Abstract
Preterm birth remains a significant global health challenge and is strongly associated with heightened risks of long-term neurodevelopmental impairments, including cognitive delays, behavioural disorders, and emotional dysregulation. In recent years, accumulating evidence has underscored the critical role of the gut microbiota in early brain development through the gut–brain axis. In preterm infants, microbial colonisation is frequently delayed or disrupted due to caesarean delivery, perinatal antibiotic exposure, formula feeding, and prolonged stays in neonatal intensive care units (NICUs), all of which contribute to gut dysbiosis during critical periods of neurodevelopment. This review synthesises current knowledge on the sources, temporal patterns, and determinants of gut microbiota colonisation in preterm infants. This review focuses on the gut bacteriome and uses faecal-sample bacteriome sequencing as its primary method of characterisation. We detail five mechanistic pathways that link microbial disturbances to adverse neurodevelopmental outcomes: immune activation and white matter injury, short-chain fatty acids (SCFAs)-mediated neuroprotection, tryptophan–serotonin metabolic signalling, hypothalamic–pituitary–adrenal (HPA) axis modulation, and the integrity of intestinal and blood–brain barriers (BBB). We also critically examine emerging microbiota-targeted interventions—including probiotics, prebiotics, human milk oligosaccharides (HMOs), antibiotic stewardship strategies, skin-to-skin contact (SSC), and faecal microbiota transplantation (FMT)—focusing on their mechanisms of action, translational potential, and associated ethical concerns. Finally, we identify key research gaps, including the scarcity of longitudinal studies, limited functional modelling, and the absence of standardised protocols across clinical settings. A comprehensive understanding of microbial–neurodevelopmental interactions may provide a foundation for the development of targeted, timing-sensitive, and ethically sound interventions aimed at improving neurodevelopmental outcomes in this vulnerable population.
1. Introduction
Preterm birth, defined as delivery before 37 weeks of gestation, remains a major global health challenge. In 2020, ~9.9% of births worldwide were classified as preterm, a rate that has improved little over the past decade and even reached 10.6% in 2014 [1,2]. Although advances in neonatal intensive care units (NICUs) have increased survival, long-term neurodevelopmental outcomes remain suboptimal—particularly in cognition, behaviour, and mental health [3]. Compared with term-born peers, preterm infants have higher risks of autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder (ADHD), mood disturbances, and internalising symptoms, as well as motor and sensory dysfunctions (e.g., vestibular imbalance, altered pain perception, hearing loss, and features of cerebral palsy) [4,5,6]. Delays in language, cognition, sensory processing, and motor skills are frequent and often translate into poorer academic performance [7,8]. These adverse outcomes impose substantial psychological and economic burdens on families and pose persistent challenges to public health systems [9,10,11].
A growing body of research links preterm birth with altered neurodevelopment from infancy through adolescence, with potential long-term effects on brain structural connectivity [12,13]. The period between 20 and 40 gestational weeks is a critical “neurodevelopmental window” marked by rapid cortical expansion, massive neuronal migration, accelerated synaptogenesis, and the onset of myelination [14]: around 30 weeks, the subplate zone peaks in structural complexity and functional activity [15]. High-resolution foetal MRI indicates that significant white-matter tracts (e.g., cortico-thalamic pathways and corpus callosum) develop rapidly and non-linearly in utero, a trajectory complex to replicate ex utero [16]. Consequently, being born before the completion of microstructural remodelling and myelination is associated with persistent cortical immaturity, retention of subplate and early synaptic networks, and delayed myelination of primary fibres—neurobiological substrates for later deficits in sensation, cognition, and behaviour [14,17,18].
In parallel, late gestation and the early postnatal period are key windows for establishing gut microbiota, which shape brain development via the gut–brain axis. Disruptions in maternal microbial profiles may reprogram foetal neurodevelopment and affect postnatal behaviours [19,20,21,22]. Microbial-derived components—such as short-chain fatty acids (SCFAs), immune mediators (e.g., cytokines), enteroendocrine signals, and neuroendocrine circuits, including the vagus nerve and hypothalamic–pituitary–adrenal axis—act in concert to influence brain maturation and plasticity [23,24]. Animal studies show that these signals regulate gene expression, neurochemistry, and behaviours, including anxiety, memory, and motor function, and have been implicated in neuropsychiatric conditions such as depression, anxiety, and Parkinson’s disease [20].
Preterm delivery, by removing the foetus from the intrauterine environment before full maturity, simultaneously disrupts central nervous system development and early microbiota–brain interactions, potentially compounding neurodevelopmental vulnerability. Elucidating how variations in gut microbiota colonisation influence brain development through the gut–brain axis is therefore critical to understanding pathophysiology and designing targeted, evidence-based interventions for this high-risk population. Unless otherwise stated, “gut microbiota” refers to the bacteriome (typically profiled from stool as a practical proxy for intestinal communities); other microbiome domains—virome, archaea and fungi—are noted where relevant but are outside the scope of this review.
This narrative review searched PubMed/MEDLINE, Embase, the Web of Science Core Collection, and the Cochrane Library; ClinicalTrials.gov was screened for ongoing or recent trials. The strategy combined keywords and controlled vocabulary (MeSH/Emtree): (preterm/premature/very-low-birth-weight [VLBW]) and (gut microbiota/microbiome/bacteriome) and (neurodevelopment/white matter injury [WMI]/periventricular leukomalacia [PVL]/ASD/ADHD/emotional disorders), plus mechanistic/intervention terms SCFA signalling; tryptophan–serotonin/kynurenine; vagus hypothalamic–pituitary–adrenal (HPA) axis; barrier integrity; probiotics; prebiotics; human milk oligosaccharides (HMOs); skin-to-skin contact (SSC); antibiotic stewardship; faecal microbiota transplantation (FMT). Inclusion criteria were English, peer-reviewed studies involving preterm/very preterm/very-low-birth-weight (VLBW) populations or neonatal/animal work directly relevant to preterm neurodevelopment, reporting microbiota–neurodevelopment associations, objective outcomes (e.g., MRI, Bayley scales), or intervention effects. Exclusions were editorials, abstract-only records, single case reports without mechanistic value, and studies lacking relevant outcomes. Evidence was synthesised thematically; no formal risk-of-bias assessment or meta-analysis was undertaken. For orientation, we provide a cross-phenotype framework in Table 1 (Section 4), a study-level evidence summary in Table 2 (at the end of Section 4), and intervention details in Table 3 (Section 5).

Table 1.
Microbiome features and functional readouts across neurodevelopmental phenotypes.

Table 2.
Summary of human studies on gut microbiota and neurodevelopment in preterm cohorts: design, population, exposures/interventions, microbiome methods, outcomes, and level of evidence.

Table 3.
Microbiota-modulating strategies in the NICUs: mechanisms, timing, evidence, and safety and regulatory considerations.
2. Patterns and Determinants of Gut Microbiota Colonisation in Preterm Infants
2.1. Intrauterine Microbial Transmission: Sources and Controversies
2.1.1. Microbial Evidence in Placenta, Amniotic Fluid, and Cord Blood
Recent studies have challenged the long-standing “sterile womb” paradigm by detecting low-abundance, low-diversity microbial communities in term placentas, amniotic fluid, and cord blood. Aagaard et al. first used shotgun metagenomic sequencing to identify low-biomass microbiota dominated by Proteobacteria and Firmicutes in 320 healthy placentas, with phylogenetic similarity to maternal oral microbiota, suggesting haematogenous translocation [44]. Similarly, Fardini et al. detected DNA from oral commensals such as Fusobacterium and Streptococcus in the basal plate of placental villi, supporting a “blood–placenta” transmission route [45].
In amniotic fluid, DiGiulio et al. employed both molecular and culture-based techniques to identify multiple bacterial species in preterm pregnancies, with microbial diversity correlating with intrauterine inflammation [46]. Collado et al. further detected Proteobacteria and Firmicutes signals in amniotic fluid and cord blood of term pregnancies [47]. Other studies reported anaerobic bacterial DNA, such as Prevotella and Methylobacterium, in human cord blood, implying potential transplacental passage into the foetal circulation [48].
Overall, low-biomass signals detected in placenta, amniotic fluid, and cord blood suggest episodic microbial DNA presence with phylogenetic ties to maternal oral sources, consistent with possible hematogenous transfer. However, the low abundance and contamination susceptibility of these samples warrant cautious interpretation.
2.1.2. Meconium Microbiota and Maternal Influences
Meconium offers a valuable window into potential prenatal microbial exposure. Jiménez et al. successfully isolated cultivable strains of Lactobacillus, Staphylococcus, and Enterobacteriaceae from the meconium of 21 healthy term neonates, and confirmed their community structure through molecular methods [49]. In preterm infants, Mshvildadze et al. detected microbial DNA in 91% of meconium samples from 22 to 32 weeks’ gestational age (GA) using non-culture-based methods, with diversity positively correlated with gestational age [50]. Hu et al. reported that maternal diabetes significantly altered meconium microbiota, with increased Proteobacteria and decreased Bacteroidetes [51], highlighting gestational age and maternal metabolic status as key determinants of meconium colonisation.
Collectively, meconium findings indicate that putative prenatal and very-early postnatal microbial exposures are detectable and shaped by gestational age and maternal metabolic status. Yet the timing and persistence of these signals relative to actual foetal colonisation remain unresolved.
2.1.3. Hypothesised Mechanisms of Maternal–Foetal Microbial Transmission
Multiple mechanisms have been postulated to explain maternal–foetal microbial transmission. In a murine model, Jiménez et al. administered GFP-tagged Enterococcus faecium to pregnant mice and later recovered genetically identical strains from the amniotic fluid and meconium of pups delivered by caesarean section, supporting a haematogenous route of transfer [52]. Alternative hypotheses propose that maternal phagocytic cells—such as macrophages and dendritic cells—may engulf gut bacteria and migrate across the placental barrier. Additionally, periodontal pathogens, including Fusobacterium nucleatum and Porphyromonas gingivalis, commonly associated with oral infections, have been observed to enter the maternal circulation and potentially access the intra-amniotic environment [53]. Nevertheless, these proposed pathways require further verification through direct microbial tracing studies.
In sum, multiple routes—hematogenous spread, trafficking of phagocytic cells, and translocation of oral pathogens—are biologically plausible and supported by animal tracing. Direct, unequivocal demonstration in humans, however, is still lacking.
2.1.4. Controversies Surrounding in Utero Colonisation
Despite accumulating supportive evidence, the in utero colonisation hypothesis remains controversial. Lauder et al. found no significant differences between placental samples and laboratory contaminants under stringent negative controls, questioning the biological relevance of prior findings [54]. Dos Santos and Kennedy et al. similarly failed to detect specific microbial lineages in meconium compared to control samples, opposing the notion of intrauterine colonisation [55,56]. These findings underscore the importance of rigorous contamination control and suggest that current evidence remains inconclusive.
Taken together, current evidence from low-biomass samples is inconsistent and sensitive to the effectiveness of contamination controls. While in utero microbial exposure remains plausible, robust proof of persistent foetal colonisation is lacking.
2.1.5. Bacterial Extracellular Vesicles (bEVs) and Prenatal Exposure
Accumulating evidence indicates that bacteria release nanoscale extracellular vesicles carrying lipids, proteins, and nucleic acids that can traverse epithelial and even the blood–brain barrier (BBB), thereby triggering and modulating host immune responses in the absence of culturable live bacteria [57,58]. Several studies further propose that maternally derived bEVs can enter the circulation via the oral or intestinal mucosa and reach the intrauterine environment, enter amniotic fluid, and “precondition” foetal immunity, thereby shaping neonatal gut colonisation and early immunoprogramming at birth [58]. In parallel, bEVs contribute to gut homeostasis through multiple mechanisms—including reinforcement of epithelial barrier function and orchestration of mucosal immunity—supporting a conceptual framework that emphasises “functional signal transmission” rather than “true colonisation” [59,60].
Collectively, bEVs constitute a biologically plausible conduit for prenatal microbial signalling in the absence of durable foetal colonisation. Yet current human evidence is inferential; causal claims will require harmonised isolation methods with rigorous contamination controls and longitudinal linkage of vesicle cargo to functional neonatal endpoints.
2.2. Temporal Succession of Gut Microbiota
Due to immature gut barrier, immune, and nervous systems—and frequent exposure to perinatal antibiotics—preterm infants display delayed and altered microbial colonisation patterns compared to term infants. During the first postnatal week, their faecal microbiota is dominated by low-diversity facultative anaerobes such as Enterobacteriaceae, Enterococcus, and Staphylococcus, while beneficial obligate anaerobes like Bifidobacterium and Bacteroides are markedly underrepresented [61,62,63].
