Resurgence of Bordetella pertussis in Lazio: A Cross-Age Surveillance Study from Two Referral Hospitals
Abstract
1. Introduction
2. Materials and Methods
2.1. Adult Population
2.2. Pediatric Population
2.3. Statistical Analyses
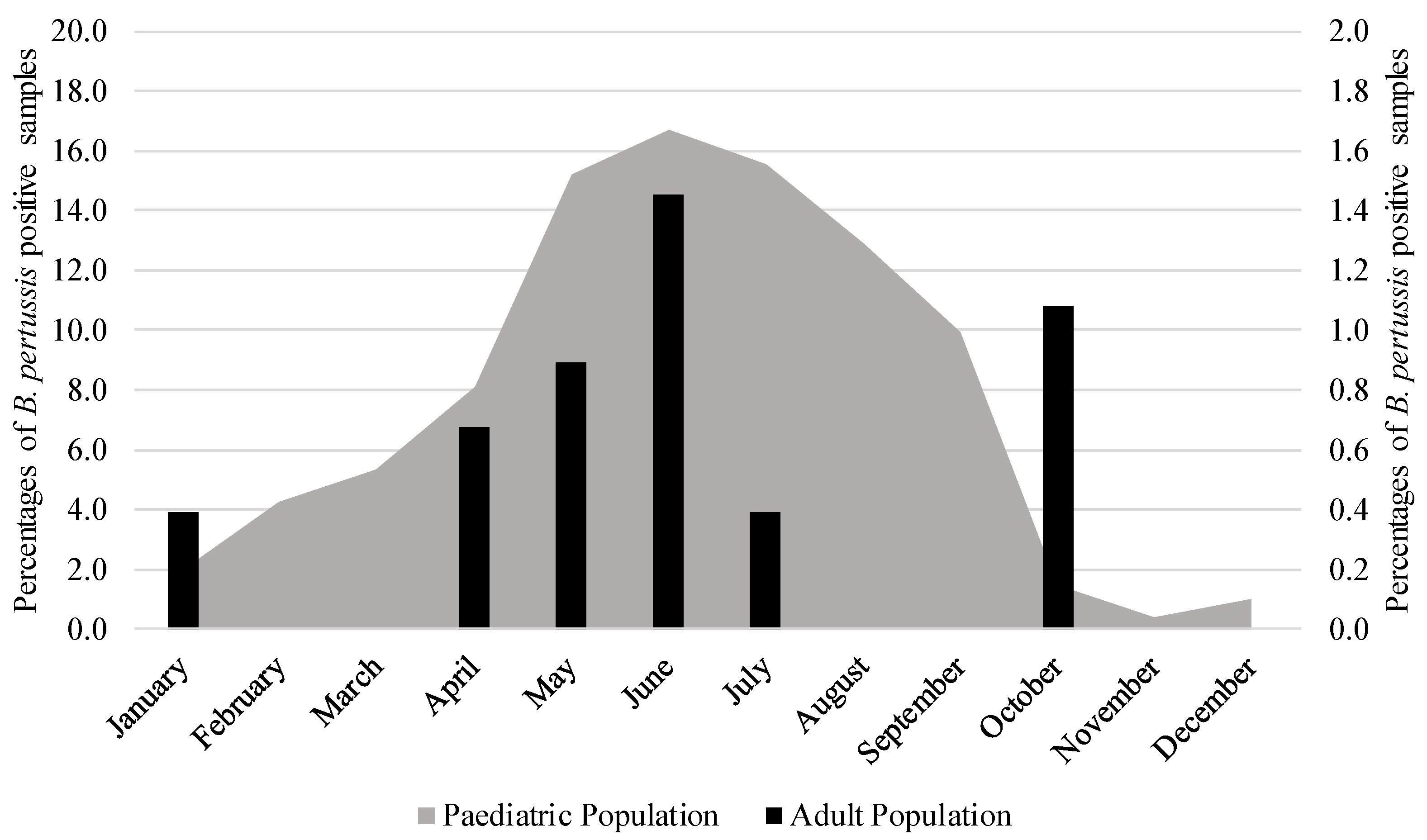
3. Results
Trend and Regression Analysis
4. Discussion
5. Limitations of This Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gabutti, G.; Azzari, C.; Bonanni, P.; Prato, R.; Tozzi, A.E.; Zanetti, A.; Zuccotti, G. Pertussis. Hum. Vaccin. Immunother. 2015, 11, 108–117. [Google Scholar] [CrossRef] [PubMed]
- UK Health Security Agency Confirmed Cases of Pertussis in England by Month. 2024. Available online: https://www.gov.uk/government/publications/pertussis-epidemiology-in-england-2024/confirmed-cases-of-pertussis-in-england-by-month (accessed on 21 July 2025).
