Abstract
Rice paddy fields are sustainable agricultural systems as soil microorganisms help maintain nitrogen fertility through generating ammonium. In these soils, dissimilatory nitrate reduction to ammonium (DNRA), nitrogen fixation, and denitrification are closely linked. DNRA and denitrification share the same initial steps and nitrogen gas, the end product of denitrification, can serve as a substrate for nitrogen fixation. However, the microorganisms responsible for these three reductive nitrogen transformations, particularly those focused on ammonium generation, have not been comprehensively characterized. In this study, we used stable isotope probing with 15NO3−, 15N2O, and 15N2, combined with 16S rRNA high-throughput sequencing and metatranscriptomics, to identify ammonium-generating microbial consortia in paddy soils. Our results revealed that several bacterial families actively contribute to ammonium generation under different nitrogen substrate conditions. Specifically, Geobacteraceae (N2O and +N2), Bacillaceae (+NO3− and +N2), Rhodocyclaceae (+N2O and +N2), Anaeromyxobacteraceae (+NO3− and +N2O), and Clostridiaceae (+NO3− and +N2) were involved. Many of these bacteria participate in key ecological processes typical of paddy environments, including iron or sulfate reduction and rice straw decomposition. This study revealed the ammonium-generating microbial consortia in paddy soil that contain several key bacterial drivers of multiple reductive nitrogen transformations and suggested their diverse functions in paddy soil metabolism.
1. Introduction
Rice is the primary food source for more than half of the global population, particularly in Asia [1,2]. Rice cultivation depends on various nutrients, making soil fertility a key determinant of both rice yield and quality. Among these nutrients, nitrogen is essential for plant growth and development, as it enhances crop yield, promotes chlorophyll II formation, and improves photosynthetic efficiency [3,4]. Among the available forms of nitrogen, nitrate (NO3−) and ammonium (NH4+) are the primary inorganic sources absorbed and utilized by plants [5]. Rice efficiently absorbs ammonium via root transporters [5,6]. In addition, as a cation, ammonium readily binds to most soil types and is less susceptible to leaching than nitrate, ensuring a more stable and continuous nitrogen supply [7]. Consequently, ammonium-based fertilizers are widely used to improve rice production.
However, nitrogen fertilizer production is resource- and energy-intensive, and excessive application releases harmful gases such as ammonia (NH3) and nitrogen oxides (NOx), which pose environmental and human health risks [8]. Moreover, only a fraction of the applied nitrogen is effectively absorbed by crops; the remainder leaches into surface water and groundwater, threatening aquatic ecosystems [9,10,11]. These concerns highlight the need to reduce nitrogen fertilizer use and adopt more sustainable agricultural practices.
Paddy soils are frequently waterlogged and anaerobic ammonium is naturally generated through microbial processes, thereby contributing to soil fertility and plant growth [12]. The primary microbial pathways responsible for ammonium generation include nitrogen fixation (N2 → NH3) and dissimilatory nitrate reduction to ammonium (DNRA; NO3− → NO2− → NH4+). Moreover, denitrification (NO3− → NO2− → NO → N2O → N2), which shares an initial pathway with DNRA, is also prevalent in paddy soils. The final product, nitrogen gas (N2), subsequently serves as a substrate for nitrogen fixation [13,14,15,16,17]. These reductive nitrogen transformation processes—DNRA, nitrogen fixation, and denitrification—interact dynamically to regulate ammonium generation in paddy soils.
Despite extensive research on nitrogen transformation in paddy soils, several limitations remain. Most studies have examined DNRA, denitrification, or nitrogen fixation as independent processes, disregarding their associations within the nitrogen cycle. Additionally, the presence of pseudo-nifH genes, which are frequently misannotated as functional nifH genes, leads to an overestimation of the nitrogen-fixing microbial abundance [18]. DNRA predominates nitrate removal in paddy soils owing to the anaerobic, carbon-rich conditions that preferentially support DNRA over denitrification; however, its ecological significance has only recently gained recognition [14,19]. Furthermore, many microorganisms involved in these processes are challenging to culture under laboratory conditions because of their specific growth requirements and sensitivity to environmental factors, which limits our understanding of their physiological and metabolic functions [20,21]. Recent advances in molecular biology and bioinformatics, such as high-throughput sequencing, isotope labeling techniques, metagenomics, and metatranscriptomics, have significantly improved our ability to study microbial communities [22,23,24]. However, no single technique can completely capture the complexity of microbial community structure and function, underscoring the need for an integrated approach to comprehensively elucidate reductive nitrogen transformation processes and the microorganisms involved.