With increasing postmenstrual age (PMA), the gut enters a “secondary succession” phase. Facultative anaerobes are gradually replaced by Bifidobacteria and Clostridia, with diversity increasing significantly by around 30 weeks PMA. Nonetheless, microbial maturity remains lagging behind that of term infants [64]. By 3–6 months post-weaning, most preterm infants partially achieve an adult-like microbiota composition, but overall community stability remains reduced [61,65]. In most cohorts, obligate anaerobes increase only after ~30 weeks PMA, and overall community stability often trails term infants by 2–3 months post-discharge [66].
Caesarean delivery and NICU-related exposures further delay the progression of gut microbial succession. Preterm infants delivered by caesarean section show reduced microbial diversity over the first six months of life, an elevated Proteobacteria-to-Firmicutes ratio, and persistently low abundances of Bifidobacterium and Bacteroides. NICUs’ colonisation is often dominated by opportunistic pathogens such as Enterobacteriaceae and Staphylococcus, with microbiota diversity showing substantial recovery only after six months [67,68].
Functionally, preterm stool samples exhibit reduced gene abundance for SCFA synthesis and enrichment in pathways related to xenobiotic metabolism, lipid degradation, and vitamin biosynthesis, suggesting immature microbial functionality. These deficits may compromise mucosal immunity and energy regulation, increasing the risk of necrotising enterocolitis (NEC), sepsis, and long-term neuroimmune-metabolic disturbances [63,64].
2.3. Key Influencing Factors
2.3.1. Impact of Delivery Mode
The mode of delivery significantly affects the initial microbial colonisation of the gut and subsequent immune and metabolic development. During vaginal birth, the infant’s stomach is seeded with maternal vaginal and intestinal microbes, including Lactobacillus, Bacteroides, Prevotella, and Sneathia. These bacteria deplete oxygen to create an anaerobic environment conducive to Bifidobacterium proliferation and promote gut–immune axis maturation through metabolites such as SCFAs [69]. In contrast, caesarean delivery bypasses maternal birth canal exposure. Instead, the infant acquires microbes from the skin and hospital environment, including Staphylococcus, Corynebacterium, Cutibacterium acnes (C. acnes), and Enterococcus, resulting in an approximately 30% reduction in key maternal-derived gut taxa such as Bifidobacterium and Bacteroides [70,71].
This “birth mode effect” contributes to persistent dysbiosis, with reduced diversity, skewed Firmicutes/Proteobacteria ratios, and higher risks of immune and metabolic disorders. C-section infants are more prone to asthma and allergic diseases, associated with diminished early microbial diversity and increased colonisation by skin-derived Staphylococcus aureus [72]. Longitudinal studies have also linked caesarean delivery to increased risk of childhood overweight and obesity, possibly due to insufficient Bifidobacteria and early microbial dysregulation of host energy metabolism [70].
2.3.2. NICUs Environment and Antibiotic Exposure
The NICUs environment and empirical use of broad-spectrum antibiotics profoundly shape early microbiota development in preterm infants. NICUs often harbour nosocomial organisms such as Klebsiella and Enterococcus, which readily colonise the immature gut [73]. Broad-spectrum antibiotics further reduce microbial diversity, with overgrowth of Proteobacteria taxa including Klebsiella and Enterobacteriaceae, and depletion of Bifidobacteria and Bacteroides [43,67].
This dual impact of environmental exposure and antibiotic pressure disrupts microbial succession, delaying the establishment of a healthy, diverse community. It also correlates with increased risks of NEC and late-onset sepsis (LOS) [43,74]. Functionally, antibiotic-driven dysbiosis reduces SCFA production, impairs mucosal barrier integrity, and promotes the enrichment of antibiotic-resistant strains, thereby further increasing susceptibility to infection and inflammation [75,76]. While microbial diversity and beneficial taxa may partially recover within weeks of antibiotic cessation, substantial structural differences persist until 3–6 months of age [77,78].
Thus, optimising antibiotic stewardship in the NICUs and integrating targeted microbial interventions may help prevent severe complications and support the healthy development of the neuroimmune axis in preterm infants.
2.3.3. Feeding Mode
Microbiota Benefits and Functional Roles of Human Milk
Breastfeeding plays a pivotal role in promoting the colonisation of beneficial gut bacteria in preterm infants, particularly strains of Bifidobacterium and Lactobacillus. This advantage is primarily attributed to the abundance of HMOs, which cannot be digested by the infant but serve as fermentable substrates for these probiotics [79]. Upon fermentation, these microbes generate SCFAs, such as acetate and propionate, which contribute to strengthening intestinal barrier function and promoting the differentiation of regulatory immune cells. These processes help suppress inflammation and reduce the likelihood of allergic diseases [80]. Furthermore, human milk contains secretory IgA, maternal immune components, and live bacteria that aid in mucosal colonisation and foster local immune tolerance. Collectively, these bioactive constituents facilitate the coordinated development of gut–brain axis signalling and neuroimmune regulation in early life [81].
Microbial Characteristics and Health Risks of Formula Feeding
Preterm infants fed with formula typically exhibit higher gut microbial diversity compared to exclusively breastfed infants; however, their dominant taxa tend to shift toward Firmicutes and Proteobacteria, including Staphylococcus, Klebsiella, and Enterobacteriaceae. In contrast, beneficial genera such as Bifidobacterium and Lactobacillus are markedly reduced [82]. This “high-diversity but low-beneficial” profile may result in decreased SCFA production, impaired gut barrier function, and suboptimal immune modulation, contributing to an increased risk of allergic and metabolic diseases [83].
Transitional Features and Interventional Potential of Mixed Feeding
Mixed-fed infants, who receive both human milk and formula, display a transitional microbial pattern. The abundance of Bifidobacteria and Lactobacillus in their gut microbiota typically falls between that of exclusively breastfed and formula-fed groups. Correspondingly, SCFA levels and immune biomarkers also show intermediate profiles [81]. This “transitional community” offers a potential window for targeted microbiota-based interventions, such as HMO supplementation or probiotic administration, especially in the NICUs setting, to optimise the coordinated development of the gut and immune systems in preterm infants.
2.3.4. Maternal and Host-Related Factors
Maternal Diet and Metabolic Status During Pregnancy
Maternal body mass index (BMI), gestational weight gain, and dietary patterns can reshape the maternal microbiome and influence vertical transmission to the foetus, thereby significantly affecting the infant’s gut colonisation. Infants born to overweight or obese mothers exhibit increased levels of Bacteroides and Staphylococcus, and decreased levels of Bifidobacterium and Akkermansia, mirroring alterations in maternal microbiota [81,84]. A high-fat, low-fibre diet during pregnancy enriches proinflammatory taxa such as Collinsella and Sutterella, correlating with maternal metabolic profiles. Animal models further suggest that such diets can induce long-term expansion of Clostridiales and systemic inflammation in offspring [85,86,87].
Preterm infants born to mothers with gestational diabetes exhibit reduced gut α-diversity and a disrupted Firmicutes/Proteobacteria ratio [88].
Host Genotype and Microbial Regulation
Host genetic background indirectly shapes early gut microbiota by modulating immune responses, mucosal barrier integrity, and metabolic functions [89,90]. Comparative studies have found higher relative abundances of Prevotella and Bifidobacteria in the faeces of African rural children, while European urban children are enriched in Bacteroides [91,92]. Similar differences are observed between children living in urban slums in Bangladesh and suburban areas in the United States [93]. The INFABIO multicenter study highlighted that even after the introduction of complementary foods, gut microbiota assembly remains influenced by ancestral diet and genetic background [94]. In a Danish cohort, children with siblings had significantly higher microbial diversity, suggesting a role for familial genetic and microbial transmission in shaping microbiota maturation [75]. Studies in term infants also indicate that individual variability in community structure may be partially explained by TLR and MHC gene polymorphisms, suggesting a similar regulatory role in preterm infants. However, this hypothesis requires further validation in preterm-specific cohorts [95].
Family Structure and Geographic Environment
Family structure significantly influences gut microbiota development via shared microbial exposures. The “sibling effect” has been shown to accelerate gut microbial maturation: infants with siblings exhibit higher Bifidobacterium abundance and overall microbial diversity compared to only children, while those without siblings are more likely to harbour Clostridium and facultative anaerobes, with a reduced anaerobe/facultative anaerobe ratio [71,94]. Geographic factors also play a pivotal role in shaping initial colonisation. Children living in sub-Saharan African rural areas exhibit higher relative abundances of Prevotella and Bifidobacterium, whereas Bacteroides dominates European urban children. Similarly, Bangladeshi urban slum children show markedly different Prevotella/Bacteroides ratios compared to their U.S. suburban counterparts [91,92,96]. The INFABIO study further confirmed that both geographic location and genetic background continue to influence microbiota trajectories after weaning [97]. For preterm infants, exposure to different microenvironments due to hospital transfers or NICUs admissions across regions may result in distinct colonisation patterns, warranting systematic investigation.
In summary, the colonisation of gut microbiota in preterm infants is influenced by multiple interrelated factors, including mode of delivery, NICUs environment and antibiotic exposure, feeding practices, maternal metabolic status and dietary patterns, host genetic background, and geographical and family atmosphere. These factors interact across prenatal and postnatal stages, collectively shaping the early establishment and developmental trajectory of the gut microbial ecosystem, as illustrated in Figure 1.
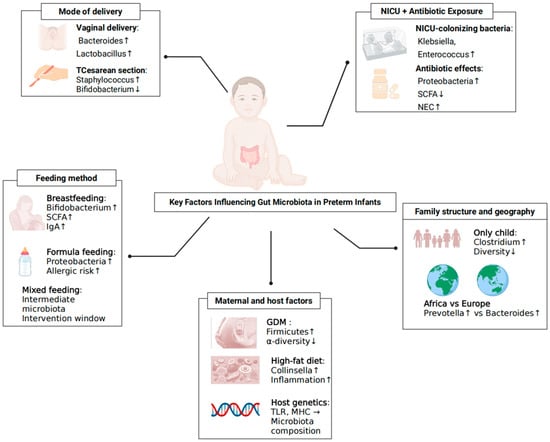
Figure 1.
Key Factors Shaping Gut Bacteriome Colonisation in Preterm Infants. Determinants span delivery mode (vaginal vs. caesarean), NICUs environment and antibiotic exposure, feeding practices (human milk, mixed, formula), maternal factors (diet, BMI, gestational diabetes), host genotype/family structure and geography. Arrows indicate the typical direction of effects on early community succession; ↑ indicates an increase (higher abundance/risk); ↓ indicates a decrease (lower abundance/risk); → indicates a directional relationship.
3. Key Mechanistic Pathways of the Gut–Brain Axis in Neurodevelopment
3.1. Immune Pathways and White Matter Injury (WMI)
Dysbiosis in preterm infants can activate TLR-dependent proinflammatory cascades (notably TLR4–MyD88–NF-κB), elevating IL-1β/IL-6/TNF-α, disrupting the BBB, priming microglia, and injuring pre-oligodendrocytes—thereby impairing myelination and exacerbating WMI [98,99]. Germ-free models demonstrate that the absence of microbiota skews microglial maturation toward inflammatory states [100], while TNF-α can drive astrocyte-mediated pre-oligodendrocyte (pre-OL) apoptosis and demyelination [101]. Dysbiosis-biassed M1 microglial polarisation and early microbiota–immune axis disruption have been linked to neuroinflammation, circuit instability, and periventricular leukomalacia (PVL) in the preterm brain [102,103].
3.2. SCFA Pathways in Cognitive and Behavioural Regulation
SCFAs (acetate, propionate, butyrate) are key microbiota-derived signals that inhibit HDACs, promote oligodendrocyte differentiation/myelination, preserve BBB integrity, and enhance neurotrophins (e.g., BDNF), collectively mitigating preterm-related brain injury [104,105,106]. In vivo, butyrate augments neurogenesis and BDNF–TrkB signalling, improving cognition after hypoxic injury [106,107]. Germ-free and antibiotic-perturbation models demonstrate that microbiota and their metabolites facilitate microglial maturation [100,107,108], and microbial metabolites modulate astrocytic AhR to mitigate neuroinflammation [109]. Behaviourally, SCFAs can reverse stress-induced prefrontal and affective alterations, and probiotics/SCFAs ameliorate social and emotional phenotypes; SCFA-driven Treg induction further supports neuroimmune homeostasis [110,111,112].
3.3. Tryptophan–5-HT Pathways and Emotional Regulation
The microbiota shapes tryptophan fate along three routes with distinct neural consequences: serotonin synthesis via TPH (mood/emotion), kynurenine via IDO (neurotoxicity), and indole derivatives that engage AhR to regulate glial activity and neuroinflammation [113]. In demyelinating models, microbial indole metabolites activate astrocytic/microglial AhR, suppress NF-κB/STAT1, lower IL-6, and lessen demyelination, underscoring a microbiota–glia–emotion axis relevant to preterm neurodevelopment [114].
