Comparison of Commercial Poultry Semen Extenders Modified for Cryopreservation Procedure in the Genetic Resource Program of Czech Golden Spotted Hen
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Semen Collection and Insemination Dose Processing
2.3. Evaluation of the Quality of Insemination Doses
2.4. Statistical Evaluation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kraus, A.; Zita, L.; Krunt, O.; Härtlová, H.; Chmelíková, E. Determination of selected biochemical parameters in blood serum and egg quality of Czech and Slovak native hens depending on the housing system and hen age. Poult. Sci. 2021, 100, 1142–1153. [Google Scholar] [CrossRef] [PubMed]
- Kraus, A.; Krunt, O.; Zita, L.; Vejvodová, K.; Drábek, O. Laying hens under smallholder conditions: Laying performance, growth and bone quality of tibia and femur including essential elements. Poult. Sci. 2022, 101, 101927. [Google Scholar] [CrossRef] [PubMed]
- Anderle, V.; Lichovníková, M.; Przywarova, A.; Dračková, E. Egg quality of gene reserve Czech golden spotted hens. Acta Fytotech. Et Zootech. 2014, 17, 84–86. [Google Scholar] [CrossRef][Green Version]
- Rubinsky, B.; Perez, P.A.; Carlson, M.E. The thermodynamic principles of isochoric cryopreservation. Cryobiology 2005, 50, 121–138. [Google Scholar] [CrossRef]
- Partyka, A.; Nizański, W. Advances in storage of poultry semen. Anim. Reprod. Sci. 2022, 106921. [Google Scholar] [CrossRef]
- Rakha, B.A.; Ansari, M.S.; Akhter, S.; Blesbois, E.; Santiago-Moreno, J. Effect of dimethylformamide on sperm quality and fertilizing ability of Indian red jungle fowl (Gallus gallus murghi). Theriogenology 2020, 149, 55–61. [Google Scholar] [CrossRef]
- Wu, B.X.; Yang, X.H.; Yan, H.F. Improving the quality of rooster semen frozen in straws by screening the glycerol concentration and freezing rate. Br. Poult. Sci. 2020, 61, 173–179. [Google Scholar] [CrossRef]
- Sasaki, K.; Tatsumi, T.; Tsutsui, M.; Niinomi, T.; Imai, T.; Naito, M.; Tajima, A.; Nishi, Y. A Method for Cryopreserving Semen from Yakido Roosters Using N-Methylacetamide as a Cryoprotective Agent. J. Poult. Sci. 2010, 47, 297–301. [Google Scholar] [CrossRef]
