Peculiarities of Diagnostic Reliability—Nested PCR Versus SAT in the Identification of Helicobacter pylori
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. SAT
2.3. DNA Isolation
2.4. Nested PCR
2.5. Analysis of PCR Products and Their DNA Sequences
2.6. Other Procedures
3. Results
3.1. Presence of H. pylori Antigen in Stool
3.2. Presence of H. pylori Long 454 bp Amplicon in Stool
3.3. Presence of H. pylori Short 148 bp Amplicon in Stool
3.4. SAT Versus NPCR for Short 148 bp Amplicon
3.5. SAT Versus NPCR for the Short 148 bp Amplicon in an Asymptomatic Population
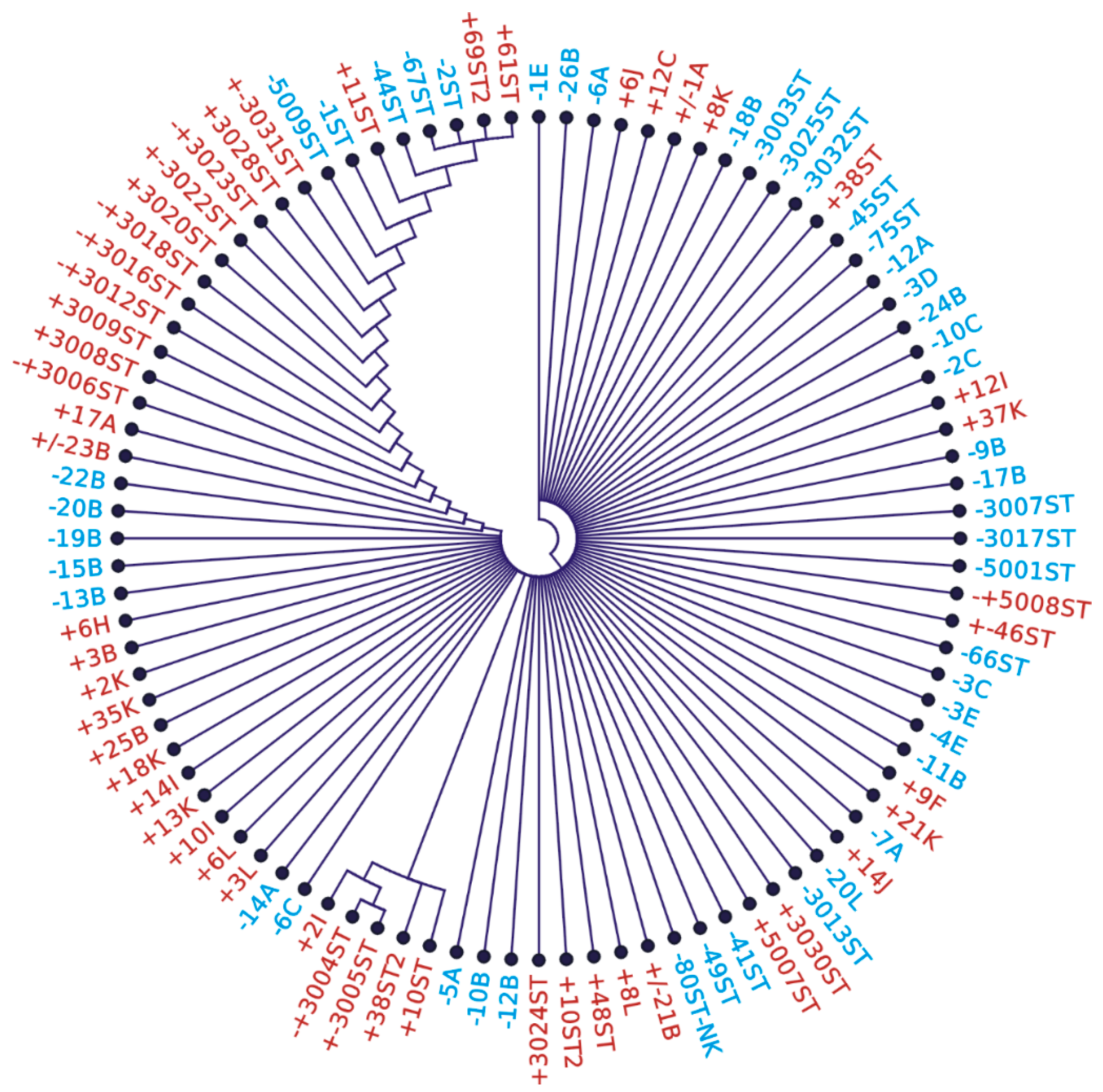
3.6. Variability of DNA Sequence from NPCR Short 148 bp Amplicons
4. Discussion
Technical Merits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NPCR | Nested polymerase chain reaction |
| SAT | Stool antigen test |
| bp | Base pairs |
| UBT | Urea breath test |
| GIT | Gastrointestinal tract |
| PPI | Proton pump inhibitor |
| DOB | Delta over baseline |
| RUT | Rapid urease test |
| ddPCR | Droplet digital PCR |
| rRNA | Ribosomal RNA |
References
- Gargano, L.M.; Hughes, J.M. Microbial origins of chronic diseases. Annu. Rev. Public Health 2014, 35, 65–82. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E. Helicobacter pylori infection. Nat. Rev. Dis. Primers 2023, 9, 19. [Google Scholar] [CrossRef]
- Duan, M.; Li, Y.; Liu, J.; Zhang, W. Transmission routes and patterns of Helicobacter pylori. Helicobacter 2023, 28, e12945. [Google Scholar] [CrossRef] [PubMed]
- Leja, M.; Grinberga-Derica, I.; Bilgilier, C.; Steininger, C. Review: Epidemiology of Helicobacter pylori infection. Helicobacter 2019, 24 (Suppl. S1), e12635. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef] [PubMed]
- Aumpan, N.; Mahachai, V.; Vilaichone, R. Management of Helicobacter pylori infection. JGH Open 2023, 7, 3–15. [Google Scholar] [CrossRef]
- Mezmale, L.; Coelho, L.G.; Bordin, D.; Leja, M. Review: Epidemiology of Helicobacter pylori. Helicobacter 2020, 25 (Suppl. S1), e12734. [Google Scholar] [CrossRef]
- Elbehiry, A.; Marzouk, E.; Aldubaib, M.; Abalkhail, A.; Anagreyyah, S.; Anajirih, N.; Almuzaini, A.M.; Rawway, M.; Alfadhel, A.; Draz, A.; et al. Helicobacter pylori Infection: Current Status and Future Prospects on Diagnostic, Therapeutic and Control Challenges. Antibiotics 2023, 12, 191. [Google Scholar] [CrossRef]
