Abstract
In the current study, we aimed to elucidate the plant growth-promoting characteristics of Pseudomonas psychrotolerans CS51 under heavy metal stress conditions (Zn, Cu, and Cd) and determine the genetic makeup of the CS51 genome using the single-molecule real-time (SMRT) sequencing technology of Pacific Biosciences. The results revealed that inoculation with CS51 induced endogenous indole-3-acetic acid (IAA) and gibberellins (GAs), which significantly enhanced cucumber growth (root shoot length) and increased the heavy metal tolerance of cucumber plants. Moreover, genomic analysis revealed that the CS51 genome consisted of a circular chromosome of 5,364,174 base pairs with an average G+C content of 64.71%. There were around 4774 predicted protein-coding sequences (CDSs) in 4859 genes, 15 rRNA genes, and 67 tRNA genes. Around 3950 protein-coding genes with function prediction and 733 genes without function prediction were identified. Furthermore, functional analyses predicted that the CS51 genome could encode genes required for auxin biosynthesis, nitrate and nitrite ammonification, the phosphate-specific transport system, and the sulfate transport system, which are beneficial for plant growth promotion. The heavy metal resistance of CS51 was confirmed by the presence of genes responsible for cobalt-zinc-cadmium resistance, nickel transport, and copper homeostasis in the CS51 genome. The extrapolation of the curve showed that the core genome contained a minimum of 2122 genes (95% confidence interval = 2034.24 to 2080.215). Our findings indicated that the genome sequence of CS51 may be used as an eco-friendly bioresource to promote plant growth in heavy metal-contaminated areas.
1. Introduction
Heavy metal stress greatly decreases crop growth and productivity. It is known as a major threat in various terrestrial ecosystems worldwide [1]. Currently, extensive industrialization directly increases the accumulation of heavy metals in soils, which consequently has detrimental effects on crop growth and productivity [2]. Although several heavy metals (e.g., Mn, Cu, Co, Zn, Mo, and Ni) are vital for important biological processes [1,3], the excessive accumulation of these heavy metals with other highly toxic heavy metals including Pb, Cd, As, Hg, Cr, and Al can limit crop productivity [4]. The accumulation of heavy metals in soils directly affects the texture and pH of the soil, which ultimately may reduce the growth of plants by exerting detrimental effects on various biological processes in plants [5].
Several studies have reported the positive role of microorganisms in plant growth and productivity in heavy metal-contaminated soils [6,7]. Plant growth-promoting rhizobacteria (PGPR) are a group of bacteria that can improve plant growth and productivity [8]. These microorganisms are found predominantly in the rhizosphere. In the literature, PGPR have been reported to alleviate heavy metal stress through several mechanisms such as mobilization, immobilization, and heavy metal transformation [9]. Furthermore, various PGPR present in the soil around plant roots can facilitate plant growth either by producing various plant growth regulators or providing and facilitating nutrients uptake [10]. PGPR could improve plants tolerance to different abiotic stresses, including heavy metal stress [11]. Several genera of heavy metal-tolerant bacteria, including Pseudomonas, Bacillus, Methylobacterium, and Streptomyces have the ability to increase the growth and production of crops by minimizing the negative effects of heavy metal stress [12]. In the last decade, various genera of PGPR including Pseudomonas, Bacillus, Rhizobium, Pantoea, Burkholderia, Paenibacillus, Enterobacter, Azospirillum, Achromobacter, Methylobacterium, Variovorax, and Microbacterium have been found to play a role in tolerance to abiotic stress [13,14].
Among the various PGPR genera, the genus Pseudomonas has attracted attention due to several unique characteristics. The genus Pseudomonas has a wide distribution, and it is very easy to culture under laboratory conditions [15]. Pseudomonas can be found in various environments, such as plants [16], straw [16], soil [17], animals [18], and saline water [19,20]. Currently, 322 valid species names have been reported in the literature (http://www.bacterio.net). The beneficial role of Pseudomonas has been described in various studies [21,22]. To further understand the traits of Pseudomonas at the genetic level, whole genome sequence analysis is widely used, and various species from the genus have been studied genetically [23,24].
P. psychrotolerans is a diverse species predominant in nature [25]. It was initially isolated from animals in 2004 [26]. The first whole genome sequence was released in 2012 from a P. psychrotolerans strain isolated from copper coins [27]. In addition, other studies have reported the whole genome sequences of P. psychrotolerans strains isolated from a clinical sample [28]. In the case of plants, Adorada, et al. [29] reported the whole genome sequences of P. psychrotolerans isolated from diseased rice. Furthermore, Xie et al. [30] characterized several Pseudomonas spp., including P. psychrotolerans PRS08-11306, from rice seeds.
The plant growth-promoting activities of various P. psychrotolerans strains have been reported previously; however, their plant growth-promoting characteristics have not been fully explored [22,31]. Similarly, P. psychrotolerans was reported for silicate solubilization and improve soybean plant growth [32]. Therefore, the identification of the plant growth-promoting characteristics of the current strain is crucial. In the current study, we investigated the CS51 whole genome sequence to determine plant growth-promoting characteristics of P. psychrotolerans. The findings of this study could help elucidate the complex biological mechanisms of the current strain responsible for plant growth and tolerance to heavy metal stress. The whole genome sequencing of CS51 would contribute to the investigation of plant growth-promoting activity and tolerance characteristics against different environmental stresses. In addition, unique traits shared among already sequenced Pseudomonas species may be identified. Hence, it will offer insight into the evolutionary changes that have occurred within this genus.
2. Material and Methods
2.1. Study Site and Microbe Isolation
Soil samples were collected from an agriculture field in Gyeongbuk province, and microbes were isolated. First, the soil was diluted, and it was plated on nutrient agar plates. Based on the morphology, different colonies were selected, which were then further purified by streaking. For each colony, the morphology and texture were determined and recorded. Finally, the colonies were randomly selected for further analyses; the selected colonies were maintained on a nutrient agar slant at 4 °C.
2.2. Solubilization of Insoluble Phosphate
The rhizospheric bacteria were assessed for potential phosphate solubilization on Pikovskaya’s (PVK) agar medium (supplemented with 1.5% Bacto Agar), as described previously [33]. The rhizospheric bacterial colonies were stabbed in triplicate on PVK agar plates using sterile toothpicks. The plates were incubated at 28 °C. Bacterial growth was evaluated every 24 h for 7 days after incubation, both the halo and colony diameters were measured. To measure the diameter of the halo zone, the colony diameter was subtracted from the halo. The data are the mean of three experiments.
2.3. Screening for Indole-3-Acetic Acid (IAA) and Gibberellins (GAs) in Cell-Free Cultures
The broth culture of the bacteria was centrifuged at 2500× g for 10 min at 4 °C to separate the cells from the supernatant. Subsequently, 0.45 μm cellulose acetate filters (DISMIC®; Frisenette ApS, Knebel, Denmark) were used to remove debris from the resulting supernatant, and the clear supernatant was used for IAA analysis. Initially, Salkowski’s reagent was used for the confirmation of IAA production [34]. The bacterial supernatant (2 mL) was mixed with 1 mL of Salkowski’s reagent (50 mL 35% HClO4, 1 mL 0.5 M FeCl3) and was placed for 30 min in the dark. After incubation, the absorbance was taken at 530 nm (pink color) (T60 UV-VIS Spectrophotometer; PG Instruments, Leicester, UK) and quantified using the calibration curve of an IAA standard with linear regression analysis.
The IAA in bacterial cell-free cultures (5 mL) was quantified by GC-MS/SIM (6890 N Network GC System and 5973 Network Mass Selective Detector; Agilent Technologies, Palo Alto, CA, USA) as described previously [35]. Similarly, the quantification of GAs in the CS51 culture was carried out according to a previous protocol [36]. Bacterial culture filtrates supplemented with [2H2] GA standards were processed for the detection, identification, and quantification of GAs by gas chromatography and mass spectroscopy.