- Partyka, A.; Nizański, W.; Łukaszewicz, E. Evaluation of fresh and frozen-thawed fowl semen by flow cytometry. Theriogenology 2010, 74, 1019–1027. [Google Scholar] [CrossRef]
- Blanco, J.M.; Long, J.A.; Gee, G.; Wildt, D.E.; Donoghue, A.M. Comparative cryopreservation of avian spermatozoa: Effects of freezing and thawing rates on turkey and sandhill crane sperm cryosurvival. Anim. Reprod. Sci. 2012, 131, 1–8. [Google Scholar] [CrossRef]
- Mosca, F.; Madeddu, M.; Abdel Sayed, A.; Zaniboni, L.; Iaffaldano, N.; Cerolini, S. Combined effect of permeant and non-permeant cryoprotectants on the quality of frozen/thawed chicken sperm. Cryobiology 2016, 73, 343–347. [Google Scholar]
- Abouelezz, F.M.K.; Sayed, M.A.M.; Santiago-Moreno, J. Fertility disturbances of dimethylacetamide and glycerol in rooster sperm diluents: Discrimination among effects produced pre and post freezing-thawing process. Anim. Reprod. Sci. 2017, 184, 228–234. [Google Scholar] [CrossRef]
- Rakha, B.A.; Ansari, M.S.; Akhter, S.; Santiago-Moreno, J.; Blesbois, E. Cryoprotectant effects of egg yolk on Indian red jungle fowl (Gallus gallus murghi) sperm. Theriogenology 2018, 119, 150–155. [Google Scholar] [CrossRef]
- Di Iorio, M.; Rusco, G.; Iampietro, R.; Maiuro, L.; Schiavone, A.; Cerolini, S.; Iaffaldano, N. Validation of the Turkey semen cryopreservation by evaluating the effect of two diluents and the inseminating doses. Animals 2020, 10, 1329. [Google Scholar] [CrossRef]
- Neville, W.J.; Macpherson, J.W.; Reinhart, B. Contraceptive action of glycerol in chickens. Poult. Sci. 1971, 50, 2047–2053. [Google Scholar] [CrossRef]
- Lake, P.E. The History and Future of the Cryopreservation of Avian Germ Plasm. Poult. Sci. 1986, 65, 1–15. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, S.K.; Jang, H.J.; Kang, K.S.; Kim, J.H.; Choi, S.B.; Han, J.Y. Cryopreservation of Korean Oge chicken semen using N-metylacetamide. CryoLetters 2012, 33, 427–434. [Google Scholar]
