Protists with Uncertain Phylogenetic Affiliations for Resolving the Deep Tree of Eukaryotes
Abstract
1. The Potential Role of Protists with Uncertain Phylogenetic Affiliations in the Elucidation of the Eukaryotic Tree of Life
2. Changes in Protist Classification and PUPAs
3. PUPAs Found Their Taxonomic Homes Through Large-Scale Phylogenetic Analyses
3.1. Barthelonids as a Deep-Branch Lineage in Metamonada
3.2. Glissandra Expanded Our Knowledge of the Diversity of CRuMs
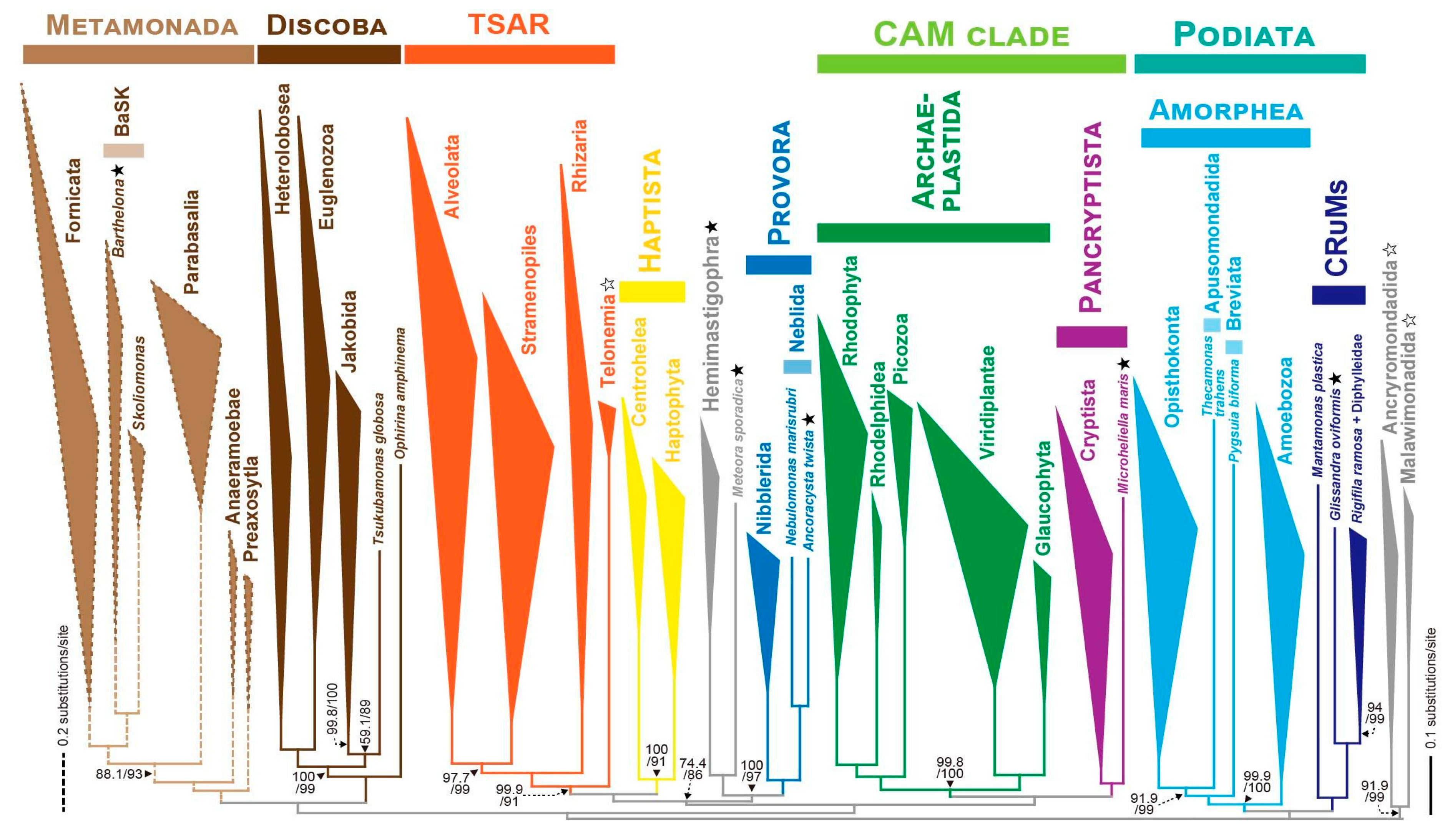
3.3. Emergence of a New Supergroup Encompassing Hemimastigophorans, Meteora, and Provora
3.4. Microheliella Acts as a “Phylogenetic Matchmaker” Between Archaeplastida and Cryptista
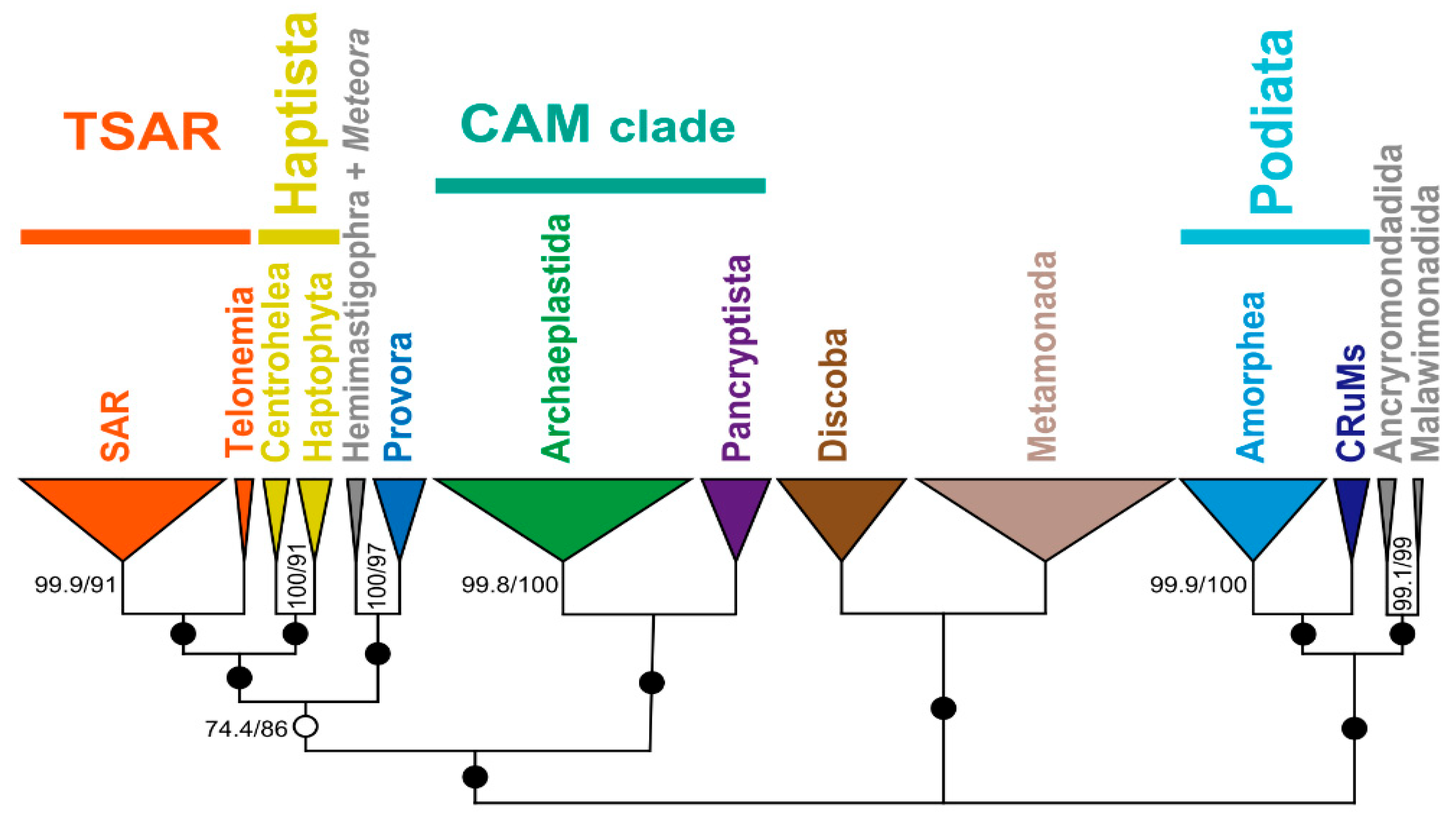
4. Uncertainties in the eToL Related to PUPAs
5. Beyond PUPAs: Novel Protists for Elucidating the eToL
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lennox, J.G. Aristotle’s Philosophy of Biology: Studies in the Origins of Life Science; Cambridge University Press: Cambridge, UK, 2001; ISBN 978-0-521-65027-4. [Google Scholar]
- Connell, S.M. (Ed.) The Cambridge Companion to Aristotle’s Biology, 1st ed.; Cambridge University Press: Cambridge, UK, 2021; ISBN 978-1-108-18179-2. [Google Scholar]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular Condensates: Organizers of Cellular Biochemistry. Nat. Rev. Mol. Cell. Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef]
- Mizushima, N. A Brief History of Autophagy from Cell Biology to Physiology and Disease. Nat. Cell Biol. 2018, 20, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Cerneckis, J.; Cai, H.; Shi, Y. Induced Pluripotent Stem Cells (iPSCs): Molecular Mechanisms of Induction and Applications. Signal Transduct. Target. Ther. 2024, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Dayrat, B. The Roots of Phylogeny: How Did Haeckel Build His Trees? Syst. Biol. 2003, 52, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Woese, C.R.; Kandler, O.; Wheelis, M.L. Towards a Natural System of Organisms: Proposal for the Domains Archaea, Bacteria, and Eucarya. Proc. Natl. Acad. Sci. USA 1990, 87, 4576–4579. [Google Scholar] [CrossRef]
- Nobs, S.-J.; MacLeod, F.I.; Wong, H.L.; Burns, B.P. Eukarya the Chimera: Eukaryotes, a Secondary Innovation of the Two Domains of Life? Trends Microbiol. 2022, 30, 421–431. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; Li, M.; Gu, J.-D. Two or Three Domains: A New View of Tree of Life in the Genomics Era. Appl. Microbiol. Biotechnol. 2018, 102, 3049–3058. [Google Scholar] [CrossRef]
- Williams, T.A.; Cox, C.J.; Foster, P.G.; Szöllősi, G.J.; Embley, T.M. Phylogenomics Provides Robust Support for a Two-Domains Tree of Life. Nat. Ecol. Evol. 2019, 4, 138–147. [Google Scholar] [CrossRef]
- Liu, Y.; Makarova, K.S.; Huang, W.-C.; Wolf, Y.I.; Nikolskaya, A.N.; Zhang, X.; Cai, M.; Zhang, C.-J.; Xu, W.; Luo, Z.; et al. Expanded Diversity of Asgard Archaea and Their Relationships with Eukaryotes. Nature 2021, 593, 553–557. [Google Scholar] [CrossRef]
- Parks, D.H.; Rinke, C.; Chuvochina, M.; Chaumeil, P.-A.; Woodcroft, B.J.; Evans, P.N.; Hugenholtz, P.; Tyson, G.W. Recovery of Nearly 8000 Metagenome-Assembled Genomes Substantially Expands the Tree of Life. Nat. Microbiol. 2017, 2, 1533–1542, Erratum in Nat. Microbiol. 2018, 3, 253. [Google Scholar] [CrossRef]
- Doolittle, W.F. Evolution: Two Domains of Life or Three? Curr. Biol. 2020, 30, R177–R179. [Google Scholar] [CrossRef] [PubMed]
- Burki, F.; Roger, A.J.; Brown, M.W.; Simpson, A.G.B. The New Tree of Eukaryotes. Trends Ecol. Evol. 2020, 35, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Williamson, K.; Eme, L.; Baños, H.; McCarthy, C.G.P.; Susko, E.; Kamikawa, R.; Orr, R.J.S.; Muñoz-Gómez, S.A.; Minh, B.Q.; Simpson, A.G.B.; et al. A Robustly Rooted Tree of Eukaryotes Reveals Their Excavate Ancestry. Nature 2025, 640, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Dudek, N.K.; Sun, C.L.; Burstein, D.; Kantor, R.S.; Aliaga Goltsman, D.S.; Bik, E.M.; Thomas, B.C.; Banfield, J.F.; Relman, D.A. Novel Microbial Diversity and Functional Potential in the Marine Mammal Oral Microbiome. Curr. Biol. 2017, 27, 3752–3762.e6. [Google Scholar] [CrossRef]