- Broutin, H.; Viboud, C.; Grenfell, B.T.; Miller, M.A.; Rohani, P. Impact of Vaccination and Birth Rate on the Epidemiology of Pertussis: A Comparative Study in 64 Countries. Proc. Biol. Sci. 2010, 277, 3239–3245. [Google Scholar] [CrossRef] [PubMed]
- PERISCOPE Consortium PERISCOPE: Road towards Effective Control of Pertussis. Lancet Infect. Dis. 2019, 19, e179–e186. [CrossRef] [PubMed]
- World Health Organization Pertussis. Available online: https://www.who.int/health-topics/pertussis#tab=tab_1 (accessed on 9 June 2025).
- World Health Organization Global Childhood Vaccination Coverage Holds Steady, yet over 14 Million Infants Remain Unvaccinated—WHO, UNICEF. Available online: https://www.who.int/news/item/15-07-2025-global-childhood-vaccination-coverage-holds-steady-yet-over-14-million-infants-remain-unvaccinated-who-unicef (accessed on 21 July 2025).
- European Centre for Disease Prevention and Control Increase of Pertussis Cases in the EU/EEA. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Increase%20in%20pertussis%20cases%20in%20the%20EU-EEA%20-%20May%202024%20FINAL.pdf (accessed on 21 July 2025).
- Istituto Superiore di Sanità Vaccini e Vaccinazioni. Available online: https://www.epicentro.iss.it/vaccini/dati_ita#pertosse (accessed on 21 July 2025).
- Poltorak, V.; Cabré-Riera, A.; Martínez-Botías, F.; Borràs López, E.; Clotet Romero, L.; Sala Farré, M.R.; Jané Checa, M. Increase of Pertussis Cases in the Vallès Region, Catalonia, Spain, September 2023 to April 2024. Euro Surveill. 2024, 29, 2400332. [Google Scholar] [CrossRef] [PubMed]
- Poeta, M.; Moracas, C.; Albano, C.; Petrarca, L.; Maglione, M.; Pierri, L.; Carta, M.; Montaldo, P.; Venturini, E.; De Luca, M.; et al. Pertussis Outbreak in Neonates and Young Infants across Italy, January to May 2024: Implications for Vaccination Strategies. Euro Surveill. 2024, 29, 2400301. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Samara, A.; Campbell, H.; Ladhani, S.N.; Amirthalingam, G. Recent Increase in Infant Pertussis Cases in Europe and the Critical Importance of Antenatal Immunizations: We Must Do Better…now. Int. J. Infect. Dis. 2024, 146, 107148. [Google Scholar] [CrossRef] [PubMed]
- Scutari, R.; Linardos, G.; Ranno, S.; Pisani, M.; Vittucci, A.C.; Coltella, L.; Colagrossi, L.; Di Maio, V.C.; Sisto, A.; Mancinelli, L.; et al. A New Epidemic Wave of Bordetella Pertussis in Paediatric Population: Impact and Role of Co-Infections in Pertussis Disease. Ital. J. Pediatr. 2025, 51, 7. [Google Scholar] [CrossRef] [PubMed]
- Qiagen QIAstat-Dx Respiratory Panel Plus. Available online: https://www.qiagen.com/us/products/diagnostics-and-clinical-research/infectious-disease/qiastat-dx-syndromic-testing/qiastat-dx-na (accessed on 9 June 2025).