2.4. Screening of Endophytes on GA-Deficient Waito-C Dwarf Rice
To examine the ability of the isolated bacteria for GA production, Waito-C, in which the biosynthesis pathway for GA is suppressed, was used. Initially, the seeds were treated with 2.5% sodium hypochlorite (NaClO) for 30 min to sterilize the surface, followed by washing with autoclave-distilled water three times. The seeds were incubated with 20 mg/L of uniconazole for 24 h to detect GA production. GA biosynthesis was arrested by treating the seeds with uniconazole to determine the effects of CS51 on mutant rice. As reported previously, pre-germinated Waito-C seeds were transferred to autoclaved pots [37]. The root and shoot lengths were calculated.
2.5. Screening of Bacteria for Heavy Metal Stress and Germination of Seeds
Initially, P. psychrotolerans CS51 was screened for resistance to three heavy metals (copper, zinc, and cadmium). P. psychrotolerans CS51 had a higher resistance to Zn and Cd compared with Cu. The bacteria were grown on plates with different concentrations (1 mM and 10 mM) of Cu, Zn, and Cd and were incubated at 28 °C. Bacterial growth was evaluated every 24 h for 7 days. The bacterial strains were streaked on plates in triplicate.
2.6. Plant–Microbe Interaction under Heavy Metal Stress
In the current study, cucumber seeds were used. The surface of approximately 100 seeds was sterilized by soaking with 75% ethanol for 2 min. Subsequently, the seeds were treated with 1% NaClO for 1 min. The seeds were then washed repeatedly with autoclave-distilled water to completely remove any residual NaClO. The seeds were placed on a pre-soaked filter paper containing 1.5 mL autoclave-distilled water in a dark environment. After 3 days, germinated seeds with equal lengths were transplanted into sterile plastic pots (22 × 15 × 7 cm). Three pots were used per replication. The pots were placed in a growth chamber. The conditions of the growth chamber were as follows: 25 ± 2 °C with a 12 h light/dark cycle and a relative humidity of 60%. At the 2 leaves stage, morphologically similar samples were selected for further analysis.
P. psychrotolerans CS51 was cultured in LB broth at 30 °C for 3 days in a shaking incubator at 200 rpm. The resulting bacterial cultures were centrifuged at 10,000× g for 10 min, and the supernatant was diluted 10 times; 50 mL of the diluted supernatant was applied to seedlings at the 2 leaves stage. The treatment conditions were as follows: (1) Control cucumber seedling without microbial cells; (2) cucumber seedling with CS51; (3) 5 mM Cu, CS51 + 5 mM Cu, 10 mM Cu, and CS51 + 10 mM Cu; (4) 5 mM Zn, CS51 + 5 mM Zn, 10 mM Zn, and CS51 + 10 mM Zn. During the experiment, the plants were exposed to the following environmental conditions: 14/10 h day/night cycle at 28/25 °C with 60–70% relative humidity. The control plant was grown only in water, and a second control was treated with bacterial cell-free culture. For statistical analysis, three plants were randomly selected. The treated and negative control samples were compared. At this stage, the root and shoot lengths of the plants were measured. Chlorophyll contents were recorded for fully expanded leaves using a chlorophyll meter. After harvesting, the fresh weight was measured immediately. To measure the dry weight, the plants were first placed in an oven at 70 °C for 72 h.
2.7. DNA Extraction, Genome Sequencing, and Genome Assembly
Qiagen™ QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) was used for the extraction of genomic DNA from the overnight cell suspension cultures of CS51 bacteria for whole genome sequencing. As described previously, the sequencing of the complete genome was performed using the single-molecule real-time (SMRT) sequencing technology of Pacific Biosciences (PacBio, Menlo Park, CA, USA) [38]. In brief, from high-molecular-weight genomic DNA (120.0 ng/μL), a PacBio large-insert library (15–20 kb) was generated, and V2 SMRT cells were used for sequencing by P4-C2 chemistry with a running movie for 4 h at the Duke Center for Genome and Computational Biology, Duke University (Durham, NC, USA). The PacBio-generated data file was in the HDF5 format (*.h5), and the corresponding input file of SMRT Analysis software was a bas.h5 file or an associated bax.h5 file. QUAST 2.3 was used to ensure the data quality of the assemblies [39]. A total of 96,384 reads were generated with a mean read length of 13,888 base pairs. The total reads were de novo assembled into the form of a circular chromosome with an average genomic coverage of 150.26 reads using the Hierarchical Genome Assembly Process (HGAP) workflow in SMRT Portal (version 2.1.1).
2.8. Genome Annotation
The NCBI Prokaryotic Genome Annotation Pipeline was used to perform complete genome annotation [40]. The coding genes were predicted using an ab initio gene prediction algorithm with homology-based methods. Furthermore, from the annotation process, functional genomic units such as structural RNAs (23S, 5S, and 16S), tRNAs, and small noncoding RNAs were determined. Rapid Annotation using Subsystem Technology (RAST) version 3.0 and the Integrated Microbial Genomes (IMG) platform were used to perform additional coding gene prediction and functional annotation [41,42,43,44]. The assembled and annotated sequences of CS51were submitted to GenBank with accession number CP021645.
2.9. Comparative Genome Analysis
To elucidate the genomic characteristics of P. psychrotolerans CS51, comparative assessments were performed with the recently reported genome sequences of P. aeruginosa, P. psychrotolerans PRS08, P. putida, and P. syringae, all of which were retrieved from the NCBI database. Gene prediction and functional annotation of CS51 were performed using the RAST subsystem [41,42,43]. A circular genomic map of each genome was generated by BioCircos using EDGAR version 2.0 for comparison [45]. Each circular genomic map was generated with BLAST+ using standard parameters (70% lower and 90% upper cutoff for identity, E-value of 10) with the CS51 genome as the “alignment reference genome”. Similarly, pan-genome and core genome analyses of CS51 against related species were carried out using EDGAR version 2.0 [45] and PGAP version 1.12 [46].
3. Results and Discussion
3.1. Isolation of PGPR from Soil
Initially, around 10 bacterial strains were isolated from soil samples (associated with the roots of the plants). All of the bacterial strains were analyzed based on morphological characteristics, such as the shape of the colony, growth on the medium, growth rate and pattern, base color, margin characteristics, and texture, which revealed the same morphotypes. However, all strains were initially screened for heavy metals resistance and the production of growth-promoting phytohormones such as IAA and GAs in their culture filtrate (Figure 1A,B). Among all the PGPR strains, P. psychrotolerans CS51 was selected for further analysis, as it produced GAs and IAA and exhibited high resistance to heavy metals such as Zn and Cu (Figure 1A–C). Previously, P. psychrotolerans CS51 was reported for silicate solubilizing and to improve soybean plant growth and development at paddy soil [32]. Similarly, one other strain psychrotolerans PRS08-11306 was also reported as a plant growth-promoting bacterium [22].
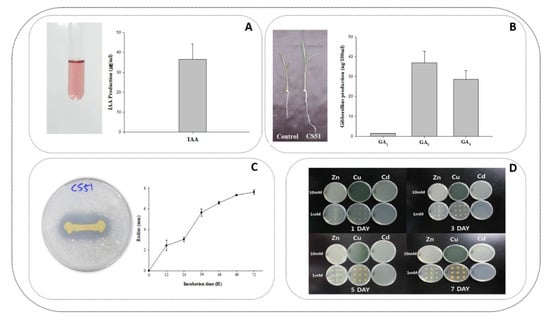
Figure 1.
(A) Salkowski test for indole-3-acetic acid (IAA) determination and the amount of IAA produced by CS51 by GC-MS/SIM spectrometry analysis, (B) growth promoting effect of CS51 inoculation on Waito-C (gibberellins (GA) deficient) rice plants and the amount of different GAs produced by CS51 by GC-MS/SIM spectrometry analysis, (C) growth pattern of CS51 in 1 mM and 10 mM Zn-supplemented, Cu-supplemented, and Cd-supplemented media, (D) phosphate-solubilizing activity of CS51 on national botanical research institute’s phosphate (NBRIP) growth media plates, and incubated for 7 days at 30°C. The clarification halos show P solubilization and the maximum size of the clarification halos was reached after 72 values given are means of three replicates. Error bars indicate standard deviations.
