Abstract
Background: Escherichia coli and Salmonella are etiologic agents of intestinal infections. A previous study showed the presence of shared epitopes between lipopolysaccharides (LPSs) of E. coli O157 and Salmonella. Aim: Using phage display, the aim of this study is to identify mimotopes of shared epitopes in different enterobacterial LPSs. Methods: We use anti-LPS IgG from E. coli O157 and Salmonella to select peptide mimotopes of the M13 phage. The amino acid sequence of the mimotopes is used to synthesize peptides, which are in turn used to immunize rabbits. The antibody response of the resulting sera against the LPSs and synthetic peptides (SPs) is analyzed by ELISA and by Western blot assays, indicating that LPS sites are recognized by the same antibody. In a complementary test, the reactions of human serum samples obtained from the general population against the SPs and LPSs are also analyzed. Results: From the last biopanning phase, sixty phagotopes are selected. The analysis of the peptide mimotope amino acid sequences shows that in 4 of them the S/N/A/PF motif is a common sequence. Antibodies from the sera of immunized rabbits with SP287/3, SP459/1, SP308/3, and SP073/14 react against both their own peptide and the different LPSs. The Western blot test shows a sera reaction against both the lateral chains and the cores of the LPSs. The analysis of the human sera shows a response against the SPs and LPSs. Conclusion: The designed synthetic peptides are mimotopes of LPS epitopes of Salmonella and E. coli that possess immunogenic capacity. These mimotopes could be considered for use in the design of vaccines against both enterobacteria.
1. Introduction
Diarrheal illnesses are an important public health problem around the world, accounting for more than 2 million deaths each year in children under 5 years of age, with those in developing countries being the most affected [1,2]. The etiology of diarrheal illness is generally associated with viruses, parasites, and bacteria such as Salmonella, Shigella, and the different Escherichia coli pathotypes. All of these bacteria are important microorganisms that participate in the pathogenesis of intestinal infections [3,4].
Enterobacteria are characterized by the cytoplasmic internal membrane, peptidoglycan, and the cell wall or external membrane, which is composed of lipopolysaccharides (LPSs) and different proteins [5]. Structurally, LPSs comprise three regions. The first presents hydrophobic characteristics that include lipid A or endotoxin. The second region is the central region or the core oligosaccharides (core OSs), while the third region is known as somatic antigen O. The O antigen is a region that provides a hydrophilic surface to the bacterial cell wall and is composed of lateral carbohydrate chains that vary in their composition. This makes the O antigen useful in identifying bacteria [6,7].
Phage display, as described by Smith [8], is a procedure that uses the filamentous bacteriophage M13, in which short peptide sequences are inserted into the gene that codes for protein III (pIII), located on the phage surface [9,10]. The peptides that are expressed at random in pIII of the filamentous phage can be captured using IgG antibodies directed against specific antigens. Phage capture is based on affinity and is carried out during rounds of peptide selection, referred to as biopanning. Phages that carry peptides that mimic epitopes are known as phagotopes and the peptides that are captured by IgG are called mimotopes of the antigen, which can be used for immunization in order to produce antibodies. The phage display procedure has identified LPS mimotopes of different enterobacteria with immunogenic properties [11,12]. The immunogenicity of mimotopes has been demonstrated by inoculating them into animal models and observing how the immune response protects the animals against the enterobacteria with which the animals were challenged [11,12].
Salmonella is an important clinical and epidemiologic microorganism that can affect both animals and humans. The illnesses that occur in infants and adults are gastroenteritis and systemic infections, such as typhoid fever related to the S. typhi serotype [2,13]. On a global level, infections caused by Salmonella are increasing in prevalence. In the United States of America in 2011, 1.2 million cases were reported, with 450 related deaths [14]; however, there was a decline in 2013, with 19,056 cases and 80 related deaths, giving an incidence rate of 15.19 per 100,000 inhabitants [15]. In 2018, the General Epidemiology Directorate in Mexico reported 79,203 cases of Salmonella infection affecting all age groups [16]. Studies looking at the prevalence of Salmonella serotypes isolated from infected patients in both the public health sector and private hospitals showed that there were 199 serotypes involved. Of those, the most frequently occurring serotypes were S. typhimurium, Salmonella serogroup B, and S. urbana [17].
Infections by E. coli O157 are also both clinically and epidemiologically important in many countries. This microorganism has a natural reservoir in bovines, from which it is transmitted to humans by various routes [18]. E. coli O157 has been associated with the etiopathogenesis of hemorrhagic colitis (HC) without fever, hemolytic–uremic syndrome (HUS), and thrombocytopenic purpura [19]. The causal strains of these conditions are able to produce one or more cytotoxins. E. coli O157:H7 is considered to be an emerging pathogen responsible for HUS outbreaks in the United States of America, Canada, Japan, and some countries in the European Union, although the country with the highest incidence of illness caused by this bacterium is Argentina [19,20,21,22]. In a previous study using serum samples obtained from rabbits against E. coli O157, S. urbana, and S. arizonae LPSs, and via performing absorption assays with homologous and heterologous antigens, it was demonstrated that the presence of common epitopes exists among the mentioned LPSs [23]. In addition to the above, an ELISA test and serial dilutions of the anti-O157 LPS serum (1:50 to 1:1600) were used to analyze the reactivity against the purified LPSs from E. coli O157, S. urbana, and S. arizonae. By the statistical analysis of the observed reactivity (A405) of this serum against the homologous O157 LPS and by comparing those obtained against the LPSs from S. urbana and S. arizonae, no significant differences (p > 0.05) were observed. A similar assay was carried out, where the reactivity of the anti-LPS O157 serum was evaluated against the E. coli O179 LPS [24] and significative differences were found, showing that E. coli O157 and O179 LPS do not share common epitopes. The presence of immunodominant epitopes in the LPSs of E. coli O157 shared with the antigenic varieties of Salmonella urbana and S. arizonae [23] is interesting, since if they could be identified then they could be proposed as alternative immunogens for a wide range of clinical and epidemiologically relevant enterobacteria without the toxic effects of the LPS endotoxin (lipid A). In this study, peptide mimotopes of shared epitopes between Salmonella spp. and E. coli O157 are shown, which have been identified by the phage display procedure. The results will offer insights as to whether synthetic peptides derived from the amino acid sequence of mimotopes could be used as immunogens with the capacity to induce a protective immune response against Salmonella spp. and E. coli O157.