- Alsina, M.; Arrazubi, V.; Diez, M.; Tabernero, J. Current developments in gastric cancer: From molecular profiling to treatment strategy. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 155–170. [Google Scholar] [CrossRef]
- Cardos, A.I.; Maghiar, A.; Zaha, D.C.; Pop, O.; Fritea, L.; Miere, F.; Cavalu, S. Evolution of Diagnostic Methods for Helicobacter pylori Infections: From Traditional Tests to High Technology, Advanced Sensitivity and Discrimination Tools. Diagnostics 2022, 12, 508. [Google Scholar] [CrossRef]
- Sabbagh, P.; Mohammadnia-Afrouzi, M.; Javanian, M.; Babazadeh, A.; Koppolu, V.; Vasigala, V.R.; Nouri, H.R.; Ebrahimpour, S. Diagnostic methods for Helicobacter pylori infection: Ideals, options, and limitations. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 55–66. [Google Scholar] [CrossRef]
- Ansari, S.; Yamaoka, Y. Helicobacter pylori Infection, Its Laboratory Diagnosis, and Antimicrobial Resistance: A Perspective of Clinical Relevance. Clin. Microbiol. Rev. 2022, 35, e00258-21. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, Y.; Wu, Y.; Zhao, X.; Gao, H.; Xie, X.; Wu, L.; Zhao, H.; Li, L.; Zhang, J.; et al. Advances in Helicobacter pylori detection technology: From pathology-based to multi-omic based methods. TrAC Trends Anal. Chem. 2024, 182, 118041. [Google Scholar] [CrossRef]
- Godbole, G.; Mégraud, F.; Bessède, E. Review: Diagnosis of Helicobacter pylori infection. Helicobacter 2020, 25, e12735. [Google Scholar] [CrossRef]
- Sulo, P.; Šipková, B. DNA diagnostics for reliable and universal identification of Helicobacter pylori. World J. Gastroenterol. 2021, 27, 7100–7112. [Google Scholar] [CrossRef] [PubMed]
- Best, L.M.; Takwoingi, Y.; Siddique, S.; Selladurai, A.; Gandhi, A.; Low, B.; Yaghoobi, M.; Gurusamy, K.S. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst. Rev. 2018, 3, CD012080. [Google Scholar]
- Sugimoto, M.; Wu, J.Y.; Abudayyeh, S.; Hoffman, J.; Brahem, H.; Al-Khatib, K.; Yamaoka, Y.; Graham, D.Y. Unreliability of results of PCR detection of Helicobacter pylori in clinical or environmental samples. J. Clin. Microbiol. 2009, 47, 738–742. [Google Scholar] [CrossRef]
- Šeligová, B.; Lukáč, Ľ.; Bábelová, M.; Vávrová, S.; Sulo, P. Diagnostic reliability of nested PCR depends on the primer design and threshold abundance of Helicobacter pylori in biopsy, stool, and saliva samples. Helicobacter 2020, 25, e12680. [Google Scholar] [CrossRef]
