Simple Summary
With regard to their high adaptability to human settlements and global distribution, corvid birds (crows, ravens, jays, etc.) are good models to understand the impacts of urbanization on wildlife. Here, we qualitatively reviewed the impacts of urbanization on corvids. At least 30 corvid species have become successfully accustomed or adapted to urbanized environments. The majority (72%, a total of 424 articles) of the studies reported positive effects of urbanization on corvids. The availability of easily accessible food and artificial nesting sites, coupled with low levels of predation, were found as the most important factors benefitting corvids in cities around the world. Studied topics ranged from population size and density, breeding biology and nesting site selection to control and management of Corvidae in cities. Despite biases in the distribution of the reviewed papers, our review attests that corvids have demonstrated high levels of adaptability to urban environments across space and time.
Abstract
Urbanization is one of the most prevalent drivers of biodiversity loss, yet few taxonomic groups are remarkably successful at adapting to urban environments. We systematically surveyed the global literature on the effects of urbanization on species of family Corvidae (crows, choughs, jackdaws, jays, magpies, nutcrackers, ravens, rooks, treepies) to assess the occurrence of corvids in urban environments and the factors affecting their success. We found a total of 424 primary research articles, and the number of articles has increased exponentially since the 1970s. Most studies were carried out in cities of Europe and North America (45.5% and 31.4%, respectively) and were directed on a single species (75.2). We found that 30 corvid species (23% of 133 total) regularly occur in urban environments. The majority (72%) of the studies reported positive effects of urbanization on corvids, with 85% of studies detecting population increases and 64% of studies detecting higher breeding success with urbanization. Of the factors proposed to explain corvids’ success (availability of nesting sites and food sources, low predation and persecution), food availability coupled with diet shifts emerged as the most important factors promoting Corvidae to live in urban settings. The breeding of corvids in urban environments was further associated with earlier nesting, similar or larger clutches, lower hatching but higher fledging success, reduced home range size and limited territoriality, increased tolerance towards humans and increasing frequency of conflicts with humans. Despite geographic and taxonomic biases in our literature sample, our review indicates that corvids show both flexibility in resource use and behavioral plasticity that enable them to exploit novel resources for nesting and feeding. Corvids can thus be urban exploiters of the large-scale modifications of ecosystems caused by urbanization.
1. Introduction
Urbanization is a spatio-temporal process of the development of cities and the increase in the concentration of populations in them, followed by a transformation of natural habitats into artificial ones [,]. In general, urbanization is strongly associated with increased cover of imperious structures (e.g., buildings, streets) and human population density, as well as the fragmentation, degradation and loss of natural habitats. An urban development is an ecological modification that often alters the functions of a given ecosystem by affecting the structure of the food chain by removing or adding species, by encouraging human tolerance and adaptation, by increasing health risks for humans and wildlife and by modifying ecological processes in relation to ecosystem services []. Urbanization leads to complex, diverse systems characterized by high levels of human disturbance, pollution and landscape and environmental changes [,,]. These changes can affect the biology, behavior, morphology and reproductive and survival traits of wildlife and can be responsible for the disappearance of native species and the appearance of non-native ones []. Therefore, understanding these effects is essential for successful wildlife conservation and management in urban habitats.
The negative impact of human-made landscapes and infrastructures on wildlife has been detected in many studies [,,]. However, numerous studies have also described how certain species, such as corvids (e.g., crows, magpies), can benefit from these infrastructures, such as using buildings, poles and power lines as nesting sites [,,]. In addition, anthropogenic food resources and milder microclimate in cities might benefit many corvid species [,,]. Urbanization has been considered as an overwhelming evolutionary force acting on the life-history traits and population genetics of species []. Currently, urbanization is still expanding at an accelerating pace [], unfortunately coinciding with a continuous increase in habitat loss. Although studies on the effects of urbanization on birds, at a community or individual species level, have been widely conducted, multi-species approaches with species belonging to the same family are still very scarce.
Corvidae is a family of mid to large-sized passerines. Many corvid species thrive in many types of urban environments, from the peripheral urban areas to highly urbanized urban core areas [,,]. Because of the wide distribution areas of many corvid species and good adaptability to many habitats, corvid species are often described as urban adaptors and even exploiters [,,]. Additionally, considering the high diversity and broad distribution of species of the Corvidae family, their spatio-temporal historical dispersal over numerous geographic and ecological areas may most likely contribute to the increase in taxonomic biodiversity []. Thus, corvids can be considered as the ideal subjects for investigating the effects of urbanization on birds. Lowry et al. (2013) have stated that the first observed adjustment shown by wildlife species in a human-made environment is a modification of behavior []. For example, numerous wild animals have been observed to alter their breeding, nesting and foraging patterns, diet composition, as well as vigilant behavior and vocalization, in response to human-made environments [,,,]. While some studies have explored the effects of urbanization on wildlife species and the adaptations of synanthropic birds (free-ranging wildlife living close to humans and benefiting from them) to urban habitats [,,,], individual studies tend to have a restricted geographical scale of inquiry, a single-species approach and usually focus only on a single trait that predicts adaptation [,,,].
In this paper, we systematically reviewed scientific papers to synthesize knowledge of corvid species in urban environments at a global scale. The main aims of the study were (i) to identify which corvid species have been studied in an urban context, and where and when they have been studied, (ii) to examine the effects of urbanization on corvid species by exploring how corvids respond to changes caused by humans, and how they evolve and survive in urbanized environments and (iii) to identify commonalities and differences between corvid species successfully colonizing urban environments around the world. We hypothesized that corvids are highly synanthropic and can adapt easily to modified environments. We predicted that corvids would display similar behavioral adjustments in different cities around the world, thanks to their high flexibility despite their different natural background. In addition, we aimed to better understand the reasons behind the successful colonization, establishment and adaptation of corvids to urban environments at a global scale. Finally, we aimed to explore the success or failure of management and conservation efforts associated with the corvids’ presence in cities worldwide. To our knowledge, this is the first comprehensive literature review specifically covering corvid species in urban areas around the world.
2. Materials and Methods
Literature Search
We first searched for studies of corvid species by using the Scopus and the Web of Science databases and summarized and assessed the findings of studies relevant to urbanization in a global context. Our extensive and systematic literature survey followed the PRISMA guidelines and checklists for literature reviews (http://prisma-statement.org/, accessed on 26 November 2020, []). We used the following search script (search date: 30 September 2020): TS (topic) = (Corvids OR Crow OR Jay OR Magpie OR Nutcracker OR Brushcrow OR Chough OR Piapiac OR Raven OR Rook OR Treepie OR Jackdaw) and TS = (cities OR city OR urban OR suburban OR sub-urban OR town OR suburbs OR residential OR man-made OR human-made OR builtup OR buildup OR built-up OR build-up OR developed OR non-rural OR metropol) AND NOT (crowd OR crowe OR crown OR jaya) and restricting hits to the English language research papers and related chapters in books only.
In addition, we later searched the Corvids Literature Database [], (search date: 17 October 2020) to check if our Web of Science and Scopus search missed any relevant publications. When using the Corvids Literature Database, we used the advanced search tools built in this database. We restricted our search to “City/Anthropogenic Impact” theme, “Normal paper” type of publications and papers from which at least an abstract was available. We did not make any search restrictions related to the year of publication or the language of publication. Therefore, our Corvids Literature Database search takes also into account non-English corvid publications that were not covered in our Scopus and Web of Science searches.
Overall, studies conducted over an urban-rural gradient (an order of settings based on the prevalence of human-mad infrastructures in association with human population density) or different urban habitats types, as well as comparative studies between urban and non-urban habitats, were included, since such studies can offer a wider perspective on urbanization’s impact on wild fauna and help to explore disturbance in urban environments [,,,].
The articles collected in the two searches were selected thoroughly by following the four phases of the systematic review flow diagram of the PRISMA guidelines. The PRISMA flow chart is given in Table S1. To be included in the analyses, the article had to deal with any of the following topics in the context of corvid species.
- 1.
- Urban colonization, establishment, abundance, population dynamics and distribution, breeding ecology, nesting success, nest site selection, nestling development and growth.
- 2.
- Territoriality and habitat use, activity, foraging, feeding, roosting behavior, diet availability and composition.
- 3.
- Corvids’ tolerance and responses to human disturbance, human tolerance of corvids, and human-corvid conflict. We also included studies about specific biological or physiological parameters as these can be important to explain the birds’ responses to human-caused changes in their environment.
- 4.
- Indirect influence of urban environmental or anthropogenic resources, such as the effect of anthropogenic food.
- 5.
- Urban wildlife management and control.
- 6.
- Pollution, metal contamination, disease transmission and zoonosis.
- 7.
- Ecosystem services, such as seed dispersal.
Finally, we extracted the following information from the filtered studies: studied species, geographic location of study (continent, country), year of study and year of publication, for a better perspective on the evolution of the different bird populations in cities around the world.
3. Results
3.1. Corvid Publications
Our Scopus literature search found a total of 2623 articles, and Web of Science search found 2565 articles, of which 972 were duplicates (Table S1). After screening and checking the eligibility of those articles, we ended up with 267 articles. Our Corvids Literature Database search found a total of 437 articles, from which 157 articles were usable after excluding duplicates (n = 57 dropped), screening (n = 338 dropped) and checking the eligibility (n = 135 dropped). Finally, our sample included 424 (267 + 157) articles for our analyses (heretofore referred to as “sample”). Additionally, 93.8% of the reviewed papers were English language publications; the additional non-English texts were mainly written in Japanese (2.36%), Polish (1.96%) and German (0.98%).
Urban corvids have been studied since the 19th century; however, most studies were published in the 21st century, with 46% of the articles published after 2010 (Figure 1).
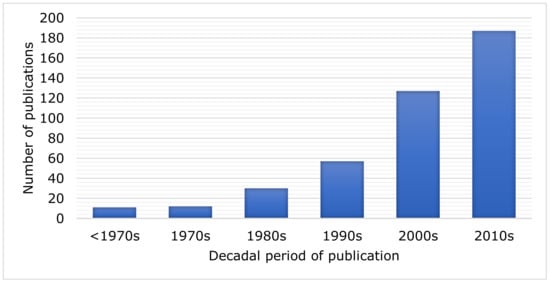
Figure 1.
Number of articles published on Corvidae species in urban environments by decade (n = 424).
Most studies were carried out in Europe or North America (45.5% and 31.4%, respectively; n = 424), followed by Asia and Australia (14.6% and 4.5%), and there were only a few studies from Africa (2.1%; Table S3). Eight studies (1.9%) had a global study range. A total of 319 articles (75.2% of all 424 articles) were on a single species, 44 (10.4%) on two species, and 60 (14.2%) on more than two species, whereas 22 (5.2%) of the articles were community-level studies (Table S3).