2. Materials and Methods
2.1. Lipopolysaccharides
We used the phenol–water method described by Westphal [25] to extract and purify the LPSs and cores from E. coli and Salmonella. The strains of E. coli that were used to obtain the LPSs and cores were as follows: O157 ATCC 700927, coded in the strain collection of the laboratory of the Faculty of Medicine UNAM (FMU) as 287 (FMU287); the cores were derived from mutant strains of E. coli, R1 (O8:K27, F470), R2 (O100:K?B:H2, F632), R3 (O111:K58:H-, F653), R4 (O14:K7, F2513), and K12 (O16, W3110) [26]; the strains of Salmonella, including the following: S. urbana (FMU459), S. arizonae (FMU308), and S. typhi ATCC 6539 (FMU073), which also came from the Faculty of Medicine, UNAM; the core Ra came from S. minnesota (List Biological Laboratories, Campbell, C A, USA). The LPSs and cores were treated with DNase, RNase, and proteinase K. Following extraction, the LPSs were lyophilized (Labconco, Kansas City, MI, USA) and kept refrigerated until use.
2.2. Anti-Sera Production Against LPS
The protocol for the research here was approved by the Research Committee (IN216417) and CONBIETICA 09CEI066201402012 of the Faculty of Medicine at the National Autonomous University of Mexico. The immunization and bleeding of rabbits was conducted in accordance with specific techniques for the production, care, and use of laboratory animals, as described in the Mexican Official Norm 062-Zoo-1999 (NOM-062-Zoo-1999; NORMA Oficial Mexicana, 1999) [27].
Before immunizing New Zealand (NZ) rabbits weighing between 2 kg and 2.5 kg, a blood sample was taken to act as a negative control for the immune reaction for the various proposed immune tests. The rabbits were obtained from the Central Animal House of the Faculty of Medicine. The rabbits were immunized with the LPSs from E. coli O157, S. urbana, S. arizonae, and S. typhi using the protocol proposed by Ewing [28]. In line with this protocol, the first dose consisted of an intradermal injection of 100 μg/mL of LPS in PBS with Freund’s adjuvant, followed by four doses in intervals of seven days. A week following the last dose, a blood sample (5 mL) was taken from the marginal vein of the rabbits and the titers against the homologous LPSs were quantified. Bloodletting was then performed on the rabbits under anesthesia. Using a ratio of 1.0 mL per kg weight, pentobarbital (Anestesal, Pfizer) was given intravenously to anesthetize the rabbits completely. The management and care of the rabbits was carried out in accordance with the Mexican official norms [27]. In accordance with the same norms, the animals were checked to be sure that they were free of transmissible diseases to humans. In addition, the blood sampling and letting was carried out according to the recommendations in Subsection 8.2 of the same Mexican official norm, which refers to the administration of fluids and substances.
2.3. Purification of the IgG Antibodies
IgG purification was carried out by affinity chromatography on agarose, as per the following protocol: Five-hundred microliters of sera was mixed with 250 μL of Protein G Agarose (Invitrogen) in a test tube and incubated for 20 min at an ambient temperature, which was shaken every two minutes in order to homogenize the suspension. The sample was centrifuged for 30 s at 500× g (Sorvall RC5B). The supernatant was eliminated and the agarose was washed 5 times with a binding buffer (0.01 M glycine with 0.15 M NaCl, pH 7.0). After the last wash, the agarose was mixed with 750 μL of an elution buffer (0.1 M glycine-HCI, pH 2.6) and centrifuged at 500× g for 30 s. The supernatant was collected with the IgG part and the pH was adjusted to 7.0 using 1 M Tris-HCI pH 8.0. The IgG concentration was determined by the Bradford method using the Coomassie Plus Kit (Thermo Scientific, 23236), according to the manufacturer’s instructions.
2.4. Biopanning and Mimotope Selection
The mimotopes were selected from a peptide library using 12 amino acid residues expressed in the filamentous phage M13 (New England BioLabs) using the method previously reported [29]. The phagotopes were selected by three rounds of selection (biopanning) with rabbit anti-LPS IgG from E. coli O157, S. urbana, S. arizonae, and S. typhi. After selecting the phagotopes, the DNA was extracted according to the Wilson method [30] and the composition of the DNA nucleotides was obtained by automated sequencing (Genetic analyzer 3100). The sequences were edited using the Chromas application and the translation of amino acids was carried out through the application of the ExPASy Proteomics Server available online http://www.expasy.ch/tools/dna.html (accessed 14-05-2017). The alignment of the amino acids and the identification of some consensual sequences were achieved by the free use of the Clustal Omega 2, available on internet (https://www.ebi.ac.uk/Tools/msa/).
2.5. Mimotope Synthesis
Having obtained the amino acid sequences, peptide synthesis in lineal sequences was carried out and conjugated by keyhole limpet hemocyanin (KLH) with a purity of more than 90% (Biosynthesis, Lewisville, TX, USA). For KLH-conjugated synthetic peptides (SP), an extension addition was included in the amino terminal group of amino acid residues with the sequence Cys-Ser-Gly-Gly-Gly (CSGGG).
2.6. Production of Anti-Sera Against Synthetic Mimoptopes by Rabbit Immunization
White New Zealand rabbits (1.5 kg) were challenged in intervals of 7 days with five doses of SP287/3, SP459/1, SP308/3, and SP073/14 conjugated with KLH. For this, 500 µg was used in the first immunization and 1.0 mg was used in the subsequent four immunizations, in accordance with the previously described method [31].
Before immunizing the rabbits, serum samples were taken for use as negative controls. One week after the final immunization, bloodletting took place and the sera were stored in aliquots at −20 °C until used. Bloodletting was carried out under anesthesia using pentobarbital (Anestesal, Pfizer). Immunization was carried out in accordance with the specific techniques for the production, care, and use of laboratory animals described in the Mexican official norms [27].
2.7. Evaluation of Antibody Reactivity Against LPS and Synthetic Mimotope Using ELISA
The capacity of the anti-peptide antibody reactions against SPs (SP287/3, SP459/1, SP308/3, and SP073/14) and LPSs were analyzed by the enzyme-linked immunosorbent assay (ELISA) method described by Navarro [32]. Briefly, this study used 96-well microplates (Nunc-Immuno Plate, MaxiSorp F96), into which SPs and the LPSs that were previously dissolved independently in a carbonate buffer at a pH of 9.6 were placed. The plates were incubated at 37 °C for 2 h and at 4 °C for 18–24 h, after which time the plates were blocked with 200 μL of PBS/1.0% low fat milk (Svelty, Nestlé) at ambient temperature for 2 h. The plates were washed three times with PBS/0.05% Tween 20 before the anti-peptide and anti-LPS serial dilutions (1:50 to 1:1600) of sera were dissolved in PBS at a pH of 7.4 and incubated at 37 °C for 2 h. The plates were washed a further three times (PBS/Tween 20) and 100 μL of rabbit anti-IgG (1:1000) conjugated with alkaline phosphatase (Invitrogen, USA) was added to each well. The plates were incubated at 37 °C for 2 h and the reaction was visualized by adding 200 µL of p-nitrophenyl phosphate (1 mg/mL, Sigma) in a diethanolamine buffer (pH 9.8, Sigma). The reaction was stopped by adding 25 µL of 3 M NaOH. An ELISA reader (BioTek EL x 800) set at 415 nm was used to read the absorbance reaction. All the tests were carried out in duplicate in two independent tests. Pre-immune rabbit sera obtained before being challenged were used as negative controls.