- Mikula, M.; Dzwonek, A.; Jagusztyn-Krynicka, K.; Ostrowski, J. Quantitative detection for low levels of Helicobacter pylori infection in experimentally infected mice by real-time PCR. J. Microbiol. Methods 2003, 55, 351–359. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Rizzi, A.; Raddadi, N.; Sorlini, C.; Nordgrd, L.; Nielsen, K.M.; Daffonchio, D. The stability and degradation of dietary DNA in the gastrointestinal tract of mammals: Implications for horizontal gene transfer and the biosafety of GMOs. Crit. Rev. Food Sci. Nutr. 2012, 52, 142–161. [Google Scholar] [CrossRef]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef] [PubMed]
- Roszczenko-Jasińska, P.; Wojtyś, M.I.; Jagusztyn-Krynicka, E.K. Helicobacter pylori treatment in the post-antibiotics era—Searching for new drug targets. Appl. Microbiol. Biotechnol. 2020, 104, 9891–9905. [Google Scholar] [CrossRef] [PubMed]
- Guinane, C.M.; Cotter, P.D. Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Ther. Adv. Gastroenterol. 2013, 6, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Skrebinska, S.; Megraud, F.; Bessede, F. Diagnosis of Helicobacter pylori infection. Helicobacter 2018, 23 (Suppl. S1), e12515. [Google Scholar] [CrossRef]
- Calik, Z.; Karamese, M.; Acar, O.; Karamese, S.A.; Dicle, Y.; Albayrak, F.; Can, S.; Guvendi, B.; Turgut, A.; Cicek, M.; et al. Investigation of Helicobacter pylori antigen in stool samples of patients with upper gastrointestinal complaints. Braz. J. Microbiol. 2016, 47, 167–171. [Google Scholar] [CrossRef]
- Gantuya, B.; El-Serag, H.B.; Matsumoto, T.; Ajami, N.J.; Oyuntsetseg, K.; Azzaya, D.; Uchida, T.; Yamaoka, Y. Gastric Microbiota in Helicobacter pylori-Negative and-Positive Gastritis Among High Incidence of Gastric Cancer Area. Cancers 2019, 11, 504. [Google Scholar] [CrossRef]
- Schrader, C.; Schielke, A.; Ellerbroek, L.; Johne, R. PCR inhibitors–occurrence, properties and removal. J. Appl. Microbiol. 2012, 113, 1014–1026. [Google Scholar] [CrossRef]
- Beer-Davidson, G.; Hindiyeh, M.; Muhsen, K. Detection of Helicobacter pylori in stool samples of young children using realtime polymerase chain reaction. Helicobacter 2018, 23, e12450. [Google Scholar] [CrossRef]
- Qiu, E.; Jin, S.; Xiao, Z.; Chen, Q.; Wang, Q.; Liu, H.; Xie, C.; Chen, C.; Li, Z.; Han, S. CRISPR-based detection of Helicobacter pylori in stool samples. Helicobacter 2021, 26, e12828. [Google Scholar] [CrossRef]