This study aimed to identify ammonium-generating microbial consortia involved in reductive nitrogen transformation in paddy soils. To achieve this goal, paddy soil microcosms were established individually using stable isotope labeled nitrate, nitrous oxide, and nitrogen. The production and consumption of nitrous oxide and nitrogen, which indicate the dynamics of the added nitrogen compounds, were monitored via gas chromatography-mass spectrometry (GC-MS). DNA-stable isotope probing (DNA-SIP), combined with 16S rRNA high-throughput sequencing, has been used to identify the microorganisms responsible for assimilatory reactions, such as ammonium or nitrate incorporation and biological nitrogen fixation. Additionally, a metatranscriptomic analysis was performed along with SIP to identify the microorganisms involved in both assimilatory and dissimilatory nitrogen transformations, including denitrification and DNRA.
2. Materials and Methods
2.1. Soil Sampling and Characterization
Soil samples were collected from an experimental paddy field at the Niigata Agricultural Research Institute, Nagaoka, Niigata Prefecture, Japan (37°44′ N, 138°87′ E), at a depth of 0–20 cm. No nitrogen fertilizer was applied to the field, as previously described [25]. During transport, the soil samples were stored in iceboxes. The samples were sieved through a 2.0 mm mesh to remove impurities and thoroughly mixed in the laboratory. The homogenized soil was then stored at 4 °C for subsequent analyses. The physicochemical properties of the samples are listed in Table S1.
2.2. Nitrate and Nitrous Oxide Concentration Gradient Experiments
The isotopically labeled compounds 15N2, 15N2O, and 15NO3 were used for the SIP analysis. Our previous SIP experiments using 15N2 established that an optimal 15N2 concentration of 80% and an incubation period of 72 h are suitable. However, the optimal concentrations for nitrate and nitrous oxide remained undetermined. Therefore, we performed a microcosm experiment using a concentration gradient of nitrate and nitrous oxide. The ambient conditions for SIP analysis were determined by quantifying nitrous oxide and ammonium generation in the nitrate gradient experiment and nitrous oxide consumption in the nitrous oxide gradient experiment. Microcosms were prepared by mixing 5 g of fresh soil with 2.5 mL of distilled water in 20 mL glass serum vials. The vials were sealed with butyl rubber stoppers and aluminum crimps. To deplete labile nitrogen and carbon sources, the microcosms were preincubated in the dark at 30 °C for 7 days. Following preincubation, rice straw (1 g/100 g mixed soil) was added as a carbon source.
For the nitrate concentration gradient experiment, sodium nitrate was supplemented at concentrations of 0, 2, 10, 30, 60, and 80 μmol/g soil based on the natural nitrate concentration (3.02 μmol/g-dry soil) (Table S1). To inhibit the reduction of nitrous oxide to nitrogen, the gas phase in the vials was replaced with a mixture of 90% Ar and 10% C2H2 [26]. For the nitrous oxide gradient experiment, the gas phase in the microcosms was first replaced with Ar, followed by the introduction of nitrous oxide at concentrations of 0, 10, 20, and 30%.
All microcosms were incubated in the dark at 30 °C for 3 days, and each treatment was performed in triplicate. The experimental design for the nitrate and nitrous oxide gradient treatments is shown in Figure S1. Gas samples were collected at 4 h intervals and stored in 15 mL glass serum vials for analysis of nitrogen dynamics. The methods for measuring nitrous oxide and ammonium concentrations are described in the Supplementary Materials Texts S1 and S2.
