Abstract
Tick-borne pathogens (TBPs) impose an important limitation to livestock production worldwide, especially in subtropical and tropical areas. Earlier studies in Korea have examined TBPs residing in ticks and animals; however, information on multiple TBPs in ticks infesting cattle is lacking. This study assessed the prevalence of TBPs in ticks parasitizing cattle. A total of 576 ticks, including 340 adults and 236 nymphs, were collected from cattle in Korea between 2014 and 2018. All ticks collected were identified as Haemaphysalis longicornis based on their morphological and molecular characteristics. Among piroplasms and other tick-associated pathogens, seven TBP genes, namely Theileria orientalis (5.0%), Anaplasma bovis (2.3%), Anaplasma capra (4.7%), Anaplasma phagocytophilum-like Anaplasma spp. (APL) clades A (1.9%) and B (0.5%), Ehrlichia canis (1.6%), and Candidatus Rickettsia longicornii (17.5%), were detected. Bartonella spp. and severe fever with thrombocytopenia syndrome virus were not found. To our knowledge, this is the first study to report the presence of the pathogens T. orientalis major piroplasm surface protein genotypes 3 and 7, A. capra, and APL in ticks from Korea. Cattle ticks may be maintenance hosts for many TBPs, and veterinary and medical clinicians should be aware of their high probability of infection and clinical complexity in humans.
1. Introduction
Many emergent tick-borne pathogens (TBPs) were circulating in animals and ticks long before their identification as causes of clinical diseases [1]. The global hazard of TBPs is increasing and fostering public health concerns, as novel pathogens have been continuously recognized during the past two decades [2]. The capability of ticks to attach to humans and transmit pathogens is affected by several factors, including human activities, geographical and climatic conditions, and tick abundance, biological stage, burden, and attachment duration [3].
TBPs impose an important limitation to livestock production across the world, especially in subtropical and tropical areas [4]. Much of the world’s cattle population is influenced by ticks and TBPs, and the damage caused by them is extremely high [5]. Due to the significance of hard ticks (Acari: Ixodidae) in veterinary medicine and the financial cost of their control, the transmission of tick-borne diseases remains an issue for the cattle industry in subtropical and tropical regions, and it is a primary concern for numerous countries in these areas [6].
Understanding the ecology of local tick species parasitizing animals and identifying the TBPs they carry are of paramount public health significance. Previous studies have examined the presence of TBPs in Korea by studying ticks and cattle using molecular methods [7,8,9,10,11,12,13,14]. The current study measured the prevalence of TBPs, including piroplasms (genera Babesia and Theileria), rickettsiae (genera Anaplasma, Ehrlichia, and Rickettsia), Bartonella spp., and severe fever with thrombocytopenia syndrome virus (SFTSV), in ticks collected from cattle in Korea.
2. Materials and Methods
2.1. Ethical Approval
Approval from Kyungpook National University’s Institutional Animal Care and Use Committee was not required for the present study conducted from 2014 to 2018. With oral permission from cattle farm owners, veterinarians from local veterinary institutes collected tick samples when the animals underwent treatment, surveillance, monitoring, or regular check-ups. Tick removal did not cause pain or physical injury to any animals in the study.
2.2. Tick Collection and Species Identification
In total, 576 ticks, including 236 nymphs and 340 adults, were collected from cattle in the northern (81 from Gyeonggi and 82 from Gangwon), central (35 from Chungbuk, 45 from Chungnam, and 77 from Gyeongbuk), and southern (75 from Jeonbuk, 88 from Jeonnam, and 93 from Gyeongnam) areas of Korea from 2014 to 2018. Four to fifteen ticks for each animal were collected by a simple random sampling method from 149 cattle (Hanwoo, native Korean cattle, Bos taurus coreanae) and then stored in tubes containing 70% ethanol. The collected ticks were primarily identified by their morphological features [15], with an additional classification by the molecular methods described below.
2.3. Molecular Detection of Ticks and TBPs
Genomic DNA was extracted from the ticks using a commercial DNeasy Blood & Tissue Kit (Qiagen, Melbourne, Australia), according to the manufacturer’s instructions. The AccuPower HotStart PCR Premix Kit (Bioneer, Daejeon, Korea) was employed for the PCR amplification. Molecular identification of tick species was performed by amplifying the sequence of the mitochondrial cytochrome c oxidase subunit I (COI) gene using specific primers as described previously [16].
The ticks were then screened for several TBPs using primer sets specific to each pathogen. To detect piroplasm 18S rRNA, samples were first examined for infection by piroplasms by PCR using a commercial AccuPower Babesia and Theileria PCR Kit (Bioneer). Positive samples were then re-amplified by PCR using primers designed from the common sequence of the 18S rRNA gene of numerous piroplasm species [17], and major piroplasm surface protein (MPSP) genes of Theileria species were amplified by PCR [18]. Infection with rickettsiae was primarily tested by PCR via a commercial AccuPower Rickettsiales 3-Plex PCR Kit (Bioneer) for the detection of rickettsiae 16S rRNA. Additionally, positive samples were submitted to amplification for species identification. Positive samples of Anaplasma spp. were confirmed by amplifying 16S rRNA fragments using nested PCR (nPCR) [12,13], while positive samples of Rickettsia spp. were confirmed by PCR, which targeted the citrate synthase gene (gltA) [19]. nPCR was employed to amplify the internal transcribed spacer region sequence of Bartonella spp. [20] and the S segment of SFTSV [21].
All primers and amplification conditions used for detecting TBPs in ticks from cattle in the current study are described in Supplementary Table S1.
2.4. DNA Cloning, Nucleotide Sequencing, and Phylogenetic Analysis
For positive PCR products, DNA cloning was performed using pGEM-T Easy vectors (Promega, Madison, WI, USA) and Escherichia coli DH5α-competent cells (Thermo Fisher Scientific, Wilmington, DE, USA). Following cloning, nucleotide sequencing was performed with the multiple sequence alignment program CLUSTAL Omega (v. 1.2.1) [22]. Sequence alignment results were modified by BioEdit (v. 7.2.5) [23]. Phylogenetic analysis was conducted using MEGA (v. 6.0) [24], according to the maximum likelihood method with the Kimura two-parameter distance model. A similarity matrix was used to analyze the aligned sequences. The trees’ stability was assessed by a bootstrap analysis using 1000 replicates.
