Carbapenem Resistance in Acinetobacter baumannii: Mechanisms, Therapeutics, and Innovations
Abstract
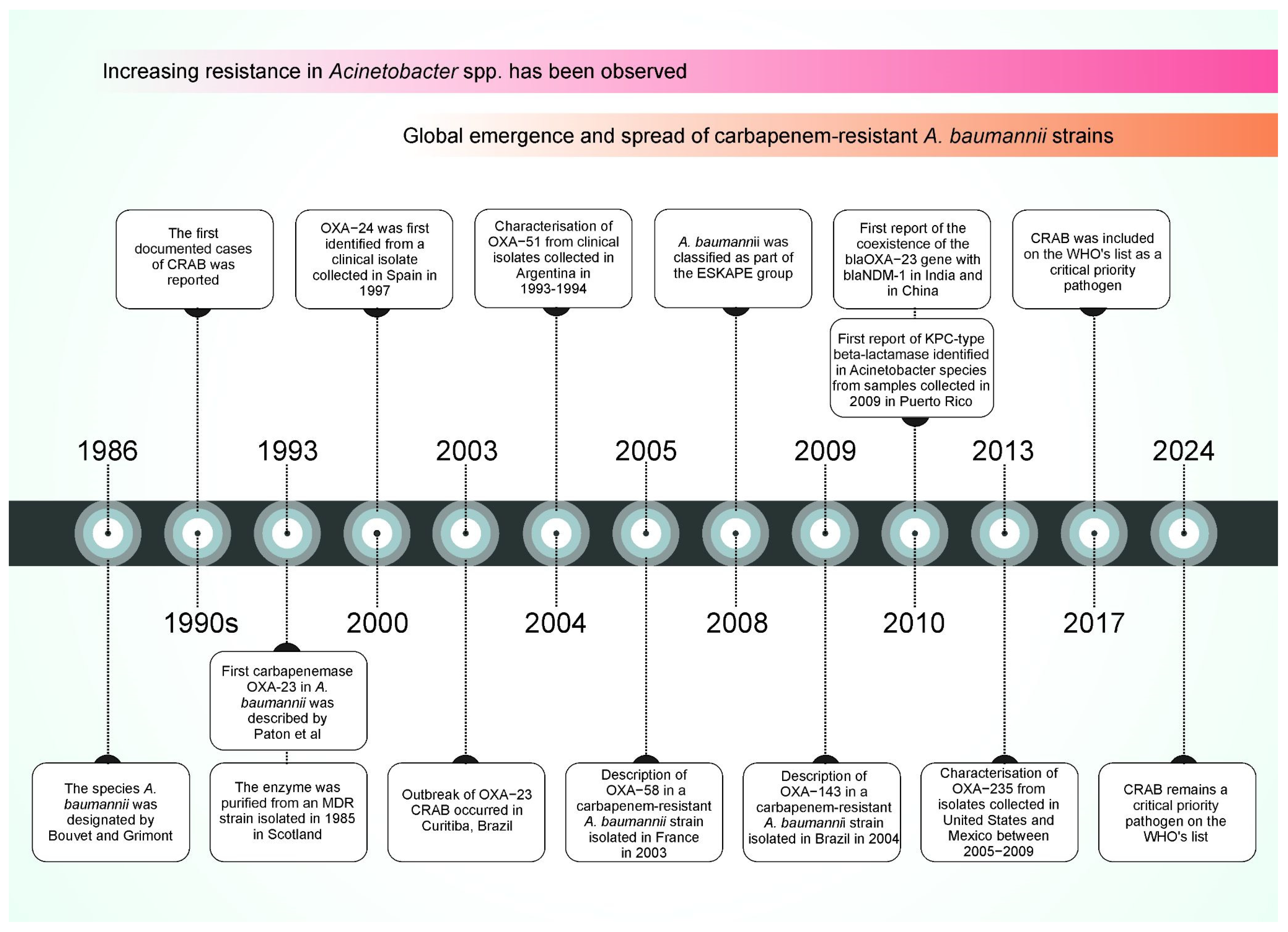
1. Emergence of Carbapenem-Resistant A. baumannii (CRAB)
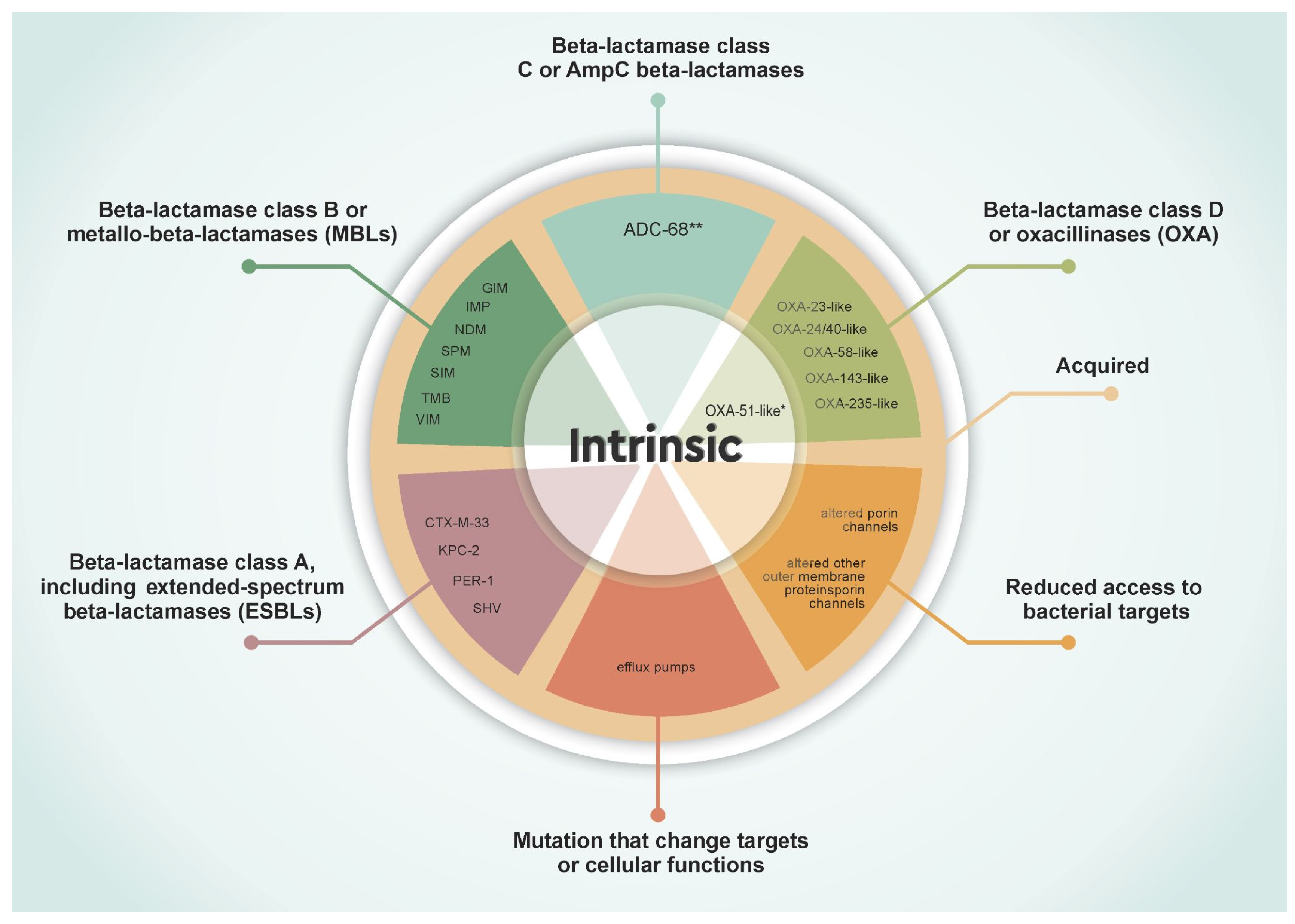
2. Molecular Mechanisms of Carbapenem Resistance in A. baumannii
2.1. Beta-Lactamases
2.1.1. Class A Beta-Lactamases, Including Extended-Spectrum Beta-Lactamases (ESBLs)
| Ambler Molecular and Structural Classification | Gene Family | Accession * | e.g., Genes | References |
|---|---|---|---|---|
| A | CTX-M beta-lactamase | ARO:3000016 | CTX-M-33 | [35,36] |
| A | KPC beta-lactamase | ARO:3000059 | KPC-2 | [31,32] |
| A | PER beta-lactamase | ARO:3000056 | PER-1 | [27,28] |
| A | SHV beta-lactamase *** | ARO:3000015 | SHV-12 | [33,34,37] |
| A | TEM beta-lactamase ** | ARO:3000014 | TEM-1 | [34] |
| A | VEB beta-lactamase | ARO:3000043 | VEB-1 | [38,39] |
| B or MBLs | GIM beta-lactamase | ARO:3003195 | GIM | [40] |
| B or MBLs | IMP beta-lactamase | ARO:3000020 | IMP-1, IMP-11, IMP-2, IMP-4, IMP-5, IMP-6, IMP-69 | [41,42,43,44,45,46,47,48] |
| B or MBLs | NDM beta-lactamase | ARO:3000057 | NDM-1, NDM-2 | [49] |
| B or MBLs | SIM beta-lactamase | ARO:3004206 | SIM-1 | [41] |
| B or MBLs | SPM beta-lactamase | ARO:3000580 | SPM | [50] |
| B or MBLs | TMB beta-lactamase | ARO:3004104 | TMB-2 | [51] |
| B or MBLs | VIM beta-lactamase | ARO:3000021 | VIM-2 | [52] |
| C or para Cephalosporins | ADC beta-lactamase with carbapenemase activity | ARO:3004545 | ADC-68 *** | [53] |
2.1.2. Class B Beta-Lactamases or MBLs
2.1.3. Class C Beta-Lactamases
| Gene Family | Variantes * | Accession ** | Associated Insertion Sequence Elements | Plasmid or Chromosomal | References |
|---|---|---|---|---|---|
| OXA-23-like beta-lactamase | 52 | ARO:3007710 | ISAba1 and ISAba4 | Chromosomal or Plasmid | [60] |
| OXA-24-like beta-lactamase (or OXA-40-like beta-lactamase) | 14 | ARO:3007711 | ISAba1 | Chromosomal or Plasmid | [61] |
| OXA-48-like beta-lactamase | 67 | ARO:3007721 | ND | ND | [62,63,64] |
| OXA-51-like beta-lactamase | 383 | ARO:3007725 | ISAba1, ISAba2, ISAba825, ISAba15, ISAba16, ISAba19 | Chromosomal | [60,65] |
| OXA-58-like beta-lactamase | 8 | ARO:3007728 | ISAba1, ISAba2, ISAba3, ISAba125 and IS18 | Chromosomal or Plasmid | [65,66] |
| OXA-134-like beta-lactamase | 28 | ARO:3007700 | ND | ND | [62,65] |
| OXA-143-like beta-lactamase | 11 | ARO:3007701 | ND | Plasmid | [11,62] |
2.1.4. Class D Beta-Lactamases or OXA-Type
2.1.5. Other Gene Families
2.2. Target Modifications and Reduced Antibiotic Access
2.2.1. Low Permeability of Its Outer Membrane (OM)
2.2.2. Altered Molecular Antibiotic Targets
2.2.3. Efflux Pumps
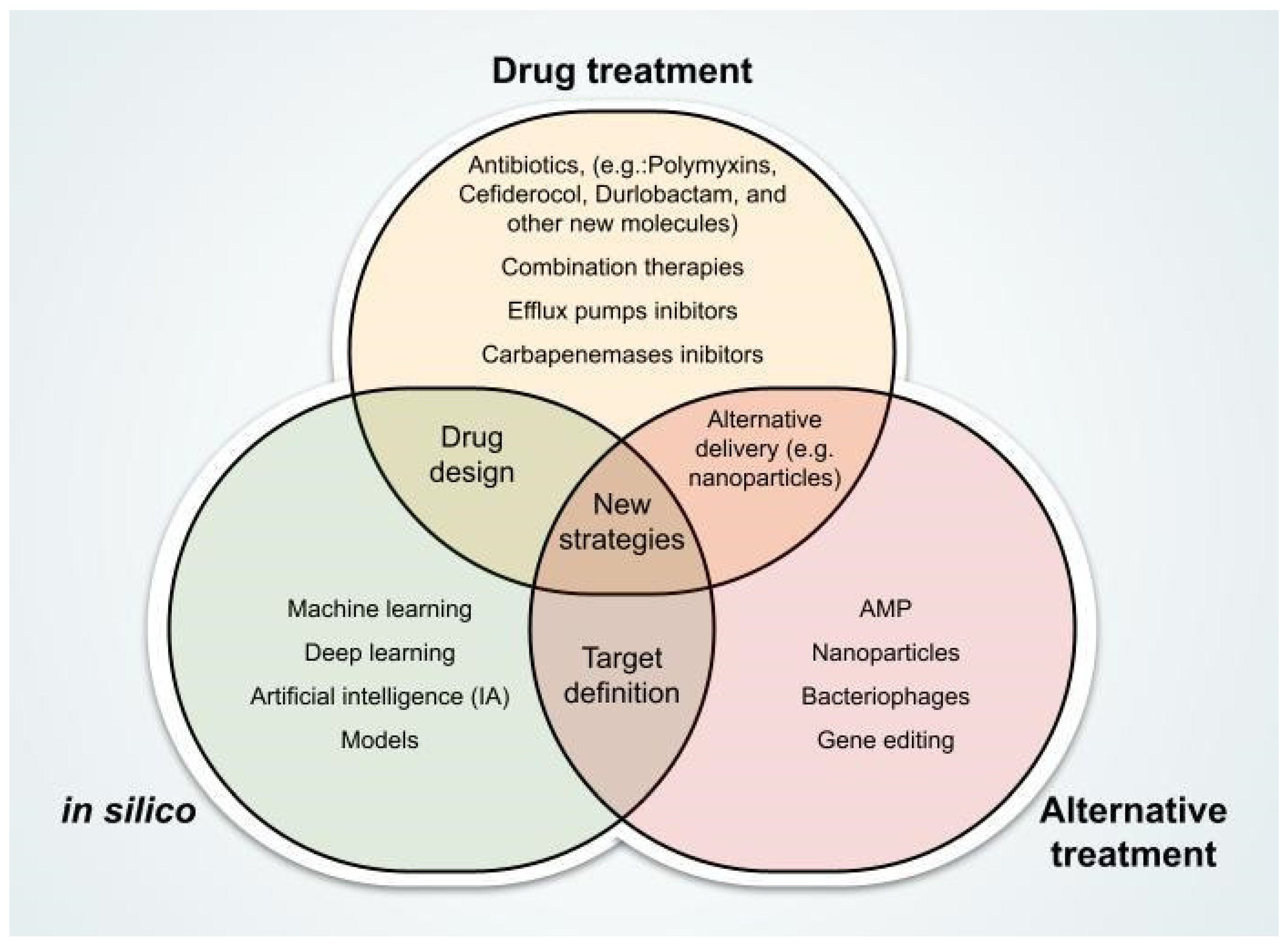
3. Promising Therapeutic Innovations Against CRAB
3.1. New Antibiotics
3.2. Combination Therapies
3.2.1. Sulbactam–Durlobactam
3.2.2. Ceftolozane–Tazobactam
3.2.3. Synergistic Use of Polymyxinswith Tetracyclines or Glycopeptides
3.2.4. Efficacy and Challenges of Combination Therapies in Resistant Strains
3.3. Development of New Carbapenemase Inhibitors
3.4. Alternative and Innovative Therapies
3.4.1. Development of New Efflux Pump Inhibitors
3.4.2. Phage Therapy
3.4.3. Antimicrobial Peptides (AMPs)
3.4.4. Gene Editing Tools
3.4.5. Nanoparticle-Based Strategies
4. Computational Approaches Supporting the Fight Against Carbapenem-Resistant A. baumannii
4.1. AI-Based Screening and Design of Antimicrobials
4.2. AI for Genomic Interpretation and Phenotype Prediction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, M.; Joshi, S.G. Carbapenem Resistance in Acinetobacter baumannii, and Their Importance in Hospital-acquired Infections: A Scientific Review. J. Appl. Microbiol. 2021, 131, 2715–2738. [Google Scholar] [CrossRef] [PubMed]
