Abstract
In Mexico, Plasmodium vivax transmission has been confined to the northwestern and southern regions since 2000. Parasites from five malaria foci were analyzed using three genetic markers. The circumsporozoite gene was examined by PCR-RFLP and sequencing, and pvs25 mutations and variants of ribosomal 18S SSU rRNA S-type were also determined. Previous data from the southernmost Pacific in Chiapas were included in the analysis. Both the VK210 and VK247 types of pvcsp were detected, and VK210 had greater haplotype diversity (0.860) than VK247 parasites (0.198). Two pvs25 mutations (Q87K and I130T) yielded three haplotypes, and two ribosomal variants were detected. Gene and multilocus haplotype frequencies varied among malarious foci (p < 0.001). An AMOVA test, FST values, and Spearman’s correlation suggested a structured P. vivax population among the malaria foci. Each malaria focus across the northwestern and southern regions retained a portion of the past countrywide P. vivax population, which seems unique in Latin America. In the Lacandon region (LR), a linkage equilibrium between pvs25 haplotypes and the ribosomal variants within the VK247 or VK210 populations was observed. This region harbored the broadest reservoir of P. vivax haplotypes, and the high adaptation of parasites in the northwestern region represents a challenge for malaria elimination. These finding are relevant for monitoring and epidemiological surveillance.
Keywords:
Plasmodium vivax; circumsporozoite; pvcsp; pvs25; S-type 18S SSU rRNA; polymorphism; malaria foci; Mexico; Nicaragua 1. Introduction
Malaria is a sickness caused by Plasmodium parasites and transmitted to humans by infected Anopheles mosquitoes. Plasmodium vivax is the second most prevalent species, causing high morbidity in tropical and subtropical regions worldwide. In Latin America, P. vivax was responsible for about 73% of the 505,600 cases reported in 2023 [1]. In Mexico, malaria case reporting began in the 1950s, with P. vivax cases predominating across the Mexican territory [2]. In this country, malaria cases fluctuated annually, ranging from ~3000 to ~55,000 during the 1960s–1970s period. In the 1980s, the number of cases increased steadily, reaching over 130,000 in a single year. Incidence rates per 100,000 inhabitants varied significantly among states, from 2497 in Quintana Roo in 1984 to just 0.1 in Guanajuato in 1982 [2]. Later, in the 1990s, the number of cases constantly decreased, and in 2009, 2636 P. vivax cases were reported [2]. Afterwards, the number of P. vivax cases has remained low, confined to regions with persistent malaria transmission. In 2017 and 2024, the country reported 551 and 342 confirmed P. vivax cases, respectively [2]. Last year, cases were reported in Chiapas, with a few others in Oaxaca and Chihuahua. Based on the WHO criteria, Mexico progressed to the pre-elimination phase in 2009 [3].
In Mexico, primaquine was introduced in the 1960s to treat P. vivax patients, and the large number of cases led a malaria program to implement a monthly and intermittent combined therapy of Chloroquine and primaquine [4]. This treatment, however, may have not fully eliminated all infections caused by hypnozoites, thereby potentially contributing to ongoing malaria transmission [5]. Since then, malaria incidence has decreased to few hundreds of cases, with treatment protocols typically involving combined CQ/PQ treatment with either 7- or 4-day regimens [6].
Molecular genetic studies are crucial for understanding the epidemiology, diversity, geographical distribution, and dynamics of P. vivax populations [7]. In Mexico’s southernmost Pacific (SMP) region, specifically in the state of Chiapas, genetic studies have been conducted using markers that encode proteins in both the sporozoite and blood stages. These studies, along with those on genes associated with drug resistance, have suggested a low-to-moderate parasite diversity with exclusive haplotypes not yet reported elsewhere on the continent [8,9,10,11,12,13]. Also, longitudinal studies revealed that the most adapted or highly frequent haplotypes of pvama1I–II and pvmsp142 persisted across 17 years [14,15]. In this region, two circumsporozoite repeat variants were detected: VK210 and VK247. While the VK247 type was highly conserved, two VK210 types differed at the variable carboxyl terminus. One variant was related to the Sal-I strain, while the other expressed the domain ANKKAEDA at the carboxyl terminus [12].
In the SMP, high compatibility was found in sympatric P. vivax–vector combinations. P. vivax from patients living in the foothills produced high infection rates in Anopheles (subgenus Anopheles) pseudopunctipennis, a species abundant in that region. These parasites were low-infectious to Anopheles (subgenus Nyssorhynchus) albimanus, a vector of the coastal regions. In contrast, P. vivax from patients coming from the coastal regions were highly infectious to Ny. albimanus and did not produce infection in An. pseudopunctipennis [16]. PvCSP VK210 and VK247 were partially associated with vector susceptibility [17,18]. Other studies showed that mutations in genes expressing ookinete proteins, which are involved in parasite development in the mosquito (such as Pvs25, Pvs28, SOAP, and CTRP), and the ribosomal 18S SSU rRNA S-type variants defined two genetic lineages [16]. Strong linkage disequilibrium was observed within these lineages, which is likely a result of geographic and vector restrictions [16].
In this study, pvcsp, pvs25, and 18S SSU rRNA were analyzed, for the first time, in different malaria foci across the northwestern and southern regions. Additionally, this study sought to determine whether P. vivax genotypes from the SMP were also predominant in other regions and whether a pattern could be detected regarding vector specificity. For comparisons, genetic data previously obtained from the SMP were incorporated into the analysis, and P. vivax samples from Central America were included as an outgroup.
2. Materials and Methods
2.1. P. vivax Samples and Geographic Origin
Three hundred and thirty-five P. vivax blood smear samples were obtained from the National Malaria Control Program in Mexico. The samples were previously fixed with methanol, stained with Giemsa, and diagnosed with P. vivax infection [19]. Table 1 indicates the number of samples per state from a period of three years: 2010–2012. This period corresponds to the early pre-elimination phase, and after malaria transmission, it was focalized. Therefore, these P. vivax samples might represent the most likely persistent haplotypes in this country.

Table 1.
Precedence and year of collection of smear blood samples randomly selected and diagnosed with P. vivax in Mexico.
Another 12 P. vivax-infected blood samples impregnated in Whatman #2 filter paper from the SMP, Mexico [16], and 58 samples from Central America (the Autonomous Region of the North Caribbean Coast (RACCN) in Nicaragua) [20,21] were included in the analysis since the Mesoamerican region shared similar anopheline species [16,22].
The spatial representation in Figure 1 delineates malaria foci as follows: NWa corresponds to the interstate regions of Sinaloa and Chihuahua, while NWb corresponds to the interstate area comprising Durango, Nayarit, and Jalisco. These are areas with difficult access where malaria risk is low, and transmission is intermittent. Malaria cases in these regions have gradually declined with some fluctuations. In Pochutla, Oaxaca (OAX) malaria transmission occurred on the coastal and foothill regions. After years of intensive control measures—which included disturbing mosquito breeding sites with community participation and patient detection and treatment—this state did not report any cases in 2014–2021. However, an outbreak was reported in 2022. These regions have ecological conditions for breeding An. pseudopunctipennis [23,24]. The SMP has a tropical wet climate, with a long rainy season, although no autochthonous cases are currently reported. Since 2015, most reported cases in Chiapas correspond to the Lacandon regions (LRs). The climate there is tropical wet with abundant rainfall from May to November, with annual precipitation ranging between 2500 and 3500 mm [25]. In Chiapas, the SMP and LRs are about 500 km apart, and they have historically been migration routes for people traveling to the United States from diverse origins [26]. These regions are about 1000 km away from Nicaragua, Central America.
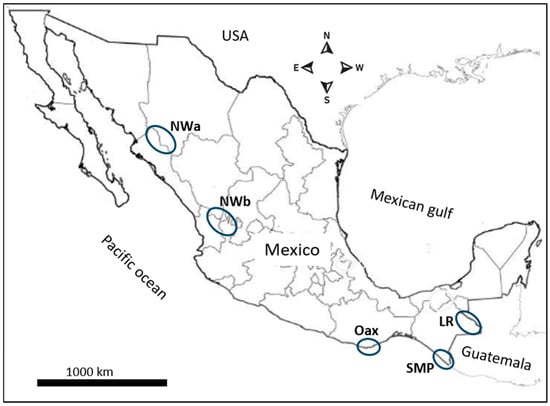
Figure 1.
The geographical distribution of the malaria foci and source of P. vivax samples in Mexico. The blue circles represent the malaria foci studied here.
2.2. Sample Preparation and DNA Extraction
Dry blood smear samples were sprayed with molecular-grade water and scraped out from a microscope slide, using the clean edge of a new glass slide. Low-density powder was meticulously transferred into a 1.5 mL Eppendorf tube. The thick smear, conversely, remained preserved on the original slide for storage. DNA extraction from the powder was conducted using the QIAmp DNA Blood Minikit (Qiagen, Redwood City, CA, USA), following the manufacturer’s protocol. Genomic DNA was eluted with 50 µL of molecular-grade water and cryopreserved at −20 °C until further analysis. From infected blood samples on filter paper (from SMP and Nicaragua), three 5 mm diameter circles from each filter paper were used to extract genomic DNA as described above.