3.4. HPA Axis and Vagal Pathways in Learning and Memory
Early colonisation calibrates stress circuitry: germ-free mice exhibit exaggerated HPA responses that normalise with conventional/probiotic colonisation [21,115]. Microbial signals (metabolites, cytokines, neurotransmitters) engage hypothalamic CRH neurons to drive ACTH release and maintain gut–brain homeostasis [116]. Early-life stress perturbs serotonergic tone and 5-HT receptor expression across multiple regions [117,118]. Vagal afferents convey gut-derived 5-HT, GLP-1, and SCFAs to limbic and hypothalamic circuits, modulating cognition, metabolism, and memory; human functional magnetic resonance imaging (fMRI) demonstrates vagally mediated hippocampal responses to gastric/nutrient cues, while vagus-nerve stimulation enhances hippocampal plasticity and memory via BDNF [119,120,121,122,123,124].
3.5. Barrier Dysfunction and Neuroinflammation
Immature intestinal and neural barriers in preterm infants heighten permeability. Commensals upregulate epithelial tight-junction proteins to limit systemic translocation [125,126]. Microbiota and SCFAs likewise fortify the BBB by increasing junctional components [105], and neonatal probiotics (e.g., Bifidobacterium infantis) reduce IL-6/TNF-α and attenuate brain injury [22]. Conversely, barrier failure permits LPS entry, activating microglial TLR4 and propagating neuroinflammation [100].
4. Associations Between Gut Microbiota and Common Neurodevelopmental Disorders in Preterm Infants
As an overview, Table 1 summarises directional taxa, functional readouts, biological plausibility, and key confounders across phenotypes (Section 4.1, Section 4.2, Section 4.3 and Section 4.4).
4.1. WMI
WMI is one of the most prevalent forms of brain injury in preterm infants. It is widely recognised as a leading cause of cerebral palsy and neurocognitive impairment [127]. Based on MRI findings, WMI can be categorised into haemorrhagic infarction, PVL, diffuse WMI, and punctate white matter lesions (PWML) [6,127,128]. Among these, PVL is the most representative pathology, occurring in approximately 5% of extremely low birth weight (ELBW)infants and often accompanied by motor, cognitive, and visual impairments [129,130].
Recent studies suggest that, in addition to hypoxia–ischemia, the initiation of inflammatory and immune signalling cascades is a major contributor to the development of white matter injury (WMI) [129,131]. Increasing evidence indicates that gut microbial dysbiosis may influence WMI pathophysiology by modulating systemic inflammation, immune activation, and neuroimmune pathways through microbial-derived metabolites [132,133]. Specifically, decreased abundance of Bifidobacteria alongside overgrowth of Clostridium and Enterobacteriaceae members has been linked to a heightened susceptibility to WMI among premature neonates [134].
Microbial metabolites such as SCFAs are key regulators of microglial development and immune homeostasis. Erny et al. found that microglial development was impaired in germ-free mice and could be partially restored with SCFA supplementation, highlighting their role in central immune regulation [100]. Furthermore, the LPS–TLR4 signalling pathway is implicated in WMI: elevated TLR4 expression and expansion of LPS-producing bacteria (e.g., Enterobacteriaceae) in preterm infants may lead to pre-oligodendrocyte apoptosis via microglial activation [98]. Conversely, Lactobacillus and Bifidobacterium species may attenuate inflammation through enhanced SCFA production and modulation of TLR signalling, thereby reducing WMI risk.
Clinically, preterm infants with Grade III–IV intraventricular haemorrhage (IVH) are at greater risk for cystic PVL and severe motor impairments [135]. Even infants with Grade I PVL may experience persistent cognitive and emotional difficulties, suggesting long-term cortical connectivity alterations associated with early WMI [130,136].
4.2. ASD
Preterm birth significantly increases the risk of neurodevelopmental disorders, particularly ASD, characterised by social deficits, repetitive behaviours, and sensory abnormalities. Meta-analyses confirm that the risk of ASD is higher in preterm infants and correlates with lower gestational age, birth weight, and perinatal complications such as PVL [4,137,138].
Gastrointestinal dysfunction is highly prevalent in children with ASD (23–70%), including symptoms such as constipation, diarrhoea, and bloating, suggesting a role for gut microbiota in the pathophysiology of ASD [139,140,141]. Numerous studies have reported distinct microbial signatures in ASD children compared to typically developing (TD) peers, with reductions in beneficial genera (Bifidobacterium, Prevotella) and increases in potential pathobionts (Clostridium, Desulfovibrio, Ruminococcus) [142,143,144]. Notably, Clostridia produce neurotoxins and high levels of SCFAs like propionate, which are implicated in repetitive behaviours and social impairment [145,146].
A study comparing preterm children with and without ASD found higher α-diversity in the ASD group, suggesting a more heterogeneous but potentially pathogenic microbiota [28]. LEfSe analysis revealed increased abundance of Firmicutes, Clostridiales, Ruminococcus gnavus, and Bifidobacterium longum, alongside a depletion of anti-inflammatory species such as Mitsuokella and Sutterella wadsworthensis, which may underlie both gastrointestinal symptoms and social dysfunction [29].
At the metabolic level, faecal concentrations of propionate, phenylpropionate, and p-cresol are elevated in ASD children with ASD and correlate positively with behavioural and emotional severity [30,146]. In high-risk infants (e.g., siblings of ASD patients), reductions in Bifidobacteria, increases in Clostridium, and decreased GABA levels at 5 months predict poorer expressive language development [31].
Perinatal factors influencing microbial colonisation—including caesarean section, lack of breastfeeding, and antibiotic exposure—are associated with reduced Bifidobacterium colonisation and increased ASD risk [37,38]. Preliminary studies suggest that FMT and dietary interventions may alleviate core ASD symptoms and gastrointestinal issues by modulating gut microbiota composition [147,148].
4.3. ADHD
Associations in ADHD and emotional disorders are based on small, heterogeneous cohorts and should be considered hypothesis-generating [149]. ADHD, affecting approximately 5.3–7.2% of children globally, is characterised by inattention, impulsivity, and hyperactivity, often accompanied by language delays, social difficulties, and psychiatric comorbidities [150,151,152]. While its aetiology is multifactorial, disruptions in gut–brain axis function have been increasingly implicated [153].
Recent studies demonstrate that the gut microbiota composition in children with ADHD differs from that of neurotypical peers. A Dutch study found increased levels of Bifidobacterium, with associated genes involved in tyrosine metabolism, a precursor of dopamine [32]. In contrast, studies from China and Taiwan reported reduced abundance of beneficial genera such as Faecalibacterium, Sutterella, and Dialister [33,34,35], which may impair SCFA synthesis and neurotransmitter regulation.
SCFAs—particularly propionate, acetate, and butyrate—not only maintain gut barrier integrity but also modulate central serotonin (5-HT) and dopamine levels [154]. Lower SCFA levels have been observed in children with ADHD, with reductions in propionate and acetate correlating negatively with symptom severity [36].
Microbial diversity and stability during infancy are critical for cognitive development. A prospective study found that higher α-diversity at age one was associated with lower early learning scores at age 2, along with altered grey matter volume in the precentral gyrus and amygdala [26]. Deficits in expressive language commonly seen in ADHD may reflect early microbial disruption of GABA and 5-HT pathways [155].
Animal models support a causal role: Tengeler et al. demonstrated that FMT from ADHD patients into germ-free mice induced hyperactivity and anxiety-like behaviours, alongside altered tryptophan–serotonin metabolism [156]. Taxonomic analyses revealed increased Bacteroides uniformis and decreased Faecalibacterium prausnitzii in ADHD children, affecting SCFA production, inflammation, and neural signalling [34,157]. Additionally, lower plasma TNF-α levels and reduced microbial diversity in ADHD children suggest an immune–microbiota interaction in pathogenesis [158].
4.4. Emotional Disorders
Findings relating gut microbiota to emotional disorders in those born preterm are likewise preliminary; current associations stem from small, heterogeneous cohorts and should be considered hypothesis-generating.
Emotional disorders—primarily anxiety and depression—are prevalent among adolescents and adults born preterm. Epidemiological data indicate that extremely preterm birth (<28 weeks) and extremely low birth weight (<1000 g) are strong risk factors for later emotional problems [159]. In recent years, gut microbiota has emerged as a critical environmental factor influencing emotional development in this population.
In general paediatric populations, several studies have linked gut microbiota composition with emotional traits. For example, Jiang et al. reported decreased diversity and lower abundance of beneficial microbes in adults with depression [160]. Aatsinki et al. found that early microbiota features in 301 infants were associated with irritability, avoidance, and fussiness at 8 months of age [25]. Carlson et al. showed that infant microbial diversity and stability could predict social and emotional behaviour at age 2 [26]. In preterm infants, He et al. reported that dysbiosis during the first 6 months—characterised by reduced Bifidobacteria—was associated with maternal reports of anxiety and depressive symptoms at age 2 [27].
Animal studies provide further support. Zheng et al. demonstrated that faecal transplantation from depressed patients induced social withdrawal and depressive-like behaviours in germ-free mice [161]. Similarly, Kelly et al. found that microbiota from chronically stressed rats transferred depressive phenotypes to recipient animals [162].
Preterm infants often experience delayed colonisation and reduced microbial diversity due to antibiotic exposure, limited breastfeeding, and prolonged NICUs stays [163]. These colonisation patterns have been linked to elevated risk of later emotional disorders, warranting further investigation and targeted early interventions.
In summary, immune modulation, SCFA signalling, tryptophan–serotonin/kynurenine metabolism, HPA–vagal regulation and barrier integrity are the principal routes by which the gut–brain axis shapes preterm neurodevelopment; these interconnected pathways are illustrated in Figure 2.
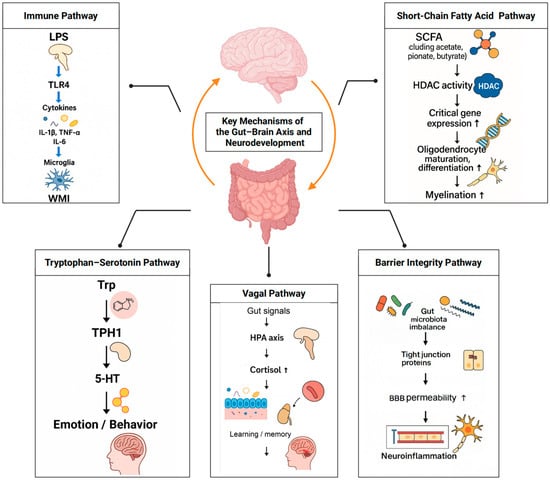
Figure 2.
Core gut–brain axis pathways relevant to preterm neurodevelopment. Five interlinked pathways link the gut bacteriome to brain outcomes: (1) immune activation and microglial maturation; (2) SCFA-mediated myelination and BBB support; (3) tryptophan–serotonin/kynurenine-AhR–AhR signalling; (4) HPA axis and vagal pathways; (5) barrier integrity of gut and BBB. Representative mediators. Arrows indicate the direction of effect/flow within each pathway; ↑ denotes an increase.
5. Microbiota-Modulating Strategies and Clinical Interventions in Preterm Infants
5.1. Probiotics, Prebiotics, and Human Milk Oligosaccharides: Clinical Evidence and Potential
5.1.1. Common Strains and Functional Components
Probiotics are viable microbes that confer health benefits when administered in adequate amounts [164]. In preterm infants, selected strains support colonisation and intestinal stability. They may offer neuroprotection through immune modulation, inflammation reduction, and effects on neurotransmitter pathways [165]—strains such as Lactobacillus rhamnosus, L. plantarum, Bifidobacterium longum subsp. Infantis (B. infantis), B. breve, and L. reuteri have demonstrated the capacity to reduce anxiety-related and depression-like symptoms in preclinical models [166,167].
Prebiotics—indigestible substrates that selectively stimulate beneficial taxa—include fructooligosaccharides (FOS) and galactooligosaccharides (GOS), which promote the growth of Bifidobacterium and modulate the activity of T cells, neutrophils, and dendritic cells [168]. HMOs, abundant in human milk, are fucosylated, non-fucosylated, or sialylated oligosaccharides [169] that act as natural prebiotics, lowering intestinal pH and increasing SCFAs [170,171]. HMO-adapted strains such as B. infantis are associated with more favourable white-matter development in preterm neonates [172,173].
Implementation notes. Candidate infants: very preterm or VLBW infants after establishment of minimal enteral feeds. Timing and duration: initiate early and continue until approximately 34–36 weeks’ PMA or until discharge. Dose and product quality: use hospital-grade preparations that provide ≥ 109 CFU/day; strain-level labelling, batch traceability, and controlled storage are required [174]. Monitoring: record feeding intolerance, sepsis evaluations, and concomitant antibiotic exposures in a unit registry.
