Fecal Microbiota Transplant in Two Ulcerative Colitis Pediatric Cases: Gut Microbiota and Clinical Course Correlations
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject Recruitment, Sample Collection and FMT Procedures
2.2. SrRNA Metagenomic Sequencing
2.3. Statistical Analyses
3. Results
3.1. Specific Lines of FMT Protocols’ Management
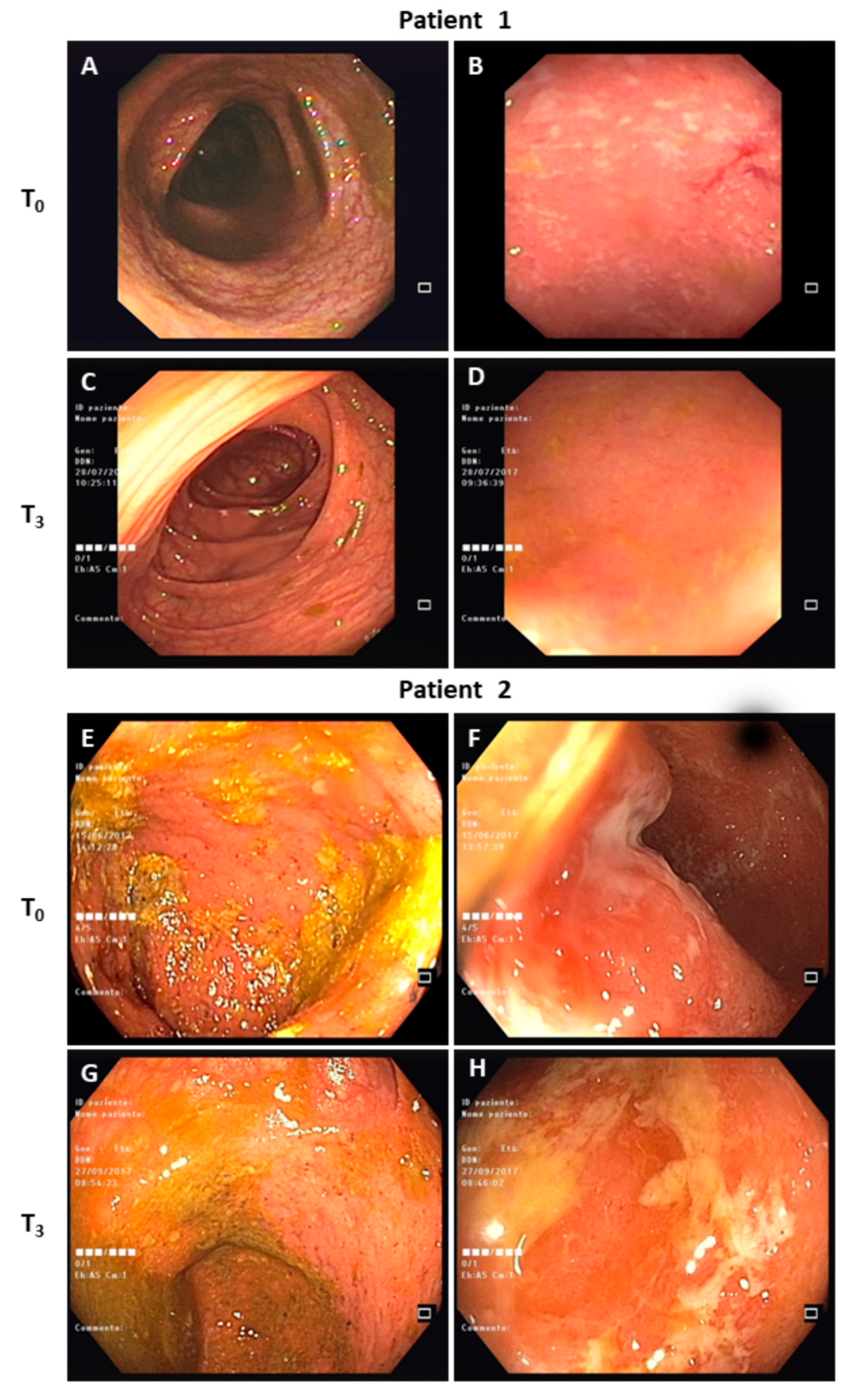
3.2. Clinical Course
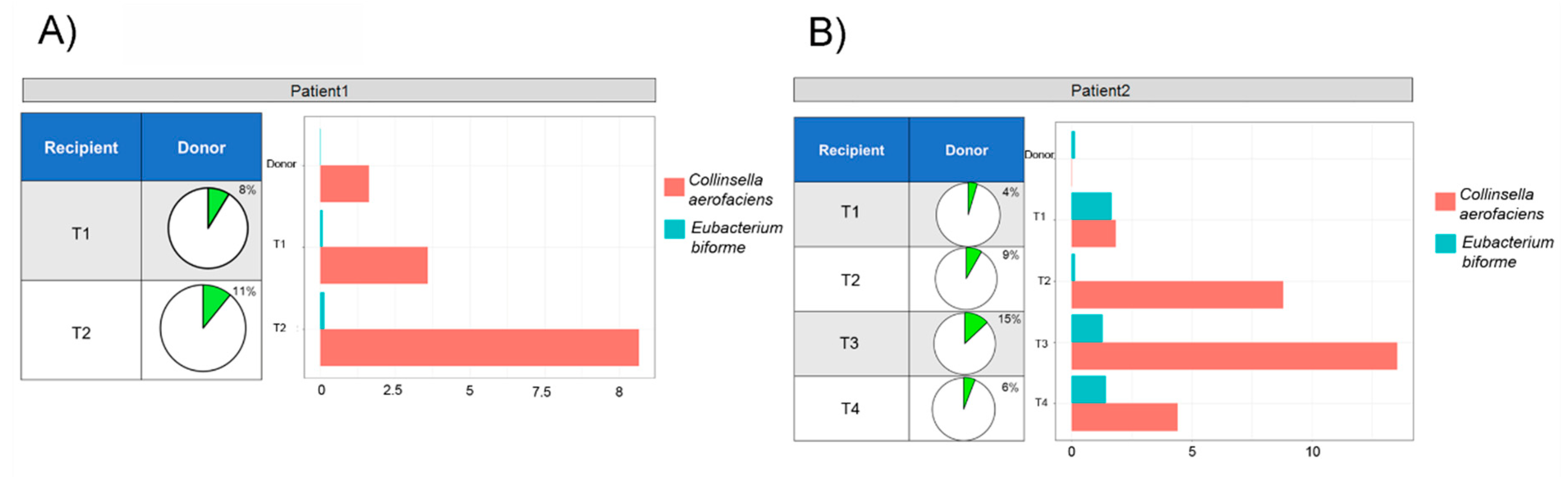
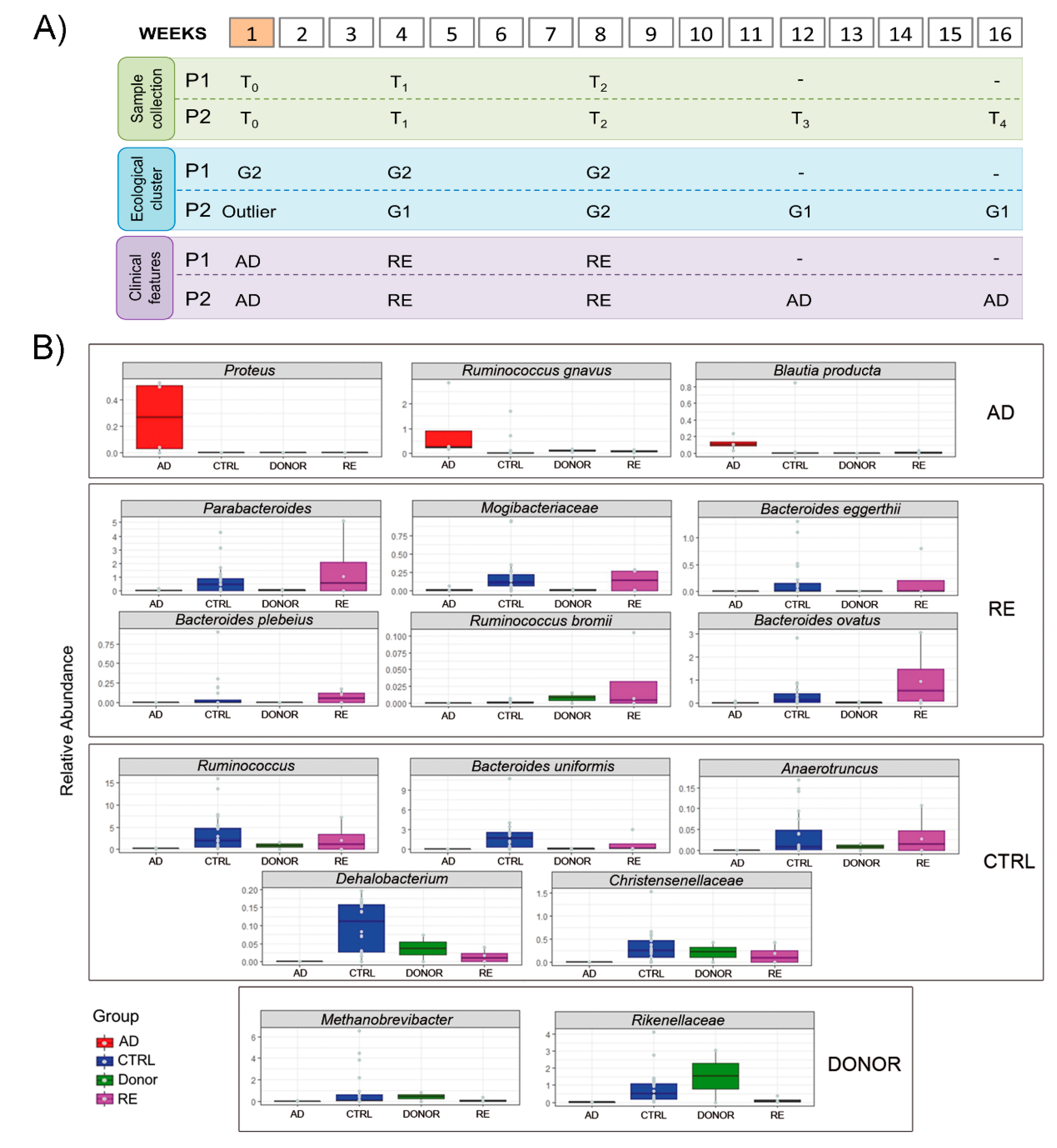
3.3. Gut Microbiota Ecology
3.4. Microbial Ecology and Clinical Course
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.-Z. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91. [Google Scholar] [CrossRef] [PubMed]
- Flamant, M.; Roblin, X. Inflammatory bowel disease: Towards a personalized medicine. Ther. Adv. Gastroenterol. 2018, 11, 1756283X1774502. [Google Scholar] [CrossRef] [PubMed]
- Sousa, P.; Allez, M. Complications of biologics in inflammatory bowel disease. Curr. Opin. Gastroenterol. 2015, 31, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Michail, S.; Ramsy, M.; Soliman, E. Advances in inflammatory bowel diseases in children. Minerva Pediatrica 2012, 64, 257–270. [Google Scholar] [PubMed]
- Putignani, L.; Del Chierico, F.; Vernocchi, P.; Cicala, M.; Cucchiara, S.; Dallapiccola, B. Gut Microbiota dysbiosis as risk and premorbid factors of IBD and IBS along the childhood–Adulthood transition. Inflamm. Bowel Dis. 2016, 22, 487–504. [Google Scholar] [CrossRef]
- Celiberto, L.S.; Graef, F.A.; Healey, G.R.; Bosman, E.S.; Jacobson, K.; Sly, L.M.; Vallance, B.A. Inflammatory bowel disease and immunonutrition: Novel therapeutic approaches through modulation of diet and the gut microbiome. Immunology 2018. [Google Scholar] [CrossRef]
- Ott, S.J.; Waetzig, G.H.; Rehman, A.; Moltzau-Anderson, J.; Bharti, R.; Grasis, J.A.; Cassidy, L.; Tholey, A.; Fickenscher, H.; Seegert, D.; et al. Efficacy of sterile fecal filtrate transfer for treating patients with clostridium difficile infection. Gastroenterology 2017, 152, 799–811.e7. [Google Scholar] [CrossRef]
- Bagdasarian, N.; Rao, K.; Malani, P.N. Diagnosis and treatment of Clostridium difficile in adults: A systematic review. JAMA 2015, 313, 398–408. [Google Scholar] [CrossRef]
