Abstract
Standard blood agar medium with 2% NaCl (BAS) and Marine Agar (MA) are commonly used in bacteriological investigations of winter ulcers in farmed Atlantic salmon (Salmo salar Linnaeus) in Norway and allow easy recovery of Moritella viscosa based on its characteristic viscous colonies and β-hemolytic activity. However, the recent increase in cases of winter ulcers involving Tenacibaculum spp. and the potential emergence of T. maritimum due to rising temperatures highlight the need for improved methods of isolation and identification. Indeed, the recovery of Tenacibaculum spp. from outbreaks of winter ulcers or tenacibaculosis can be challenging. Despite the development of several agar media over the years to overcome this issue, such as Flexibacter maritimus medium (FMM), it remains difficult to differentiate Tenacibaculum species. We evaluated the growth dynamics and phenotypic characteristics of 13 bacterial isolates commonly associated with ulcer outbreaks on five different agar media, including two new formulations: kanamycin-supplemented marine blood agar for the selective isolation of Tenacibaculum spp. (KABAMA) and general blood agar for marine bacteria (BAMA). These new media facilitate the identification of Tenacibaculum spp., including T. maritimum, by distinguishing colonies based on their specific color, shape, and hemolytic activity.
1. Introduction
Tenacibaculosis is a significant disease in many economically important marine fish species, including salmonids, and is caused by bacteria of the genus Tenacibaculum [1,2]. This disease is characterized by frayed fins, tail rot, mouth erosion, skin lesions, and ulcers [1]. Several Tenacibaculum spp., including Tenacibaculum dicentrarchi, T. finnmarkense, T. maritimum, and T. piscium, have been shown to induce tenacibaculosis in challenge studies [3,4,5,6,7,8].The psychrotrophic T. finnmarkense is mostly associated with tenacibaculosis at lower seawater temperatures [7,9], while increased prevalence and severity of tenacibaculosis associated with T. maritimum have been reported at higher temperatures, above 15 °C [1,10]. The clinical manifestations of diseases caused by these bacteria are also different in that T. finnmarkense and T. dicentrarchi typically affect non-scaled skin, while T. maritimum may affect the entire body, including the gills, producing a distinct slimy bacterial mat on body surfaces [2,10,11,12,13].
Ulcerative skin diseases occurring in lower temperature seawater (e.g., ‘winter ulcers’) are a major health and welfare problem for the global salmonid-farming industry [14,15] and have been reported in Norway since the late 1980s [16,17,18]. While the bacterium Moritella viscosa is considered the main etiological agent of ‘winter ulcers’, several other bacterial species contribute to ulcerative diseases. The most frequently associated bacteria are Aliivibrio wodanis and Tenacibaculum