- bioMérieux BIOFIRE® Respiratory 2.1 and 2.1plus. Available online: https://www.biomerieux.com/corp/en/our-offer/clinical-products/biofire-respiratory-2-1-panels.html#tabs-8cd7ba93fe-item-51e9655c24 (accessed on 9 June 2025).
- Qiagen Genomic DNA Extraction. Available online: https://www.qiagen.com/us/product-categories/discovery-and-translational-research/dna-rna-purification/dna-purification/genomic-dna?cmpid=PC_QF_NON_dna-purification-sales_0321_SEA_GA&gad_source=1&gad_campaignid=12498785447&gbraid=0AAAAAD-RrCwyJwRpBrq2bF66oai2IPCw7&gclid=EAIaIQobChMI2aKikZbOjgMVqo-DBx2moAs7EAAYASAAEgJdePD_BwE (accessed on 21 July 2025).
- Seegene AllplexTM Respiratory Panel 4. Available online: https://www.seegene.com/assays/allplex_respiratory_panel_4 (accessed on 9 June 2025).
- Bio-Rad CFX96 Dx Real-Time PCR Detection Systems for In Vitro Diagnostics (IVD). Available online: https://www.bio-rad.com/it-it/product/cfx96-dx-real-time-pcr-detection-systems-for-vitro-diagnostics-ivd?ID=NQH35P15 (accessed on 9 June 2025).
- bioMérieux ARGENE®. Available online: https://www.biomerieux.com/content/dam/biomerieux-com/03----our-offer/clinical/in-hospital--in-lab/products/argene-respiratory-range/documents/9313606-007-gb-a%20argene%20respiratory-hd.pdf (accessed on 21 July 2025).
- Moraga-Llop, F.; Garcés-Sánchez, M.; González-López, J.J. Reemergence of Pertussis: Strategies and Challenges in Its Control in Spain. An. Pediatr. Engl. Ed. 2024, 101, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Nordholm, A.C.; Emborg, H.-D.; Nørgaard, S.K.; Nygaard, U.; Ronayne, A.; Nielsen, L.B.; Søborg, B.; Andersen, P.H.; Dalby, T. Pertussis Epidemic in Denmark, August 2023 to February 2024. Euro Surveill. 2024, 29, 2400160. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Bouchez, V.; Soares, A.; Trombert-Paolantoni, S.; Aït El Belghiti, F.; Cohen, J.F.; Armatys, N.; Landier, A.; Blanchot, T.; Hervo, M.; et al. Resurgence of Bordetella Pertussis, Including One Macrolide-Resistant Isolate, France, 2024. Euro Surveill. 2024, 29, 2400459. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Wu, Y. Co-Infection in Unvaccinated Infants with Acute Pertussis in Western China (2018–2019): Pathogen Distribution and Impact on Disease Severity. Ital. J. Pediatr. 2025, 51, 111. [Google Scholar] [CrossRef] [PubMed]
- Sberna, G.; Bordi, L.; Mija, C.; Girardi, E.; Maggi, F.; Lalle, E. A Retrospective Study of Respiratory Viruses in a Four-Year Study of Nasal Swabs from Patients with Severe Influenza-like Symptoms in the Lazio Region, Italy. Viruses 2025, 17, 452. [Google Scholar] [CrossRef]
- Istituto Superiore di Sanità La Pertosse, Una Malattia Prevenibile Con La Vaccinazione: Priorità Diagnostiche. Available online: https://www.iss.it/documents/20126/45616/18_23_web.pdf/d84c010e-b06d-3f1f-28f7-a2e7598b4325?t=1581095816428 (accessed on 9 June 2025).
- Centers for Disease Control and Prevention Treatment of Pertussis. Available online: https://www.cdc.gov/pertussis/hcp/clinical-care/index.html (accessed on 21 July 2025).