- Juul, F.E.; Garborg, K.; Bretthauer, M.; Skudal, H.; Øines, M.N.; Wiig, H.; Rose, Ø.; Seip, B.; Lamont, J.T.; Midtvedt, T.; et al. Fecal Microbiota transplantation for primary Clostridium difficile infection. N. Engl. J. Med. 2018, 378, 2535–2536. [Google Scholar] [CrossRef]
- Borody, T.; Fischer, M.; Mitchell, S.; Campbell, J. Fecal Microbiota transplantation in gastrointestinal disease: 2015 update and the road ahead. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 1379–1391. [Google Scholar] [CrossRef]
- Kang, D.-W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Kunde, S.; Pham, A.; Bonczyk, S.; Crumb, T.; Duba, M.; Conrad, H.; Cloney, D.; Kugathasan, S. Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J. Pediatric Gastroenterol. Nutr. 2013, 56, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; de Groot, P.F.; Geerlings, S.E.; Hodiamont, C.J.; Belzer, C.; Berge, I.J.M.T.; de Vos, W.M.; Bemelman, F.J.; Nieuwdorp, M. Fecal microbiota transplantation against intestinal colonization by extended spectrum beta-lactamase producing Enterobacteriaceae: A proof of principle study. BMC Res. Notes 2018, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Costello, S.P.; Hughes, P.A.; Waters, O.; Bryant, R.V.; Vincent, A.D.; Blatchford, P.; Katsikeros, R.; Makanyanga, J.; Campaniello, M.A.; Mavrangelos, C.; et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: A randomized clinical trial. JAMA 2019, 321, 156. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Ianiro, G.; Allegretti, J.R.; Bibbò, S.; Gasbarrini, A.; Scaldaferri, F.; Cammarota, G. Fecal transplantation for ulcerative colitis: Current evidence and future applications. Expert Opin. Biol. Ther. 2020, 20, 343–351. [Google Scholar] [CrossRef]
- Sood, A.; Singh, A.; Mahajan, R.; Midha, V.; Mehta, V.; Gupta, Y.K.; Narang, V.; Kaur, K. Acceptability, tolerability, and safety of fecal microbiota transplantation in patients with active ulcerative colitis (AT&S Study). J. Gastroenterol. Hepatol. 2020, 35, 418–424. [Google Scholar] [CrossRef]
- Chen, H.; Huang, H.; Xu, H.; Luo, Q.; He, J.; Li, Y.; Zhou, Y.; Nie, Y.; Zhou, Y. Fecal microbiota transplantation ameliorates active ulcerative colitis. Exp. Ther. Med. 2020. [Google Scholar] [CrossRef]