2.3. Molecular Markers
Three markers positioned on different chromosomes were analyzed: csp variable regions (CRR and carboxyl variable terminus), a pvs25 polymorphism at a DNA segment containing Q87K and I130T variable codons [16], and ribosomal variants.
2.4. PCR Amplification and Genotyping
Circumsporozoite gene (pvcsp). All P. vivax samples were screened for pvcsp gene amplification, which included the full central repeat region (CRR) and the variable carboxyl terminus, using primers and PCR conditions as previously reported [12]. Supplementary Figure S1 shows that the quantity of stained blood smears and parasitemia were not the sole determinants for PCR amplification success in a batch of samples. For RFLP analysis, amplified products were digested with AluI (New England Biolabs, Beverly, MA, USA) and BstOI (Promega, Madison, WI, USA) restriction enzymes as previously described [12]. Negative controls (uninfected blood samples) and positive controls (P. vivax-infected blood samples) were included in each run as amplification references. Furthermore, smear blood samples from different states, years, and PCR-RFLP patterns were selected for sequencing. Only isolates exhibiting pvcsp amplification were examined for pvs25 and the ribosomal variants.
Pv25 gene. A gene fragment of ~250 bp encompassing variable codons Q87K and I130T was amplified employing primers Pvs25-F23 (5′-GTG TAT GTG TAA CGA AGG GCT-3′) and Pvs25-R214 (5′-TAC CAA AAC GGG AGA AAC TG-3′). The PCR master mix composition and reaction conditions were previously reported [26].
Ribosomal 18S SSU rRNA S-type. A segment of the P. vivax ribosomal was amplified using forward (SSU-F) and reverse (SSU-R) primers as previously reported [16]. The two variants were distinguished by differences in the size of the amplified product: 450 bp for rVar2 (Sal-I) and 480 bp for rVar1 (Thai) [27].
2.5. Sanger Sequencing
For pvcsp gene sequencing, groups of samples were selected at random based on their PCR-RFLP patterns and geographic origin. The pvcsp and pvs25 gene segments were resolved on 1% agarose gels. Fragments of the appropriate molecular size were then purified using the Wizard Plus Minipreps DNA Purification System (Promega, Madison, WI, USA). The purified fragments were sequenced by Macrogen Inc. (Seoul, Republic of Korea), using the same forward and reverse primers that were used for amplification. The quality of pherograms was manually revised using BioEdit v7 [28], and consensus sequences were generated. The sequences were deposited in GenBank (NCBI) with accession numbers PX225108–PX225182 for pvcsp and PX236386–PX236514 for pvs25.
2.6. Data Analysis
Previous results obtained in the SMP for pvs25, pvcsp, and ribosomal variants were included in the analysis [13,16]. Pvs25: EU024410.1, EU024411.1, EU024414.1, EU024416.1, EU024417.1, EU024419.1, EU024420.1, EU024437.1, EU024438.1, EU024449, EU024455.1, EU024458.1, EU024466.1-EU024472.1 [13], MN015361-MN015369 [16]. Another 11 sequences were extracted from plasmoDB (SRP046167, SRP046170, SRP046174, SRP046175, SRP046181-SRP046184, SRP046187, SRP046194, SRP046198) [29]. The Sal-I sequence (XM_001608410.1) was employed as a reference. Nucleotide sequences were aligned utilizing CLUSTALW within BioEdit v7 [28].
For each pvcsp PCR-RFLP pattern, a code was assigned according to previous studies in the SMP and Nicaragua [12]. The Sal-I sequence (XM001614511.1) and others from the SMP were used to make comparisons: Mxch1-VK210a (JQ511263.1), Mxch5-VK210d (KF437876.1), Mxch4-VK210b (JQ511267), Mxch6-VK247_I (JQ511270), and Mxch13-VK210aII (JQ511280.1) [12].
For pvs25, genetic parameters were estimated for each malaria focus and the total sequences from Mexico, such as the number of mutations (n) and segregating sites (S), the mean number of pairwise differences (k), haplotype number (h), haplotype diversity (Hd), and both nucleotide (π) and genetic (θ) diversities. Neutrality tests, such as Fu’s Fs and Tajima’s D, were calculated to determine departure from the neutral theory of evolution, in DnaSP v5.1 [30]. To analyze the genetic differentiation among parasite groups, pairwise comparisons were made by calculating a matrix of FST; values range from 0 (no difference) to 1 (completely isolated). The Nm values were also estimated as an indirect measure of gene flow based on the calculated FST values. To infer population history, the mismatch distribution’s sum of squared deviations (SSD) and Raggedness index were estimated by running 10,000 simulations in Arlequin v3.5.2 [31]. Low values support the expansion model, whereas high and significant values suggest a deviation from it. Pvs25 mutations and haplotype frequencies were compared across malaria foci utilizing the χ2 test (Kruskal–Wallis non-parametric test) at the 95% confidence level in Stata v14.
The genetic variance for different hypothesized population groupings was estimated using an analysis of molecular variance (AMOVA) [32] for pvs25 and multilocus haplotypes, using the distance method of Kimura 2P, with 10,000 permutations. These estimations were performed in Arlequin v3.5.2 [31]. To assess association patterns in the allele distribution of P. vivax from different malaria foci across Mexico, pairwise Spearman correlation analyses were conducted [33]. The analysis utilized 10 allelic variants from three molecular markers: pvcsp, pvs25, and the ribosomal variants. Procedures on binary data and visualizations were carried out using RStudio version 2023.03.1, incorporating the stats v4.4.1, corrplot v0.95, FactoMineR v2.11, and ggplot2 v3.5.2 packages. Correlations were visualized via heatmaps, with color gradients and circle size indicating direction and strength, respectively. A value of −1 denoted strong negative correlation, +1 a strong positive correlation, and 0 no association. The size of the circle encoded the absolute value of the correlation coefficient, so larger circles indicated stronger correlations regardless of sign, while smaller circles indicated weaker correlations closer to zero.
The frequencies of multilocus haplotypes were estimated per malaria focus using three markers and between the pvs25 polymorphism and the ribosomal variants for each pvcsp type. To estimate linkage disequilibrium (LD) between two or three loci, LIAN was used to simulate random allele assortment at each locus. The null hypothesis (H0) VD = Ve was tested by calculating the statistical difference between the observed variance (VD) and the expected variance (Ve)—that is, LD—by computing a p-value using Monte Carlo (PMC) simulations with 10,000 re-samplings without replacement in LInkage ANalysis v3.7. For the IAS index, a measure of multilocus LD, high values suggest limited recombination and clonal expansion, while low values suggest more frequent recombination [34].
3. Results
3.1. Circumsporozoite Gene Polymorphism
3.1.1. PCR-RFLP of Pvcsp
Information regarding parasitemia, amount in smear samples, and successful pvcsp amplification is shown in Figure S1. For NWa, Sinaloa contributed 64.3% of the samples (three, seven, and eight from 2010, 2011, and 2012, respectively), and Chihuahua contributed 35.7% (four and six from 2010 and 2012, respectively). Meanwhile, for NWb, Durango contributed 64% of the samples (4, 3, and 34 from 2010, 2011, and 2012, respectively), Nayarit contributed 28.1% (6, 5, and 9 from 2010, 2011, and 2012, respectively), and Jalisco contributed 7.8% (5 from 2012). In 183 samples (54.3% of the total), PCR-RFLP analysis was successfully achieved (Table S1). This analysis revealed the presence of both pvcsp CRR types, VK210 and VK247, with their frequencies varying significantly among malaria foci in Mexico (χ2 = 35.8, p < 0.001). The VK247 type was identified in 79.8% of isolates (n = 222) and showed clear predominance in the NWa (92.9%, n = 28), NWb (100%; n = 64), and Oaxaca foci (100%; n = 28). In contrast, the LR and SMP foci showed VK247 prevalence rates of 54% (n = 63) and 53% (n = 39), respectively, with the remaining isolates being VK210. Nationwide, three VK247 types were defined by PCR-RFLP (Figure S2): VK247_I was predominant and present in all malaria foci. Another variant, VK247_III, was detected in a single isolate in NWb (sample form Nayarit state, collected in 2011), while VK247_II was exclusive to the LR (six isolates from 2012).
Pvcsp PCR-RFLP pattern VK210a was identified in 21 isolates, representing 11.5% of the total. Additionally, five isolates harbored VK210b and two isolates VK210d. Two distinct PCR-RFLP patterns were also detected, each in a single isolate: VK210g, which exhibited a smaller molecular size than VK210a but a similar restriction pattern, suggesting variation in the number of repeats; and VK210h, which displayed a molecular size comparable to VK210a but a different restriction pattern, suggesting polymorphism within the carboxyl variable terminus (Figure S2). Moreover, two samples demonstrated mixed genotype infections (VK210a/VK247 and VK210a/b). The highest number of genotypes were detected in the LR. The geographical distribution of pvcsp genotypes is delineated in Table 2.

Table 2.
Pvcsp genotype resolved by PCR-RFLP in parasites from different malaria foci in Mexico and from Nicaragua.