- Ministero della Sulute Adulti 19–60 Anni. Available online: https://www.salute.gov.it/new/it/tema/vaccinazioni/adulti-19-60-anni/#:~:text=Nel%20corso%20dell’et%C3%A0%20adulta%20%C3%A8%20opportuna%20la,modo%20attivo%2C%20trovando%20anche%20le%20occasioni%20opportune (accessed on 21 July 2025).

| 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NPS 1 | LRTS 2 | NPS | LRTS | NPS | LRTS | NPS | LRTS | NPS | LRTS | NPS | LRTS | |
| P 3/T 4 | P/T | P/T | P/T | P/T | P/T | P/T | P/T | P/T | P/T | P/T | P/T | |
| January | 1/80 | 0/16 | 1/158 | 0/10 | 0/92 | 0/10 | 0/76 | 0/14 | 0/159 | 0/25 | 1/168 | 0/53 |
| February | 0/74 | 0/22 | 0/332 | 0/7 | 0/170 | 0/11 | 0/62 | 0/17 | 2/151 | 0/17 | 0/34 | 0/39 |
| March | 0/55 | 0/8 | 2/398 | 0/11 | 0/307 | 0/10 | 0/83 | 0/17 | 1/102 | 0/19 | 0/60 | 0/46 |
| April | 0/30 | 0/10 | 0/224 | 0/6 | 0/139 | 0/12 | 0/90 | 0/17 | 0/83 | 0/18 | 1/65 | 0/45 |
| May | 0/23 | 0/12 | 1/213 | 0/14 | 0/109 | 0/9 | 0/52 | 0/35 | 0/81 | 0/20 | 2/99 | 0/38 |
| June | 1/25 | 0/7 | 0/78 | 0/1 | 0/87 | 0/15 | 0/38 | 0/13 | 0/70 | 0/15 | 4/113 | 0/19 |
| July | 0/13 | 0/3 | 0/85 | 0/9 | 0/63 | 0/13 | 0/63 | 0/9 | 0/68 | 0/7 | 1/106 | 0/9 |
| August | 0/10 | 0/4 | 0/29 | 0/4 | 0/62 | 0/7 | 0/56 | 0/14 | 0/64 | 0/17 | 0/112 | 0/22 |
| September | 0/18 | 0/1 | 0/97 | 0/3 | 0/52 | 0/12 | 0/55 | 0/13 | 0/60 | 0/11 | 0/116 | 0/20 |
| October | 0/32 | 0/6 | 0/114 | 0/1 | 0/71 | 0/12 | 0/91 | 0/7 | 0/66 | 0/5 | 2/154 | 1/18 |
| November | 0/44 | 0/1 | 0/101 | 0/0 | 0/106 | 0/9 | 0/76 | 0/12 | 0/86 | 0/16 | 0/145 | 0/24 |
| December | 1/54 | 0/7 | 0/123 | 0/0 | 0/93 | 0/9 | 0/106 | 0/15 | 0/143 | 0/17 | 0/237 | 0/21 |
| Total | 3/458 | 0/97 | 4/1952 | 0/66 | 0/1351 | 0/129 | 0/848 | 0/183 | 3/1133 | 0/187 | 11/1408 | 1/355 |
| 3/555 | 4/2018 | 0/1480 | 0/1031 | 3/1320 | 12/1763 | |||||||
| 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NPS 1 | LRTS 2 | NPS | LRTS | NPS | LRTS | NPS | LRTS | NPS | LRTS | NPS | LRTS | |
| P 3/T 4 | P/T | P/T | P/T | P/T | P/T | P/T | P/T | P/T | P/T | P/T | P/T | |
| January | 0/5 | 4/208 | 0/28 | 8/133 | 0/1 | 0/7 | 0/0 | 0/18 | 0/6 | 0/37 | 0/7 | 5/29 |
| February | 0/4 | 1/152 | 2/57 | 3/83 | 0/1 | 0/12 | 0/0 | 0/12 | 0/1 | 0/11 | 0/1 | 4/20 |
| March | 1/6 | 3/121 | 0/30 | 2/42 | 0/3 | 0/10 | 0/6 | 0/9 | 0/3 | 0/18 | 1/8 | 7/36 |