- Mosca, F.; Zaniboni, L.; Abdel Sayed, A.; Madeddu, M.; Iaffaldano, N.; Cerolini, S. Effect of dimethylacetamide and N-methylacetamide on the quality and fertility of frozen/thawed chicken semen. Poult. Sci. 2019, 98, 6071–6077. [Google Scholar]
- Mosca, F.; Zaniboni, L.; Sayed, A.A.; Iaffaldano, N.; Soglia, D.; Schiavone, A.; Cerolini, S. Effect of N-Methylacetamide Concentration and Thawing Rate on Chicken Sperm Quality after Cryopreservation. Animals 2020, 10, 824. [Google Scholar]
- Hanzawa, S.; Niinomi, T.; Miyata, T.; Tsutsui, M.; Tajima, A. Cryopreservation of chicken semen using N-methylacetamide as cryoprotective agent. Jpn. J. Poult. Sci. 2010, 47, J27–J32. [Google Scholar]
- Choi, J.S.; Shin, D.B.; Ko, Y.G.; Do, Y.J.; Seong, H.H.; Kim, D.H.; Kong, I.K.; Kim, S.W. Evaluation of cryopreserved chicken semen with different cryoprotectants. Reprod Fertil. Dev. 2013, 26, 143. [Google Scholar] [CrossRef]
- Kim, S.W.; Choi, J.S.; Ko, Y.; Do, Y.; Byun, M.; Park, S.; Seong, H.; Kim, C. Effect of N-methylacetamide concentration on the fertility and hatchability of cryopreserved Oyge rooster semen. Korean J. Poult. Sci. 2014, 41, 21–27. [Google Scholar] [CrossRef]
- Burrows, W.H.; Quinn, J.P. A Method of Obtaining Spermatozoa from the Domestic Fowl. Poult. Sci. 1935, 14, 251–253. [Google Scholar] [CrossRef]
- Madeddu, M.; Mosca, F.; Abdel Sayed, A.; Zaniboni, L.; Mangiagalli, M.G.; Colombo, E.; Cerolini, S. Effect of cooling rate on the survival of cryopreserved rooster sperm: Comparison of different distances in the vapor above the surface of the liquid nitrogen. Anim. Reprod. Sci. 2016, 171, 58–64. [Google Scholar] [CrossRef]
- Augustin, N.H.; Sauleau, E.A.; Wood, S.N. On quantile quantile plots for generalized linear models. Computational Stat. Data Anal. 2012, 56, 2404–2409. [Google Scholar] [CrossRef]
- Blesbois, E.; Seigneurin, F.; Grasseau, I.; Limouzin, C.; Besnard, J.; Gourichon, D.; Coquerelle, G.; Rault, P.; Tixier-Boichard, M. Semen Cryopreservation for Ex Situ Management of Genetic Diversity in Chicken: Creation of the French Avian Cryobank. Poult. Sci. 2007, 86, 555–564. [Google Scholar] [CrossRef]