2.8. Human Serum Analysis Against LPS and Synthetic Mimotopes Using ELISA
Forty-six human serum samples from adults (age range = 15–40 years) obtained from a previous study [32] were evaluated against SP287/3, SP459/1, SP308/3, and SP073/14; and the LPSs from E. coli O157, S. urbana, S. arizonae, and S. typhi. The SPs and LPSs (10 µg/mL) dissolved in carbonate buffer (pH 9.6) were fixed in 96-well microplates (Nunc-Immuno Plate, MaxiSorp F96) and incubated at 37 °C for 2 h and at 4 °C for 18–24 h, after which time the plates were blocked with 200 μL of PBS/1.0% low fat milk (Svelty, Nestlé) at ambient temperature for 2 h.
The plates were washed three times with PBS/0.05% Tween 20 before adding human serum samples to a dilution of 1:100 in PBS at a pH of 7.4 and then incubating these at 37 °C for 2 h. The plates were washed a further three times (PBS/Tween 20) and 100 μL of human anti-IgG (1:1000) antibodies obtained in goats conjugated with alkaline phosphatase (Invitrogen, USA) was added to each well. The plates were incubated at 37 °C for 2 h and the reaction was visualized as described above. Preimmunized rabbit serum was used as negative control.
2.9. Interaction Site of Synthetic Antipeptide Sera
Using Western blotting, the interaction site of anti-SP287/3, anti-SP459/1, anti-SP308/3, and anti-SP073/14 sera with the LPS from S. urbana, S. arizonae, S. typhi, and E. coli O157 was analyzed, as well as the Ra, Rd, and Re cores from Salmonella minnesota (List Biological) and the Ra, Rb, Rc, Rd, and Re cores from E. coli. Using SDS-PAGE in a gel of polyacrylamide (15%) with 4 M of urea, 10 µL samples of different LPSs and cores were separated and then transferred to PVDF membranes (BioRad). Visualization was carried out using rabbit anti-IgG marked with phosphatase (Invitrogen).
2.10. Statistical Analysis
All ELISA tests were carried out in duplicate and the arithmetic means were compared by X2 proportional analysis, with a statistical significance of < 0.05, as reported previously [32].
3. Results
3.1. Mimotopes Selection
In total, 60 phagotopes were selected from the biopanning selection; 15 from each one were obtained with IgG anti-LPSs of E. coli O157, S. urbana, S. arizonae, and S. typhi LPSs. The amino acid sequences and the alignments of the 60 peptide mimotopes are presented in Table S1. In the analysis, we observed the sequence S/N/A/PF as a consensus motif shared by the SP287/3, SP459/1, SP308/3, and SP073/14 peptides selected with IgG anti-LPSs of E. coli O157, S. urbana, S. arizonae, and S. typhi, respectively (Table 1). The composition of the consensus sequence showed serine (S) and asparagine (N) as uncharged polar amino acids and alanine (A) and phenylalanine (F) as hydrophobic amino acids. Proline (P), a non-polar cyclic amino acid, was present in three of the four peptide mimotopes mentioned above, while tyrosine (Y), an aromatic amino acid, was found in two of the peptides.

Table 1.
Alignment and consensus sequence of the selected phagotopes by phage display and anti-lipopolysaccharide (LPS) IgG response.
3.2. Anti-Peptide Sera Response
The synthesis of each peptide that presented the S/N/A/PF sequence was carried out and the resulting synthetic peptides (SPs) were used to immunize the rabbits to obtain the anti-SP287/3, SP459/1, SP308/3, and SPS073/14 sera. The anti-synthetic peptide sera as well as the anti-LPS sera were evaluated using double serial dilutions (Tables S2 and S3). The obtained results show that in all dilutions with a 1:50 dilution ratio, the anti-peptide sera reactivity is suitable (Figure 1). In this context, the ELISA test with the anti-SP287/3 serum showed OD (415 nm) lecture values of 1.65 with the homologous peptide, 0.89 with the O157 LPS, and 0.55 with the Ra core. Additionally, the serum showed reactivity with the R3 core. The same analysis of anti-SP459/1 serum reported values of 1.13 with the homologous SP, 0.62 with the S. urbana LPS, and 0.45 with the Ra core. This serum also recognized the R1 core (0.60). With the anti-SP308/3 serum, the obtained values were 1.19 with the homologous SP, 0.45 with the S. arizonae LPS, 0.51 with the Ra core, and 0.28 with R1 core. Finally, the results with anti-SP073/14 were 1.01 with the homologous SP, 0.65 with the S. typhi 073 LPS, and 0.55 with the Ra core. Additionally, the serum showed a response of 0.25 with the R3 core. The average values obtained with the results of the different assays with the pre-immune sera were of 0.11 OD.
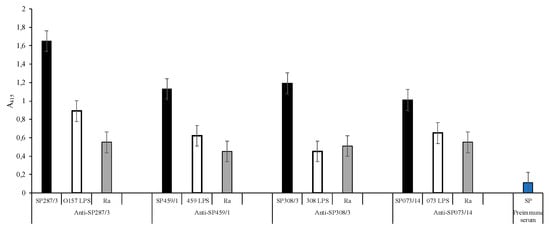
Figure 1.
Anti-synthetic peptide sera response. Reactivity of sera anti-SP287/3, anti-SP459/1, anti-SP308/3, and anti-SP073/14 against synthetic peptides ( ); E. coli O157, S. urbana, S. arizonae, and S. typhi LPSs (
); E. coli O157, S. urbana, S. arizonae, and S. typhi LPSs ( ); and the Ra core (
); and the Ra core ( ) was evaluated by the ELISA test, as was previously mentioned in the Methods section. The pre-immune sera (
) was evaluated by the ELISA test, as was previously mentioned in the Methods section. The pre-immune sera ( ) response is the average of the obtained results in the different assays.
) response is the average of the obtained results in the different assays.