- Urban, C.; Go, E.; Mariano, N.; Berger, B.J.; Avraham, I.; Rubin, D.; Rahal, J.J. Effect of Sulbactam on Infections Caused by Imipenem-Resistant Acinetobacter calcoaceticus Biotype Anitratus. J. Infect. Dis. 1993, 167, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, V.; Sanchaita, S.; Singh, N. Multidrug Resistant Acinetobacter. J. Glob. Infect. Dis. 2010, 2, 291. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, P.J.M.; Grimont, P.A.D. Taxonomy of the Genus Acinetobacter with the Recognition of Acinetobacter baumannii sp. Nov., Acinetobacter haemolyticus sp. Nov., Acinetobacter johnsonii sp. Nov., and Acinetobacter junii sp. Nov. and Emended Descriptions of Acinetobacter calcoaceticus and Acinetobacter lwoffii. Int. J. Syst. Bacteriol. 1986, 36, 228–240. [Google Scholar] [CrossRef]
- Paton, R.; Miles, R.S.; Hood, J.; Amyes, S.G.B.; Miles, R.S.; Amyes, S.G.B. ARI 1: β-Lactamase-Mediated Imipenem Resistance in Acinetobacter baumannii. Int. J. Antimicrob. Agents 1993, 2, 81–87. [Google Scholar] [CrossRef]
- Bou, G.; Oliver, A.; Martínez-Beltrán, J. OXA-24, a Novel Class D β-Lactamase with Carbapenemase Activity in an Acinetobacter baumannii Clinical Strain. Antimicrob. Agents Chemother. 2000, 44, 1556–1561. [Google Scholar] [CrossRef]
- Dalla-Costa, L.M.; Coelho, J.M.; Souza, H.A.P.H.M.; Castro, M.E.S.; Stier, C.J.N.; Bragagnolo, K.L.; Rea-Neto, A.; Penteado-Filho, S.R.; Livermore, D.M.; Woodford, N. Outbreak of Carbapenem-Resistant Acinetobacter baumannii Producing the OXA-23 Enzyme in Curitiba, Brazil. J. Clin. Microbiol. 2003, 41, 3403–3406. [Google Scholar] [CrossRef]
- Brown, S.; Amyes, S. OXA β-Lactamases in Acinetobacter: The Story so Far. J. Antimicrob. Chemother. 2006, 57, 1–3. [Google Scholar] [CrossRef]
- Poirel, L.; Marqué, S.; Héritier, C.; Segonds, C.; Chabanon, G.; Nordmann, P. OXA-58, a Novel Class D β-Lactamase Involved in Resistance to Carbapenems in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2005, 49, 202–208. [Google Scholar] [CrossRef]
- Rice, L.B. Federal Funding for the Study of Antimicrobial Resistance in Nosocomial Pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- Higgins, P.G.; Poirel, L.; Lehmann, M.; Nordmann, P.; Seifert, H. OXA-143, a Novel Carbapenem-Hydrolyzing Class D β-Lactamase in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2009, 53, 5035–5038. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, K.; Thirunarayan, M.A.; Krishnan, P. Coexistence of blaOXA-23 with blaNDM-1 and armA in Clinical Isolates of Acinetobacter Baumannii from India. J. Antimicrob. Chemother. 2010, 65, 2253–2254. [Google Scholar] [CrossRef] [PubMed]
- Higgins, P.G.; Pérez-Llarena, F.J.; Zander, E.; Fernández, A.; Bou, G.; Seifert, H. OXA-235, a Novel Class D β-Lactamase Involved in Resistance to Carbapenems in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2013, 57, 2121–2126. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug Resistant Bacterial Infections, Including Tuberculosis. Essential Medicines and Health Products. Available online: https://apps.who.int/iris/handle/10665/311820 (accessed on 14 April 2025).
- World Health Organization (WHO). WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance. Available online: https://iris.who.int/bitstream/handle/10665/376776/9789240093461-eng.pdf (accessed on 14 April 2025).
- Ayobami, O.; Willrich, N.; Harder, T.; Okeke, I.N.; Eckmanns, T.; Markwart, R. The Incidence and Prevalence of Hospital-Acquired (Carbapenem-Resistant) Acinetobacter baumannii in Europe, Eastern Mediterranean and Africa: A Systematic Review and Meta-Analysis. Emerg. Microbes Infect. 2019, 8, 1747–1759. [Google Scholar] [CrossRef]
- ECDC—European Centre for Disease Prevention and Control. Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Acute Care Hospitals, 2022–2023; Publications Office: Luxembourg, 2024. [Google Scholar]
- Mathu, R.; Diago-Navarro, E.; Lynch, E.; Degail, M.-A.; Ousley, J.; Kanapathipillai, R.; Michel, J.; Gastellu-Etchegorry, M.; Malou, N. Antibiotic Resistance in the Middle East and Southern Asia: A Systematic Review and Meta-Analysis. JAC-Antimicrob. Resist. 2024, 7, dlaf010. [Google Scholar] [CrossRef]
- GLASS. GLASS Dashboard. Available online: https://worldhealthorg.shinyapps.io/glass-dashboard/ (accessed on 11 April 2025).