5.1.2. Clinical Evidence and Target Populations
RCTs in term infants suggest cognitive and motor benefits with probiotics or HMO-enriched formulas [41,175], whereas evidence in preterm populations remains limited and heterogeneous [42]. Innovative approaches (e.g., maternal vaginal microbiota transfer, FMT) are being explored (SECFLOR, PREFLORE) [40,176]. The efficacy of prebiotics for neurodevelopment is uncertain and requires larger trials and mechanistic validation [98,177].
Translational perspective. Pragmatic use should be tailored to the feeding context (exclusive human milk versus fortified or formula feeding), individual variability (e.g., secretor status for human milk oligosaccharides), and institutional product quality assurance. Units should prespecify primary endpoints—NEC and LOS—and secondary neurodevelopmental outcomes (e.g., the Bayley Scales of Infant and Toddler Development) [178]. Key strains/doses and practical considerations are summarised in Table 3.
5.2. Antibiotic Stewardship and Skin-to-Skin Care in NICUs Settings
5.2.1. Optimising Antibiotic Use and Preserving the Microbiota
Antibiotic exposure in NICUs is associated with reduced microbial diversity, delayed colonisation of beneficial taxa, expansion of resistant genes and opportunistic pathogens, and compromised intestinal and immune barriers, thereby increasing risks such as NEC [179,180]. Studies have shown that antibiotic-treated infants exhibit higher Klebsiella abundance and elevated expression of antibiotic resistance genes [181,182]. Notably, the occurrence of dysbiosis appears more closely linked to the presence of antibiotic exposure rather than its duration, underscoring the sensitivity of the microbiota to antimicrobial perturbations. Animal studies have further suggested that antibiotics may impair cognition by altering hippocampal neurogenesis and BDNF expression [183,184]. Therefore, narrow-spectrum antibiotics and shortened treatment durations are critical strategies. Recent RCTs highlight that rapid strain-level and resistome analysis can guide precise antibiotic selection while minimising microbiota disruption [182,185].
Implementation considerations. Initiate narrow-spectrum antibiotics only when clinically indicated; reassess at 48–72 h using culture and rapid resistome results to de-escalate or discontinue therapy; avoid prolonged empiric courses; and integrate stewardship checkpoints into feeding-advancement protocols to preserve early gut colonisation [186]. Stewardship checkpoints and typical de-escalation timelines are outlined in Table 3.
5.2.2. Clinical and Long-Term Benefits of SSC
SSC, including kangaroo care, improves early gut colonisation and neurodevelopment by supporting gut barrier maturation and maternal microbial transfer; it modulates HPA axis activity, improves sleep architecture, and promotes prefrontal maturation [63,187], with associations to better neurobehavioural outcomes up to 10 years [39]. SSC also promotes breastfeeding and strengthens bonding, supporting neurodevelopment through microbiota-immune pathways and reducing maternal anxiety and depression [188,189,190].
Implementation considerations for SSC dosing: initiate as soon as the infant is clinically stable; set a daily cumulative-duration target and document minutes/hours as a reportable dose in the medical record; and embed SSC as a standard, recorded component of daily NICUs care with routine auditing [191,192]. Suggested SSC “dose” tracking and implementation tips appear in Table 3.
5.3. FMT: Frontiers and Ethical Considerations
5.3.1. Emerging Applications and Target Populations
FMT has been shown to modulate neurodevelopment by enhancing SCFA production, maintaining microglial homeostasis, and reducing proinflammatory cytokine expression [100,193]. In germ-free mice colonised with stool from preterm infants, microbiota from donors of higher gestational age improved associative learning in adulthood [194]. Infant microbiota enriched in Bifidobacteria and Bacteroides is associated with more favourable cognitive phenotypes [195,196]. In ASD models, FMT improved behavioural outcomes and enhanced social interaction [147,197].
5.3.2. Safety, Consent, and Ethical Implementation
For pregnant women and preterm neonates—vulnerable populations—microbiome-based interventions (probiotics, vaginal seeding, faecal microbiota transplantation, FMT) should occur under heightened protection thresholds and standardised oversight to reduce adverse events and procedural heterogeneity [198,199,200]. Ethical safeguards include prospective registration with transparent protocols to support reproducibility [201,202]; stratified eligibility by gestational age, delivery mode, and feeding type to reduce confounding [199,203,204]; predefined primary and safety endpoints with adverse-event reporting and long-term follow-up for rigorous evaluation [205,206]; establishment of standardised donor banks with resistome surveillance and end-to-end traceability to mitigate risks of pathogen transmission and antimicrobial resistance [204,205]; and multidisciplinary ethics review to ensure that regulation and clinical translation are grounded in the best available evidence [202]. Additional governance components include risk–benefit assessment with pre-specified stopping rules and independent data and safety monitoring board (DSMB) oversight, deferred consent with prompt countersignature in emergent NICUs contexts, stringent donor governance (pathogen/AMR screening, manufacturing/release compliance), attention to data governance and equity, and precise legal classification with staged lot release and post-marketing-style surveillance [199,200,207]. Regulatory exemplars include the FDA biologics pathway for FMT with adult recurrent Clostridioides difficile infection (rCDI) approvals—Rebyota (2022) and VOWST (2023) [208,209]—and, in the UK, NICE recommendations for FMT in rCDI with MHRA manufacturing requirements while FMT lies outside HTA oversight [210]. Animal data linking birth canal microbiota to neonatal health add biological plausibility to vaginal seeding [211].
NICUs priorities: stabilise early colonisation, protect the gut–brain axis, and avoid iatrogenic dysbiosis. Practice bundle: quality-assured, context-aligned probiotics; closed-loop antibiotic stewardship with 48–72 h review; SSC with a recorded daily dose; and FMT/vaginal seeding only under research/regulated pathways with donor governance and follow-up. See Table 3 for an at-a-glance checklist and implementation details.
6. Research Challenges and Future Directions
Despite growing interest in the gut–brain axis of preterm infants, clinical translation still faces multiple obstacles. Most studies continue to rely on 16S rRNA sequencing, which lacks sub-genus resolution and is susceptible to technical and sampling biases [212,213]. Although metagenomics and metabolomics are increasingly applied, protocols remain insufficiently standardised and are seldom embedded within longitudinal, mechanism-anchored frameworks. Environmental heterogeneity across NICUs (clinical settings, sampling windows, interventions) further drives inconsistent findings, and there is no consensus definition of a “high-risk” microbiome profile. Current animal models do not recapitulate disrupted intrauterine development or the complex, multi-exposure NICU milieu, underscoring the need for human-derived gut–brain organoids and co-culture systems. Knowledge gaps also persist for microbiota-targeted interventions—including optimal timing, dosing, and durability of effects across gestational ages and feeding contexts—whilst ethical and regulatory considerations, particularly for infant FMT, continue to evolve [214].
Looking ahead, progress will hinge on harmonised, longitudinal multi-omics cohorts with standardised sampling and data sharing; human gut–brain organoid platforms that better mirror early-life exposures; and rigorously designed clinical trials that prioritise neurodevelopmental outcomes. Trials should pre-specify standardised, developmentally meaningful endpoints and adopt objective, reproducible assessments, ensure adequate follow-up into early childhood, and include independent safety oversight. In parallel, development of a translational biomarker framework that integrates microbial, metabolic, and host inflammatory/barrier measures, together with mechanism-anchored trial designs and multi-centre collaboration under proportionate regulation, will be essential for responsible and effective clinical translation.
Author Contributions
K.D. and L.D. contributed equally to this work and shared the first authorship. K.D. led the initial drafting and multiple rounds of manuscript revision, coordinated the team’s collaboration, and integrated feedback from co-authors to finalise the content. He also took responsibility for organising the case data and ensuring consistency across sections. L.D. played a pivotal role in developing the study’s conceptual framework and research rationale, conducted an in-depth literature review, and synthesised existing evidence to inform the structure of the paper. She also participated in drafting the background and discussion sections. X.Y. was responsible for comprehensive literature screening, data extraction, and preliminary analysis of selected studies. She also provided input on editing and formatting the reference materials. S.W. contributed critical revisions to the manuscript, especially in refining argumentation and improving logical coherence. She helped restructure several sections to enhance readability and academic rigour. Z.R. conceptualised and supervised the entire study, including defining the research scope and objectives. She provided strategic guidance, reviewed multiple manuscript drafts, and ensured methodological soundness and scholarly quality throughout the process. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ohuma, E.O.; Moller, A.B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: A systematic analysis. Lancet 2023, 402, 1261–1271. [Google Scholar] [CrossRef]
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, e37–e46. [Google Scholar] [CrossRef] [PubMed]
- Spittle, A.; Orton, J.; Anderson, P.J.; Boyd, R.; Doyle, L.W. Early developmental intervention programmes provided post-hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst. Rev. 2015, 2015, Cd005495. [Google Scholar] [CrossRef]
- Rogers, C.E.; Lean, R.E.; Wheelock, M.D.; Smyser, C.D. Aberrant structural and functional connectivity and neurodevelopmental impairment in preterm children. J. Neurodev. Disord. 2018, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Pascal, A.; Naulaers, G.; Ortibus, E.; Oostra, A.; De Coen, K.; Michel, S.; Cloet, E.; Casaer, A.; D'HAese, J.; Laroche, S.; et al. Neurodevelopmental outcomes of very preterm and very-low-birthweight infants in a population-based clinical cohort with a definite perinatal treatment policy. Eur. J. Paediatr. Neurol. 2020, 28, 133–141. [Google Scholar] [CrossRef]
- Woodward, L.J.; Anderson, P.J.; Austin, N.C.; Howard, K.; Inder, T.E. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N. Engl. J. Med. 2006, 355, 685–694. [Google Scholar] [CrossRef]
- Twilhaar, E.S.; de Kieviet, J.F.; Aarnoudse-Moens, C.S.; van Elburg, R.M.; Oosterlaan, J. Academic performance of children born preterm: A meta-analysis and meta-regression. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F322–F330. [Google Scholar] [CrossRef]