 ); E. coli O157, S. urbana, S. arizonae, and S. typhi LPSs (
); E. coli O157, S. urbana, S. arizonae, and S. typhi LPSs ( ); and the Ra core (
); and the Ra core ( ) was evaluated by the ELISA test, as was previously mentioned in the Methods section. The pre-immune sera (
) was evaluated by the ELISA test, as was previously mentioned in the Methods section. The pre-immune sera ( ) response is the average of the obtained results in the different assays.
) response is the average of the obtained results in the different assays.
3.3. Interaction Site of Anti-Peptide Antibodies on the LPSs
The Western blot analysis of the interaction site of anti-SP antibodies on the different LPSs showed that the anti-SP287/3 reacted with repeating carbohydrate subunits and the core region of the LPSs from E. coli O157. A similar response was obtained with the anti-LPS O157 antibodies (Figure 2). On the other hand, anti-SP459/1 antibody recognized a 10–15 kDa fraction corresponding to the core region and lipid A from S. urbana and the R1 core of E. coli (Figure 3). Anti-SP308/3 serum reacted with the R1 core and a 10 kDa region of the LPS from S. arizonae (Figure 4). Finally, anti-SP073/14 reacted with a 10 kDa region of the LPS from S. typhi and the R3 core of E. coli (Figure 5).
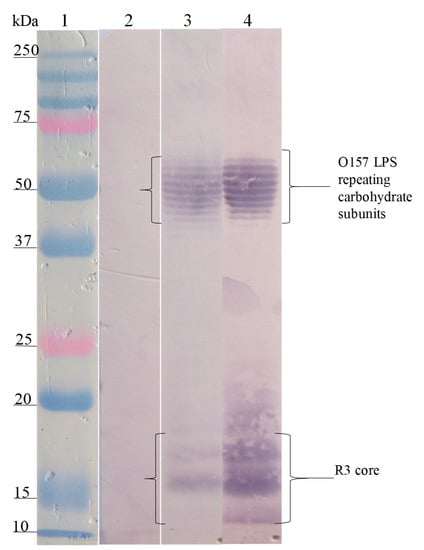
Figure 2.
Recognition of the O157 LPS (FMU287) and R3 core by the anti-O157 LPS and the anti-SP 287/3 antibodies. Western blotting was carried out using a purified O157 LPS and R3 core. Lane 1, molecular weight marker; lane 2, O157 LPS revealed with pre-immune serum; lane 3, O157 LPS revealed with anti-O157 LPS serum; lane 4, O157 LPS revealed with anti-SP287/3 serum. The anti-O157 LPS and anti-SP287/3 antibodies recognized fractions of repeating carbohydrate subunits (stairway pattern) and the polysaccharide of the R3 core of the O157 LPS.
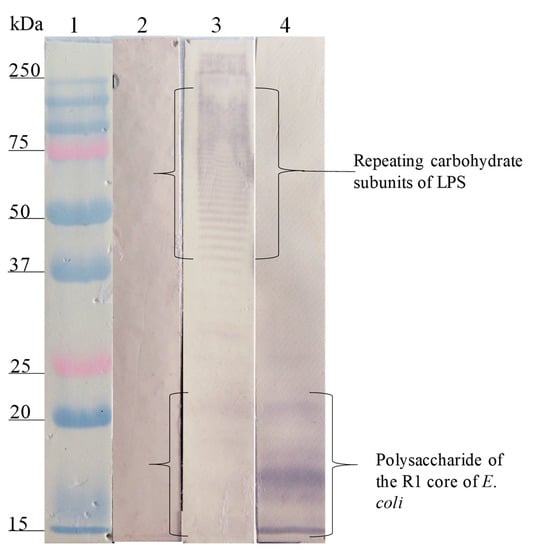
Figure 3.
Recognition of the LPS of S. urbana (FMU459) and SP459/1 by the anti-LPS of S. urbana and the anti-SP459/1 antibodies. Western blotting was carried out using a purified LPS of S. urbana and the R1 core of E. coli. Lane 1, molecular weight marker; lane 2, LPS of S. urbana revealed with pre-immune antibodies; lane 3, LPS S. urbana revealed with anti-LPS S. urbana serum; lane 4, R1 core of E. coli revealed with anti-SP459/1 serum. The anti-LPS S. urbana antibodies recognized fractions of repeating carbohydrates subunits and of the core of LPS S. urbana. The anti-SP459/1 antibodies only recognized the R1 core of E. coli.
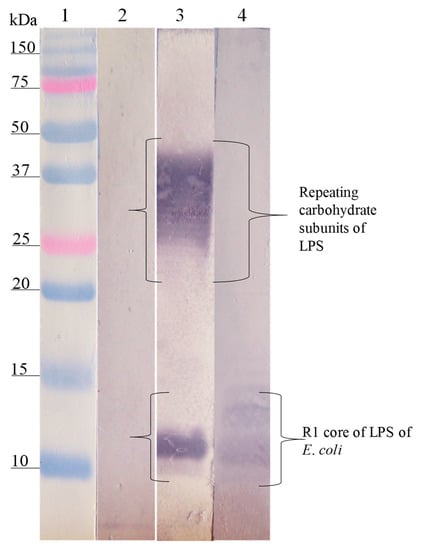
Figure 4.
Recognition of the LPS of S. arizonae (FMU308) and of the R1 core of E. coli by the anti-LPS antibodies of S. arizonae and anti-SP308/3. Western blotting was carried out using a purified LPS of S. arizonae and the R1 core. Lane 1, molecular weight marker; lane 2, LPS of S. arizonae revealed with pre-immune serum; lane 3, LPS of S. arizonae revealed with the anti-LPS of S. arizonae serum; lane 4, R1 core revealed with anti-SP308/3 antibodies. The anti-LPS of S. arizonae antibodies recognized fractions of repeating carbohydrate subunits and the core of the LPS of S. arizonae. The anti-SP308/3 antibodies recognized the polysaccharide of the R1 core of E. coli.
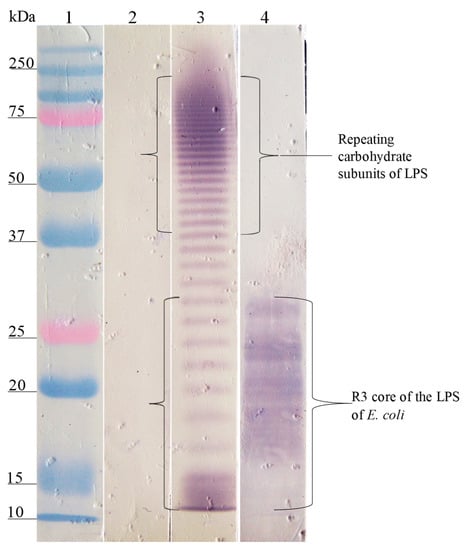
Figure 5.