- Müller, C.; Reuter, S.; Wille, J.; Xanthopoulou, K.; Stefanik, D.; Grundmann, H.; Higgins, P.G.; Seifert, H. A Global View on Carbapenem-Resistant Acinetobacter baumannii. mBio 2023, 14, e02260-23. [Google Scholar] [CrossRef]
- Li, S.; Jiang, G.; Wang, S.; Wang, M.; Wu, Y.; Zhang, J.; Liu, X.; Zhong, L.; Zhou, M.; Xie, S.; et al. Emergence and Global Spread of a Dominant Multidrug-Resistant Clade within Acinetobacter baumannii. Nat. Commun. 2025, 16, 2787. [Google Scholar] [CrossRef]
- Mack, A.R.; Hujer, A.M.; Mojica, M.F.; Taracila, M.A.; Feldgarden, M.; Haft, D.H.; Klimke, W.; Prasad, A.B.; Bonomo, R.A. β-Lactamase Diversity in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2025, 69, e00784-24. [Google Scholar] [CrossRef]
- Migliaccio, A.; Bray, J.; Intoccia, M.; Stabile, M.; Scala, G.; Jolley, K.A.; Brisse, S.; Zarrilli, R. Phylogenomics of Acinetobacter Species and Analysis of Antimicrobial Resistance Genes. Front. Microbiol. 2023, 14, 1264030. [Google Scholar] [CrossRef]
- Poirel, L.; Bonnin, R.A.; Nordmann, P. Genetic Basis of Antibiotic Resistance in Pathogenic Acinetobacter Species. IUBMB Life 2011, 63, 1061–1067. [Google Scholar] [CrossRef]
- Ouellette, M.; Bhattacharya, A. Exploiting Antimicrobial Resistance: Better Knowledge of Resistance Mechanisms Can Inform the Search for and Development of New Antibiotics. EMBO Rep. 2020, 21, e50249. [Google Scholar] [CrossRef] [PubMed]
- Ambler, R.P. The Structure of β-Lactamases. Phil. Trans. R. Soc. Lond. B 1980, 289, 321–331. [Google Scholar] [CrossRef]
- Sechi, L.A.; Karadenizli, A.; Deriu, A.; Zanetti, S.; Kolayli, F.; Balikci, E.; Vahaboglu, H. PER-1 Type Beta-Lactamase Production in Acinetobacter baumannii Is Related to Cell Adhesion. Med. Sci. Monit. 2004, 10, BR180–BR184. [Google Scholar] [PubMed]
- Vahaboglu, H.; Oztürk, R.; Aygün, G.; Coşkunkan, F.; Yaman, A.; Kaygusuz, A.; Leblebicioglu, H.; Balik, I.; Aydin, K.; Otkun, M. Widespread Detection of PER-1-Type Extended-Spectrum Beta-Lactamases among Nosocomial Acinetobacter and Pseudomonas aeruginosa Isolates in Turkey: A Nationwide Multicenter Study. Antimicrob. Agents Chemother. 1997, 41, 2265–2269. [Google Scholar] [CrossRef]
- Poirel, L.; Sadek, M.; Nordmann, P. Contribution of PER-Type and NDM-Type β-Lactamases to Cefiderocol Resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2021, 65, e00877-21. [Google Scholar] [CrossRef]
- Liu, X.; Lei, T.; Yang, Y.; Zhang, L.; Liu, H.; Leptihn, S.; Yu, Y.; Hua, X. Structural Basis of PER-1-Mediated Cefiderocol Resistance and Synergistic Inhibition of PER-1 by Cefiderocol in Combination with Avibactam or Durlobactam in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2022, 66, e00828-22. [Google Scholar] [CrossRef]
- Robledo, I.E.; Aquino, E.E.; Santé, M.I.; Santana, J.L.; Otero, D.M.; León, C.F.; Vázquez, G.J. Detection of KPC in Acinetobacter spp. in Puerto Rico. Antimicrob. Agents Chemother. 2010, 54, 1354–1357. [Google Scholar] [CrossRef]
- Robledo, I.E.; Aquino, E.E.; Vázquez, G.J. Detection of the KPC Gene in Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii during a PCR-Based Nosocomial Surveillance Study in Puerto Rico. Antimicrob. Agents Chemother. 2011, 55, 2968–2970. [Google Scholar] [CrossRef]
- Ordoni, R.; Amini, Y.; Ranayi, M.A.; Avestan, Z.; Mohagheghi-Fard, A.H.; Zandhaghighi, M.; Bameri, Z.; Kord, E.; Shahraki-Zahedani, S.; Dehvari, A. Prevalence of the per, Tem, Veb, Shv Genes in Acinetobacter baumannii Isolated from Educational Hospital of Zahedan, Iran. Iran. J. Med. Microbiol. 2022, 16, 566–572. [Google Scholar] [CrossRef]
- Zhu, L.-J.; Pan, Y.; Gao, C.-Y.; Hou, P.-F. Distribution of Carbapenemases and Efflux Pump in Carbapenem-Resistance Acinetobacter baumannii. Ann. Clin. Lab. Sci. 2020, 50, 241–246. [Google Scholar]
- Ibrahimagić, A.; Kamberović, F.; Uzunović, S.; Bedenić, B.; Idrizović, E. Molecular Characteristics and Antibiotic Resistance of Acinetobacter baumannii Beta-Lactamase-Producing Isolates, a Predominance of Intrinsic BlaOXA-51, and Detection of TEM and CTX-M Genes. Turk. J. Med. Sci. 2017, 47, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; De La Rosa, J.-M.O.; Richard, A.; Aires-de-Sousa, M.; Nordmann, P. CTX-M-33 Is a CTX-M-15 Derivative Conferring Reduced Susceptibility to Carbapenems. Antimicrob. Agents Chemother. 2019, 63, e01515-19. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Héritier, C.; Podglajen, I.; Sougakoff, W.; Gutmann, L.; Nordmann, P. Emergence in Klebsiella pneumoniae of a Chromosome-Encoded SHV β-Lactamase That Compromises the Efficacy of Imipenem. Antimicrob. Agents Chemother. 2003, 47, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Menuteau, O.; Agoli, N.; Cattoen, C.; Nordmann, P. Outbreak of Extended-Spectrum β-Lactamase VEB-1-Producing Isolates of Acinetobacter baumannii in a French Hospital. J. Clin. Microbiol. 2003, 41, 3542–3547. [Google Scholar] [CrossRef]
- Naas, T.; Coignard, B.; Carbonne, A.; Blanckaert, K.; Bajolet, O.; Bernet, C.; Verdeil, X.; Astagneau, P.; Desenclos, J.-C.; Nordmann, P.; et al. VEB-1 Extended-Spectrum β-Lactamase–Producing Acinetobacter baumannii, France1. Emerg. Infect. Dis. 2006, 12, 1214–1222. [Google Scholar] [CrossRef]
- Girija, S.A.; Jayaseelan, V.P.; Arumugam, P. Prevalence of VIM- and GIM-Producing Acinetobacter baumannii from Patients with Severe Urinary Tract Infection. Acta Microbiol. Immunol. Hung. 2018, 65, 539–550. [Google Scholar] [CrossRef]
- Lee, K.; Yum, J.H.; Yong, D.; Lee, H.M.; Kim, H.D.; Docquier, J.-D.; Rossolini, G.M.; Chong, Y. Novel Acquired Metallo-β-Lactamase Gene, BlaSIM-1, in a Class 1 Integron from Acinetobacter baumannii Clinical Isolates from Korea. Antimicrob. Agents Chemother. 2005, 49, 4485–4491. [Google Scholar] [CrossRef]
- Shakibaie, M.R.; Azizi, O.; Shahcheraghi, F. Insight into Stereochemistry of a New IMP Allelic Variant (IMP-55) Metallo-β-Lactamase Identified in a Clinical Strain of Acinetobacter baumannii. Infect. Genet. Evol. 2017, 51, 118–126. [Google Scholar] [CrossRef]
- Cornaglia, G.; Riccio, M.; Mazzariol, A.; Lauretti, L.; Fontana, R.; Rossolini, G. Appearance of IMP-1 Metallo-β-Lactamase in Europe. Lancet 1999, 353, 899–900. [Google Scholar] [CrossRef]
- Walsh, T.R.; Toleman, M.A.; Poirel, L.; Nordmann, P. Metallo-β-Lactamases: The Quiet before the Storm? Clin. Microbiol. Rev. 2005, 18, 306–325. [Google Scholar] [CrossRef]
- Riccio, M.L.; Franceschini, N.; Boschi, L.; Caravelli, B.; Cornaglia, G.; Fontana, R.; Amicosante, G.; Rossolini, G.M. Characterization of the Metallo-β-Lactamase Determinant of Acinetobacter baumannii AC-54/97 Reveals the Existence of BlaIMP Allelic Variants Carried by Gene Cassettes of Different Phylogeny. Antimicrob. Agents Chemother. 2000, 44, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-W.; Afzal-Shah, M.; Houang, E.T.S.; Palepou, M.-F.I.; Lyon, D.J.; Woodford, N.; Livermore, D.M. IMP-4, a Novel Metallo-β-Lactamase from Nosocomial Acinetobacter spp. Collected in Hong Kong between 1994 and 1998. Antimicrob. Agents Chemother. 2001, 45, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G. Molecular Characterization of BlaIMP-5, a New Integron-Borne Metallo-β-Lactamase Gene from an Acinetobacter baumannii Nosocomial Isolate in Portugal. FEMS Microbiol. Lett. 2002, 215, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Gales, A.C.; Tognim, M.C.B.; Reis, A.O.; Jones, R.N.; Sader, H. élio S. Emergence of an IMP-like Metallo-Enzyme in an Acinetobacter baumannii Clinical Strain from a Brazilian Teaching Hospital. Diagn. Microbiol. Infect. Dis. 2003, 45, 77–79. [Google Scholar] [CrossRef]
- Bonnin, R.A.; Naas, T.; Poirel, L.; Nordmann, P. Phenotypic, Biochemical, and Molecular Techniques for Detection of Metallo-β-Lactamase NDM in Acinetobacter baumannii. J. Clin. Microbiol. 2012, 50, 1419–1421. [Google Scholar] [CrossRef]