All samples from RACCN, Nicaragua, were VK210; 95% of them had a PCR-RFLP pattern similar to VK210a, and the other three samples had a different pattern (VK210e).
3.1.2. Pvcsp Sequence Polymorphism
Gene sequences were obtained from 75 isolates, revealing greater genetic polymorphism among VK210 sequences than was detected by PCR-RFLP. Across all sequences, the RI (KLKQP) amino acid domain, which flanks the amino-side repeat region, was conserved. Alignment was performed using the post-repeat carboxyl variable side, extending up to the GQGQ domain. Polymorphism was detected within either the repeat region, the variable carboxyl region, or both. Forty-seven VK247 sequences from different malaria foci yielded three haplotypes (as determined by PCR-RFLP and sequencing). Forty-two of the VK247_I sequences, from different malaria foci, were resolved by PCR-RFLP and had identical nucleotide sequence. Because of this, and due to limited financial resources, no further sequencing was performed. An additional four isolates showing the same PCR-RFLP VK247_III pattern were similar to VK247_I, except the deletion of one repeat at the CRR (Figure S3A). Likewise, the VK247_II sequence was distinguished from VK247_I only based on the absence of the amino acid segment GAGGQAAGGNAANKKAGDA at the carboxyl terminus (Figure S3B).
VK210 parasites displayed five distinct PCR-RFLP patterns. Nucleotide sequencing for two samples with PCR-RFLP VK210g (in LR) and VK210a (in NWa) was unsuccessful. Because gene variation was observed in the first group of VK210a sequences, the remaining VK210 samples were also sequenced. In 20 sequences classified as VK210a (resembling Sal-I), six subvariants were identified: IA (n = 6), IB (n = 4), IC (n = 1), ID (n = 1). The VK210a_II variant was identical to Mxch13 (JQ511280.1) from SMP (Figure S3A). Variant IE (n = 1) shares identity with IA and II and had 19 repeat units (Figure S3A).
One sample from Chihuahua (NWa) identified as VK210h had two copies of the GGNAANKKAEDA domain flanked by one copy of GGNA at the carboxyl terminus. This isolate corresponded to the ANKKAEDA domain group, along with VK210b and VK210d (Figure S3A,B). These differed in their pattern of peptide repeat motifs (PRMs) from VK210a isolates. Most of the VK210 sequences were from LR, and all VK210 sequences yielded nine haplotypes and exhibited a higher Hd (0.860) compared to the VK247 parasites (Hd = 0.198; n = 47).
Two PRMs predominated in the CRR VK210: GDRADGQPA and GDRAAGQPA. Other less frequent PRMs were detected in the PRM flanking the carboxyl region (Figure 2), with the exception of VK210a_IE, which had 19 repeats. In the VK247 CRR, the amino acid repeat ANGAGNQPG predominated (Figure 2).
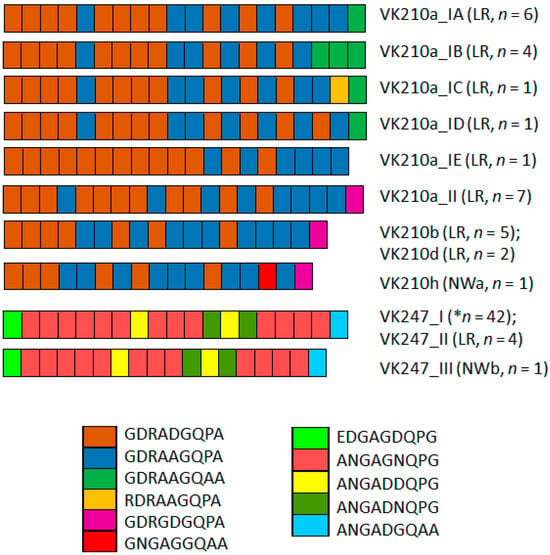
Figure 2.
P. vivax circumsporozoite peptide repeat motif (PRM) variation in CRR of P. vivax VK210 and VK247 sequences. Two main PRM types predominated in VK210a: subtypes IA, IB, IC, and ID. Notably, last PRM in VK210a_II, VK210b, VK210d, and VK210h was different (pink). Conversely, VK210a_IE lacked this PRM. Overall, VK210 variants exhibited 2–4 distinct PRMs and VK247 5 PRMs. LR, Lacandon region; NWa, far northwestern; NWb, northwestern. * indicates that this type was from NWa, NWb, OAX, and LR.
3.2. Pvs25 Polymorphism and Genetic Structure
For the 129 blood smear samples from different malaria foci in Mexico (NWa, NWb, OAX, and LR), a pvs25 sequence of 249 bp (nt 175–423) was obtained. Compared to the Sal-I strain, two polymorphisms were detected at codons 87 (cag/Q → aag/K) and 130 (atc/I → acc/T).
At the country level (including 39 sequences from SMP), the amino acid change Q87K was present in 28.1% of the samples. Its frequencies varied significantly among malaria foci: 4.4%, 77%, 0%, 4.3%, and 35.9% in NWa, NWb, OAX, LR, and SMP, respectively (χ2 = 68.5, p < 0.001). Conversely, the amino acid change I130T was at a high frequency (86.2%) countrywide, with no significant difference observed among foci (74–100%, χ2 = 4.13, p = 0.388). Among P. vivax isolates, 15.4% had haplotype QI (wild haplotype), 56.5% had QT, and 28% had KT. These three haplotypes were found in all malaria foci except in OAX, and their frequencies varied significantly (χ2 = 37.7; p < 0.001, 95% confidence) (Figure 3). The QI haplotype ranged from 0% in OAX to 23.1% in SMP; QT was found at a high frequency in four malaria foci, varying from 19.5% in NWb to 100% in OAX; and KT varied from 0% in OAX to 73.2% in NWb. One sample exhibited a mixed Q-I/T genotype infection. All 55 samples from Nicaragua had wild-type sequences (Figure 3).
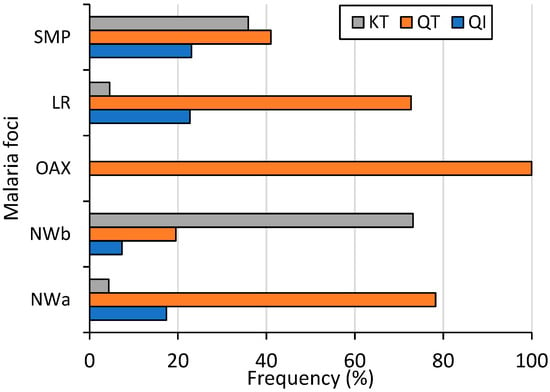
Figure 3.
Frequency of Pvs25 haplotypes across malaria foci in Mexico (χ2 = 37.7, p < 0.001). NWa (n = 23), NWb (n = 41), OAX (n = 21), LR (n = 44), SMP (n = 39).
Table 3 compares the genetic diversity parameters using pvs25 among malaria foci in Mexico. SMP exhibited the highest k value, nucleotide diversity, and haplotype diversity. Conversely, the lowest diversity was detected in LR. The highest and positive Fu’s Fs and Tajima’s D values were observed in SMP, although these values were not statistically significant. In this region, the values for mismatch distribution SSD and Raggedness indexes were significant: 0.0175 (p = 0.04) and 0.1622 (p = 0.02), respectively.

Table 3.
Parameters of diversity and neutrality tests in P. vivax among malaria foci in Mexico, using pvs25.
The AMOVA using pvs25 (n = 168) revealed that the percentage of variation was higher within populations (70%) than among malaria foci (30%). The FST index, based on pvs25, was the highest between foci NWb and OAX, followed by LR or NWa. The lowest FST index was observed between NWa and OAX (Table 4). In accordance, the lowest Nm values were observed between NWb and OAX or LR or NWa.

Table 4.
FST and Nm values of P. vivax between malaria foci in Mexico, using pvs25.
3.3. Frequencies of 18S rRNA S-Type Variants
Both ribosomal variants were detected; 87 and 76 had rVar2 and rVar1, respectively, and their frequencies varied among malaria foci (χ2 = 30.32, p < 0.001, 95% confidence) (Figure 4). A distinct latitudinal pattern was evident: as the frequency of one variant increased, the other’s decreased from the northwestern to southern foci. Specifically, the rVar2 variant progressively declined from 90.1% in NWa to 23.1% in SMP. Variant rVar1 had its highest frequency in SMP (76.9%) before gradually decreasing to 9.1% in NWa. All 55 samples from Nicaragua had the rVar2 variant.
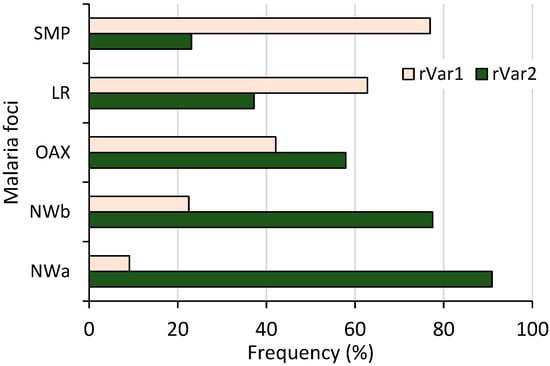
Figure 4.