Recognition of the LPS of S. typhi (FMU073) and the R3 core of E. coli by the anti-LPS antibodies of S. typhi and anti-SP073/14. Western blotting was carried out using a purified LPS of S. typhi and the R3 core of E. coli. Lane 1, molecular weight marker; lane 2, LPS of S. typhi FMU073 revealed with pre-immune serum; lane 3, LPS of S. typhi revealed with anti-LPS S. typhi antibodies; lane 4, R3 core of E. coli revealed with anti-SP073/14 antibodies. The anti-LPS S. typhi antibodies recognized fractions of repeating carbohydrate subunits and the core of S. typhi. The anti-SP073/14 antibodies recognized the polysaccharide of the R3 core of E. coli.
3.4. Sera Response of General Population Against Synthetic Peptides and LPSs
The ELISA assay with different serum samples obtained from the general population tested against SP287/3, SP459/1, SP308/3, and SP073/14 showed reactivity between 17% and 28% of the samples (Figure 6); however, no significant differences (p > 0.05) were found in any of the cases. The analysis evaluating the LPSs showed reactivity in 43.5%, 32.6%, 37.0%, and 23.9% against E. coli O157, S. urbana, S. arizonae, and S. typhi, respectively (Figure 6). The statistical analyses did not show significant differences.
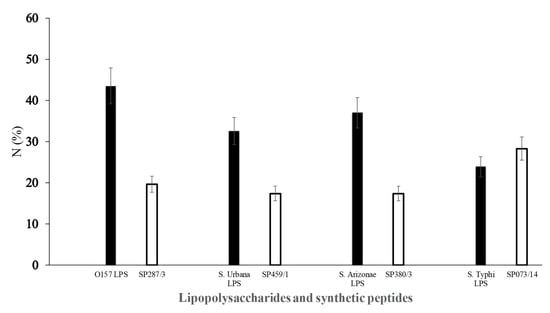
Figure 6.
Response of human serum samples (1:100) against lipopolysaccharides and synthetic peptides. The LPSs ( ) of E. coli O157 (FMU287), S. urbana (FMU459), S. arizonae (FMU308), and S. typhi (FMU073), as well as the synthetic peptides (
) of E. coli O157 (FMU287), S. urbana (FMU459), S. arizonae (FMU308), and S. typhi (FMU073), as well as the synthetic peptides ( ) SP287/3, SP459/1, SP308/3, and SP073/14 were analyzed independently in an ELISA test assay against community serum samples, as described previously in the Methods section.
) SP287/3, SP459/1, SP308/3, and SP073/14 were analyzed independently in an ELISA test assay against community serum samples, as described previously in the Methods section.
 ) of E. coli O157 (FMU287), S. urbana (FMU459), S. arizonae (FMU308), and S. typhi (FMU073), as well as the synthetic peptides (
) of E. coli O157 (FMU287), S. urbana (FMU459), S. arizonae (FMU308), and S. typhi (FMU073), as well as the synthetic peptides ( ) SP287/3, SP459/1, SP308/3, and SP073/14 were analyzed independently in an ELISA test assay against community serum samples, as described previously in the Methods section.
) SP287/3, SP459/1, SP308/3, and SP073/14 were analyzed independently in an ELISA test assay against community serum samples, as described previously in the Methods section.
4. Discussion
Despite the fact that mortality by intestinal infections has decreased in the general population, and more specifically in children, these infections continue to be a public health problem, representing increased morbidity [3]. Viruses are the main etiological agent of intestinal infections; however, bacteria participate in an important area of the disease. Of these infections, the enterobacteria appear with greater frequency in the pathogenesis of disease. E. coli O157 and Salmonella are bacteria that have greater clinical and epidemiologic impacts and are responsible for diarrhea outbreaks caused by consumption of contaminated food [33]. To date, there are no effective vaccines that reduce the number of people susceptible to infection by these bacteria. For this reason, it is necessary to identify specific immunogens that could protect susceptible populations.
Phage display is a versatile procedure that expresses peptides at random in protein III of the M13 phage. Some of these peptides may be mimotopes of epitopes of polysaccharides, proteins, and other molecules from microorganisms that could be used as potential immunogens in the development of vaccines [34,35].
The lipopolysaccharides of E. coli and Salmonella feature a complex structure of three regions, where the O antigen, which is constituted by repeating carbohydrate subunits, provides antigenic variability to the LPSs. The core OS, although having less variability, is also constituted by carbohydrates [6]. E. coli presents different core OSs, described as K12, R1, R2, R3, and R4 [26]. Additionally, for Salmonella, these have been described as different OS cores named Arizonae IIIA and Ra-type LPS with the chemotypes Ra, Rb, Rc, Rd, and Re. Of all of these, Ra is more commonly identified in Salmonella strains isolated from clinical samples [36,37,38,39]. In the present study, we utilized the phage display procedure to identify immunodominant sites present on the lipopolysaccharides of S. typhi, S. urbana, S. arizonae, and E. coli O157. For the epitope detection, we utilized IgG antibodies of immunized rabbits with the LPSs of the aforementioned bacteria. Finally, with the anti-LPS antibodies, we selected the phages expressing the peptide mimotopes. The analysis of peptide mimotopes showed the consensual sequence of S/N/A/PF in four of them. Serine, asparagine, alanine, phenylalanine, and proline residues formed a common motif in the four selected mimotopes, where each one was obtained with the different IgG anti-LPSs that were utilized. The characteristics of the motif amino acids are important, such that proline is a non-polar cyclic amino acid, where its α-amine group binds to a side chain, resulting in the formation of rigid turns into the peptide chain and causing the formation of β folds in proteins [40]. The presence of proline in the peptide mimotopes influences in the formation of curvatures in these, which could be important in the recognition of such peptide mimotopes by antibodies. Two of the selected peptides that were later synthesized (SP287/3 and SP308/3) show proline forming with the phenylalanine dipeptides (PF), a configuration that has been identified in the mimotope group A Streptococcus cell wall, Vibrio cholerae O1 Ogawa LPS, and also that of the E. coli O157:H7 LPS [24,41,42,43]. All these observations enables us to understand why there are cross-reactions between microorganisms of distinct genera and species, and to underline the feasibility of using mimotopes as potential immunogens.