- Massik, A.; Hibaoui, L.; Moussa, B.; Yahyaoui, G.; Oumokhtar, B.; Mahmoud, M. First Report of SPM Metallo-β-Lactamases Producing Acinetobacter baumannii Isolates in Morocco. Iran. J. Microbiol. 2022, 14, 438. [Google Scholar] [CrossRef]
- Suzuki, S.; Matsui, M.; Suzuki, M.; Sugita, A.; Kosuge, Y.; Kodama, N.; Ichise, Y.; Shibayama, K. Detection of Tripoli Metallo-Beta-Lactamase 2 (TMB-2), a Variant of BlaTMB-1, in Clinical Isolates of Acinetobacter spp. in Japan. J. Antimicrob. Chemother. 2013, 68, 1441–1442. [Google Scholar] [CrossRef][Green Version]
- Yum, J.H. Molecular Characterization of Metallo-b-Lactamase-Producing Acinetobacter baumannii and Acinetobacter Genomospecies 3 from Korea: Identification of Two New Integrons Carrying the blaVIM-2 Gene Cassettes. J. Antimicrob. Chemother. 2002, 49, 837–840. [Google Scholar] [CrossRef]
- Jeon, J.H.; Hong, M.-K.; Lee, J.H.; Lee, J.J.; Park, K.S.; Karim, A.M.; Jo, J.Y.; Kim, J.H.; Ko, K.S.; Kang, L.-W.; et al. Structure of ADC-68, a Novel Carbapenem-Hydrolyzing Class C Extended-Spectrum β-Lactamase Isolated from Acinetobacter baumannii. Acta Crystallogr. D Biol. Crystallogr. 2014, 70, 2924–2936. [Google Scholar] [CrossRef]
- Gaillot, S.; Oueslati, S.; Vuillemenot, J.-B.; Bour, M.; Iorga, B.I.; Triponney, P.; Plésiat, P.; Bonnin, R.A.; Naas, T.; Jeannot, K.; et al. Genomic Characterization of an NDM-9-Producing Acinetobacter baumannii Clinical Isolate and Role of Glu152Lys Substitution in the Enhanced Cefiderocol Hydrolysis of NDM-9. Front. Microbiol. 2023, 14, 1253160. [Google Scholar] [CrossRef]
- Chen, Z.; Qiu, S.; Wang, Y.; Wang, Y.; Liu, S.; Wang, Z.; Du, X.; Wang, L.; Guo, J.; Wang, Z.; et al. Coexistence of blaNDM-1 with the Prevalent blaOXA23 and blaIMP in Pan-Drug Resistant Acinetobacter Baumannii Isolates in China. Clin. Infect. Dis. 2011, 52, 692–693. [Google Scholar] [CrossRef] [PubMed]
- Acman, M.; Wang, R.; Van Dorp, L.; Shaw, L.P.; Wang, Q.; Luhmann, N.; Yin, Y.; Sun, S.; Chen, H.; Wang, H.; et al. Role of Mobile Genetic Elements in the Global Dissemination of the Carbapenem Resistance Gene BlaNDM. Nat. Commun. 2022, 13, 1131. [Google Scholar] [CrossRef] [PubMed]
- Toleman, M.A.; Spencer, J.; Jones, L.; Walsh, T.R. BlaNDM-1 Is a Chimera Likely Constructed in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2012, 56, 2773–2776. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, L.L.; Kraychete, G.B.; Rezende, A.M.; Campana, E.H.; Lima-Morales, D.; Wink, P.L.; Picão, R.C. NDM-1-Encoding Plasmid in Acinetobacter chengduensis Isolated from Coastal Water. Infect. Genet. Evol. 2021, 93, 104926. [Google Scholar] [CrossRef]
- Wang, B.; Sun, D. Detection of NDM-1 Carbapenemase-Producing Acinetobacter calcoaceticus and Acinetobacter junii in Environmental Samples from Livestock Farms. J. Antimicrob. Chemother. 2015, 70, 611–613. [Google Scholar] [CrossRef]
- Turton, J.F.; Ward, M.E.; Woodford, N.; Kaufmann, M.E.; Pike, R.; Livermore, D.M.; Pitt, T.L. The Role of ISAba1 in Expression of OXA Carbapenemase Genes in Acinetobacter baumannii: Role of ISAba1 in Expression of OXA Carbapenemase Genes. FEMS Microbiol. Lett. 2006, 258, 72–77. [Google Scholar] [CrossRef]
- Hashemizadeh, Z.; Hatam, G.; Fathi, J.; Aminazadeh, F.; Hosseini-Nave, H.; Hadadi, M.; Shakib, N.H.; Kholdi, S.; Bazargani, A. The Spread of Insertion Sequences Element and Transposons in Carbapenem Resistant Acinetobacter baumannii in a Hospital Setting in Southwestern Iran. Infect. Chemother. 2022, 54, 275. [Google Scholar] [CrossRef]
- Poirel, L.; Naas, T.; Nordmann, P. Diversity, Epidemiology, and Genetics of Class D β-Lactamases. Antimicrob. Agents Chemother. 2010, 54, 24–38. [Google Scholar] [CrossRef]
- Weng, X.; Shi, Q.; Wang, S.; Shi, Y.; Sun, D.; Yu, Y. The Characterization of OXA-232 Carbapenemase-Producing ST437 Klebsiella pneumoniae in China. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 5626503. [Google Scholar] [CrossRef]
- Potron, A.; Rondinaud, E.; Poirel, L.; Belmonte, O.; Boyer, S.; Camiade, S.; Nordmann, P. Genetic and Biochemical Characterisation of OXA-232, a Carbapenem-Hydrolysing Class D β-Lactamase from Enterobacteriaceae. Int. J. Antimicrob. Agents 2013, 41, 325–329. [Google Scholar] [CrossRef]
- Evans, B.A.; Amyes, S.G.B. OXA β-Lactamases. Clin. Microbiol. Rev. 2014, 27, 241–263. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Nordmann, P. Carbapenem Resistance in Acinetobacter baumannii: Mechanisms and Epidemiology. Clin. Microbiol. Infect. 2006, 12, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Donald, H.M.; Scaife, W.; Amyes, S.G.B.; Young, H.-K. Sequence Analysis of ARI-1, a Novel OXA β-Lactamase, Responsible for Imipenem Resistance in Acinetobacter baumannii 6B92. Antimicrob. Agents Chemother. 2000, 44, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Capodimonte, L.; Meireles, F.T.P.; Bahr, G.; Bonomo, R.A.; Dal Peraro, M.; López, C.; Vila, A.J. OXA β-Lactamases from Acinetobacter spp. Are Membrane Bound and Secreted into Outer Membrane Vesicles. mBio 2025, 16, e03343-24. [Google Scholar] [CrossRef]
- Zahn, M.; Bhamidimarri, S.P.; Baslé, A.; Winterhalter, M.; van den Berg, B. Structural Insights into Outer Membrane Permeability of Acinetobacter Baumannii. Structure 2016, 24, 221–231. [Google Scholar] [CrossRef]
- Mussi, M.A.; Limansky, A.S.; Viale, A.M. Acquisition of Resistance to Carbapenems in Multidrug-Resistant Clinical Strains of Acinetobacter baumannii : Natural Insertional Inactivation of a Gene Encoding a Member of a Novel Family of β-Barrel Outer Membrane Proteins. Antimicrob. Agents Chemother. 2005, 49, 1432–1440. [Google Scholar] [CrossRef]
- Cayô, R.; Rodríguez, M.-C.; Espinal, P.; Fernández-Cuenca, F.; Ocampo-Sosa, A.A.; Pascual, Á.; Ayala, J.A.; Vila, J.; Martínez-Martínez, L. Analysis of Genes Encoding Penicillin-Binding Proteins in Clinical Isolates of Acinetobacter Baumannii. Antimicrob. Agents Chemother. 2011, 55, 5907–5913. [Google Scholar] [CrossRef]
- Fernandez-Cuenca, F. Relationship between Beta-Lactamase Production, Outer Membrane Protein and Penicillin-Binding Protein Profiles on the Activity of Carbapenems against Clinical Isolates of Acinetobacter Baumannii. J. Antimicrob. Chemother. 2003, 51, 565–574. [Google Scholar] [CrossRef]
- Russo, T.A.; Carlino-MacDonald, U.; Alvarado, C.L.; Davies, C.J.; Barnes, O.; Trivedi, G.; Mathur, P.; Hutson, A.; Adams, F.G.; Zang, M.; et al. Penicillin Binding Protein 7/8 Is a Potential Drug Target in Carbapenem-Resistant Acinetobacter Baumannii. Antimicrob. Agents Chemother. 2023, 67, e01033-22. [Google Scholar] [CrossRef]
- Principe, L.; Di Bella, S.; Conti, J.; Perilli, M.; Piccirilli, A.; Mussini, C.; Decorti, G. Acinetobacter baumannii Resistance to Sulbactam/Durlobactam: A Systematic Review. Antibiotics 2022, 11, 1793. [Google Scholar] [CrossRef]
- Abdi, S.N.; Ghotaslou, R.; Ganbarov, K.; Mobed, A.; Tanomand, A.; Yousefi, M.; Asgharzadeh, M.; Kafil, H.S. Acinetobacter Baumannii Efflux Pumps and Antibiotic Resistance. Infect. Drug Resist. 2020, 13, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.J.; Courvalin, P.; Grillot-Courvalin, C. RND-Type Efflux Pumps in Multidrug-Resistant Clinical Isolates of Acinetobacter Baumannii: Major Role for AdeABC Overexpression and Aders Mutations. Antimicrob. Agents Chemother. 2013, 57, 2989–2995. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-Y.; Chang, K.-C.; Kuo, J.-W.; Yueh, H.-W.; Liou, M.-L. Imipenem: A Potent Inducer of Multidrug Resistance in Acinetobacter Baumannii. Int. J. Antimicrob. Agents 2012, 39, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). Drug Approval Package: FETROJA (Cefiderocol). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/209445Orig1s000TOC.cfm (accessed on 10 April 2025).
- European Medicines Agency (EMA). Fetcroja—European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/fetcroja (accessed on 10 April 2025).