- Shiratori, T.; Thakur, R.; Ishida, K. Pseudophyllomitus vesiculosus (Larsen and Patterson 1990) Lee, 2002, a Poorly Studied Phagotrophic Biflagellate Is the First Characterized Member of Stramenopile Environmental Clade MAST-6. Protist 2017, 168, 439–451. [Google Scholar] [CrossRef]
- Keeling, P.J.; Burki, F. Progress towards the Tree of Eukaryotes. Curr. Biol. 2019, 29, R808–R817. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Phillips, R.; Milo, R. The Biomass Distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef]
- Van De Peer, Y.; De Wachter, R. Evolutionary Relationships among the Eukaryotic Crown Taxa Taking into Account Site-to-Site Rate Variation in 18S rRNA. J. Mol. Evol. 1997, 45, 619–630. [Google Scholar] [CrossRef]
- Sogin, M.L.; Silberman, J.D. Evolution of the Protists and Protistan Parasites from the Perspective of Molecular Systematics. Int. J. Parasitol. 1998, 28, 11–20. [Google Scholar] [CrossRef]
- Ben Ali, A.; De Baere, R.; De Wachter, R.; Van De Peer, Y. Evolutionary Relationships among Heterokont Algae (the Autotrophic Stramenopiles) Based on Combined Analyses of Small and Large Subunit Ribosomal RNA. Protist 2002, 153, 123–132. [Google Scholar] [CrossRef]
- Cavalier-Smith, T. The Phagotrophic Origin of Eukaryotes and Phylogenetic Classification of Protozoa. Int. J. Syst. Evol. Microbiol. 2002, 52, 297–354. [Google Scholar] [CrossRef]
- Adl, S.M.; Simpson, A.G.B.; Farmer, M.A.; Andersen, R.A.; Anderson, O.R.; Barta, J.R.; Bowser, S.S.; Brugerolle, G.; Fensome, R.A.; Fredericq, S.; et al. The New Higher Level Classification of Eukaryotes with Emphasis on the Taxonomy of Protists. J. Eukaryot. Microbiol. 2005, 52, 399–451. [Google Scholar] [CrossRef]
- Adl, S.M.; Simpson, A.G.B.; Lane, C.E.; Lukeš, J.; Bass, D.; Bowser, S.S.; Brown, M.W.; Burki, F.; Dunthorn, M.; Hampl, V.; et al. The Revised Classification of Eukaryotes. J. Eukaryot. Microbiol. 2012, 59, 429–514. [Google Scholar] [CrossRef]
- Adl, S.M.; Bass, D.; Lane, C.E.; Lukeš, J.; Schoch, C.L.; Smirnov, A.; Agatha, S.; Berney, C.; Brown, M.W.; Burki, F.; et al. Revisions to the Classification, Nomenclature, and Diversity of Eukaryotes. J. Eukaryot. Microbiol. 2019, 66, 4–119. [Google Scholar] [CrossRef] [PubMed]
- Torruella, G.; Galindo, L.J.; Moreira, D.; López-García, P. Phylogenomics of Neglected Flagellated Protists Supports a Revised Eukaryotic Tree of Life. Curr. Biol. 2025, 35, 198–207.e4. [Google Scholar] [CrossRef] [PubMed]
- Zlatogursky, V.; Boscaro, V.; Lax, G.; Wanntorp, M.; Pohl, N.; Burki, F.; Keeling, P.J. Phylogenetic Position and Mitochondrial Genome Evolution of “Orphan” Eukaryotic Lineages. iScience 2025, 28, 113184. [Google Scholar] [CrossRef]
- Janouškovec, J.; Tikhonenkov, D.V.; Burki, F.; Howe, A.T.; Rohwer, F.L.; Mylnikov, A.P.; Keeling, P.J. A New Lineage of Eukaryotes Illuminates Early Mitochondrial Genome Reduction. Curr. Biol. 2017, 27, 3717–3724.e5. [Google Scholar] [CrossRef] [PubMed]
- Lax, G.; Eglit, Y.; Eme, L.; Bertrand, E.M.; Roger, A.J.; Simpson, A.G.B. Hemimastigophora Is a Novel Supra-Kingdom-Level Lineage of Eukaryotes. Nature 2018, 564, 410–414. [Google Scholar] [CrossRef]
- Tikhonenkov, D.V.; Mikhailov, K.V.; Gawryluk, R.M.R.; Belyaev, A.O.; Mathur, V.; Karpov, S.A.; Zagumyonnyi, D.G.; Borodina, A.S.; Prokina, K.I.; Mylnikov, A.P.; et al. Microbial Predators Form a New Supergroup of Eukaryotes. Nature 2022, 612, 714–719. [Google Scholar] [CrossRef]