- Çiftci, H.B.; Aygün, A. Poultry Semen Cryopreservation Technologies; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar]
- Long, J.A.; Bongalhardo, D.C.; Pelaéz, J.; Saxena, S.; Settar, P.; O’Sullivan, N.P.; Fulton, J.E. Rooster semen cryopreservation: Effect of pedigree line and male age on postthaw sperm function. Poult. Sci. 2010, 89, 966–973. [Google Scholar] [CrossRef]
- Sonseeda, P.; Vongpralub, T.; Laopaiboon, B. Effects of environmental factors, ages and breeds on semen characteristics in thai indigenous chickens: A one-year study. Thai Vet. Med. 2013, 43, 347–352. [Google Scholar]
- Long, J.A.; Purdy, P.H.; Zuidberg, K.; Hiemstra, S.J.; Velleman, S.G.; Woelders, H. Cryopreservation of turkey semen: Effect of breeding line and freezing method on post-thaw sperm quality, fertilization, and hatching. Cryobiology 2014, 68, 371–378. [Google Scholar] [CrossRef]
- Meamar, M.; Shahneh, A.Z.; Zamiri, M.J.; Zeinoaldini, S.; Kohram, H.; Hashemi, M.R.; Asghari, S. Preservation effects of melatonin on the quality and fertility of native Fars rooster semen during liquid storage. Czech J. Anim. Sci. 2016, 61, 42–48. [Google Scholar] [CrossRef]
- Rakha, B.A.; Ansari, M.S.; Akhter, S.; Zafar, Z.; Naseer, A. Dimethyleacetamide improves the cryosurvivability of Indian red jungle fowl (Gallus gallus murghi) sperm. Theriogenology 2017, 103, 83–89. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pérez-Marín, C.C.; Arando, A.; Mora, C.; Cabello, A. Fertility after insemination with frozen-thawed sperm using N-methylacetamide extender on the Combatiente Español avian breed. Anim. Reprod. Sci. 2019, 208, 106111. [Google Scholar] [CrossRef] [PubMed]
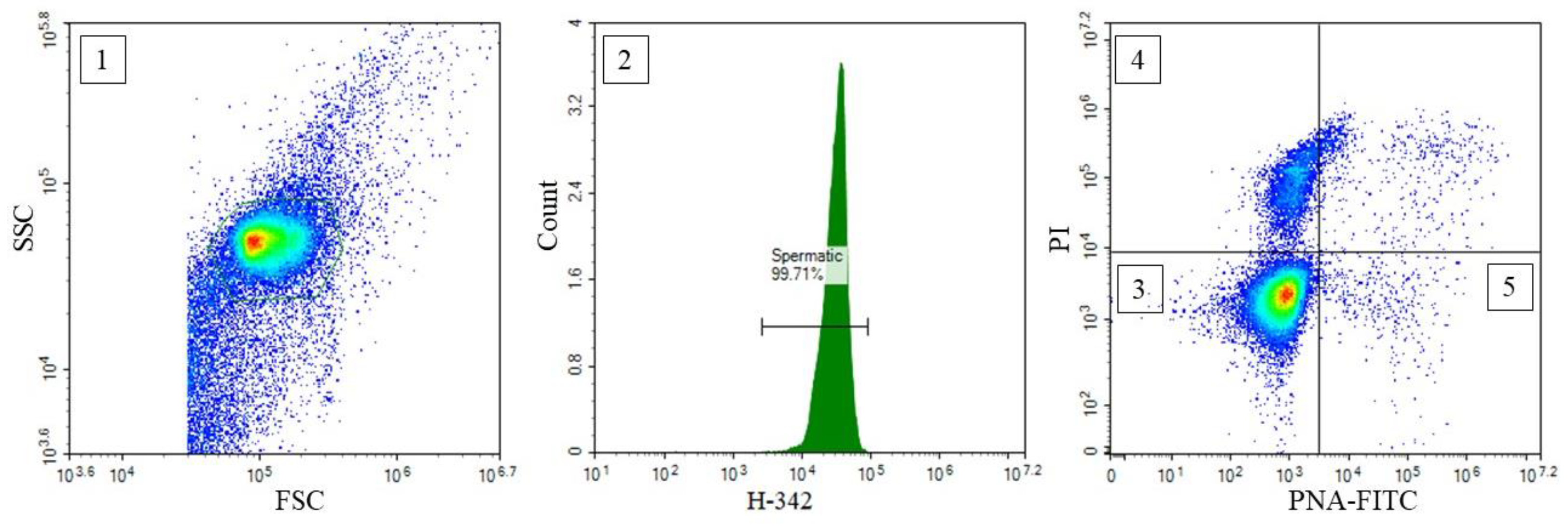
| Variable | n | AM | SD | Minimum | Maximum |
|---|---|---|---|---|---|
| MOT | 90 | 17.23 | 18.52 | 0.00 | 68.00 |
| PAI | 90 | 36.08 | 21.83 | 3.04 | 58.65 |
| PMD | 90 | 58.96 | 21.98 | 27.50 | 94.96 |
| ACRD | 90 | 2.95 | 4.06 | 0.12 | 13.56 |
| DAY | MOT (%) | PAI (%) | PMD (%) | ACRD (%) |
|---|---|---|---|---|