3.2. GA Production and Phosphate Solubilization Potential of P. psychrotolerans CS51
The P. psychrotolerans CS51 culture filtrate was evaluated to determine its ability to secrete GAs in the growth medium. Different GAs (physiologically active and nonactive) were observed in the culture filtrate using a GC/MS selected ion monitor. The most predominantly detected GAs were GA3 (35 ng/100 mL), GA4 (28 ng/100 mL), and GA1 (3 ng/100 mL) in the different HPLC fractions. Among the different bioactive GAs, the concentration of GA3 and GA4 was significantly higher than that of other GAs (Figure 1B). In addition, the culture filtrate was analyzed for the presence of IAA (Figure 1A). The level of IAA was 35 ng/mL. A number of plant growth-promoting bacteria have been reported to produce GAs and IAA previously by various researchers [47,48,49,50]. The ability of CS51 to produce IAA and GAs (Figure 1AB) was similar to previously reported plant growth promoting bacterial species [51,52,53]. Previous research revealed that the production of IAA and GAs is considered the most important attribute of plant growth-promoting bacteria [37,47]. Furthermore, the bacteria were assessed for their ability to solubilize inorganic phosphate. The formation of a clear zone around the colony indicated the phosphate-solubilizing ability of the CS51 bacteria (Figure 1D). Previous studies have reported that different endophytic Pseudomonas species can produce GAs and insoluble phosphates [54,55]. Furthermore, CS51 has demonstrated phosphate-solubilizing ability, which is considered one of the most crucial abilities of plant growth-promoting bacteria [55,56]. Phosphate-solubilizing bacteria may enhance the uptake of phosphorus by converting inorganic phosphorus to a more available form [57]. Phosphate solubilization is mainly associated with the production of microbial metabolites, including organic acids, which can decrease the pH of the culture media [56,58]. Phosphate-solubilizing microbial populations are present in soils, and the microbes may be used as biofertilizers for crop production and would be beneficial for sustainable agriculture.
3.3. Promotion of Cucumber Plant Growth by P. Psychrotolerans CS51 under Heavy Metal Stress
The use of metal-resistant plant growth-promoting bacteria constitutes an important technology for enhancing biomass production as well as the tolerance of plants to heavy metals. In the present study, P. psychrotolerans CS51 was initially grown on culture plates with various heavy metals (Zn, Cu, and Cd). CS51 exhibited high resistance to Zn and Cu compared with Cd (Figure 1C). In order to examine its contribution to plant growth, isolates of CS51 were used for in vivo experiments. Based on the initial selection, we only used the heavy metals Zn and Cu to investigate the potential of CS51 PGPR in attenuating heavy metal effects.
Inoculation with CS51 increased the length of the shoot and root of cucumber plants. Under Cu and Zn (5 mM and 10 mM) stress, the shoot and root lengths significantly decreased in both inoculated and non-inoculated plants compared with control plants (Figure 2 and Figure 3A,B). The shoot and root lengths of the CS51-treated plants were 28.24 ± 1.90 cm and 27.86 ± 1.24 cm, respectively, compared with those of the control plants (25.78 ± 1.37 cm and 24.14 ± 3.14 cm, respectively) (Figure 2 and Figure 3A,B). Similarly, CS51 application increased the stem diameter under normal and heavy metal stress conditions (Figure 2 and Figure 3C). The Soil Plant Analysis Development (SPAD) value indicated that the chlorophyll content was significantly higher in cucumber plants inoculated with CS51 than in non-inoculated control plants (46.16 ± 0.74 cm and 42.54 ± 1.28 cm, respectively) (Figure 2 and Figure 3D). Similarly, there was a significant increase in shoot and root fresh weight (18.25 ± 1.55 and 3.81 ± 0.24, respectively) of the inoculated (with CS51) plants compared with the control (13.82 ± 2.42 and 3.16 ± 0.13 respectively) (Figure 2 and Figure 3A,B). Hence, the results reveal that the application of CS51 promote plant growth under normal and heavy metals stress conditions. Previously, several strains of bacteria have been reported to improve plant growth by regulating endogenous hormones [35,53,59,60]; however, there is still a need to identify novel strains. Several studies have demonstrated that metal-tolerant rhizobacteria can stimulate plant growth in the presence or absence of toxic concentrations of heavy metals, such as Ni [61], Cd [62], Pb [63], and Cr (VI) [64,65]. Kang et al. [59] reported that the inoculation of Brassica rapa with Burkholderia cepacia could increase the tolerance to Zn+ toxicity. The results of the current study indicated that the inoculation of plants with CS51 increased heavy metal tolerance, improving plant growth parameters.
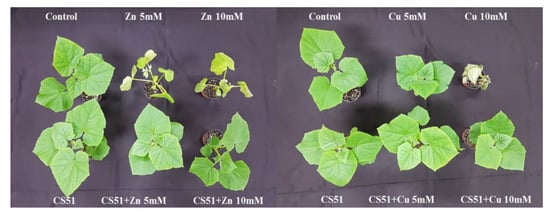
Figure 2.
Effect of CS51 on the growth of cucumber plant, under control conditions, copper (5 mM and 10 mM Cu) and zinc (5 mM and 10 mM Zn) stress. Control, untreated plants; CS51, P. psychrotolerans-treated; Cu 5 mM and Cu 10 mM, copper treated plants; Zn 5 mM and Zn 10 mM, zinc treated plants; Cu 5 mM and Cu 10 mM + CS51, copper + CS51-treated plants; Zn 5 mM and Zn 10 mM + CS51, zinc + CS51-treated plants.
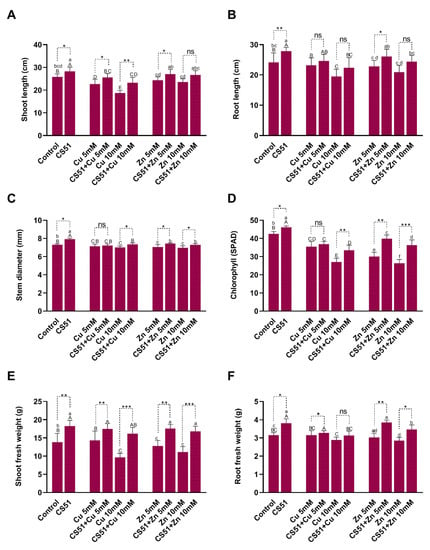
Figure 3.
Effect of CS51 on the growth of cucumber plant growth, under control conditions, Cu (5 mM and 10 mM Cu), and Zn (5 mM and 10 mM Zn) stress. Growth parameters include (A) shoot length, (B) root length, (C) stem diameter, (D) chlorophyll contents (SPAD), (E) shoot fresh weight, and (F) root fresh weight. Data are means of five replicates along with standard error bars. Mean bars labeled with different letters (Capital letters = Control vs. Cu-stress; Small letters = Control vs. Zn-stress) are significantly different as evaluated by Duncan multiple range test (DMRT) analysis. ns, *, **, *** indicates non-significance or significance at 5%, 1%, and 0.1% probability levels, respectively. Data were analyzed with an unpaired student t test at 95% confidence intervals in cucumber plants treated with and without CS51 under both Cu and Zn stress.
3.4. General Genomic Features of P. psychrotolerans CS51
The genome of the P. psychrotolerans CS51 strain consisted of a circular chromosome of 5,364,174 base pairs with an average G+C content of 64.71% (Figure 4). Previously, the similar genome sizes of different Pseudomonas species isolated from the endosphere and rhizosphere have been reported [66]. There were around 4774 predicted protein-coding sequences (CDSs) in 4859 genes, 15 rRNA genes, and 67 tRNA genes (Table 1). Around 3950 protein-coding genes with function prediction and 733 genes without function prediction were identified. Proteins, rRNAs, and tRNAs were encoded by 82.81%, 0.31%, and 1.40% of the complete genome, respectively. Among the predicted CDSs, around 3,683 CDSs (77.21%) could be assigned to the Clusters of Orthologous Groups (COG) database. The major COG categories were amino acid transport and metabolism (10.93%), general function and prediction only (7.98%), transcription (7.98%), and signal transduction and mechanisms (6.71%) (Figure S1). Similarly, the number of CDSs assigned to the Kyoto Encyclopedia of Genes and Genomes (KEGG) database was 1577, and the major pathways were global and overview maps (26.15%), amino acid metabolism (8.66%), carbohydrate metabolism (8.5%), and membrane transport (7.94%) (Figure S2).