2.5. Statistical Analysis
The two-sided Fisher’s exact test was performed to analyze significant differences between pathogens for each tick stage, and a value of p < 0.05 was considered to indicate statistical significance. GraphPad Prism (v. 8.0; GraphPad Software Inc., La Jolla, CA, USA) was employed for the statistical analyses.
3. Results
3.1. Identification of Ticks
In total, 576 ticks were collected in the study. Most ticks were partially fed. All of them, including 340 adults and 236 nymphs, were identified as Haemaphysalis longicornis by their morphological characteristics. Tick species were also molecularly identified using universal primers for the COI gene (expected size 710 bp) to avoid potential mistakes in the morphological identification. Both the morphological and molecular analyses identified all the ticks as H. longicornis. Furthermore, the nucleotide sequences from the representative ticks based on the developmental stage and collected region were assessed for the data analysis. The COI gene sequences obtained in this study shared close genetic relationships with H. longicornis (97.7–99.9% nucleotide identity). A phylogenetic tree was created according to the COI genes documented from several tick sequences deposited in GenBank (Figure 1).
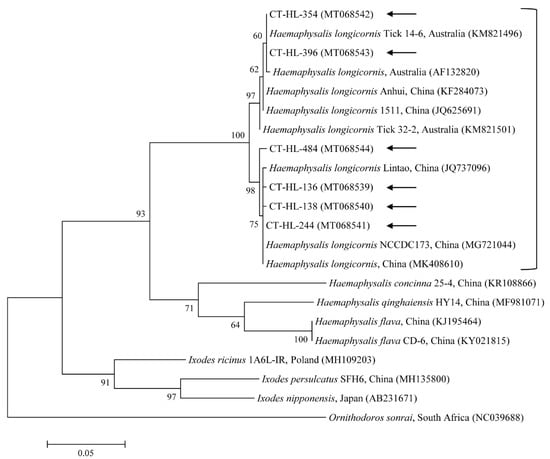
Figure 1.
Molecular identification of ticks according to phylogenetic analysis using the maximum likelihood method with the mitochondrial cytochrome COI gene. Ornithodoros sonrai was employed as the outgroup. Black arrows show the sequences analyzed in this study. GenBank accession numbers for other sequences are presented with the sequence name. Branch numbers signify the bootstrap support levels (1000 replicates), and the scale bar shows the number of substitutions for each nucleotide.
3.2. Identification of TBPs
In total, 33.5% (193/576) of ticks, including 26.7% (63/236) of the nymphs and 38.2% (130/340) of the adults, were PCR-positive for at least one TBP (Table 1). TBPs were significantly more abundant (p = 0.0004) in the adult stage compared with the nymph stage. The 18S rRNA sequences of Theileria orientalis (29/576, 5.0%) were detected in the ticks. Among the positive samples, an additional genetic analysis revealed that the ticks were also positive for T. orientalis MPSP (29/576, 5.0%). The 16S rRNA sequences of Anaplasma bovis (13/576, 2.3%), Anaplasma capra (27/576, 4.7%), Anaplasma phagocytophilum-like Anaplasma spp. (APL) clades A (11/576, 1.9%) and B (3/576, 0.5%), and Ehrlichia canis (9/576, 1.6%) were detected in the ticks. Additionally, the 16S rRNA sequences of Candidatus Rickettsia longicornii (101/576, 17.5%) were discovered in the ticks. Among the positive samples, a supplemental genetic analysis revealed that the ticks were also positive for Candidatus R. longicornii gltA (101/576, 17.5%). Candidatus R. longicornii was the most abundant TBP in both developmental stages: 17.4% (41/236) of the nymphs and 17.6% (60/340) of the adults were PCR-positive. A. bovis (p = 0.0011), A. capra (p = 0.00001), and APL clade A (p = 0.0318) were significantly more abundant in the adult stage compared with the nymph stage. A. bovis was only identified in adults. TBPs were detected in varying proportions in different areas: in the nymph stage, 16.3% (13/80), 28.3% (17/60), and 34.4% (33/96) were detected in the northern, central, and southern areas, respectively, and in the adult stage, 16.9% (14/83), 35.1% (34/97), and 51.3% (82/160) were detected in the northern, central, and southern areas, respectively. A. bovis from the southern area (p = 0.0150) and A. capra from the central (p = 0.0444) and southern (p = 0.0039) areas were significantly more abundant in the adult stage compared with the nymph stage.

Table 1.
Prevalence of tick-borne pathogens (TBPs) detected in H. longicornis ticks parasitizing cattle in Korea, 2014–2018.
With regard to infection, 2.8% (16/576) of ticks represented multiple infections (Table 2). Ticks infected with more than one pathogen constituted 1.3% (3/236) of the nymphs and 3.8% of the adults (13/340). There were no significant differences in multiple infections between pathogens for each tick stage. However, multiple infections tended to be more abundant in the adult stage compared with the nymph stage. Among the infected ticks, 4.8% of the nymphs (3/63) and 10% (13/130) of the adults carried more than one pathogen species. Three (0.5%) were coinfected with T. orientalis and Candidatus R. longicornii, one (0.2%) was coinfected with T. orientalis and APL clade A, one (0.2%) was coinfected with E. canis and A. capra, three (0.5%) were coinfected with Candidatus R. longicornii and A. bovis, four (0.7%) were coinfected with Candidatus R. longicornii and A. capra, three (0.5%) were coinfected with Candidatus R. longicornii and APL clade A, and one (0.2%) was coinfected with T. orientalis, A. bovis, and Candidatus R. longicornii. Bartonella spp. and SFTSV were not detected in this study.

Table 2.
Multiple infections of TBPs detected in H. longicornis ticks parasitizing cattle in Korea, 2014–2018.