- Twilhaar, E.S.; de Kieviet, J.F.; van Elburg, R.M.; Oosterlaan, J. Academic trajectories of very preterm born children at school age. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F419–F423. [Google Scholar] [CrossRef]
- Gath, M.E.; Lee, S.J.; Austin, N.C.; Woodward, L.J. Increased risk of parental instability for children born very preterm and impacts on neurodevelopmental outcomes at age 12. Children 2022, 9, 304. [Google Scholar] [CrossRef]
- Shaw, R.J.; Givrad, S.; Poe, C.; Loi, E.C.; Hoge, M.K.; Scala, M. Neurodevelopmental, mental health, and parenting issues in preterm infants. Children 2023, 10, 1565. [Google Scholar] [CrossRef] [PubMed]
- Blencowe, H.; Lee, A.C.; Cousens, S.; Bahalim, A.; Narwal, R.; Zhong, N.; Chou, D.; Say, L.; Modi, N.; Katz, J.; et al. Preterm birth-associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatr. Res. 2013, 74, 17–34. [Google Scholar] [CrossRef]
- Nosarti, C.; Giouroukou, E.; Healy, E.; Rifkin, L.; Walshe, M.; Reichenberg, A.; Chitnis, X.; Williams, S.C.R.; Murray, R.M. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain 2008, 131, 205–217. [Google Scholar] [CrossRef]
- Nagy, Z.; Ashburner, J.; Andersson, J.; Jbabdi, S.; Draganski, B.; Skare, S.; Böhm, B.; Smedler, A.-C.; Forssberg, H.; Lagercrantz, H. Structural correlates of preterm birth in the adolescent brain. Pediatrics 2009, 124, e964–e972. [Google Scholar] [CrossRef]
- Kostovic, I.; Vasung, L. Insights from in vitro fetal magnetic resonance imaging of cerebral development. Semin. Perinatol. 2009, 33, 220–233. [Google Scholar] [CrossRef]
- Vasung, L.; Lepage, C.; Radoš, M.; Pletikos, M.; Goldman, J.S.; Richiardi, J.; Raguž, M.; Fischi-Gómez, E.; Karama, S.; Huppi, P.S.; et al. Quantitative and qualitative analysis of transient fetal compartments during prenatal human brain development. Front. Neuroanat. 2016, 10, 11. [Google Scholar] [CrossRef]
- Wilson, S.; Pietsch, M.; Cordero-Grande, L.; Price, A.N.; Hutter, J.; Xiao, J.; McCabe, L.; Rutherford, M.A.; Hughes, E.J.; Counsell, S.J.; et al. Development of human white matter pathways in utero over the second and third trimester. Proc. Natl. Acad. Sci. USA 2021, 118, e2023598118. [Google Scholar] [CrossRef]
- Hüppi, P.S.; Warfield, S.; Kikinis, R.; Barnes, P.D.; Zientara, G.P.; Jolesz, F.A.; Tsuji, M.K.; Volpe, J.J. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann. Neurol. 1998, 43, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Kostović, I.; Judas, M. The development of the subplate and thalamocortical connections in the human foetal brain. Acta Paediatr. 2010, 99, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Borre, Y.E.; Moloney, R.D.; Clarke, G.; Dinan, T.G.; Cryan, J.F. The impact of microbiota on brain and behavior: Mechanisms & therapeutic potential. Adv. Exp. Med. Biol. 2014, 817, 373–403. [Google Scholar] [PubMed]
- Borre, Y.E.; O'Keeffe, G.W.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends Mol. Med. 2014, 20, 509–518. [Google Scholar] [CrossRef]
- Diaz Heijtz, R.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef]
- Kim, Y.K.; Shin, C. The microbiota-gut-brain axis in neuropsychiatric disorders: Pathophysiological mechanisms and novel treatments. Curr. Neuropharmacol. 2018, 16, 559–573. [Google Scholar] [CrossRef]
- Borkent, J.; Ioannou, M.; Laman, J.D.; Haarman, B.C.M.; Sommer, I.E.C. Role of the gut microbiome in three major psychiatric disorders. Psychol. Med. 2022, 52, 1222–1242. [Google Scholar] [CrossRef] [PubMed]
- Aatsinki, A.K.; Lahti, L.; Uusitupa, H.M.; Munukka, E.; Keskitalo, A.; Nolvi, S.; O‘MAhony, S.; Pietilä, S.; Elo, L.L.; Eerola, E.; et al. Gut microbiota composition is associated with temperament traits in infants. Brain Behav. Immun. 2019, 80, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Carlson, A.L.; Xia, K.; Azcarate-Peril, M.A.; Goldman, B.D.; Ahn, M.; Styner, M.A.; Thompson, A.L.; Geng, X.; Gilmore, J.H.; Knickmeyer, R.C. Infant gut microbiome associated with cognitive development. Biol. Psychiatry 2018, 83, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lan, Y.; Zhang, J.; Cao, M.; Yang, X.; Wang, X. Effects of early-life gut microbiota on the neurodevelopmental outcomes of preterm infants: A multi-center, longitudinal observational study in China. Eur. J. Pediatr. 2024, 183, 1733–1740. [Google Scholar] [CrossRef]
- Wan, Y.; Zuo, T.; Xu, Z.; Zhang, F.; Zhan, H.; Chan, D.; Leung, T.-F.; Yeoh, Y.K.; Chan, F.K.L.; Chan, R.; et al. Underdevelopment of the gut microbiota and bacteria species as non-invasive markers of prediction in children with autism spectrum disorder. Gut 2022, 71, 910–918. [Google Scholar] [CrossRef]
- Parracho, H.M.; Bingham, M.O.; Gibson, G.R.; McCartney, A.L. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J. Med. Microbiol. 2005, 54, 987–991. [Google Scholar] [CrossRef]
- Needham, B.D.; Adame, M.D.; Serena, G.; Rose, D.R.; Preston, G.M.; Conrad, M.C.; Campbell, A.S.; Donabedian, D.H.; Fasano, A.; Ashwood, P.; et al. Plasma and fecal metabolite profiles in autism spectrum disorder. Biol. Psychiatry 2021, 89, 451–462. [Google Scholar] [CrossRef]
- Zuffa, S.; Schimmel, P.; Gonzalez-Santana, A.; Belzer, C.; Knol, J.; Bölte, S.; Falck-Ytter, T.; Forssberg, H.; Swann, J.; Heijtz, R.D. Early-life differences in the gut microbiota composition and functionality of infants at elevated likelihood of developing autism spectrum disorder. Transl. Psychiatry 2023, 13, 257. [Google Scholar] [CrossRef] [PubMed]
- Aarts, E.; Ederveen, T.H.A.; Naaijen, J.; Zwiers, M.P.; Boekhorst, J.; Timmerman, H.M.; Smeekens, S.P.; Netea, M.G.; Buitelaar, J.K.; Franke, B.; et al. Gut microbiome in ADHD and its relation to neural reward anticipation. PLoS ONE 2017, 12, e0183509. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Zhou, Y.Y.; Zhou, G.L.; Li, Y.C.; Yuan, J.; Li, X.H.; Ruan, B. Gut microbiota profiles in treatment-naïve children with attention deficit hyperactivity disorder. Behav. Brain Res. 2018, 347, 408–413. [Google Scholar] [CrossRef]
- Wang, L.J.; Yang, C.Y.; Chou, W.J.; Lee, M.J.; Chou, M.C.; Kuo, H.C.; Yeh, Y.-M.; Lee, S.-Y.; Huang, L.-H.; Li, S.-C. Gut microbiota and dietary patterns in children with attention-deficit/hyperactivity disorder. Eur. Child Adolesc. Psychiatry 2020, 29, 287–297. [Google Scholar] [CrossRef]
- Wang, L.J.; Li, S.C.; Li, S.W.; Kuo, H.C.; Lee, S.Y.; Huang, L.H.; Chin, C.-Y.; Yang, C.-Y. Gut microbiota and plasma cytokine levels in patients with attention-deficit/hyperactivity disorder. Transl. Psychiatry 2022, 12, 76. [Google Scholar] [CrossRef]
- Boonchooduang, N.; Louthrenoo, O.; Likhitweerawong, N.; Kunasol, C.; Thonusin, C.; Sriwichaiin, S.; Nawara, W.; Chattipakorn, N.; Chattipakorn, S.C. Impact of psychostimulants on microbiota and short-chain fatty acids alterations in children with attention-deficit/hyperactivity disorder. Sci. Rep. 2025, 15, 3034. [Google Scholar] [CrossRef]
- Azad, M.B.; Konya, T.; Persaud, R.R.; Guttman, D.S.; Chari, R.S.; Field, C.J.; Sears, M.R.; Mandhane, P.; Turvey, S.; Subbarao, P.; et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: A prospective cohort study. BJOG 2016, 123, 983–993. [Google Scholar] [CrossRef]
- Chen, J.; Cai, W.; Feng, Y. Development of intestinal bifidobacteria and lactobacilli in breast-fed neonates. Clin. Nutr. 2007, 26, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Bigelow, A.E.; Power, M. Mother-infant skin-to-skin contact: Short- and long-term effects for mothers and their children born full-Term. Front. Psychol. 2020, 11, 1921. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Qiu, W.; Wang, J.; Zhao, A.; Zhou, C.; Sun, T.; Xiong, Z.; Cao, P.; Shen, W.; Chen, J.; et al. Effects of vaginal microbiota transfer on the neurodevelopment and microbiome of cesarean-born infants: A blinded randomized controlled trial. Cell Host Microbe 2023, 31, 1232–1247.e5. [Google Scholar] [CrossRef]
- Colombo, J.; Harris, C.L.; Wampler, J.L.; Zhuang, W.; Shaddy, D.J.; Liu, B.Y.; Wu, S.S. Improved neurodevelopmental outcomes at 5.5 years of age in children who received bovine milk fat globule membrane and lactoferrin in infant formula through 12 months: A randomized controlled trial. J. Pediatr. 2023, 261, 113483. [Google Scholar] [CrossRef] [PubMed]
- Colaizy, T.T.; Poindexter, B.B.; McDonald, S.A.; Bell, E.F.; Carlo, W.A.; Carlson, S.J.; DeMauro, S.; Kennedy, K.; Nelin, L.; Sanchez, P.; et al. Neurodevelopmental outcomes of extremely preterm infants fed donor milk or preterm infant formula: A randomized clinical trial. JAMA 2024, 331, 582–591. [Google Scholar] [CrossRef]
- Cotten, C.M.; Taylor, S.; Stoll, B.; Goldberg, R.N.; Hansen, N.I.; Sánchez, P.J.; Ambalavanan, N.; Benjamin, D.K., Jr. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics 2009, 123, 58–66. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef]
- Fardini, Y.; Chung, P.; Dumm, R.; Joshi, N.; Han, Y.W. Transmission of diverse oral bacteria to murine placenta: Evidence for the oral microbiome as a potential source of intrauterine infection. Infect. Immun. 2010, 78, 1789–1796. [Google Scholar] [CrossRef]
- DiGiulio, D.B. Diversity of microbes in amniotic fluid. Semin. Fetal Neonatal Med. 2012, 17, 2–11. [Google Scholar] [CrossRef]
- Collado, M.C.; Rautava, S.; Aakko, J.; Isolauri, E.; Salminen, S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep. 2016, 6, 23129. [Google Scholar] [CrossRef] [PubMed]
- Vander Haar, E.L.; Wu, G.; Gyamfi-Bannerman, C.; Thomas, C.; Wapner, R.J.; Reddy, U.M.; Zhao, L.; Silver, R.M.; Goldenberg, R.L.; Han, Y.W.; et al. Microbial analysis of umbilical cord blood reveals novel pathogens associated with stillbirth and early preterm birth. mBio 2022, 13, e0203622. [Google Scholar] [CrossRef]
- Jiménez, E.; Marín, M.L.; Martín, R.; Odriozola, J.M.; Olivares, M.; Xaus, J.; Fernández, L.; Rodríguez, J.M. Is meconium from healthy newborns actually sterile? Res. Microbiol. 2008, 159, 187–193. [Google Scholar] [CrossRef]
- Mshvildadze, M.; Neu, J.; Shuster, J.; Theriaque, D.; Li, N.; Mai, V. Intestinal microbial ecology in premature infants assessed with non-culture-based techniques. J. Pediatr. 2010, 156, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Nomura, Y.; Bashir, A.; Fernandez-Hernandez, H.; Itzkowitz, S.; Pei, Z.; Stone, J.; Loudon, H.; Peter, I.; Tse, H. Diversified microbiota of meconium is affected by maternal diabetes status. PLoS ONE 2013, 8, e78257. [Google Scholar] [CrossRef]
- Jiménez, E.; Fernández, L.; Marín, M.L.; Martín, R.; Odriozola, J.M.; Nueno-Palop, C.; Narbad, A.; Olivares, M.; Xaus, J.; Rodríguez, J.M. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr. Microbiol. 2005, 51, 270–274. [Google Scholar] [CrossRef]
- Funkhouser, L.J.; Bordenstein, S.R. Mom knows best: The universality of maternal microbial transmission. PLoS Biol. 2013, 11, e1001631. [Google Scholar] [CrossRef] [PubMed]
- Lauder, A.P.; Roche, A.M.; Sherrill-Mix, S.; Bailey, A.; Laughlin, A.L.; Bittinger, K.; Leite, R.; Elovitz, M.A.; Parry, S.; Bushman, F.D. Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome 2016, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, S.J.; Pakzad, Z.; Elwood, C.N.; Albert, A.Y.K.; Gantt, S.; Manges, A.R.; Dumonceaux, T.J.; Maan, E.J.; Hill, J.E.; Money, D.M.; et al. Early neonatal meconium does not have a demonstrable microbiota determined through use of robust negative controls with cpn60-based microbiome profiling. Microbiol. Spectr. 2021, 9, e0006721. [Google Scholar] [CrossRef]