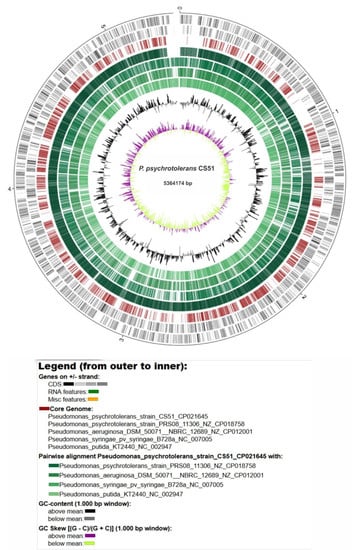
Figure 4.
Circular representation of the P. psychrotolerans CS51 genome and comparison with related species. The green color intensity shows the pairwise alignment of P. psychrotolerans CS51 with related species. The inner two circles show the G+C content and skew.

Table 1.
Genomic features, gene prediction, and annotation summary of the CS51 genome.
3.5. Plant Growth-Promoting Potential of P. psychrotolerans CS51
Various species of bacteria have been reported to promote plant growth by providing phytohormones or enzymes [55,67,68]. The current study revealed that CS51 promoted plant growth through the production of GAs and IAA (Figure 1A,B). Furthermore, we identified genes related to auxin biosynthesis, which included tryptophan synthase beta chain (TSb) (CCZ28_16130), tryptophan synthase alpha chain (TSa) (CCZ28_16125), phosphoribosylanthranilate isomerase (PRAI), and anthranilate phosphoribosyltransferase (APRT) (Table 2). Previously, a study reported [67] the presence of tryptophan-related genes may be associated with IAA production in the bacteria. Similarly, genes related to phosphorus (P), sulfur (S), and nitrogen (N) in CS51 were analyzed (Table 2). Cyanase is an inducible enzyme of E. coli found in CS51 (CynS and CynX), which catalyzes the bicarbonate-dependent decomposition of cyanate. For the N cycle, genes encoding nitrite reductase (nirB), nitrate reductase (napA), and nitric oxide reductase (norB) were detected; these enzymes are involved in the denitrification process, catalyzing the conversion of nitrate to nitrite to nitric oxide, followed by nitric oxide to nitrous oxide [69,70].

Table 2.
Genes related to plant growth-promoting activities in the CS51 genome.
Bacteria such as E. coli and B. subtilis use the phosphate-specific transport (pst) system for the transport of free inorganic phosphate. A previous study [71] has described the structure of the pst operon in E. coli and B. subtilis. The study indicated that the pst operon is composed of pstS, pstC, pstA, and pstB as well as a two-component signal transduction system for phosphate uptake consisting of the phoP/phoR genes. Based on the analysis of the CS51 complete genome, we found that the pst operon included the pstA, pstB, pstC, and pstS genes as well as the phoB, phoP, and phoR genes. CS51 bacteria could regulate the concentration of phosphorus sources via polyphosphate kinases (ppk1 and ppk2) in the extracellular or intracellular environment by polyphosphate degradation and inorganic phosphate transport (pst system and pitA), which could ensure sufficient inorganic phosphate uptake despite the influence of the extracellular environment. These results are in agreement with the results of another study showing a positive activity for alkaline phosphatase [72,73].
In the genome of CS51, genes related to sulfate transporters (CysA, CysP, CysQ, CysW, and CysT) were observed (Table 2). Previously, in the superfamily of transport proteins (PiT), CysP is the only characterized sulfate transport protein, operated by sulfate: H+ symport. Subsequently, the CysP gene function was confirmed by expressing B. subtilis CysP in E. coli with a mutated sulfate transport system [74]. The operon encoded by CysP in B. subtilis is composed of genes responsible for sulfur metabolism, such as the sulfate adenylyltransferase gene (sat) [75]. CysP in B. subtilis has been reported to have 10–12 TMHs and homologous domains due to internal gene duplication [74].
3.6. Phytoremediation Strategies and Resistance to Heavy Metals
Several mechanisms have been reported in the literature regarding the heavy metal stress tolerance of different bacterial species [76]. Analysis of the CS51 genome revealed the presence of several genes involved in the homeostasis of heavy metals. Specifically, the Cop operon, which is a membrane cation transporter, was identified. This operon was composed of structural genes, such as the copper resistance genes CopC, CopD, CopA, and CopB (Table 3). Similarly, the structure of this copper-inducible operon has been found in different bacterial species such as P. syringae, Xanthomonas campestris, and E. coli [77,78,79,80,81]. The cop operon encodes for proteins responsible for copper sequestration and compartmentalization in the periplasm and outer membrane [82,83]. Furthermore, other copper uptake functions have been associated with this operon [82]. CopA encodes for periplasmic proteins, which bind with multiple copper atoms, whereas CopB encodes for an outer membrane protein. In a previous study, among different isolated bacteria, 62% of bacterial strains were found to exhibit substantial resistance against copper [84]. Further analysis revealed the presence of cop or cop-like gene systems in 49% of isolates. A subsequent study [85] reported that the main mechanism underlying copper resistance in the plant pathogen P. syringae may be attributed to four types of proteins (CopB, CopC, CopA, and CopD), which are responsible for copper accumulation in the periplasm and outer membrane.

Table 3.
Genes potentially involved in heavy metal resistance in the CS51 genome.
Furthermore, the magnesium and cobalt efflux resistance genes CorA and CorC were found in the CS51 genome (Table 3). These genes have been reported to be involved in Mg2+ transport. CorA and MgtE, which are considered the primary Mg2+ transporters in bacteria, have a wide phylogenetic distribution, and the corresponding genes have been reported to be transcribed from constitutive promoters [86,87,88]. The current study revealed the presence of the cbtA and cbtC genes, which are putatively involved in Zn homeostasis. Previously, the cbtA gene was found in Agrobacterium tumefaciens, which is thought to be involved in Ni transport. However, the existence of this gene was not predicted in the genome of S. meliloti [89].
In addition, analysis of the CS51 genome revealed the presence of genes (CzcD, CobW, CcmF, and CutE) involved in cobalt-zinc-cadmium resistance and Cu homeostasis (Table 3). Most of the bacterial genes that confer copper resistance are organized in operons [90,91,92]. Various bacterial genes including copA, copB, copC, and copD are thought to be involved in Cu resistance [93]. The copA gene was identified in the genome of CS51, which encodes multi-copper oxidase, an important copper resistance protein in Gram-negative bacteria [94,95,96].
3.7. Comparison with Related Species
The size of the genome of CS51 was within the expected range (based on related genomes); it was larger than the P. psychrotolerans PRSO8 strain and smaller than other related species (Table S1). The GC content was similar to that of P. psychrotolerans PRSO8, lower than that of P. aeruginosa, and higher than that of P. putida and P. syringae. In addition, variation was observed in the total number of genes detected in these genomes; a higher number of genes (5,980) were identified in P. aeruginosa (Table S1). Furthermore, visual examination of the circular mapping of genomes revealed that the P. psychrotolerans PRSO8 strain was more syntenic and showed a higher similarity to CS51; however, it also showed variations in some regions. The other three genomes showed variations in various regions (Figure 4). Whole genome sequencing has delivered new taxonomic metrics, e.g., average nucleotide identity (ANI) and average amino acid identity (AAI), calculated from pairwise comparisons of all sequences shared between any two strains (Figure 5A,B). These are measures of genetic relatedness based on sequences conserved among compared genomes and have gained acceptance as a method for defining bacterial species [97,98]. The results are consistent with circular mapping, and CS51 was more similar to P. psychrotolerans PRSO8 with 98.52% ANI and 9.35 % AAI (Figure 5A-B). Furthermore, the synteny plot showed the conservation of the gene order of CS51 with related genomes, and the continuous line in the dot plots of CS51 and P. psychrotolerans PRSO8 revealed 4,038 orthologs. Many parallel and discontinuous lines were also observed in the dot plots of CS51 (Figure 5C). Genomic rearrangements are important for bacterial evaluation, such as genome reduction processes [99].