However, James and Shin [44,45] mention that an excess of proline in the mimotopes can lead to the production of autoantibodies, so they suggest that such a situation may favor the creation of unstable configurations, and thereby the presence of multiple epitopes. However, in our study, we did not find an excess of proline in the selected peptides. It is important to carry out further studies to discard the idea that proline could have a damaging effect if used as an immunogen. Regarding other amino acids identified in the peptide mimotopes (F and Y), previous reports suggest that phenylalanine in conjunction with tyrosine and tryptophan (W) mimics structures formed by carbohydrates [41,46]. Recently, Shin [45] found phenylalanine to be an important component in 23 peptide mimotopes of the capsular polysaccharide of Streptococcus pneumoniae. In his study, Shin observed that the presence of this amino acid formed a dipeptide with lysin (KF). Recently, Smith [47] reported that immunized rats with peptides containing the KF dimer were protected when challenged with S. pneumoniae. Meanwhile, Thomas [48] identified mimotopes of lipid A in E. coli. In the current study, a dipeptide formed by proline and phenylalanine (PF) was identified in the peptides 287/3 and 308/3. This could be the explanation for the anti-SP287/3 and anti-SP308/3 sera response against the LPSs.
In addition to the PF dimer, the SP287/3 peptide presents another motif comprised of YxY amino acids. In a study by Oldenburg and Westerink [46,49], it was shown that the amino acid motif YxY is a carbohydrate mimotope and that the antibodies generated against the mimotope recognize concanavalin A and polysaccharides of the Neisseria meningitidis capsule. The presence of the YxY amino acid motif in the SP287/3 peptide suggests that the production of antibodies against this motif could be an explanation of the ability of the anti-SP287/3 serum to react against the lateral chains and core OSs of LPSs. Serine was another amino acid present in the peptide mimotopes SP287/3, SP073/14, and SP459/1. This is relevant since serine, in conjunction with proline, phenylalanine, and tyrosine, has been identified in mimotopes of carbohydrate structures of the cell wall from various microorganisms [50]. Some other dipeptides integrated by asparagine–isoleucine (NI) and isoleucine–threonine (IT) were also identified in the SP073/14 and SP459/1 peptides. In a study by Wu [51], NI and IT dipeptides were identified in the mimotope EIFTNIT of the Streptococcus agalactiae and Neisseria meningitidis capsules. The immunization of animals with the motif induced antibody production against the capsule of these microorganisms, and also against of other Gram-positive bacteria. We considered that the presence of NI and IT dipeptides in SP073/14 and SP459/1 stimulates antibody production against the LPSs evaluated in the study. In order to know the possible interaction site in the LPSs of the anti-LPS and anti-SP antibodies, we used a Western blotting assay. The results showed that each one of the different sera recognize specific LPS sites. It was observed that the anti-SP287/3 serum obtained with the corresponding SP that was selected with the anti-LPS O157 antibodies reacted with both the repeating carbohydrate subunits of the LPS and the R3 core of E. coli O157. Similar observations were previously reported in our laboratory [24]. In the study, it was also was observed that the anti-SP073/14 serum obtained from rabbits immunized with the SP selected with the anti-S. typhi LPS antibodies recognized lipid A of the S. typhi LPS and the R3 core of E. coli. In this regard, it is important to mention that the K12 and R2 cores of E. coli and the Ra core of Salmonella are conserved between both bacteria, showing a similar carbohydrate composition [52]. The existence of common epitopes between the E. coli and Salmonella LPSs was confirmed in the ELISA assay when the anti-SP287/3, anti-SP459/1, anti-SP308/3, and anti-SP073/14 antibodies were analyzed against the Ra core of E. coli. A possible explanation for this aspect is the fact that the Ra core was obtained from the E. coli EH100 strain, which presents the R2 core similarly to the Salmonella enterica Ra core [6,53].
To understand if previous observations occur in the human population, a collection of sera obtained previously was evaluated against the designed SPs and the LPSs of E. coli O157, S. urbana, S. arizonae, and S. typhi. The results showed that some of the population sera reacted with both SPs and LPSs, as was observed in a previous study [23]. The existence of shared epitopes that are present in the LPSs of different enterobacteria can explain the presence of antibodies against non-common bacteria, such as E. coli O157, as was reported in Mexico. Another interesting result observed in the study is that the peptide mimotope SP073/14 was selected with the anti-LPS sera of S. typhi for the serum response of anti-SP073/14 against both the SP and the S. typhi LPS, which suggests the potential of this peptide as a vaccine to protect against the infection of this important clinical and epidemiological bacteria. The study’s conclusion is that the response of antibodies against S. typhi, S. urbana, S. arizonae, and E. coli O157 LPS obtained from immunized rabbits with SP287/3, SP459/1, SP308/3, and SP073/14 confirms that synthetic peptides are immunogenic mimotopes of the evaluated LPSs and that it is feasible to consider them as an alternative system to protect against infections caused by Salmonella and E. coli O157; however, further studies with animal models are necessary to confirm their protective capacity.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/8/5/780/s1, Table S1: Selected phagotope clones, their peptide mimotope sequence and IgG anti-Phage clone response. Table S2: Reactivity of sera anti-SP287/3, anti-SP459/1, anti-SP308/3 and anti-SP073/14 by serial dilutions against synthetic peptides and the LPSs of E. coli O157, S. urbana, S. arizonae, S. typhi and Ra core. Table S3: Antigenic cross reaction between LPS and mimotope peptides, determined by the ELISA assay and serial dilutions of the antisera.
Author Contributions
Conceptualization, A.N. and C.A.C.E.; investigation, U.H.-C.; methodology, D.L.-M. and A.M.-R.; writing—review and editing, A.N. and C.A.E.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Dirección General de Asuntos del Personal Académico (DGAPA) and the Programa de Innovación Tecnológica (PAPIIT) IN216417, Universidad Nacional Autónoma de México (UNAM); and Secretaria de Educación, Ciencia, Tencología e Innovación (SECTEI), project 9200c19.
Acknowledgments
We thank Gabriel Pérez and Luis Antonio León (Faculty of Medicine, UNAM) for their technical assistance in the laboratory.
Conflicts of Interest
The authors declare that there are no competing interest associated with the manuscript.