2.3. Soil Microcosms for DNA-SIP Incubation
A randomized controlled experimental design was employed, including three nitrogen source treatments (+15NO3− or 14NO3−, +15N2O or 14N2O, and +15N2 or 14N2) and one soil-only control (CK). Each treatment was conducted in triplicate to ensure reproducibility and to allow for statistical analysis.
Microcosms were prepared using the procedure as described in Section 2.2. In total, three experimental treatments with different nitrogen sources (NO3, N2O, and N2) and one soil control treatment were used: (i) soil +Na15NO3 (+15NO3−) or Na14NO3 (+14NO3−) (30 μmol/g mixed soil); (ii) soil +15N2O (+15N2O) or 14N2O (+14N2O) (15 or 14N2O/Ar = 20:80, v/v); (iii) soil +15N2 (+15N2) or 14N2 (+14N2) (15 or 14N2/Ar = 80:20, v/v); and (iv) a soil-only control (CK). Pure 15N2, 15N2O (>99.9%, GL Sciences, Inc., Tokyo, Japan), and 15NaNO3 (>99.8%, SI Science, Saitama, Japan) were used in this study. The vials were filled with Ar for treatments (i) and (iv). All microcosms were incubated in the dark at 30 °C for 24, 48, or 72 h. Gas samples from all four treatments were collected and stored in 15 mL glass serum vials for nitrogen dynamics analysis. Soil samples from the three experimental treatments were destructively collected for DNA-SIP analysis.
2.4. Determination of 15N-Labeled Gas
To quantify the consumption or generation of nitrous oxide and nitrogen gas in the microcosms containing nitrate, nitrous oxide, or nitrogen as substrates, headspace gas from each vial was collected using a syringe and stored in 15 mL glass serum vials. Prior to analysis, the gas samples were diluted with ultrapure helium (He). Gas chromatography-mass spectrometry (GC-MS) was performed using a GC-MS system (GC-2014 gas chromatograph and HS-20 headspace sampler, Shimadzu, Kyoto, Japan) equipped with an SH-Rt-Q-BOND column (32.5 m, 0.32 mm i.d., 10 µm film thickness, Shimadzu, Kyoto, Japan). Ultrapure helium was used as the carrier gas at a flow rate of 2 mL/min. A 1 mL gas sample was injected via the sample loop into the separation column, which was heated at 50 °C, with a split ratio of 30. For MS detection following electron-impact ionization, the detection voltage was set to 0.8 kV, the injector temperature was 250 °C, and the ion-source temperature was 200 °C. Mass spectra were obtained in the selected ion monitoring mode. Other details regarding gas sample injection, GC separation, and MS detection were consistent with a previous study [27]. Pure 15N2 and 15N2O were diluted in ultrapure He (1–2 × 104 ppm) to generate standard curves for the GC-MS analysis.
2.5. DNA Extraction, SIP Gradient Fractionation, and Quantitative PCR
Soil DNA and RNA were extracted from 0.5 g of soil collected from each microcosm in the three experimental treatments on days 0–3, following a previously described method [25]. The collected nucleic acid solution was purified to remove the PCR inhibitors using a OneStep PCR Inhibitor Removal Kit (Zymo Research, Orange, CA, USA) according to the manufacturer’s protocol. RNA was removed using RNase A (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer’s instructions. The resulting RNA-free DNA solution was purified using a QIAquick PCR Purification Kit (Qiagen, Venlo, The Netherlands) and quantified using a NanoDrop One (Thermo Fisher Scientific).