- Hourigan, S.K.; Oliva-Hemker, M. Fecal microbiota transplantation in children: A brief review. Pediatric Res. 2016, 80, 2–6. [Google Scholar] [CrossRef]
- Imdad, A.; Nicholson, M.R.; Tanner-Smith, E.E.; Zackular, J.P.; Gomez-Duarte, O.G.; Beaulieu, D.B.; Acra, S. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst. Rev. 2018, 11, CD012774. [Google Scholar] [CrossRef]
- Pigneur, B.; Sokol, H. Fecal microbiota transplantation in inflammatory bowel disease: The quest for the holy grail. Mucosal Immunol. 2016, 9, 1360–1365. [Google Scholar] [CrossRef]
- Wang, A.Y.; Popov, J.; Pai, N. Fecal microbial transplant for the treatment of pediatric inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 10304–10315. [Google Scholar] [CrossRef] [PubMed]
- Suskind, D.L.; Brittnacher, M.J.; Wahbeh, G.; Shaffer, M.L.; Hayden, H.S.; Qin, X.; Singh, N.; Damman, C.J.; Hager, K.R.; Nielson, H.; et al. Fecal microbial transplant effect on clinical outcomes and fecal microbiome in active Crohn’s disease. Inflamm. Bowel Dis. 2015, 21, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Davidovics, Z.H.; Michail, S.; Nicholson, M.R.; Kociolek, L.K.; Pai, N.; Hansen, R.; Schwerd, T.; Maspons, A.; Shamir, R.; Szajewska, H.; et al. Fecal microbiota transplantation for recurrent Clostridium difficile infection and other conditions in children: A joint position paper from the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology and Nutrition. J. Pediatric Gastroenterol. Nutr. 2019, 68, 130–143. [Google Scholar] [CrossRef]
- Pai, N.; Popov, J. Protocol for a randomised, placebo-controlled pilot study for assessing feasibility and efficacy of faecal microbiota transplantation in a paediatric ulcerative colitis population: PediFETCh trial. BMJ Open 2017, 7, e016698. [Google Scholar] [CrossRef]
- Karolewska-Bochenek, K.; Grzesiowski, P.; Banaszkiewicz, A.; Gawronska, A.; Kotowska, M.; Dziekiewicz, M.; Albrecht, P.; Radzikowski, A.; Lazowska-Przeorek, I. A Two-Week fecal microbiota transplantation course in pediatric patients with inflammatory bowel disease. In Clinical Investigation; Pokorski, M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; Volume 1047, pp. 81–87. ISBN 978-3-319-74079-9. [Google Scholar]