References
- Bryce, J.; Boschi-Pinto, C.; Shibuya, K.; Black, R.E. WHO estimates of the causes of death in children. Lancet 2005, 365, 1147–1152. [Google Scholar] [CrossRef]
- GBD Diarrhoeal Diseases Collaborators. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 909–948. [Google Scholar] [CrossRef]
- Troeger, C.; Blacker, B.F.; Khalil, I.A.; Rao, P.C.; Cao, S.; Zimsen, S.R.M.; Albertson, S.B.; Stanaway, J.D.; Deshpande, A.; Abebe, Z.; et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1211–1228. [Google Scholar] [CrossRef]
- Anderson, J.D., IV; Bagamian, K.H.; Muhib, F.; Amaya, M.P.; Laytner, L.A.; Wierzba, T.; Rheingans, R. Burden of enterotoxigenic Escherichia coli and Shigella non-fatal diarrhoeal infections in 79 low-income and lower middle-income countries: A modelling analysis. Lancet Glob. Health 2019, 7, e321–e330. [Google Scholar] [CrossRef]
- Caroff, M.; Karibian, D. Structure of bacterial lipopolysaccharides. Carbohydr. Res. 2003, 338, 2431–2447. [Google Scholar] [CrossRef]
- Raetz, C.R.H.; Whitfield, C. Lipopolysaccharide Endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef]
- Beutler, B.; Rietschel, E.T. Innate immune sensing and its roots: The story of endotoxin. Nat. Rev. Immunol. 2003, 3, 169–176. [Google Scholar] [CrossRef]
- Smith, G.P. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science 1985, 228, 1315–1317. [Google Scholar] [CrossRef]
- Scott, J.K.; Smith, G.P. Searching for peptide ligands with an epitope library. Science 1990, 249, 386–390. [Google Scholar] [CrossRef]
- Smith, G.P.; Scott, J.K. Libraries of peptides and proteins displayed on filamentous phage. Methods Enzymol. 1993, 217, 228–257. [Google Scholar] [CrossRef]
- Meyer, T.; Schirrmann, T.; Frenzel, A.; Miethe, S.; Stratmann-Selke, J.; Gerlach, G.F.; Strutzberg-Minder, K.; Dübel, S.; Hust, M. Identification of immunogenic proteins and generation of antibodies against Salmonella Typhimurium using phage display. BMC Biotechnol. 2012, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, S.; Hossany, R.B.; Pinto, B.M. Immunological Evidence for Functional Rather than Structural Mimicry by a Shigella flexneri Y Polysaccharide-Mimetic Peptide. Clin. Vaccine Immunol. 2008, 15, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M.; International Collaboration on Enteric Disease “Burden of Illness” Studies. The Global Burden of Nontyphoidal Salmonella Gastroenteritis. Clin. Microbiol. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Crim, S.M.; Iwamoto, M.; Huang, J.Y.; Griffin, P.M.; Gilliss, D.; Cronquist, A.B.; Cartter, M.; Tobin-D’Angelo, M.; Blythe, D.; Smith, K. Incidence and trends of infection with pathogens transmitted commonly through food—Foodborne Diseases Active Surveillance Network, 10 US sites, 2006–2013. Morb. Mortal Wkly. Rep. 2014, 63, 328–332. [Google Scholar]
- DGE-SINAVE. Salud/Sistema de Notificación Semanal de casos nuevos de enfermedades. Boletín Epiedemiológico 2019, 36, 68. [Google Scholar]
- Gutiérrez-Cogco, L.; Montiel-Vázquez, E.; Aguilera-Pérez, P.; González-Andrade, M.D.C. Serotipos de Salmonella identificados en los servicios de salud de México. Salud Pública Méx. 2000, 42, 490–495. [Google Scholar] [CrossRef]
- Callaway, T.R.; Carr, M.A.; Edrington, T.S.; Anderson, R.C.; Nisbet, D.J. Diet, Escherichia coli O157:H7, and cattle: A review after 10 years. Curr. Issues Mol. Biol. 2009, 11, 67–79. [Google Scholar]
- Tarr, P.I.; Gordon, C.A.; Chandler, W.L. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 2005, 365, 1073–1086. [Google Scholar] [CrossRef]
- Noftall, K.; Taylor, M.; Hoang, L.; Galanis, E. Outbreaks: Shiga toxin–producing Escherichia coli in British Columbia, 2011–2017: Analysis to inform exclusion guidelines. Can. Commun. Dis. Rep. 2019, 45, 238–243. [Google Scholar] [CrossRef]
- Nielsen, E.M.; Scheutz, F. Characterisation of Escherichia coli O157 isolates from Danish cattle and human patients by genotyping and presence and variants of virulence genes. Vet. Microbiol. 2002, 88, 259–273. [Google Scholar] [CrossRef]
- Lopez, E.L.; Diaz, M.; Grinstein, S.; Devoto, S.; Mendilaharzu, F.; Murray, B.E.; Ashkenazi, S.; Rubeglio, E.; Woloj, M.; Vasquez, M.; et al. Hemolytic Uremic Syndrome and Diarrhea in Argentine Children: The Role of Shiga-like Toxins. J. Infect. Dis. 1989, 160, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Eslava, C.; de la Torre, G.G.; Leon, L.A.; Licona, D.; Leon, L.; Zarco, L.A.; Cravioto, A. Common epitopes in LPS of different Enterobacteriaceae are associated with an immune response against Escherichia coli O157 in bovine serum samples. J. Med. Microbiol. 2007, 56, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Navarro, A.; Hernández-Chiñas, U.; Licona-Moreno, D.; Zenteno, E.; Cravioto, A.; Eslava-Campos, C.A. Immunogenic peptide mimotopes from an epitope of Escherichia coli O157 LPS. Biochem. J. 2016, 473, 3791–3804. [Google Scholar] [CrossRef] [PubMed]
- Westphal, O.; Jann, K. Bacterial lipopolysaccharide: Extraction with phenol-water and further applications of the procedure. Meth. Carbohydr. Chem. 1965, 5, 83–91. [Google Scholar]
- Amor, K.; Heinrichs, D.E.; Frirdich, E.; Ziebell, K.; Johnson, R.P.; Whitfield, C. Distribution of Core Oligosaccharide Types in Lipopolysaccharides from Escherichia coli. Infect. Immun. 2000, 68, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- NOM NOM-062-ZOO-1999; ETP LA PRODUCCION. Norma Oficial Mexicana NOM-062-ZOO-1999: Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio. D. Of. Fed. México 1999. [Google Scholar]
- Ewing, W.H. Edwards and Ewing’s Identification of Enterobacteriaceae; Elsevier Science Publishing Co., Inc.: New York, NY, USA, 1986; 536p. [Google Scholar]
- Ulises, H.-C.; Tatiana, G.; Karlen, G.; Guillermo, M.-H.; Juan, X.-C.; Carlos, E. Peptide sequences identified by phage display are immunodominant functional motifs of Pet and Pic serine proteases secreted by Escherichia coli and Shigella flexneri. Peptides 2009, 30, 2127–2135. [Google Scholar] [CrossRef]