Isopycnic gradient centrifugation and fractionation were performed as previously described using a saturated CsCl solution with a buoyant density of 1.88 g/mL [28,29]. A total of 2.5 μg of extracted DNA was dissolved in a mixed solution of CsCl and gradient buffer (density: 1.690 g/mL) and transferred to a 5PA seal tube (Eppendorf Himac Technologies Co., Ltd., Hitachinaka, Ibaraki, Japan). The DNA mixture was ultracentrifuged at 20 °C and 55,000 rpm (RCF, 172,750× g) for 66 h using a Himac Micro Ultracentrifuge CS100 FNX (Eppendorf Himac Technologies Co., Ltd., Japan) equipped with a Himac S110AT-2611 rotor (Eppendorf Himac Technologies Co., Ltd.; k-factor = 15). To improve the resolution and ensure adequate separation of labeled and unlabeled DNA, each seal tube was immediately fractionated into 25 equal fractions using a model SPDC-1 syringe pump (Asone, Osaka, Japan) after ultracentrifugation. The buoyant density (BD) of each fraction was measured using a model PR-RI digital refractometer (ATAGO, Co., Ltd., Tokyo, Japan). The DNA recovered from each fraction was precipitated using a polyethylene glycol solution (30% PEG, 1.6 M NaCl) with 20 μg glycogen and washed with 70% ethanol, then resuspended in 30 μL TE buffer (pH 8.0).
Quantitative PCR (qPCR) was performed in triplicate using the 27F/520R primer set, TB Green Premix Ex Taq (TaKaRa Bio, Shiga, Japan) and the StepOnePlus System (Applied Biosystems, Foster City, CA, USA) to quantify the 16S rRNA gene copy number in each collected fraction. Standard curves were generated using a 10-fold serial dilution of standard DNA (8.883 × 105 to 8.883 × 1011). The 16S rRNA gene copy numbers were calculated from the Ct values using the standard curve equation. The PCR conditions and the standard curve for each target gene were consistent with those described previously [25].
2.6. 16S rRNA Gene Amplicon Sequencing
On the basis of the qPCR results, DNA samples collected from the heavy and light gradient fractions in each selected treatment were prepared for 16S rRNA gene (V3–V4) amplicon sequencing to analyze the bacterial community composition using primers 341F (5′-CCTACGGGNGGCWGCAG-3′) and 805R (5′-GACTACHVGGGTATCTAATCC-3′). Fractions were selected based on evident contrasts between the labeled and unlabeled samples at the same buoyant DNA densities. The selected treatments and corresponding fractions were as follows: (i) 15NO3− treatment (heavy fractions H0, H1, and H2) or 14NO3− treatment (light fractions L1, L2, and L3) at 24 h of culture; (ii) 15N2O treatment (heavy fractions H1 and H2) or 14N2O treatment (light fractions L2 and L3) at 48 h of culture; and (iii) 15N2 treatment (heavy fractions H0, H1, and H3) or 14N2O treatment (light fractions L1, L2, and L3) at 72 h of culture. Each fraction was analyzed in triplicate. The details of the 16S rRNA gene amplicon sequencing method are described in Table S2. Raw 16S rRNA gene sequences were generated using the Illumina MiSeq platform (2 × 300 bp paired-end). Reads whose 5′ ends exactly matched the primer sequences were extracted using the FASTX-Toolkit (ver. 0.0.14), and the primer sequences were removed. Low-quality reads with a quality score < 20 were discarded using Sickle (ver. 1.33). Paired-end reads were then merged using FLASH (ver. 1.2.11) with the following parameters: merged read length = 310 bp, read length = 230 bp, and overlap length = 10 bp. After removing chimeric and noisy sequences using the DADA2 plugin in QIIME 2 (ver. 2021.2), representative sequences and an ASV table were generated. Taxonomic classification was performed using the feature-classifier plugin by comparing the representative sequences with 99% of the operational taxonomic unit (OTU) reference sequences from the SILVA database (ver. 138.1; Supplementary Table S3 [30]).