- Kellermayer, R.; Nagy-Szakal, D.; Harris, R.A.; Luna, R.A.; Pitashny, M.; Schady, D.; Mir, S.A.V.; Lopez, M.E.; Gilger, M.A.; Belmont, J.; et al. Serial fecal microbiota transplantation alters mucosal gene expression in pediatric ulcerative colitis. Am. J. Gastroenterol. 2015, 110, 604–606. [Google Scholar] [CrossRef]
- Suskind, D.L.; Singh, N.; Nielson, H.; Wahbeh, G. Fecal microbial transplant via nasogastric tube for active pediatric ulcerative colitis. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 27–29. [Google Scholar] [CrossRef]
- Goyal, A.; Yeh, A.; Bush, B.R.; Firek, B.A.; Siebold, L.M.; Rogers, M.B.; Kufen, A.D.; Morowitz, M.J. Safety, clinical response, and microbiome findings following fecal microbiota transplant in children with inflammatory bowel disease. Inflamm. Bowel Dis. 2018, 24, 410–421. [Google Scholar] [CrossRef]
- Turner, D.; Hyams, J.; Markowitz, J.; Lerer, T.; Mack, D.R.; Evans, J.; Pfefferkorn, M.; Rosh, J.; Kay, M.; Crandall, W.; et al. Appraisal of the pediatric ulcerative colitis activity index (PUCAI). Inflamm. Bowel Dis. 2009, 15, 1218–1223. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; DeSantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, T.Z.; Hugenholtz, P.; Keller, K.; Brodie, E.L.; Larsen, N.; Piceno, Y.M.; Phan, R.; Andersen, G.L. NAST: A multiple sequence alignment server for comparative analysis of 16S rRNA genes. Nucleic Acids Res. 2006, 34, W394–W399. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Kellermayer, R. Fecal microbiota transplantation: Great potential with many challenges. Transl. Gastroenterol. Hepatol. 2019, 4, 40. [Google Scholar] [CrossRef]
- Bag, S.; Ghosh, T.S.; Das, B. Complete genome sequence of Collinsella aerofaciens isolated from the gut of a healthy indian subject. Genome Announc. 2017, 5. [Google Scholar] [CrossRef]
- Kassinen, A.; Krogius-Kurikka, L.; Mäkivuokko, H.; Rinttilä, T.; Paulin, L.; Corander, J.; Malinen, E.; Apajalahti, J.; Palva, A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology 2007, 133, 24–33. [Google Scholar] [CrossRef]
- Malinen, E.; Krogius-Kurikka, L.; Lyra, A.; Nikkilä, J.; Jääskeläinen, A.; Rinttilä, T.; Vilpponen-Salmela, T.; von Wright, A.J.; Palva, A. Association of symptoms with gastrointestinal microbiota in irritable bowel syndrome. World J. Gastroenterol. 2010, 16, 4532–4540. [Google Scholar] [CrossRef]