- Paul, M.; Carrara, E.; Retamar, P.; Tängdén, T.; Bitterman, R.; Bonomo, R.A.; De Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Guidelines for the Treatment of Infections Caused by Multidrug-Resistant Gram-Negative Bacilli (Endorsed by European Society of Intensive Care Medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar] [CrossRef]
- Tamma, P.D.; Heil, E.L.; Justo, J.A.; Mathers, A.J.; Satlin, M.J.; Bonomo, R.A. Infectious Diseases Society of America 2024 Guidance on the Treatment of Antimicrobial-Resistant Gram-Negative Infections. Clin. Infect. Dis. 2024, ciae403. [Google Scholar] [CrossRef]
- Yousefi, B.; Kashanipoor, S.; Mazaheri, P.; Alibabaei, F.; Babaeizad, A.; Asli, S.; Mohammadi, S.; Gorgin, A.H.; Alipour, T.; Oksenych, V.; et al. Cefiderocol in Combating Carbapenem-Resistant Acinetobacter Baumannii: Action and Resistance. Biomedicines 2024, 12, 2532. [Google Scholar] [CrossRef]
- Onorato, L.; De Luca, I.; Monari, C.; Coppola, N. Cefiderocol Either in Monotherapy or Combination versus Best Available Therapy in the Treatment of Carbapenem-Resistant Acinetobacter baumannii Infections: A Systematic Review and Meta-Analysis. J. Infect. 2024, 88, 106113. [Google Scholar] [CrossRef]
- Zhan, Y.; Mao, W.; Zhao, C.; Lu, D.; Chen, C.; Hu, W.; Yang, Q. Comparison of Cefiderocol and Colistin-Based Regimens for the Treatment of Severe Infections Caused by Carbapenem-Resistant Acinetobacter baumannii: A Systematic Review with Meta-Analysis and Trial Sequential Analysis. BMC Infect. Dis. 2024, 24, 967. [Google Scholar] [CrossRef]
- Gatti, M.; Cosentino, F.; Giannella, M.; Viale, P.; Pea, F. Clinical Efficacy of Cefiderocol-Based Regimens in Patients with Carbapenem-Resistant Acinetobacter Baumannii Infections: A Systematic Review with Meta-Analysis. Int. J. Antimicrob. Agents 2024, 63, 107047. [Google Scholar] [CrossRef]
- Zampaloni, C.; Mattei, P.; Bleicher, K.; Winther, L.; Thäte, C.; Bucher, C.; Adam, J.-M.; Alanine, A.; Amrein, K.E.; Baidin, V.; et al. A Novel Antibiotic Class Targeting the Lipopolysaccharide Transporter. Nature 2024, 625, 566–571. [Google Scholar] [CrossRef]
- Arshad, N.; Azzam, W.; Zilberberg, M.D.; Shorr, A.F. Acinetobacter baumannii Complex Infections: New Treatment Options in the Antibiotic Pipeline. Microorganisms 2025, 13, 356. [Google Scholar] [CrossRef] [PubMed]
- Guenther, A.; Millar, L.; Messer, A.; Giraudon, M.; Patel, K.; Deurloo, E.J.; Lobritz, M.; Gloge, A. 2126. Safety, Tolerability, and Pharmacokinetics (PK) in Healthy Participants Following Single Dose Administration of Zosurabalpin, a Novel Pathogen-Specific Antibiotic for the Treatment of Serious Acinetobacter Infections. Open Forum Infect. Dis. 2023, 10, ofad500.1749. [Google Scholar] [CrossRef]
- Trebosc, V.; Kemmer, C.; Lociuro, S.; Gitzinger, M.; Dale, G.E. Rifabutin for Infusion (BV100) for the Treatment of Severe Carbapenem-Resistant Acinetobacter baumannii Infections. Drug Discov. Today 2021, 26, 2099–2104. [Google Scholar] [CrossRef] [PubMed]
- Jean, S.-S.; Liu, C.-Y.; Huang, T.-Y.; Lai, C.-C.; Liu, I.-M.; Hsieh, P.-C.; Hsueh, P.-R. Potentially Effective Antimicrobial Treatment for Pneumonia Caused by Isolates of Carbapenem-Resistant and Extensively Drug-Resistant Acinetobacter baumannii Complex Species: What Can We Expect in the Future? Expert. Rev. Anti-Infect. Ther. 2024, 22, 1171–1187. [Google Scholar] [CrossRef]
- ESCMID Global. Abstract Book 2025. Proceedings of the 35th Congress of the European Society of Clinical Microbiology and Infectious Diseases, Vienna, Austria, 11–15 April 2025, ESCMID Global: Basel, Switzerland, 2015. [Google Scholar]
- Morgan, C.E.; Zhang, Z.; Bonomo, R.A.; Yu, E.W. An Analysis of the Novel Fluorocycline TP-6076 Bound to Both the Ribosome and Multidrug Efflux Pump AdeJ from Acinetobacter baumannii. mBio 2022, 13, e03732-21. [Google Scholar] [CrossRef]
- Seifert, H.; Stefanik, D.; Olesky, M.; Higgins, P.G. In Vitro Activity of the Novel Fluorocycline TP-6076 against Carbapenem-Resistant Acinetobacter baumannii. Int. J. Antimicrob. Agents 2020, 55, 105829. [Google Scholar] [CrossRef]
- Tsai, L.; Moore, A. 1371. Safety, Tolerability, and Pharmacokinetics of Multiple Doses of TP-6076, a Novel, Fully Synthetic Tetracycline, in a Phase 1 Study. Open Forum Infect. Dis. 2018, 5, S420. [Google Scholar] [CrossRef][Green Version]
- Shein, A.M.S.; Hongsing, P.; Smith, O.K.; Phattharapornjaroen, P.; Miyanaga, K.; Cui, L.; Ishikawa, H.; Amarasiri, M.; Monk, P.N.; Kicic, A.; et al. Current and Novel Therapies for Management of Acinetobacter baumannii -Associated Pneumonia. Crit. Rev. Microbiol. 2024, 51, 441–462. [Google Scholar] [CrossRef]
- Jangra, M.; Travin, D.Y.; Aleksandrova, E.V.; Kaur, M.; Darwish, L.; Koteva, K.; Klepacki, D.; Wang, W.; Tiffany, M.; Sokaribo, A.; et al. A Broad-Spectrum Lasso Peptide Antibiotic Targeting the Bacterial Ribosome. Nature 2025, 640, 1022–1030. [Google Scholar] [CrossRef]
- Durante-Mangoni, E.; Utili, R.; Zarrilli, R. Combination Therapy in Severe Acinetobacter baumannii Infections: An Update on the Evidence to Date. Future Microbiol. 2014, 9, 773–789. [Google Scholar] [CrossRef]
- Richards, G.A.; Perovic, O.; Brink, A.J. The Challenges of Difficult-to-Treat Acinetobacter Infections. Clin. Microbiol. Rev. 2024, 37, e00093-24. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shu, Y.; Zhu, F.; Feng, B.; Zhang, Z.; Liu, L.; Wang, G. Comparative Efficacy and Safety of Combination Therapy with High-Dose Sulbactam or Colistin with Additional Antibacterial Agents for Multiple Drug-Resistant and Extensively Drug-Resistant Acinetobacter Baumannii Infections: A Systematic Review and Network Meta-Analysis. J. Glob. Antimicrob. Resist. 2021, 24, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Mosaed, R.; Haghighi, M.; Kouchak, M.; Miri, M.M.; Salarian, S.; Shojaei, S.; Javadi, A.; Taheri, S.; Nazirzadeh, P.; Foroumand, M.; et al. Interim Study: Comparison Of Safety And Efficacy of Levofloxacin Plus Colistin Regimen With Levofloxacin Plus High Dose Ampicillin/Sulbactam Infusion In Treatment of Ventilator-Associated Pneumonia Due To Multi Drug Resistant Acinetobacter. Iran. J. Pharm. Res. 2018, 17, 206–213. [Google Scholar] [PubMed]
- Betrosian, A.P.; Frantzeskaki, F.; Xanthaki, A.; Douzinas, E.E. Efficacy and Safety of High-Dose Ampicillin/Sulbactam vs. Colistin as Monotherapy for the Treatment of Multidrug Resistant Acinetobacter Baumannii Ventilator-Associated Pneumonia. J. Infect. 2008, 56, 432–436. [Google Scholar] [CrossRef]
- Betrosian, A.P.; Frantzeskaki, F.; Xanthaki, A.; Georgiadis, G. High-Dose Ampicillin-Sulbactam as an Alternative Treatment of Late-Onset VAP from Multidrug-Resistant Acinetobacter Baumannii. Scand. J. Infect. Dis. 2007, 39, 38–43. [Google Scholar] [CrossRef]
- Shields, R.K.; Paterson, D.L.; Tamma, P.D. Navigating Available Treatment Options for Carbapenem-Resistant Acinetobacter baumannii-Calcoaceticus Complex Infections. Clin. Infect. Dis. 2023, 76, S179–S193. [Google Scholar] [CrossRef]
- Karruli, A.; Migliaccio, A.; Pournaras, S.; Durante-Mangoni, E.; Zarrilli, R. Cefiderocol and Sulbactam-Durlobactam against Carbapenem-Resistant Acinetobacter Baumannii. Antibiotics 2023, 12, 1729. [Google Scholar] [CrossRef]
- Keam, S.J. Sulbactam/Durlobactam: First Approval. Drugs 2023, 83, 1245–1252. [Google Scholar] [CrossRef]
- Granata, G.; Taglietti, F.; Schiavone, F.; Petrosillo, N. Durlobactam in the Treatment of Multidrug-Resistant Acinetobacter Baumannii Infections: A Systematic Review. J. Clin. Med. 2022, 11, 3258. [Google Scholar] [CrossRef]