- Yazaki, E.; Harada, R.; Isogai, R.; Bamba, K.; Ishida, K.; Inagaki, Y.; Shiratori, T. Glissandra oviformis n. sp.: A Novel Predatory Flagellate Illuminates the Character Evolution within the Eukaryotic Clade CRuMs. Open Biol. 2025, 15, 250057. [Google Scholar] [CrossRef]
- Čepička, I.; Valt, M.; Pánek, T.; Mirzoyan, S.; Tice, A.; Jones, R.; Dohnálek, V.; Dolezal, P.; Mikšátko, J.; Rotterová, J.; et al. Rare Microbial Relict Sheds Light on an Ancient Eukaryotic Supergroup. Res. Sq. 2025. [Google Scholar] [CrossRef]
- Jamy, M.; Huber, T.; Antoine, T.; Ruscheweyh, H.-J.; Paoli, L.; Pelletier, E.; Delmont, T.O.; Burki, F. New Deep-Branching Environmental Plastid Genomes on the Algal Tree of Life. bioRxiv 2025. [Google Scholar] [CrossRef]
- Eglit, Y.; Shiratori, T.; Jerlström-Hultqvist, J.; Williamson, K.; Roger, A.J.; Ishida, K.; Simpson, A.G.B. Meteora sporadica, a Protist with Incredible Cell Architecture, Is Related to Hemimastigophora. Curr. Biol. 2024, 34, 451–459.e6. [Google Scholar] [CrossRef] [PubMed]
- Müller, O.F.; Otto, F. Animalcula Infusoria Fluviatilia et Marina; Typis Nicolai Mölleri: Copenhagen, Danmark, 1786. [Google Scholar]
- Ehrenberg, C.G. Die Infusionsthierchen Als Vollkommene Organismen; Ein Blick in das Tiefere Organische Leben der Natur.: Leipzig, Germany, 1838. [Google Scholar]
- Pritchard, A.; Arlidge, J.T. A History of Infusoria: Including the Dismidiaceœ and Diatomaceœ, British and Foreign; Whitaker and Company: London, UK, 1861. [Google Scholar]
- Hogg, J. On the Distinctions of a Plant and an Animal, and on a Fourth Kingdom of Nature. Edinb. New Philos. J. New Ser. 1860, 12, 216–225. [Google Scholar]
- Haeckel, E. Generelle Morphologie der Organismen; Georg Reimer: Berlin, Germany, 1866. [Google Scholar]
- Goldfuss, G.A. Handbuch Der Zoologie: Anatomie Der Thiere; Arnz & Comp.: Düsseldorf, Germany, 1820. [Google Scholar]
- Von Siebold, C.T.E.; Stannius, H.F. Lehrbuch der Vergleichenden Anatomie; Verlag von Friedrich Vieweg und Sohn: Braunschweig, Germany, 1845. [Google Scholar]
- Bütschli, O. Protozoa. In Bronn’s Klassen und Ordnungen des Thierreichs; C. F. Winter: Leipzig, Germany, 1880; Volume I. [Google Scholar]
- Hall, R.P. Protozoology; Prentice-Hall, Inc.: New York, NY, USA, 1953. [Google Scholar]
- Cavalier-Smith, T. Eukaryote Kingdoms: Seven or Nine? Biosystems 1981, 14, 461–481. [Google Scholar] [CrossRef]
- Moestrup, Ø. Phycological Reviews 7: Flagellar Structure in Algae: A Review, with New Observations Particularly on the Chrysophyceae, Phaeophyceae (Fucophyceae), Euglenophyceae, and Reckertia. Phycologia 1982, 21, 427–528. [Google Scholar] [CrossRef]
- Patterson, D.J. The Fine Structure of Opalina ranarum (Family Opalinidae): Opalinid Phylogeny and Classification. Protistologica 1985, 21, 413–428. [Google Scholar]
- Moestrup, Ø.; Andersen, R.A. Organization of Heterotrophic Heterokonts. In The Biology of Free-Living Heterotrophic Flagellates; Patterson, D.J., Larsen, J., Eds.; Oxford University Press: Oxford, UK, 1992; pp. 333–360. ISBN 978-0-19-857747-8. [Google Scholar]
- Cavalier-Smith, T. The Kingdom Chromista: Origin and Systematics. Prog. Phycol. Res. 1986, 4, 309–347. [Google Scholar]
- Leedale, G.F. Phylogenetic Aspects of Nuclear Cytology in the Algae. Ann. N. Y. Acad. Sci. 1970, 175, 429–453. [Google Scholar] [CrossRef]
- Corliss, J.O. Nuclear Characteristics and Phylogeny in the Protistan Phylum Ciliophora. Biosystems 1975, 7, 338–349. [Google Scholar] [CrossRef]
- Taylor, F.J.R. Flagellate Phylogeny: A Study in Conflicts*. J. Protozool. 1976, 23, 28–40. [Google Scholar] [CrossRef]
- Bremer, K. Summary of Green Plant Phylogeny and Classification. Cladistics 1985, 1, 369–385. [Google Scholar] [CrossRef]
- Patterson, D.J.; Zölffel, M. Heterotrophic Flagellates of Uncertain Taxonomic Position. In The Biology of Free-Living Heterotrophic Flagellates; Oxford University Press: Oxford, UK, 1992; pp. 427–476. ISBN 978-0-19-857747-8. [Google Scholar]