3.3. Molecular and Phylogenetic Analyses
Phylogenetic analyses showed that the 18S rRNA (Figure 2) and MPSP (Figure 3) nucleotide sequences of Theileria spp., 16S rRNA nucleotide sequences of Anaplasma spp. (Figure 4) and E. canis (Figure 5), and 16S rRNA (Figure 6) and gltA (Figure 7) nucleotide sequences of Rickettsia spp. were clustered with previously documented sequences.
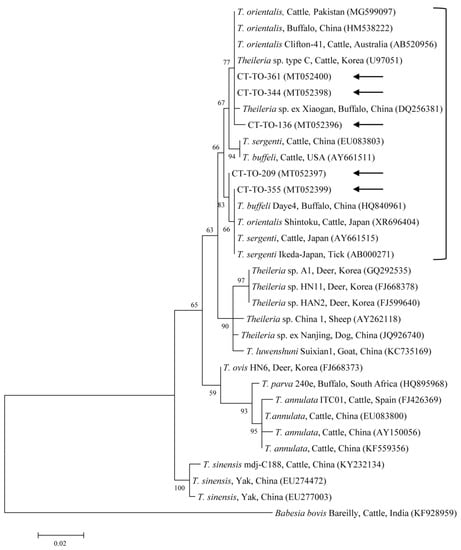
Figure 2.
Phylogenetic tree of Theileria spp. based on sequences of the 18S rRNA gene. The tree was created using the maximum likelihood method. Black arrows show the sequences analyzed in the present study. Babesia bovis was employed as the outgroup. GenBank accession numbers of other sequences are presented with the sequence name. Branch numbers signify the bootstrap support levels (1000 replicates), and the scale bar shows the number of substitutions for each nucleotide.
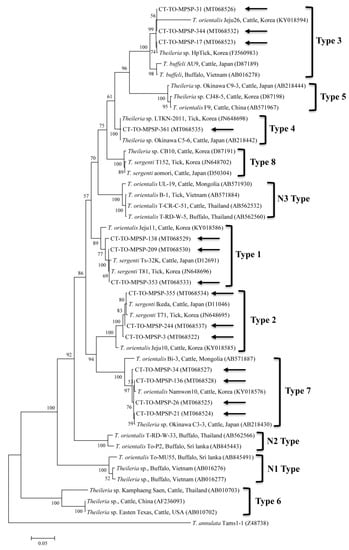
Figure 3.
Phylogenetic tree of Theileria spp. based on sequences of the major piroplasm surface protein (MPSP) gene. The tree was created using the maximum likelihood method. Black arrows show the sequences analyzed in the present study. Theileria annulata was employed as the outgroup. GenBank accession numbers of other sequences are presented with the sequence name. Branch numbers signify the bootstrap support levels (1000 replicates), and the scale bar shows the number of substitutions for each nucleotide.
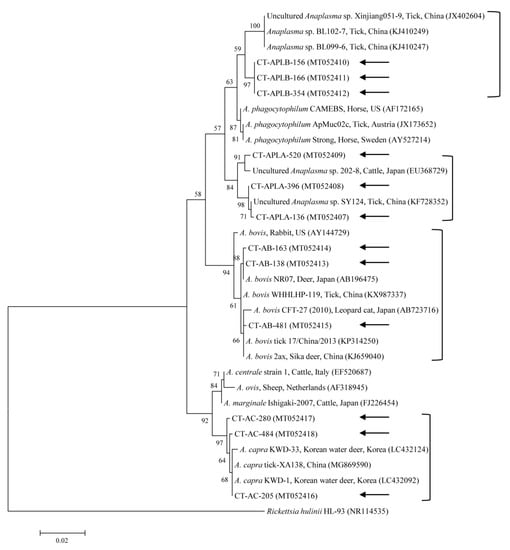
Figure 4.
Phylogenetic tree of Anaplasma spp. based on sequences of the 16S rRNA gene. The tree was created using the maximum likelihood method. Black arrows show the sequences analyzed in the present study. Rickettsia hulinii was employed as the outgroup. GenBank accession numbers of other sequences are presented with the sequence name. Branch numbers signify the bootstrap support levels (1000 replicates), and the scale bar shows the number of substitutions for each nucleotide.
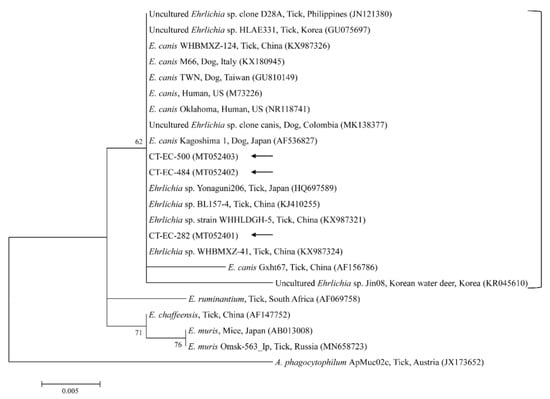
Figure 5.
Phylogenetic tree of Ehrlichia spp. based on sequences of the 16S rRNA gene. The tree was created using the maximum likelihood method. Black arrows show the sequences analyzed in the present study. Anaplasma phagocytophilum was employed as the outgroup. GenBank accession numbers of other sequences are presented with the sequence name. Branch numbers signify the bootstrap support levels (1000 replicates), and the scale bar shows the number of substitutions for each nucleotide.
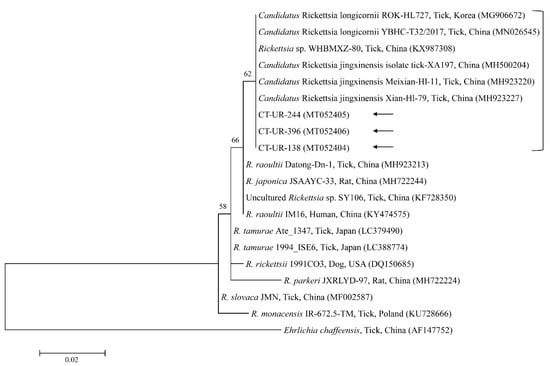
Figure 6.