Frequency of P. vivax ribosomal rRNA 18S S-type variants in different malaria foci from Mexico. Both variants showed inverse frequency trend, differing from northwestern to southernmost regions (n = 163, χ2 = 44.12, p < 0.001).
3.4. P. vivax Multilocus Haplotype Analyses
An AMOVA test, performed using multilocus data from 159 P. vivax isolates, revealed that a greater variation occurred within malaria foci (73%) compared to between foci (27%) (p < 0.001). Haplotype frequencies were estimated per malaria focus, and categorized by their pvcsp type (Figure 5). VK210 parasites were further subdivided into those either expressing or lacking the ANKKAEDA domain at the carboxyl terminus (Figure 5A,B). Diverse pairings among pvcsp types, pvs25 genotypes, and ribosomal variants were observed at different frequencies (Figure 5). Pvcsp VK247 was the most geographically widespread among the foci and exhibited all possible combinations with pvs25 genotypes and ribosomal variants (Figure 5C).
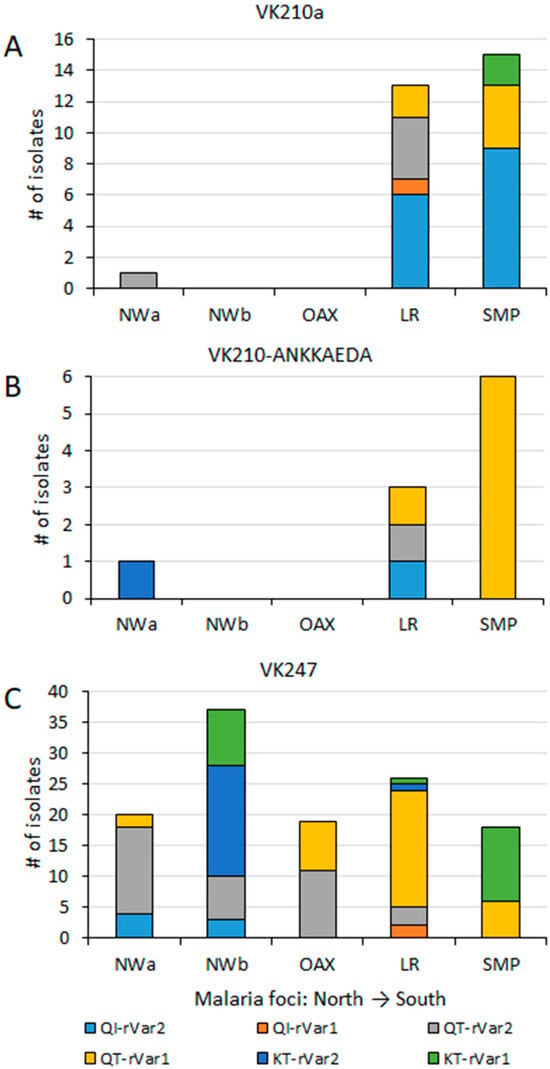
Figure 5.
Geographical distribution of haplotypes by pvcsp type, pvs25, and ribosomal variants across malaria foci in Mexico. Pvs25, and ribosomal variants haplotypes are indicated per pvcsp type: (A) VK210a, without ANKKAEDA domain; (B) VK210 expressing ANKKAEA domain; (C) isolates VK247. Significant differences in haplotype frequency were observed among foci (χ2 = 29.0, p < 0.001). These differences remained significant among VK247 populations (χ2 = 37.9, p < 0.001).
Many haplotypes, specially the most frequent ones, were shared between two or more malaria foci, extending from northwestern to southern Mexico or vice versa. In the far northwestern (NWa) region, two highly frequent haplotypes were found, VK247_I-QT-rVar1 at 18.2% and VK247_I-QT-rVar2 at 63.6%, both present in Chihuahua and Sinaloa. They were also present in other foci, except in NWb and SMP, respectively. Haplotype VK247_I-QT-rV1 was at high frequency in OAX (42.1%), LR (38%), and SMP (15.4%). Meanwhile, VK247_I-QT-rV2 was also highly frequent in OAX (57.9%), NWb (18.9%), and LR (4.7%) (Figure 5). In the NWb focus, the haplotypes VK247_I-QT-rV2 (at 18.9%) and VK247_I-KT-rVar2 (at 48.6%) were detected in Durango, Nayarit, and Jalisco (NWb). The latter haplotype was also detected in LR at 2.4%. Another haplotype VK247_I-KT-rVar1 (24%), detected in NWb (Durango and Jalisco), was also detected in SMP at 30.8%. Of the two haplotypes detected in Oaxaca, one was shared with NWb and the other with SMP. The SMP and LR foci were the only ones that shared haplotype Vk210a-QI-rVar2, at 23.1% and 14.3%, respectively, and VK210b-QT-rVar1, at 15.3% and 2.3%, respectively. NWa, NWb, and LF foci had exclusive haplotypes as 210h-KT-rVar2 in Chihuahua, 247_I-QI-rVar2 in Durango and Nayarit, and VK247_II-QI-rVar2 in Nayarit. Several haplotypes in LR were exclusive (Figure 5). In LR, the VK247_II haplotype was found within three distinct multilocus haplotypes. In contrast, P. vivax from Nicaragua displayed high homogeneity, having only VK210a/e-QI-rVar2 haplotypes.
The Spearman correlation analysis revealed site-specific genetic variant associations and overlapping correlation patterns in geographically proximal foci (Figure 6). The SMP focus exhibited a positive association with both the VK210-ANKKAEDA and VK210a types. In contrast, the LR focus was primarily associated with VK210a. Malaria foci in the northwestern regions and Oaxaca were associated with VK247_I, whereas LR correlated with VK247_II. The strongest positive correlation was observed between LR and Pvs25–87K, followed by the VK247_I type with NWb, VK210a and ribosomal rVar1 with SMP, ribosomal rVar2 with NWa 1, and VK427_II and Pvs25 87Q with LR.
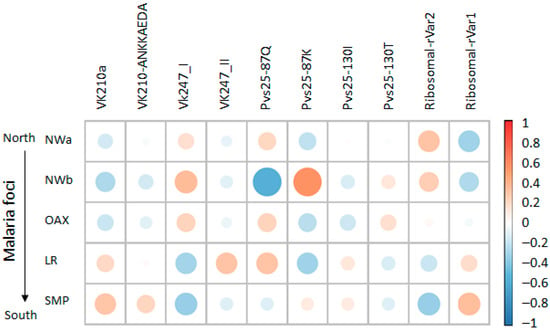
Figure 6.
Heatmap of correlation patterns between genetic variants and Plasmodium vivax foci across Mexico. Coefficients range from −1 for strong negative correlation (shown in blue) to +1 for strong positive correlation (shown in orange), with 0 representing no association (white). Size of circles represents absolute value of correlation coefficient, with larger circles indicating stronger correlations (negative or positive), while smaller circles indicate weaker correlations closer to zero. Both displays include genetic variants and focus identifiers, revealing site-specific marker correlations and shared haplotype patterns among geographically proximal areas.
A linkage disequilibrium (LD) analysis was conducted by defining three csp types (VK210a, VK210-ANKKAEDA, and VK247), three Pvs25 haplotypes (QI, QT, KT), and two ribosomal variants (rVar2/rVar1) (Table 5). As anticipated [16], the southernmost Pacific population (SMP) exhibited the highest Association Index (p = 0.583). For the overall population, the IAS value was low, and H0:VD = Ve was significant (p = 0.0004). Conversely, no evidence of LD was detected between pvs25 genotypes and ribosomal variants within the nationwide VK247 population (p = 0.583). Similarly, LD between pvs25 and the ribosomal variants was not evident when analyzing, independently, the VK210 and VK247 populations from LR (Table 5).

Table 5.
Linkage disequilibrium analysis using loci: pvcsp, pvs25, and the ribosomal variants.
4. Discussion
In this study, pvcsp, pvs25, and the ribosomal polymorphism found in smear samples from various malaria foci (NWa, NWb, OAX, and LR) were consistent with previous findings in SMP [12,13,16,18]. All pvcsp types VK210a, VK210-ANKAEDA, and the conserved VK247_I; Pvs25 haplotypes QI, QT, and KT; and ribosomal variants were observed in SMP [13,16]. In SMP, a PCR-RFLP analysis of 379 samples showed that 48% were VK247_I, while 52% belonged to the VK210a group [12]. The VK247_I predominated in NWa/b and OAX foci and remain highly frequent in SMP and LR foci in Chiapas, and the presence of both VK210 types in NWa, SMP, and LR foci points to a history of substantial parasite circulation across Mexico before malaria transmission became focalized. In contrast, the lack of detection of VK247 and VK210-ANKKAEDA in Nicaragua might imply limited parasite exchange between Mexico and Central America beyond the immediate border areas.