- Baldauf, S.L.; Roger, A.J.; Wenk-Siefert, I.; Doolittle, W.F. A Kingdom-Level Phylogeny of Eukaryotes Based on Combined Protein Data. Science 2000, 290, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Sogin, M.L. Evolution of Eukaryotic Microorganisms and Their Small Subunit Ribosomal RNAs. Am. Zool. 1989, 29, 487–499. [Google Scholar] [CrossRef]
- Sogin, M.L.; Elwood, H.J.; Gunderson, J.H. Evolutionary Diversity of Eukaryotic Small-Subunit rRNA Genes. Proc. Natl. Acad. Sci. USA 1986, 83, 1383–1387. [Google Scholar] [CrossRef]
- Mikrjukov, K.A.; Mylnikov, A.P. Protist Multicilia marina Cienk. Flagellate or a Heliozoon? Dokl. Akad. Nauk 1996, 346, 136–139. [Google Scholar]
- Cienkowski, L. An Account on the White Sea Excursion in 1880. Tr. Leningr. Obs. Estestvoispyt. 1881, 12, 130–171. [Google Scholar]
- Mikrjukov, K.A.; Mylnikov, A.P. The Fine Structure of a Carnivorous Multiflagellar Protist Multicilia marina Cienkowski, 1881 (Flagellata Incertae Sedis). Eur. J. Protistol. 1998, 34, 391–401. [Google Scholar] [CrossRef]
- Nikolaev, S.I.; Berney, C.; Petrov, N.B.; Mylnikov, A.P.; Fahrni, J.F.; Pawlowski, J. Phylogenetic Position of Multicilia marina and the Evolution of Amoebozoa. Int. J. Syst. Evol. Microbiol. 2006, 56, 1449–1458. [Google Scholar] [CrossRef]
- Cavalier-Smith, T. The Protozoan Phylum Opalozoa. J. Eukaryot. Microbiol. 1993, 40, 609–615. [Google Scholar] [CrossRef]
- Sournia, A. Atlas du Phytoplancton Marin; Éditions du CNRS: Paris, France, 1986; Volume 1. [Google Scholar]
- Hoppenrath, M.; Leander, B.S. Dinoflagellate, Euglenid, or Cercomonad? The Ultrastructure and Molecular Phylogenetic Position of Protaspis grandis n. sp. J. Eukaryot. Microbiol. 2006, 53, 327–342. [Google Scholar] [CrossRef]
- Lee, W.J.; Park, J.S. Placement of the Unclassified Cyranomonas australis Lee 2002 within a Novel Clade of Cercozoa. Eur. J. Protistol. 2016, 56, 60–66. [Google Scholar] [CrossRef]
- Zlatogursky, V.V.; Shɨshkin, Y.; Drachko, D.; Burki, F. The Long-time Orphan Protist Meringosphaera mediterranea Lohmann, 1902 [1903] Is a Centrohelid Heliozoan. J. Eukaryot. Microbiol. 2021, 68, e12860. [Google Scholar] [CrossRef] [PubMed]
- Ntakou, E.; Siemensma, F.; Bonkowski, M.; Dumack, K. The Dancing Star: Reinvestigation of Artodiscus saltans (Variosea, Amoebozoa) Penard 1890. Protist 2019, 170, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Ichinomiya, M.; Dos Santos, A.L.; Gourvil, P.; Yoshikawa, S.; Kamiya, M.; Ohki, K.; Audic, S.; De Vargas, C.; Noël, M.-H.; Vaulot, D.; et al. Diversity and Oceanic Distribution of the Parmales (Bolidophyceae), a Picoplanktonic Group Closely Related to Diatoms. ISME J. 2016, 10, 2419–2434. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, A.; Ishida, K. An Orphan Protist Quadricilia rotundata Finally Finds Its Phylogenetic Home in Cercozoa. J. Eukaryot. Microbiol. 2018, 65, 729–732. [Google Scholar] [CrossRef]
- Gómez, F.; Moreira, D.; Benzerara, K.; López-García, P. Solenicola setigera Is the First Characterized Member of the Abundant and Cosmopolitan Uncultured Marine Stramenopile Group MAST-3. Environ. Microbiol. 2011, 13, 193–202. [Google Scholar] [CrossRef]
- Bass, D. Polyubiquitin Insertions and the Phylogeny of Cercozoa and Rhizaria. Protist 2005, 156, 149–161. [Google Scholar] [CrossRef]
- Park, J.S.; Simpson, A.G.B.; Lee, W.J.; Cho, B.C. Ultrastructure and Phylogenetic Placement within Heterolobosea of the Previously Unclassified, Extremely Halophilic Heterotrophic Flagellate Pleurostomum flabellatum (Ruinen 1938). Protist 2007, 158, 397–413. [Google Scholar] [CrossRef]
- Bass, D.; Chao, E.E.; Nikolaev, S.; Yabuki, A.; Ishida, K.; Berney, C.; Pakzad, U.; Wylezich, C.; Cavalier-Smith, T. Phylogeny of Novel Naked Filose and Reticulose Cercozoa: Granofilosea cl. n. and Proteomyxidea Revised. Protist 2009, 160, 75–109. [Google Scholar] [CrossRef]