Phylogenetic tree of Rickettsia spp. based on sequences of the 16S rRNA gene. The tree was created using the maximum likelihood method. Black arrows show the sequences analyzed in the present study. Ehrlichia chaffeensis was employed as the outgroup. GenBank accession numbers of other sequences are presented with the sequence name. Branch numbers signify the bootstrap support levels (1000 replicates), and the scale bar shows the number of substitutions for each nucleotide.
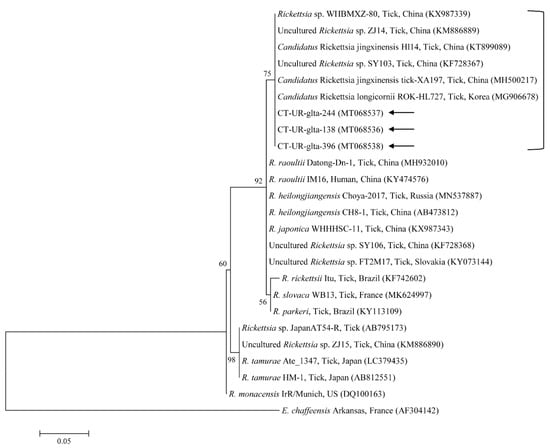
Figure 7.
Phylogenetic tree of Rickettsia spp. based on sequences of the gltA gene. The tree was created using the maximum likelihood method. Black arrows show the sequences analyzed in the present study. Ehrlichia chaffeensis was employed as the outgroup. GenBank accession numbers of other sequences are presented with the sequence name. Branch numbers signify the bootstrap support levels (1000 replicates), and the scale bar shows the number of substitutions for each nucleotide.
The five sequences of T. orientalis found in the present study shared a 97.4–98.8% identity with the 18S rRNA sequence. They also shared a 97.6–99.7% identity with the 18S rRNA sequences in previously reported T. orientalis isolates. The T. orientalis MPSP gene sequences were classified into five genotypes: types 1, 2, 3, 4, and 7. Among the 29 sequences, 11, 10, 3, 1, and 4 isolates were assigned to types 1, 2, 3, 4, and 7, respectively. The three representative sequences of types 1 and 2 found in the present study shared a 98.7–99.8% and 98.8–99.3% identity with the MPSP sequence, respectively. They also shared a 99.5–99.8% and 99.3–99.5% identity with the MPSP sequences in previously reported T. orientalis isolates, respectively. The three, one, and four sequences of types 3, 4, and 7 that we found each shared a 99.6–100%, 100%, and 97.4–99.9% identity with the MPSP sequence, respectively. Each of them also shared a 98.1–99.8%, 98.3–99.8%, and 97.9–99.3% identity with the MPSP sequences in previously reported T. orientalis isolates, respectively.
The three representative sequences of A. capra herein shared a 99.7–99.8% identity with the 16S rRNA sequence. They also shared a 99.7–100% identity with the 16S rRNA sequences in previously reported A. capra isolates. We determined that the three representative sequences of APL clade A shared a 98.7–99.8% identity with the 16S rRNA sequences and a 98.7–99.8% identity with the 16S rRNA sequences in previously reported APL clade A isolates. Our three sequences of APL clade B shared a 100% and 98.6% identity with the 16S rRNA sequence and reported APL clade B isolates, respectively. Similarly, the three representative sequences of A. bovis shared a 99.4–99.9% identity with the 16S rRNA sequences and a 99.2–100% identity with other A. bovis isolates.
The three representative sequences of E. canis observed in our study shared a 100% identity with the 16S rRNA sequence and a 99.0–100% identity with previously reported E. canis isolates. Our three representative sequences of Candidatus R. longicornii shared a 100% identity with 16S rRNA and gltA sequences and a 100% and 98.9–99.2% identity with 16S rRNA and gltA sequences in previously reported isolates, respectively.
The representative sequences ascertained in this study were submitted to GenBank. The accession numbers are as follows: MT068539–MT068544 (H. longicornis), MT052396–MT052400 (Theileria spp. 18S rRNA), MT068522–MT068535 (Theileria spp. MPSP), MT052407–MT052418 (Anaplasma spp.), MT052401–MT052403 (E. canis), MT052404–MT052406 (Rickettsia spp. 16S rRNA), and MT068536–MT068538 (Rickettsia spp. gltA).
4. Discussion
In our study, only H. longicornis, including 340 adults and 236 nymphs, was found in cattle by both morphological and molecular methods. These findings are consistent with the result of a previous Korean study, which found H. longicornis (900/903, 99.7%) and Ixodes spp. (3/903, 0.3%) in cattle [10]. In another study [25], H. longicornis (15,020/19,821, 75.8%), H. flava (3889/19,821, 19.6%), and I. nipponensis (912/19,821, 4.6%) were identified from various habitats. H. longicornis is the most frequently identified species in Korea. The climate of Korea is steadily becoming subtropical due to global warming. The emergence of endemic TBPs might be associated with climate-driven changes to their geographic ecology and range. In the present study, TBPs were more prevalent in the southern area in the nymph and adult tick stages. This biogeoclimatic difference may clarify the observed differences in the prevalence of ticks and TBPs. These findings show that an additional geographical study is needed to fully understand the tick populations and clarify the distribution of TBPs in animals.
In the present study, the prevalence of TBPs was 1.4-times higher in the adults (38.2%) than in the nymphs (26.7%). This result is similar to that of a previous study [26] in which the overall infection rate was 2.7-times higher in adults compared with nymphs, most likely due to the transstadial accumulation in the mature ticks. In addition, the prevalence of multiple infections was higher in the adults than in the nymphs. In total, 1.3% of the collected H. longicornis nymphs and 3.8% of the adults were infected with more than one disease agent, constituting 4.8% and 10% of all the infected nymphs and adults, respectively. The COI sequences from the collected H. longicornis showed a 97.6–99.7% nucleotide identity with known COI sequences of H. longicornis (Figure 1). H. longicornis is typically collected from grasslands and herbaceous vegetation throughout Korea [25].