We found evidence of occurrence in urban environments in 30 species/subspecies/races of Corvidae (Table S3). Most (76%, n = 424) of the reviewed studies considered the adaptation of crows and magpies to the urban environment. Additionally, population trends, breeding biology, nesting site selection, and human–corvid interactions have been often monitored. (Table S3). The Eurasian Magpie (Pica pica; n = 93), the Rook (Corvus frugilegus), the Western Jackdaw (Coloeus monedula), the Hooded Crow (Corvus corone cornix), the American Crow (Corvus brachyrhynchos), the Scrub Jays (Aphelocoma spps.), the Common Raven (Corvus corax), and the Carrion Crow (Corvus cororne corone) were the most often studied Corvidae species.
3.2. Effects of Urbanization on Corvids
From a total of 245 articles addressing the topic of the urban effect on corvid species, 177 articles (72.2% of the 245 articles) demonstrated positive effects of urbanization on the Corvidae, as various corvid species correlate positively with the proportion of built up establishments, demonstrating a continuous increase in population rates, and 42 articles (17.1%) reported negative effects, showing a decrease in the population density of the birds in question, and 26 articles (10.6%) reported no effect, reflecting thus no display of any changes in population trends (Figure 2). Specifically, the Eurasian Magpie, the Common Raven, the Rook, the Hooded Crow and the Carrion Crow have been reported to benefit from urbanization (Figure 2).
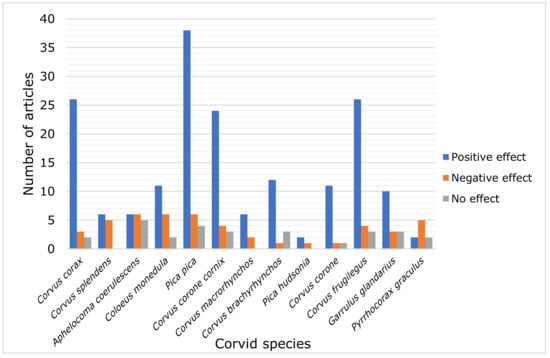
Figure 2.
Number of articles reporting positive, negative or no effect of urbanization on the most frequently studied corvid species (n = 245).
3.2.1. Breeding Abundance/Density of Corvids
From 92 articles addressing the topic of the breeding abundance of corvid species in urban areas, 81 articles (88%) indicated that the abundance or density of corvid species increased with urbanization (Table 1). At least six corvids (the American Crow, the Common Raven, the Hooded Crow, the Carrion Crow, the Eurasian Magpie, the Rook and the House Crow (Corvus splendens) were unambiguously demonstrated to show an increase in abundance or density with increasing urbanization. In contrast, only a few species were observed to show a decrease with urbanization, and even in these species, the number of studies showing increases usually exceeded the number of studies showing decreases (e.g., the Western (also known as Eurasian) Jackdaw; Table 1).

Table 1.
Articles reporting positive, negative or no effects of urbanization on the abundance/density of populations in different corvid species (total n = 92). The reference numbers in the table refers to the individual publication numbers given in Table S2.
3.2.2. Breeding Success
We found 31 articles that studied the impact of urbanization on breeding success in ten corvid species (Table 2). The breeding success of the Eurasian Magpie, Common Raven and American Crow increased with urbanization, whereas opposite results were reported for the Scrub Jay (Table 2).

Table 2.
Articles reporting lower, higher or no effects on breeding success in urban areas relative to rural areas in different corvid species (n = 31). The reference numbers in the table refer to the individual publication numbers given in Table S2.
3.3. Factors Influencing Corvids
3.3.1. Artificial Food
Fourteen corvid species were reported to use artificial food resources in urban habitats (Table 3). Ten species were reported to use garbage cans and dumps, and five species used bird feeders, while four species used carrion. The Magpie and the Carrion Crow were reported to use all of these kinds of artificial food resources (Table 3).

Table 3.
List of articles in relation to artifical food resource use of corvids in urban environments (n = 28). The reference numbers in the table refer to the individual publication numbers given in Table S2.
3.3.2. Artificial Nest Site Use
Fourteen species were reported to use some kind of artificial nest site; of these species, 10 use exotic trees, 6 use poles and 5 use buildings, roofs and attics (Table 4). Specifically, the Common Raven uses poles, whereas the Eurasian Magpie uses exotic trees for nesting in urban areas (Table 4).

Table 4.
List of articles in relation to artificial nest site selection (n = 47). The reference numbers in the table refer to the individual publication numbers given in Table S2.
3.3.3. Roosting within Urban Settings
A total of 23 articles reported that at least 11 corvid species use urban areas as nocturnal roosting areas (Table 5). Rooks, Eurasian Magpies and Western Jackdaws were most often reported to roost in urban settings (Table 5).

Table 5.
List of articles reporting roosting corvids in urban areas (n = 23). The reference numbers in the table refers to the individual publication numbers given in Table S2.
3.3.4. Predation and Persecution
We found 30 articles that indicated either predation on or persecution of corvids (Table 6). Ten corvid species were reported as victims of predation events in 13 articles and, nine species were reported to be persecuted (Table 6). Many studies reported that the Eurasian Magpie suffers from predation and persecution.

Table 6.
List of articles reporting predation events and persecution towards to corvids in urban areas (n = 30). The reference numbers in the table refer to the individual publication numbers given in Table S2.
3.3.5. Human Related Infections and Contaminations
We found 17 articles reporting different kinds of viral, bacterial or fungal pathogens of human importance and 12 articles about the contamination of heavy metals in 9 corvid species (Table 7).

Table 7.
List of articles about human-related infection and contaminations of corvids (n = 29). The reference numbers in the table refers to the individual publication numbers given in Table S2.
3.3.6. Artificial Light and Noise
We found only six articles reporting on the responses of corvids to artificial light or noise in seven species. Artificial light was reported to negatively influence the House Crow and the Western Scrub Jay (Aphelocoma californica), but to positively influence the Rook, the Hooded Crow and the Western Jackdaw. Three species (Rook, Hooded Crow and Western Jackdaw) were reported to suffer from artificial noise.
3.4. Bird Responses to Human Developments
3.4.1. Reproduction
Several articles demonstrated that breeding parameters, such as egg-laying date, clutch size, hatching and fledging success, were reported to differ between urban and rural corvid populations (Table 8). Fifteen studies of seven species demonstrated that egg-laying starts earlier in urban corvid populations than in non-urban populations. A total of 11 articles reported larger clutches in urban than non-urban corvid populations. A lower hatching success but a greater fledging success in urban than in non-urban corvid populations were reported in six and four articles, respectively. Nevertheless, four other articles have demonstrated lower fledging success in urban than in non-urban corvid populations. Such differences were most frequently reported in the Eurasian Magpie and the Florida Scrub Jay (Aphelocoma coerulescens), with eight and seven articles, respectively (Table 8).

Table 8.
Number of articles reporting differences between urban and rural areas in egg-laying date, clutch size, hatching success and fledging success in ten corvid species (n = 31). The reference numbers in the table refer to the individual publication numbers given in Table S2.
Brood reduction, repeated nesting after nesting failure and nest reuse of the Eurasian Magpie, have been reported in four, two, and two studies, respectively. Brood reduction and repeated nesting were reported in one study each in the Florida Scrub Jay.
3.4.2. Corvids’ Behavioral Responses to the Urban Environment
We found 26 articles reporting behaviors of 16 urban corvid species (Table 9). All 16 species were reported to show some kind of behavioral change or adjustment in response to the urban conditions, whereas only 3 species did not show such changes. The Common Raven was particularly often reported to change its behavior in urban settings.

Table 9.
List of articles reporting behavioral change or adjustment of corvid species in urban environments (n = 26). The reference numbers in the table refer to the individual publication numbers given in Table S2.
3.4.3. Corvids’ Responses to Human Presence
We found nine articles addressing the level of tolerance or vigilance in eight different corvid species. Seven out of eight species were reported to show greater tolerance towards humans, whereas only three species were reported to have an opposite reaction (Table 10).

Table 10.
List of papers about corvids’ tolerance towards humans (n = 9). The reference numbers in the table refer to the individual publication numbers given in Table S2.
3.4.4. Human–Corvid Conflicts in Urban Settlements
We found 15 articles that described some kinds of corvid-human conflicts in urban areas (Table 11). Eight species were reported to cause noise, five species were reported to foul infrastructure and showed aggressive behavior, four species caused problems via fecal droppings and three species by scavenging on garbage (Table 11).

Table 11.
List of articles reporting different kinds of human-crow conflicts (n = 15). The reference numbers in the table refer to the individual publication numbers given in Table S2.
3.4.5. Ecosystem Services of Urban Corvids
A total of 16 articles demonstrated the potential role of the corvids providing ecosystem services (Table 12). Seven species were identified as seed dispersers, and six species provided services with implications on human health. Corvids may be used as biosensors for different types of contaminations that can potentially result in the transmission of agents hazardous to humans, such as the West Nile Virus (WNV), a zoonotic viral infection often detected in crows and ravens [,].

Table 12.
List of articles reporting on ecosystem services provided by urban corvids (n = 16). The reference numbers in the table refer to the individual publication numbers given in Table S2.
4. Discussion
Our results indicate that corvids have been widely successful in adapting to urbanized environments over recent decades over a worldwide geographic scale. At least 23% of the 130 corvid species in the world are reported to live in urbanized environments, attesting this group’s extreme flexibility in resource use and highly opportunistic and plastic behavior, often enabling them to reach high abundances in various cities around the world. In contrast, we found no evidence of occurrence in urban environments for 100 corvid species. However, this does not mean that these species avoid cities and towns; rather, it reflects the lack of detailed studies documenting the occurrence and breeding of corvids in urban environments.
Accordingly, while the establishment and colonization of corvids in urban environments have drawn interest from researchers since the early 1990s, there has been a recent surge of scientific interest in corvids in urban environments in many areas of the world, and research on urban corvids is a rapidly emerging field of inquiry. Most of these studies address population trends, breeding biology and nest site selection in particular, in cities and towns, and a lower number of studies investigated the habitat use and movements, including home range estimates, of urban corvids. An overwhelming majority of the reviewed articles reported positive effects of urbanization on the population density or abundance of corvid species, and a majority reported higher breeding success. While several factors, such as nest site availability and low abundance of predators, have been suggested to explain the success of corvids in cities, easily accessible food sources, coupled with shifts in the diet of corvids seem to be the single most important factor promoting the success of corvids in cities. Many urban breeding corvid species start their breeding earlier, have larger or equal clutch sizes, lower hatching success, greater fledging success, reduced home range size, less territoriality and increased tolerance towards humans, as well as more conflicts with humans than their corresponding rural populations.