- Zadrobílková, E.; Smejkalová, P.; Walker, G.; Čepička, I. Morphological and Molecular Diversity of the Neglected Genus Rhizomastix Alexeieff, 1911 (Amoebozoa: Archamoebae) with Description of Five New Species. J. Eukaryot. Microbiol. 2016, 63, 181–197. [Google Scholar] [CrossRef]
- Chantangsi, C.; Leander, B.S. Ultrastructure, Life Cycle and Molecular Phylogenetic Position of a Novel Marine Sand-Dwelling Cercozoan: Clautriavia biflagellata n. sp. Protist 2010, 161, 133–147. [Google Scholar] [CrossRef]
- Shiratori, T.; Yokoyama, A.; Ishida, K. Phylogeny, Ultrastructure, and Flagellar Apparatus of a New Marimonad Flagellate Abollifer globosa sp. nov. (Imbricatea, Cercozoa). Protist 2014, 165, 808–824. [Google Scholar] [CrossRef]
- Gomaa, F.; Mitchell, E.A.D.; Lara, E. Amphitremida (Poche, 1913) Is a New Major, Ubiquitous Labyrinthulomycete Clade. PLoS ONE 2013, 8, e53046. [Google Scholar] [CrossRef] [PubMed]
- Cavalier-Smith, T.; Chao, E.E. Phylogeny and Megasystematics of Phagotrophic Heterokonts (Kingdom Chromista). J. Mol. Evol. 2006, 62, 388–420. [Google Scholar] [CrossRef] [PubMed]
- Bass, D.; Yabuki, A.; Santini, S.; Romac, S.; Berney, C. Reticulamoeba Is a Long-Branched Granofilosean (Cercozoa) That Is Missing from Sequence Databases. PLoS ONE 2012, 7, e49090. [Google Scholar] [CrossRef] [PubMed]
- Galindo, L.J.; López-García, P.; Moreira, D. First Molecular Characterization of the Elusive Marine Protist Meteora sporadica. Protist 2022, 173, 125896. [Google Scholar] [CrossRef]
- Brugerolle, G.; Bricheux, G.; Philippe, H.; Coffe, G. Collodictyon triciliatum and Diphylleia rotans (=Aulacomonas submarina) Form a New Family of Flagellates (Collodictyonidae) with Tubular Mitochondrial Cristae That Is Phylogenetically Distant from Other Flagellate Groups. Protist 2002, 153, 59–70. [Google Scholar] [CrossRef]
- Klaveness, D.; Shalchian-Tabrizi, K.; Thomsen, H.A.; Eikrem, W.; Jakobsen, K.S. Telonema antarcticum sp. nov., a Common Marine Phagotrophic Flagellate. Int. J. Syst. Evol. Microbiol. 2005, 55, 2595–2604. [Google Scholar] [CrossRef]
- Katara, A.; Chand, S.; Chaudhary, H.; Chaudhry, V.; Chandra, H.; Dubey, R.C. Evolution and Applications of Next Generation Sequencing and Its Intricate Relations with Chromatographic and Spectrometric Techniques in Modern Day Sciences. J. Chromatogr. Open 2024, 5, 100121. [Google Scholar] [CrossRef]
- Yabuki, A.; Chao, E.E.; Ishida, K.; Cavalier-Smith, T. Microheliella maris (Microhelida Ord. n.), an Ultrastructurally Highly Distinctive New Axopodial Protist Species and Genus, and the Unity of Phylum Heliozoa. Protist 2012, 163, 356–388. [Google Scholar] [CrossRef] [PubMed]
- Rokas, A.; Williams, B.L.; King, N.; Carroll, S.B. Genome-Scale Approaches to Resolving Incongruence in Molecular Phylogenies. Nature 2003, 425, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Wascher, M.; Kubatko, L. Consistency of SVDQuartets and Maximum Likelihood for Coalescent-Based Species Tree Estimation. Syst. Biol. 2021, 70, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Steenwyk, J.L.; Li, Y.; Zhou, X.; Shen, X.-X.; Rokas, A. Incongruence in the Phylogenomics Era. Nat. Rev. Genet. 2023, 24, 834–850. [Google Scholar] [CrossRef]
- Bernard, C.; Simpson, A.G.B.; Patterson, D.J. Some Free-Living Flagellates (Protista) from Anoxic Habitats. Ophelia 2000, 52, 113–142. [Google Scholar] [CrossRef]
- Lee, W.J. Some Free-living Heterotrophic Flagellates from Marine Sediments of Inchon and Ganghwa Island, Korea. Korean J. Biol. Sci. 2002, 6, 125–143. [Google Scholar] [CrossRef]
- Lee, W.J. Some Free-Living Heterotrophic Flagellates from Marine Sediments of Tropical Australia. Ocean Sci. J. 2006, 41, 75–95. [Google Scholar] [CrossRef]