VK210 parasites expressing the ANKKAEDA domain have been reported persistently in Middle East and Asia, for instance, in Vietnam [35] and Thailand [36], where its presence was attributed to parasite circulation across the Thai–Myanmar border. In Thailand, this polymorphic form was reported circulating in the 1990s [37]. In Myanmar, a country with high malaria transmission, P. vivax VK210 exhibited great diversity, as well as the presence of the ANKKAEDA domain and other diversified forms such as ANKKAENA and ANEAENA [38]. A recent resurgence of malaria cases in Pakistan revealed that all VK210 isolates were low-diverse and harbored the ANKKAEDA domain [39]. The authors attributed this to the high cross-border exchanges between Pakistan, Iran, and Afghanistan. In southern Iran, the presence of VK210-ANKKAEDA was reported decades earlier [40,41]. Similarly, VK247 parasites in Pakistan were identical to those reported earlier in Iran [40]. The Pvcsp VK247_1 found to be predominant in Mexico and across time in SMP [12] resembles a sequence from South America (isolated in 2008) and Iran [12,40]. This highlights the importance of molecular surveillance across time to understand genotype persistence and parasite evolution in regions with different eco-epidemiological conditions.
Pvs25 haplotype (QI, QT, and KT) frequencies varied among malaria foci in Mexico, likely due to specific ecological conditions, differences in vector species, and varying intensities of evolutionary forces. All Nicaraguan parasites had the Pvs25_QI haplotype, which matches strains from Central America and South America [13,29]. In South America, only the amino acid change Q87K has been reported across different regions (e.g., Brazil [42], Colombia, Peru [29], and Venezuela [43]), suggesting the presence of two genotypes: QI and KI. Beyond the Americas, the Q87K mutation has also been documented in Iran [44] and Mauritania [45]. In China, a different change (Q87L) was reported [46]. Pvs25 I130T polymorphism, which was found to be highly frequent in all malaria foci in Mexico, predominates in P. vivax populations outside Latin America, e.g., in China [46], Iran [44], Thailand [47], Myanmar [48], the Thai–Myanmar border [49], India [27,50], Bangladesh [51], and South Korea [52]. Additionally, two recent studies have indicated that the Pvs25 QT haplotype is highly frequent across Asia. A haplotype network analysis revealed that this haplotype might be more ancestral than the KT or QI haplotype, which likely diversified through distinct evolutionary routes from haplotype QT. Furthermore, any of these haplotypes might have given rise to the KI haplotype, most likely from QI in South America [47,48]. Using pvs25 sequences, parasites from Central and South America clustered with those from Africa [53]. For pvs25, the highest nucleotide and haplotype diversity was detected in SMP, and the mismatch distribution SSD and Raggedness indexes suggest that SMP population has a complex demographic history.
In Mexico, the genetic characteristics pvcsp and pvs25 suggest distinct introductions of P. vivax compared to those in Central or Southern America, with a limited circulation of some haplotypes across the continent. Parasite dispersal is influenced by compatibility and/or P. vivax adaptation to local vector species. Li et al. [54] found two ribosomal variants associated with vector infectivity. Parasites from Central America with the rVar2 variant produced high infections in Ny. albimanus, while parasites from other geographical sites expressing the rVar1 variant produced very low infection in this vector. The ribosomal rVar2 variant was at a higher frequency in northwestern foci, while rVar1 predominated in SMP and LR foci. In SMP, Pvs25 QI was found to be exclusively associated with ribosomal rVar2 and pvcsp VK210a, a haplotype found in the LR focus. Other haplotypes comprising any pvcsp type, Pvs25 QT/KT haplotypes, which combined exclusively with ribosomal rVar1, were highly infectious to An. pseudopuntipennis [16]. In SMP, these haplotypes were in linkage disequilibrium likely due to geographic and/or vector restriction.
Following the implementation of intensive control measures in the 1990s, malaria transmission became focalized [2]. The results from the AMOVA test, FST, and Spearman correlation analysis using pvs25 and/or multilocus analysis support the existence of a genetic structure among P. vivax populations in Mexico. The haplotype sharing among malaria foci and the Nm values using pvs25 suggest that the genetic flow observed in Mexican P. vivax populations is a remnant of a past period, when malaria transmission was spread across the country. The persistence and evolution of parasites in each focus are likely due to their adaptation to local ecological conditions. This process may have led to the loss of haplotypes as the number of cases declined, a phenomenon previously documented in SMP [14,15]. The presence of several novel multilocus (CSP–Pvs25–ribosomal) haplotypes in malaria foci outside SMP was a notable finding. In northwestern (NWa and NWb) and OAX, An. pseudopuntipennis is the main malaria vector [24,55]. This species thrives in mountainous regions, with larvae commonly found along river margins amidst filamentous algae [24]. In these foci, the presence of several multilocus haplotypes suggests a high degree of adaptation of An. pseudopuntipennis to transmit a wide variety of P. vivax haplotypes (VK247/VK210a-QT-rVar2, VK247-QI/KT-rVar2, and VK210h-KT-rVar2), not reported in the SMP [16]. The multilocus LD encountered in P. vivax from Mexico suggests minimal recombination and a pattern of clonal transmission. However, the lack of linkage disequilibrium between the pvs25 genotypes and the ribosomal variants within VK247 populations (nationwide, LR and NWb) suggests that free recombination happened in the past. These findings align with studies demonstrating that An. pseudopunctipennis from various regions in Mexico and Guatemala were highly susceptible to VK247 parasites from southern Mexico [26].
In the Lacandon region, both An. pseudopunctipennis and Ny. albimanus were reported as abundant [24]. Unlike in SMP, these vector species were found to be coexisting with other anopheline species and sharing breeding sites [24]. This region harbors numerous multilocus P. vivax haplotypes, and various were exclusive (e.g., VK247/VK210a-QI-rVar1, VK210b-QI-rVar2, VK210d-QT-rVar2). Conversely, low and non-significant IAS values might suggest potential recombination events within the VK247 or VK210 population in LR and within the VK247 population in NWb. The possibility that these findings propose different vector specificities for the VK247 and VK210 populations warrants further investigation. The presence of VK210a-QI-rVar2 haplotypes in the LR and SMP foci and their predominance in Nicaragua (like Sal-I strain [56]) suggest that parasite exchange may be occurring between Mexico’s southern border and Central America.
In Mexico, the Anopheles genus includes 27 species, widely distributed across the country. LR has ecological conditions conducive to the year-round vector breeding of at least 11 species [24]. Of these, only three have been identified as primary malaria vectors: An. pseudopunctipennis, Ny. albimanus, and Anopheles (subgenus Anopheles) vestitipennis [57]. These species converge with other secondary vectors, including Anopheles (subgenus Anopheles) punctimacula, Anopheles (subgenus Anopheles) apicimacula, and Anopheles (subgenus Nyssorhynchus) darlingi [23,58]. In Brazil, mosquitoes Ny. darlingi transmits both VK210 [59,60] and VK247 parasites [61]. High parasite diversity is typically observed when numerous Anopheles species and high transmission intensity take place, such as in Brazil, Colombia, or Myanmar [23,62]. Vector control in the Lacandon region has been scarce or nonexistent due to a high level of insecurity, a lack of roads, and limited attention from the vector control program. Various indigenous ethnic groups have historically inhabited this geographic area. Between the 1970s and 1980s, the region experienced a significant migration of people from central and northern Mexico [63]. Additionally, people from Central American countries, primarily Guatemala, migrated to the area to flee violence, which created new conflicts with local residents and authorities over land ownership. Furthermore, the persistence of malaria in some regions in Mexico has been associated with several factors, including villages with poor infrastructure, reduced knowledge about malaria prevention, cultural factors, population movement, restricted access to primary health services, and environments with conflict [64,65,66].
This study represents the first genetic analysis of P. vivax from malaria foci outside SMP, demonstrating the feasibility of using smear samples for population-based studies. However, certain limitations must be considered, including potential bias from the non-uniform quality of the samples. Notwithstanding, all states contributed to the parasite diversity in NWa and NWb. In addition, several key findings were notable, e.g., the higher diversity in LR compared to other foci, the predominance of VK247 parasites in the northwestern foci and OAX, the high presence of VK210 parasites in LR, and the discovery of multiple novel multilocus haplotypes, among others.
P. vivax samples correspond to a period immediately following Mexico’s pre-elimination phase accreditation by the WHO in 2009 [3]. This parasite population might not entirely represent current genetic status of P. vivax in this country. However, when parasite transmission is mostly clonal, the highly frequent haplotypes would persist over time, as observed earlier in SMP [12,13,14,15]. These findings provide a baseline for parasite surveillance to evaluate persistent or emerging genotypes causing outbreaks in Mexico [2] or other regions in Latin America. In 2020, a total of 56 active microfoci were identified in 406 localities, as well as 71 residual foci in 320 localities [67]. This represents a powerful reminder that “malaria-free” status might not be permanent if receptivity and vulnerability to transmission remain. LR is currently the most challenging malaria focus [2], as it harbors the broadest reservoir of P. vivax haplotypes. This wide genetic diversity and high vector adaptation of P. vivax in different malaria foci might represent a significant challenge for malaria surveillance and elimination efforts.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microorganisms13092221/s1, Figure S1: Concordance between P. vivax parasitemia, sample amount, and successful pvcsp gene amplification; Figure S2: PCR-RFLP of pvcsp gene from isolates in Mexico; Figure S3A: Pvcsp CRR polymorphism from malaria foci, Mexico; Figure S3B: Pvcsp nucleotide and amino acid carboxyl variable terminus in isolates from Mexico; Table S1: Percentage of samples with successful amplification of pvcsp gene, per geographical site and year, Mexico.