4.1. Corvids in Urban Environments: Change of Research Interests with Time
Most of the articles published in the early 1900s focused on the observation and description of corvid populations in the green spaces in cities of North America [,,,]. Similar studies were also conducted in Europe at this time [,,,]. One of the main goals of these early studies was to establish factors affecting the colonization of towns and cities by corvids, concentrating mainly on the availability of food sources and green spaces in the city. By the 1970s, with the continuous increase in corvids in urban areas, abundance censuses of the urban populations [,,] and breeding ecology studies became the core subject of urban corvid research [,,,,,]. In the early1990s, the interest of researchers took a twisting turn to the rapid increase in human development around the world and its usually negative effect on wild fauna. Understanding how these synanthropic corvids adapt and benefit from urbanization became an important puzzle to be solved [,,,]. By the 21st century, earlier qualitative studies were supplemented by quantitative ones [,,,] that included experiments on the behavior of urban crows [,,,] and mathematical models to follow the movements of these birds in urban settlements [,,,].
4.2. Factors Explaining the Adaptation of Corvids to Urban Environments
Our literature search results indicated that most corvids are generalist species (able to consume a variety of foods and survive in different types of habitats) with a high degree of behavioral plasticity that makes them easily adaptable to environmental changes [,,,]. It has been widely established that many corvid species have flexible survival modes in order to benefit increasingly from anthropogenic habitats in different areas of the world. The most commonly assessed factor is food availability []. In every study assessing movement patterns and/or nest site selection, the impact of anthropogenic food sources on individuals’ choices was explored. For example, while it has been reported that non-breeding Common Ravens adapt their space use patterns to benefit from human food sources over large areas [,,,], breeding pairs choose to nest in areas with good food availability nearby their nests [,,]. Indeed, earlier studies have indicated that breeding Ravens forage mostly around their nest [,]. Moreover, movement patterns of the American Crow are strongly correlated with the abundance of anthropogenic resources [,,], as easily accessible food in urban areas was described as the main cause for the regular movement of rural American Crow fledglings to the cities, thus resulting in annual increases of the urban populations [,,]. Furthermore, food availability highly influenced the abundance and habitat choice of urban Carrion Crows and Jungle Crows (Corvus macrorhynchos) [,,,,]. Even the Alpine Chough (Pyrrhocorax graculus), one of the least explored corvids, was observed to alter its foraging behavior and to increase its survival based on the availability of anthropogenic foods [,,]. Winter bird feeding in cities and towns by humans also further increases foraging opportunities to corvids, such as for the Eurasian Magpie and Hooded Crow in northern latitudes []. The role of food availability is also supported by indirect evidence; for example, the decline of the Western Jackdaw populations in several European cities is partially caused by the lack of food [,].
In contrast, some studies suggested that the role of anthropogenic food availability in explaining the corvid colonization of cities could be overrated. Vuorisalo et al. (2003) suggested that while easily available food is certainly important, the local overabundance of food does not explain the sudden increase in the Hooded Crow population density in the 1960s in Finland []. Instead, they suggested that less persecution by hunting and the absence of natural predators, as well as the availability of novel nesting opportunities in the city also played an important role in this expansion. In addition, studies on the Little Raven (Corvus mellori), in Australia reported that variation in home range size was mainly related to the habitat type associated with the distribution of food sources [,,].
The second most frequently mentioned factor influencing corvid presence in urban habitats is the availability of novel nesting sites in the cities, which, along with their highly behavioral flexibility, can also explain the colonization of cities by corvids. For example, the Common Raven is almost exclusively nesting on anthropogenic structures, such as electric poles, in cities [,,,]. Magpies and Hooded Crows were also reported to change their nesting habits in urban settings. For example, Magpies tend to nest on the highest trees available in response to high levels of disturbance [,,,]. However, with decreased persecution, Magpies can nest on a less preferred site and build their nests at lower heights []. Hooded Crows will also nest on non-preferred tree species and at lower heights as the urban population increases and preferred nesting sites become scarce []. Again, cavity nesters, such as the Western Jackdaws offer indirect evidence for the importance of nesting sites because jackdaw populations declined in some European cities mainly due to the loss of suitable nest sites caused by renovations and modernizations of buildings that had been previously suitable for cavity-nesting [,,,].
Additionally, access to better nesting and feeding resources in the cities often translates into shifts in reproductive behaviors. For example, Scrub Jays in urban areas start breeding about three weeks earlier than Scrub Jays in rural areas, mostly due to the higher availability of food [,]. Bagyura et al. (2017) also observed unusually early breeding of Common Ravens nesting on electric poles in Hungary []. Nesting earlier does not necessarily translate into higher success; for example, earlier breeding in the Alpine Chough resulted in an increase in nest failure in urban settlements [].
The third most frequently mentioned factor influencing urban corvids is their high adaptability in behavior, physiology and breeding biology. Corvids’ responses to environmental change have been shown to be highly flexible, and it is suspected to be correlated with specific biological traits shared by corvid species [,,,,,,]. For instance, breeding biology parameters of several corvid species were reported to differ between urban and non-urban populations in several corvid species, including the Eurasian Magpie, American Crow, Common Raven and Hooded Crow, and to a lesser extent, the Steller’s Jays (Cyanocitta stelleri) and Jackdaws. In addition to earlier breeding, urban birds produce smaller clutches (fewer eggs) and rear their young longer until fledging, as well as produce smaller fledglings and fewer potential future breeders than non-urban birds [,,,,]. While some of these differences appear to imply disadvantages of nesting in urban environments, they did not seem to affect the size or growth of the urban populations of these species. This discrepancy may be explained by two factors. First, the increasing population sizes of some urban corvids may be due to the immigration of young crows from adjacent rural populations into the city [], as the higher breeding success in rural areas induced movements of rural dispersers into the city, creating a net immigration of crows into cities []. Second, Marzluff et al. (2001) demonstrated that urban crow populations tend to increase partially because survival rates are higher in urban than in rural corvid populations in North America []. It is important to note that most studies carried out during non-breeding seasons suggested that chances of survival are probably higher in cities than in non-urban habitats, as attested by large numbers of individuals that move from rural areas to cities for overwintering, at least in the Northern Hemisphere [,,,].
In addition, some corvids can adapt to changing light and noise conditions caused by urbanization. For example, Ciach and Frohlich (2017) indicated that the density of wintering Rooks and Magpies in southern Poland increased with increasing artificial light, but decreased with increasing noise []. Nevertheless, Ciach and Frohlich (2017) also pointed out that food availability during winter is probably the primary factor explaining corvid densities in urban areas []. It is plausible that artificial lights in cities may increase the time that the corvids can spend foraging, as has been observed in the case of urban pigeons [].
4.3. Corvids’ Responses to Urbanization
Since access to anthropogenic resources in the cities and high levels of adaptation to novel environments often translate into shifts in corvids’ activities, as stated above, many corvids change their behavior and get accustomed to human presence. The birds’ responses to human presence and proximity have been extensively studied, often in experiments, in the American Crow, the Carrion Crow, Torresian Crow (Corvus orru) and Jackdaws. The various behavioral experiments aimed to explore the crows’ tolerance towards humans and their danger recognition ability in urban settlements [,,], while other studies aimed to investigate social learning in these birds [,,,]. Decreased persecution in cities in the last few decades as opposed to rural areas appears to be an important factor promoting the corvids’ tolerance and habituation to humans and traffic; this tolerance is a pre-requisite of colonization of city centers as breeding habitat [,]. Moreover, such habituation has been widely documented in the American Crow [,,].
In addition, several studies demonstrated that corvids can depend on social cues to learn about dangers in a given area and that they are able to communicate this information to each other [,,]. An extreme case of habituation to human presence has been observed in a Carrion Crow captured in Vienna Zoo, which did not show any sign of struggle during handling by researchers []. Finally, studies based on measurements of crows’ flight initiation distance upon human approach have indicated that escape distances are considerably shorter in urban than in non-urban areas in many corvid species [,].
4.4. Corvid Human Interactions
The increased abundance of corvids due to urbanization means that corvid-human interactions will also increase. Corvids have been reported to cause conflicts due to their disturbing noise, fecal droppings, garbage scattering, damages to infrastructures and aggressive behavior towards humans and pets. For that reason, corvids have often been perceived as nuisance birds and have intensively been persecuted or even hunted in many cases [,,]. However, in relation to wildlife and nature conservation, for example, the EU Birds Directive [], the disturbance of birds during their spring migratory and breeding periods is banned, as well as prohibiting the large scale and non-selective means of bird killing.
Because corvids often forage on communal waste found in household garbage dens, on commercial refuse in dumpsters, near outdoor restaurants and food stands, trashcans in parking lots, and landfills [,,], corvids can serve as vectors of disease transmission. In particular, communal roosts and the feeding location of corvids in cities greatly increase the chances of disease transmission, which is of great concern for human and animal health [,]. Several studies addressed the potential role of wild birds as vectors and spreaders of pathogens of important zoonotic and other human-related diseases in urban areas. Most studies of zoonosis in crow species focused on the west Nile virus (WNV), which can cause high mortality in corvids, as demonstrated in the American Crow [,,,,]. Due to their high susceptibility to WNV, crows and ravens can thus be used as biosensors or early indicators of the presence of this virus in a given area. Similarly, corvids have been proposed or already been used to detect other pathogens of public health concern [,,,].
In addition to diseases, contamination by human-made pollutants has been documented worldwide in various urban corvids. For example, increased lead concentrations and high levels of dioxins have been detected, and other environmental chemicals have been observed in Eurasian Magpies [,,,,], Common Ravens [], Rooks [,,] and Jungle Crows [,,]. While these studies emphasize the detrimental impacts of these pollutants on urban wildlife, their biological and physiological implications on the survival or reproduction of wild animals in urban areas are not yet fully understood. Finally, high corvid abundance in cities often leads to the homogenization and/or depauperating of the urban bird fauna [,]. Some studies have indicated that the expansion of corvids, or their increase, in urban habitats have caused decreases in the richness or abundance of other species mainly via increased nest predation rate [,,].
Based on the artificial nest predation experiments, corvids are often perceived as efficient nest predators that directly impact the populations of other bird species [,]. Despite this perception, relatively few experimental removal or population control studies have been performed in corvids [,,,]. Most of these studies found that the overall impact of corvid species on nest predation rate of other species is rather small, and that the populations of other bird species are less likely to be limited by corvid predation than suspected before. Predation by corvids is thus likely an effect that influences other bird species in synergy with other negative impacts on those species. Finally, although corvids are often perceived as a nuisance, recent studies shed light on their potential role to provide supporting ecosystem services, such as helping seed dispersal of fugacious tree species through transporting fruits and seeds per caching trips over long distances [,], and regulating ecosystem services, such as health-based services by acting as a surveillance tool of important zoonotic pathogens and by reducing animal remains by scavenging within urban settings [,,,,].