In this study, T. orientalis, A. bovis, A. capra, APL clades A and B, E. canis, and Candidatus R. longicornii were detected in cattle ticks by the molecular analysis. Theileriosis, one of the most important tick-borne hemoprotozoan diseases, can affect domestic animals, most frequently sheep and cattle in subtropical and tropical zones, and it causes great economic losses [27]. The members of the taxonomic group encompassing T. buffeli, T. sergenti, and T. orientalis are very similar, and the separate taxonomy of this group is controversial. Based on molecular studies, the three parasites are classified as one species, T. orientalis [28]. Recently in Korea, 18S rRNA genes were detected as T. orientalis in ticks (3.7%, 21/566 pools) from cattle [10] and T. orientalis in cattle (23.2%, 69/298) [29]. Here, 15 H. longicornis nymphs and 14 H. longicornis adults were positive for the T. orientalis 18S rRNA gene. The prevalence of T. orientalis in ticks herein (5.0%) was lower than that in a cattle study (23.2%) in Korea [29]. Based on the sequence analysis of the MPSP gene, T. orientalis consists of at least 11 different MPSP genotypes, including types 1–8 and N1–N3 [28]. Of the 11 MPSP genotypes, the Ikeda group consists of types 2 and 7, and the Chitose group consists of types 1, 3, 4, 5, 8, and N-3 [30]. Types N-1 and N-2 appear to have low sequence homology to each other [30]. Type 6 has been found in yaks and cattle, and it is classified as T. sinensis [31]. Recently in Korea, types 1, 2, 4, and 8 of the Theileria MPSP gene (2.7%, 15/556) were identified in ticks from grazing cattle [9], and types 1–3 and 7 (41.3%, 57/138) were identified in cattle [32]. In the present study, types 1–4 and 7 of the Theileria MPSP gene (5%, 29/576) were identified in cattle ticks. To our knowledge, this is the first study to report the presence of Theileria MPSP genotypes 3 and 7 in ticks in Korea. Of the five MPSP genotypes identified in this study, types 1 (37.9%, 11/29) and 2 (34.5%, 10/29) were the most commonly detected in ticks. In previous studies, type 2 in cattle [32] and types 2 and 4 in ticks from cattle [9] were also predominant. Types 1 (Ikeda) and 2 (Chitose) have been most commonly linked to clinical diseases. Of these, type 2 is more pathogenic [28], and it causes high parasitemia, severe anemia, and sometimes death. Therefore, it is imperative to conduct additional studies to identify the relationship between clinical signs and pathogenic types by determining the MPSP genotypes of T. orientalis that are related to animals and ticks.
The genus Anaplasma contains obligate intracellular Gram-negative bacteria belonging to the order Rickettsiales and the family Anaplasmataceae [33]. There are seven formally recognized species of Anaplasma (A. phagocytophilum, A. centrale, A. marginale, A. platys, A. bovis, A. ovis, and A. caudatum). A. capra represents a probable eighth species, and it has been argued as a new Anaplasma species whose name has not yet been formally recognized [34]. A. capra was recently identified from goats in China as a new tick-transmitted emerging zoonotic pathogen owing to its isolation from human blood (5.9%, 28/477) after tick bites [35]. It remains uncertain whether A. capra is pathogenic to both humans and animals, but if it is confirmed as such, it could pose a significant public health risk, the same as A. phagocytophilum [35]. In Korea, A. capra was formerly identified in cattle (0.4%, 5/1219) [12] and in Korean water deer (17.7%, 35/198) [36]. In the present study, one H. longicornis nymph and 26 H. longicornis adults tested positive for A. capra. The prevalence of A. capra in ticks herein (4.7%) was higher than that in a cattle study (0.4%) in Korea [12]. To our knowledge, this is the first study to report the presence of A. capra in ticks in Korea. A. capra is an emerging human pathogen in ticks parasitizing cattle and other animals in Korea. However, the vector capability of ticks for the transmission of A. capra is still unclear and needs additional evaluation for public health control.
A. phagocytophilum is the causative pathogen of granulocytic anaplasmosis in various species, such as humans, dogs, goats, horses, sheep, and cattle [37]. The rapid and accurate diagnosis of pathogenic and zoonotic diseases, such as human granulocytic anaplasmosis, is required for the risk estimation in TBP control programs [38]. Therefore, it is meaningful to differentiate between pathogenic A. phagocytophilum and APL species that do not cause clinical signs in infected animals and that are presently considered non-pathogenic [38]. APL clade A was detected in cattle (2.6%, 20/764) in Korea [13]. Several APL clades have also been reported in other countries: APL clade A was detected in cattle (2.0%, 1/50) and ticks (2.4%, 2/85) in Japan [39], APL clade B was found in ticks (0.8%, 3/388 pools) in China [40], and APL clades A and B were detected in cattle (1.9%, 7/367; 0.5%, 2/367), sheep (7.0%, 25/355; 5.4%, 19/355), and goats (13.3%, 32/241; 5.0%, 12/241) in Tunisia [38]. In the present study, APL clade A was detected in one H. longicornis nymph and 10 H. longicornis adults, and one H. longicornis nymph and two H. longicornis adults were positive for APL clade B. The prevalence of APL clade A in ticks herein (1.9%) was lower than that in a cattle study (2.6%) in Korea [13]. To our knowledge, this is the first study to report the presence of both APL clades A and B in ticks from Korea. Additional studies are required to provide more information on the molecular background and to trace the evolutionary tree of novel Anaplasma species. A. bovis, a monocytotropic species, has been reported in ruminants in numerous countries [41]. A. bovis was formerly detected in Korea in cattle (1.0%, 12/1219) [12], in ticks (7.5%, 20/266 pools) from Korean water deer [11], in H. longicornis ticks (2.5%, 1/40 pools) from native Korean goats [14], and in H. longicornis ticks (1.0%, 5/506 pools) [8]. In the present study, 13 H. longicornis adults were positive for A. bovis. The prevalence of A. bovis in ticks herein (2.3%) was higher than that in a cattle study (1.0%) in Korea [12]. A. bovis was the only species of TBPs that was not found in the nymph stage. Meanwhile, in our previous study [14], A. bovis was only detected in the nymph stage of H. longicornis ticks from goats. In another study [42], A. bovis was detected in all tick stages, including larvae, nymphs, and adults. Thus, this appears to be an issue of the number of ticks tested. If we were to collect more ticks, we would be more likely to detect A. bovis in nymphs.