spp. [18,19]. Several studies have shown that T. finnmarkense genomovar (gv) finnmarkense is the dominant Tenacibaculum species associated with ulcerative disease in farmed salmon in Norway [6,7,9]. In the Norwegian salmon farming industry, the recovery of Tenacibaculum spp. from field outbreaks of ulcerative diseases using standard nutrient agar media has proven challenging despite the presence of a large number of bacteria in the ulcerative lesions with Tenacibaculum morphology (long slender rods) [18]. This is likely due to the historical use of blood agar media containing 1.5–2.0% NaCl (BAS) as the main medium for investigating bacterial diseases in saltwater salmon life stages. The increased use of Marine Agar (MA) (e.g., Difco 2216 or Zobell 2216), which contains sea salts and therefore mimics the natural composition of seawater, has improved the recovery of Tenacibaculum spp. from field samples [18,20]. Some Tenacibaculum spp. grow only in the presence of varying concentrations of sea salts and exhibit poor growth with NaCl alone [7,18,21]. Despite these improvements, recovering Tenacibaculum spp. from skin lesions or ulcers in farmed Atlantic salmon (Salmo salar Linnaeus) remains challenging, particularly in cases of mixed infections [18].
MA supports the growth of a wide range of heterotrophic marine bacteria, which can make the isolation of Tenacibaculum spp. challenging, as these may be outcompeted, inhibited, or overgrown by fast-growing marine bacteria like Aliivibrio, Vibrio, Alteromonas, and Pseudoalteromonas [22,23,24,25,26,27]. To circumvent this issue for T. maritimum, the specific medium Flexibacter maritimus medium (FMM) was developed to limit the growth of other heterotrophic marine bacteria (e.g., Vibrio spp., Alteromonas spp.) [28]. In addition to improving the recovery of T. maritimum from mixed infections, FMM may help indicate the serotype of a certain isolate [25,28]. Marine Shieh’s Selective Medium (MSSM), originally developed for isolating Flavobacterium columnare [29], has also proven effective for recovering Tenacibaculum spp., including T. maritimum, particularly in New Zealand, where it is routinely used for disease surveillance [27,30]. The intrinsic filamentous morphology of T. maritimum and its ability to adhere to substrates pose challenges for experimental infection models and can hinder the recognition of T. maritimum colonies [10,31,32].
Another option for creating a selective nutrient agar is to add an antimicrobial agent to the medium, which limits the growth of extraneous bacteria. For Tenacibaculum, several different agents have proven effective, most of which belong to the class of aminoglycoside antibiotics (e.g., kanamycin and neomycin), to which Tenacibaculum spp. appear to be innately resistant [20,22,24,26,33].