4.5. Management Efforts
Corvids in urban areas have often been considered as pests and sources of nuisance, thus have become the target of management efforts around the world [,,]. Human-corvid conflicts emerge because of the corvids’ garbage scattering, fouling infrastructures, roosting in high numbers on roofs and in parks, unpleasant vocalization, attacks on pets and humans during chick-rearing, use of sensitive infrastructure for nesting and predation on birds and other animal species dear to humans. These conflicts have initiated a large number of studies discussing the management of these birds in urban areas [,,,,]. Many studies focused on the House Crow, a highly invasive corvid originally from Southeast Asia (mainly Pakistan and India) that has recently colonized and been thriving in cities of the sub-Saharan region and in the Middle East, and on the Pied Crow (Corvus albus) in Brazil. The wide range of these birds and their high flexibility makes them targets to persecution by shooting [,,,,]. Other examples of management include the destruction of Chihuahuan (Corvus cryptoleucus) and Common Ravens nests on electric poles [,,], scaring away winter roosts of American Crows in U.S. cities [,] and trapping and removal of Hooded Crow and Carrion Crow individuals in cities in Europe [,,]. However, the current population status of these species indicates that the success of these management efforts so far is limited. This implies that controlling the resources vital for urban corvid populations may be more successful at reducing their populations than direct population control. For example, Chong et al. (2012) believe that efficient waste management contributed largely to the reduction in the House Crow population in Singapore []. Restani, Marzluff and Yates (2001) also demonstrated the role of controlling access to food sources on corvid population growth in North America by focusing on the necessity of increased attention to garbage storage, animal husbandry practices and bird feeding around residential areas [].
4.6. Study Limitations
Our review has geographical and taxonomical biases that must be considered in the correct interpretation of the patterns reported. The studies in our literature sample had some geographical biases, with the majority of studies coming from the Northern Hemisphere, North America and Europe in particular. This was mainly because of a language bias, as even though we were able to assess materials published in different languages, most corvids’ studies were published in English. Yet, it is important to note that only foreign papers with an English abstract were accepted for this review. Nevertheless, we found no substantial differences in the effect of urbanization on the reviewed corvid species from the different cities around the world. Although these cities and towns represent various biogeographical and environmental characteristics, anthropogenic changes in urban environments are rather similar worldwide, which increases the external validity of our results.
The geographical bias also leads to a taxonomic (species) bias in our sample because corvid species in our literature sample are limited to those species that are distributed in the areas where studies were conducted and published in English. With numerous studies carried out in Europe and North America since the beginning of the previous century, we found only nine and two studies on the colonization/invasion of a non-native corvid (House Crow) in sub-Saharan Africa and Latin America, respectively. In addition, some species have been the object of numerous management studies, especially the House Crow [,,,,], the American Crow [,,], the Eurasian Magpie [,,] and, to a smaller extent, the Common Raven [,], the Hooded Crow [,,] and the Rook []. Studies of management have reported on the use of different methods ranging from direct shooting [], trapping [,,], egg removal [], poisoning [], pruning roost trees [], acoustic scaring of birds [,], dispersing roosts with lasers [] and refuse management in cities [].
In addition, by following the PRISMA guidelines, we may have missed some publications in relation to our topic. For example, bird community studies broadly including corvid species might not have been fully detected. This could potentially be an additional shortcoming of our analysis. While many of our results reported are from the given subset of corvid species, the patterns found and the conclusions drawn will likely be interpretable and useful for researchers studying species other than the well-studied ones.
4.7. Research Needs and Avenues
Our review also identified several gaps or shortcomings in our knowledge and thus suggests new avenues for research. First, we found a substantial geographical and taxonomical bias in studies of urban corvids (see above), and there is a dire need to initiate or report results from studies conducted in little-known geographic areas and corvid species, e.g., the Little Raven, the Chihuahuan Raven and the Jungle Crow. In the case of rapidly spreading species, such as the House Crow, there is a need to know more about the biology of species in their native range as opposed to their invaded, non-native range to understand the factors influencing their success and to provide information for their management.
Second, while we found a diversity of methods applied to study corvid species, certain species were studied by a limited set of methods. Our sample includes studies applying many different methods, such as: (i) experimental studies of corvid behavior to human presence or of responses of corvid populations to management efforts, such as removal experiments for predation impact studies, (ii) spatial methods, such as transect surveys and radio/satellite tracking methods, for home range and space use studies [,,] and (iii) correlative studies to collect biological and ecological parameters using existing data over time and space (e.g., [,]), which can provide informative comparisons of urban and rural populations of corvid species to understand the factors facilitating the colonization and increase in corvids in urban environments. This methodological diversity was not independent of species; for example, the Eurasian Magpie was the most studied Corvidae, mostly because it is native to Europe, and was most often studied by correlative methods primarily to understand nest site selection. Similarly, studies of American Crows and Common Ravens were mostly initiated for understanding the role of anthropogenic food sources and for monitoring of West Nile Virus. Moreover, while only a few studies explored the biological aspects of urban House Crows in their native range, numerous studies discussed management tools and methods to eradicate this bird in their non-native areas. While the methodological approaches will lead to interesting results on their own, we stress that their combined use can probably further increase the applicability of results for both science and practice.
Third, we need to know more about whether and how exposure to pollutants widespread in the urban environments affects the biology or physiology of corvids. Pollution is likely to have a harmful impact on all wildlife, but this likelihood probably varies between species and by the type or concentration of the pollutant.
Finally, we need more information on management and conservation. Management of crow populations that have exceeded the acceptable level of abundance has become a necessity in many parts of the world. However, we have little information on successful long-term methods of population control and removal of corvids from urban environments. While direct methods, such as hunting bag data, in the case of control by shooting can be informative in many cases, indirect methods, such as control of resources (e.g., reducing food availability), are much less frequently studied or reported, and thus little is known on the efficiency of these approaches. More studies are needed to understand the presumed negative impact of corvids on assemblages of urban species of birds and other taxa (e.g., rodents) for future management of corvids, for the conservation of urban wildlife and for city planning. One interesting study topic might be why some corvid species are urbanized, whereas others, such as the Siberian Jay (Perisoreus infaustus), are not. Evidently, the habitat needs of some corvid species are not fulfilled in cities. For example, the Siberian Jay is an old-growth bird species living in old-aged and large-sized coniferous forests []; therefore, this species does not breed or over-winter in cities. Since urban green areas are normally quite small-sized, fragmented and deciduous tree-dominated [].
5. Conclusions
Our review shows that corvids have long been associated with the development of urban environments, and their worldwide distribution makes them the perfect model system to study the effects of urbanization on wildlife. A considerable proportion of species in the Corvidae family have already been shown to adapt to urban environments, and, with consideration to the geographical and taxonomical bias in our literature sample, it appears likely that many more species will be shown to be successful in the adaptation process. The primary traits of corvids that enable them to exploit new, urban environments are their high behavioral plasticity and flexible resource use. With easily accessible food being the most important resource attracting these birds into cities; influencing different traits of habitat selection (e.g., use of new nesting sites) and life history (e.g., earlier nesting, larger clutches, higher fledging success, reduced home ranges and territoriality), as well as behavior (increased tolerance of humans); understanding the relative importance of these changes in each species will be fundamental to better understand the adaptation process and human–wildlife interactions and to develop efficient management applications. While the effects of urbanization on numerous corvid species have been relatively well explored, there are important gaps in our knowledge, calling for a more diversified approach to study this process with different, complementary methods and a focus on the potential benefits of this process, such as the ecosystem services that corvid species provide. We encourage researchers to also address these aspects for a more balanced view of corvids in urban environments.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ani11113226/s1, Table S1: Prisma Check List, Table S2: List of reviewed articles with the reference numbers used in the tables, Table S3: Number of species-specific articles by species and continent (n = 424). The reference numbers in the table refers to the individual publication numbers given in Table S2.
Author Contributions
Conceptualization, I.B. and L.K.; Methodology, J.J.; Investigation, I.B. and P.P.; Writing—Original Draft Preparation, I.B. and L.K.; Writing—Review and Editing, I.B., J.J., L.K., M.-L.K.-J. and S.L.; Supervision, J.J., L.J. and G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Tempus Public Foundation—Stipendium Hungaricum Program, grant number: SH-00355-004/2019. The publication is supported by the EFOP-3.6.1-16-2016-0002 project. The project is co-financed by the European Union and the European Social Fund. S.L. was supported by a grant from the National Research, Development, and Innovation Office of Hungary (NKFIH-OTKA K134391).
Data Availability Statement
Reference list of all publications used in this review is available in Supplementary Table S2.
Acknowledgments
We are grateful to three anonymous reviewers whose comments and suggestions greatly contributed to the improvement of the manuscript.
Conflicts of Interest
The authors state that there are no conflicts of interest. J.J. is the Editor-in-Chief of Animals, Section Birds. However, he did not participate in any way in the review process of this manuscript.