- Kennedy, K.M.; Gerlach, M.J.; Adam, T.; Heimesaat, M.M.; Rossi, L.; Surette, M.G.; Sloboda, D.M.; Braun, T. Fetal meconium does not have a detectable microbiota before birth. Nat. Microbiol. 2021, 6, 865–873. [Google Scholar] [CrossRef]
- Cuesta, C.M.; Guerri, C.; Ureña, J.; Pascual, M. Role of microbiota-derived extracellular vesicles in gut-brain communication. Int. J. Mol. Sci. 2021, 22, 4235. [Google Scholar] [CrossRef]
- Doré, E.; Boilard, E. Bacterial extracellular vesicles and their interplay with the immune system. Pharmacol. Ther. 2023, 247, 108443. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Chen, P.; Xi, Y.; Sheng, J. From trash to treasure: The role of bacterial extracellular vesicles in gut health and disease. Front. Immunol. 2023, 14, 1274295. [Google Scholar] [CrossRef]
- Melo-Marques, I.; Cardoso, S.M.; Empadinhas, N. Bacterial extracellular vesicles at the interface of gut microbiota and immunity. Gut Microbes 2024, 16, 2396494. [Google Scholar] [CrossRef]
- Arboleya, S.; Binetti, A.; Salazar, N.; Fernández, N.; Solís, G.; Hernández-Barranco, A.; Margolles, A.; de los Reyes-Gavilán, C.G.; Gueimonde, M. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol. Ecol. 2012, 79, 763–772. [Google Scholar] [CrossRef]
- Jacquot, A.; Neveu, D.; Aujoulat, F.; Mercier, G.; Marchandin, H.; Jumas-Bilak, E.; Picaud, J.-C. Dynamics and clinical evolution of bacterial gut microflora in extremely premature patients. J. Pediatr. 2011, 158, 390–396. [Google Scholar] [CrossRef]
- Cong, X.; Xu, W.; Janton, S.; Henderson, W.A.; Matson, A.; McGrath, J.M.; Maas, K.; Graf, J. Gut microbiome developmental patterns in early life of preterm infants: Impacts of feeding and gender. PLoS ONE 2016, 11, e0152751. [Google Scholar] [CrossRef]
- Korpela, K.; Blakstad, E.W.; Moltu, S.J.; Strømmen, K.; Nakstad, B.; Rønnestad, A.E.; Brække, K.; Iversen, P.O.; Drevon, C.A.; de Vos, W. Intestinal microbiota development and gestational age in preterm neonates. Sci. Rep. 2018, 8, 2453. [Google Scholar] [CrossRef] [PubMed]
- Rougé, C.; Goldenberg, O.; Ferraris, L.; Berger, B.; Rochat, F.; Legrand, A.; Göbel, U.B.; Vodovar, M.; Voyer, M.; Rozé, J.-C.; et al. Investigation of the intestinal microbiota in preterm infants using different methods. Anaerobe 2010, 16, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Zwittink, R.D.; van Zoeren-Grobben, D.; Martin, R.; van Lingen, R.A.; Groot Jebbink, L.J.; Boeren, S.; Renes, I.B.; van Elburg, R.M.; Belzer, C.; Knol, J. Metaproteomics reveals functional differences in intestinal microbiota development of preterm infants. Mol. Cell Proteomics 2017, 16, 1610–1620. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Lieber, A.D.; Wu, F.; Perez-Perez, G.I.; Chen, Y.; et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 2016, 8, 343ra82. [Google Scholar] [CrossRef]
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A.; Simpson, N.; Kumar, N.; Stares, M.D.; Rodger, A.; Brocklehurst, P.; et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019, 574, 117–121. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Rutayisire, E.; Huang, K.; Liu, Y.; Tao, F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: A systematic review. BMC Gastroenterol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; Van den Brandt, P.A.; Stobberingh, E.E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef]
- Bager, P.; Wohlfahrt, J.; Westergaard, T. Caesarean delivery and risk of atopy and allergic disease: Meta-analyses. Clin. Exp. Allergy 2008, 38, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, C.; Morrow, A.L.; Lagomarcino, A.J.; Altaye, M.; Taft, D.H.; Yu, Z.; Newburg, D.S.; Ward, D.V.; Schibler, K.R. Early empiric antibiotic use in preterm infants is associated with lower bacterial diversity and higher relative abundance of Enterobacter. J. Pediatr. 2014, 165, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Hagan, T.; Cortese, M.; Rouphael, N.; Boudreau, C.; Linde, C.; Maddur, M.S.; Das, J.; Wang, H.; Guthmiller, J.; Zheng, N.-Y.; et al. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in Humans. Cell 2019, 178, 1313–1328.E13. [Google Scholar] [CrossRef]
- Arboleya, S.; Sánchez, B.; Milani, C.; Duranti, S.; Solís, G.; Fernández, N.; de Los Reyes-Gavilan, C.G.; Ventura, M.; Margolles, A.; Gueimonde, M. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J. Pediatr. 2015, 166, 538–544. [Google Scholar] [CrossRef]
- Chen, X.; Shi, Y. Determinants of microbial colonization in the premature gut. Mol. Med. 2023, 29, 90. [Google Scholar] [CrossRef]
- Diamond, L.; Wine, R.; Morris, S.K. Impact of intrapartum antibiotics on the infant gastrointestinal microbiome: A narrative review. Arch. Dis. Child. 2022, 107, 627–634. [Google Scholar] [CrossRef]
- Graspeuntner, S.; Waschina, S.; Künzel, S.; Twisselmann, N.; Rausch, T.K.; Cloppenborg-Schmidt, K.; Zimmermann, J.; Viemann, D.; Herting, E.; Göpel, W.; et al. Gut dysbiosis with bacilli dominance and accumulation of fermentation products precedes late-onset sepsis in preterm infants. Clin. Infect. Dis. 2019, 69, 268–277. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015, 17, 852. [Google Scholar] [CrossRef] [PubMed]
- Le Huërou-Luron, I.; Blat, S.; Boudry, G. Breast-v. formula-feeding: Impacts on the digestive tract and immediate and long-term health effects. Nutr. Res. Rev. 2010, 23, 23–36. [Google Scholar] [CrossRef]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef]
- Turroni, F.; Ventura, M.; Buttó, L.F.; Duranti, S.; O’Toole, P.W.; Motherway, M.O.C.; van Sinderen, D. Molecular dialogue between the human gut microbiota and the host: A Lactobacillus and Bifidobacterium perspective. Cell. Mol. Life Sci. 2014, 71, 183–203. [Google Scholar] [CrossRef] [PubMed]
- O‘Sullivan, A.; Farver, M.; Smilowitz, J.T. The influence of early infant-feeding practices on the intestinal microbiome and body composition in Infants. Nutr. Metab. Insights. 2016, 8, 1–9, Erratum in Nutr. Metab. Insights. 2016, 8 (Suppl. 1), 87. [Google Scholar]
- Collado, M.C.; Isolauri, E.; Laitinen, K.; Salminen, S. Effect of mother’s weight on infant’s microbiota acquisition, composition, and activity during early infancy: A prospective follow-up study initiated in early pregnancy. Am. J. Clin. Nutr. 2010, 92, 1023–1030. [Google Scholar] [CrossRef]
- Mandal, S.; Godfrey, K.M.; McDonald, D.; Treuren, W.V.; Bjørnholt, J.V.; Midtvedt, T.; Moen, B.; Rudi, K.; Knight, R.; Brantsæter, A.L.; et al. Fat and vitamin intakes during pregnancy have stronger relations with a pro-inflammatory maternal microbiota than does carbohydrate intake. Microbiome 2016, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Myles, I.A.; Fontecilla, N.M.; Janelsins, B.M.; Vithayathil, P.J.; Segre, J.A.; Datta, S.K. Parental dietary fat intake alters offspring microbiome and immunity. J. Immunol. 2013, 191, 3200–3209. [Google Scholar] [CrossRef]
- Ma, J.; Prince, A.L.; Bader, D.; Hu, M.; Ganu, R.; Baquero, K.; Blundell, P.; Harris, R.A.; Frias, A.E.; Grove, K.L.; et al. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat. Commun. 2014, 5, 3889. [Google Scholar] [CrossRef]
- Chen, T.; Qin, Y.; Chen, M.; Zhang, Y.; Wang, X.; Dong, T.; Chen, G.; Sun, X.; Lu, T.; White, R.A.; et al. Gestational diabetes mellitus is associated with the neonatal gut microbiota and metabolome. BMC Med. 2021, 19, 120. [Google Scholar] [CrossRef]
- Rodríguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015, 26, 26050. [Google Scholar] [CrossRef]
- Fouhy, F.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C.; Cotter, P.D. Composition of the early intestinal microbiota: Knowledge, knowledge gaps and the use of high-throughput sequencing to address these gaps. Gut Microbes 2012, 3, 203–220. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Grześkowiak, Ł.; Collado, M.C.; Mangani, C.; Maleta, K.; Laitinen, K.; Ashorn, P.; Isolauri, E.; Salminen, S. Distinct gut microbiota in southeastern African and northern European infants. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 812–816. [Google Scholar] [CrossRef]
- Subramanian, S.; Huq, S.; Yatsunenko, T.; Haque, R.; Mahfuz, M.; Alam, M.A.; Benezra, A.; DeStefano, J.; Meier, M.F.; Muegge, B.D.; et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 2014, 510, 417–421. [Google Scholar] [CrossRef]
- Laursen, M.F.; Zachariassen, G.; Bahl, M.I.; Bergström, A.; Høst, A.; Michaelsen, K.F.; Licht, T.R. Having older siblings is associated with gut microbiota development during early childhood. BMC Microbiol. 2015, 15, 154. [Google Scholar] [CrossRef]
- Spor, A.; Koren, O.; Ley, R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat. Rev. Microbiol. 2011, 9, 279–290. [Google Scholar] [CrossRef]
- Lin, A.; Bik, E.M.; Costello, E.K.; Dethlefsen, L.; Haque, R.; Relman, D.A.; Singh, U.; Aziz, R.K. Distinct distal gut microbiome diversity and composition in healthy children from Bangladesh and the United States. PLoS ONE 2013, 8, e53838. [Google Scholar] [CrossRef]
- Fallani, M.; Amarri, S.; Uusijarvi, A.; Adam, R.; Khanna, S.; Aguilera, M.; Gil, A.; Vieites, J.M.; Norin, E.; Young, D.; et al. Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiology 2011, 157, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Lehnardt, S.; Lachance, C.; Patrizi, S.; Lefebvre, S.; Follett, P.L.; Jensen, F.E.; Rosenberg, P.A.; Volpe, J.J.; Vartanian, T. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J. Neurosci. 2002, 22, 2478–2486. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.; Golenbock, D.; Bowie, A.G. The history of toll-like receptors—Redefining innate immunity. Nat. Rev. Immunol. 2013, 13, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Kim, S.; Steelman, A.J.; Koito, H.; Li, J. Astrocytes promote TNF-mediated toxicity to oligodendrocyte precursors. J. Neurochem. 2011, 116, 53–66. [Google Scholar] [CrossRef]
- Bokobza, C.; Van Steenwinckel, J.; Mani, S.; Mezger, V.; Fleiss, B.; Gressens, P. Neuroinflammation in preterm babies and autism spectrum disorders. Pediatr. Res. 2019, 85, 155–165. [Google Scholar] [CrossRef]
- Volpe, J.J. Encephalopathy of prematurity includes neuronal abnormalities. Pediatrics 2005, 116, 221–225. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158, Erratum in Sci. Transl. Med. 2014, 6, 266er7. [Google Scholar] [CrossRef]
- Lan, Z.; Tang, X.; Lu, M.; Hu, Z.; Tang, Z. The role of short-chain fatty acids in central nervous system diseases: A bibliometric and visualized analysis with future directions. Heliyon 2024, 10, e26377. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, J.; Zalewska, T.; Sypecka, J.; Ziemka-Nalecz, M. Effect of the HDAC inhibitor, sodium butyrate, on neurogenesis in a rat model of neonatal hypoxia-ischemia: Potential mechanism of action. Mol. Neurobiol. 2019, 56, 6341–6370. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, L.; Qu, Y.; Zhou, Y.; Zhu, J.; Li, Y.; Zhu, T.; Zhao, F.; Tang, J.; Mu, D. Histone acetylation of oligodendrocytes protects against white matter injury induced by inflammation and hypoxia-ischemia through activation of BDNF-TrkB signaling pathway in neonatal rats. Brain Res. 2018, 1688, 33–46. [Google Scholar] [CrossRef]