- El-Ghali, A.; Kunz Coyne, A.J.; Caniff, K.; Bleick, C.; Rybak, M.J. Sulbactam-durlobactam: A Novel Β-lactam-β-lactamase Inhibitor Combination Targeting Carbapenem-resistant Acinetobacter baumannii Infections. Pharmacotherapy 2023, 43, 502–513. [Google Scholar] [CrossRef]
- Seifert, H.; Müller, C.; Stefanik, D.; Higgins, P.G.; Miller, A.; Kresken, M. In Vitro Activity of Sulbactam/Durlobactam against Global Isolates of Carbapenem-Resistant Acinetobacter Baumannii. J. Antimicrob. Chemother. 2020, 75, 2616–2621. [Google Scholar] [CrossRef] [PubMed]
- Kaye, K.S.; Shorr, A.F.; Wunderink, R.G.; Du, B.; Poirier, G.E.; Rana, K.; Miller, A.; Lewis, D.; O’Donnell, J.; Chen, L.; et al. Efficacy and Safety of Sulbactam–Durlobactam versus Colistin for the Treatment of Patients with Serious Infections Caused by Acinetobacter Baumannii–Calcoaceticus Complex: A Multicentre, Randomised, Active-Controlled, Phase 3, Non-Inferiority Clinical Trial (ATTACK). Lancet Infect. Dis. 2023, 23, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Beganovic, M.; Daffinee, K.E.; Luther, M.K.; LaPlante, K.L. Minocycline Alone and in Combination with Polymyxin B, Meropenem, and Sulbactam against Carbapenem-Susceptible and -Resistant Acinetobacter Baumannii in an In Vitro Pharmacodynamic Model. Antimicrob. Agents Chemother. 2021, 65, e01680-20. [Google Scholar] [CrossRef] [PubMed]
- Bowers, D.R.; Cao, H.; Zhou, J.; Ledesma, K.R.; Sun, D.; Lomovskaya, O.; Tam, V.H. Assessment of Minocycline and Polymyxin B Combination against Acinetobacter Baumannii. Antimicrob. Agents Chemother. 2015, 59, 2720–2725. [Google Scholar] [CrossRef]
- Greig, S.L.; Scott, L.J. Intravenous Minocycline: A Review in Acinetobacter Infections. Drugs 2016, 76, 1467–1476. [Google Scholar] [CrossRef]
- He, S.; He, H.; Chen, Y.; Chen, Y.; Wang, W.; Yu, D. In Vitro and in Vivo Analysis of Antimicrobial Agents Alone and in Combination against Multi-Drug Resistant Acinetobacter Baumannii. Front. Microbiol. 2015, 6, 507. [Google Scholar] [CrossRef]
- Bartal, C.; Rolston, K.V.I.; Nesher, L. Carbapenem-Resistant Acinetobacter Baumannii: Colonization, Infection and Current Treatment Options. Infect. Dis. Ther. 2022, 11, 683–694. [Google Scholar] [CrossRef]
- Rao, G.G.; Ly, N.S.; Diep, J.; Forrest, A.; Bulitta, J.B.; Holden, P.N.; Nation, R.L.; Li, J.; Tsuji, B.T. Combinatorial Pharmacodynamics of Polymyxin B and Tigecycline against Heteroresistant Acinetobacter Baumannii. Int. J. Antimicrob. Agents 2016, 48, 331–336. [Google Scholar] [CrossRef]
- Ni, W.; Han, Y.; Zhao, J.; Wei, C.; Cui, J.; Wang, R.; Liu, Y. Tigecycline Treatment Experience against Multidrug-Resistant Acinetobacter Baumannii Infections: A Systematic Review and Meta-Analysis. Int. J. Antimicrob. Agents 2016, 47, 107–116. [Google Scholar] [CrossRef]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Robles Aguilar, G.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global Burden of Bacterial Antimicrobial Resistance 1990–2021: A Systematic Analysis with Forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Bassetti, M.; Magnè, F.; Giacobbe, D.R.; Bini, L.; Vena, A. New Antibiotics for Gram-Negative Pneumonia. Eur. Respir. Rev. 2022, 31, 220119. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Xu, N.; Wang, X. Activity of Cefiderocol in Combination with Tetracycline Analogues against Carbapenem-Resistant Acinetobacter Baumannii. J. Antibiot. 2024, 78, 190–196. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, J.A.; Ambe, L.A.; Casella, L.G.; Townsend, B.M.; Pelletier, M.R.; Ernst, R.K.; Shanks, R.M.Q.; Doi, Y. Activities of Vancomycin-Containing Regimens against Colistin-Resistant Acinetobacter baumannii Clinical Strains. Antimicrob. Agents Chemother. 2013, 57, 2103–2108. [Google Scholar] [CrossRef] [PubMed]
- Hornsey, M.; Wareham, D.W. In Vivo Efficacy of Glycopeptide-Colistin Combination Therapies in a Galleria mellonella Model of Acinetobacter baumannii Infection. Antimicrob. Agents Chemother. 2011, 55, 3534–3537. [Google Scholar] [CrossRef]
- Wareham, D.W.; Gordon, N.C.; Hornsey, M. In Vitro Activity of Teicoplanin Combined with Colistin versus Multidrug-Resistant Strains of Acinetobacter baumannii. J. Antimicrob. Chemother. 2011, 66, 1047–1051. [Google Scholar] [CrossRef]
- Hornsey, M.; Longshaw, C.; Phee, L.; Wareham, D.W. In Vitro Activity of Telavancin in Combination with Colistin versus Gram-Negative Bacterial Pathogens. Antimicrob. Agents Chemother. 2012, 56, 3080–3085. [Google Scholar] [CrossRef]
- Hornsey, M.; Phee, L.; Longshaw, C.; Wareham, D.W. In Vivo Efficacy of Telavancin/Colistin Combination Therapy in a Galleria Mellonella Model of Acinetobacter baumannii Infection. Int. J. Antimicrob. Agents 2013, 41, 285–287. [Google Scholar] [CrossRef]
- Ceccarelli, G.; Oliva, A.; d’Ettorre, G.; D’Abramo, A.; Caresta, E.; Barbara, C.S.; Mascellino, M.T.; Papoff, P.; Moretti, C.; Vullo, V.; et al. The Role of Vancomycin in Addition with Colistin and Meropenem against Colistin-Sensitive Multidrug Resistant Acinetobacter baumannii Causing Severe Infections in a Paediatric Intensive Care Unit. BMC Infect. Dis. 2015, 15, 393. [Google Scholar] [CrossRef]
- Choi, S.J.; Kim, E.S. Optimizing Treatment for Carbapenem-Resistant Acinetobacter baumannii Complex Infections: A Review of Current Evidence. Infect. Chemother. 2024, 56, 171. [Google Scholar] [CrossRef]
- Cahill, S.T.; Cain, R.; Wang, D.Y.; Lohans, C.T.; Wareham, D.W.; Oswin, H.P.; Mohammed, J.; Spencer, J.; Fishwick, C.W.G.; McDonough, M.A.; et al. Cyclic Boronates Inhibit All Classes of β-Lactamases. Antimicrob. Agents Chemother. 2017, 61, e02260-16. [Google Scholar] [CrossRef]
- Liu, B.; Trout, R.E.L.; Chu, G.-H.; McGarry, D.; Jackson, R.W.; Hamrick, J.C.; Daigle, D.M.; Cusick, S.M.; Pozzi, C.; De Luca, F.; et al. Discovery of Taniborbactam (VNRX-5133): A Broad-Spectrum Serine- and Metallo-β-Lactamase Inhibitor for Carbapenem-Resistant Bacterial Infections. J. Med. Chem. 2020, 63, 2789–2801. [Google Scholar] [CrossRef] [PubMed]
- Hecker, S.J.; Reddy, K.R.; Lomovskaya, O.; Griffith, D.C.; Rubio-Aparicio, D.; Nelson, K.; Tsivkovski, R.; Sun, D.; Sabet, M.; Tarazi, Z.; et al. Discovery of Cyclic Boronic Acid QPX7728, an Ultrabroad-Spectrum Inhibitor of Serine and Metallo-β-Lactamases. J. Med. Chem. 2020, 63, 7491–7507. [Google Scholar] [CrossRef] [PubMed]
- Wagenlehner, F.M.; Gasink, L.B.; McGovern, P.C.; Moeck, G.; McLeroth, P.; Dorr, M.; Dane, A.; Henkel, T. Cefepime–Taniborbactam in Complicated Urinary Tract Infection. N. Engl. J. Med. 2024, 390, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.T.; Leiris, S.; Zalacain, M.; Sprynski, N.; Castandet, J.; Bousquet, J.; Lozano, C.; Llanos, A.; Alibaud, L.; Vasa, S.; et al. Discovery of ANT3310, a Novel Broad-Spectrum Serine β-Lactamase Inhibitor of the Diazabicyclooctane Class, Which Strongly Potentiates Meropenem Activity against Carbapenem-Resistant Enterobacterales and Acinetobacter baumannii. J. Med. Chem. 2020, 63, 15802–15820. [Google Scholar] [CrossRef]
- Li, Y.; Yan, M.; Xue, F.; Zhong, W.; Liu, X.; Chen, X.; Wu, Y.; Zhang, J.; Wang, Q.; Zheng, B.; et al. In Vitro and in Vivo Activities of a Novel β-Lactamase Inhibitor Combination Imipenem/XNW4107 against Recent Clinical Gram-Negative Bacilli from China. J. Glob. Antimicrob. Resist. 2022, 31, 1–9. [Google Scholar] [CrossRef]
- Evopoint Has Submitted the Pre-NDA Application for Its Novel Drug Funobactam (XNW4107), Offering New Hope for HABP/VABP Patients. Available online: https://www.evopointbio.com/en/news/detail?id=185 (accessed on 10 May 2025).