References
- Shochat, E.; Warren, P.S.; Faeth, S.H.; McIntyre, N.E.; Hope, D. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol. Evol. 2006, 21, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, D.E.; Cannon, A.R.; Toms, M.P.; Leech, D.I.; Hatchwell, B.J.; Gaston, K.J. Avian productivity in urban landscapes: A review and meta-analysis. Ibis 2009, 151, 1–18. [Google Scholar] [CrossRef]
- Kristan, W.B., III.; Boarman, W.I. Effects of anthropogenic developments on common raven nesting biology in the west Mojave Desert. Ecol. Appl. 2007, 17, 1703–1713. [Google Scholar]
- McDonnell, M.J.; Pickett, S.T. Ecosystem structure and function along urban-rural gradients: An unexploited opportunity for ecology. Ecology 1990, 71, 1232–1237. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, Z.; Wang, B. Effect of urbanization intensity on nest-site selection by Eurasian Magpies (Pica pica). Urban Ecosyst. 2020, 23, 1099–1105. [Google Scholar] [CrossRef]
- Peterson, M.N.; Peterson, M.J.; Peterson, T.R.; Liu, J. A household perspective for biodiversity conservation. J. Wildl. Manag. 2007, 71, 1243–1248. [Google Scholar] [CrossRef]
- Stoyanov, G.P.; Ivanova, T.; Petrov, B.P.; Gueorguieva, A. Past and present breeding distribution of the alpine chough (Pyrrhocorax graculus) in Western Stara Planina and Western Predbalkan Mts.(Bulgaria). Acta Zool. Bulg. Suppl. 2008, 2, 119–132. [Google Scholar]
- Fenoglio, M.S.; Rossetti, M.R.; Videla, M. Negative effects of urbanization on terrestrial arthropod communities: A meta-analysis. Glob. Ecol. Biogeogr. 2020, 29, 1412–1429. [Google Scholar] [CrossRef]
- Withey, J.C.; Marzluff, J.M. Multi-scale use of lands providing anthropogenic resources by American Crows in an urbanizing landscape. Landsc. Ecol. 2009, 24, 281–293. [Google Scholar] [CrossRef]
- Dutta, S.K.; Raut, S.K. Nesting Site of House Crow: Tree Versus Light-Post—An Impact Assessment. Proc. Zool. Soc. 2013, 66, 141–148. [Google Scholar] [CrossRef]
- Szala, K.; Kubicka, A.M.; Sparks, T.H.; Tryjanowski, P. Birds using tram tracks in Poznań (Poland): Species, infrastructure use and behaviour. Transp. Res. D Transp. Environ. 2020, 81, 102282. [Google Scholar] [CrossRef]
- United Nations. World Population Prospects: Highlights; UN DESA: New York, NY, USA, 2019. [Google Scholar]
- Salvati, L. Distribution and size of Jackdaw Corvus monedula colonies in Inner Rome Central Italy. Alauda 2002, 70, 347–349. [Google Scholar]
- Lim, H.C.; Sodhi, N. Space use and habitat selection of house crows in a tropical urban environment: A radio-tracking study. Raffles Bull. Zool. 2009, 57, 561–568. [Google Scholar]
- Matsyura, A.V.; Jankowski, K. Spatial patterns of habitat distribution of Corvidae (the case of urban-rural gradient). Biosyst. Divers. 2016, 24, 451–458. [Google Scholar] [CrossRef]
- Kark, S.; Iwaniuk, A.; Schalimtzek, A.; Banker, E. Living in the city: Can anyone become an ‘urban exploiter’? J. Biogeogr. 2007, 34, 638–651. [Google Scholar] [CrossRef]
- Jokimäki, J.; Suhonen, J.; Vuorisalo, T.; Kövér, L.; Kaisanlahti-Jokimäki, M.L. Urbanization and nest-site selection of the Black-billed Magpie (Pica pica) populations in two Finnish cities: From a persecuted species to an urban exploiter. Landsc. Urban Plan. 2017, 157, 577–585. [Google Scholar] [CrossRef]
- Šálek, M.; Grill, S.; Riegert, J. Nest-site selection of an avian urban exploiter, the Eurasian magpie Pica pica, across the urban-rural gradient. J. Vertebr. Biol. 2020, 70, 20086-1. [Google Scholar] [CrossRef]
- Kennedy, J.D.; Borregaard, M.K.; Jønsson, K.A.; Holt, B.; Fjeldså, J.; Rahbek, C. Does the colonization of new biogeographic regions influence the diversification and accumulation of clade richness among the Corvides (Aves: Passeriformes)? Evolution 2017, 71, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Lowry, H.; Lill, A.; Wong, B.B. Behavioural responses of wildlife to urban environments. Biol. Rev. Biol. 2013, 88, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Górski, W.; Kotlarz, B. Changes and breeding ecology in an urban population of the Magpie Pica pica in Słupsk, NW Poland. Acta Ornithol. 1997, 32, 61–67. [Google Scholar]
- Schoech, S.J.; Bowman, R. Variation in the timing of breeding between suburban and wildland Florida Scrub-Jays: Do physiologic measures reflect different environments? In Avian Ecology and Conservation in an Urbanizing World; Springer: Boston, MA, USA, 2001; pp. 289–306. [Google Scholar]
- O’Brien, R.C.; Larcombe, A.; Meyer, J.; Forbes, S.L.; Dadour, I. The scavenging behaviour of the Australian raven (Corvus coronoides): Patterns and influencing factors. Sylvia 2010, 46, 133–148. [Google Scholar]
- Vasilev, V.; Marinov, M.P. Oological Characteristics of a Colony of Eurasian Jackdaw (Corvus monedula L.) in the Town of Shumen, NE Bulgaria. Acta Zool. Bulg. 2017, 69, 143–147. [Google Scholar]
- Evans, K.L.; Newson, S.E.; Gaston, K.J. Habitat influences on urban avian assemblages. Ibis 2009, 151, 19–39. [Google Scholar] [CrossRef]
- Chong, K.Y.; Teo, S.; Kurukulasuriya, B.; Chung, Y.F.; Rajathurai, S.; Lim, H.C.; Tan, H.T. Decadal changes in urban bird abundance in Singapore. Raffles Bull. Zool. 2012, 25, 189–196. [Google Scholar]
- Antonov, A.; Atanasova, D. Re-use of old nests versus the construction of new ones in the Magpie Pica pica in the city of Sofia (Bulgaria). Acta Ornithol. 2003, 38, 1–4. [Google Scholar] [CrossRef]
- Allan, D.G.; Davies, G.B. Breeding biology of house crows (Corvus splendens) in Durban, South Africa. Ostrich 2005, 76, 21–31. [Google Scholar] [CrossRef]
- Clucas, B.; Marzluff, J.M.; Mackovjak, D.; Palmquist, I. Do A merican Crows Pay Attention to Human Gaze and Facial Expressions? Ethology 2013, 119, 296–302. [Google Scholar] [CrossRef]
- Bagyura, J.; Fidlóczky, J.; Schwartz, V.; Tóth, L. Interesting breeding cases of the Raven (Corvus corax) in Hungary. Ornis Hung. 2017, 25, 39–43. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Droege, G.; Töpfer, T. The Corvids Literature Database—500 years of ornithological research from a crow’s perspective. Database 2016, 2016, bav122. Available online: http://www.corvids.de/cld/search.php (accessed on 17 October 2020). [CrossRef] [PubMed]
- Vigallon, S.M.; Marzluff, J.M. Abundance, nest sites, and nesting success of Steller’s Jays along a gradient of urbanization in western Washington. Northwest Sci. 2005, 79, 22. [Google Scholar]
- Carreiro, M.M. Using the urban–rural gradient approach to determine the effects of land use on forest remnants. In Ecology, Planning, and Management of Urban Forests; Springer: New York, NY, USA, 2008; pp. 169–186. [Google Scholar]
- Kroll, J.F.; Van Hell, J.G.; Tokowicz, N.; Green, D.W. The Revised Hierarchical Model: A critical review and assessment. Biling. Lang. Cogn. 2010, 13, 373. [Google Scholar] [CrossRef]
- Julian, K.G.; Eidson, M.; Kipp, A.M.; Weiss, E.; Petersen, L.R.; Miller, J.R.; Marfin, A.A. Early season crow mortality as a sentinel for West Nile virus disease in humans, northeastern United States. Vector Borne Zoonotic Dis. 2002, 2, 145–155. [Google Scholar] [CrossRef]
- LaDeau, S.L.; Calder, C.A.; Doran, P.J.; Marra, P.P. West Nile virus impacts in American crow populations are associated with human land use and climate. Ecol. Res. 2011, 26, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Hix, G.E. A Year with the Birds in New York City. Wilson Bull. 1905, 17, 35–43. [Google Scholar]
- Emlen, J.T. Midwinter distribution of the American Crow in New York State. Ecology 1938, 19, 264–275. [Google Scholar] [CrossRef]
- Thurston, H.; Boyle, H.S. Some Seasonal Notes on Long Island Birds. Auk 1913, 30, 542–545. [Google Scholar] [CrossRef]
- Bowles, J.H.; Decker, F.R. The ravens of the State of Washington. Condor 1930, 32, 192–201. [Google Scholar] [CrossRef]
- Macpherson, A.H. A list of the birds of Inner London. Br. Birds 1929, 22, 222–244. [Google Scholar]
- Burns, P.S. Rook and Jackdaw roosts around Bishop’s Stortford. Bird Study 1957, 4, 62–71. [Google Scholar] [CrossRef]
- Selous, E. An observational diary on the domestic habits of the Carrion-Crow (Pica pica). Zoologist 1912, 16, 321–337. [Google Scholar]
- Slagsvold, T. Hooded Crow Corvus corone cornix census 1973–78 at Trondheim, Central Norway. Fauna Norv. Ser. C. Cinclus. 1979, 2, 1–6. [Google Scholar]
- McNair, D.B. Status and distribution of the Fish Crow in the Carolinas and Georgia. Oriole 1987, 52, 28–45. [Google Scholar]
- Jefferson, B. Observations of Common Ravens in metropolitan Totonto. Ont. Birds 1989, 7, 15–20. [Google Scholar]
- Batten, L.A. Breeding bird species diversity in relation to increasing urbanisation. Bird Study 1972, 19, 157–166. [Google Scholar] [CrossRef]
- Campbell, B.; Turner, G.E.S. Ravens breeding on city buildings. Br. Birds 1976, 69, 229–230. [Google Scholar]
- Altevogi, R.; Davis, T.A. Urbanization in nest building of Indian House Crow (Corvus splendens Vieillot). J. Bombay Nat. Hist. Soc. 1979, 76, 283–290. [Google Scholar]
- Tatner, P. The breeding biology of magpies Pica pica in an urban environment. J. Zool. 1982, 197, 559–581. [Google Scholar] [CrossRef]
- Antikainen, E. The Breeding Success of the Jackdaw Corvus monedula in Nesting Cells. Ornis Fennica 1981, 58, 72–77. [Google Scholar]