This study investigated the benefits, including the ease of colony selection, of a modified nutrient agar medium for the isolation and growth of Tenacibaculum spp. The growth of 11 bacterial strains in the genus Tenacibaculum and two bacterial species commonly associated with ‘winter ulcer’ outbreaks in Norwegian salmonids was compared on five different agar media.
2. Materials and Methods
2.1. Nutrient Agar Media Preparation
BAMA was formulated as shown in Table 1. All components, except defibrinated sheep blood, were mixed and sterilized at 121 °C for 15 min. The agar medium was then cooled down to 50 °C before blood was aseptically added. Selective agar (KABAMA) was achieved by adding kanamycin to a final concentration of 50 µg mL−1 concurrent with the addition of blood (Table 1) to improve the primary isolation of Tenacibaculum spp. by restricting the growth of faster-growing extraneous bacteria. FMM (Condalab, Madrid, Spain) and MA (Difco 2216, BD Difco, Franklin Lakes, NJ, USA) were prepared according to the manufacturers’ protocols. BAS was ordered from the Norwegian Veterinary Institute (http://vetinst.no, accessed on 30 June 2025). Plates were stored at 4 °C and used before the expiration date.

Table 1.
List of components and formula for 1 L of BAMA and KABAMA agar media.
2.2. Comparison of Bacterial Growth on Five Different Agar Media
The growth of 13 bacterial strains was compared using BAMA, KABAMA, BAS, FMM, and MA agar media. The study included 11 Tenacibaculum strains: T. adriaticum strain B390T, T. dicentrarchi strain NCIMB 14598T, T. finnmarkense gv. finnmarkense strain HFJ, T. finnmarkense gv. ulcerans strain TNO010T, T. maritimum strains NCIMB 2154T, CAN 15-1, NLF-15, and Ch-2402, T. ovolyticum strain NCIMB 13127T, T. piscium strain TNO020T, and T. soleae strain LL04 12.1.7T.
Tenacibaculum-type strains were obtained from the National Collection of Industrial, Food, and Marine Bacteria (http://ncimb.com, accessed on 1 June 2024). Upon receipt, the identity of each bacterial strain was verified by 16S rRNA gene sequencing and compared with reference sequences. T. maritimum strains CAN 15-1, Ch-2402, and NLF-15 were isolated from Atlantic salmon in Canada [26], Chile [34], and lumpsuckers (Cyclopterus lumpus, Linnaeus) in Norway [35], respectively. T. finnmarkense gv finnmarkense strain HFJ was isolated from skin lesions of diseased Atlantic salmon [7]. Two strains of bacterial species commonly associated with ‘winter ulcers’ were also included in the study: Moritella viscosa type strain NCIMB 13584T and an isolate of Aliivibrio wodanis recovered from a farmed Atlantic salmon suffering from skin disease in Norway. Bacterial inocula were cultured directly from cryo stocks. After thawing, an inoculum of 400 µL was added to either 30 mL of Marine Broth (MB, Difco) in a 50 mL tube or, for the T. maritimum strains, to 1 L of MB in a 2 L Erlenmeyer flask. Cultures were incubated at 16 °C and 140 rpm for approximately 48 h or at 16 °C and 230 rpm for approximately 