- Iregui, A.; Khan, Z.; Landman, D.; Quale, J. Activity of Meropenem with a Novel Broader-Spectrum β-Lactamase Inhibitor, WCK 4234, against Gram-Negative Pathogens Endemic to New York City. Antimicrob. Agents Chemother. 2019, 64, e01666-19. [Google Scholar] [CrossRef]
- Mushtaq, S.; Vickers, A.; Woodford, N.; Livermore, D.M. WCK 4234, a Novel Diazabicyclooctane Potentiating Carbapenems against Enterobacteriaceae, Pseudomonas and Acinetobacter with Class A, C and D β-Lactamases. J. Antimicrob. Chemother. 2017, 72, 1688–1695. [Google Scholar] [CrossRef][Green Version]
- Lasarte-Monterrubio, C.; Vázquez-Ucha, J.C.; Maneiro, M.; Arca-Suárez, J.; Alonso, I.; Guijarro-Sánchez, P.; Buynak, J.D.; Bou, G.; González-Bello, C.; Beceiro, A. Activity of Imipenem, Meropenem, Cefepime, and Sulbactam in Combination with the β-Lactamase Inhibitor LN-1-255 against Acinetobacter spp. Antibiotics 2021, 10, 210. [Google Scholar] [CrossRef]
- Vázquez-Ucha, J.C.; Martínez-Guitián, M.; Maneiro, M.; Conde-Pérez, K.; Álvarez-Fraga, L.; Torrens, G.; Oliver, A.; Buynak, J.D.; Bonomo, R.A.; Bou, G.; et al. Therapeutic Efficacy of LN-1-255 in Combination with Imipenem in Severe Infection Caused by Carbapenem-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2019, 63, e01092-19. [Google Scholar] [CrossRef]
- Sivarajan, K.; Ravindhiran, R.; Sekar, J.N.; Murugesan, R.; Chidambaram, K.; Dhandapani, K. Deciphering the Impact of Acinetobacter baumannii on Human Health, and Exploration of Natural Compounds as Efflux Pump Inhibitors to Treat Multidrug Resistance. J. Med. Microbiol. 2024, 73, 001867. [Google Scholar] [CrossRef] [PubMed]
- Tambat, R.; Kinthada, R.K.; Saral Sariyer, A.; Leus, I.V.; Sariyer, E.; D’Cunha, N.; Zhou, H.; Leask, M.; Walker, J.K.; Zgurskaya, H.I. AdeIJK Pump-Specific Inhibitors Effective against Multidrug Resistant Acinetobacter baumannii. ACS Infect. Dis. 2024, 10, 2239–2249. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Herbal Products and Their Active Constituents Used Alone and in Combination with Antibiotics against Multidrug-Resistant Bacteria. Planta Med. 2023, 89, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Tiwari, M.; Tiwari, V. Potentiate the Activity of Current Antibiotics by Naringin Dihydrochalcone Targeting the AdeABC Efflux Pump of Multidrug-Resistant Acinetobacter baumannii. Int. J. Biol. Macromol. 2022, 217, 592–605. [Google Scholar] [CrossRef]
- Lee, T.; Lee, S.; Kim, M.K.; Ahn, J.H.; Park, J.S.; Seo, H.W.; Park, K.-H.; Chong, Y. 3- O -Substituted Quercetin: An Antibiotic-Potentiating Agent against Multidrug-Resistant Gram-Negative Enterobacteriaceae through Simultaneous Inhibition of Efflux Pump and Broad-Spectrum Carbapenemases. ACS Infect. Dis. 2024, 10, 1624–1643. [Google Scholar] [CrossRef] [PubMed]
- Singkham-In, U.; Phuengmaung, P.; Makjaroen, J.; Saisorn, W.; Bhunyakarnjanarat, T.; Chatsuwan, T.; Chirathaworn, C.; Chancharoenthana, W.; Leelahavanichkul, A. Chlorhexidine Promotes Psl Expression in Pseudomonas Aeruginosa That Enhances Cell Aggregation with Preserved Pathogenicity Demonstrates an Adaptation against Antiseptic. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.M.; Ezzat, H.; El-Sayyad, G.S.; Zedan, H. Regulation of Overexpressed Efflux Pump Encoding Genes by Cinnamon Oil and Trimethoprim to Abolish Carbapenem-Resistant Acinetobacter Baumannii Clinical Strains. BMC Microbiol 2024, 24, 52. [Google Scholar] [CrossRef] [PubMed]
- Plé, C.; Tam, H.-K.; Vieira Da Cruz, A.; Compagne, N.; Jiménez-Castellanos, J.-C.; Müller, R.T.; Pradel, E.; Foong, W.E.; Malloci, G.; Ballée, A.; et al. Pyridylpiperazine-Based Allosteric Inhibitors of RND-Type Multidrug Efflux Pumps. Nat Commun 2022, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Castellanos, J.-C.; Pradel, E.; Compagne, N.; Vieira Da Cruz, A.; Flipo, M.; Hartkoorn, R.C. Characterization of Pyridylpiperazine-Based Efflux Pump Inhibitors for Acinetobacter Baumannii. JAC-Antimicrobial Resistance 2023, 5, dlad112. [Google Scholar] [CrossRef] [PubMed]
- Leite, G.C.; Perdigão-Neto, L.V.; Ruedas Martins, R.C.; Rizek, C.; Levin, A.S.; Costa, S.F. Genetic Factors Involved in Fosfomycin Resistance of Multidrug-Resistant Acinetobacter Baumannii. Infection, Genetics and Evolution 2021, 93, 104943. [Google Scholar] [CrossRef] [PubMed]
- Saini, M.; Gaurav, A.; Hussain, A.; Pathania, R. Small Molecule IITR08367 Potentiates Antibacterial Efficacy of Fosfomycin against Acinetobacter Baumannii by Efflux Pump Inhibition. ACS Infect. Dis. 2024, 10, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Jernigan, D.A.; Hentish, R.D. Successful Treatment of a Patient With Chronic Bronchiectasis Using an Induced Native Phage Cocktail: A Case Report. Cureus 2025, 17, e77681. [Google Scholar] [CrossRef]
- Qu, J.; Zou, J.; Zhang, J.; Qu, J.; Lu, H. Phage Therapy for Extensively Drug Resistant Acinetobacter Baumannii Infection: Case Report and in Vivo Evaluation of the Distribution of Phage and the Impact on Gut Microbiome. Front. Med. 2024, 11, 1432703. [Google Scholar] [CrossRef]
- Rastegar, S.; Skurnik, M.; Niaz, H.; Tadjrobehkar, O.; Samareh, A.; Hosseini-Nave, H.; Sabouri, S. Isolation, Characterization, and Potential Application of Acinetobacter Baumannii Phages against Extensively Drug-Resistant Strains. Virus Genes 2024, 60, 725–736. [Google Scholar] [CrossRef]
- Wang, R.; You, X.; Liu, X.; Fei, B.; Li, Y.; Wang, D.; Zhu, R.; Li, Y. Characterization of Phage HZY2308 against Acinetobacter Baumannii and Identification of Phage-Resistant Bacteria. Virol. J. 2024, 21, 283. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, M.; Li, P.; Xu, S.; Chen, L.; Xu, G.; Pang, X.; Du, H.; Zheng, Y.; Huo, X.; et al. Antibacterial Activity Evaluation of a Novel K3-Specific Phage against Acinetobacter Baumannii and Evidence for Receptor-Binding Domain Transfer across Morphologies. Virol. Sin. 2024, 39, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Borzilov, A.I.; Volozhantsev, N.V.; Korobova, O.V.; Kolupaeva, L.V.; Pereskokova, E.S.; Kombarova, T.I.; Shneider, M.M.; Miroshnikov, K.A.; Dyatlov, I.A.; Popova, A.V. Bacteriophage and Phage-Encoded Depolymerase Exhibit Antibacterial Activity Against K9-Type Acinetobacter Baumannii in Mouse Sepsis and Burn Skin Infection Models. Viruses 2025, 17, 70. [Google Scholar] [CrossRef] [PubMed]
- Rathor, N.; Bahadur, T.; Thakur, C.K.; Bamola, V.D.; Das, B.K.; Chaudhry, R. Bacteriophages as Therapeutic & Disinfectant Agents to Tackle Multidrug-Resistant Acinetobacter Baumannii. Indian. J. Med. Res. 2023, 157, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Pu, M.; Li, Y.; Wang, Y.; Li, M.; Song, L.; Li, M.; An, X.; Fan, H.; Tong, Y. Acinetobacter Baumannii Phages: Past, Present and Future. Viruses 2023, 15, 673. [Google Scholar] [CrossRef]
- Azam, A.H.; Tanji, Y. Bacteriophage-Host Arm Race: An Update on the Mechanism of Phage Resistance in Bacteria and Revenge of the Phage with the Perspective for Phage Therapy. Appl. Microbiol. Biotechnol. 2019, 103, 2121–2131. [Google Scholar] [CrossRef]
- Laanto, E.; Mäkelä, K.; Hoikkala, V.; Ravantti, J.J.; Sundberg, L.-R. Adapting a Phage to Combat Phage Resistance. Antibiotics 2020, 9, 291. [Google Scholar] [CrossRef]
- Malik, S.; Ahsan, O.; Muhammad, K.; Munawar, N.; Waheed, Y. Phagetherapy Updates: New Frontiers against Antibiotic Resistance. Eur. J. Microbiol. Immunol. 2025, 15, 1–12. [Google Scholar] [CrossRef]
- De Mandal, S.; Panda, A.K.; Murugan, C.; Xu, X.; Senthil Kumar, N.; Jin, F. Antimicrobial Peptides: Novel Source and Biological Function With a Special Focus on Entomopathogenic Nematode/Bacterium Symbiotic Complex. Front. Microbiol. 2021, 12, 555022. [Google Scholar] [CrossRef]
- Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.-M.; Bechinger, B.; Naas, T. Antimicrobial Peptides: A Potent Alternative to Antibiotics. Antibiotics 2021, 10, 1095. [Google Scholar] [CrossRef]
- Thennarasu, S.; Tan, A.; Penumatchu, R.; Shelburne, C.E.; Heyl, D.L.; Ramamoorthy, A. Antimicrobial and Membrane Disrupting Activities of a Peptide Derived from the Human Cathelicidin Antimicrobial Peptide LL37. Biophys. J. 2010, 98, 248–257. [Google Scholar] [CrossRef]
- Vila-Farrés, X.; López-Rojas, R.; Pachón-Ibáñez, M.E.; Teixidó, M.; Pachón, J.; Vila, J.; Giralt, E. Sequence-Activity Relationship, and Mechanism of Action of Mastoparan Analogues against Extended-Drug Resistant Acinetobacter baumannii. Eur. J. Med. Chem. 2015, 101, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, J.; Yin, Y.; Wang, G.; Yang, M.; Li, Y.; Zhang, Z.; Lai, R. The Antimicrobial Peptide ZY4 Combats Multidrug-Resistant Pseudomonas aeruginosa and Acinetobacter baumannii Infection. Proc. Natl. Acad. Sci. USA 2019, 116, 26516–26522. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, Y.; Nie, D.; Li, N.; Chen, Z.; Zhou, S.; Li, M.; Xue, X. A Peptide Targeting Outer Membrane Protein A of Acinetobacter baumannii Exhibits Antibacterial Activity by Reducing Bacterial Pathogenicity. Antimicrob. Agents Chemother. 2024, 68, e00565-24. [Google Scholar] [CrossRef]