- Yazaki, E.; Kume, K.; Shiratori, T.; Eglit, Y.; Tanifuji, G.; Harada, R.; Simpson, A.G.B.; Ishida, K.; Hashimoto, T.; Inagaki, Y. Barthelonids Represent a Deep-Branching Metamonad Clade with Mitochondrion-Related Organelles Predicted to Generate No ATP. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201538. [Google Scholar] [CrossRef]
- Eglit, Y.; Williams, S.K.; Roger, A.J.; Simpson, A.G.B. Characterization of Skoliomonas gen. nov., a Haloalkaliphilic Anaerobe Related to Barthelonids (Metamonada). J. Eukaryot. Microbiol. 2024, 71, e13048. [Google Scholar] [CrossRef]
- Williams, S.K.; Jerlström Hultqvist, J.; Eglit, Y.; Salas-Leiva, D.E.; Curtis, B.; Orr, R.J.S.; Stairs, C.W.; Atalay, T.N.; MacMillan, N.; Simpson, A.G.B.; et al. Extreme Mitochondrial Reduction in a Novel Group of Free-Living Metamonads. Nat. Commun. 2024, 15, 6805. [Google Scholar] [CrossRef]
- Patterson, D.J.; Simpson, A.G.B. Heterotrophic Flagellates from Coastal Marine and Hypersaline Sediments in Western Australia. Eur. J. Protistol. 1996, 32, 423–448. [Google Scholar] [CrossRef]
- Brown, M.W.; Heiss, A.A.; Kamikawa, R.; Inagaki, Y.; Yabuki, A.; Tice, A.K.; Shiratori, T.; Ishida, K.; Hashimoto, T.; Simpson, A.G.B.; et al. Phylogenomics Places Orphan Protistan Lineages in a Novel Eukaryotic Super-Group. Genome Biol. Evol. 2018, 10, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, A.; Ishida, K.; Cavalier-Smith, T. Rigifila ramosa n. gen., n. sp., a Filose Apusozoan with a Distinctive Pellicle, Is Related to Micronuclearia. Protist 2013, 164, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Brugerolle, G. Description of a New Freshwater Heterotrophic Flagellate Sulcomonas lacustris Affiliated to the Collodictyonids. Acta Protozool. 2006, 45, 175–182. [Google Scholar]
- Tice, A.K.; Žihala, D.; Pánek, T.; Jones, R.E.; Salomaki, E.D.; Nenarokov, S.; Burki, F.; Eliáš, M.; Eme, L.; Roger, A.J.; et al. PhyloFisher: A Phylogenomic Package for Resolving Eukaryotic Relationships. PLoS Biol. 2021, 19, e3001365. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Criscuolo, A.; Gribaldo, S. BMGE (Block Mapping and Gathering with Entropy): A New Software for Selection of Phylogenetic Informative Regions from Multiple Sequence Alignments. BMC Evol. Biol. 2010, 10, 210. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Foissner, W.; Blatterer, H.; Foissner, I. The Hemimastigophora (Hemimastix amphikineta nov. gen., nov. spec.), a New Protistan Phylum from Gondwanian Soils. Eur. J. Protistol. 1988, 23, 361–383. [Google Scholar] [CrossRef]
- Foissner, I.; Foissner, W. Revision of the Family Spironemidae Doflein (Protista, Hemimastigophora), With Description of Two New Species, Spironema terricola N. Sp. and Stereonema geiseri n, g., n. ap. J. Eukaryot. Microbiol. 1993, 40, 422–438. [Google Scholar] [CrossRef]
- Hausmann, K.; Weitere, M.; Wolf, M.; Arndt, H. Meteora sporadica gen. nov. et sp. nov. (Protista Incertae Sedis)—An Extraordinary Free-Living Protist from the Mediterranean Deep Sea. Eur. J. Protistol. 2002, 38, 171–177. [Google Scholar] [CrossRef]
- Cavalier-Smith, T.; Chao, E.E. Molecular Phylogeny of Centrohelid Heliozoa, a Novel Lineage of Bikont Eukaryotes That Arose by Ciliary Loss. J. Mol. Evol. 2003, 56, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Cavalier-Smith, T.; Chao, E.E.; Lewis, R. Multiple Origins of Heliozoa from Flagellate Ancestors: New Cryptist Subphylum Corbihelia, Superclass Corbistoma, and Monophyly of Haptista, Cryptista, Hacrobia and Chromista. Mol. Phylogenet. Evol. 2015, 93, 331–362. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, E.; Yabuki, A.; Imaizumi, A.; Kume, K.; Hashimoto, T.; Inagaki, Y. The Closest Lineage of Archaeplastida Is Revealed by Phylogenomics Analyses That Include Microheliella maris. Open Biol. 2022, 12, 210376. [Google Scholar] [CrossRef]