74 h for the T. maritimum strains. The most probable number (MPN) method was used to estimate the number of cells per inoculum [36]. A 30 µL aliquot of the bacterial culture was subsequently streaked in triplicate onto each agar medium and incubated at different temperatures. Relative growth and colony morphology were observed and documented daily for seven days. The β-hemolytic activity of the strains was readily observed by visualizing the plates under a light source. Images were captured using a Nikon D80 digital camera with an AF-S Micro Nikkor 60 mm 1:2.8G ED lens (Nikon Corporation, Tokyo, Japan) in a portable photo studio. Each culture was performed in triplicates.
The Details of the bacterial strains included in the study, incubation temperatures, and estimated number of bacteria streaked onto the agar plates for the tests are included in Table 2.

Table 2.
List of the 13 bacterial strains included in this study. The number of cells per agar plate was estimated from the most probable number (MPN) of the inoculum.
2.3. Phenotypic Characterization
To thoroughly characterize the morphology of the 11 Tenacibaculum strains on the different agar media, three plates per medium (BAMA, KABAMA, FMM, and MA) were inoculated with a bacterial suspension to yield approximately one to 20 colonies per plate. After inoculation, the plates were incubated at 16 °C, and daily observations were recorded over seven days to document growth patterns and phenotypic characteristics, such as colony color, shape, margin, and elevation. The number of colonies counted and the average colony diameter are detailed in Supplementary Material Table S1.
3. Results
3.1. Growth Comparison
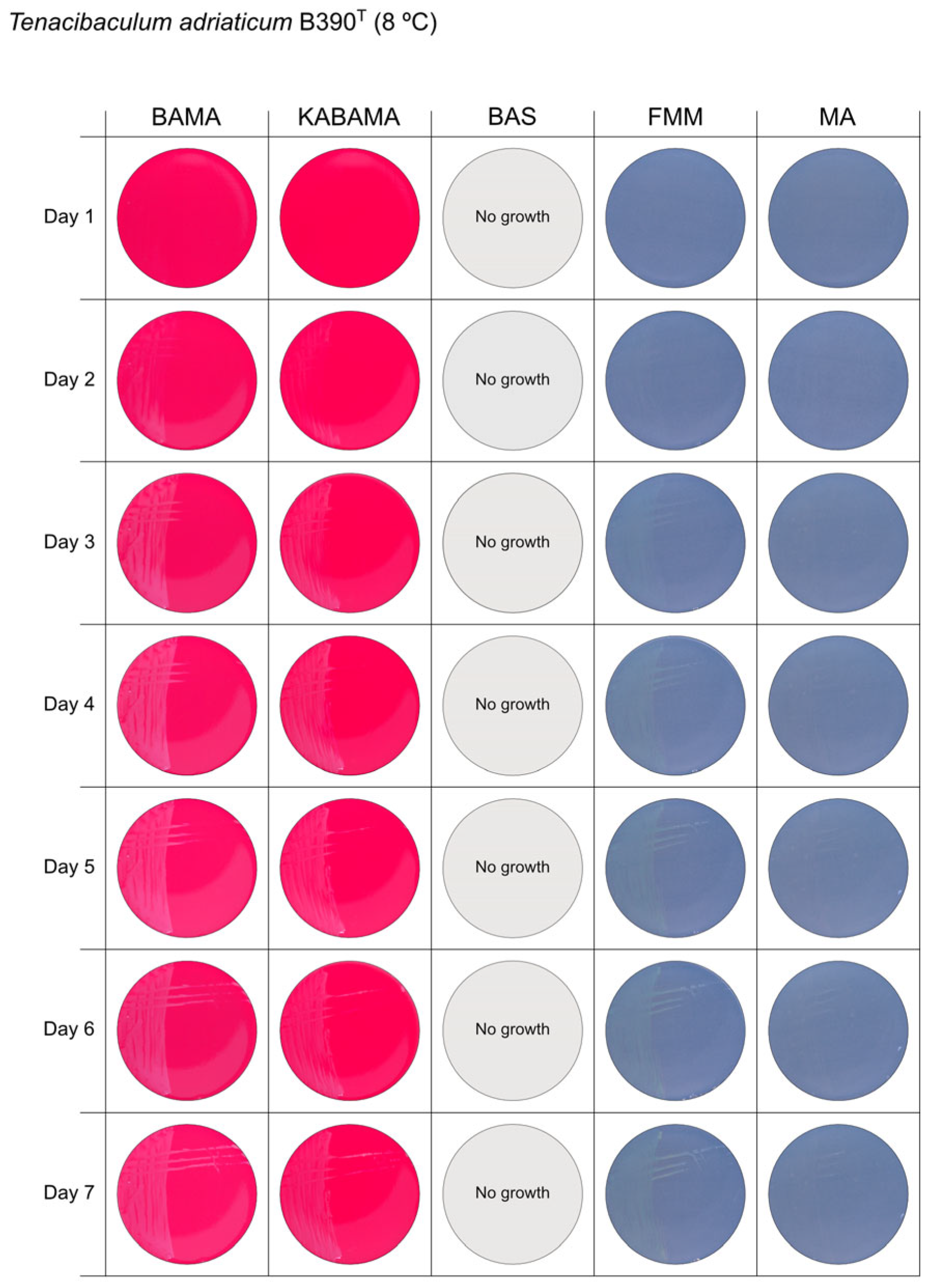
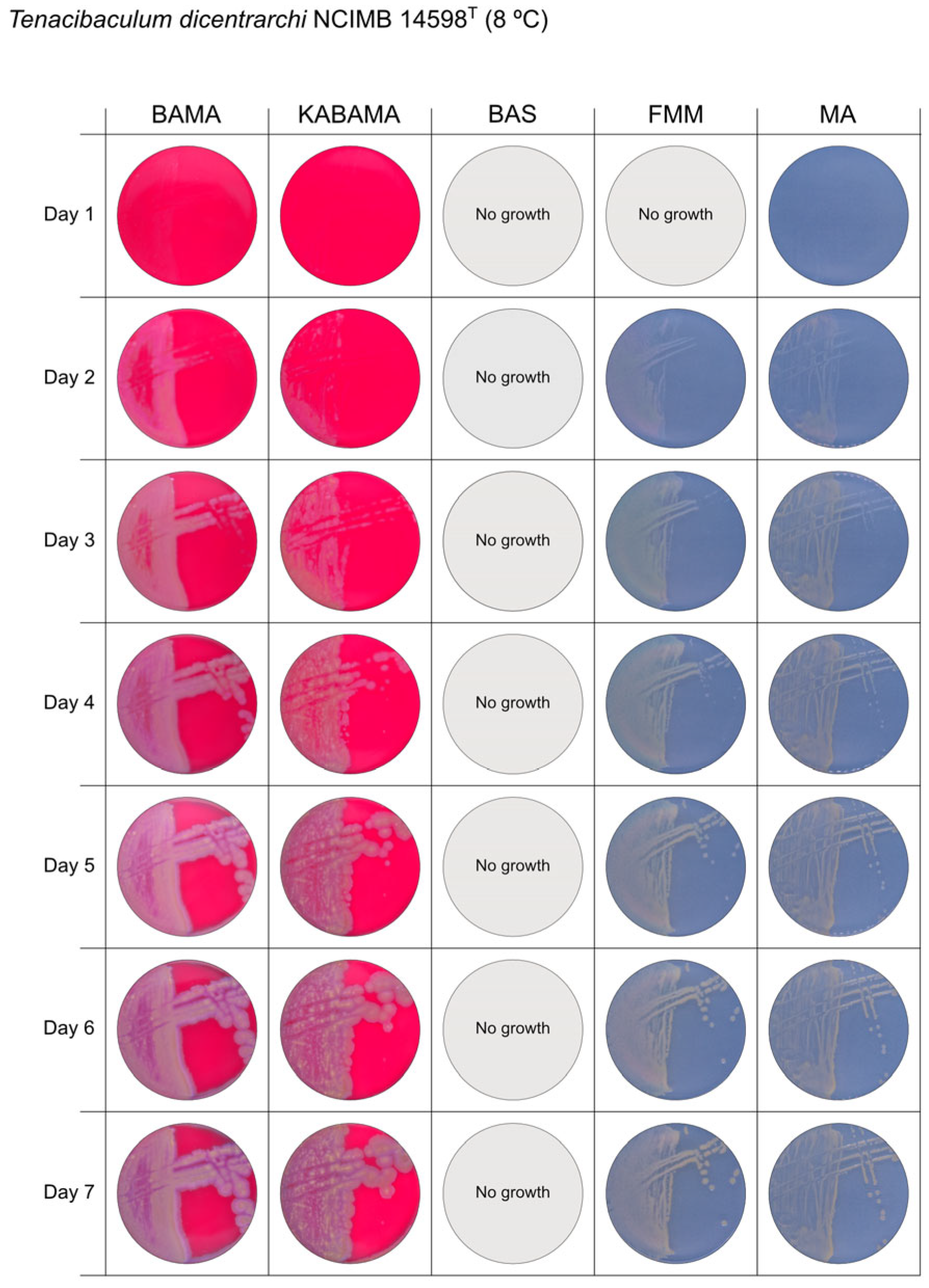
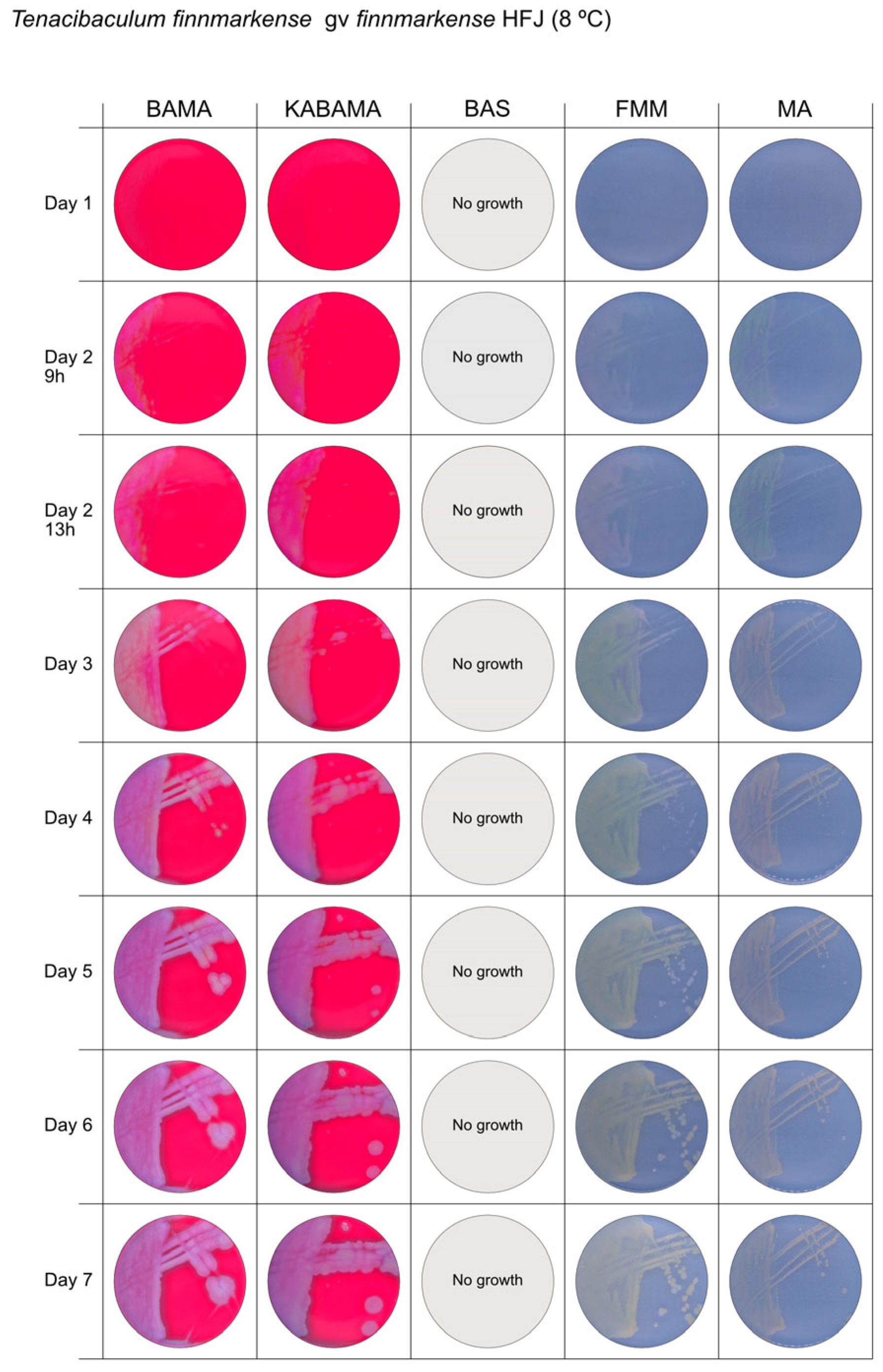
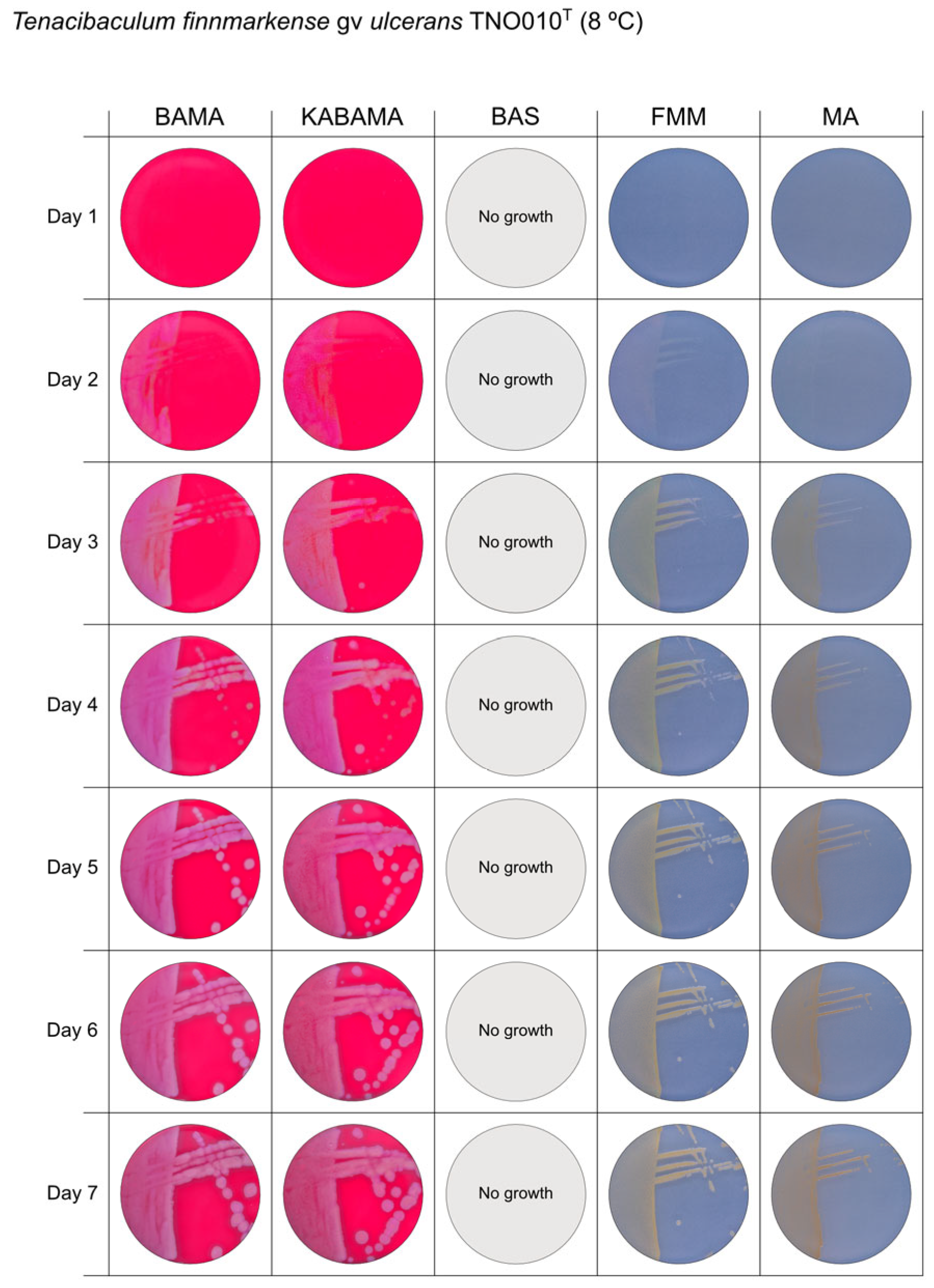
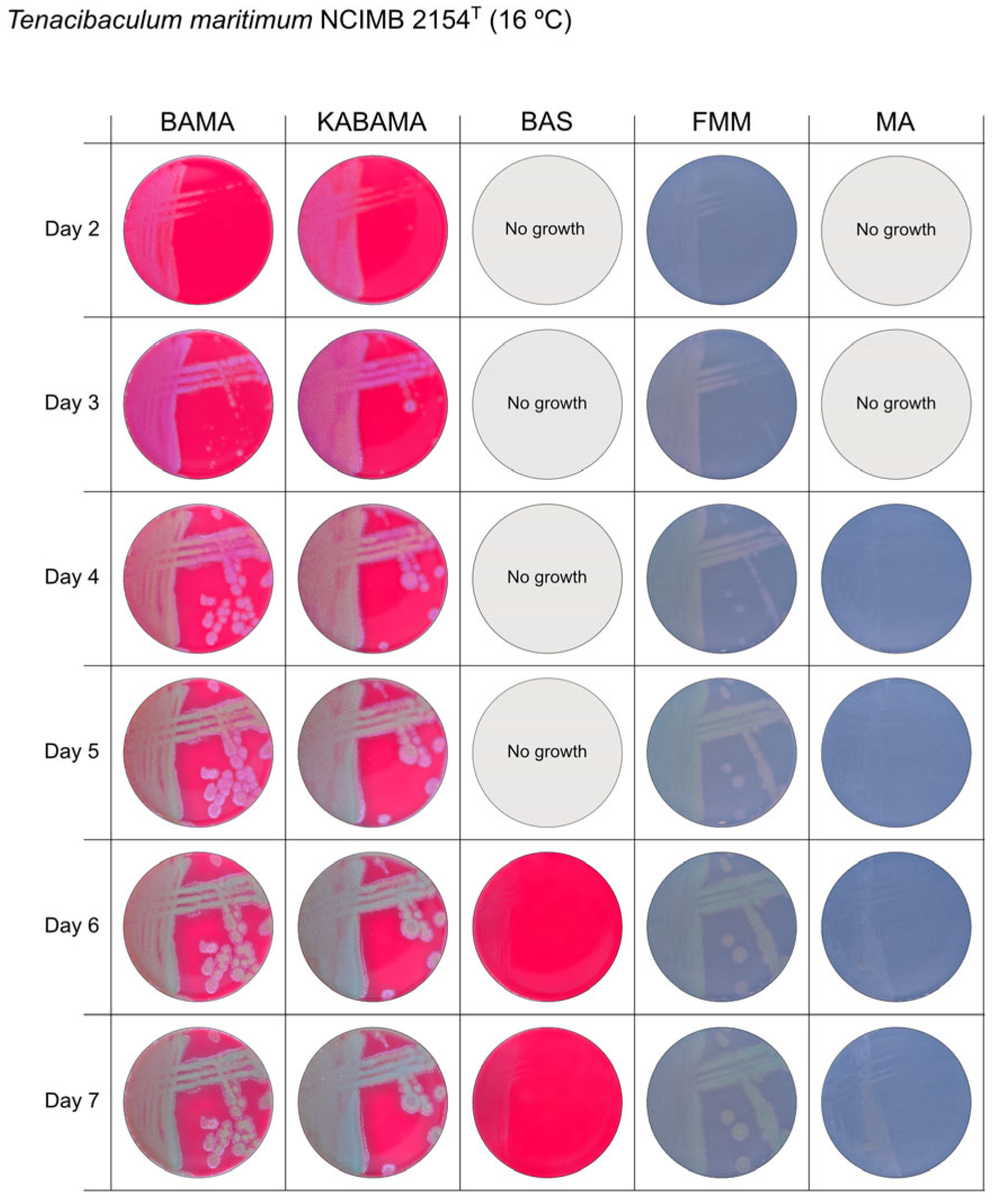
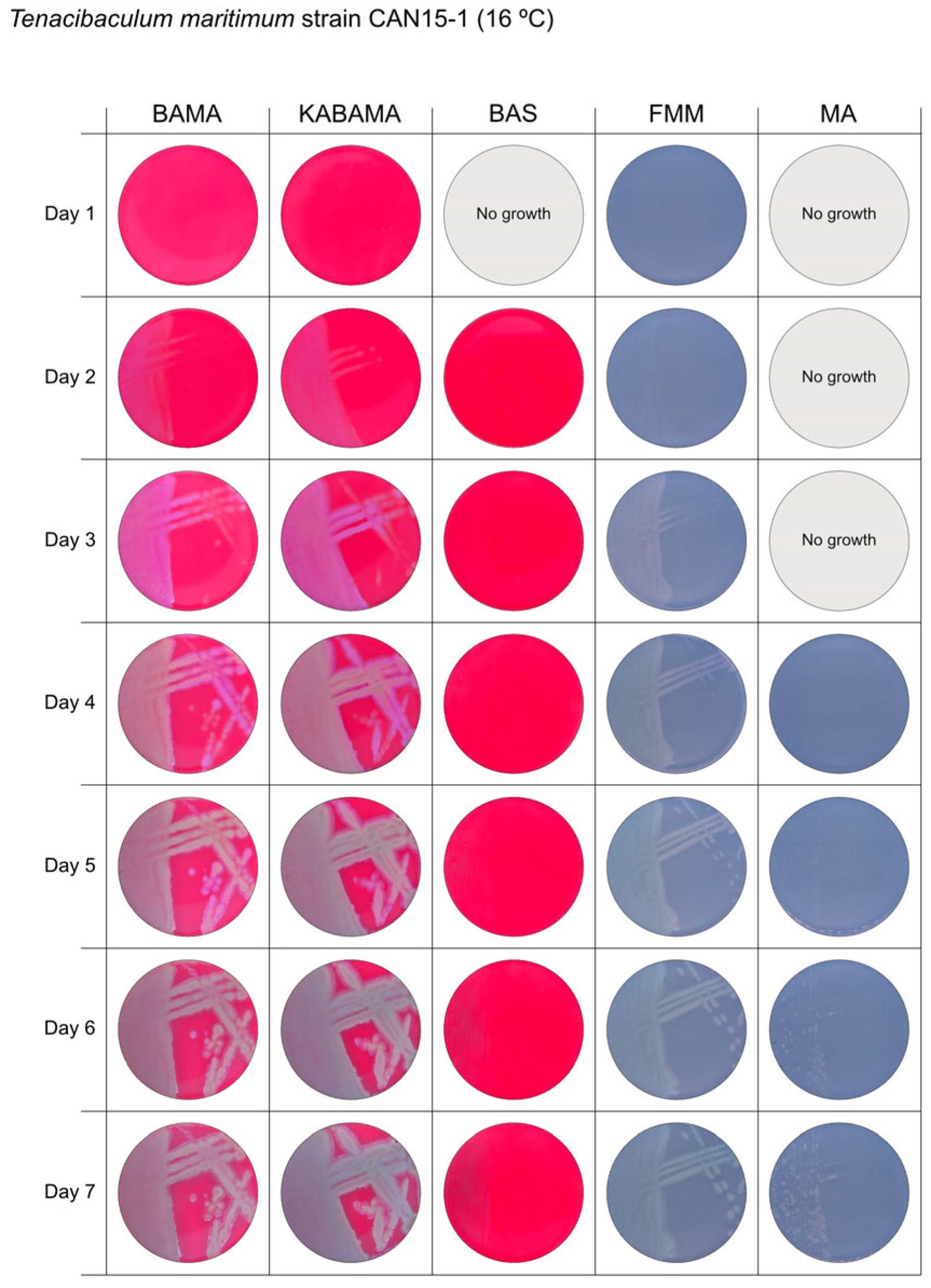
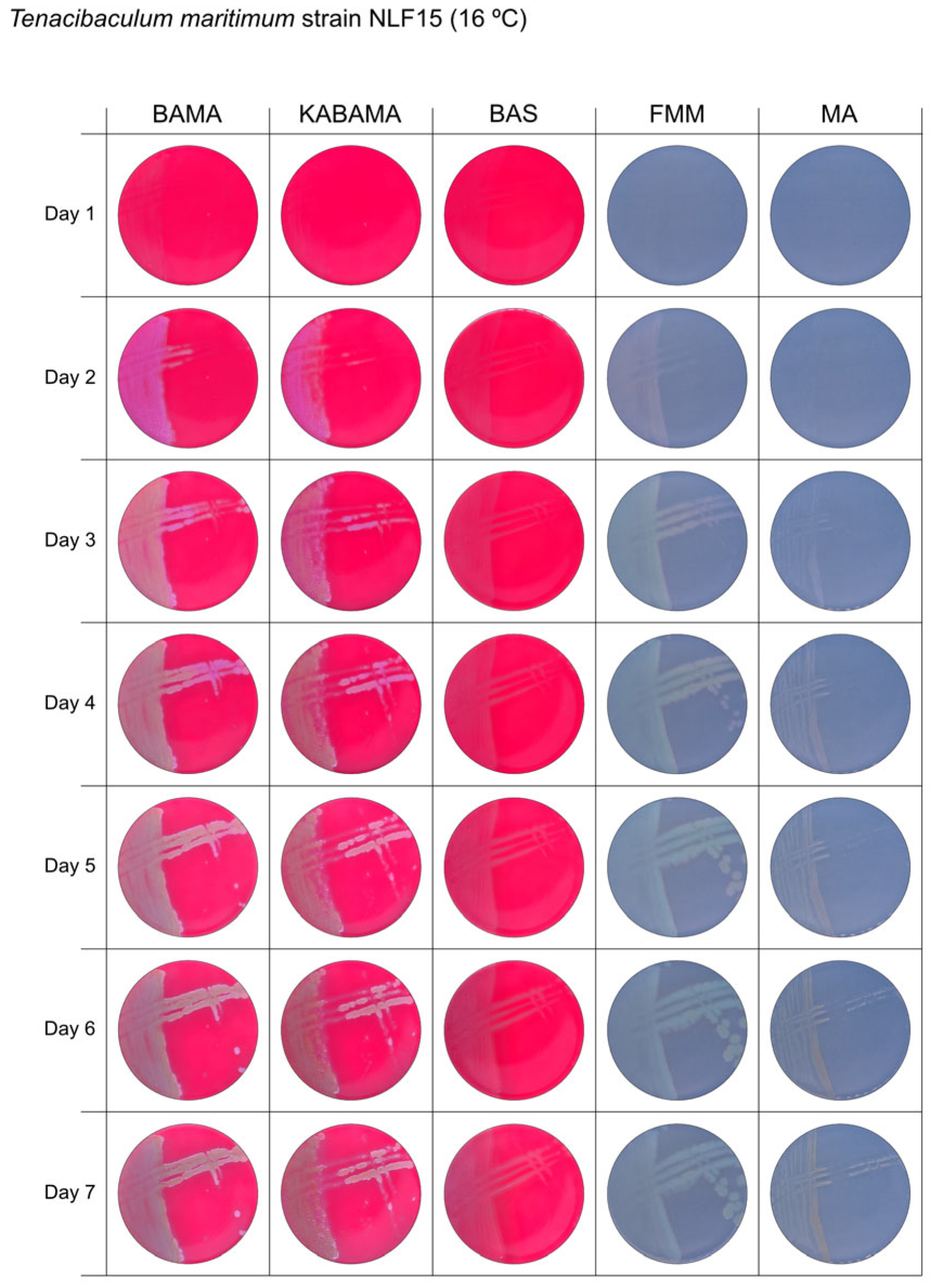
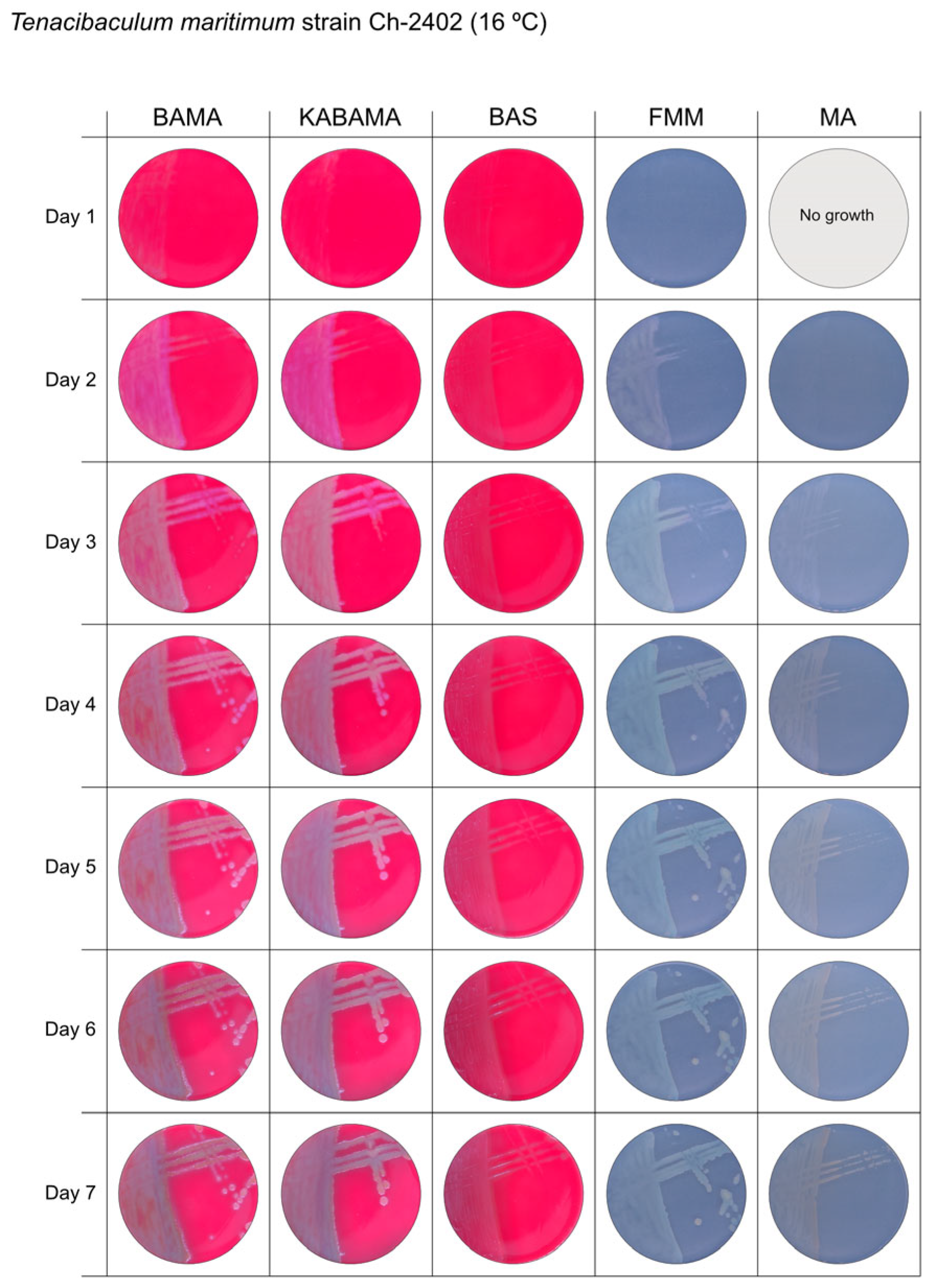
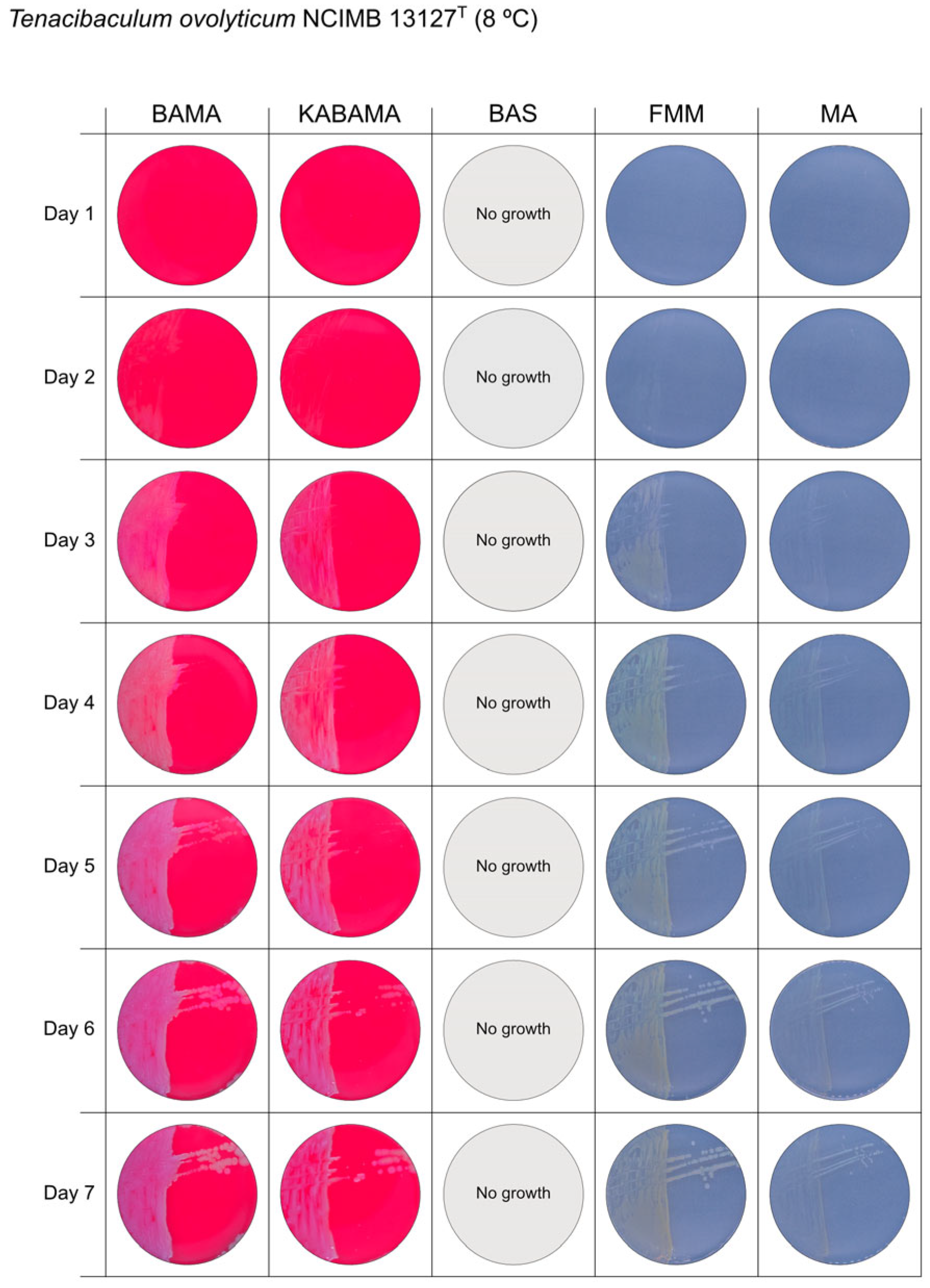
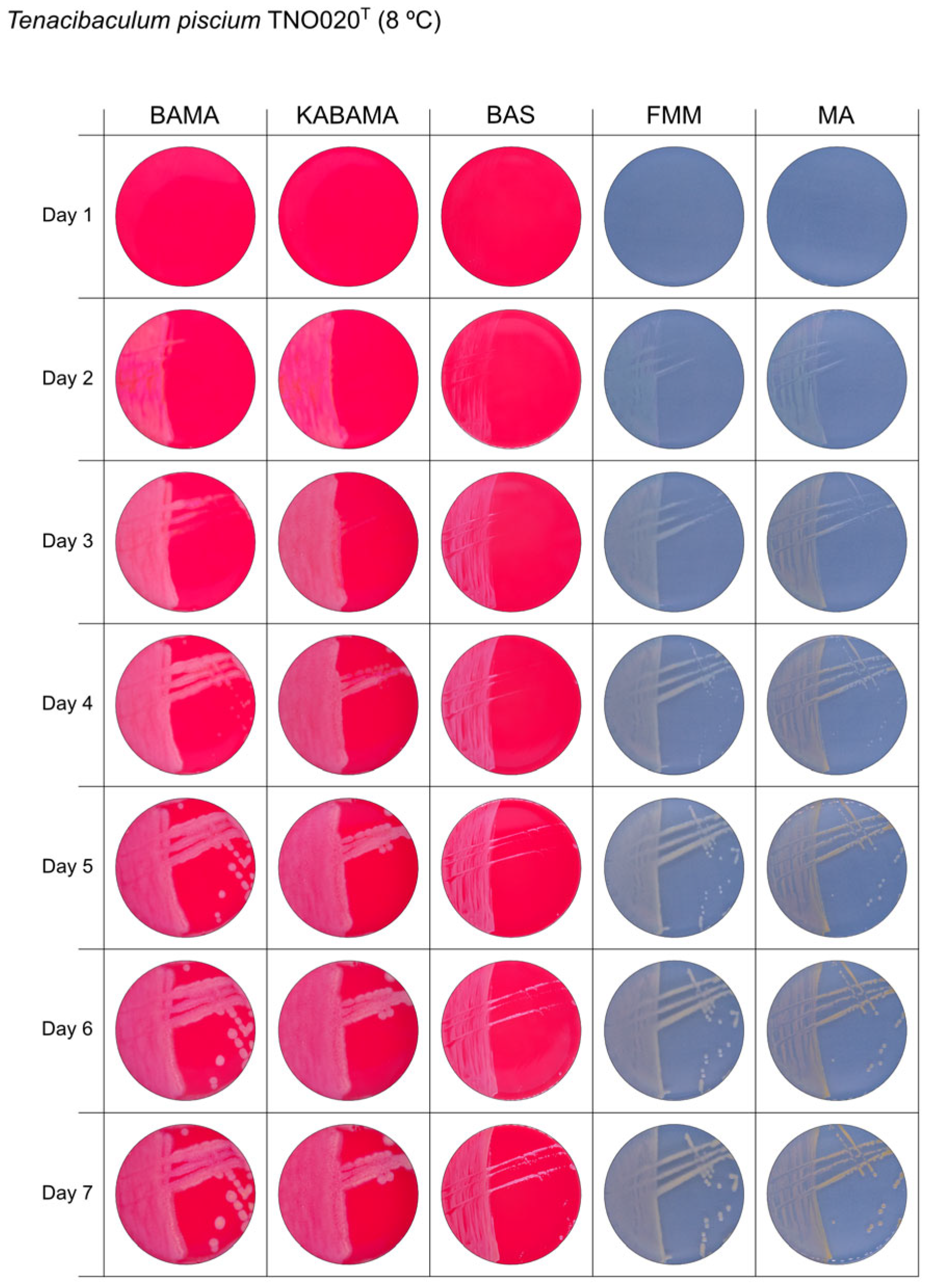
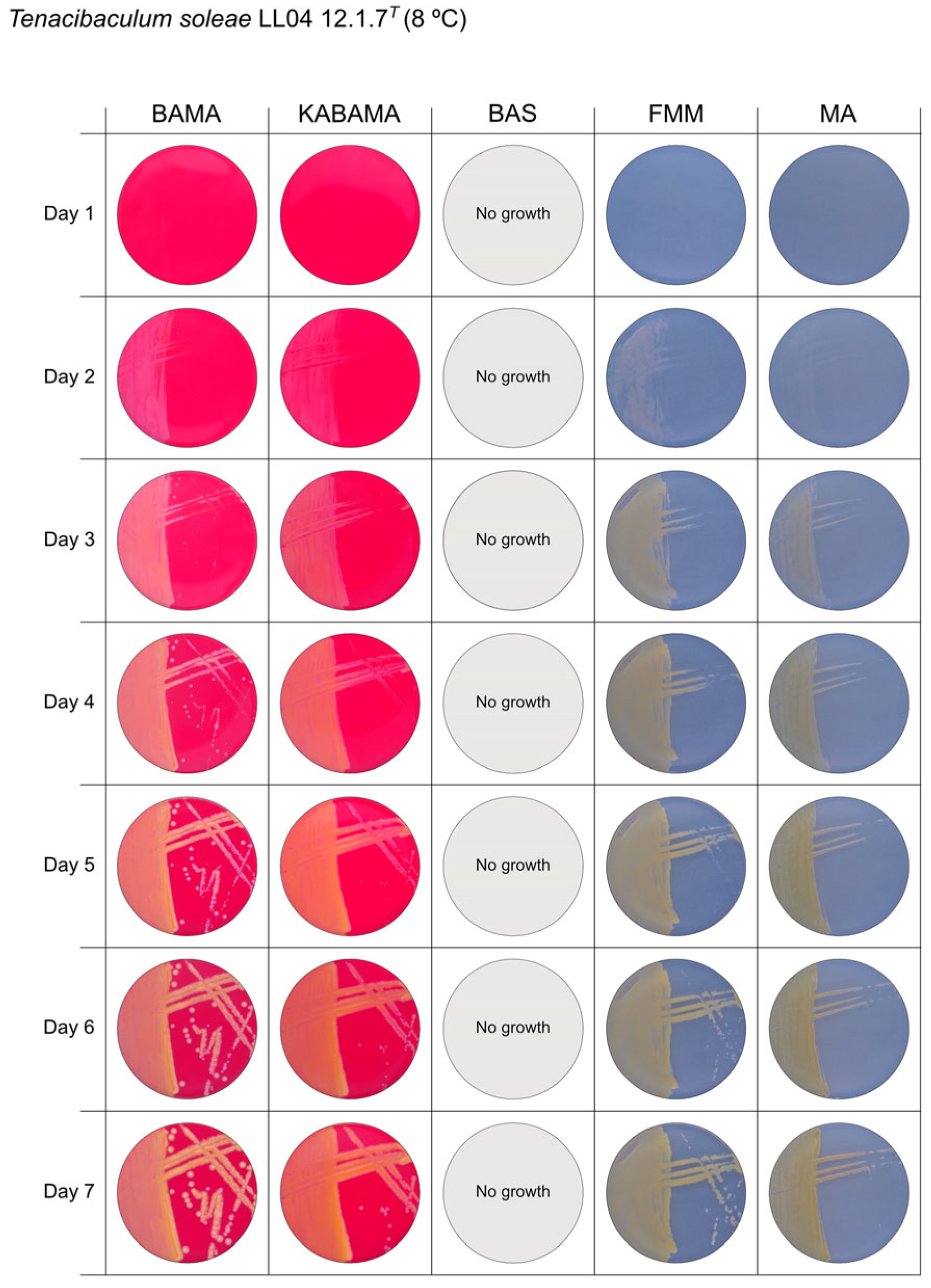
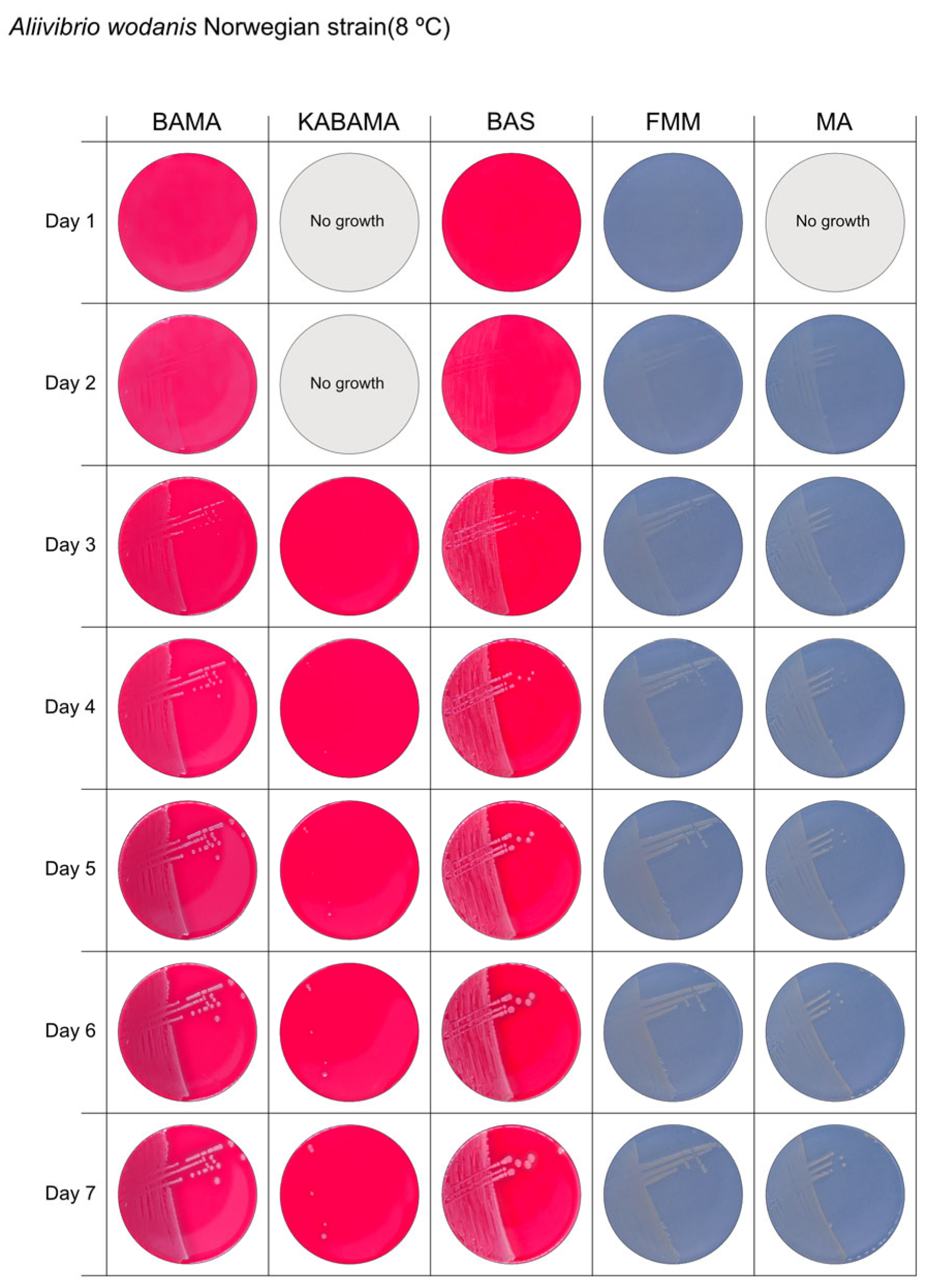
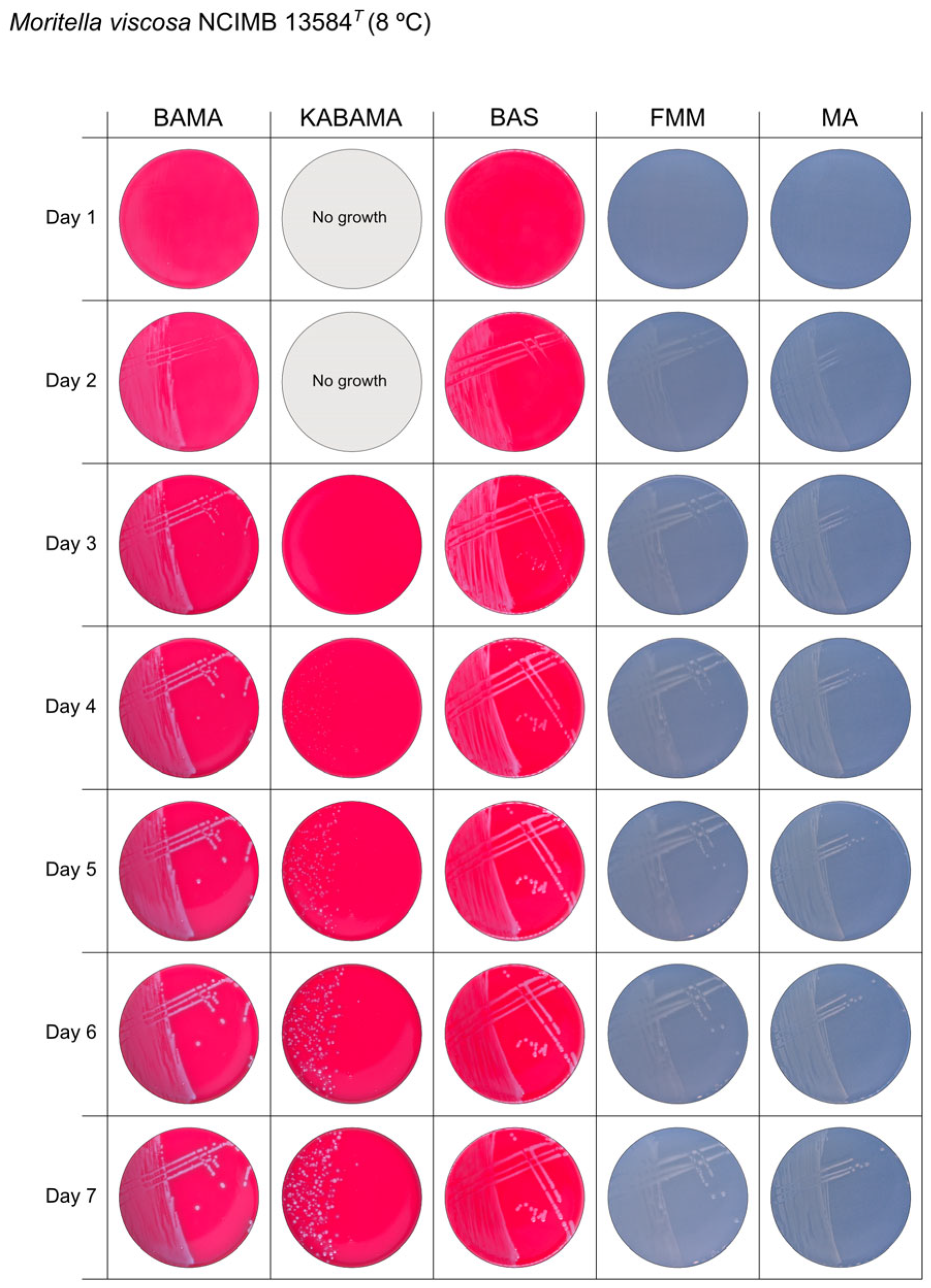
All Tenacibaculum strains included in this study demonstrated stronger growth on BAMA and KABAMA, and to a lesser extent on FMM, than on MA and BAS under identical conditions (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10 and Figure 11). Notably, the four T. maritimum strains exhibited slow and sparse growth on both MA and BAS media (Figure 5, Figure 6, Figure 7 and Figure 8). Among the other Tenacibaculum strains, none grew on BAS except for T. piscium strain TNO020T (Figure 10). In contrast, the A. wodanis field strain and M. viscosa strain NCIMB 13584T grew well on BAS, BAMA, MA, and FMM but not on kanamycin-supplemented KABAMA (Figure 12 and Figure 13, respectively).