Author Contributions
Conceptualization, L.G.-C.; Data curation, D.d.J.G.-P.; Formal analysis, L.G.-C., A.P.-M., and G.R.A.; Funding acquisition, L.G.-C. and A.M.-P.; Investigation, L.G.-C. and C.V.-T.; Methodology, D.d.J.G.-P., F.S.-V., M.O.-M., C.G.-B. and A.M.-P.; Resources, L.G.-C., C.G.-B. and A.M.-P.; Supervision, L.G.-C.; Validation, L.G.-C., F.S.-V. and M.O.-M.; Visualization, L.G.-C.; Writing—original draft, L.G.-C., F.S.-V., A.P.-M., G.R.A. and C.V.-T.; Writing—review and editing, L.G.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CONACyT (SECIHTI)—Mexico: grant number CB-2009-131247.
Institutional Review Board Statement
The Ethics Committee of the National Institute of Public Health in Mexico approved the protocol (CI1042; 15 December 2011). This study followed the bioethics guidelines (CITI program); all participants above 18 years old gave their written informed consent, and minors between 7 and 17 years of age gave their assent accompanied by the written informed consent of one parent or guardian, in accordance with the Declaration of Helsinki [9,23,24].
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study. Blood smear samples diagnosed with P. vivax and from the national malaria program had no personal identifier, only the year of collection and the origin (municipality and/or the state) in Mexico.
Data Availability Statement
Gene sequences were deposited in GenBank with accession numbers PX225108–PX225182 for pvcsp and PX236386–PX236514 for pvs25. All other relevant data are contained within the article or in the Supplementary Material.
Acknowledgments
Gemini 1.5 Flash AI was used as an assistant for grammar, spelling, and typo review. Mendeley Reference Manager version 2.135 was used for referencing the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations were used in this manuscript:
| P. vivax | Plasmodium vivax |
| CSP | Circumsporozoite protein |
| rRNA | Ribosomal RNA |
| PRM | Peptide repeat motif |
| CRR | Central repeat region |
| pvcsp | Circumsporozoite gene of P. vivax |
| RACCN | Autonomous Region of the North Caribbean Coast |
| PCR | Polymerase chain reaction |
| RFLP | Restriction fragment length polymorphism |
| AMOVA | Analysis of molecular variance |
| NWa | Far northwestern foci (Chihuahua, Sinaloa), Mexico |
| NWb | Northwestern foci (Nayarit, Durango, Jalisco), Mexico |
| OAX | Pochutla, Oaxaca, Mexico |
| SMP | Southernmost Pacific region in Chiapas, Mexico |
| LR | Lacandon region (Palenque, Ocosingo in Chiapas), Mexico |
References
- World Health Organization. World Malaria Report. 2024. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024 (accessed on 3 July 2025).
- Secretaria de Salud. Boletin Epidemiologico. Available online: https://www.gob.mx/salud/acciones-y-programas/direccion-general-de-epidemiologia-boletin-epidemiologico (accessed on 3 June 2025).
- World Health Organization. World Malaria Report 2009; World Health Organization: Geneva, Switzerland, 2009; Available online: https://www.who.int/publications/i/item/9789241563901 (accessed on 20 August 2025).
- Gonzalez-Ceron, L.; Rodriguez, M.H.; Sandoval, M.A.; Santillan, F.; Galindo-Virgen, S.; Betanzos, A.F.; Rosales, A.F.; Palomeque, O.L. Effectiveness of Combined Chloroquine and Primaquine Treatment in 14 Days versus Intermittent Single Dose Regimen, in an Open, Non-Randomized, Clinical Trial, to Eliminate Plasmodium vivax in Southern Mexico. Malar. J. 2015, 14, 426. [Google Scholar] [CrossRef][Green Version]
- Gonzalez-Ceron, L.; Mu, J.; Santillan, F.; Joy, D.; Sandoval, M.A.; Camas, G.; Su, X.; Choy, E.V.; Torreblanca, R. Molecular and Epidemiological Characterization of Plasmodium vivax Recurrent Infections in Southern Mexico. Parasites Vectors 2013, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Secretaria de Salud/Cenaprece. Manual De Tratamientos Médicos Para La Atención De Casos Confirmados De Paludismo En México. Available online: https://www.gob.mx/cms/uploads/attachment/file/805562/Manual_de_Tratamientos_para_Paludismo_Definitivo.pdf (accessed on 3 July 2025).
- Alicia, A.; Barry, A.E.; Reeder, J.C. Understanding the Population Genetics of Plamodium vivax Is Essential for Malaria Control and Elimination. Malar. J. 2012, 11, 1–10. [Google Scholar] [CrossRef]
- Gonzalez-Ceron, L.; Cerritos, R.; Corzo-Mancilla, J.; Santillan, F. Diversity and Evolutionary Genetics of the Three Major Plasmodium vivax Merozoite Genes Participating in Reticulocyte Invasion in Southern Mexico. Parasites Vectors 2015, 8, 651. [Google Scholar] [CrossRef][Green Version]
- Gonzalez-Ceron, L.; Montoya, A.; Corzo-Gomez, J.C.; Cerritos, R.; Santillan, F.; Sandoval, M.A. Genetic Diversity and Natural Selection of Plasmodium vivax Multi-Drug Resistant Gene (Pvmdr1) in Mesoamerica. Malar. J. 2017, 16, 261. [Google Scholar] [CrossRef]
- Gonzalez-Ceron, L.; Rodriguez, M.H.; Montoya, A.; Santillan-Valenzuela, F.; Corzo-Gomez, J.C. Molecular Variation of Plasmodium vivax Dihydrofolate Reductase in Mexico and Nicaragua Contrasts with That Occurring in South America. Salud Publica Mex. 2020, 62, 364–371. [Google Scholar] [CrossRef]
- Gonzalez-Ceron, L.; Cebrian-Carmona, J.; Mesa-Valle, C.M.; Garcia-Maroto, F.; Santillan-Valenzuela, F.; Garrido-Cardenas, J.A. Plasmodium vivax Cysteine-Rich Protective Antigen Polymorphism at Exon-1 Shows Recombination and Signatures of Balancing Selection. Genes 2020, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ceron, L.; Martinez-Barnetche, J.; Montero-Solis, C.; Santillan, F.; Soto, A.M.; Rodriguez, M.H.; Espinosa, B.J.; Chavez, O.A. Molecular Epidemiology of Plasmodium vivax in Latin America: Polymorphism and Evolutionary Relationships of the Circumsporozoite Gene. Malar. J. 2013, 12, 243. [Google Scholar] [CrossRef]
- Gonzalez-Ceron, L.; Alvarado-Delgado, A.; Martinez-Barnetche, J.; Rodriguez, M.H.; Ovilla-Munoz, M.; Perez, F.; Hernandez-Avila, J.E.; Sandoval, M.A.; Rodriguez Mdel, C.; Villarreal-Trevino, C. Sequence Variation of Ookinete Surface Proteins Pvs25 and Pvs28 of Plasmodium vivax Isolates from Southern Mexico and Their Association to Local Anophelines Infectivity. Infect. Genet. Evol. 2010, 10, 645–654. [Google Scholar] [CrossRef]
- Flores-Alanis, A.; Gonzalez-Ceron, L.; Santillan, F.; Ximenez, C.; Sandoval, M.A.; Cerritos, R. Temporal Genetic Changes in Plasmodium vivax Apical Membrane Antigen 1 over 19 Years of Transmission in Southern Mexico. Parasites Vectors 2017, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- Flores-Alanis, A.; Gonzalez-Ceron, L.; Santillan-Valenzuela, F.; Ximenez, C.; Sandoval-Bautista, M.A.; Cerritos, R. Spatiotemporal Changes in Plasmodium vivax Msp142 Haplotypes in Southern Mexico: From the Control to the Pre-Elimination Phase. Microorganisms 2022, 10, 186. [Google Scholar] [CrossRef]
- González-Cerón, L.; Rodríguez, M.H.; Ovilla-Muñoz, M.T.; Santillán-Valenzuela, F.; Hernández-Ávila, J.E.; Rodríguez, M.C.; Martínez- Barnetche, J.; Villarreal-Treviño, C. Ookinete-Specific Genes and 18S SSU RRNA Evidenced in Plasmodium vivax Selection and Adaptation by Sympatric Vectors. Front. Genet. 2020, 10, 1362. [Google Scholar] [CrossRef]