Canine monocytic ehrlichiosis is a systemic infection in dogs caused by E. canis, which is transmitted by Rhipicephalus sanguineus sensu lato, known as the brown dog tick [43]. E. canis was previously identified in Korea in H. longicornis and Ixodes turdus (1.1%, 18/1638 pools) and small mammals (12.0%, 51/424) [7], in H. longicornis ticks (1.2%, 6/506 pools) [8], and in H. longicornis from cattle (22.3%, 126/566 pools) [10]. Moreover, reports have revealed human infections of E. canis in Venezuela [44] and a novel genotype of E. canis in a human from Costa Rica [45]. The presence of E. canis in human samples is likely associated with the high prevalence of this pathogen in ticks and dogs [45]. In our study, four H. longicornis nymphs and five H. longicornis adults tested positive for E. canis. Continuous monitoring for infected ticks and reservoir hosts is required to ensure the health and safety of animals and the public against the risks of TBP exposure.
Rickettsia spp. are emerging or re-emerging pathogens with public health importance [46]. Obligate intracellular bacteria belong to the spotted fever group (SFG) and cause tick-borne rickettsioses [47]. In the present study, one SFG rickettsia with the Candidatus status was identified in the ticks. A potentially new SFG rickettsia classified into the putative novel subgroup “Candidatus R. longicornii” has been detected in Korea, including in H. longicornis ticks (43.2%, 79/183) [48], in H. longicornis ticks (16.7%, 52/311 pools) [49], and in H. longicornis ticks (45%, 18/40 pools) from native Korean goats [14]. These pathogens were clustered together in a subgroup that represented a sister taxon separate from the known subgroups of SFG rickettsiae. Gene fragment sequences reported in GenBank for rickettsial isolates from H. longicornis in Korea, Japan, and China have uncertain taxonomic statuses and unidentified pathogenicity that are most likely correlated to Candidatus R. longicornii or to a very closely associated species [48]. In addition, the XY118 (KU853023) isolate was detected from a patient, representing its possible pathogenicity in humans. Further studies are required to determine the pathogenicity of this novel pathogen. Here, Candidatus R. longicornii was detected in 41 H. longicornis nymphs and 60 H. longicornis adults. Additional research is necessary to identify other novel Rickettsia spp. in ticks and animals in Korea.
The bootstrap support levels need to be evaluated cautiously. As a limitation in this study, some bootstrap values were low. This could be due to using small amplification fragments of genes for the phylogenetic analysis. Therefore, further studies are needed to analyze longer fragments of genes in a phylogenetic analysis for a better presentation of the data.
5. Conclusions
In the present study, seven TBPs were detected in cattle H. longicornis ticks from Korea, including human pathogens of A. capra, E. canis, and Candidatus R. longicornii. Overall, 33.5% of ticks harbored at least one TBP. In 1.3% of the nymphs and 3.8% of the adults, we found more than one TBP. Among them, Candidatus R. longicornii was the most prevalent. To our knowledge, this is the first study to report the presence of the pathogens T. orientalis MPSP genotypes 3 and 7, A. capra, and APL in ticks from Korea. Cattle ticks may be maintenance hosts for many TBPs, and veterinary and medical clinicians should be aware of their high probability of infection and clinical complexity in humans. Our results show that ticks parasitizing cattle could be possible maintenance hosts for TBPs, and because of the zoonotic pathogenic importance of TBPs, we need to increase the awareness of their wide distribution and adopt measures to prevent their spread. Moreover, this study shows that coinfections are represented and thus should be considered in the diagnosis of TBPs, and that additional studies in Korea are needed in the future.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/8/5/728/s1, Table S1: Primers used for the detection of tick-borne pathogens in ticks from cattle in the present study.
Author Contributions
Conceptualization, M.-G.S. and D.K.; Formal analysis, O.-D.K. and D.K.; Funding acquisition, D.K.; Methodology, M.-G.S.; Supervision, D.K.; Validation, O.-D.K. and D.K.; Writing—original draft, M.-G.S.; Writing—editing and review, O.-D.K. and D.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education (Grant No. NRF-2016R1D1A1B02015366).
Acknowledgments
This research was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baneth, G. Tick-borne infections of animals and humans: A common ground. Int. J. Parasitol. 2014, 44, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.E.; et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Raoult, D. Ticks and tickborne bacterial diseases in humans: An emerging infectious threat. Clin. Infect. Dis. 2001, 32, 897–928. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Chomel, B.B.; Otranto, D. Ticks and tick-borne diseases: A One Health perspective. Trends. Parasitol. 2012, 28, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Alp, H.; Aksin, M.; Seitzer, U. Current status of ticks in Asia. Parasitol. Res. 2007, 101, 159–162. [Google Scholar] [CrossRef]
- Lodos, J.; Boue, O.; de la Fuente, J. A model to simulate the effect of vaccination against Boophilus ticks on cattle. Vet. Parasitol. 2000, 87, 315–326. [Google Scholar] [CrossRef]