Figure 5.
(A) Average nucleotide identity (ANI), (B) average amino acid identity (AAI) values of P. psychrotolerans CS51 and four Pseudomonas species, and (C) synteny matrix of P. psychrotolerans CS51 with related species.
A complement of genes in a clade is often defined by the pan-genome. In this study, with a focus on the pan-genome and core genome of the Pseudomonas genus, CS51 was sequenced with four other Pseudomonas species. The genomes analyzed in this study were plotted against the pan-genome and core genome sizes. When an extra genome was added, the unique gene number, the number of analogous gene clusters that comprise the core genome, was slightly decreased (Figure 6A). However, in the pan-genome, the number of unique genes was increased. Curve extrapolation showed that the core genome was composed of 2122 genes (95% confidence interval = 2034.24 to 2080.215) when the genomes of P. syringae, P. putida, P. psychrotolerans PRS08, and P. aeruginosa were added. Owing to duplicated and paralogous genes, the number of shared genes was different in each genome. Moreover, analysis of the pan-genome showed that for each sequenced genome of the Pseudomonas species, 1500 new genes (average) were added to the pan-genome (Figure 6B).
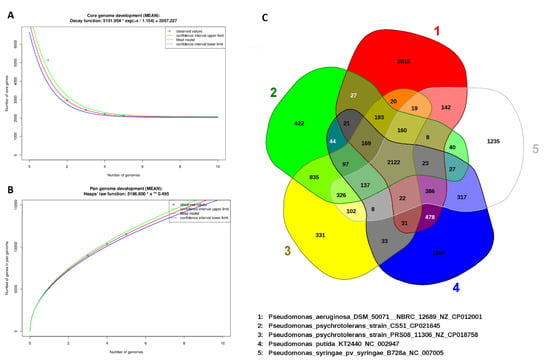
Figure 6.
(A) The number of gene clusters in the core genome, (B) the number of gene clusters in the pan-genome was plotted against the number of Pseudomonas species genomes, and (C) Venn diagram illustrating the orthologous gene complements of P. psychrotolerans CS51, P. aeruginosa, P. psychrotolerans PRS08, P. putida, and P. syringae. The number in the center represents orthologous sequences common to all five genomes, whereas the unique genes identified in the genomes are in the outer circles.
Similarly, the curve of the pan-genome showed that the characteristic species of the Pseudomonas genus had an open pan-genome. The genome number examined was insufficient for the description of gene sets; thus, to describe all genes in this genus, the sequencing of additional Sphingomonas species would be required. In the same species or genus, conserved genes are present in the genome of the bacteria. The core genome contains genes that are conserved, particularly those that are similar and found across the genome in the bacteria. The core genome is often present at both the genus and species level [100], and it is usually used for the identification of variable genes in the genome [101]. Generally, conserved genes are slower to evolve and may be used to determine bacterial associations [102]. The 2122 genes in the Venn diagram are common among all the five species of Pseudomonas. The CS51 species shares 27 genes with P. aeruginosa, 835 genes with P. psychrotolerans PRS08, 44 genes with P. putida, and 40 genes with P. syringae. On the other hand, there are 422 unique genes in P. aeruginosa, 2015 unique genes in P. psychrotolerans PRS08, 331 unique genes in P. putida, and 1590 unique genes in P. syringae (Figure 6C).
4. Conclusions
Genome sequencing of P. psychrotolerans CS51 has opened up a number of opportunities to study this potential plant growth promoting bacterium in the future. The results of this sequence will benefit the development of a more complete understanding of the mechanisms used by this bacterium to promote plant growth and alleviate heavy metal stress. From the results, it was concluded that CS51 inoculation induced endogenous IAA and GA, to promote plant growth under heavy metal stress. Similarly, genomics analysis revealed that CS51 genome consisted of a circular chromosome of 5,364,174 base pairs having 4774 CDSs genes, 15 rRNA genes, and 67 tRNA genes. Furthermore, the heavy metal resistance of CS51 was confirmed by the detection of cobalt-zinc-cadmium resistant genes in its genome sequence. This work aims to initiate a more comprehensive study and will provide a fundamental basis for future studies towards fully understanding the functioning of this bacterium. Similarly, the availability of the whole genome contents of CS51 will help to provide more insight in unraveling the complex biological mechanisms which indicated that CS51 may be used as an eco-friendly bioresource to promote plant growth in heavy metal-contaminated areas.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/8/3/382/s1.
Author Contributions
Experiments performed: S.-M.K., L., B.-G.M., A.K., M.A.K.; Bioinformatics analysis: S.A., A.L.K.; Manuscript drafting and editing: A.L.K., H.G., I.-J.L. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1D1A1B04035601).
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Shahid, M.; Khalid, S.; Abbas, G.; Shahid, N.; Nadeem, M.; Sabir, M.; Aslam, M.; Dumat, C. Heavy metal stress and crop productivity. In Crop Production and Global Environmental Issues; Springer: Berlin, Germany, 2015; pp. 1–25. [Google Scholar]
- Förstner, U.; Wittmann, G.T. Metal Pollution in the Aquatic Environment; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Salla, V.; Hardaway, C.J.; Sneddon, J. Preliminary investigation of Spartina alterniflora for phytoextraction of selected heavy metals in soils from Southwest Louisiana. Microchem. J. 2011, 97, 207–212. [Google Scholar] [CrossRef]
- Xiong, T.; Leveque, T.; Shahid, M.; Foucault, Y.; Mombo, S.; Dumat, C. Lead and cadmium phytoavailability and human bioaccessibility for vegetables exposed to soil or atmospheric pollution by process ultrafine particles. J. Environ. Qual. 2014, 43, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Moftah, A. Physiological responses of lead-polluted tomato and eggplant to the antioxidant ethylendiure. Minufiya J. Agric. Res. (Egypt) 2000, 25, 933–955. [Google Scholar]
- Chen, M.; Xu, P.; Zeng, G.; Yang, C.; Huang, D.; Zhang, J. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: Applications, microbes and future research needs. Biotechnol. Adv. 2015, 33, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, M.; Sandhya, S.; Prasad, M.; Freitas, H. Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol. Adv. 2012, 30, 1562–1574. [Google Scholar] [CrossRef] [PubMed]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Hassan, T.U.; Bano, A.; Naz, I. Alleviation of heavy metals toxicity by the application of plant growth promoting rhizobacteria and effects on wheat grown in saline sodic field. Int. J. Phytoremediation 2017, 19, 522–529. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef]
- Dary, M.; Chamber-Pérez, M.; Palomares, A.; Pajuelo, E. “In Situ” phytostabilisation of heavy metal polluted soils using Lupinus luteus inoculated with metal resistant plant-growth promoting rhizobacteria. J. Hazard. Mater. 2010, 177, 323–330. [Google Scholar] [CrossRef]