- Gonzalez-Ceron, L.; Rodriguez, M.H.; Nettel, J.C.; Villarreal, C.; Kain, K.C.; Hernandez, J.E. Differential Susceptibilities of Anopheles albimanus and Anopheles pseudopunctipennis to Infections with Coindigenous Plasmodium vivax Variants VK210 and VK247 in Southern Mexico. Infect. Immun. 1999, 67, 410–412. [Google Scholar] [CrossRef]
- Rodriguez, M.H.; Gonzalez-Ceron, L.; Hernandez, J.E.; Nettel, J.A.; Villarreal, C.; Kain, K.C.; Wirtz, R.A. Different Prevalences of Plasmodium vivax Phenotypes VK210 and VK247 Associated with the Distribution of Anopheles albimanus and Anopheles pseudopunctipennis in Mexico. Am. J. Trop. Med. Hyg. 2000, 62, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ceron, L.; Rodriguez, M.H.; Betanzos, A.F.; Abadia, A. Efficacy of a rapid test to diagnose Plasmodium vivax in symptomatic patients of Chiapas, Mexico. Salud Publica Mex. 2005, 47, 282–287. [Google Scholar] [PubMed][Green Version]
- Gutierrez, S.; Gonzalez-Ceron, L.; Montoya, A.; Sandoval, M.A.; Torres, M.E.; Cerritos, R. Genetic Structure of Plasmodium vivax in Nicaragua, a Country in the Control Phase, Based on the Carboxyl Terminal Region of the Merozoite Surface Protein-1. Infect. Genet. Evol. 2016, 40, 324–330. [Google Scholar] [CrossRef]
- Gonzalez Ceron, L.; Piedra-Arevalo, F.O.; Casanova-Hernandez, D.; Santillan-Valenzuela, F.; Montoya Perez, A. Plasmodium vivax apical membrane antigen 1 I-II from Nicaragua (2012–2013): Genetic and antigenic polymorphism. Salud Publica Mex. 2023, 65, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Joy, D.A.; Gonzalez-Ceron, L.; Carlton, J.M.; Gueye, A.; Fay, M.; McCutchan, T.F.; Su, X.Z. Local Adaptation and Vector-Mediated Population Structure in Plasmodium vivax Malaria. Mol. Biol. Evol. 2008, 25, 1245–1252. [Google Scholar] [CrossRef]
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Rubio-Palis, Y.; Chareonviriyaphap, T.; Coetzee, M.; Mbogo, C.M.; Hemingway, J.; Patil, A.P.; Temperley, W.H.; et al. A Global Map of Dominant Malaria Vectors. Parasites Vectors 2012, 5, 69. [Google Scholar] [CrossRef]
- Villarreal-Treviño, C.; Ríos-Delgado, J.C.; Penilla-Navarro, R.P.; Rodríguez, A.D.; López, J.H.; Nettel-Cruz, J.A.; Moo-Llanes, D.A.; Fuentes-Maldonado, G. Composition and Abundance of Anopheline Species According to Habitat Diversity in Mexico. Salud Publica Mex. 2020, 62, 388–401. [Google Scholar] [CrossRef]
- Semarnat/Conagua. Servicio Meteorológico Nacional. Available online: https://smn.conagua.gob.mx/es/index.php (accessed on 27 August 2025).
- Gonzalez-Ceron, L.; Rodriguez, M.H.; Nettel-Cruz, J.A.; Hernandez-Avila, J.E.; Malo-Garcia, I.R.; Santillan-Valenzuela, F.; Villarreal-Trevino, C. Plasmodium vivax CSP-Pvs25 Variants from Southern Mexico Produce Distinct Patterns of Infectivity for Anopheles albimanus versus An. pseudopunctipennis, in Each Case Independent of Geographical Origin. Parasites Vectors 2019, 12, 86. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Joshi, H.; Shalini, S.; Patarroyo, M.A.; Suwanarusk, R.; Kumar, A.; Sharma, S.K.; Eapen, A.; Dev, V.; Bhatt, R.M.; et al. Plasmodium vivax Lineages: Geographical Distribution, Tandem Repeat Polymorphism, and Phylogenetic Relationship. Malar. J. 2011, 10, 374. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids. Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Hupalo, D.N.; Luo, Z.; Melnikov, A.; Sutton, P.L.; Rogov, P.; Escalante, A.; Vallejo, A.F.; Herrera, S.; Arevalo-Herrera, M.; Fan, Q.; et al. Population Genomics Studies Identify Signatures of Global Dispersal and Drug Resistance in Plasmodium vivax. Nat. Genet. 2016, 48, 953–958. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A Software for Comprehensive Analysis of DNA Polymorphism Data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E.L. Arlequin Suite Ver 3.5: A New Series of Programs to Perform Population Genetics Analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Weir, B.S. Genetic Data Analysis II. Methods for Discrete Population Genetic Data; Sinauer Associates, Inc.: Sunderland, MA, USA, 1996. [Google Scholar]
- Bohonak, A.J. Dispersal, Gene Flow, and Population Structure. Q. Rev. Biol. 1999, 74, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Haubold, B.; Hudson, R.R. LIAN 3.0: Detecting Linkage Disequilibrium in Multilocus Data. Linkage Analysis. Bioinformatics 2000, 16, 847–848. [Google Scholar] [CrossRef] [PubMed]
- Võ, T.C.; Trinh, N.T.M.; Lê, H.G.; Kang, J.-M.; Yoo, W.G.; Quang, H.H.; Na, B.-K. Genetic Diversity of Circumsporozoite Surface Protein of Plasmodium vivax from the Central Highlands, Vietnam. Pathogens 2022, 11, 1158. [Google Scholar] [CrossRef] [PubMed]
- Khulmanee, T.; Thita, T.; Kritsiriwutinan, K.; Boonyuen, U.; Saai, A.; Inkabjan, K.; Chakrabarti, R.; Rathod, P.K.; Krudsood, S.; Mungthin, M.; et al. Low Genetic Diversity of Plasmodium vivax Circumsporozoite Surface Protein in Clinical Isolates from Southern Thailand. Trop. Med. Infect. Dis. 2024, 9, 94. [Google Scholar] [CrossRef] [PubMed]
- Rongnoparut, P.; Supsamran, N.; Sattabongkot, J.; Suwanabun, N.; Rosenberg, R. Phenotype and Genotype Diversity in the Circumsporozoite Proteins of Plasmodium vivax in Thailand. Mol. Biochem. Parasitol. 1995, 74, 201–210. [Google Scholar] [CrossRef]
- Võ, T.C.; Lê, H.G.; Kang, J.M.; Moe, M.; Naw, H.; Myint, M.K.; Lee, J.; Sohn, W.M.; Kim, T.S.; Na, B.K. Genetic Polymorphism and Natural Selection of Circumsporozoite Protein in Myanmar Plasmodium vivax. Malar. J. 2020, 19, 303. [Google Scholar] [CrossRef]
- Bibi, Z.; Fatima, A.; Rani, R.; Maqbool, A.; Khan, S.; Naz, S.; Waseem, S. Genetic Characterization of Plasmodium vivax Isolates from Pakistan Using Circumsporozoite Protein (Pvcsp) and Merozoite Surface Protein-1 (Pvmsp-1) Genes as Genetic Markers. Malar. J. 2021, 20, 112. [Google Scholar] [CrossRef]
- Zakeri, S.; Abouie Mehrizi, A.; Djadid, N.D.; Snounou, G. Circumsporozoite Protein Gene Diversity among Temperate and Tropical Plasmodium vivax Isolates from Iran. Trop. Med. Int. Health 2006, 11, 729–737. [Google Scholar] [CrossRef]
- Shabani, S.H.; Zakeri, S.; Mehrizi, A.A.; Mortazavi, Y.; Djadid, N.D. Population Genetics Structure of Plasmodium vivax Circumsporozoite Protein during the Elimination Process in Low and Unstable Malaria Transmission Areas, Southeast of Iran. Acta Trop. 2016, 160, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.B.; Perce-Da-Silva, D.d.S.; Totino, P.R.R.; Riccio, E.K.P.; Baptista, B.d.O.; de Souza, A.B.L.; Rodrigues-Da-Silva, R.N.; Machado, R.L.D.; de Souza, R.M.; Daniel-Ribeiro, C.T.; et al. Plasmodium vivax Ookinete Surface Protein (Pvs25) Is Highly Conserved among Field Isolates from Five Different Regions of the Brazilian Amazon. Infect. Genet. Evol. 2019, 73, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Chaurio, R.A.; Pacheco, M.A.; Cornejo, O.E.; Durrego, E.; Stanley, C.E., Jr.; Castillo, A.I.; Herrera, S.; Escalante, A.A. Evolution of the Transmission-Blocking Vaccine Candidates Pvs28 and Pvs25 in Plasmodium vivax: Geographic Differentiation and Evidence of Positive Selection. PLoS Neglected Trop. Dis. 2016, 10, e0004786. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, S.; Razavi, S.; Djadid, N.D. Genetic Diversity of Transmission Blocking Vaccine Candidate (Pvs25 and Pvs28) Antigen in Plasmodium vivax Clinical Isolates from Iran. Acta Trop. 2009, 109, 176–180. [Google Scholar] [CrossRef]