| April | 0/1 | 6/81 | 0/10 | 1/13 | 0/1 | 0/8 | 0/0 | 0/12 | 0/1 | 0/12 | 3/13 | 9/25 |
| May | 0/2 | 5/62 | 0/0 | 0/5 | 0/0 | 0/17 | 0/1 | 0/12 | 0/3 | 0/13 | 23/58 | 11/29 |
| June | 0/1 | 3/43 | 0/4 | 1/17 | 0/0 | 0/11 | 0/2 | 0/6 | 0/1 | 0/8 | 38/115 | 8/29 |
| July | 1/3 | 4/20 | 0/2 | 0/11 | 0/2 | 0/6 | 0/1 | 0/3 | 0/7 | 0/6 | 38/122 | 2/20 |
| August | 4/6 | 2/21 | 0/1 | 0/21 | 0/1 | 0/7 | 0/0 | 0/6 | 1/6 | 1/5 | 22/66 | 7/25 |
| September | 1/3 | 3/30 | 0/5 | 0/15 | 0/1 | 0/4 | 0/1 | 0/9 | 0/8 | 0/10 | 17/73 | 6/22 |
| October | 0/2 | 3/36 | 0/3 | 0/18 | 0/9 | 0/22 | 0/1 | 0/14 | 1/10 | 0/5 | 4/87 | 0/18 |
| November | 0/13 | 0/63 | 0/1 | 0/18 | 0/25 | 0/64 | 0/5 | 0/25 | 0/3 | 0/11 | 1/71 | 0/22 |
| December | 1/28 | 2/140 | 0/2 | 0/15 | 0/19 | 0/35 | 0/17 | 0/50 | 0/10 | 0/26 | 2/26 | 1/24 |
| Total | 8/74 | 36/977 | 2/143 | 15/391 | 0/64 | 0/203 | 0/34 | 0/176 | 2/59 | 1/162 | 149/648 | 58/298 |
| 44/1051 | 17/534 | 0/267 | 0/210 | 3/221 | 207/946 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sberna, G.; Linardos, G.; Lalle, E.; Scutari, R.; Vulcano, A.; Mija, C.; Bordi, L.; Bartolini, B.; Maggi, F.; Perno, C.F.; et al. Resurgence of Bordetella pertussis in Lazio: A Cross-Age Surveillance Study from Two Referral Hospitals. Microorganisms 2025, 13, 1808. https://doi.org/10.3390/microorganisms13081808
Sberna G, Linardos G, Lalle E, Scutari R, Vulcano A, Mija C, Bordi L, Bartolini B, Maggi F, Perno CF, et al. Resurgence of Bordetella pertussis in Lazio: A Cross-Age Surveillance Study from Two Referral Hospitals. Microorganisms. 2025; 13(8):1808. https://doi.org/10.3390/microorganisms13081808
Chicago/Turabian StyleSberna, Giuseppe, Giulia Linardos, Eleonora Lalle, Rossana Scutari, Antonella Vulcano, Cosmina Mija, Licia Bordi, Barbara Bartolini, Fabrizio Maggi, Carlo Federico Perno, and et al. 2025. "Resurgence of Bordetella pertussis in Lazio: A Cross-Age Surveillance Study from Two Referral Hospitals" Microorganisms 13, no. 8: 1808. https://doi.org/10.3390/microorganisms13081808
APA StyleSberna, G., Linardos, G., Lalle, E., Scutari, R., Vulcano, A., Mija, C., Bordi, L., Bartolini, B., Maggi, F., Perno, C. F., & Fontana, C. (2025). Resurgence of Bordetella pertussis in Lazio: A Cross-Age Surveillance Study from Two Referral Hospitals. Microorganisms, 13(8), 1808. https://doi.org/10.3390/microorganisms13081808



_Di_Marco.png)