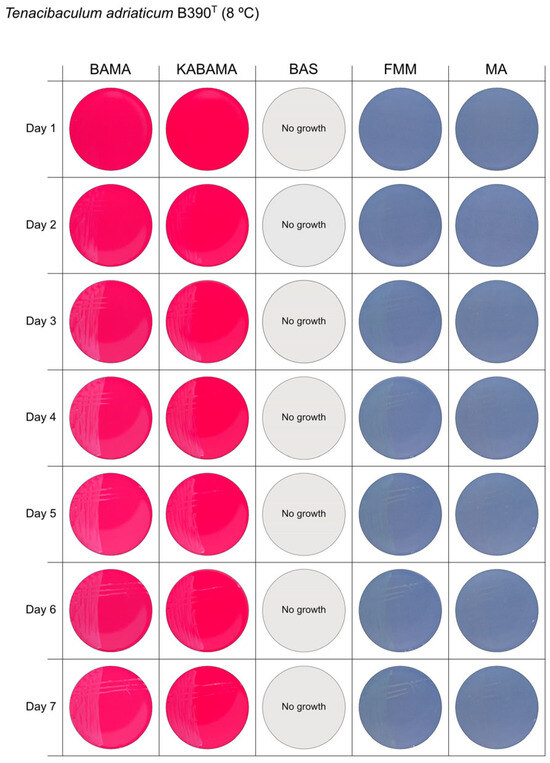
Figure 1.
Seven-day growth of Tenacibaculum adriaticum strain B390T at 8 °C on BAMA, KABAMA, BAS, FMM, and MA.
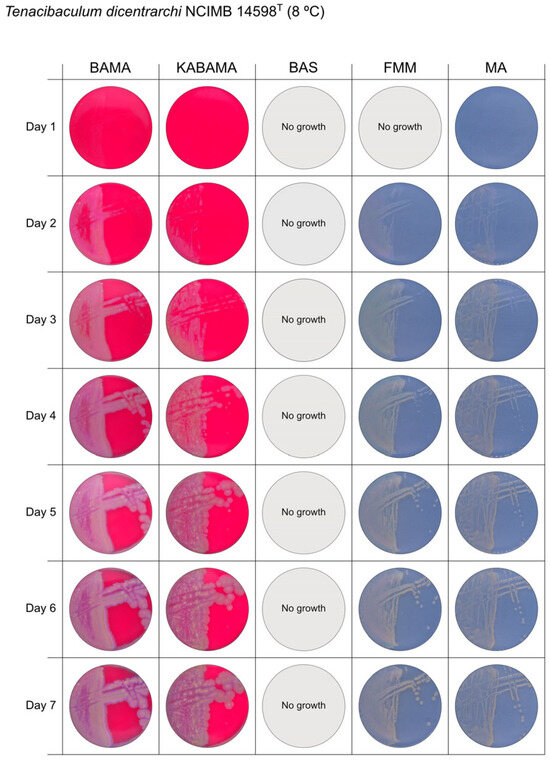
Figure 2.
Seven-day growth of Tenacibaculum dicentrarchi strain NCIMB 14598T at 8 °C on BAMA, KABAMA, BAS, FMM, and MA.
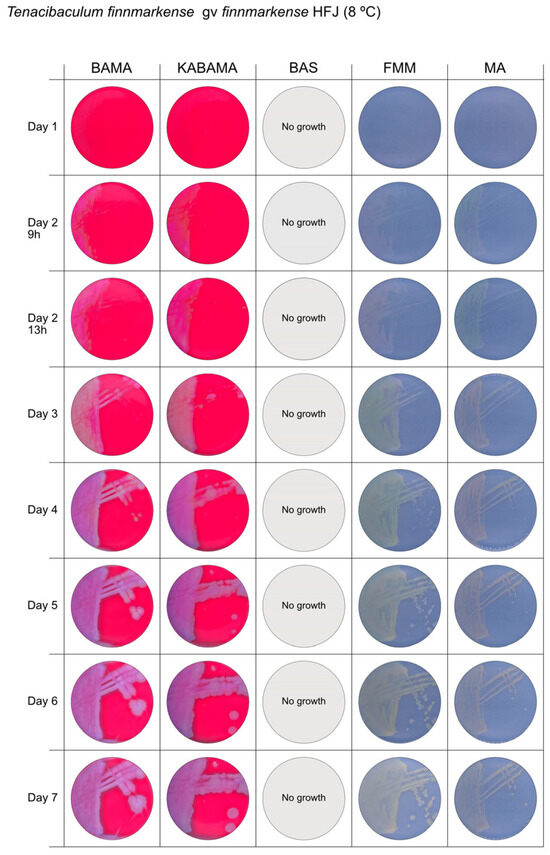
Figure 3.
Seven-day growth of Tenacibaculum finnmarkense genomovar finnmarkense strain HFJ at 8 °C on BAMA, KABAMA, BAS, FMM, and MA.
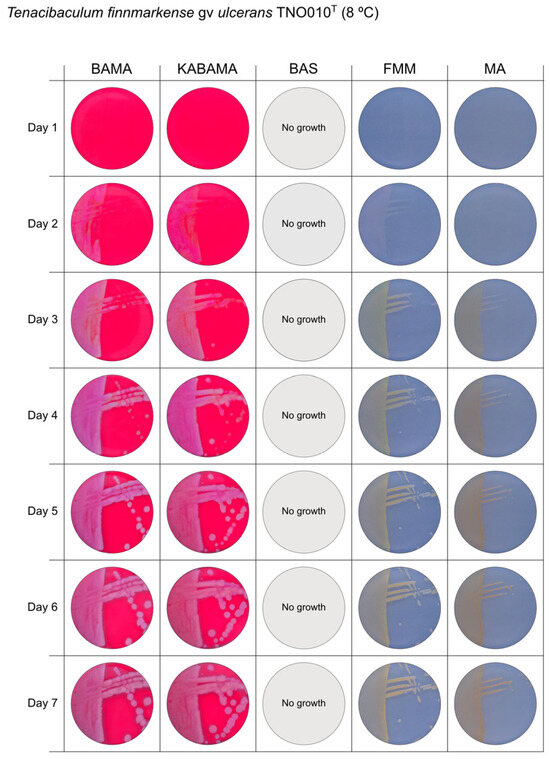
Figure 4.
Seven-day growth of Tenacibaculum finnmarkense genomovar ulcerans strain TNO010T at 8 °C on BAMA, KABAMA, BAS, FMM, and MA.
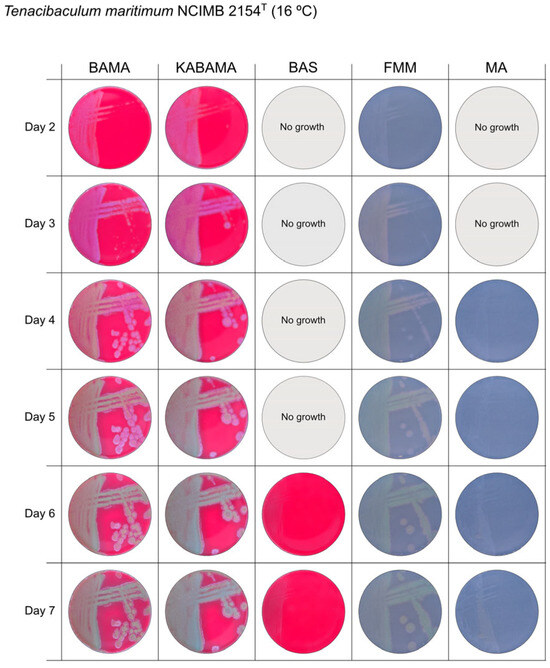
Figure 5.
Seven-day growth of Tenacibaculum maritimum strain NCIMB 2151T at 16 °C on BAMA, KABAMA, BAS, FMM, and MA.
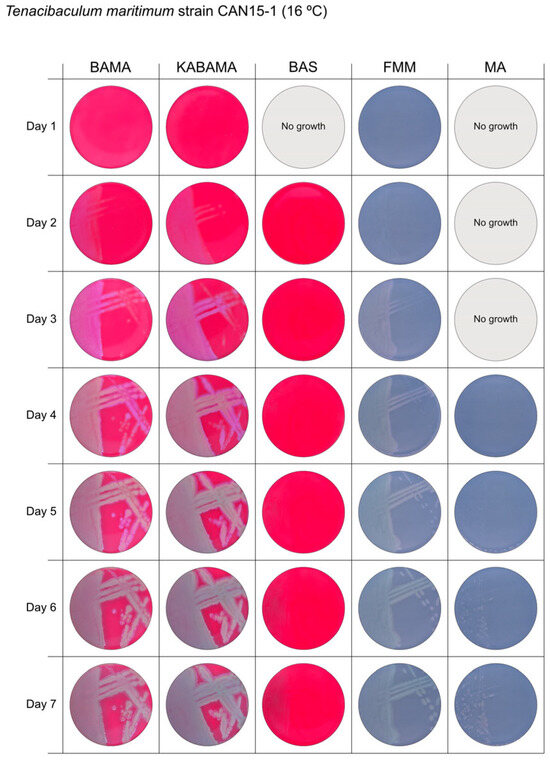
Figure 6.
Seven-day growth of Tenacibaculum maritimum strain CAN 15-1 at 16 °C on BAMA, KABAMA, BAS, FMM, and MA.
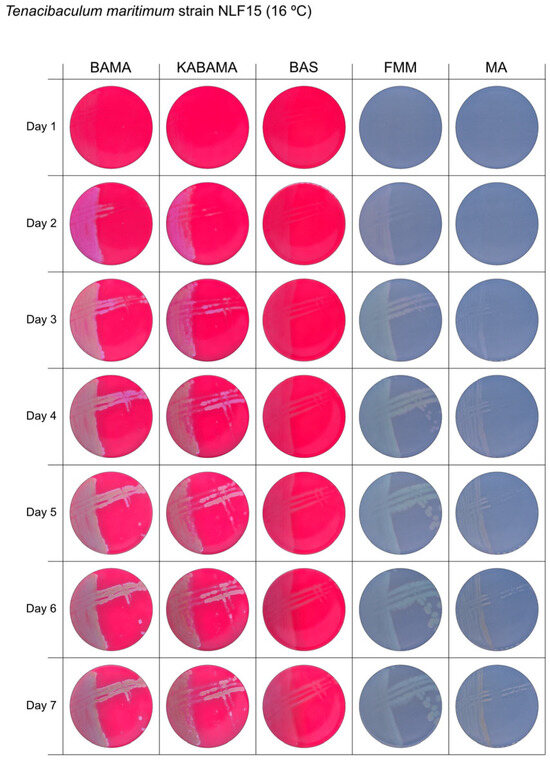
Figure 7.
Seven-day growth of Tenacibaculum maritimum strain NLF-15 at 16 °C on BAMA, KABAMA, BAS, FMM, and MA.
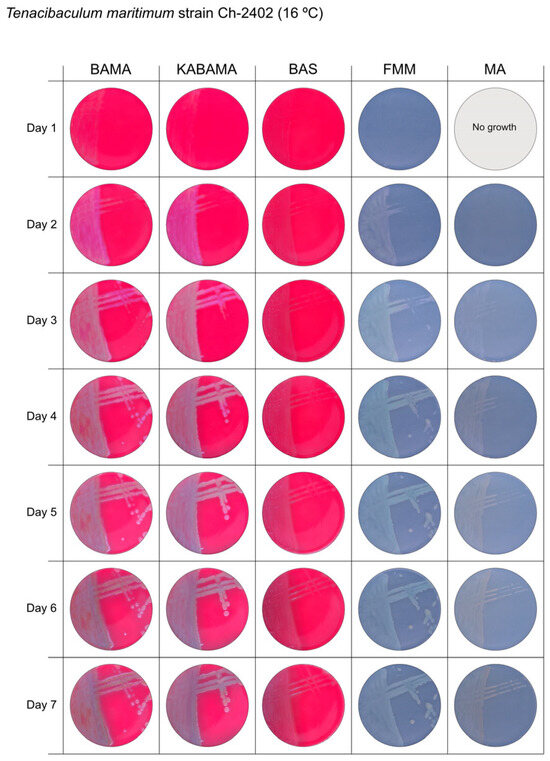
Figure 8.
Seven-day growth of Tenacibaculum maritimum strain Ch-2402 at 16 °C on BAMA, KABAMA, BAS, FMM, and MA.
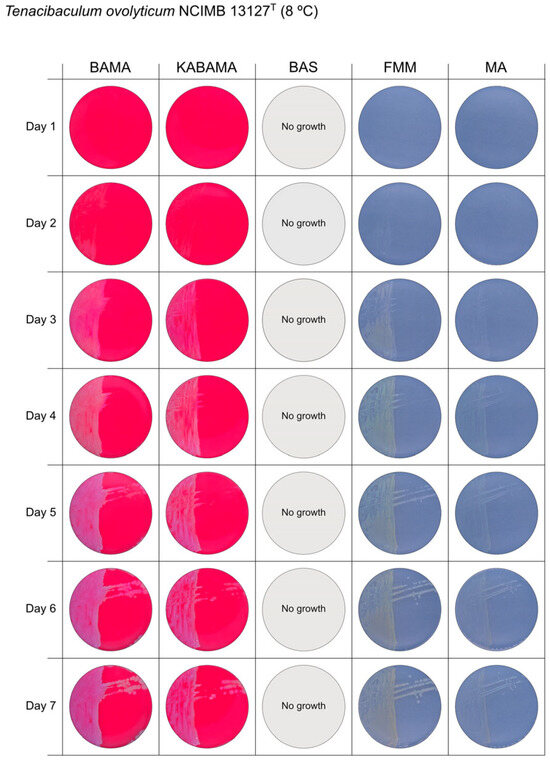
Figure 9.
Seven-day growth of Tenacibaculum ovolyticum strain NCIMB 13127T at 8 °C on BAMA, KABAMA, BAS, FMM, and MA.