- Eden, S.F. The comparative breeding biology of magpies Pica pica in an urban and a rural habitat (Aves: Corvidae). J. Zool. 1985, 205, 325–334. [Google Scholar] [CrossRef]
- Knight, R.L.; Knight, H.A.; Camp, R.J. Raven populations and land-use patterns in the Mojave Desert, California. Wildl. Soc. Bull. 1993, 469–471. [Google Scholar]
- Rolando, A.; Patterson, I.J. Range and movements of the Alpine Chough Pyrrhocorax graculus in relation to human developments in the Italian Alps in summer. J. Ornithol. 1993, 134, 338–344. [Google Scholar] [CrossRef]
- Nowakowski, J.J. Changes in the breeding avifauna of Olsztyn [NE Poland] in the years 1968–1993. Acta Ornithol. 1996, 31, 39–44. [Google Scholar]
- Breininger, D.R.; Burgman, M.A.; Stith, B.M. Influence of habitat quality, catastrophes, and population size on extinction risk of the Florida scrub-jay. Wildl. Soc. Bull. 1999, 810–822. [Google Scholar]
- Jokimäki, J.; Kaisanlahti-Jokimäki, M.L. Spatial similarity of urban bird communities: A multiscale approach. J. Biogeogr. 2003, 30, 1183–1193. [Google Scholar] [CrossRef]
- Mazgajski, T.D.; Woźniak, A.; Żmihorski, M. Changes in the numbers of corvids wintering in Warsaw over years 1974–2004. In Corvids of Poland; Jerzak, L., Kavanagh, B.P., Tryjanowski, P., Eds.; Bogucki Wyd. Nauk.: Poznań, Poland, 2005. [Google Scholar]
- Anderies, J.M.; Katti, M.; Shochat, E. Living in the city: Resource availability, predation, and bird population dynamics in urban areas. J. Theor. Biol. 2007, 247, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Webb, W.C.; Marzluff, J.M.; Hepinstall-Cymerman, J. Linking resource use with demography in a synanthropic population of common ravens. Biol. Conser. 2011, 144, 2264–2273. [Google Scholar] [CrossRef]
- Nihei, Y.; Higuchi, H. When and where did crows learn to use automobiles as nutcrackers. Tohoku Psychol. Folia 2001, 60, 93–97. [Google Scholar]
- Bílá, K.; Beránková, J.; Veselý, P.; Bugnyar, T.; Schwab, C. Responses of urban crows to con-and hetero-specific alarm calls in predator and non-predator zoo enclosures. Anim. Cogn. 2017, 20, 43–51. [Google Scholar] [CrossRef]
- Taufique, S.T.; Prabhat, A.; Kumar, V. Illuminated night alters hippocampal gene expressions and induces depressive-like responses in diurnal corvids. Eur. J. Neurosci. 2018, 48, 3005–3018. [Google Scholar] [CrossRef]
- Lee, V.E.; Régli, N.; McIvor, G.E.; Thornton, A. Social learning about dangerous people by wild jackdaws. R. Soc. Open Sci. 2019, 6, 191031. [Google Scholar] [CrossRef]
- Breininger, D.R.; Stolen, E.D.; Carter, G.M.; Oddy, D.M.; Hunt, D.K. A model-selection approach to predicting whether Florida scrub-jays delay breeding. Condor 2010, 112, 378–389. [Google Scholar] [CrossRef]
- Baltensperger, A.P.; Mullet, T.C.; Schmid, M.S.; Humphries, G.R.W.; Kövér, L.; Huettmann, F. Seasonal observations and machine-learning-based spatial model predictions for the common raven (Corvus corax) in the urban, sub-arctic environment of Fairbanks, Alaska. Polar Biol. 2013, 36, 1587–1599. [Google Scholar] [CrossRef]
- McLane, A.J.; Semeniuk, C.; McDermid, G.J.; Tomback, D.F.; Lorenz, T.; Marceau, D. Energetic behavioural-strategy prioritization of clark’s nutcrackers in whitebark pine communities: An agent-based modeling approach. Ecol. Modell. 2017, 354, 123–139. [Google Scholar] [CrossRef]
- Le Louarn, M.; Clergeau, P.; Strubbe, D.; Deschamps-Cottin, M. Dynamic species distribution models reveal spatiotemporal habitat shifts in native range-expanding versus non-native invasive birds in an urban area. J. Avian Biol. 2018, 49, jav-01527. [Google Scholar] [CrossRef]
- Rolando, A.; Laiolo, P.; Carisio, l. Urbanization and the flexibility of the foraging ecology of the Alpine Chough Pyrrhocorax graculus in winter. Rev. Ecol. 2003, 58, 337–352. [Google Scholar]
- Marzluff, J.M.; Neatherlin, E. Corvid response to human settlements and campgrounds: Causes, consequences, and challenges for conservation. Biol. Conserv. 2006, 130, 301–314. [Google Scholar] [CrossRef]
- Vines, A.; Lill, A. Boldness and urban dwelling in little ravens. Wildl. Res. 2016, 42, 590–597. [Google Scholar] [CrossRef]
- Kövér, L.; Lengyel, S.; Takenaka, M.; Kirchmeir, A.; Uhl, F.; Miller, R.; Schwab, C. Why do zoos attract crows? A comparative study from Europe and Asia. Ecol. Evol. 2019, 9, 14465–14475. [Google Scholar] [CrossRef]
- Boarman, W.I.; Camp, R.J.; Hagan, M.; Deal, W. Raven Abundance at Anthropogenic Resources in the Western Mojave Desert, California; Report to Edwards Air Force Base, CA; National Biological Service: Riverside, CA, USA, 1995. [Google Scholar]
- Restani, M.; Marzluff, J.M.; Yates, R.E. Effects of anthropogenic food sources on movements, survivorship, and sociality of Common Ravens in the Arctic. Condor 2001, 103, 399–404. [Google Scholar] [CrossRef]
- Bui, T.V.D.; Marzluff, J.M.; Bedrosian, B. Common raven activity in relation to land use in western Wyoming: Implications for greater sage-grouse reproductive success. Condor 2010, 112, 65–78. [Google Scholar] [CrossRef]
- Harju, S.M.; Olson, C.V.; Hess, J.E.; Bedrosian, B. Common raven movement and space use: Influence of anthropogenic subsidies within greater sage-grouse nesting habitat. Ecosphere 2018, 9, e02348. [Google Scholar] [CrossRef]
- Coates, P.S.; Brussee, B.E.; Howe, K.B.; Gustafson, K.B.; Casazza, M.L.; Delehanty, D.J. Landscape characteristics and livestock presence influence common ravens: Relevance to greater sage-grouse conservation. Ecosphere 2016, 7, e01203. [Google Scholar] [CrossRef]
- Kristan III, W.B.; Boarman, W.I.; Crayon, J.J. Diet composition of common ravens across the urban-wildland interface of the West Mojave Desert. Wildl. Soc. Bull. 2004, 32, 244–253. [Google Scholar] [CrossRef]
- Loretto, M.C.; Reimann, S.; Schuster, R.; Graulich, D.M.; Bugnyar, T. Shared space, individually used: Spatial behaviour of non-breeding ravens (Corvus corax) close to a permanent anthropogenic food source. J. Ornithol. 2016, 157, 439–450. [Google Scholar] [CrossRef]
- Marzluff, J.M.; McGowan, K.J.; Donnelly, R.; Knight, R.L. Causes and Consequences of Expanding American Crow Populations. In Avian Ecology and Conservation in an Urbanizing World; Springer: Boston, MA, USA, 2001; pp. 331–363. [Google Scholar]
- Withey, J.C.; Marzluff, J.M. Dispersal by juvenile American crows (Corvus brachyrhynchos) influences population dynamics across a gradient of urbanization. Auk 2005, 122, 205–221. [Google Scholar] [CrossRef]
- McGowan, K.J. Demographic and behavioral comparisons of suburban and rural American Crows. In Avian Ecology and Conservation in an Urbanizing World; Springer: Boston, MA, USA, 2001; pp. 365–381. [Google Scholar]
- Matsubara, H. Comparative study of territoriality and habitat use in syntopic Jungle Crow (Corvus macrorhynchos) and Carrion Crow (C. corone). Ornithol. Sci. 2003, 2, 103–111. [Google Scholar] [CrossRef]
- Tuljapurkar, V.B.; Bhagwat, V. Avifauna of a waste disposal site. Indian Birds 2007, 3, 87–90. [Google Scholar]
- Preininger, D.; Schoas, B.; Kramer, D.; Boeckle, M. Waste Disposal Sites as All-You-Can Eat Buffets for Carrion Crows (Pica pica). Animals 2019, 9, 215. [Google Scholar] [CrossRef]
- Uhl, F.; Ringler, M.; Miller, R.; Deventer, S.A.; Bugnyar, T.; Schwab, C. Counting crows: Population structure and group size variation in an urban population of crows. Behav. Ecol. 2019, 30, 57–67. [Google Scholar] [CrossRef]
- Yoda, T. Managing urban crow populations in Japan. HUM-WILDL Interact. 2019, 13, 12. [Google Scholar]
- Vallino, C.; Caprio, E.; Genco, F.; Chamberlain, D.; Palestrini, C.; Roggero, A.; Rolando, A. Behavioural responses to human disturbance in an alpine bird. J. Ornithol. 2019, 160, 763–772. [Google Scholar] [CrossRef]
- Väisänen, R.A. Frequency and abundance of 61 bird species at feeding sites in Finland in 1880/1991—2019/2020. Linnut-Vuosikirja 2020, 30–45. [Google Scholar]
- Czechowski, P.; Bocheński, M.; Ciebiera, O. Decline of Jackdaws Corvus monedula in the city of Zielona Góra. Int. Stud. Sparrows 2013, 37, 32–36. [Google Scholar] [CrossRef]
- Meyrier, E.; Jenni, L.; Bötsch, Y.; Strebel, S.; Erne, B.; Tablado, Z. Happy to breed in the city? Urban food resources limit reproductive output in Western Jackdaws. Ecol. Evol. 2017, 7, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Vuorisalo, T.; Andersson, H.; Hugg, T.; Lahtinen, R.; Laaksonen, H.; Lehikoinen, E. Urban development from an avian perspective: Causes of hooded crow (Pica pica cornix) urbanisation in two Finnish cities. Landsc. Urban Plan. 2003, 62, 69–87. [Google Scholar] [CrossRef]
- Whisson, D.A.; Weston, M.A.; Shannon, K. Home range, habitat use and movements by the little raven (Corvus mellori) in a coastal peri-urban landscape. Wildl. Res. 2015, 42, 500–508. [Google Scholar] [CrossRef]
- Bender, H. Caching or coating? An interesting behaviour by the Little Raven Corvus mellori in an urban setting. Vic. Nat. 2018, 135, 18. [Google Scholar]
- Bednorz, J. Ravens Corvus corax LINNAEUS, 1758, nesting on electricity pylons in the Wielkopolska region. Acta Zool. Cracov. 2000, 43, 177–184. [Google Scholar]
- Jurčević Agić, I. Ravens, Corvus corax (L. 1758), nesting on high-voltage transmission line pylons in Croatia. Belg. J. Zool. 2006, 136, 167–171. [Google Scholar]