- Escalante, A.A.; Cornejo, O.E.; Freeland, D.E.; Poe, A.C.; Durrego, E.; Collins, W.E.; Lal, A.A. A Monkey’s Tale: The Origin of Plasmodium vivax as a Human Malaria Parasite. Proc. Natl. Acad. Sci. USA 2005, 102, 1980–1985. [Google Scholar] [CrossRef]
- Wang, S.; Tian, P.; Li, S.; Liu, H.; Guo, X.; Huang, F. Genetic Diversity of Transmission-Blocking Vaccine Candidate Antigens Pvs25 and Pvs28 in Plasmodium vivax Isolates from China. BMC Infect. Dis. 2022, 22, 944. [Google Scholar] [CrossRef]
- Kuesap, J.; Suphakhonchuwong, N.; Rungsihirunrat, K. Genetic Polymorphisms of Plasmodium vivax Ookinete (Sexual Stage) Surface Proteins (Pvs25 and Pvs28) from Thailand. Infect. Genet. Evol. 2024, 118, 105558. [Google Scholar] [CrossRef] [PubMed]
- Lê, H.G.; Kang, J.M.; Jun, H.; Lee, J.; Moe, M.; Thái, T.L.; Lin, K.; Myint, M.K.; Yoo, W.G.; Sohn, W.M.; et al. Genetic Diversity and Natural Selection of Transmission-Blocking Vaccine Candidate Antigens Pvs25 and Pvs28 in Plasmodium vivax Myanmar Isolates. Acta Trop. 2019, 198, 105104. [Google Scholar] [CrossRef]
- Guled, B.A.; Na-Bangchang, K.; Chaijaroenkul, W. Exploring Genetic Polymorphisms among Plasmodium vivax Isolates from the Thai-Myanmar Borders Using Circumsporozoite Protein (Pvcsp) and Ookinete Surface Protein (Pvs25) Encoding Genes. Parasitol. Res. 2024, 123, 91. [Google Scholar] [CrossRef]
- Kaur, H.; Sehgal, R.; Kumar, A.; Sehgal, A.; Bharti, P.K.; Bansal, D.; Mohapatra, P.K.; Mahanta, J.; Sultan, A.A. Exploration of Genetic Diversity of Plasmodium vivax Circumsporozoite Protein (Pvcsp) and Plasmodium vivax Sexual Stage Antigen (Pvs25) among North Indian Isolates. Malar. J. 2019, 18, 308. [Google Scholar] [CrossRef]
- Kibria, M.G.; Elahi, R.; Mohon, A.N.; Khan, W.A.; Haque, R.; Alam, M.S. Genetic Diversity of Plasmodium vivax in Clinical Isolates from Bangladesh. Malar. J. 2015, 14, 267. [Google Scholar] [CrossRef]
- Han, E.T.; Lee, W.J.; Sattabongkot, J.; Jang, J.W.; Nam, M.H.; An, S.S.A.; Suh, I.; Lim, C.S. Sequence Polymorphisms of Plasmodium vivax Ookinete Surface Proteins (Pvs25 and Pvs28) from Clinical Isolates in Korea. Trop. Med. Int. Health 2010, 15, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.; Kepple, D.; Williams, J.; Kolesar, G.; Ford, C.T.; Abebe, A.; Golassa, L.; Janies, D.A.; Yewhalaw, D.; Lo, E. Gene Polymorphisms Among Plasmodium vivax Geographical Isolates and the Potential as New Biomarkers for Gametocyte Detection. Front. Cell. Infect. Microbiol. 2022, 11, 789417. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Collins, W.E.; Wirtz, R.A.; Rathore, D.; Lal, A.; McCutchan, T.F. Geographic Subdivision of the Range of the Malaria Parasite Plasmodium vivax. Emerg. Infect. Dis. 2001, 7, 35–42. [Google Scholar] [CrossRef]
- Bond, J.G.; Rojas, J.C.; Arredondo–Jiménez, J.I.; Quiroz-Martínez, H.; Valle, J.; Williams, T. Population Control of the Malaria Vector Anopheles pseudopunctipennis by Habitat Manipulation. Proc. R. Soc. B Biol. Sci. 2004, 271, 2161–2169. [Google Scholar] [CrossRef]
- Carlton, J.M.; Adams, J.H.; Silva, J.C.; Bidwell, S.L.; Lorenzi, H.; Caler, E.; Crabtree, J.; Angiuoli, S.V.; Merino, E.F.; Amedeo, P.; et al. Comparative Genomics of the Neglected Human Malaria Parasite Plasmodium vivax. Nature 2008, 455, 757–763. [Google Scholar] [CrossRef]
- Villarreal-Treviño, C.; Arredondo-Jiménez, J.I.; Rodriguez, M.H. Bionomía de Los Principales Vectores Del Paludismo En México. In A cien Años del Descubrimiento de Ross. El Paludismo en México; Kumate, J., Martínez-Palomo, A., Eds.; El Colegio Nacional: Mexico City, Mexico, 1998; pp. 149–165. [Google Scholar]
- Villarreal-Treviño, C.; Penilla-Navarro, R.P.; Vázquez-Martínez, M.G.; Moo-Llanes, D.A.; Ríos-Delgado, J.C.; Fernández-Salas, I.; Rodríguez, A.D. Larval Habitat Characterization of Anopheles darlingi from Its Northernmost Geographical Distribution in Chiapas, Mexico. Malar. J. 2015, 14, 517. [Google Scholar] [CrossRef]
- Almeida-De-Oliveira, N.K.; de Abreu-Fernandes, R.; Lima-Cury, L.; de Lavigne, A.R.; de Pina-Costa, A.; de Souza Perce-Da-Silva, D.; Catanho, M.; Rossi, A.D.; Brasil, P.; Daniel-Ribeiro, C.T.; et al. Balancing Selection and High Genetic Diversity of Plasmodium vivax Circumsporozoite Central Region in Parasites from Brazilian Amazon and Rio de Janeiro Atlantic Forest. PLoS ONE 2020, 15, e0241426. [Google Scholar] [CrossRef]
- de Abreu, F.V.S.; dos Santos, E.; Lavigne Mello, A.R.; Gomes, L.R.; de Alvarenga, D.A.M.; Gomes, M.Q.; Vargas, W.P.; Bianco-Júnior, C.; de Pina-Costa, A.; Teixeira, D.S.; et al. Howler Monkeys Are the Reservoir of Malarial Parasites Causing Zoonotic Infections in the Atlantic Forest of Rio de Janeiro. PLoS Neglected Trop. Dis. 2019, 13, e0007906. [Google Scholar] [CrossRef]
- Santos, E.d.A.; Sucupira, I.M.C.; de Oliveira Martins, B.M.; de Paula Souza e Guimarães, R.J.; Catete, C.P.; de Souza, R.T.L.; dos Santos, A.C.F.; Póvoa, M.M. VK210 and VK247 Genotypes of Plasmodium vivax in Anopheline Mosquitoes from Brazilian Amazon. Sci. Rep. 2019, 9, 9391. [Google Scholar] [CrossRef]
- Ool, T.T.; Storch, V.; Becker, N. Review of the Anopheline Mosquitoes of Myanmar. J. Vector Ecol. 2004, 29, 21–40. [Google Scholar] [PubMed]
- Villafuerte Solís, D.; Del Carmen García Aguilar, M. Tres Ciclos Migratorios En Chiapas: Interno, Regional e Internacional. Available online: https://www-scielo.org.mx/pdf/myd/v12n22/v12n22a1.pdf (accessed on 25 August 2025).
- Hernández-Avila, J.E.; Rodríguez, M.H.; Betanzos-Reyes, A.F.; Danis-Lozano, R.; Méndez-Galván, J.F.; Velázquez-Monroy, O.J.; Tapia-Conyer, R. Determinant Factors for Malaria Transmission on the Coast of Oaxaca State, the Main Residual Transmission Focus in Mexico. Salud Publica Mex. 2006, 48, 405–417. [Google Scholar] [CrossRef]
- Danis-Lozano, R.; Rodriguez, M.H.; Betanzos-Reyes, A.F.; Hernandez-Avila, J.E.; Gonzalez-Ceron, L.; Mendez-Galvan, J.F.; Velazquez-Monroy, O.J.; Tapia-Conyer, R. Individual Risk Factors for Plasmodium vivax Infection in the Residual Malaria Transmission Focus of Oaxaca, Mexico. Salud Publica Mex. 2007, 49, 199–209. [Google Scholar] [CrossRef]
- Betanzos, A.F. La Malaria En México. Progresos y Desafíos Hacia Su Eliminación Challenges and Progress in the Elimination of Malaria in Mexico [Challenges and Progress in the Elimination of Malaria in Mexico]. Bol. Med. Hosp. Infant Mex. 2011, 68, 159–168. [Google Scholar]
- Santos-Luna, R.; Román-Pérez, S.; Reyes-Cabrera, G.; Sánchez-Arcos, M.d.R.; Correa-Morales, F.; Pérez-Solano, M.A. Web Geographic Information System: A Support Tool for the Study, Evaluation, and Monitoring of Foci of Malaria Transmission in Mexico. Int. J. Environ. Res. Public Health 2023, 20, 3282. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).