- Yabuki, A.; Kamikawa, R.; Ishikawa, S.A.; Kolisko, M.; Kim, E.; Tanabe, A.S.; Kume, K.; Ishida, K.; Inagki, Y. Palpitomonas bilix Represents a Basal Cryptist Lineage: Insight into the Character Evolution in Cryptista. Sci. Rep. 2014, 4, 4641. [Google Scholar] [CrossRef]
- Gawryluk, R.M.R.; Tikhonenkov, D.V.; Hehenberger, E.; Husnik, F.; Mylnikov, A.P.; Keeling, P.J. Non-Photosynthetic Predators Are Sister to Red Algae. Nature 2019, 572, 240–243. [Google Scholar] [CrossRef]
- Burki, F.; Kaplan, M.; Tikhonenkov, D.V.; Zlatogursky, V.; Minh, B.Q.; Radaykina, L.V.; Smirnov, A.; Mylnikov, A.P.; Keeling, P.J. Untangling the Early Diversification of Eukaryotes: A Phylogenomic Study of the Evolutionary Origins of Centrohelida, Haptophyta and Cryptista. Proc. R. Soc. B Biol. Sci. 2016, 283, 20152802. [Google Scholar] [CrossRef]
- Bamba, K.; Harada, R.; Inagaki, Y. AUTOEB: A Software for Systematically Evaluating Bipartitions in a Phylogenetic Tree Employing an Approximately Unbiased Test. IPSJ Trans. Bioinform. 2024, 17, 72–82. [Google Scholar] [CrossRef]
- Yabuki, A.; Eikrem, W.; Takishita, K.; Patterson, D.J. Fine Structure of Telonema subtilis Griessmann, 1913: A Flagellate with a Unique Cytoskeletal Structure among Eukaryotes. Protist 2013, 164, 556–569. [Google Scholar] [CrossRef]
- Tikhonenkov, D.V.; Jamy, M.; Borodina, A.S.; Belyaev, A.O.; Zagumyonnyi, D.G.; Prokina, K.I.; Mylnikov, A.P.; Burki, F.; Karpov, S.A. On the Origin of TSAR: Morphology, Diversity and Phylogeny of Telonemia. Open Biol. 2022, 12, 210325. [Google Scholar] [CrossRef]
- Strassert, J.F.H.; Jamy, M.; Mylnikov, A.P.; Tikhonenkov, D.V.; Burki, F. New Phylogenomic Analysis of the Enigmatic Phylum Telonemia Further Resolves the Eukaryote Tree of Life. Mol. Biol. Evol. 2019, 36, 757–765. [Google Scholar] [CrossRef]
- Schön, M.E.; Zlatogursky, V.V.; Singh, R.P.; Poirier, C.; Wilken, S.; Mathur, V.; Strassert, J.F.H.; Pinhassi, J.; Worden, A.Z.; Keeling, P.J.; et al. Single Cell Genomics Reveals Plastid-Lacking Picozoa Are Close Relatives of Red Algae. Nat. Commun. 2021, 12, 6651. [Google Scholar] [CrossRef]
- Harada, R.; Hirakawa, Y.; Yabuki, A.; Kim, E.; Yazaki, E.; Kamikawa, R.; Nakano, K.; Eliáš, M.; Inagaki, Y. Encyclopedia of Family A DNA Polymerases Localized in Organelles: Evolutionary Contribution of Bacteria Including the Proto-mitochondrion. Mol. Biol. Evol. 2024, 41, msae014. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, H. An Approximately Unbiased Test of Phylogenetic Tree Selection. Syst. Biol. 2002, 51, 492–508. [Google Scholar] [CrossRef] [PubMed]
- O’Kelly, C.J.; Nerad, T.A. Malawimonas Jakobiformis n. gen., n. sp. (Malawimonadidae n. Fam.): A Jakoba-like Heterotrophic Nanoflagellate with Discoidal Mitochondrial Cristae. J. Eukaryot. Microbiol. 1999, 46, 522–531. [Google Scholar] [CrossRef]
- Heiss, A.A.; Kolisko, M.; Ekelund, F.; Brown, M.W.; Roger, A.J.; Simpson, A.G.B. Combined Morphological and Phylogenomic Re-Examination of Malawimonads, a Critical Taxon for Inferring the Evolutionary History of Eukaryotes. R. Soc. Open Sci. 2018, 5, 171707. [Google Scholar] [CrossRef]
- Heiss, A.A.; Warring, S.D.; Lukacs, K.; Favate, J.; Yang, A.; Gyaltshen, Y.; Filardi, C.; Simpson, A.G.B.; Kim, E. Description of Imasa heleensis, gen. nov., sp. nov. (Imasidae, fam. nov.), a Deep-Branching Marine Malawimonad and Possible Key Taxon in Understanding Early Eukaryotic Evolution. J. Eukaryot. Microbiol. 2021, 68, e12837. [Google Scholar] [CrossRef]
- Simpson, A.G.B. Cytoskeletal Organization, Phylogenetic Affinities and Systematics in the Contentious Taxon Excavata (Eukaryota). Int. J. Syst. Evol. Microbiol. 2003, 53, 1759–1777. [Google Scholar] [CrossRef]
- Karpov, S.A. The Flagellar Apparatus Structure of Apusomonas poboscidea and Apusomonad Relationships. Protistology 2007, 5, 146–155. [Google Scholar]