| 10 January (n = 18) | 22.18 ± 2.89 ab | 42.79 ± 0.86 a | 44.98 ± 0.98 c | 2.73 ± 0.22 b |
| 14 January (n = 18) | 23.61 ± 2.89 a | 38.85 ± 0.86 ab | 58.80 ± 0.98 b | 0.99 ± 0.22 c |
| 17 January (n = 18) | 8.39 ± 2.89 b | 35.80 ± 0.86 b | 63.33 ± 0.98 a | 0.25 ± 0.22 c |
| 20 January (n = 18) | 15.67 ± 2.89 ab | 28.98 ± 0.86 c | 59.23 ± 0.98 b | 10.19 ± 0.22 a |
| 24 January (n = 18) | 14.39 ± 2.89 ab | 37.30 ± 0.86 b | 61.42 ± 0.98 ab | 0.56 ± 0.22 c |
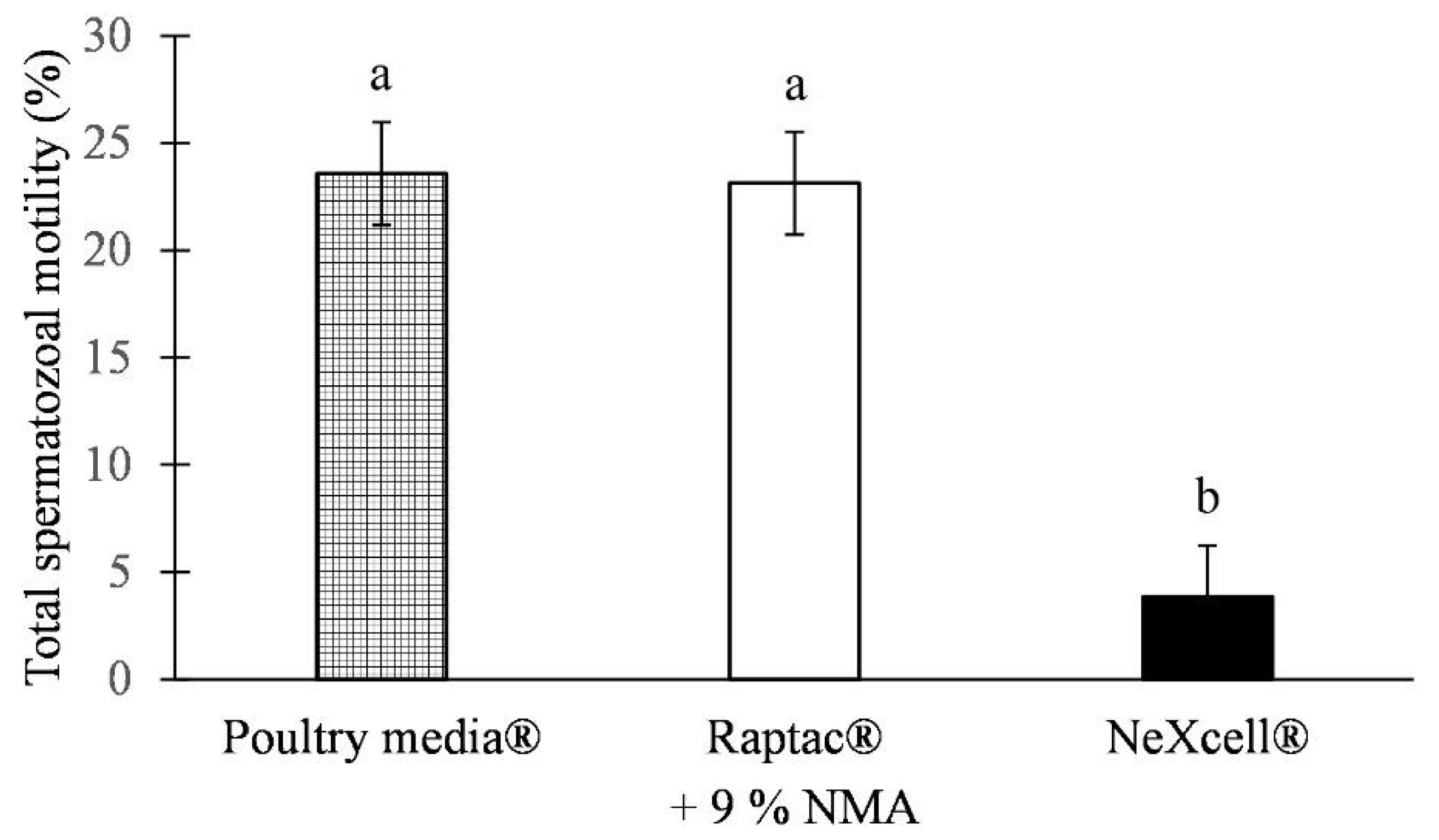
| Extender | PAI (%) | PMD (%) | ACRD (%) |
|---|---|---|---|
| Poultry media® + 9% NMA (n = 30) | 51.11 ± 0.72 a | 43.06 ± 0.82 b | 3.36 ± 0.18 |
| Raptac® + 9% NMA (n = 30) | 52.04 ± 0.72 a | 42.57 ± 0.82 b | 2.87 ± 0.18 |
| NeXcell® + 9 % NMA (n = 30) | 7.07 ± 0.72 b | 87.03 ± 0.82 a | 2.59 ± 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petričáková, K.; Janošíková, M.; Ptáček, M.; Zita, L.; Savvulidi, F.G.; Partyka, A. Comparison of Commercial Poultry Semen Extenders Modified for Cryopreservation Procedure in the Genetic Resource Program of Czech Golden Spotted Hen. Animals 2022, 12, 2886. https://doi.org/10.3390/ani12202886
Petričáková K, Janošíková M, Ptáček M, Zita L, Savvulidi FG, Partyka A. Comparison of Commercial Poultry Semen Extenders Modified for Cryopreservation Procedure in the Genetic Resource Program of Czech Golden Spotted Hen. Animals. 2022; 12(20):2886. https://doi.org/10.3390/ani12202886
Chicago/Turabian StylePetričáková, Kristýna, Martina Janošíková, Martin Ptáček, Lukáš Zita, Filipp Georgijevič Savvulidi, and Agnieszka Partyka. 2022. "Comparison of Commercial Poultry Semen Extenders Modified for Cryopreservation Procedure in the Genetic Resource Program of Czech Golden Spotted Hen" Animals 12, no. 20: 2886. https://doi.org/10.3390/ani12202886
APA StylePetričáková, K., Janošíková, M., Ptáček, M., Zita, L., Savvulidi, F. G., & Partyka, A. (2022). Comparison of Commercial Poultry Semen Extenders Modified for Cryopreservation Procedure in the Genetic Resource Program of Czech Golden Spotted Hen. Animals, 12(20), 2886. https://doi.org/10.3390/ani12202886