- Rothhammer, V.; Mascanfroni, I.D.; Bunse, L.; Takenaka, M.C.; Kenison, J.E.; Mayo, L.; Chao, C.-C.; Patel, B.; Yan, R.; Blain, M.; et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 2016, 22, 586–597. [Google Scholar] [CrossRef]
- Desbonnet, L.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. Microbiota is essential for social development in the mouse. Mol. Psychiatry 2014, 19, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-chain fatty acids: Microbial metabolites that alleviate stress-induced brain-gut axis alterations. J. Physiol. 2018, 596, 4923–4944. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Ajuwon, K.M. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS ONE 2017, 12, e0179586. [Google Scholar]
- Liang, S.; Wu, X.; Jin, F. Gut-brain psychology: Rethinking psychology from the microbiota-gut-brain axis. Front. Integr. Neurosci. 2018, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Rothhammer, V.; Borucki, D.M.; Tjon, E.C.; Takenaka, M.C.; Chao, C.C.; Ardura-Fabregat, A.; de Lima, K.A.; Gutiérrez-Vázquez, C.; Hewson, P.; Staszewski, O.; et al. Microglial control of astrocytes in response to microbial metabolites. Nature 2018, 557, 724–728. [Google Scholar] [CrossRef]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef]
- Misiak, B.; Łoniewski, I.; Marlicz, W.; Frydecka, D.; Szulc, A.; Rudzki, L.; Samochowiec, J. The HPA axis dysregulation in severe mental illness: Can we shift the blame to gut microbiota? Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 102, 109951. [Google Scholar] [CrossRef]
- O'Mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.M.; Quigley, E.M.; Cryan, J.F.; Dinan, T.G. Early life stress alters behavior, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef]
- Williams, B.B.; Van Benschoten, A.H.; Cimermancic, P.; Donia, M.S.; Zimmermann, M.; Taketani, M.; Ishihara, A.; Kashyap, P.C.; Fraser, J.S.; Fischbach, M.A. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe 2014, 16, 495–503. [Google Scholar] [CrossRef]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011, 141, 599–609.e1-3. [Google Scholar] [CrossRef]
- Min, D.K.; Tuor, U.I.; Chelikani, P.K. Gastric distention induced functional magnetic resonance signal changes in the rodent brain. Neuroscience 2011, 179, 151–158. [Google Scholar] [CrossRef]
- Clark, K.B.; Smith, D.C.; Hassert, D.L.; Browning, R.A.; Naritoku, D.K.; Jensen, R.A. Posttraining electrical stimulation of vagal afferents with concomitant vagal efferent inactivation enhances memory storage processes in the rat. Neurobiol. Learn Mem. 1998, 70, 364–373. [Google Scholar] [CrossRef]
- Follesa, P.; Biggio, F.; Gorini, G.; Caria, S.; Talani, G.; Dazzi, L.; Puligheddu, M.; Marrosu, F.; Biggio, G. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. 2007, 1179, 28–34. [Google Scholar] [CrossRef]
- O'Leary, O.F.; Ogbonnaya, E.S.; Felice, D.; Levone, B.R.; Conroy, L.C.; Fitzgerald, P.; Bravo, J.A.; Forsythe, P.; Bienenstock, J.; Dinan, T.G.; et al. The vagus nerve modulates BDNF expression and neurogenesis in the hippocampus. Eur. Neuropsychopharmacol. 2018, 28, 307–316. [Google Scholar] [CrossRef]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier Function. Cell Host Microbe. 2015, 17, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.C.; Stiemsma, L.T.; Amenyogbe, N.; Brown, E.M.; Finlay, B. The intestinal microbiome in early life: Health and disease. Front. Immunol. 2014, 5, 427. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J.J. (Ed.) Neurology of the Newborn, 5th ed.; W.B. Saunders: Philadelphia, PA, USA, 2008; pp. 1055–1094. [Google Scholar]
- Raybaud, C.; Ahmad, T.; Rastegar, N.; Shroff, M.; Al Nassar, M. The premature brain: Developmental and lesional anatomy. Neuroradiology 2013, 55, 23–40. [Google Scholar] [CrossRef]
- Khwaja, O.; Volpe, J.J. Pathogenesis of cerebral white matter injury of prematurity. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F153–F161. [Google Scholar] [CrossRef]
- Cioni, G.; Bertuccelli, B.; Boldrini, A.; Canapicchi, R.; Fazzi, B.; Guzzetta, A.; Mercuri, E. Correlation between visual function, neurodevelopmental outcome, and magnetic resonance imaging findings in infants with periventricular leucomalacia. Arch. Dis. Child. Fetal Neonatal Ed. 2000, 82, F134–F140. [Google Scholar] [CrossRef] [PubMed]
- Banker, B.Q.; Larroche, J.C. Periventricular leukomalacia of infancy: A form of neonatal anoxic encephalopathy. Arch. Neurol. 1962, 7, 386–410. [Google Scholar] [CrossRef]
- Renwick, V.L.; Stewart, C.J. Exploring functional metabolites in preterm infants. Acta Paediatr. 2022, 111, 45–53. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Mai, V.; Young, C.M.; Ukhanova, M.; Wang, X.; Sun, Y.; Casella, G.; Theriaque, D.; Li, N.; Sharma, R.; Hudak, M.; et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS ONE 2011, 6, e20647. [Google Scholar] [CrossRef]
- Kusters, C.D.; Chen, M.L.; Follett, P.L.; Dammann, O. “Intraventricular” hemorrhage and cystic periventricular leukomalacia in preterm infants: How are they related? J. Child Neurol. 2009, 24, 1158–1170. [Google Scholar] [CrossRef] [PubMed]
- Judas, M.; Rados, M.; Jovanov-Milosevic, N.; Hrabac, P.; Stern-Padovan, R.; Kostovic, I. Structural, immunocytochemical, and mr imaging properties of periventricular crossroads of growing cortical pathways in preterm infants. AJNR Am. J. Neuroradiol. 2005, 26, 2671–2684. [Google Scholar] [PubMed]
- Agrawal, S.; Rao, S.C.; Bulsara, M.K.; Patole, S.K. Prevalence of autism spectrum disorder in preterm infants: A meta-analysis. Pediatrics 2018, 142, e20180134. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Marlow, N. Preterm birth and childhood psychiatric disorders. Pediatr. Res. 2011, 69, 11–18. [Google Scholar] [CrossRef]
- Molloy, C.A.; Manning-Courtney, P. Prevalence of chronic gastrointestinal symptoms in children with autism and autistic spectrum disorders. Autism 2003, 7, 165–171. [Google Scholar] [CrossRef]
- Buie, T.; Campbell, D.B.; Fuchs, G.J.; 3rd Furuta, G.T.; Levy, J.; Vandewater, J.; Whitaker, A.H.; Atkins, D.; Bauman, M.L.; Beaudet, A.L.; et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: A consensus report. Pediatrics 2010, 125, S1–S18. [Google Scholar] [CrossRef]
- Hsiao, E.Y. Gastrointestinal issues in autism spectrum disorder. Harv. Rev. Psychiatry 2014, 22, 104–111. [Google Scholar] [CrossRef]
- Finegold, S.M.; Molitoris, D.; Song, Y.; Liu, C.; Vaisanen, M.L.; Bolte, E.; McTeague, M.; Sandler, R.; Wexler, H.; Marlowe, E.M.; et al. Gastrointestinal microflora studies in late-onset autism. Clin. Infect. Dis. 2002, 35, S6–S16. [Google Scholar]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol. Autism 2013, 4, 42. [Google Scholar] [CrossRef]
- Dan, Z.; Mao, X.; Liu, Q.; Guo, M.; Zhuang, Y.; Liu, Z.; Chen, K.; Chen, J.; Xu, R.; Tang, J.; et al. Altered gut microbial profile is associated with abnormal metabolism activity of autism spectrum disorder. Gut Microbes 2020, 11, 1246–1267. [Google Scholar] [CrossRef]
- Macfabe, D.F. Short-chain fatty acid fermentation products of the gut microbiome: Implications in autism spectrum disorders. Microb. Ecol. Health Dis. 2012, 23, 19260. [Google Scholar] [CrossRef]
- Fattorusso, A.; Di Genova, L.; Dell'Isola, G.B.; Mencaroni, E.; Esposito, S. Autism spectrum disorders and the gut microbiota. Nutrients 2019, 11, 521. [Google Scholar] [CrossRef]
- Kang, D.W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef]
- Stewart Campbell, A.; Needham, B.D.; Meyer, C.R.; Tan, J.; Conrad, M.; Preston, G.M.; Bolognani, F.; Rao, S.G.; Heussler, H.; Griffith, R.; et al. Safety and target engagement of an oral small-molecule sequestrant in adolescents with autism spectrum disorder: An open-label phase 1b/2a trial. Nat. Med. 2022, 28, 528–534. [Google Scholar] [CrossRef]
- Jakobi, B.; Vlaming, P.; Mulder, D.; Ribases, M.; Richarte, V.; Ramos-Quiroga, J.A.; Tendolkar, I.; van Eijndhoven, P.; Vrijsen, J.N.; Buitelaar, J.; et al. The gut-microbiome in adult attention-deficit/hyperactivity disorder—A meta-analysis. medRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Thapar, A.; Cooper, M. Attention deficit hyperactivity disorder. Lancet 2016, 387, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- First, M.B. Diagnostic and statistical manual of mental disorders, 5th edition, and clinical utility. J. Nerv. Ment. Dis. 2013, 201, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.F.; Rubin, G.J.; Rogers, M.B.; Wessely, S.; Greenberg, N.; Hall, C.E.; Pitt, A.; Logan, P.E.; Lucas, R.; Brooks, S.K. The changing prevalence of ADHD? A systematic review. J. Affect. Disord. 2025, 388, 119427. [Google Scholar] [CrossRef]
- Stilling, R.M.; Dinan, T.G.; Cryan, J.F. Microbial genes, brain & behaviour—Epigenetic regulation of the gut-brain axis. Genes Brain Behav. 2014, 13, 69–86. [Google Scholar]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef]
- Korrel, H.; Mueller, K.L.; Silk, T.; Anderson, V.; Sciberras, E. Research review: Language problems in children with attention-deficit hyperactivity disorder—A systematic meta-analytic review. J. Child Psychol. Psychiatry 2017, 58, 640–654. [Google Scholar] [CrossRef]
- Tengeler, A.C.; Dam, S.A.; Wiesmann, M.; Naaijen, J.; van Bodegom, M.; Belzer, C.; Dederen, P.J.; Verweij, V.; Franke, B.; Kozicz, T.; et al. Gut microbiota from persons with attention-deficit/hyperactivity disorder affects the brain in mice. Microbiome 2020, 8, 44. [Google Scholar] [CrossRef]
- Tillisch, K.; Mayer, E.A.; Gupta, A.; Gill, Z.; Brazeilles, R.; Le Nevé, B.; Vlieg, J.E.v.H.; Guyonnet, D.; Derrien, M.; Labus, J.S. Brain structure and response to emotional stimuli as related to gut microbial profiles in healthy women. Psychosom. Med. 2017, 79, 905–913. [Google Scholar] [CrossRef]
- Chang, J.P.; Mondelli, V.; Satyanarayanan, S.K.; Chiang, Y.J.; Chen, H.T.; Su, K.P.; Pariante, C.M. Cortisol, inflammatory biomarkers and neurotrophins in children and adolescents with attention deficit hyperactivity disorder (ADHD) in Taiwan. Brain Behav. Immun. 2020, 88, 105–113. [Google Scholar] [CrossRef]
- Burnett, A.C.; Mainzer, R.M.; Doyle, L.W.; Lee, K.J.; Anderson, P.J.; Zannino, D.; Duff, J.; Patton, G.C.; Cheong, J.L.Y.; for the Victorian Infant Collaborative Study Group. Mental health in young adults born extremely preterm or extremely low birthweight with contemporary neonatal intensive care. Psychol. Med. 2023, 53, 5227–5234. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Borre, Y.; O'Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Groer, M.W.; Gregory, K.E.; Louis-Jacques, A.; Thibeau, S.; Walker, W.A. The very low birth weight infant microbiome and childhood health. Birth Defects Res. C Embryo Today 2015, 105, 252–264. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Keunen, K.; van Elburg, R.M.; van Bel, F.; Benders, M.J. Impact of nutrition on brain development and its neuroprotective implications following preterm birth. Pediatr. Res. 2015, 77, 148–155. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef]
- Eiwegger, T.; Stahl, B.; Haidl, P.; Schmitt, J.; Boehm, G.; Dehlink, E.; Urbanek, R.; Szépfalusi, Z. Prebiotic oligosaccharides: In vitro evidence for gastrointestinal epithelial transfer and immunomodulatory properties. Pediatr. Allergy Immunol. 2010, 21, 1179–1188. [Google Scholar] [CrossRef]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef]