- Kim, C.M.; Yi, Y.H.; Yu, D.H.; Lee, M.J.; Cho, M.R.; Desai, A.R.; Shringi, S.; Klein, T.A.; Kim, H.C.; Song, J.W.; et al. Tick-borne rickettsial pathogens in ticks and small mammals in Korea. Appl. Environ. Microbiol. 2006, 72, 5766–5776. [Google Scholar] [CrossRef]
- Oh, J.Y.; Moon, B.C.; Bae, B.K.; Shin, E.H.; Ko, Y.H.; Kim, Y.J.; Park, Y.H.; Chae, J.S. Genetic identification and phylogenetic analysis of Anaplasma and Ehrlichia species in Haemaphysalis longicornis collected from Jeju island, Korea. J. Bacteriol. Virol. 2009, 39, 257–267. [Google Scholar] [CrossRef]
- Kang, S.W.; Nguyen, L.T.; Noh, J.H.; Reddy, K.E.; Kweon, C.H.; Choe, S.E. Phylogenetic analysis of benign Theileria species based on major piroplasm surface protein (MPSP) genes from ticks of grazing cattle in Korea. Vet. Parasitol. 2012, 189, 145–152. [Google Scholar] [CrossRef]
- Kang, S.W.; Doan, H.T.; Choe, S.E.; Noh, J.H.; Yoo, M.S.; Reddy, K.E.; Kim, Y.H.; Kweon, C.H.; Jung, S.C.; Chang, K.Y. Molecular investigation of tick-borne pathogens in ticks from grazing cattle in Korea. Parasitol. Int. 2013, 62, 276–282. [Google Scholar] [CrossRef]
- Kang, J.G.; Ko, S.; Kim, H.C.; Chong, S.T.; Klein, T.A.; Chae, J.B.; Jo, Y.S.; Choi, K.S.; Yu, D.H.; Park, B.K.; et al. Prevalence of Anaplasma and Bartonella spp. in ticks collected from Korean water deer (Hydropotes inermis argyropus). Korean J. Parasitol. 2016, 54, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.G.; Ouh, I.O.; Lee, H.; Geraldino, P.J.L.; Rhee, M.H.; Kwon, O.D.; Kwak, D. Differential identification of Anaplasma in cattle and potential of cattle to serve as reservoirs of Anaplasma capra, an emerging tick-borne zoonotic pathogen. Vet. Microbiol. 2018, 226, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.G.; Ouh, I.O.; Kwon, O.D.; Kwak, D. Molecular detection of Anaplasma phagocytophilum-like Anaplasma spp. and pathogenic A. Phagocytophilum in cattle from South Korea. Mol. Phylogenet. Evol. 2018, 126, 23–30. [Google Scholar] [CrossRef]
- Seo, M.G.; Kwon, O.D.; Kwak, D. Molecular and phylogenetic analysis of tick-borne pathogens in ticks parasitizing native Korean goats (Capra hircus coreanae) in South Korea. Pathogens 2020, 9, 71. [Google Scholar] [CrossRef]
- Barker, S.C.; Walker, A.R. Ticks of Australia. The species that infest domestic animals and humans. Zootaxa 2014, 3816, 1–144. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Seo, M.G.; Yun, S.H.; Choi, S.K.; Cho, G.J.; Park, Y.S.; Cho, K.H.; Kwon, O.D.; Kwak, D. Molecular and phylogenetic analysis of equine piroplasms in the Republic of Korea. Res. Vet. Sci. 2013, 94, 579–583. [Google Scholar] [CrossRef]
- Kakuda, T.; Shiki, M.; Kubota, S.; Sugimoto, C.; Brown, W.C.; Kosum, C.; Nopporn, S.; Onuma, M. Phylogeny of benign Theileria species from cattle in Thailand, China and the U.S.A. based on the major piroplasm surface protein and small subunit ribosomal RNA genes. Int. J. Parasitol. 1998, 28, 1261–1267. [Google Scholar] [CrossRef]
- Reis, C.; Cote, M.; Paul, R.E.; Bonnet, S. Questing ticks in suburban forest are infected by at least six tick-borne pathogens. Vector Borne Zoonotic Dis. 2011, 11, 907–916. [Google Scholar] [CrossRef]
- Ko, S.; Kim, S.J.; Kang, J.G.; Won, S.; Lee, H.; Shin, N.S.; Choi, K.S.; Youn, H.Y.; Chae, J.S. Molecular detection of Bartonella grahamii and B. schoenbuchensis-related species in Korean water deer (Hydropotes inermis argyropus). Vector Borne Zoonotic Dis. 2013, 13, 415–418. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Fukushi, S.; Tani, H.; Fukuma, A.; Taniguchi, S.; Toda, S.; Shimazu, Y.; Yano, K.; Morimitsu, T.; Ando, K.; et al. Sensitive and specific PCR systems for detection of both Chinese and Japanese severe fever with thrombocytopenia syndrome virus strains and prediction of patient survival based on viral load. J. Clin. Microbiol. 2014, 52, 3325–3333. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Higgins, D.G. Clustal Omega for making accurate alignments of many protein sciences. Protein Sci. 2018, 27, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids. Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.T.; Kim, H.C.; Lee, I.Y.; Kollars, T.M., Jr.; Sancho, A.R.; Sames, W.J.; Chae, J.S.; Klein, T.A. Seasonal distribution of ticks in four habitats near the demilitarized zone, Gyeonggi-do (Province), Republic of Korea. Korean J. Parasitol. 2013, 51, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Klitgaard, K.; Kjær, L.J.; Isbrand, A.; Hansen, M.F.; Bødker, R. Multiple infections in questing nymphs and adult female Ixodes ricinus ticks collected in a recreational forest in Denmark. Ticks Tick Borne Dis. 2019, 10, 1060–1065. [Google Scholar] [CrossRef]
- Uilenberg, G. International collaborative research: Significance of tick-borne hemoparasitic diseases to world animal health. Vet. Parasitol. 1995, 57, 19–41. [Google Scholar] [CrossRef]
- Sivakumar, T.; Hayashida, K.; Sugimoto, C.; Yokoyama, N. Evolution and genetic diversity of Theileria. Infect. Genet. Evol. 2014, 27, 250–263. [Google Scholar] [CrossRef]
- Kim, S.; Yu, D.H.; Kang, S.W.; Chae, J.B.; Choi, K.S.; Kim, H.C.; Park, B.K.; Chae, J.S.; Park, J. Hematological changes associated with Theileria orientalis infection in Korean indigenous cattle. Korean J. Parasitol. 2017, 55, 481–489. [Google Scholar] [CrossRef]