- Antonov, A.; Atanasova, D. Nest-site selection in the Magpie Pica pica in a high-density urban population of Sofia (Bulgaria). Acta Ornithol. 2002, 37, 55–66. [Google Scholar] [CrossRef]
- McIvor, G.E.; Healy, S.D. Nest site selection and patterns of nest re-use in the Hooded Crow Corvus cornix. Bird Study 2017, 64, 374–385. [Google Scholar] [CrossRef]
- Kövér, L.; Gyüre, P.; Balogh, P.; Huettmann, F.; Lengyel, S.; Juhász, L. Recent colonization and nest site selection of the Hooded Crow (Pica pica cornix L.) in an urban environment. Landsc. Urban Plan. 2015, 133, 78–86. [Google Scholar] [CrossRef]
- Salvati, L. Nest site and breeding habitat characteristics in urban jackdaws Corvus monedula in Rome (Italy). Acta Ornithol. 2002, 37, 15–19. [Google Scholar] [CrossRef]
- Božič, L. Numbers, distribution and nest site characteristics of Jackdaw Corvus monedula in Slovenia and its conservation status. Acrocephalus 2016, 37, 123–150. [Google Scholar] [CrossRef]
- Fleischer Jr, A.L.; Bowman, R.; Woolfenden, G.E. Variation in foraging behavior, diet, and time of breeding of Florida scrub-jays in suburban and wildland habitats. Condor 2003, 105, 515–527. [Google Scholar] [CrossRef]
- Richner, H. The effect of extra food on fitness in breeding carrion crows. Ecol. 1992, 73, 330–335. [Google Scholar] [CrossRef]
- Schoech, S.J.; Bowman, R. Does differential access to protein influence differences in timing of breeding of Florida scrub-jays (Aphelocoma coerulescens) in suburban and wildland habitats? Auk 2003, 120, 1114–1127. [Google Scholar] [CrossRef]
- Yamac, E.; Kirazli, C. Road Effect on the Breeding Success and Nest Characteristics of the Eurasian Magpie (Pica pica). Ekoloji 2012, 21, 1–10. [Google Scholar] [CrossRef]
- Aldredge, R.A.; LeClair, S.C.; Bowman, R. Declining egg viability explains higher hatching failure in a suburban population of the threatened Florida scrub-jay Aphelocoma coerulescens. J. Avian Biol. 2012, 43, 369–375. [Google Scholar] [CrossRef]
- Jadczyk, P.; Drzeniecka-Osiadacz, A. Feeding strategy of wintering rooks Corvus frugilegus L. in urban habitats. Pol. J. Ecol. 2013, 61, 587–596. [Google Scholar]
- Miller, A.E.; Goad, E.; Gallo, T.; Reed, S.E.; Bailey, L.L.; Pejchar, L. The effect of exurban development on wintering birds in Colorado. Wilson J. Ornithol. 2017, 129, 85–97. [Google Scholar] [CrossRef]
- Brauze, T.; Zieliński, J. Intrapersonal and Interpersonal Differences in Results of Quantitative Winter Studies on Selected Species of Corvids in Urban Green Areas. Russ. J. Ecol. 2019, 50, 75–79. [Google Scholar] [CrossRef]
- Szala, K.; Dylewski, Ł.; Tobolka, M. Winter habitat selection of Corvids in an urban ecosystem. Urban Ecosyst. 2020, 23, 483–493. [Google Scholar] [CrossRef]
- Ciach, M.; Fröhlich, A. Habitat type, food resources, noise and light pollution explain the species composition, abundance and stability of a winter bird assemblage in an urban environment. Urban Ecosyst. 2017, 20, 547–559. [Google Scholar] [CrossRef]
- Leveau, L.M. Artificial light at night (ALAN) is the main driver of nocturnal feral pigeon (Columba livia f. domestica) foraging in urban areas. Animals 2020, 10, 554. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.D.; Diez-Méndez, D.; Kempenaers, B. Effects of experimental night lighting on the daily timing of winter foraging in common European songbirds. J. Avian Biol. 2017, 48, 862–871. [Google Scholar] [CrossRef]
- Rockwell, C.; Gabriel, P.O.; Black, J.M. Bolder, older, and selective: Factors of individual-specific foraging behaviors in Steller’s jays. Behav. Ecol. 2012, 23, 676–683. [Google Scholar] [CrossRef]
- Miller, R.; Schiestl, M.; Whiten, A.; Schwab, C.; Bugnyar, T. Tolerance and social facilitation in the foraging behaviour of free-ranging crows (Pica pica corone; C. c. cornix). Ecology 2014, 120, 1248–1255. [Google Scholar]
- Brown, M.J.; Jones, D.N. Cautious crows: Neophobia in Torresian crows (Corvus orru) compared with three other corvoids in suburban Australia. Ecology 2016, 122, 726–733. [Google Scholar] [CrossRef]
- Poddubnaya, N.; Korotkova, T.; Vanicheva, P. Increasing corvid tolerance to humans in urban ecosystems with increasing latitude. Commun. Biol. 2019, 64, 252–259. [Google Scholar] [CrossRef][Green Version]
- Neatherlin, E.A.; Marzluff, J.M. Responses of American crow populations to campgrounds in remote native forest landscapes. J. Wildl. Manag. 2004, 68, 708–718. [Google Scholar] [CrossRef]
- Cornell, H.N.; Marzluff, J.M.; Pecoraro, S. Social learning spreads knowledge about dangerous humans among American crows. Proc. R. Soc. B. 2012, 279, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Deventer, S.A.; Uhl, F.; Bugnyar, T.; Miller, R.; Fitch, W.T.; Schiestl, M.; Schwab, C. Behavioural type affects space use in a wild population of crows (Corvus corone). Ecology 2016, 122, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Matsyura, A.; Jankowski, K.; Zimaroeva, A. Escape behaviours of Corvidae in an urban ecosystem of Zhytomyr (Ukraine). Rom. J. Biol. Zool. 2015, 60, 125–134. [Google Scholar]
- Tätte, K.; Møller, A.P.; Mänd, R. Corvids exhibit dynamic risk assessment during escape. Behav. Process. 2020, 170, 104017. [Google Scholar] [CrossRef]
- Gorenzel, W.P.; Salmon, T.P. Tape-recorded calls disperse American crows from urban roosts. Wildl. Soc. Bull. 1993, 21, 334–338. [Google Scholar]
- Brook, B.W.; Sodhi, N.S.; Soh, M.C.; Lim, H.C. Abundance and projected control of invasive house crows in Singapore. J. Wildl. Manag. 2003, 808–817. [Google Scholar] [CrossRef]
- Directive 2009/147/EC of the European Parliament and of the Council of 30 November 2009 on the Conservation of Wild Birds [2010]. OJ 2010, L20, 7–25.
- Haemig, P.D.; de Luna, S.S.; Blank, H.; Lundqvist, H. Ecology and phylogeny of birds foraging at outdoor restaurants in Sweden. Biodivers. Data J. 2015, 3, e6360. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Glahn, J.F. European starlings. In The Handbook: Prevention and Control of Wildlife Damage; Hygnstrom, S.E., Timm, R.M., Larson, G.E., Eds.; University of Nebraska: Lincoln, NE, USA, 1994; p. 72. [Google Scholar]
- Smith, H.T.; Engeman, R.M. An Extraordinary Raccoon, Procyon Lotor, Density at an Urban Park; USDA-NWRC: Fort Collins, CO, USA, 2002; p. 487. [Google Scholar]
- Yaremych, S.A.; Novak, R.J.; Raim, A.J.; Mankin, P.C.; Warner, R.E. Home range and habitat use by American Crows in relation to transmission of West Nile virus. Wilson J. Ornithol. 2004, 116, 232–239. [Google Scholar] [CrossRef]
- Ludwig, A.; Bigras-Poulin, M.; Michel, P.; Bélanger, D. Risk factors associated with West Nile virus mortality in American Crow populations in Southern Quebec. J. Wildl. Dis. 2010, 46, 195–208. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bingham, J.; Lunt, R.A.; Green, D.J.; Davies, K.R.; Stevens, V.; Wong, F.Y.K. Experimental studies of the role of the little raven (Corvus mellori) in surveillance for West Nile virus in Australia. Aust. Vet. J. 2010, 88, 204–210. [Google Scholar] [CrossRef]
- Vlahović, K.; Prukner-Radovčić, E.; Horvatek, D.; Pavlak, M.; Gomerčić, T.; Rumiha, Z.; Dovč, A. Bacterial and fungal flora in faecal samples from rooks (Corvus frugilegus) in the City of Zagreb, Croatia. Vet Arh 2010, 80, 81–92. [Google Scholar]
- Weis, A.M.; Miller, W.A.; Byrne, B.A.; Chouicha, N.; Boyce, W.M.; Townsend, A.K. Prevalence and pathogenic potential of Campylobacter isolates from free-living, human-commensal American crows. Appl. Environ. Microbiol. 2014, 80, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Perec-Matysiak, A.; Wesołowska, M.; Leśniańska, K.; Buńkowska-Gawlik, K.; Hildebrand, J.; Kicia, M. Survey for zoonotic microsporidian pathogens in wild living urban rooks (Corvus frugilegus). J. Eukaryot. Microbiol. 2017, 64, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Sadraei, J.; Dalimi, A.; Pirestani, M. Isolation of Encephalitozoon intestinalis from crows living in urban parks of Tehran, Iran: An investigation with zoonotic aspect. J. Parasit. Dis. 2018, 42, 494–499. [Google Scholar]
- Dmowski, K.; Golimowski, J. Feathers of the magpie (Pica pica) as a bioindicator material for heavy metal pollution assessment. Sci. Total Environ. 1993, 139, 251–258. [Google Scholar] [CrossRef]
- Dmowski, K. Environmental monitoring of heavy metals with magpie (Pica pica) feathers—An example of Polish polluted and control areas. In Trace Metals in the Environment; Elsevier: Amsterdam, The Netherlands, 2000; pp. 455–477. [Google Scholar]
- Zarrintab, M.; Mirzaei, R.; Mostafaei, G.; Dehghani, R.; Akbari, H. Concentrations of metals in feathers of magpie (Pica pica) from Aran-O-Bidgol city in Central Iran. Bull. Environ. Contam. Toxicol. 2016, 96, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Zarrintab, M.; Mirzaei, R. Tissue distribution and oral exposure risk assessment of heavy metals in an urban bird: Magpie from Central Iran. Environ. Sci. Pollut. Res. 2018, 25, 17118–17127. [Google Scholar] [CrossRef] [PubMed]
- Girişgin, A.O.; Demirer, A.A.; Büyükcangaz, E.; Khider, M.; Birlik, S.; İpek, V. Postmortem findings on a group of Pica pica (Passeriformes: Corvidae). Ankara Univ. Vet. Fak. Derg. 2019, 66, 155–161. [Google Scholar]