- Sprenger, N.; Tytgat, H.L.P.; Binia, A.; Austin, S.; Singhal, A. Biology of human milk oligosaccharides: From basic science to clinical evidence. J. Hum. Nutr. Diet. 2022, 35, 280–299. [Google Scholar] [CrossRef] [PubMed]
- Goehring, K.C.; Marriage, B.J.; Oliver, J.S.; Wilder, J.A.; Barrett, E.G.; Buck, R.H. Similar to Those who are breastfed, infants fed a formula containing 2'-Fucosyllactose have lower inflammatory cytokines in a randomized controlled trial. J. Nutr. 2016, 146, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Bifidobacterium longum subspecies infantis: Champion colonizer of the infant gut. Pediatr. Res. 2015, 77, 229–235. [Google Scholar] [CrossRef]
- Sela, D.A.; Chapman, J.; Adeuya, A.; Kim, J.H.; Chen, F.; Whitehead, T.R.; Lapidus, A.; Rokhsar, D.S.; Lebrilla, C.B.; German, J.B.; et al. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc. Natl. Acad. Sci. USA 2008, 105, 18964–18969. [Google Scholar] [CrossRef]
- Manzoni, P.; Lista, G.; Gallo, E.; Marangione, P.; Priolo, C.; Fontana, P.; Guardione, R.; Farina, D. Routine Lactobacillus rhamnosus GG administration in VLBW infants: A retrospective, 6-year cohort study. Early Hum. Dev. 2011, 87, S35–S38. [Google Scholar] [CrossRef]
- Lin, F.L.; Chen, C.M.; Sun, C.K.; Cheng, Y.S.; Tzang, R.F.; Chiu, H.J.; Wang, M.-Y.; Cheng, Y.-C.; Hung, K.-C. Effects of probiotics on neurocognitive outcomes in infants and young children: A meta-analysis. Front. Public Health. 2023, 11, 1323511. [Google Scholar] [CrossRef] [PubMed]
- Carpén, N.; Brodin, P.; de Vos, W.M.; Salonen, A.; Kolho, K.L.; Andersson, S.; Helve, O. Transplantation of maternal intestinal flora to the newborn after elective cesarean section (SECFLOR): Study protocol for a double blinded randomized controlled trial. BMC Pediatr. 2022, 22, 565. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, J.P.; Westerbeek, E.A.; Bröring-Starre, T.; Garssen, J.; van Elburg, R.M. Neurodevelopment of preterm infants at 24 months after neonatal supplementation of a prebiotic mix: A randomized trial. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Sari, F.N.; Eras, Z.; Dizdar, E.A.; Erdeve, O.; Oguz, S.S.; Uras, N.; Dilmen, U. Do oral probiotics affect growth and neurodevelopmental outcomes in very low-birth-weight preterm infants? Am. J. Perinatol. 2012, 29, 579–586. [Google Scholar] [CrossRef]
- Gewolb, I.H.; Schwalbe, R.S.; Taciak, V.L.; Harrison, T.S.; Panigrahi, P. Stool microflora in extremely low birthweight infants. Arch. Dis. Child. Fetal Neonatal Ed. 1999, 80, F167–F173. [Google Scholar] [CrossRef]
- Wang, Y.; Hoenig, J.D.; Malin, K.J.; Qamar, S.; Petrof, E.O.; Sun, J.; Antonopoulos, D.A.; Chang, E.B.; Claud, E.C. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009, 3, 944–954. [Google Scholar] [CrossRef]
- Gasparrini, A.J.; Wang, B.; Sun, X.; Kennedy, E.A.; Hernandez-Leyva, A.; Ndao, I.M.; Tarr, P.I.; Warner, B.B.; Dantas, G. Persistent metagenomic signatures of early-life hospitalization and antibiotic treatment in the infant gut microbiota and resistome. Nat. Microbiol. 2019, 4, 2285–2297. [Google Scholar] [CrossRef]
- Gibson, M.K.; Wang, B.; Ahmadi, S.; Burnham, C.A.; Tarr, P.I.; Warner, B.B.; Dantas, G. Developmental dynamics of the preterm infant gut microbiota and antibiotic resistome. Nat. Microbiol. 2016, 1, 16024. [Google Scholar] [CrossRef]
- Bordiuk, O.L.; Smith, K.; Morin, P.J.; Semënov, M.V. Cell proliferation and neurogenesis in adult mouse brain. PLoS ONE 2014, 9, e111453. [Google Scholar] [CrossRef]
- Hill, W.D.; Marioni, R.E.; Maghzian, O.; Ritchie, S.J.; Hagenaars, S.P.; McIntosh, A.M.; Gale, C.R.; Davies, G.; Deary, I.J. A combined analysis of genetically correlated traits identifies 187 loci and a role for neurogenesis and myelination in intelligence. Mol. Psychiatry 2019, 24, 169–181. [Google Scholar] [CrossRef]
- Mahoney, T.F.; Silhavy, T.J. The Cpx stress response confers resistance to some, but not all, bactericidal antibiotics. J. Bacteriol. 2013, 195, 1869–1874. [Google Scholar] [CrossRef] [PubMed]
- Lamba, V.; D’souza, S.; Carafa, C.; Zepf, A.; Bassel, C.L.; Gutierrez, M.; Balakrishnan, M. Standardizing the approach to late onset sepsis in neonates through antimicrobial stewardship: A quality improvement initiative. J. Perinatol. 2020, 40, 1433–1440. [Google Scholar] [CrossRef]
- Feldman, R.; Eidelman, A.I. Skin-to-skin contact (kangaroo care) accelerates autonomic and neurobehavioural maturation in preterm infants. Dev. Med. Child Neurol. 2003, 45, 274–281. [Google Scholar] [CrossRef]
- Moore, E.R.; Anderson, G.C.; Bergman, N.; Dowswell, T. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst. Rev. 2012, 5, Cd003519. [Google Scholar] [PubMed]
- Bigelow, A.; Power, M.; MacLellan-Peters, J.; Alex, M.; McDonald, C. Effect of mother/infant skin-to-skin contact on postpartum depressive symptoms and maternal physiological stress. J. Obs. Gynecol. Neonatal Nurs. 2012, 41, 369–382. [Google Scholar] [CrossRef]
- Tessier, R.; Cristo, M.; Velez, S.; Giron, M.; de Calume, Z.F.; Ruiz-Palaez, J.G.; Charpak, Y.; Charpak, N. Kangaroo mother care and the bonding hypothesis. Pediatrics 1998, 102, e17. [Google Scholar] [CrossRef] [PubMed]
- Clarke-Sather, A.R.; Compton, C.; Roberts, K.; Brearley, A.; Wang, S.G. Systematic review of kangaroo care duration’s impact in neonatal intensive care units on infant-maternal health. Am. J. Perinatol. 2024, 41, 975–987. [Google Scholar] [CrossRef]
- Boukydis, Z. Parent-infant skin-to-skin contact: Parents’ views versus nurses’ views. Acta Paediatr. 2011, 100, 638–640. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, J.; Liu, Y.; Xiao, N.; Suo, H.; Xie, K.; Yang, C.; Wu, C. Short-chain fatty acids suppress lipopolysaccharide-induced production of nitric oxide and proinflammatory cytokines through inhibition of NF-κB pathway in RAW264.7 cells. Inflammation 2012, 35, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Lu, L.; Yu, Y.; Oliphant, K.; Drobyshevsky, A.; Claud, E.C. Early preterm infant microbiome impacts adult learning. Sci. Rep. 2022, 12, 3310. [Google Scholar] [CrossRef]
- Cerdó, T.; Ruiz-Rodríguez, A.; Acuña, I.; Torres-Espínola, F.J.; Menchén-Márquez, S.; Gámiz, F.; Gallo, M.; Jehmlich, N.; Haange, S.-B.; von Bergen, M.; et al. Infant gut microbiota contributes to cognitive performance in mice. Cell Host Microbe 2023, 31, 1974–1988.e4. [Google Scholar] [CrossRef]
- Guzzardi, M.A.; Ederveen, T.H.A.; Rizzo, F.; Weisz, A.; Collado, M.C.; Muratori, F.; Gross, G.; Alkema, W.; Iozzo, P. Maternal pre-pregnancy overweight and neonatal gut bacterial colonization are associated with cognitive development and gut microbiota composition in pre-school-age offspring. Brain. Behav. Immun. 2022, 100, 311–320. [Google Scholar] [CrossRef]
- Goo, N.; Bae, H.J.; Park, K.; Kim, J.; Jeong, Y.; Cai, M.; Cho, K.; Jung, S.Y.; Kim, D.-H.; Ryu, J.H. The effect of fecal microbiota transplantation on autistic-like behaviors in Fmr1 KO mice. Life Sci. 2020, 262, 118497. [Google Scholar] [CrossRef]
- Orłowska, D.; Olszak, J.; Zalewa, K.; Bartoszek, L.; Kapłan, W.; Starownik, J. Assessing the safety and efficacy of probiotics in improving the gut microbiome of premature infants. Qual. Sport 2024, 18, 53454. [Google Scholar] [CrossRef]
- van den Akker, C.H.P.; Embleton, N.D.; Lapillonne, A.; Mihatsch, W.A.; Salvatore, S.; Canani, R.B.; Dinleyici, E.C.; Domellöf, M.; Guarino, A.; Gutiérrez-Castrellón, P.; et al. Reevaluating the FDA’s warning against the use of probiotics in preterm neonates: A societal statement by ESPGHAN and EFCNI. J. Pediatr. Gastroenterol. Nutr. 2024, 78, 1403–1408. [Google Scholar] [CrossRef]
- DuPont, H.L.; Salge, M.M.H. The importance of a healthy microbiome in pregnancy and infancy and microbiota treatment to reverse dysbiosis for improved health. Antibiotics 2023, 12, 1617. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Z.; Chen, X.; Qin, F.; Zhou, W. Association between vaginal microbiomes and neonatal septicemia in pregnant women with preterm premature rupture of membranes based on metagenome sequencing. Am. J. Transl. Res. 2023, 15, 4544–4557. [Google Scholar]
- Fransson, E.; Gudnadottir, U.; Hugerth, L.W.; Itzel, E.W.; Hamsten, M.; Boulund, F.; Pennhag, A.; Du, J.; Schuppe-Koistinen, I.; Brusselaers, N.; et al. Cohort profile: The swedish maternal microbiome project (SweMaMi)—Assessing the dynamic associations between the microbiome and maternal and neonatal adverse events. BMJ Open 2022, 12, e065825. [Google Scholar] [CrossRef]
- Leo, S.; Cetiner, O.F.; Pittet, L.F.; Messina, N.L.; Jakob, W.; Falquet, L.; Curtis, N.; Zimmermann, P. Metagenomics analysis of the neonatal intestinal resistome. Front. Pediatr. 2023, 11, 1169651. [Google Scholar] [CrossRef] [PubMed]
- Leo, S.; Curtis, N.; Zimmermann, P. The neonatal intestinal resistome and factors that influence it-a systematic review. Clin. Microbiol. Infect. 2022, 28, 1539–1546. [Google Scholar] [CrossRef]
- Hourigan, S.K.; Subramanian, P.; Hasan, N.A.; Ta, A.; Klein, E.; Chettout, N.; Huddleston, K.; Deopujari, V.; Levy, S.; Baveja, R.; et al. Comparison of infant gut and skin microbiota, resistome and virulome between neonatal intensive care unit (NICU) environments. Front. Microbiol. 2018, 9, 1361. [Google Scholar] [CrossRef] [PubMed]
- Cason, C.; D'Accolti, M.; Campisciano, G.; Soffritti, I.; Ponis, G.; Mazzacane, S.; Maggiore, A.; Risso, F.M.; Comar, M.; Caselli, E. Microbial contamination in hospital environment has the potential to colonize preterm newborns’ nasal cavities. Pathogens 2021, 10, 615. [Google Scholar] [CrossRef]
- Sachs, R.E.; Edelstein, C.A. Ensuring the safe and effective FDA regulation of fecal microbiota transplantation. J. Law Biosci. 2015, 2, 396–415. [Google Scholar] [CrossRef]
- Goldsmith, J.; Tomkovich, S.; Auniņš, J.G.; McGovern, B.H.; Mahoney, J.C.; Hasson, B.R.; McChalicher, C.W.J.; Ege, D.S. End-to-end donor screening and manufacturing controls: Complementary quality-based strategies to minimize patient risk for donor-derived microbiome therapeutics. Gut Microbes 2024, 16, 2402550. [Google Scholar] [CrossRef]
- Live fecal microbiota (Rebyota) for prevention of CDI recurrence. Med. Lett. Drugs Ther. 2023, 65, 35–36. [CrossRef]
- Deepti, I.; Chettri, B.; Mehra, A.; Pinheiro, A.M.; Ravi, R. Faecal microbiota transplantation for recurrent Clostridiodes difficile infection & its global regulatory landscape. Indian J. Med. Res. 2025, 161, 113–119. [Google Scholar] [PubMed]
- Garrigues, Q.; Apper, E.; Mercier, F.; Rodiles, A.; Rovere, N.; Chastant, S.; Mila, H. Composition of the fecal, vaginal and colostrum microbiotas of dams at parturition and their relationship with neonatal outcomes in dogs. Anim. Microbiome 2025, 7, 23. [Google Scholar] [CrossRef]
- Westaway, J.A.F.; Huerlimann, R.; Miller, C.M.; Kandasamy, Y.; Norton, R.; Rudd, D. Methods for exploring the faecal microbiome of premature infants: A review. Matern. Health Neonatol. Perinatol. 2021, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Devarajalu, P.; Kumar, J.; Dutta, S.; Attri, S.V.; Kabeerdoss, J. Gut microbiota alteration in healthy preterm infants: An observational study from tertiary care center in India. Microorganisms 2025, 13, 577. [Google Scholar] [CrossRef] [PubMed]
- Daloiso, V.; Minacori, R.; Refolo, P.; Sacchini, D.; Craxì, L.; Gasbarrini, A.; Spagnolo, A.G. Ethical aspects of fecal microbiota transplantation (FMT). Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3173–3180. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).