- Sessitsch, A.; Kuffner, M.; Kidd, P.; Vangronsveld, J.; Wenzel, W.W.; Fallmann, K.; Puschenreiter, M. The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol. Biochem. 2013, 60, 182–194. [Google Scholar] [CrossRef]
- Yang, J.; Kloepper, J.W.; Ryu, C.-M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009, 14, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Bharti, N.; Yadav, D.; Barnawal, D.; Maji, D.; Kalra, A. Exiguobacterium oxidotolerans, a halotolerant plant growth promoting rhizobacteria, improves yield and content of secondary metabolites in Bacopa monnieri (L.) Pennell under primary and secondary salt stress. World J. Microbiol. Biotechnol. 2013, 29, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.V.; Bogino, P.C.; Nocelli, N.; Cappellari Ldel, R.; Giordano, W.F.; Banchio, E. Analysis of Plant Growth-Promoting Effects of Fluorescent Pseudomonas Strains Isolated from Mentha piperita Rhizosphere and Effects of Their Volatile Organic Compounds on Essential Oil Composition. Front. Microbiol. 2016, 7, 1085. [Google Scholar] [CrossRef] [PubMed]
- Anwar, N.; Abaydulla, G.; Zayadan, B.; Abdurahman, M.; Hamood, B.; Erkin, R.; Ismayil, N.; Rozahon, M.; Mamtimin, H.; Rahman, E. Pseudomonas populi sp. nov., an endophytic bacterium isolated from Populus euphratica. Int. J. Syst. Evol. Microbiol. 2016, 66, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pan, Y.; Wang, K.; Zhang, X.; Zhang, C.; Zhang, S.; Fu, X.; Jiang, J. Pseudomonas zhaodongensis sp. nov., isolated from saline and alkaline soils. Int. J. Syst. Evol. Microbiol. 2015, 65, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Menéndez, E.; Ramírez-Bahena, M.H.; Fabryová, A.; Igual, J.M.; Benada, O.; Mateos, P.F.; Peix, A.; Kolařík, M.; García-Fraile, P. Pseudomonas coleopterorum sp. nov., a cellulase-producing bacterium isolated from the bark beetle Hylesinus fraxini. Int. J. Syst. Evol. Microbiol. 2015, 65, 2852–2858. [Google Scholar] [CrossRef]
- Pascual, J.; Lucena, T.; Ruvira, M.A.; Giordano, A.; Gambacorta, A.; Garay, E.; Arahal, D.R.; Pujalte, M.J.; Macián, M.C. Pseudomonas litoralis sp. nov., isolated from Mediterranean seawater. Int. J. Syst. Evol. Microbiol. 2012, 62, 438–444. [Google Scholar] [CrossRef]
- Zhong, Z.-P.; Liu, Y.; Hou, T.-T.; Liu, H.-C.; Zhou, Y.-G.; Wang, F.; Liu, Z.-P. Pseudomonas salina sp. nov., isolated from a salt lake. Int. J. Syst. Evol. Microbiol. 2015, 65, 2846–2851. [Google Scholar] [CrossRef]
- Kohler, J.; Caravaca, F.; Carrasco, L.; Roldán, A. Contribution of Pseudomonas mendocina and Glomus intraradices to aggregate stabilization and promotion of biological fertility in rhizosphere soil of lettuce plants under field conditions. Soil Use Manag. 2006, 22, 298–304. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Chen, P.; Lin, H.; Ye, G.; Wang, Z.; Ge, C.; Zhu, B.; Ren, D. Genomic and phenotypic analyses of Pseudomonas psychrotolerans PRS08-11306 reveal a turnerbactin biosynthesis gene cluster that contributes to nitrogen fixation. J. Biotechnol. 2017, 253, 10–13. [Google Scholar] [CrossRef]
- Roca, A.; Pizarro-Tobías, P.; Udaondo, Z.; Fernández, M.; Matilla, M.A.; Molina-Henares, M.A.; Molina, L.; Segura, A.; Duque, E.; Ramos, J.L. Analysis of the plant growth-promoting properties encoded by the genome of the rhizobacterium P seudomonas putida BIRD-1. Environ. Microbiol. 2013, 15, 780–794. [Google Scholar] [CrossRef] [PubMed]
- Yanzhen, M.; Yang, L.; Xiangting, X.; Wei, H. Complete genome sequence of a bacterium Pseudomonas fragi P121, a strain with degradation of toxic compounds. J. Biotechnol. 2016, 224, 68–69. [Google Scholar] [CrossRef] [PubMed]
- Spiers, A.J.; Buckling, A.; Rainey, P.B. The causes of Pseudomonas diversity. Microbiology 2000, 146, 2345–2350. [Google Scholar] [CrossRef] [PubMed]
- Hauser, E.; Kämpfer, P.; Busse, H.-J. Pseudomonas psychrotolerans sp. nov. Int. J. Syst. Evol. Microbiol. 2004, 54, 1633–1637. [Google Scholar] [CrossRef]
- Santo, C.E.; Lin, Y.; Hao, X.; Wei, G.; Rensing, C.; Grass, G. Draft genome sequence of Pseudomonas psychrotolerans L19, isolated from copper alloy coins. Am. Soc. Microbiol. 2012. [Google Scholar] [CrossRef]
- Simmon, K.E.; Croft, A.C.; Petti, C.A. Application of SmartGene IDNS software to partial 16S rRNA gene sequences for a diverse group of bacteria in a clinical laboratory. J. Clin. Microbiol. 2006, 44, 4400–4406. [Google Scholar] [CrossRef][Green Version]
- Adorada, D.L.; Stodart, B.J.; Tpoi, R.P.; Costa, S.S.; Ash, G.J. Bacteria associated with sheath browning and grain discoloration of rice in East Timor and implications for Australia’s biosecurity. Australas. Plant Dis. Notes 2013, 8, 43–47. [Google Scholar] [CrossRef]
- Xie, G.-L.; Soad, A.; Swings, J.; Mew, T. Diversity of Gram negative bacteria antagonistic against major pathogens of rice from rice seed in the tropic environment. J. Zhejiang Univ. Sci. A 2003, 4, 463–468. [Google Scholar] [CrossRef]
- Karthik, M.; Pushpakanth, P.; Krishnamoorthy, R.; Senthilkumar, M. Endophytic bacteria associated with banana cultivars and their inoculation effect on plant growth. J. Hortic. Sci. Biotechnol. 2017, 92, 568–576. [Google Scholar] [CrossRef]
- Lee, K.E.; Kang, S.M.; Adhikari, A.; Lee, I.J. Effect of Silicate Solubilizing Bacteria Pseudomonas psychrotolerans CS51 Treatment on Soybean Crops at Paddy Soil. Proc. Korean Soc. Crop Sci. Conf. 2019, 2019, 46. [Google Scholar]
- Gaur, A. Phosphate Solubilizing Micro-Organisms as Biofertilizer; Omega Scientific Publishers: New Delhi, India, 1990. [Google Scholar]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef] [PubMed]
- Asaf, S.; Khan, M.A.; Khan, A.L.; Waqas, M.; Shahzad, R.; Kim, A.-Y.; Kang, S.-M.; Lee, I.-J. Bacterial endophytes from arid land plants regulate endogenous hormone content and promote growth in crop plants: An example of Sphingomonas sp. and Serratia marcescens. J. Plant Interact. 2017, 12, 31–38. [Google Scholar] [CrossRef]
- Kang, S.-M.; Asaf, S.; Kim, S.-J.; Yun, B.-W.; Lee, I.-J. Complete genome sequence of plant growth-promoting bacterium Leifsonia xyli SE134, a possible gibberellin and auxin producer. J. Biotechnol. 2016, 239, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Bilal, S.; Shahzad, R.; Khan, A.L.; Kang, S.-M.; Imran, Q.M.; Al-Harrasi, A.; Yun, B.-W.; Lee, I.-J. Endophytic microbial consortia of phytohormones-producing fungus Paecilomyces formosus LHL10 and bacteria Sphingomonas sp. LK11 to Glycine max L. regulates physio-hormonal changes to attenuate aluminum and zinc stresses. Front. Plant Sci. 2018, 9, 1273. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.G.; Yin, W.F.; Lim, Y.L. Complete Genome Sequence of Pseudomonas aeruginosa Strain YL84, a Quorum-Sensing Strain Isolated from Compost. Genome Announc. 2014, 2. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, D206–D214. [Google Scholar] [CrossRef]