- Lopez-Perea, J.J.; Camarero, P.R.; Sanchez-Barbudo, I.S.; Mateo, R. Urbanization and cattle density are determinants in the exposure to anticoagulant rodenticides of non-target wildlife. Environ. Pollut. 2019, 244, 801–808. [Google Scholar] [CrossRef]
- Orłowski, G.; Kasprzykowski, Z.; Dobicki, W.; Pokorny, P.; Polechoński, R. Geographical and habitat differences in concentrations of copper, zinc and arsenic in eggshells of the Rook Corvus frugilegus in Poland. J. Ornithol. 2010, 151, 279–286. [Google Scholar] [CrossRef]
- Orłowski, G.; Kasprzykowski, Z.; Dobicki, W.; Pokorny, P.; Wuczyński, A.; Polechoński, R.; Mazgajski, T.D. Trace-element interactions in rook Corvus frugilegus eggshells along an urbanisation gradient. Arch. Environ. Contam. Toxicol. 2014, 67, 519–528. [Google Scholar] [CrossRef]
- Luo, J.; Li, X.; Wang, Y.; Li, H. Characterization of Pb and Cd contamination in the feces and feathers of rook (Corvus frugilegus) and the scalp hair of residents in Qiqihar, northeastern China. Human and Ecological Risk Assessment. Int. J. Res. 2018, 24, 494–508. [Google Scholar]
- Watanabe, M.X.; Iwata, H.; Okamoto, M.; Kim, E.Y.; Yoneda, K.; Hashimoto, T.; Tanabe, S. Induction of cytochrome P450 1A5 mRNA, protein and enzymatic activities by dioxin-like compounds, and congener-specific metabolism and sequestration in the liver of wild jungle crow (Corvus macrorhynchos) from Tokyo, Japan. Toxicol. Sci. 2005, 88, 384–399. [Google Scholar] [CrossRef][Green Version]
- Kobayashi, M.; Kashida, Y.; Yoneda, K.; Iwata, H.; Watanabe, M.; Tanabe, S.; Mitsumori, K. Thyroid lesions and dioxin accumulation in the livers of jungle crows (Corvus macrorhynchos) in urban and suburban Tokyo. Arch. Environ. Contam. Toxicol. 2005, 48, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Janaydeh, M.; Ismail, A.; Omar, H.; Zulkifli, S.Z.; Bejo, M.H.; Aziz, N.A.A. Relationship between Pb and Cd accumulations in house crow, their habitat, and food content from Klang area, Peninsular Malaysia. Environ. Monit. Assess. 2018, 190, 47. [Google Scholar] [CrossRef] [PubMed]
- Rush, S.A.; Romito, T.; Robison, T.L. Avian diversity in a suburban park system: Current conditions and strategies for dealing with anticipated change. Urban Ecosyst. 2014, 17, 45–60. [Google Scholar] [CrossRef]
- Diquelou, M.C.; Griffin, A.S.; Sol, D. The role of motor diversity in foraging innovations: A cross-species comparison in urban birds. Behav. Ecol. 2016, 27, 584–591. [Google Scholar] [CrossRef]
- Biaduñ, W. Winter avifauna of Lublin species composition, distribution and numbers. Berkut 2005, 14, 123. [Google Scholar]
- Tomiałojć, L. Changes in breeding bird communities of two urban parks in Wrocław across 40 years (1970–2010): Before and after colonization by important predators. Ornis Polonica 2011, 52, 1–25. [Google Scholar]
- Jokimäki, J.; Huhta, E. Artificial nest predation and abundance of birds along an urban gradient. Condor 2000, 102, 838–847. [Google Scholar] [CrossRef]
- Jokimäki, J.; Kaisanlahti-Jokimäki, M.L.; Sorace, A.; Fernández-Juricic, E.; Rodriguez-Prieto, I.; Jimenez, M.D. Evaluation of the “safe nesting zone” hypothesis across an urban gradient: A multi-scale study. Ecography 2005, 28, 59–70. [Google Scholar] [CrossRef]
- Hanmer, H.J.; Thomas, R.L.; Fellowes, M.D. Provision of supplementary food for wild birds may increase the risk of local nest predation. Ibis 2017, 159, 158–167. [Google Scholar] [CrossRef]
- Shields, T.; Currylow, A.; Hanley, B.; Boland, S.; Boarman, W.; Vaughn, M. Novel management tools for subsidized avian predators and a case study in the conservation of a threatened species. Ecosphere 2019, 10, e02895. [Google Scholar] [CrossRef]
- Jokimäki, J.; Suhonen, J.; Benedetti, Y.; Diaz, M.; Kaisanlahti-Jokimäki, M.L.; Morelli, F.; Ibánez-Álamo, J.D. Land-sharing vs. land-sparing urban development modulate predator–prey interactions in Europe. Ecol. Appl. 2020, 30, e02049. [Google Scholar] [CrossRef]
- Czarnecka, J.; Kitowski, I.; Sugier, P.; Mirski, P.; Krupiński, D.; Pitucha, G. Seed dispersal in urban green space–Does the rook Corvus frugilegus L. contribute to urban flora homogenization? Urban For. Urban Green. 2013, 12, 359–366. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Higuchi, H. Invasion of the loquat Eriobotrya japonica into urban areas of central Tokyo facilitated by crows. Ornithol. Sci. 2018, 17, 165–172. [Google Scholar] [CrossRef]
- Schwartz, A.L.; Williams, H.F.; Chadwick, E.; Thomas, R.J.; Perkins, S.E. Roadkill scavenging behaviour in an urban environment. J. Urban Ecol. 2018, 4, juy006. [Google Scholar] [CrossRef]
- Sazima, I. Australian Raven (Corvus coronoides) Scavenges on all Five Major Vertebrate Groups at Urban Sydney, Southeast Australia. Trop. Nat. Hist. 2020, 20, 89–94. [Google Scholar]
- Feare, C.J.; Mungroo, Y. The status and management of the House Crow Corvus splendens (Vieillot) in Mauritius. Biol. Conserv. 1990, 51, 63–70. [Google Scholar] [CrossRef]
- Adelino, J.R.P.; dos Anjos, L.; Lima, M.R. Invasive potential of the pied crow (Corvus albus) in eastern Brazil: Best to eradicate before it spreads. PECON 2017, 15, 227–233. [Google Scholar] [CrossRef]
- Archer, A.L. Control of the Indian house crow Corvus splendens in eastern Africa. Ostrich 2001, 147–152. [Google Scholar]
- Gorenzel, W.P.; Blackwell, B.F.; Simmons, G.D.; Salmon, T.P.; Dolbeer, R.A. Evaluation of lasers to disperse American crows, Corvus brachyrhynchos, from urban night roosts. Int. J. Pest Manag. 2002, 48, 327–331. [Google Scholar] [CrossRef][Green Version]
- Ljung, P.E.; Widemo, F.; Ericsson, G. Trapping in predator management: Catching the profile of trap users in Sweden. Eur. J. Wildl. Res. 2014, 60, 681–689. [Google Scholar] [CrossRef]
- Kövér, L.; Tóth, N.; Lengyel, S.; Juhász, L. Corvid control in urban environments: A comparison of trap types. North-West. J. Zool. 2018, 14, 85–90. [Google Scholar]
- Krüger, T.; Heckenroth, H.; Prior, N.; Seitz, J.; Zang, H. Persecution and statutory protection have driven Rook Corvus frugilegus population dynamics over the past 120 years in NW-Germany. J. Ornithol. 2020, 161, 569–584. [Google Scholar] [CrossRef]
- Dwyer, J.F.; Leiker, D.L. Managing nesting by Chihuahuan ravens on H-frame electric transmission structures. Wildl. Soc. Bull. 2012, 36, 336–341. [Google Scholar] [CrossRef]
- Dwyer, J.F.; Leiker, D.L.; King, S.N. Testing nest deterrents for Chihuahuan ravens on H-frame transmission structures. Wildl. Soc. Bull. 2015, 39, 603–609. [Google Scholar] [CrossRef]
- Wilson, R.F.; Sarim, D.; Rahman, S. Factors influencing the distribution of the invasive house crow (Corvus splendens) in rural and urban landscapes. Urban Ecosyst. 2015, 18, 1389–1400. [Google Scholar] [CrossRef]
- Ludwig, A.; Bigras-Poulin, M.; Michel, P. The analysis of crow population dynamics as a surveillance tool. Transboun. Emerg. Dis. 2009, 56, 337–345. [Google Scholar] [CrossRef]
- Jones, D.N.; Thomas, L.K. Managing to live with Brisbane’s wildlife: Magpies and the management of positive and negative interactions. Proc. R. Soc. Qld. 1998, 107, 45. [Google Scholar]
- Chiron, F.; Julliard, R. Assessing the effects of trapping on pest bird species at the country level. Biol. Conserv. 2013, 158, 98–106. [Google Scholar] [CrossRef]
- Špur, N.; Pokorny, B.; Šorgo, A. Attitudes toward and acceptability of management strategies for a population of hooded crows (Corvus cornix) in Slovenia. Anthrozoös 2016, 29, 669–682. [Google Scholar] [CrossRef]
- Shcherbinin, V.V.; Ponkina, E.V.; Ulanov, P.N.; Matsyura, A.V. Effectiveness of bio-acoustic birdscarers for bird management in the mucipipal landfill of Barnaul City. Biol. Bull. Bogdan Chmelnitskiy Melitopol State Pedagog. Univ. 2016, 6, 365–376. [Google Scholar] [CrossRef]
- Kurosawa, R.; Kanai, Y.; Matsuda, M.; Okuyama, M. Conflict between humans and crows in greater Tokyo-garbage management as a possible solution. Glob. Environ. Res. 2003, 7, 139–148. [Google Scholar]
- Townsend, A.K.; Frett, B.; McGarvey, A.; Taff, C.C. Where do winter crows go? Characterizing partial migration of American Crows with satellite telemetry, stable isotopes, and molecular markers. Auk. Ornithol. Adv. 2018, 135, 964–974. [Google Scholar] [CrossRef]
- Gianpasquale, C.; Alberto, M. The occurrence and density of three sympatric corvids in a Mediterranean agroecosystem explained by land use. J. Ornithol. 2019, 160, 1133–1150. [Google Scholar] [CrossRef]
- Newson, S.E.; Rexstad, E.A.; Baillie, S.R.; Buckland, S.T.; Aebischer, N.J. Population change of avian predators and grey squirrels in England: Is there evidence for an impact on avian prey populations? J. Appl. Ecol. 2010, 47, 244–252. [Google Scholar] [CrossRef]
- Tharme, A.P.; Green, R.E.; Baines, D.; Bainbridge, I.P.; O’brien, M. The effect of management for red grouse shooting on the population density of breeding birds on heather-dominated moorland. J. Appl. Ecol 2001, 38, 439–457. [Google Scholar] [CrossRef]
- Edenius, L.; Brodin, T.; White, N. Occurrence of Siberian jay Perisoreus infaustus in relation to amount of old forest at landscape and home range scales. Ecolog. Bull. 2004, 51, 241–247. [Google Scholar]
- Fernandez-Juricic, E.; Jokimäki, J. A habitat island approach to conserving birds in urban landscapes: Case studies from southern and northern Europe. Biodivers. Conserv. 2001, 10, 2023–2043. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).