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B.K.H.L. Review: Lessons Learned From Clinical Trials Using Antimicrobial Peptides (AMPs). Front. Microbiol. 2021, 12, 616979. [Google Scholar] [CrossRef]
- Kumar, N.; Sood, D.; Tomar, R.; Chandra, R. Antimicrobial Peptide Designing and Optimization Employing Large-Scale Flexibility Analysis of Protein-Peptide Fragments. ACS Omega 2019, 4, 21370–21380. [Google Scholar] [CrossRef] [PubMed]
- Anwer, F.; Navid, A.; Faiz, F.; Haider, U.; Nasir, S.; Farooq, M.; Zahra, M.; Bano, A.; Bashir, H.H.; Ahmad, M.; et al. AbAMPdb: A Database of Acinetobacter baumannii Specific Antimicrobial Peptides. Database 2024, 2024, baae096. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, P.; Köse, Ş.; Dao, S.; Ganbarov, K.; Tanomand, A.; Dal, T.; Aghazadeh, M.; Ghotaslou, R.; Ahangarzadeh Rezaee, M.; Yousefi, B.; et al. How CRISPR-Cas System Could Be Used to Combat Antimicrobial Resistance. Infect. Drug Resist. 2020, 13, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, J.; Sun, X.; Li, M.; Zhang, P.; Zhu, Z.; Jiao, H.; Guo, T.; Li, G. CRISPR-Cas in Acinetobacter Baumannii Contributes to Antibiotic Susceptibility by Targeting Endogenous AbaI. Microbiol. Spectr. 2022, 10, e00829-22. [Google Scholar] [CrossRef]
- Bai, J.; Dai, Y.; Farinha, A.; Tang, A.Y.; Syal, S.; Vargas-Cuebas, G.; Van Opijnen, T.; Isberg, R.R.; Geisinger, E. Essential Gene Analysis in Acinetobacter Baumannii by High-Density Transposon Mutagenesis and CRISPR Interference. J. Bacteriol. 2021, 203, e00565-20. [Google Scholar] [CrossRef]
- Li, X.; Gui, S.; Gui, R.; Li, J.; Huang, R.; Hu, M.; Luo, X.; Nie, X. Multifunctional Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-Cas9-Based Nanobomb against Carbapenem-Resistant Acinetobacter baumannii Infection through Cascade Reaction and Amplification Synergistic Effect. ACS Nano 2023, 17, 24632–24653. [Google Scholar] [CrossRef]
- De La Fuente Tagarro, C.; Martín-González, D.; De Lucas, A.; Bordel, S.; Santos-Beneit, F. Current Knowledge on CRISPR Strategies Against Antimicrobial-Resistant Bacteria. Antibiotics 2024, 13, 1141. [Google Scholar] [CrossRef]
- Junaid, M.; Thirapanmethee, K.; Khuntayaporn, P.; Chomnawang, M.T. CRISPR-Based Gene Editing in Acinetobacter Baumannii to Combat Antimicrobial Resistance. Pharmaceuticals 2023, 16, 920. [Google Scholar] [CrossRef]
- Saha, U.; Gondi, R.; Patil, A.; Saroj, S.D. CRISPR in Modulating Antibiotic Resistance of ESKAPE Pathogens. Mol. Biotechnol. 2023, 65, 1–16. [Google Scholar] [CrossRef]
- Hetta, H.F.; Al-Kadmy, I.M.S.; Khazaal, S.S.; Abbas, S.; Suhail, A.; El-Mokhtar, M.A.; Ellah, N.H.A.; Ahmed, E.A.; Abd-ellatief, R.B.; El-Masry, E.A.; et al. Antibiofilm and Antivirulence Potential of Silver Nanoparticles against Multidrug-Resistant Acinetobacter Baumannii. Sci. Rep. 2021, 11, 10751. [Google Scholar] [CrossRef] [PubMed]
- Hetta, H.F.; Ramadan, Y.N.; Al-Harbi, A.I.; Ahmed, E.A.; Battah, B.; Abd Ellah, N.H.; Zanetti, S.; Donadu, M.G. Nanotechnology as a Promising Approach to Combat Multidrug Resistant Bacteria: A Comprehensive Review and Future Perspectives. Biomedicines 2023, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Siddique, M.H.; Sadia, M.; Muzammil, S.; Saqalein, M.; Ashraf, A.; Hayat, S.; Saba, S.; Khan, A.M.; Hashem, A.; Avila-Qezada, G.D.; et al. Biofabrication of Copper Oxide Nanoparticles Using Dalbergia Sisso Leaf Extract for Antibacterial, Antibiofilm and Antioxidant Activities. Sci. Rep. 2024, 14, 31867. [Google Scholar] [CrossRef] [PubMed]
- McNeilly, O.; Mann, R.; Cummins, M.L.; Djordjevic, S.P.; Hamidian, M.; Gunawan, C. Development of Nanoparticle Adaptation Phenomena in Acinetobacter Baumannii: Physiological Change and Defense Response. Microbiol. Spectr. 2023, 11, e02857-22. [Google Scholar] [CrossRef]
- Kuntz, I.D. Structure-Based Strategies for Drug Design and Discovery. Science 1992, 257, 1078–1082. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate Structure Prediction of Biomolecular Interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Shen, T.; Guo, J.; Han, Z.; Zhang, G.; Liu, Q.; Si, X.; Wang, D.; Wu, S.; Xia, J. AutoMolDesigner for Antibiotic Discovery: An AI-Based Open-Source Software for Automated Design of Small-Molecule Antibiotics. J. Chem. Inf. Model. 2024, 64, 575–583. [Google Scholar] [CrossRef]
- Wan, F.; Wong, F.; Collins, J.J.; De La Fuente-Nunez, C. Machine Learning for Antimicrobial Peptide Identification and Design. Nat. Rev. Bioeng. 2024, 2, 392–407. [Google Scholar] [CrossRef]
- Brindangnanam, P.; Ashokkumar, K.; Kamaraj, S.; Coumar, M.S. Exploring Imidazo [4,5-g]Quinoline-4,9-Dione Derivatives as Acinetobacter baumannii Efflux Pump Inhibitor: An in Silico Approach. J. Biomol. Struct. Dyn. 2025, 43, 53–72. [Google Scholar] [CrossRef]
- Liu, G.; Catacutan, D.B.; Rathod, K.; Swanson, K.; Jin, W.; Mohammed, J.C.; Chiappino-Pepe, A.; Syed, S.A.; Fragis, M.; Rachwalski, K.; et al. Deep Learning-Guided Discovery of an Antibiotic Targeting Acinetobacter Baumannii. Nat. Chem. Biol. 2023, 19, 1342–1350. [Google Scholar] [CrossRef]
- Boulaamane, Y.; Molina Panadero, I.; Hmadcha, A.; Atalaya Rey, C.; Baammi, S.; El Allali, A.; Maurady, A.; Smani, Y. Antibiotic Discovery with Artificial Intelligence for the Treatment of Acinetobacter baumannii Infections. mSystems 2024, 9, e00325-24. [Google Scholar] [CrossRef] [PubMed]
- Salih, T.; Ali, P.G. Rational Design of Novel Compounds to Serve as Potential NDM-1 Inhibitors Using Molecular Docking, Molecular Dynamics Simulation, and Physicochemical Studies. Mol. Simul. 2023, 49, 1373–1387. [Google Scholar] [CrossRef]
- Bagdad, Y.; Sisquellas, M.; Arthur, M.; Miteva, M.A. Machine Learning and Deep Learning Models for Predicting Noncovalent Inhibitors of AmpC β-Lactamase. ACS Omega 2024, 9, 41334–41342. [Google Scholar] [CrossRef] [PubMed]
- Power, R.A.; Parkhill, J.; De Oliveira, T. Microbial Genome-Wide Association Studies: Lessons from Human GWAS. Nat. Rev. Genet. 2017, 18, 41–50. [Google Scholar] [CrossRef]
- Javkar, K.; Rand, H.; Hoffmann, M.; Luo, Y.; Sarria, S.; Thirunavukkarasu, N.; Pillai, C.A.; McGann, P.; Johnson, J.K.; Strain, E.; et al. Whole-Genome Assessment of Clinical Acinetobacter Baumannii Isolates Uncovers Potentially Novel Factors Influencing Carbapenem Resistance. Front. Microbiol. 2021, 12, 714284. [Google Scholar] [CrossRef]
- Nguyen, M.; Olson, R.; Shukla, M.; VanOeffelen, M.; Davis, J.J. Predicting Antimicrobial Resistance Using Conserved Genes. PLoS Comput. Biol. 2020, 16, e1008319. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, M.; Chen, F.; Wang, L.; Han, P.; Jiang, Z.; Shen, S.; Rao, G.; Yang, F. Prediction of Antimicrobial Resistance in Klebsiella pneumoniae Using Genomic and Metagenomic next-Generation Sequencing Data. J. Antimicrob. Chemother. 2024, 79, 2509–2517. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, Y.; Han, P.; Liu, S.; Liu, W.; Mai, C.; Deng, Q.; Ren, J.; Luo, J.; Chen, F.; et al. Novel Clinical mNGS-Based Machine Learning Model for Rapid Antimicrobial Susceptibility Testing of Acinetobacter Baumannii. J. Clin. Microbiol. 2023, 61, e01805-22. [Google Scholar] [CrossRef]
- Youn, J.; Rai, N.; Tagkopoulos, I. Knowledge Integration and Decision Support for Accelerated Discovery of Antibiotic Resistance Genes. Nat. Commun. 2022, 13, 2360. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, X.; Wang, Y.; Yu, Y.; Huang, N.; Li, G.; Li, X.; Wu, J.C.; Yang, S. Artificial Intelligence in Drug Development. Nat. Med. 2025, 31, 45–59. [Google Scholar] [CrossRef]
- Pearcy, N.; Hu, Y.; Baker, M.; Maciel-Guerra, A.; Xue, N.; Wang, W.; Kaler, J.; Peng, Z.; Li, F.; Dottorini, T. Genome-Scale Metabolic Models and Machine Learning Reveal Genetic Determinants of Antibiotic Resistance in Escherichia Coli and Unravel the Underlying Metabolic Adaptation Mechanisms. mSystems 2021, 6, e0091320. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, J.; D’Espindula, H.R.S.; Ribeiro, I.d.F.; Gonçalves, G.A.; Pillonetto, M.; Faoro, H. Carbapenem Resistance in Acinetobacter baumannii: Mechanisms, Therapeutics, and Innovations. Microorganisms 2025, 13, 1501. https://doi.org/10.3390/microorganisms13071501
de Souza J, D’Espindula HRS, Ribeiro IdF, Gonçalves GA, Pillonetto M, Faoro H. Carbapenem Resistance in Acinetobacter baumannii: Mechanisms, Therapeutics, and Innovations. Microorganisms. 2025; 13(7):1501. https://doi.org/10.3390/microorganisms13071501
Chicago/Turabian Stylede Souza, Joyce, Helena Regina Salomé D’Espindula, Isabel de Farias Ribeiro, Geiziane Aparecida Gonçalves, Marcelo Pillonetto, and Helisson Faoro. 2025. "Carbapenem Resistance in Acinetobacter baumannii: Mechanisms, Therapeutics, and Innovations" Microorganisms 13, no. 7: 1501. https://doi.org/10.3390/microorganisms13071501
APA Stylede Souza, J., D’Espindula, H. R. S., Ribeiro, I. d. F., Gonçalves, G. A., Pillonetto, M., & Faoro, H. (2025). Carbapenem Resistance in Acinetobacter baumannii: Mechanisms, Therapeutics, and Innovations. Microorganisms, 13(7), 1501. https://doi.org/10.3390/microorganisms13071501