- Rajilić-Stojanović, M.; de Vos, W.M. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev. 2014, 38, 996–1047. [Google Scholar] [CrossRef]
- Sjöberg, F.; Barkman, C.; Nookaew, I.; Östman, S.; Adlerberth, I.; Saalman, R.; Wold, A.E. Low-complexity microbiota in the duodenum of children with newly diagnosed ulcerative colitis. PLoS ONE 2017, 12, e0186178. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.N.; Forbes, J.D. Gut microbiome in inflammatory bowel disease and other chronic immune-mediated inflammatory diseases. Inflamm. Intest. Dis. 2017, 2, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2013, 2, e01202. [Google Scholar] [CrossRef] [PubMed]
- Garrett, W.S.; Gallini, C.A.; Yatsunenko, T.; Michaud, M.; DuBois, A.; Delaney, M.L.; Punit, S.; Karlsson, M.; Bry, L.; Glickman, J.N.; et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe 2010, 8, 292–300. [Google Scholar] [CrossRef]
| Features | P1 | P2 |
|---|---|---|
| UC extension | Left-sided colitis | Pancolitis |
| Stool Donor | Single | Single |
| Patient Age (years) | 16 | 15 |
| Donor Age (years) | 55 | 48 |
| Family Relationship | Father | Father |
| Stool processing | Fresh | Fresh |
| Route of delivery | Colonoscopy | Colonoscopy |
| FMT regimen | One infusion | One infusion |
| Patient condition before FMT (screening) | Recurrent episodes of bloody diarrhea and abdominal pain, needing multiple cycles of steroids/antibiotics | Recurrent episodes of bloody diarrhea and abdominal pain, needing multiple cycles of steroids/antibiotics |
| Maintenance therapy before FMT | Mesalazine | Mesalazine + Azathioprine |
| Antibiotic treatment before FMT (4 weeks) | No | No |
| Patient condition at the time of FMT (T0) | Diarrhea without blood | Semi-formed stool with occasional blood |
| PUCAI pre-FMT | 15–20 | 3–35 |
| MAYO Score pre-FMT | Mayo score: 0 in the ascending-transverse-descending colon, 2 in the sigmoid-rectum | Mayo score: 1 in the ascending-transverse colon, 2 in the descending colon-sigmoid-rectum |
| Calprotectin pre-FMT | 81–187 g/gr | >500 g/gr |
| PUCAI post-FMT | 0 | 5–25 |
| MAYO post-FMT | Mayo score: 0 in the ascending-transverse-descending colon, 1 in the sigmoid-rectum | Mayo score: 1 in the ascending-transverse colon, 2 in the descending colon-sigmoid-rectum |
