.; Kim, J.; Kim, S.; Park, J.; Kim, S.K.; Jung, D.I.; Yu, D. Comparison of clinical and inflammatory parameters in dogs with pyometra before and after ovariohysterectomy. Can. J. Vet. Res. 2021, 85, 271–278. [Google Scholar] [PubMed]
- Kjelgaard-Hansen, M.; Strom, H.; Mikkelsen, L.F.; Eriksen, T.; Jensen, A.L.; Luntang-Jensen, M. Canine serum C-reactive protein as a quantitative marker of the inflammatory stimulus of aseptic elective soft tissue surgery. Vet. Clin. Pathol. 2013, 42, 342–345. [Google Scholar] [CrossRef]
- Tvarijonaviciute, A.; Martínez-Subiela, S.; Carrillo-Sanchez, J.D.; Tecles, F.; Ceron, J.J. Effects of Orchidectomy in Selective Biochemical Analytes in Beagle Dogs. Reprod. Domest. Anim. 2011, 46, 957–963. [Google Scholar] [CrossRef]
- Michelsen, J.; Heller, J.; Wills, F.; Noble, G.K. Effect of surgeon experience on postoperative plasma cortisol and C-reactive protein concentrations after ovariohysterectomy in the dog: A randomised trial. Aust. Vet. J. 2012, 90, 474–478. [Google Scholar] [CrossRef]
- Christensen, M.B.; Eriksen, T.; Kjelgaard-Hansen, M. C-reactive protein: Quantitative marker of surgical trauma and post-surgical complications in dogs: A systematic review. Acta Vet. Scand. 2015, 57, 71. [Google Scholar] [CrossRef]
- Sibanda, S.; Hughes, J.M.; Pawson, P.E.; Kelly, G.; Bellenger, C.R. The effects of preoperative extradural bupivacaine and morphine on the stress response in dogs undergoing femoro-tibial joint surgery. Vet. Anaesth. Analg. 2009, 36, 246–257. [Google Scholar] [CrossRef]
- Saunders, A.B.; Hanzlicek, A.S.; Martinez, E.A.; Stickney, M.J.; Steiner, J.M.; Suchodolski, J.S.; Fosgate, G.T. Assessment of cardiac troponin I and C-reactive protein concentrations associated with anesthetic protocols using sevoflurane or a combination of fentanyl, midazolam, and sevoflurane in dogs. Vet. Anaesth. Analg. 2009, 36, 449–456. [Google Scholar] [CrossRef]
- Foreman, M.; Vettorato, E.; Caine, A.; Monti, P.; Cherubini, G.B.; Eminaga, S. Serum C-reactive protein in dogs with paraplegia secondary to acute intervertebral disc extrusion. J. Vet. Intern. Med. 2021, 35, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Jervan, M.; Szlosek, D.A.; Friis, H.; Coyne, M.J.; DeNicola, D.; Johnsen, O.H. Characterization of C-reactive protein in dogs undergoing medial patellar luxation surgery. PLoS ONE 2020, 15, e0231445. [Google Scholar] [CrossRef]
- Trub, S.A.; Bush, W.W.; Paek, M.; Cuff, D.E. Use of C-reactive protein concentration in evaluation of diskospondylitis in dogs. J. Vet. Intern. Med. 2021, 35, 209–216. [Google Scholar] [CrossRef]
- Smith, S.A. Hemostatic complications of canine IMHA. In Proceedings of the 25th ACVIM Forum, Seattle, DC, USA, 6–9 July 2007. [Google Scholar]
- Andersen-Ranberg, E.; Berendt, M.; Gredal, H. Biomarkers of non-infectious inflammatory CNS diseases in dogs—Where are we now? Part I: Meningoencephalitis of unknown origin. Vet. J. 2021, 273, 105678. [Google Scholar] [CrossRef] [PubMed]
- Andersen-Ranberg, E.; Berendt, M.; Gredal, H. Biomarkers of non-infectious inflammatory CNS diseases in dogs: Where are we now? Part 2—Steroid responsive meningitis-arteritis. Vet. J. 2021, 273, 105692. [Google Scholar] [CrossRef]
- Lowrie, M.; Penderis, J.; Eckersall, P.D.; McLaughlin, M.; Mellor, D.; Anderson, T.J. The role of acute phase proteins in diagnosis and management of steroid-responsive meningitis arteritis in dogs. Vet. J. 2009, 182, 125–130. [Google Scholar] [CrossRef]
- Severo, J.S.; Santana, A.E.; Aoki, V.; Michalany, N.S.; Mantovani, M.M.; Larsson, C.E., Jr.; Larsson, C.E. Evaluation of C-reactive protein as an inflammatory marker of pemphigus foliaceus and superficial pyoderma in dogs. Vet. Dermatol. 2018, 29, 128-e51. [Google Scholar] [CrossRef] [PubMed]
- Szalai, A.J. C-reactive protein (CRP) and autoimmune disease: Facts and conjectures. Clin. Dev. Immunol. 2004, 11, 221–226. [Google Scholar] [CrossRef]
- Tecles, F.; Caldín, M.; Zanella, A.; Membiela, F.; Tvarijonaviciute, A.; Subiela, S.M.; Cerón, J.J. Serum acute phase protein concentrations in female dogs with mammary tumors. J. Vet. Diagn. Investig. 2009, 21, 214–219. [Google Scholar] [CrossRef]
- Jergens, A.E.; Schreiner, C.A.; Frank, D.E.; Niyo, Y.; Ahrens, F.E.; Eckersall, P.D.; Benson, T.J.; Evans, R.A. A scoring index for disease activity in canine inflammatory bowel disease. J. Vet. Intern. Med. 2003, 17, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Galezowski, A.M.; Snead, E.C.; Kidney, B.A.; Jackson, M.L. C-reactive protein as a prognostic indicator in dogs with acute abdomen syndrome. J. Vet. Diagn. Investig. 2010, 22, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Bjørnkjær-Nielsen, K.A.; Bjørnvad, C.R. Corticosteroid treatment for acute/acute-on-chronic experimental and naturally occurring pancreatitis in several species: A scoping review to inform possible use in dogs. Acta Vet. Scand. 2021, 63, 28. [Google Scholar] [CrossRef]
- Brunner, A.; Schuller, S.; Hettlich, B.; Marti, E.; Lehmann, A.; Peters, L.M.; Adamik, K.N. Kinetics of Plasma Cytokines, Angiopoietin-2, and C-Reactive Protein in Dogs With Gastric Dilatation Volvulus. Front. Vet. Sci. 2021, 16, 652479. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).