2.7. RNA Extraction and Metatranscriptomic Analysis
Samples from the +15NO3− and +15N2O treatments incubated for 24 and 48 h, respectively, were selected for metatranscriptome sequencing. Each treatment was analyzed in triplicate. DNA and RNA were extracted from the soil (0.5 g) using the protocol described in Section 2.5, including purification with the OneStep PCR Inhibitor Removal Kit (Zymo Research, Orange, CA, USA). Genomic DNA was removed from the extracted DNA and RNA solutions (the same as those used for SIP analysis) using DNase I (Nippon Gene, Tokyo, Japan), following the manufacturer’s instructions. The GeneJET RNA Cleanup and Concentration Kit (Thermo Fisher Scientific, Waltham, MA, USA) was used to remove DNA degradation products and concentrate RNA. The quality, concentration, and integrity of the extracted RNA were assessed using a NanoDrop™ 1000 spectrophotometer (Thermo Fisher Scientific), agarose gel electrophoresis, and a model 2100 bioanalyzer instrument (Agilent 2100, Agilent Technologies, Santa Clara, CA, USA).
High-quality RNA samples (optical density [OD] 260/280 = 1.8–2.2; OD260/230 ≥ 2.0, RNA Integrity Number [RIN] ≥ 6.5, 28S:18S ≥ 1.0) were used for library construction. Detailed concentrations and volumes are provided in Supplementary Table S4. RNA libraries were prepared using the MGIEasy Fast RNA Library Prep Set, according to the manufacturer’s instructions (MGI Tech, Shenzhen, China). Library quantification was performed using a Synergy H1 microplate reader (Agilent Technologies, CA, USA) in combination with a QuantiFluor dsDNA System (Promega, Madison, WI, USA). Metatranscriptomic sequencing was performed using the DNBSEQ platform with a 150 bp paired-end configuration at the Bioengineering Laboratory Co. (Kanagawa, Japan). Quality metrics of the raw metatranscriptomic reads are summarized in Supplementary Table S5. Raw paired-end reads were subjected to quality-trimmed using Trimmomatic v0.36 (https://github.com/usadellab/Trimmomatic, (accessed on 10 May 2024)) with the following parameters: SLIDINGWINDOW/4:15 MINLEN/75 to remove adaptor contaminants and low-quality reads. The clean reads were then aligned to the SILVA SSU (16S/18S) and SILVA LSU (23S/28S) databases using SortMeRNA v2.1b (https://github.com/sortmerna/sortmerna, (accessed on 10 May 2024)) software to remove rRNA-related reads. Clean data were assembled using MEGAHIT v1.1.1-2-g02102e1 (https://github.com/voutcn/megahit, (accessed on 10 May 2024)). Genes were predicted using METAProdigal (https://github.com/hyattpd/Prodigal, (accessed on 10 May 2024)). A nonredundant gene catalog was constructed with 95% identity and 90% coverage using CD-HIT (https://github.com/weizhongli/cdhit/releases [31], (accessed on 10 May 2024)). Open reading frames (ORFs) of the assembled contigs were predicted using Prodigal (v2.6.3), and all ORFs were clustered into unique genes using CD-HIT V4.8.1 (https://github.com/weizhongli/cdhit/releases (accessed on 15 May 2024)) [31]. The unique transcriptome gene set was annotated using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database with Kofam v1.2.0 [32]. Functional protein sequences were compared using NCBI Protein BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastp (accessed on 10 June 2024)) (Basic Local Alignment Search Tool) to identify the microorganisms responsible for nitrogen transformation. Transcript abundance was normalized to transcripts per million (TPM) using SAMtools version 1.18.
2.8. Statistical Analyses
The relative abundances of the 16S rRNA gene sequences in the +15N and +14N treatment fractions was calculated by normalizing the number of amplicon sequence reads to the copy numbers of the 16S rRNA genes obtained using qPCR. Linear discriminant analysis (LDA) effect size (LEfSe) was used to identify taxa that were significantly more abundant in the +15N treatments than in the +14N treatments [33]. A nonparametric factorial Kruskal–Wallis sum-rank test (p < 0.05) was performed to detect significantly different features. Bioinformatics analyses, including LEfSe V2.1 and bubble diagram visualization V1.0, were performed using OmicStudio tools (https://www.omicstudio.cn/tool/) [34]. Based on the KEGG annotations, the key nitrogen transformation pathways and their corresponding functional genes are summarized in Supplementary Table S6.