- de Dieu Habimana, J.; Mukama, O.; Amissah, O.B.; Sun, Y.; Karangwa, E.; Liu, Y.; Mugisha, S.; Cheng, N.; Wang, L.; Chen, J.; et al. A rationally designed CRISPR/Cas12a assay using a multimodal reporter for various readouts. Anal. Chem. 2023, 95, 11741–11750. [Google Scholar] [CrossRef] [PubMed]
- Khera, H.K.; Mishra, R. Nucleic acid based testing (NABing): A game changer technology for public health. Mol. Biotechnol. 2024, 66, 2168–2200. [Google Scholar] [CrossRef] [PubMed]

| Patient Number | SAT | NPCR 148 bp Amplicon | NPCR 448 bp Amplicon |
|---|---|---|---|
| 13 | + | + | + |
| 38 | + | + | - |
| 7 | + | - | - |
| 55 | - | + | - |
| 95 | - | - | - |
| 208 | 58/208 | 106/208 | 13/208 |
| Sensitivity (%) | 54.7 |
| Specificity (%) | 93.6 |
| Positive predictive value | 100.0 |
| Negative predictive value | 48.7 |
| Number | SAT | NPCR 148 bp Amplicon | NPCR 448 bp Amplicon |
|---|---|---|---|
| 22 | + | + | + |
| 13 | + | + | - |
| 31 | - | + | - |
| 34 | - | - | - |
| 100 | 35/100 | 66/100 | 22/100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šipková, B.; Abrahamovská, M.; Klingová, J.; Prokopová, B.; Krčmáriková, J.; Cihová, I.; Sulo, P. Peculiarities of Diagnostic Reliability—Nested PCR Versus SAT in the Identification of Helicobacter pylori. Microorganisms 2025, 13, 1498. https://doi.org/10.3390/microorganisms13071498
Šipková B, Abrahamovská M, Klingová J, Prokopová B, Krčmáriková J, Cihová I, Sulo P. Peculiarities of Diagnostic Reliability—Nested PCR Versus SAT in the Identification of Helicobacter pylori. Microorganisms. 2025; 13(7):1498. https://doi.org/10.3390/microorganisms13071498
Chicago/Turabian StyleŠipková, Barbora, Michaela Abrahamovská, Janka Klingová, Bianka Prokopová, Jana Krčmáriková, Iveta Cihová, and Pavol Sulo. 2025. "Peculiarities of Diagnostic Reliability—Nested PCR Versus SAT in the Identification of Helicobacter pylori" Microorganisms 13, no. 7: 1498. https://doi.org/10.3390/microorganisms13071498
APA StyleŠipková, B., Abrahamovská, M., Klingová, J., Prokopová, B., Krčmáriková, J., Cihová, I., & Sulo, P. (2025). Peculiarities of Diagnostic Reliability—Nested PCR Versus SAT in the Identification of Helicobacter pylori. Microorganisms, 13(7), 1498. https://doi.org/10.3390/microorganisms13071498