- Wilson, R.K. High-throughput purification of M13 templates for DNA sequencing. Biotechniques 1993, 15, 414–416, 418–420, 422. [Google Scholar]
- Galfrè, G.; Monaci, P.; Nicosia, A.; Luzzago, A.; Felici, F.; Cortese, R. Immunization with phage-displayed mimotopes. In Methods in Enzymology; Elsevier; Academic Press: New York, NY, USA, 1996; Volume 267, pp. 109–115. [Google Scholar]
- Navarro, A.; Eslava, C.; Hernandez, U.; Navarro-Henze, J.L.; Aviles, M.; Garcia-de la Torre, G.; Cravioto, A. Antibody responses to Escherichia coli O157 and other lipopolysaccharides in healthy children and adults. Clin. Diagn. Lab. Immunol. 2003, 10, 797–801. [Google Scholar] [CrossRef]
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Döpfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar] [CrossRef]
- Aghebati-Maleki, L.; Bakhshinejad, B.; Baradaran, B.; Motallebnezhad, M.; Aghebati-Maleki, A.; Nickho, H.; Yousefi, M.; Majidi, J. Phage display as a promising approach for vaccine development. J. Biomed. Sci. 2016, 23, 66. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.N. Peptide-displaying phage technology in glycobiology. Glycobiology 2011, 22, 318–325. [Google Scholar] [CrossRef]
- Tsang, R.S.W.; Nielsen, K.; Diane Henning, M.; Schlecht, S.; Aleksić, S. A Murine Monoclonal Antibody that Recognizes a Genus-Specific Epitope in the Salmonella Lipopolysaccharid Outer Core. Zbl. Bakt. 1991, 274, 446–455. [Google Scholar] [CrossRef]
- Mansfield, L.P.; Forsythe, S.J. Demonstration of the Rb1 lipopolysaccharide core structure in Salmonella strains with the monoclonal antibody M105. J. Med. Microbiol. 2001, 50, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, C.; Kaniuk, N.; Frirdich, E. Molecular insights into the assembly and diversity of the outer core oligosaccharide in lipopolysaccharides from Escherichia coli and Salmonella. J. Endotoxin Res. 2003, 9, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Littlejohn, J.R.; da Silva, R.F.; Neale, W.A.; Smallcombe, C.C.; Clark, H.W.; Mackay, R.-M.A.; Watson, A.S.; Madsen, J.; Hood, D.W.; Burns, I.; et al. Structural definition of hSP-D recognition of Salmonella enterica LPS inner core oligosaccharides reveals alternative binding modes for the same LPS. PLoS ONE 2018, 13, e0199175. [Google Scholar] [CrossRef]
- MacArthur, M.W.; Thornton, J.M. Influence of proline residues on protein conformation. J. Mol. Biol. 1991, 218, 397–412. [Google Scholar] [CrossRef]
- Harris, S.L.; Craig, L.; Mehroke, J.S.; Rashed, M.; Zwick, M.B.; Kenar, K.; Toone, E.J.; Greenspan, N.; Auzanneau, F.-I.; Marino-Albernas, J.-R.; et al. Exploring the basis of peptide–carbohydrate crossreactivity: Evidence for discrimination by peptides between closely related anti-carbohydrate antibodies. Proc. Natl. Acad. Sci. USA 1997, 94, 2454–2459. [Google Scholar] [CrossRef]
- Dharmasena, M.N.; Jewell, D.A.; Taylor, R.K. Development of peptide mimics of a protective epitope of Vibrio cholerae Ogawa O-antigen and investigation of the structural basis of peptide mimicry. J. Biol. Chem. 2007, 282, 33805–33816. [Google Scholar] [CrossRef]
- Borrelli, S.; Hossany, R.B.; Findlay, S.; Pinto, B.M. Group A Streptococcus Polysaccharide-Mimetic Peptide. Am. J. Immuna. 2006, 2, 77–87. [Google Scholar]
- James, J.A.; Gross, T.; Scofield, R.H.; Harley, J.B. Immunoglobulin epitope spreading and autoimmune disease after peptide immunization: Sm B/B′-derived PPPGMRPP and PPPGIRGP induce spliceosome autoimmunity. J. Exp. Med. 1995, 181, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-S.; Yu, J.; Lin, J.; Zhong, L.; Bren, K.L.; Nahm, M.H. Peptide mimotopes of pneumococcal capsular polysaccharide of 6B serotype: A peptide mimotope can bind to two unrelated antibodies. J. Immunol. 2002, 168, 6273–6278. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, K.R.; Loganathan, D.; Goldstein, I.J.; Schultz, P.; Gallop, M.A. Peptide ligands for a sugar-binding protein isolated from a random peptide library. Proc. Natl. Acad. Sci. USA 1992, 89, 5393–5397. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Passo, C.L.; Scuderi, A.; Kolberg, J.; Baxendale, H.; Goldblatt, D.; Oggioni, M.R.; Felici, F.; Andrew, P.W. Peptide mimics of two pneumococcal capsular polysaccharide serotypes (6B and 9V) protect mice from a lethal challenge with Streptococcus pneumoniae. Eur. J. Immunol. 2009, 39, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.J.; Sharma, S.; Kumar, G.; Visweswariah, S.S.; Surolia, A. Biopanning of endotoxin-specific phage displayed peptides. Biochem. Biophys. Res. Commun. 2003, 307, 133–138. [Google Scholar] [CrossRef]
- Westerink, M.A.; Giardina, P.C.; Apicella, M.A.; Kieber-Emmons, T. Peptide mimicry of the meningococcal group C capsular polysaccharide. Proc. Natl. Acad. Sci. USA 1995, 92, 4021–4025. [Google Scholar] [CrossRef]
- Agostino, M.; Sandrin, M.S.; Thompson, P.E.; Farrugia, W.; Ramsland, P.A.; Yuriev, E. Carbohydrate-mimetic peptides: Structural aspects of mimicry and therapeutic implications. Expert Opin. Biol. Ther. 2011, 11, 211–224. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Q.; Sales, D.; Bianco, A.E.; Craig, A. Vaccination with peptide mimotopes produces antibodies recognizing bacterial capsular polysaccharides. Vaccine 2010, 28, 6425–6435. [Google Scholar] [CrossRef]
- Heinrichs, D.E.; Yethon, J.A.; Whitfield, C. Molecular basis for structural diversity in the core regions of the lipopolysaccharides of Escherichia coli and Salmonella enterica. Mol. Microbiol. 1998, 30, 221–232. [Google Scholar] [CrossRef]
- Jansson, P.-E.; Lindberg, B.; Lindberg, A.A.; Wollin, R. Structural Studies on the Hexose Region of the Core in Lipopolysaccharides from Enterobacteriaceae. Eur. J. Biochem. 1981, 115, 571–577. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).