| Calprotectin post-FMT | 61–248 g/gr | 138–237 g/gr |
| Antibiotic treatment post FMT | No | Yes (at 12 weeks, Metronidazole) |
| Clinical remission post FMT | Yes | No (at 6 weeks) |
| Maintenance therapy post-FMT | Mesalazine | Mesalazine + Azathioprine |
| Patient condition at T1 (4 weeks) | Absence of symptoms | Absence of symptoms |
| Patient condition at T2 (8 weeks) | Absence of symptoms | Absence of symptoms |
| Patient condition at T3 (12 weeks) | Absence of symptoms | Relapse of bloody diarrhea (brief cycle of Metronidazole) |
| Patient condition at T4 (16 weeks) | Absence of symptoms | Semi-formed stool without blood |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quagliariello, A.; Del Chierico, F.; Reddel, S.; Russo, A.; Onetti Muda, A.; D’Argenio, P.; Angelino, G.; Romeo, E.F.; Dall’Oglio, L.; De Angelis, P.; et al. Fecal Microbiota Transplant in Two Ulcerative Colitis Pediatric Cases: Gut Microbiota and Clinical Course Correlations. Microorganisms 2020, 8, 1486. https://doi.org/10.3390/microorganisms8101486
Quagliariello A, Del Chierico F, Reddel S, Russo A, Onetti Muda A, D’Argenio P, Angelino G, Romeo EF, Dall’Oglio L, De Angelis P, et al. Fecal Microbiota Transplant in Two Ulcerative Colitis Pediatric Cases: Gut Microbiota and Clinical Course Correlations. Microorganisms. 2020; 8(10):1486. https://doi.org/10.3390/microorganisms8101486
Chicago/Turabian StyleQuagliariello, Andrea, Federica Del Chierico, Sofia Reddel, Alessandra Russo, Andrea Onetti Muda, Patrizia D’Argenio, Giulia Angelino, Erminia Francesca Romeo, Luigi Dall’Oglio, Paola De Angelis, and et al. 2020. "Fecal Microbiota Transplant in Two Ulcerative Colitis Pediatric Cases: Gut Microbiota and Clinical Course Correlations" Microorganisms 8, no. 10: 1486. https://doi.org/10.3390/microorganisms8101486
APA StyleQuagliariello, A., Del Chierico, F., Reddel, S., Russo, A., Onetti Muda, A., D’Argenio, P., Angelino, G., Romeo, E. F., Dall’Oglio, L., De Angelis, P., Putignani, L., & all the other FMT OPBG Committee Collaborators. (2020). Fecal Microbiota Transplant in Two Ulcerative Colitis Pediatric Cases: Gut Microbiota and Clinical Course Correlations. Microorganisms, 8(10), 1486. https://doi.org/10.3390/microorganisms8101486