- Khukhuu, A.; Lan, D.T.; Long, P.T.; Ueno, A.; Li, Y.; Luo, Y.; Macedo, A.C.; Matsumoto, K.; Inokuma, H.; Kawazu, S.; et al. Molecular epidemiological survey of Theileria orientalis in Thua Thien Hue Province, Vietnam. J. Vet. Med. Sci. 2011, 73, 701–705. [Google Scholar] [CrossRef]
- Liu, A.; Guan, G.; Liu, Z.; Liu, J.; Leblanc, N.; Li, Y.; Gao, J.; Ma, M.; Niu, Q.; Ren, Q.; et al. Detecting and differentiating Theileria sergenti and Theileria sinensis in cattle and yaks by PCR based on major piroplasm surface protein (MPSP). Exp. Parasitol. 2010, 126, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Han, Y.J.; Han, D.G.; Chae, J.B.; Chae, J.S.; Yu, D.H.; Lee, Y.S.; Park, B.K.; Kim, H.C.; Choi, K.S. Genetic characterization of Theileria orientalis from cattle in the Republic of Korea. Parasitol. Res. 2017, 116, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Rar, V.; Golovljova, I. Anaplasma, Ehrlichia, and “Candidatus Neoehrlichia” bacteria: Pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infect. Genet. Evol. 2011, 11, 1842–1861. [Google Scholar] [CrossRef]
- Khumalo, Z.T.H.; Brayton, K.A.; Collins, N.E.; Chaisi, M.E.; Quan, M.; Oosthuizen, M.C. Evidence confirming the phylogenetic position of Anaplasma centrale (ex. Theiler 1911) Ristic and Kreier 1984. Int. J. Syst. Evol. Microbiol. 2018, 68, 2682–2691. [Google Scholar] [CrossRef]
- Li, H.; Zheng, Y.C.; Ma, L.; Jia, N.; Jiang, B.G.; Jiang, R.R.; Huo, Q.B.; Wang, Y.W.; Liu, H.B.; Chu, Y.L.; et al. Human infection with a novel tick-borne Anaplasma species in China: A surveillance study. Lancet Infect. Dis. 2015, 15, 663–670. [Google Scholar] [CrossRef]
- Amer, S.; Kim, S.; Yun, Y.; Na, K.J. Novel variants of the newly emerged Anaplasma capra from Korean water deer (Hydropotes inermis argyropus) in South Korea. Parasit. Vectors 2019, 12, 365. [Google Scholar] [CrossRef]
- Stuen, S.; Granquist, E.G.; Silaghi, C. Anaplasma phagocytophilum—a widespread multi-host pathogen with highly adaptive strategies. Front. Cell. Infect. Microbiol. 2013, 3, 31. [Google Scholar] [CrossRef]
- Ben Said, M.; Belkahia, H.; El Mabrouk, N.; Saidani, M.; Ben Hassen, M.; Alberti, A.; Zobba, R.; Bouattour, S.; Bouattour, A.; Messadi, L. Molecular typing and diagnosis of Anaplasma spp. closely related to Anaplasma phagocytophilum in ruminants from Tunisia. Ticks Tick Borne Dis. 2017, 8, 412–422. [Google Scholar] [CrossRef]
- Ybañez, A.P.; Tagawa, M.; Matsumoto, K.; Kishimoto, T.; Yokoyama, N.; Inokuma, H. Specific molecular detection of Anaplasma sp. closely related to Anaplasma phagocytophilum in ixodid ticks and cattle in a pastureland in Hokkaido, Japan. Vector Borne Zoonotic Dis. 2013, 13, 6–11. [Google Scholar] [CrossRef]
- Kang, Y.J.; Diao, X.N.; Zhao, G.Y.; Chen, M.H.; Xiong, Y.; Shi, M.; Fu, W.M.; Guo, Y.J.; Pan, B.; Chen, X.P.; et al. Extensive diversity of Rickettsiales bacteria in two species of ticks from China and the evolution of the Rickettsiales. BMC Evol. Biol. 2014, 14, 167. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, M.; Wang, Z.; Wang, J.; Peng, Y.; Li, Y.; Guan, G.; Luo, J.; Yin, H. Molecular survey and genetic identification of Anaplasma species in goats from central and southern China. Appl. Environ. Microbiol. 2012, 78, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Palomar, A.M.; Portillo, A.; Santibáñez, P.; Mazuelas, D.; Roncero, L.; García-Álvarez, L.; Santibáñez, S.; Gutiérrez, Ó.; Oteo, J.A. Detection of tick-borne Anaplasma bovis, Anaplasma phagocytophilum and Anaplasma centrale in Spain. Med. Vet. Entomol. 2015, 29, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Rikihisa, Y.; Wen, B. Ehrlichia canis-like agent isolated from a man in Venezuela: Antigenic and genetic characterization. J. Clin. Microbiol. 1996, 34, 2133–2139. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Bodor, M.; Zhang, C.; Xiong, Q.; Rikihisa, Y. Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Ann. N. Y. Acad. Sci. 2006, 1078, 110–117. [Google Scholar] [CrossRef]
- Bouza-Mora, L.; Dolz, G.; Solorzano-Morales, A.; Romero-Zuniga, J.J.; Salazar-Sanchez, L.; Labruna, M.B.; Aguiar, D.M. Novel genotype of Ehrlichia canis detected in samples of human blood bank donors in Costa Rica. Ticks Tick Borne Dis. 2017, 8, 36–40. [Google Scholar] [CrossRef]
- Merhej, V.; Angelakis, E.; Socolovschi, C.; Raoult, D. Genotyping, evolution and epidemiological findings of Rickettsia species. Infect. Genet. Evol. 2014, 25, 122–137. [Google Scholar] [CrossRef]
- Parola, P.; Paddock, C.D.; Raoult, D. Tick-borne rickettsioses around the world: Emerging diseases challenging old concepts. Clin. Microbiol. Rev. 2005, 18, 719–756. [Google Scholar] [CrossRef]
- Jiang, J.; An, H.; Lee, J.S.; O’Guinn, M.L.; Kim, H.C.; Chong, S.T.; Zhang, Y.; Song, D.; Burrus, R.G.; Bao, Y.; et al. Molecular characterization of Haemaphysalis longicornis-borne rickettsiae, Republic of Korea and China. Ticks Tick Borne Dis. 2018, 9, 1606–1613. [Google Scholar] [CrossRef]
- Noh, Y.; Lee, Y.S.; Kim, H.C.; Chong, S.T.; Klein, T.A.; Jiang, J.; Richards, A.L.; Lee, H.K.; Kim, S.Y. Molecular detection of Rickettsia species in ticks collected from the southwestern provinces of the Republic of Korea. Parasit. Vectors 2017, 10, 20. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).