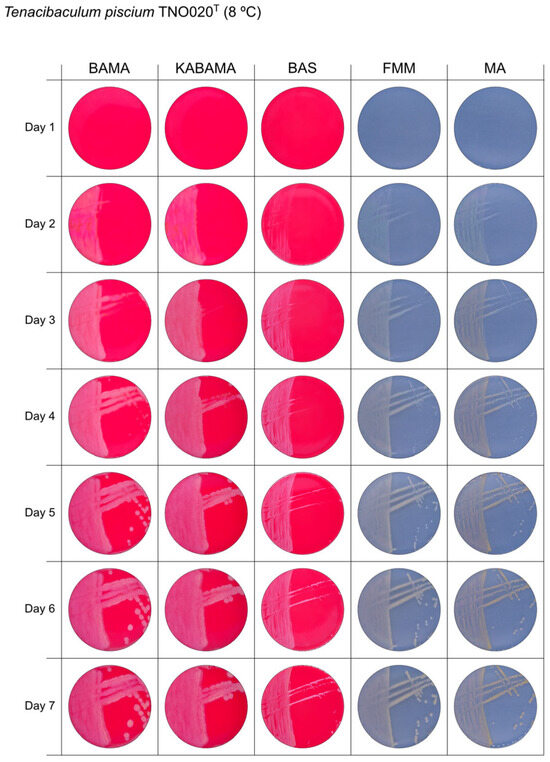
Figure 10.
Seven-day growth of Tenacibaculum piscium strain TNO020T at 8 °C on BAMA, KABAMA, BAS, FMM, and MA.

Figure 11.
Seven-day growth of Tenacibaculum soleae strain LL04 12.1.7T at 8 °C on BAMA, KABAMA, BAS, FMM, and MA.
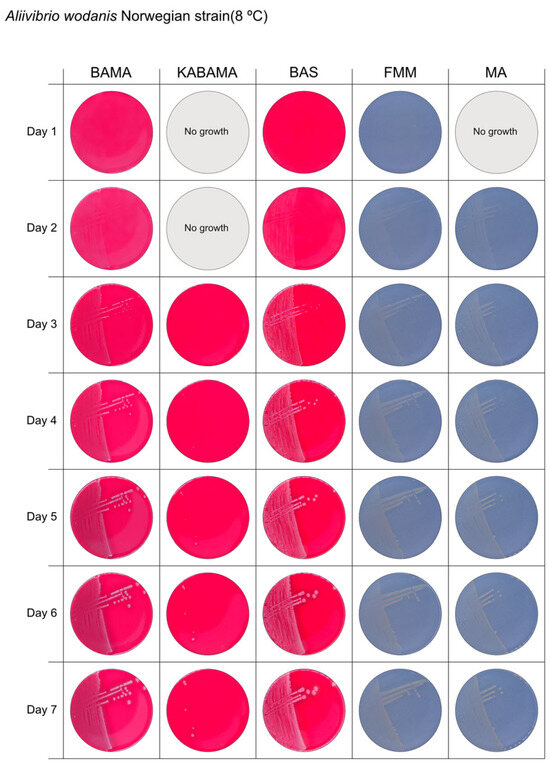
Figure 12.
Seven-day growth of Aliivibrio wodanis Norwegian field strain at 8 °C on BAMA, KABAMA, BAS, FMM, and MA.
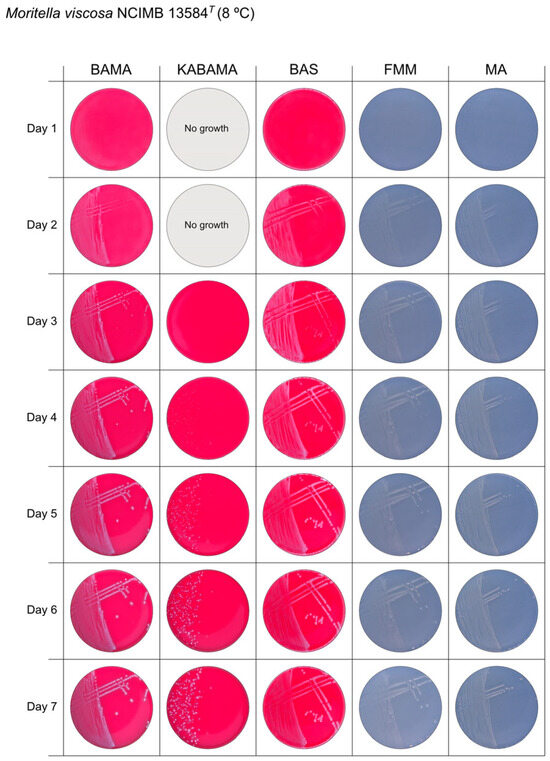
Figure 13.
Seven-day growth of Moritella viscosa strain NCIMB 13584T at 8 °C on BAMA, KABAMA, BAS, FMM, and MA.
3.2. Phenotypical Characteristics
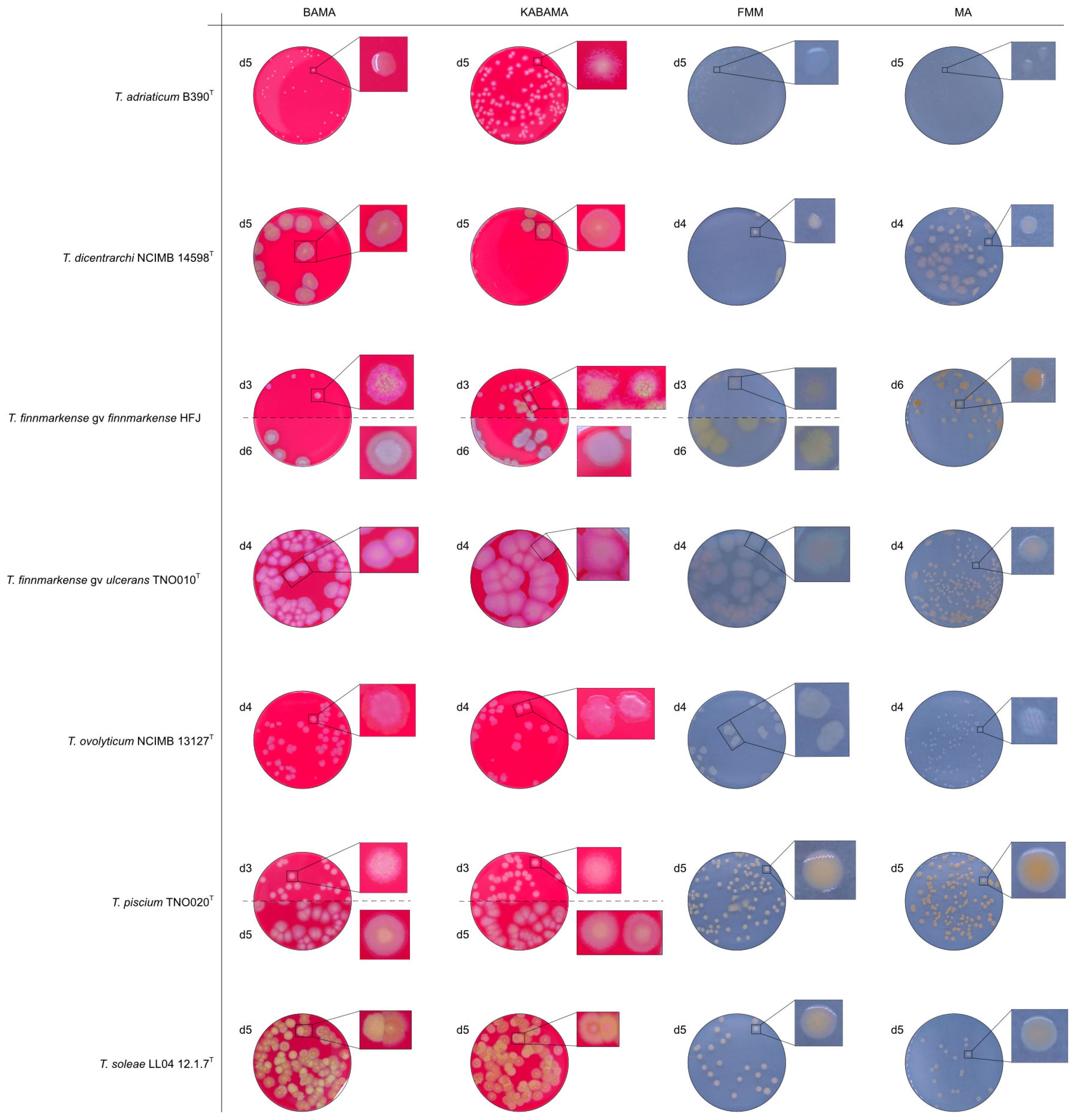
The Tenacibaculum strains displayed distinct morphological characteristics on BAMA and KABAMA, allowing for clear differentiation based on size, shape, margin, color, iridescence, and hemolytic properties (Figure 14 and Figure 15, Table 3). On both BAMA and KABAMA, a marked change in color between the center and margin of the colonies was observed for T. dicentrarchi strain NCIMB 14598T, T. finnmarkense gv finnmarkense strain HFJ, T. finnmarkense gv ulcerans strain TNO10T, T. piscium strain TNO020T, and T. soleae strain LL04 12.1.7T. These strains, along with T. maritimum strain NCIMB 2154T, exhibited varying degrees of iridescence on BAMA and KABAMA. In contrast, differentiation between the strains of Tenacibaculum on MA and FMM was challenging, as the colonies displayed a more uniform appearance with relatively similar shapes and colors.
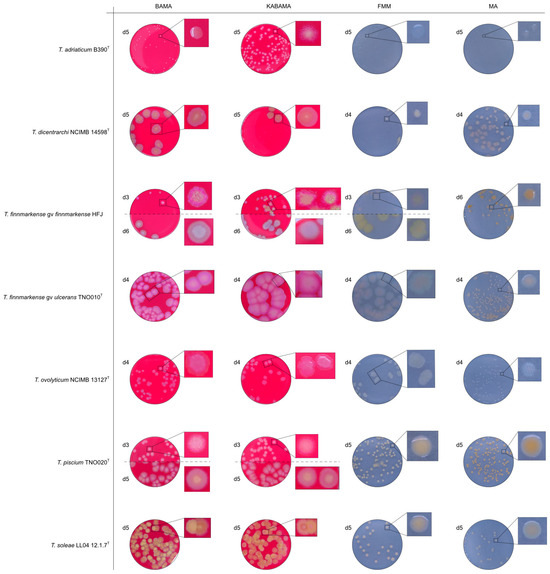
Figure 14.
Comparison of colony characteristics for Tenacibaculum adriaticum strain B390T, T. dicentrarchi strain NCIMB 14598T, T. finnmarkense genomovar finnmarkense strain HFJ, T. finnmarkense genomovar ulcerans strain TNO010T, T. ovolyticum strain NCIMB 13127T, T. piscium strain TNO020T, and T. soleae strain LL04 12.1.7T grown at 16 °C on BAMA, KABAMA, FMM, and MA. ‘d’ indicates days of growth. Dilution factors used for plating may differ between agar media for the same strain, to achieve optimal colony density for imaging.
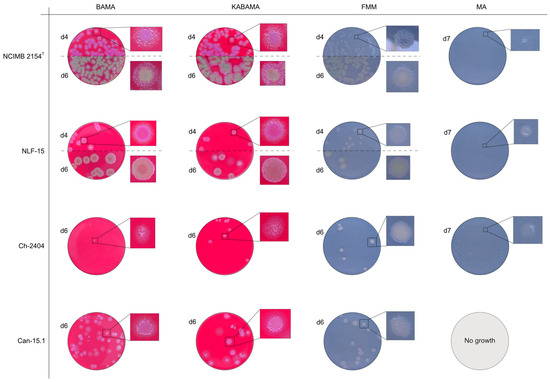
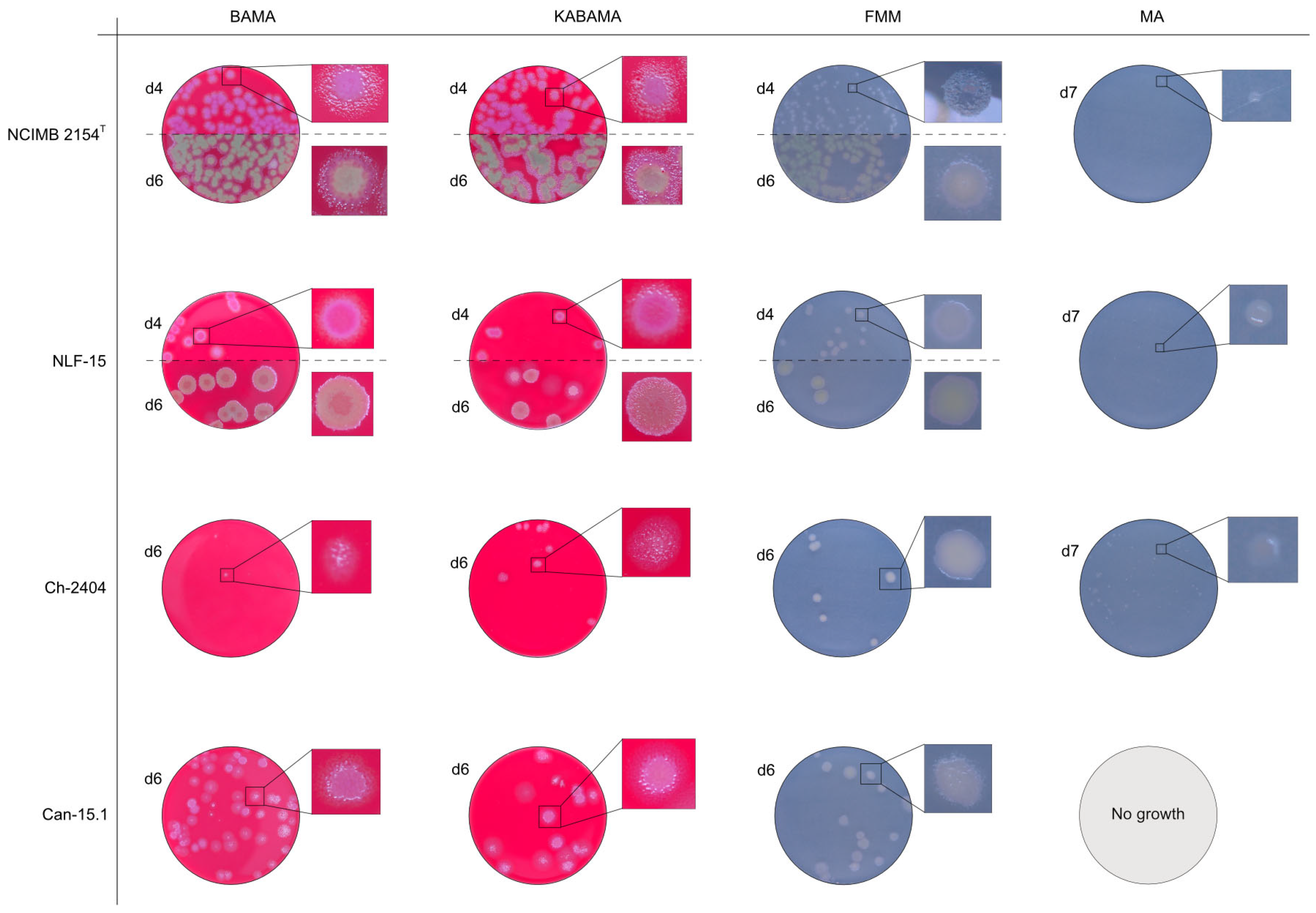
Figure 15.