- Heiss, A.A.; Walker, G.; Simpson, A.G.B. The Ultrastructure of Ancyromonas, a Eukaryote without Supergroup Affinities. Protist 2011, 162, 373–393. [Google Scholar] [CrossRef]
- Yubuki, N.; Torruella, G.; Galindo, L.J.; Heiss, A.A.; Ciobanu, M.C.; Shiratori, T.; Ishida, K.; Blaz, J.; Kim, E.; Moreira, D.; et al. Molecular and Morphological Characterization of Four New Ancyromonad Genera and Proposal for an Updated Taxonomy of the Ancyromonadida. J. Eukaryot. Microbiol. 2023, 70, e12997. [Google Scholar] [CrossRef]
- Cavalier-Smith, T. Early Evolution of Eukaryote Feeding Modes, Cell Structural Diversity, and Classification of the Protozoan Phyla Loukozoa, Sulcozoa, and Choanozoa. Eur. J. Protistol. 2013, 49, 115–178. [Google Scholar] [CrossRef]
- Isogai, R.; Harada, R.; Nakayama, T.; Inagaki, Y. Phylogenetic Dissection Provides Insights into the Incongruity in the Tree of Archaeplastida between the Analyses of Nucleus- and Plastid-Encoded Proteins. bioRxiv 2025. [Google Scholar] [CrossRef]


| Name | Taxa Assumed by Morphological Data | Affiliations Identified by SSU rDNA Phylogeny |
|---|---|---|
| Cyranomonas | Unclassified | Novel clade CU (Cercozoa) [65] |
| Ebriida | Dinoflagellata Silicoflagellata Unclassified | Thecofilosea (Cercozoa) [64] |
| Meringosphaera | Chrysophyceae (Stramenopiles) Centroplasthelida Unclassified | Environmental marine clade NC5 (Centroplasthelida) [66] |
| Multicilia | Amoebozoa Rhizomastigina Unclassified | Variosea (Amoebozoa) [61,67] |
| Parmales | Chrysophyceae (stramenopiles) | Bolidophyceae (Stramenopiles) [68] |
| Pseudophyllomitus | Unclassified | MAST-6 (Stramenopiles) [17] |
| Quadricilia | Unclassified | Novel clade 2 (Cercozoa) [69] |
| Solenicola | Xanthophyceae (Stramenopiles) Unclassified | MAST-3 (Stramenopiles) [70] |
| Metopion | Unclassified | Metopiida (Cercozoa) [71] |
| Pleurostomum | Unclassified | Heterolobosea (Discoba) [72] |
| Vampyrellidae | Sarcodina | Novel clade 8 (Endomyxa) [73] |
| Rhizomastix | Mastigophora | Archamoebae (Amoebozoa) [74] |
| Protaspa | Dinoflagella Cercozoa Euglenozoa | Thecofilosea (Cercozoa) [64] |
| Artodiscus | Amoebozoa Cercozoa Sarcodina | Variosea (Amoebozoa) [67] |
| Clautriavia | Unclassified | Imbricatea (Cercozoa) [75] |
| Abollifer | Unclassified | Imbricatea (Cercozoa) [76] |
| Amphitremida | Sarcodina Thecofilosea (Cercozoa) | Labyrinthulomycetes (Stramenopiles) [77] |
| Paramonas | Unclassified | Bicosoecida (Stramenopiles) [78] |
| Reticulamoeba | Granofilosea (Cercozoa) Proteomyxidea Unclassified | Granofilosea (Cercozoa) [79] |
| Environment | Percentage of ASVs with Similarity < 85.0% |
|---|---|
| Marine water | 16.73% |
| Marine sediment | 19.24% |
| Marine organism | 13.16% |
| Marine ice | 5.70% |
| Land water | 8.50% |
| Land soil | 29.84% |
| Land sediment | 22.00% |
| Land organism | 11.04% |
| Land freshwater | 17.36% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yazaki, E.; Shiratori, T.; Inagaki, Y. Protists with Uncertain Phylogenetic Affiliations for Resolving the Deep Tree of Eukaryotes. Microorganisms 2025, 13, 1926. https://doi.org/10.3390/microorganisms13081926
Yazaki E, Shiratori T, Inagaki Y. Protists with Uncertain Phylogenetic Affiliations for Resolving the Deep Tree of Eukaryotes. Microorganisms. 2025; 13(8):1926. https://doi.org/10.3390/microorganisms13081926
Chicago/Turabian StyleYazaki, Euki, Takashi Shiratori, and Yuji Inagaki. 2025. "Protists with Uncertain Phylogenetic Affiliations for Resolving the Deep Tree of Eukaryotes" Microorganisms 13, no. 8: 1926. https://doi.org/10.3390/microorganisms13081926
APA StyleYazaki, E., Shiratori, T., & Inagaki, Y. (2025). Protists with Uncertain Phylogenetic Affiliations for Resolving the Deep Tree of Eukaryotes. Microorganisms, 13(8), 1926. https://doi.org/10.3390/microorganisms13081926