- Brettin, T.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Olsen, G.J.; Olson, R.; Overbeek, R.; Parrello, B.; Pusch, G.D.; et al. RASTtk: A modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci. Rep. 2015, 5, 8365. [Google Scholar] [CrossRef]
- Markowitz, V.M.; Chen, I.M.A.; Palaniappan, K.; Chu, K.; Szeto, E.; Grechkin, Y.; Ratner, A.; Jacob, B.; Huang, J.; Williams, P.; et al. IMG: The integrated microbial genomes database and comparative analysis system. Nucleic Acids Res. 2012, 40, D115–D122. [Google Scholar] [CrossRef] [PubMed]
- Blom, J.; Albaum, S.P.; Doppmeier, D.; Pühler, A.; Vorhölter, F.-J.; Zakrzewski, M.; Goesmann, A. EDGAR: A software framework for the comparative analysis of prokaryotic genomes. BMC Bioinform. 2009, 10, 154. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, J.; Yang, J.; Sun, S.; Xiao, J.; Yu, J. PGAP: Pan-genomes analysis pipeline. Bioinformatics 2012, 28, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Waqas, M.; Kang, S.-M.; Al-Harrasi, A.; Hussain, J.; Al-Rawahi, A.; Al-Khiziri, S.; Ullah, I.; Ali, L.; Jung, H.-Y. Bacterial endophyte Sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. J. Microbiol. 2014, 52, 689–695. [Google Scholar] [CrossRef]
- Kang, S.-M.; Waqas, M.; Hamayun, M.; Asaf, S.; Khan, A.L.; Kim, A.-Y.; Park, Y.-G.; Lee, I.-J. Gibberellins and indole-3-acetic acid producing rhizospheric bacterium Leifsonia xyli SE134 mitigates the adverse effects of copper-mediated stress on tomato. J. Plant Interact. 2017, 12, 373–380. [Google Scholar] [CrossRef]
- Tsukanova, K.; Meyer, J.; Bibikova, T. Effect of plant growth-promoting Rhizobacteria on plant hormone homeostasis. S. Afr. J. Bot. 2017, 113, 91–102. [Google Scholar] [CrossRef]
- Kang, S.-M.; Waqas, M.; Khan, A.L.; Lee, I.-J. Plant-growth-promoting rhizobacteria: Potential candidates for gibberellins production and crop growth promotion. In Use of Microbes for the Alleviation of Soil Stresses; Springer: New York, NY, USA, 2014; Volume 1, pp. 1–19. [Google Scholar]
- Kim, M.-J.; Radhakrishnan, R.; Kang, S.-M.; You, Y.-H.; Jeong, E.-J.; Kim, J.-G.; Lee, I.-J. Plant growth promoting effect of Bacillus amyloliquefaciens H-2-5 on crop plants and influence on physiological changes in soybean under soil salinity. Physiol. Mol. Biol. Plants 2017, 23, 571–580. [Google Scholar] [CrossRef]
- Kang, S.-M.; Khan, A.L.; You, Y.-H.; Kim, J.-G.; Kamran, M.; Lee, I.-J. Gibberellin production by newly isolated strain Leifsonia soli SE134 and its potential to promote plant growth. J. Microbiol. Biotechnol. 2014, 24, 106–112. [Google Scholar] [CrossRef]
- Kang, S.-M.; Khan, A.L.; Waqas, M.; Asaf, S.; Lee, K.-E.; Park, Y.-G.; Kim, A.-Y.; Khan, M.A.; You, Y.-H.; Lee, I.-J. Integrated phytohormone production by the plant growth-promoting rhizobacterium Bacillus tequilensis SSB07 induced thermotolerance in soybean. J. Plant Interact. 2019, 14, 416–423. [Google Scholar] [CrossRef]
- Duan, J.; Jiang, W.; Cheng, Z.; Heikkila, J.J.; Glick, B.R. The Complete Genome Sequence of the Plant Growth-Promoting Bacterium Pseudomonas sp. UW4. PLoS ONE 2013, 8, e58640. [Google Scholar] [CrossRef]
- Otieno, N.; Lally, R.D.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef] [PubMed]
- Şahin, F.; Çakmakçi, R.; Kantar, F. Sugar beet and barley yields in relation to inoculation with N 2-fixing and phosphate solubilizing bacteria. Plant Soil 2004, 265, 123–129. [Google Scholar] [CrossRef]
- Bar-Yosef, B.; Rogers, R.; Wolfram, J.; Richman, E. Pseudomonas cepacia–mediated rock phosphate solubilization in kaolinite and montmorillonite suspensions. Soil Sci. Soc. Am. J. 1999, 63, 1703–1708. [Google Scholar] [CrossRef]
- Puente, M.; Bashan, Y.; Li, C.; Lebsky, V. Microbial populations and activities in the rhizoplane of rock-weathering desert plants. I. Root colonization and weathering of igneous rocks. Plant Biol. 2004, 6, 629–642. [Google Scholar] [CrossRef]
- Kang, S.-M.; Shahzad, R.; Bilal, S.; Khan, A.L.; You, Y.-H.; Lee, W.-H.; Ryu, H.-L.; Lee, K.-E.; Lee, I.-J. Metabolism-mediated induction of zinc tolerance in Brassica rapa by Burkholderia cepacia CS2-1. J. Microbiol. 2017, 55, 955–965. [Google Scholar] [CrossRef]
- Shahzad, R.; Waqas, M.; Khan, A.L.; Al-Hosni, K.; Kang, S.-M.; Seo, C.-W.; Lee, I.-J. Indoleacetic acid production and plant growth promoting potential of bacterial endophytes isolated from rice (Oryza sativa L.) seeds. Acta Biol. Hung. 2017, 68, 175–186. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Freitas, H. Improvement of plant growth and nickel uptake by nickel resistant-plant-growth promoting bacteria. J. Hazard. Mater. 2009, 166, 1154–1161. [Google Scholar] [CrossRef]
- Belimov, A.; Hontzeas, N.; Safronova, V.; Demchinskaya, S.; Piluzza, G.; Bullitta, S.; Glick, B. Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.). Soil Biol. Biochem. 2005, 37, 241–250. [Google Scholar] [CrossRef]
- Hasnain, S.; Yasmin, S.; Yasmin, A. The effects of lead-resistant Pseudomonads on the growth of Triticum aestivum seedlings under lead stress. Environ. Pollut. 1993, 81, 179–184. [Google Scholar] [CrossRef]
- Rajkumar, M.; Nagendran, R.; Lee, K.J.; Lee, W.H.; Kim, S.Z. Influence of plant growth promoting bacteria and Cr6+ on the growth of Indian mustard. Chemosphere 2006, 62, 741–748. [Google Scholar] [CrossRef]
- Hemambika, B.; Balasubramanian, V.; Rajesh Kannan, V.; Arthur James, R. Screening of chromium-resistant bacteria for plant growth-promoting activities. Soil Sediment Contam. Int. J. 2013, 22, 717–736. [Google Scholar] [CrossRef]
- Brown, S.D.; Utturkar, S.M.; Klingeman, D.M.; Johnson, C.M.; Martin, S.L.; Land, M.L.; Lu, T.Y.; Schadt, C.W.; Doktycz, M.J.; Pelletier, D.A. Twenty-one genome sequences from Pseudomonas species and 19 genome sequences from diverse bacteria isolated from the rhizosphere and endosphere of Populus deltoides. J. Bacteriol. 2012, 194, 5991–5993. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gopal, M.; Thomas, G.V.; Manikandan, V.; Gajewski, J.; Thomas, G.; Seshagiri, S.; Schuster, S.C.; Rajesh, P.; Gupta, R. Whole genome sequencing and analysis of plant growth promoting bacteria isolated from the rhizosphere of plantation crops coconut, cocoa and arecanut. PLoS ONE 2014, 9, e104259. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Shi, H.; Du, Z.; Wang, T.; Liu, X.; Chen, S. Comparative genomic and functional analysis reveal conservation of plant growth promoting traits in Paenibacillus polymyxa and its closely related species. Sci. Rep. 2016, 6, 21329. [Google Scholar] [CrossRef]
- Smith, C.J.; Nedwell, D.B.; Dong, L.F.; Osborn, A.M. Diversity and abundance of nitrate reductase genes (narG and napA), nitrite reductase genes (nirS and nrfA), and their transcripts in estuarine sediments. Appl. Environ. Microbiol. 2007, 73, 3612–3622. [Google Scholar] [CrossRef]