Comparison of colony characteristics for Tenacibaculum maritimum strains NCIMB 2151T, CAN 15-1, NLF-15, and Ch-2402 grown at 16 °C on BAMA, KABAMA, FMM, and MA. ‘d’ indicates days of growth. Dilution factors used for plating may differ between agar media for the same strain, to achieve optimal colony density for imaging.

Table 3.
Hemolytic activity and morphological characteristics of the 13 strains included in the study. All bacteria were grown at 16 °C. Hemolytic activity is described as ‘ND’ (not detectable on the medium), ‘-’ (γ-hemolysis), ‘+’ (α-hemolysis or weak β-hemolysis), ‘++’ (strong β-hemolysis), ‘+++’ (very strong β-hemolysis).
Generally, Tenacibaculum spp. colonies often showed greater spreading activity, with larger and often merged colonies on BAMA and KABAMA than on MA, FMM, and BAS.
Colonies of T. maritimum grown on BAMA and KABAMA displayed characteristic ruggedness and uneven margins, contrasting with the smooth colony surfaces and often even margins observed for all the other bacteria tested (Figure 15). These noticeable characteristics were less distinct on FMM and not visible on MA. T. maritimum strains NCIMB 2154T, Ch-2402, and NLF-15 developed a dark green coloration after prolonged incubation on BAMA and KABAMA (and, to a lesser extent, on FMM), which was not observed in any other Tenacibaculum spp. tested. The four T. maritimum strains exhibited sufficient phenotypic characteristics on BAMA and KABAMA for clear differentiation.
β-hemolytic activity was evaluated on blood-supplemented agar media BAMA, KABAMA, and BAS, and it was found that T. dicentrarchi strain NCIMB 14598T, T. finnmarkense gv. finnmarkense strain HFJ, and all T. maritimum strains exhibited strong β-hemolytic activity. In contrast, T. ovolyticum strain NCIMB 13127T exhibited weak β-hemolytic activity, while no β-hemolysis was observed for T. adriaticum strain B390T, T. finnmarkense gv ulcerans strain TNO10T, T. piscium strain TNO020T, and T. soleae strain LL04 12.1.7T. Notably, M. viscosa strain NCIMB 13584T and A. wodanis field strain also demonstrated strong β-hemolytic activity.
A summary comparison of the five media used for the cultivation and differentiation of Tenacibaculum spp. is included in Supplementary Material Table S2.
4. Discussion
This study demonstrates that KABAMA and BAMA are more specific and effective for Tenacibaculum growth than MA and BAS. Previous studies comparing growth media for Tenacibaculum similarly reported limited growth on MA compared to FMM and MSSM [27,28]. Although FMM is also suitable for Tenacibaculum growth, strain differentiation is less distinct on FMM than on BAMA and KABAMA due to the absence of blood in its composition.
The temperatures chosen for the documentation of bacterial growth (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13, Figure 14 and Figure 15) were selected to best illustrate the colony morphology under conditions representative of each strain’s optimal growth range; however, this may have introduced a bias that should be considered when interpreting visual comparisons.
Most Tenacibaculum spp. did not grow on BAS, as BAS only contains NaCl (2%), whereas Tenacibaculum spp. generally require sea salt in their growth media. Both BAMA and KABAMA included coral pro salt, which has a higher calcium content and may support better growth of T. maritimum; calcium-rich tissues are known to be preferred colonization sites for this bacterium [43]. Given the potential for batch-to-batch variability in commercial coral pro salt, future work should investigate the effects of defined calcium concentrations in the medium. This could contribute to the development of a standardized, chemically defined medium for Tenacibaculum spp.
The non-Tenacibaculum strains tested in this study exhibited limited growth on KABAMA medium. In contrast, both Flavobacterium and Tenacibaculum species in the Family Flavobacteriaceae are intrinsically resistant to kanamycin, as noted in the species descriptions for both genera. The addition of 50 μg/mL kanamycin to agar media has been shown to selectively support the growth of these bacteria [26,44].
Several Tenacibaculum species associated with tenacibaculosis outbreaks in aquaculture exhibit strong β-hemolytic activity (e.g., T. dicentrarchi, T. finnmarkense, and T. maritimum). In contrast, non-hemolytic strains of T. adriaticum and T. soleae have not been associated with disease in farmed Atlantic salmon. Whole-genome sequencing has confirmed that Tenacibaculum type strains displaying β-hemolytic activity contain genes encoding hemolysins [45,46,47]. Interestingly, although T. soleae also possesses hemolysin genes [48], no β-hemolytic activity was observed in this study. Additionally, the non-hemolytic species T. piscium has been associated with skin ulcer development in several sea-farmed fish species [6], and scale loss and frayed fins were observed in dead fish during a bath-challenge experiment [8]. Although β-hemolytic bacteria are typically considered pathogenic and β-hemolysis is often a strong predictor of bacterial pathogenicity [49], this correlation warrants further investigation of Tenacibaculum spp. isolated from outbreaks associated with skin lesions or ulcers in Norwegian farmed Atlantic salmon.
Several Tenacibaculum strains exhibit variable motility characteristics depending on their growth medium. T. dicentrarchi strain NCIMB 14598T, T. finnmarkense genomovars, T. maritimum strain NLF-15, and to a lesser extent T. piscium strain TNO020T, T. soleae strain LL04 12.1.7T, and T. maritimum strain NCIMB 2154T, show increased motility on BAMA and KABAMA compared to growth on MA and BAS. A previous study by Pazos and Santos [28] demonstrated that MA does not induce gliding motility as effectively as other agar media, such as FMM. Gliding motility has been observed in Flavobacterium columnare when cultured in low-nutrient content [50], which may explain the enhanced gliding motility observed in FMM, containing half the yeast extract of MA. In contrast, BAMA and KABAMA had nutrient contents equivalent to MA, suggesting that the addition of blood and/or Red Sea Salt in the agar media could stimulate the observed gliding motility (evident from the colony shapes) in the Tenacibaculum strains listed above. Blood components may activate virulence genes under conditions that mimic fish ulcer environments [51,52]. Genes encoding virulence factors, including hemolysins and the T9SS-mediated secretion system, are important for gliding motility and have been identified in both T. finnmarkense and T. dicentrarchi [6,18]. T. maritimum also contains genes encoding hemolysins [10]; however, it exhibited unique gliding motility characteristics in this study, with smaller colonies and a rougher surface than other Tenacibaculum spp. in this study, making it easily distinguishable. The size difference in T. adriaticum strain B390T observed on BAMA or KABAMA may result from the presence of kanamycin and potentially be related to antibiotic resistance expression, particularly if gliding motility genes are present on the same plasmid, as suggested by Schroeder and Brooks [53]. Johansen, Catón [54] further noted that gliding motility is crucial for structural coloring in colonies within the Flavobacteriaceae family, which may explain why BAMA and KABAMA, which support effective gliding motility, induced intense bacterial colony coloration.
In the Norwegian salmon industry, BAS and MA are the most commonly used media for investigating skin lesions and ulcerations. The historic reliance on BAS for diagnosing such outbreaks may partly explain why Tenacibaculum spp. have not been recognized as significant pathogens associated with these diseases. Since most Tenacibaculum spp. do not grow on media supplemented solely with NaCl, BAS tends to favor the growth of other bacteria present in skin lesions and ulcers, such as M. viscosa and A. wodanis, which grow well on this medium. This likely introduced bias in earlier bacteriological investigations, potentially overestimating M. viscosa as the primary etiological agent in skin lesion outbreaks. The challenges associated with detecting or isolating certain Tenacibaculum sp., particularly T. maritimum, and the resulting underestimation of their role in disease development have already been reported, notably in New Zealand [27,55].
The use of KABAMA provides a selective advantage for recovering pathogenic Tenacibaculum spp. associated with skin lesions and ulcers in Atlantic salmon. This medium is particularly beneficial for isolating T. maritimum, which typically grows more slowly than other bacteria found in skin lesions, including other Tenacibaculum species. Both KABAMA and BAMA revealed specific characteristics of T. maritimum colonies, such as distinct ruggedness and uneven margins, β-hemolytic activity, and distinct green coloration upon prolonged incubation. These characteristics aid in the accurate identification and recovery of T. maritimum strains in the future. Additionally, KABAMA and BAMA enhanced the recovery of other Tenacibaculum spp., revealing typical colony characteristics like smooth surfaces, β-hemolytic activity, and distinct color differences between strains. Field investigations confirmed the benefits of using KABAMA, which facilitated the rapid detection and isolation of Tenacibaculum colonies (see Supplemental Material, Figures S1–S3).
Overall, the use of KABAMA and BAMA enables the effective differentiation and identification of Tenacibaculum spp. associated with skin lesions and ulcer outbreaks in farmed Atlantic salmon and can reveal the presence of concurrent Tenacibaculum spp. in the same sample. Identifying multiple Tenacibaculum strains from a single outbreak provides valuable insights into the genetic diversity of Tenacibaculum spp.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13071567/s1. Figure S1. Bacterial cultivation from a skin ulcer on an Atlantic salmon (Salmo salar) in a facility in Vestland, Norway. Figure S2. Bacterial cultivation from Fucus vesiculosus collected in Vestland, Norway. Figure S3. Bacterial cultivation from 2 filter tanks at a fish production site in Vestland, Norway. Table S1. Colony development of 11 Tenacibaculum bacterial strains on four different agar media. Table S2. Summary comparison of media performance for the cultivation and differentiation of Tenacibaculum spp. References [56,57] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, K.F. and S.B.S.; methodology, E.L., K.F. and S.B.S.; software, E.L.; validation, A.N., E.L. and S.B.S.; formal analysis, E.L., I.E.B.K. and S.B.S.; investigation, E.L., I.E.B.K. and S.B.S.; resources, A.N. and H.D.; data curation, E.L., I.E.B.K. and S.B.S.; writing—original draft preparation, E.L., K.F. and S.B.S.; writing—review and editing, A.N., E.L., H.D., I.E.B.K., K.F. and S.B.S.; visualization, E.L. and S.B.S.; supervision, E.L. and S.B.S.; project administration, A.N. and H.D.; funding acquisition, A.N. and H.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by a FHF grant (Fiskeri og Havbruksnæringens Forskningsfond, LimiT project #901433).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors deeply thank Per Gunnar Espedal and Lars Hamre from the Department of Biological Sciences at Bergen University for their help during field sampling.
Conflicts of Interest
Authors Sverre Bang Småge and Henrik Duesund were employed by Cermaq Group AS. Author Kathleen Frisch was employed by Cermaq Canada. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript.
| BAS | Blood agar medium supplemented with 2% NaCl |
| FMM | Flexibacter maritimus medium |
| MA | Marine Agar |
References
- Toranzo, A.E.; Magariños, B.; Romalde, J.L. A review of the main bacterial fish diseases in mariculture systems. Aquaculture 2005, 246, 37–61. [Google Scholar] [CrossRef]
- Avendaño-Herrera, R.; Toranzo, A.E.; Magariños, B. Tenacibaculosis infection in marine fish caused by Tenacibaculum maritimum: A review. Dis. Aquat. Org. 2006, 71, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Avendaño-Herrera, R.; Irgang, R.; Magariños, B.; Romalde, J.L.; Toranzo, A.E. Use of microcosms to determine the survival of the fish pathogen Tenacibaculum maritimum in seawater. Environ. Microbiol. 2006, 8, 921–928. [Google Scholar] [CrossRef]
- Avendaño-Herrera, R.; Irgang, R.; Sandoval, C.; Moreno-Lira, P.; Houel, A.; Duchaud, E.; Poblete-Morales, M.; Nicolas, P.; Ilardi, P. Isolation, Characterization and Virulence Potential of Tenacibaculum dicentrarchi in Salmonid Cultures in Chile. Transbound. Emerg. Dis. 2016, 63, 121–126. [Google Scholar] [CrossRef]
- Klakegg, Ø.; Abayneh, T.; Fauske, A.K.; Fülberth, M.; Sørum, H. An outbreak of acute disease and mortality in Atlantic salmon (Salmo salar) post-smolts in Norway caused by Tenacibaculum dicentrarchi. J. Fish Dis. 2019, 42, 789–807. [Google Scholar] [CrossRef]
- Olsen, A.B.; Spilsberg, B.; Nilsen, H.K.; Lagesen, K.; Gulla, S.; Avendaño-Herrera, R.; Irgang, R.; Duchaud, E.; Colquhoun, D.J. Tenacibaculum piscium sp. nov., isolated from skin ulcers of sea-farmed fish, and description of Tenacibaculum finnmarkense sp. nov. with subdivision into genomovars finnmarkense and ulcerans. Int. J. Syst. Evol. Microbiol. 2020, 70, 6079–6090. [Google Scholar] [CrossRef]
- Småge, S.B.; Brevik, O.J.; Duesund, H.; Ottem, K.F.; Watanabe, K.; Nylund, A. Tenacibaculum finnmarkense sp. nov., a fish pathogenic bacterium of the family Flavobacteriaceae isolated from Atlantic salmon. Antonie Van Leeuwenhoek 2016, 109, 273–285. [Google Scholar] [CrossRef]
- Avendaño-Herrera, R.; Olsen, A.B.; Saldarriaga-Cordoba, M.; Colquhoun, D.J.; Reyes, V.; Rivera-Bohle, J.; Duchaud, E.; Irgang, R. Isolation, identification, virulence potential and genomic features of Tenacibaculum piscium isolates recovered from Chilean salmonids. Transbound. Emerg. Dis. 2022, 69, e3305–e3315. [Google Scholar] [CrossRef]
- Lagadec, E.; Småge, S.B.; Trösse, C.; Nylund, A. Phylogenetic analyses of Norwegian Tenacibaculum strains confirm high bacterial diversity and suggest circulation of ubiquitous virulent strains. PLoS ONE 2021, 16, e0259215. [Google Scholar] [CrossRef]
- Mabrok, M.; Algammal, A.M.; Sivaramasamy, E.; Hetta, H.F.; Atwah, B.; Alghamdi, S.; Fawzy, A.; Avendaño-Herrera, R.; Rodkhum, C. Tenacibaculosis caused by Tenacibaculum maritimum: Updated knowledge of this marine bacterial fish pathogen. Front. Cell. Infect. Microbiol. 2022, 12, 1068000. [Google Scholar] [CrossRef]
- Frisch, K.; Småge, S.B.; Johansen, R.; Duesund, H.; Brevik, Ø.J.; Nylund, A. Pathology of experimentally induced mouthrot caused by Tenacibaculum maritimum in Atlantic salmon smolts. PLoS ONE 2018, 13, e0206951. [Google Scholar] [CrossRef] [PubMed]
- Handlinger, J.; Soltani, M.; Percival, S. The pathology of Flexibacter maritimus in aquaculture species in Tasmania, Australia. J. Fish Dis. 1997, 20, 159–168. [Google Scholar] [CrossRef]
- Escribano, M.P.; Balado, M.; Toranzo, A.E.; Lemos, M.L.; Magariños, B. The secretome of the fish pathogen Tenacibaculum maritimum includes soluble virulence-related proteins and outer membrane vesicles. Front. Cell. Infect. Microbiol. 2023, 13, 1197290. [Google Scholar] [CrossRef] [PubMed]
- Løvoll, M.; Wiik-Nielsen, C.R.; Tunsjø, H.S.; Colquhoun, D.; Lunder, T.; Sørum, H.; Grove, S. Atlantic salmon bath challenged with Moritella viscosa--pathogen invasion and host response. Fish Shellfish. Immunol. 2009, 26, 877–884. [Google Scholar] [CrossRef]
- Tingbø, M.G.; Haugen Tunheim, S.; Klevan, A.; Kamisinska, A.; Behzaad, H.; Sandtrø, A.; Furevik, A. Antigenic similarities and clinical cross-protection between variant and classic non-viscous strains of Moritella viscosa in Atlantic salmon in Norway. Fish Shellfish. Immunol. 2024, 145, 109306. [Google Scholar] [CrossRef] [PubMed]
- Lillehaug, A.; Lunestad, B.T.; Grave, K. Epidemiology of bacterial diseases in Norwegian aquaculture--a description based on antibiotic prescription data for the ten-year period 1991 to 2000. Dis. Aquat. Org. 2003, 53, 115–125. [Google Scholar] [CrossRef]
- Lunder, T.; Evensen, O.; Holstad, G.; Hastein, T.; Evensen, O. ‘Winter ulcer’ in the Atlantic salmon Salmo salar. Pathological and bacteriological investigations and transmission experiments. Dis. Aquat. Org. 1995, 23, 39–49. [Google Scholar] [CrossRef]
- Olsen, A.B.; Nilsen, H.; Sandlund, N.; Mikkelsen, H.; Sorum, H.; Colquhoun, D.J. Tenacibaculum sp. associated with winter ulcers in sea-reared Atlantic salmon Salmo salar. Dis. Aquat. Organ. 2011, 94, 189–199. [Google Scholar] [CrossRef]
- Benediktsdottir, E.; Verdonck, L.; Sproer, C.; Helgason, S.; Swings, J. Characterization of Vibrio viscosus and Vibrio wodanis isolated at different geographical locations: A proposal for reclassification of Vibrio viscosus as Moritella viscosa comb. nov. Int. J. Syst. Evol. Microbiol. 2000, 50, 479–488. [Google Scholar] [CrossRef]
- Småge, S.B.; Brevik, O.J.; Frisch, K.; Watanabe, K.; Duesund, H.; Nylund, A. Concurrent jellyfish blooms and tenacibaculosis outbreaks in Northern Norwegian Atlantic salmon (Salmo salar) farms. PLoS ONE 2017, 12, e0187476. [Google Scholar] [CrossRef]
- Suzuki, M.; Nakagawa, Y.; Harayama, S.; Yamamoto, S. Phylogenetic analysis and taxonomic study of marine Cytophaga-like bacteria: Proposal for Tenacibaculum gen. nov. with Tenacibaculum maritimum comb. nov. and Tenacibaculum ovolyticum comb. nov., and description of Tenacibaculum mesophilum sp. nov. and Tenacibaculum amylolyticum sp. nov. Int. J. Syst. Evol. Microbiol. 2001, 51 Pt 5, 1639–1652. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.E.; Henry-Ford, D.; Groff, J.M. Isolation and Characterization of Flexibacter maritimus from Marine Fishes of California. J. Aquat. Anim. Health 1995, 7, 318–326. [Google Scholar] [CrossRef]
- Kent, M.L.; Dungan, C.; Elston, R.A.; Holt, R.A. Cytophaga sp. (Cytophagales) infection in seawater pen-reared Atlantic salmon Salmo salar. Dis. Aquat. Org. 1988, 4, 173–179. [Google Scholar] [CrossRef]
- Hahnke, R.L.; Harder, J. Phylogenetic diversity of Flavobacteria isolated from the North Sea on solid media. Syst. Appl. Microbiol. 2013, 36, 497–504. [Google Scholar] [CrossRef]
- Toranzo, A.E. Tenacibaculosis of Farmed Fish in Southern Europe; Tenacibaculum Maritimum Workshop Maritimum Heritage Center: Campbell River, BC, Canada, 2015. [Google Scholar]
- Frisch, K.; Småge, S.B.; Brevik, O.J.; Duesund, H.; Nylund, A. Genotyping of Tenacibaculum maritimum isolates from farmed Atlantic salmon in Western Canada. J. Fish Dis. 2018, 41, 131–137. [Google Scholar] [CrossRef]
- Kumanan, K.; von Ammon, U.; Fidler, A.; Symonds, J.E.; Walker, S.P.; Carson, J.; Hutson, K.S. Advantages of selective medium for surveillance of Tenacibaculum species in marine fish aquaculture. Aquaculture 2022, 558, 738365. [Google Scholar] [CrossRef]
- Pazos, F.; Santos, Y.; Macías, A.R.; Núñez, S.; Toranzo, A.E. Evaluation of media for the successful culture of Flexibacter maritimus. J. Fish Dis. 1996, 19, 193–197. [Google Scholar] [CrossRef]
- Shieh, H. Studies on the nutrition of a fish pathogen, Flexibacter columnaris. Microbios Lett. 1980, 13, 129–133. [Google Scholar]
- Wilson, T.K.; Douglas, M.; Dunn, V. First identification in Tasmania of fish pathogens Tenacibaculum dicentrarchi and T. soleae and multiplex PCR for these organisms and T. maritimum. Dis. Aquat. Org. 2019, 136, 219–226. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kawai, K.; Oshima, S.-i. Evaluation of an Experimental Immersion Infection Method with Tenacibaculum maritimum in Japanese Flounder Paralichthys olivaceus. Aquac. Sci. 2010, 58, 481–489. [Google Scholar] [CrossRef]
- Mabrok, M.; Machado, M.; Serra, C.R.; Afonso, A.; Valente, L.M.; Costas, B. Tenacibaculosis induction in the Senegalese sole (Solea senegalensis) and studies of Tenacibaculum maritimum survival against host mucus and plasma. J. Fish Dis. 2016, 39, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.; Suga, K.; Kanai, K.; Sugihara, Y. Biological and Serological Characterization of a Non-gliding Strain of Tenacibaculum maritimum Isolated from a Diseased Puffer Fish Takifugu rubripes. Fish Pathol. 2014, 49, 121–129. [Google Scholar] [CrossRef]
- Apablaza, P.; Frisch, K.; Brevik Ø, J.; Småge, S.B.; Vallestad, C.; Duesund, H.; Mendoza, J.; Nylund, A. Primary Isolation and Characterization of Tenacibaculum maritimum from Chilean Atlantic Salmon Mortalities Associated with a Pseudochattonella spp. Algal Bloom. J. Aquat. Anim. Health 2017, 29, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Småge, S.B.; Frisch, K.; Brevik, Ø.J.; Watanabe, K.; Nylund, A. First isolation, identification and characterisation of Tenacibaculum maritimum in Norway, isolated from diseased farmed sea lice cleaner fish Cyclopterus lumpus L. Aquaculture 2016, 464, 178–184. [Google Scholar] [CrossRef]
- Blodgett, R. BAM Appendix 2: Most Probable Number from Serial Dilutions. In Bacteriological Analytical Manual, 8th ed.; Revision A; FDA: Silver Spring, MD, USA, 1998. [Google Scholar]
- Heindl, H.; Wiese, J.; Imhoff, J.F. Tenacibaculum adriaticum sp. nov., from a bryozoan in the Adriatic Sea. Int. J. Syst. Evol. Microbiol. 2008, 58, 542–547. [Google Scholar] [CrossRef]
- Piñeiro-Vidal, M.; Gijón, D.; Zarza, C.; Santos, Y. Tenacibaculum dicentrarchi sp. nov., a marine bacterium of the family Flavobacteriaceae isolated from European sea bass. Int. J. Syst. Evol. Microbiol. 2012, 62, 425–429. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Hikida, M.; Masumura, K. Flexibacter maritimus sp. nov., a pathogen of marine fishes. Int. J. Syst. Evol. Microbiol. 1986, 36, 396–398. [Google Scholar] [CrossRef]
- Hansen, G.H.; Bergh, O.; Michaelsen, J.; Knappskog, D. Flexibacter ovolyticus sp. nov., a pathogen of eggs and larvae of Atlantic halibut, Hippoglossus hippoglossus L. Int. J. Syst. Evol. Microbiol. 1992, 42, 451–458. [Google Scholar] [CrossRef]
- Piñeiro-Vidal, M.; Carballas, C.G.; Gómez-Barreiro, O.; Riaza, A.; Santos, Y. Tenacibaculum soleae sp. nov., isolated from diseased sole (Solea senegalensis Kaup). Int. J. Syst. Evol. Microbiol. 2008, 58, 881–885. [Google Scholar] [CrossRef]
- Lunder, T.; Sørum, H.; Holstad, G.; Steigerwalt, A.G.; Mowinckel, P.; Brenner, D.J. Phenotypic and genotypic characterization of Vibrio viscosus sp. nov. and Vibrio wodanis sp. nov. isolated from Atlantic salmon (Salmo salar) with ‘winter ulcer’. Int. J. Syst. Evol. Microbiol. 2000, 50, 427–450. [Google Scholar] [CrossRef]
- Frelier, F.P.; Elton, A.R.; Loy, K.J.; Mincher, C. Macroscopic and microscopic features of ulcerative stomatitis in farmed Atlantic salmon Salmo salar. Dis. Aquat. Org. 1994, 18, 227–231. [Google Scholar] [CrossRef]
- Flint, K.P. A note on a selective agar medium for the enumeration of Flavobacterium species in water. J. Appl. Bacteriol. 1985, 59, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Bridel, S.; Olsen, A.B.; Nilsen, H.; Bernardet, J.F.; Achaz, G.; Avendaño-Herrera, R.; Duchaud, E. Comparative genomics of Tenacibaculum dicentrarchi and “Tenacibaculum finnmarkense” highlights intricate evolution of fish-pathogenic species. Genome Biol. Evol. 2018, 10, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pascual, D.; Lunazzi, A.; Magdelenat, G.; Rouy, Z.; Roulet, A.; Lopez-Roques, C.; Larocque, R.; Barbeyron, T.; Gobet, A.; Michel, G.; et al. The Complete Genome Sequence of the Fish Pathogen Tenacibaculum maritimum Provides Insights into Virulence Mechanisms. Front. Microbiol. 2017, 8, 1542. [Google Scholar] [CrossRef]
- Teramoto, M.; Zhai, Z.; Komatsu, A.; Shibayama, K.; Suzuki, M. Genome Sequence of the Psychrophilic Bacterium Tenacibaculum ovolyticum Strain da5A-8 Isolated from Deep Seawater. Genome Announc. 2016, 4, 10–1128. [Google Scholar] [CrossRef]
- Lujan, K.M.; Eisen, J.A.; Coil, D.A. Draft Genome Sequence of Tenacibaculum soleae UCD-KL19. Genome Announc. 2016, 4, e01120-16. [Google Scholar] [CrossRef]
- Sum, R.; Swaminathan, M.; Rastogi, S.K.; Piloto, O.; Cheong, I. Beta-Hemolytic bacteria selectively trigger liposome lysis, enabling rapid and accurate pathogen detection. ACS Sens. 2017, 2, 1441–1451. [Google Scholar] [CrossRef]
- Penttinen, R.; Hoikkala, V.; Sundberg, L.-R. Gliding Motility and Expression of Motility-Related Genes in Spreading and Non-spreading Colonies of Flavobacterium columnare. Front. Microbiol. 2018, 9, 525. [Google Scholar] [CrossRef]
- Litwin, C.M.; Calderwood, S.B. Role of iron in regulation of virulence genes. Clin. Microbiol. Rev. 1993, 6, 137–149. [Google Scholar] [CrossRef]
- Saldarriaga-Córdoba, M.; Irgang, R.; Avendaño-Herrera, R. Comparison between genome sequences of Chilean Tenacibaculum dicentrarchi isolated from red conger eel (Genypterus chilensis) and Atlantic salmon (Salmo salar) focusing on bacterial virulence determinants. J. Fish Dis. 2021, 44, 1843–1860. [Google Scholar] [CrossRef]
- Schroeder, M.; Brooks, B.D.; Brooks, A.E. The Complex Relationship between Virulence and Antibiotic Resistance. Genes 2017, 8, 39. [Google Scholar] [CrossRef]
- Johansen, V.E.; Catón, L.; Hamidjaja, R.; Oosterink, E.; Wilts, B.D.; Rasmussen, T.S.; Sherlock, M.M.; Ingham, C.J.; Vignolini, S. Genetic manipulation of structural color in bacterial colonies. Proc. Natl. Acad. Sci. USA 2018, 115, 2652–2657. [Google Scholar] [CrossRef] [PubMed]
- Brosnahan, C.L.; Munday, J.S.; Ha, H.J.; Preece, M.; Jones, J.B. New Zealand rickettsia-like organism (NZ-RLO) and Tenacibaculum maritimum: Distribution and phylogeny in farmed Chinook salmon (Oncorhynchus tshawytscha). J. Fish Dis. 2019, 42, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, S.J.; Rappé, M.S.; Vergin, K.L.; Adair, N.L. 16S rRNA genes reveal stratified open ocean bacterioplankton populations related to the Green Non-Sulfur bacteria. Proc. Natl. Acad. Sci. USA 1996, 93, 7979–7984. [Google Scholar] [CrossRef] [PubMed]
- Habib, C.; Houel, A.; Lunazzi, A.; Bernardet, J.F.; Olsen, A.B.; Nilsen, H.; Toranzo, A.E.; Castro, N.; Nicolas, P.; Duchaud, E. Multilocus sequence analysis of the marine bacterial genus Tenacibaculum suggests parallel evolution of fish pathogenicity and endemic colonization of aquaculture systems. Appl. Environ. Microbiol. 2014, 80, 5503–5514. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).