- Wang, H.; Deng, N.; Wu, D.; Hu, S. Quantitative response relationships between net nitrogen transformation rates and nitrogen functional genes during artificial vegetation restoration following agricultural abandonment. Sci. Rep. 2017, 7, 7752. [Google Scholar] [CrossRef]
- Qi, Y.; Kobayashi, Y.; Hulett, F.M. The pst operon of Bacillus subtilis has a phosphate-regulated promoter and is involved in phosphate transport but not in regulation of the pho regulon. J. Bacteriol. 1997, 179, 2534–2539. [Google Scholar] [CrossRef]
- Sakurai, M.; Wasaki, J.; Tomizawa, Y.; Shinano, T.; Osaki, M. Analysis of bacterial communities on alkaline phosphatase genes in soil supplied with organic matter. Soil Sci. Plant Nutr. 2008, 54, 62–71. [Google Scholar] [CrossRef]
- Ram, B.; Fartyal, D.; Sheri, V.; Varakumar, P.; Borphukan, B.; James, D.; Yadav, R.; Bhatt, A.; Agrawal, P.K.; Achary, V.M.M. Characterization of phoA, a Bacterial Alkaline Phosphatase for Phi Use Efficiency in Rice Plant. Front. Plant Sci. 2019, 10, 37. [Google Scholar] [CrossRef]
- Mansilla, M.C.; de Mendoza, D. The Bacillus subtilis cysP gene encodes a novel sulphate permease related to the inorganic phosphate transporter (Pit) family. Microbiology 2000, 146, 815–821. [Google Scholar] [CrossRef]
- Aguilar-Barajas, E.; Díaz-Pérez, C.; Ramírez-Díaz, M.I.; Riveros-Rosas, H.; Cervantes, C. Bacterial transport of sulfate, molybdate, and related oxyanions. Biometals 2011, 24, 687–707. [Google Scholar] [CrossRef] [PubMed]
- Kunito, T.; Kusano, T.; Oyaizu, H.; Senoo, K.; Kanazawa, S.; Matsumoto, S. Cloning and sequence analysis of czc genes in Alcaligenes sp. strain CT14. Biosci. Biotechnol. Biochem. 1996, 60, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Cooksey, D.A. Molecular mechanisms of copper resistance and accumulation in bacteria. FEMS Microbiol. Rev. 1994, 14, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Voloudakis, A.E.; Bender, C.L.; Cooksey, D.A. Similarity between copper resistance genes from Xanthomonas campestris and Pseudomonas syringae. Appl. Environ. Microbiol. 1993, 59, 1627–1634. [Google Scholar] [CrossRef]
- Duncan, R.; Camakaris, J.; Lee, B.; Luke, R. Inducible plasmid-mediated copper resistance in Escherichia coli. Microbiology 1985, 131, 939–943. [Google Scholar] [CrossRef]
- Lee, Y.; Hendson, M.; Schroth, M. Cloning and characterization of copper-resistance genes from Xanthomonas campestris pv. juglandis. Phytopathology 1992, 82, 1125. [Google Scholar]
- Silver, S. Bacterial plasmid resistances to copper, cadmium, and zinc. Chem. Copp. Zinc Triads 1993, 39–53. [Google Scholar]
- Cha, J.-S.; Cooksey, D.A. Copper resistance in Pseudomonas syringae mediated by periplasmic and outer membrane proteins. Proc. Natl. Acad. Sci. USA 1991, 88, 8915–8919. [Google Scholar] [CrossRef]
- Cooksey, D.A.; Azad, H.R. Accumulation of copper and other metals by copper-resistant plant-pathogenic and saprophytic pseudomonads. Appl. Environ. Microbiol. 1992, 58, 274–278. [Google Scholar] [CrossRef]
- Lin, C.; Olson, B.H. Occurrence of cop-like copper resistance genes among bacteria isolated from a water distribution system. Can. J. Microbiol. 1995, 41, 642–646. [Google Scholar] [CrossRef]
- Cooksey, D.A. Copper uptake and resistance in bacteria. Mol. Microbiol. 1993, 7. [Google Scholar] [CrossRef] [PubMed]
- Hmiel, S.; Snavely, M.; Florer, J.; Maguire, M.; Miller, C. Magnesium transport in Salmonella typhimurium: Genetic characterization and cloning of three magnesium transport loci. J. Bacteriol. 1989, 171, 4742–4751. [Google Scholar] [CrossRef] [PubMed]
- Hmiel, S.; Snavely, M.; Miller, C.; Maguire, M. Magnesium transport in Salmonella typhimurium: Characterization of magnesium influx and cloning of a transport gene. J. Bacteriol. 1986, 168, 1444–1450. [Google Scholar] [CrossRef] [PubMed]
- Groisman, E.A.; Hollands, K.; Kriner, M.A.; Lee, E.-J.; Park, S.-Y.; Pontes, M.H. Bacterial Mg2+ homeostasis, transport, and virulence. Annu. Rev. Genet. 2013, 47, 625–646. [Google Scholar] [CrossRef] [PubMed]
- Eitinger, T.; Suhr, J.; Moore, L.; Smith, J.A.C. Secondary transporters for nickel and cobalt ions: Theme and variations. Biometals 2005, 18, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C.L.; Grass, G.; Rensing, C. Copper toxicity and the origin of bacterial resistance—New insights and applications. Metallomics 2011, 3, 1109–1118. [Google Scholar] [CrossRef]
- Magnani, D.; Solioz, M. How bacteria handle copper. In Molecular Microbiology of Heavy Metals; Springer: Berlin/Heidelberg, Germany, 2007; pp. 259–285. [Google Scholar]
- Wei, G.; Fan, L.; Zhu, W.; Fu, Y.; Yu, J.; Tang, M. Isolation and characterization of the heavy metal resistant bacteria CCNWRS33-2 isolated from root nodule of Lespedeza cuneata in gold mine tailings in China. J. Hazard. Mater. 2009, 162, 50–56. [Google Scholar] [CrossRef]
- Mellano, M.A.; Cooksey, D.A. Nucleotide sequence and organization of copper resistance genes from Pseudomonas syringae pv. tomato. J. Bacteriol. 1988, 170, 2879–2883. [Google Scholar] [CrossRef]
- Lejon, D.P.; Nowak, V.; Bouko, S.; Pascault, N.; Mougel, C.; Martins, J.M.; Ranjard, L. Fingerprinting and diversity of bacterial copA genes in response to soil types, soil organic status and copper contamination. FEMS Microbiol. Ecol. 2007, 61, 424–437. [Google Scholar] [CrossRef]
- Nies, D.H. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 1999, 51, 730–750. [Google Scholar] [CrossRef]
- Rensing, C.; Grass, G. Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol. Rev. 2003, 27, 197–213. [Google Scholar] [CrossRef]
- Konstantinidis, K.T.; Ramette, A.; Tiedje, J.M. The bacterial species definition in the genomic era. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Z.; Halachev, M.R.; Loman, N.J.; Constantinidou, C.; Pallen, M.J. Defining bacterial species in the genomic era: Insights from the genus Acinetobacter. BMC Microbiol. 2012, 12, 302. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Ke, R.; Hughes, D.; Nilsson, M.; Andersson, D.I. Genome-wide detection of spontaneous chromosomal rearrangements in bacteria. PLoS ONE 2012, 7, e42639. [Google Scholar] [CrossRef] [PubMed]
- Leekitcharoenphon, P.; Lukjancenko, O.; Friis, C.; Aarestrup, F.M.; Ussery, D.W. Genomic variation in Salmonella enterica core genes for epidemiological typing. BMC Genom. 2012, 13, 88. [Google Scholar] [CrossRef]
- Adékambi, T.; Butler, R.W.; Hanrahan, F.; Delcher, A.L.; Drancourt, M.; Shinnick, T.M. Core Gene Set As the Basis of Multilocus Sequence Analysis of the Subclass Actinobacteridae. PLoS ONE 2011, 6, e14792. [Google Scholar] [CrossRef]
- Urwin, R.; Maiden, M.C.J. Multi-locus sequence typing: A tool for global epidemiology. Trends Microbiol. 2003, 11, 479–487. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).