3. Results
3.1. Determination of SIP Experimental Conditions
Our previous study demonstrated that an incubation period of approximately 72 h under 80% 15N2 is suitable for SIP studies targeting nitrogen fixation [35]. To ensure consistent reaction times across experimental groups during DNA-SIP while balancing labeling efficiency, microbial activity, and environmental relevance, we optimized the incubation duration and the substrate concentrations of nitrate (2, 10, 30, 60, and 80 μmol NaNO3−/g soil) and nitrous oxide (10, 20, and 30%).
In the nitrate concentration gradient experiment, N2O generation in the +2 μmol NaNO3/g soil treatment ceased within 8 h, contributing to <1% of the total gas content owing to rapid nitrate depletion (Figure S2a), which was insufficient for effective 15N labeling of microbial DNA in the DNA-SIP experiments. In contrast, in the +10 μmol NaNO3/g soil treatment, N2O generation ceased within 24 h, accounting for approximately 5% of the total gas content. This coincided with near-complete nitrate depletion and a high conversion rate of nitrate to ammonium (Figure S3). In the +30, +60, and +80 μmol NaNO3/g soil treatments, N2O was continuously generated for up to 48 h, stabilizing at 12–14% of the total gas content (Figure S2a). The +30 μmol treatment maintained nitrate availability for over 48 h, albeit with a marginally lower ammonium conversion efficiency compared with the +10 μmol treatment (Figure S3). In the +60 and +80 μmol treatments, nitrate remained even after 96 h of incubation, indicating that excessive nitrate levels significantly deviated from natural paddy field conditions (Figure S2b).
In the nitrate gradient experiment, the maximum N2O concentration generated was approximately 12–14%. To match the maximum N2O concentration generated in the nitrate experiment (Figure S2a) and ensure the continuous availability of N2O during the incubation period, a concentration range of 10–30% N2O was selected for the N2O concentration gradient experiment. As shown in Figure S4, N2O was fully depleted after 48 h in the +10% N2O treatment, whereas in the +20% N2O treatment, depletion was nearly complete by 72 h. In contrast, the +30% N2O treatment resulted in excess residual N2O.
To maintain consistency with the previous 80% 15N2 conditions and the newly optimized +NO3− and +N2O conditions, the DNA-SIP incubation times were set to 0, 24, 48, and 72 h. A nitrate concentration of 30 μmol/g was selected, as this value sustained the reaction for over 48 h while maintaining ammonium conversion efficiency. Additionally, 20% N2O was chosen as the optimal condition for the 72 h incubation. These conditions ensured effective 15N labeling while preventing excessive substrate accumulation.
3.2. Inorganic Nitrogen Reduction and 15N-Labeled Gas Generation
In the +15NO3 treatment, 15N2O accumulated at 24 h (Figure 1a), indicating that denitrification reactions occurred before this time. Additionally, 15N2 accumulation was observed at 72 h, suggesting that the generated 15N2O was converted to 15N2 between 48 and 72 h (Figure 1a).
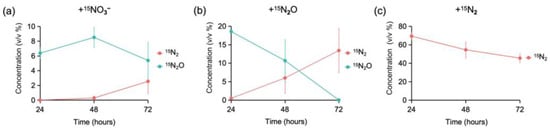
Figure 1.
Concentration (v/v %, volume/volume percent) of 15N-labeled gas (15N2 and 15N2O, >99.9 atom%) produced and consumed in the microcosm during 1–3 days of incubation across the three treatments: (a) 15N2 generation, 15N2O generation, and 15N2O consumption in the +15NO3− treatment; (b) 15N2O consumption and 15N2 production in the +15N2O treatment; (c) 15N2 consumption in the +15N2 treatment. The x-axis shows the incubation time, and the y-axis indicates the gas concentration (%). Error bars represent the range of values from three duplicate batch tests.
In the +15N2O treatment, 15N2O was consumed and a portion of it was converted to 15N2 between 24 and 48 h (Figure 1b). Interestingly, the total amount of 15N2O consumed exceeded that of 15N2 generated, suggesting that either the generated 15N2 was partially fixed by microorganisms or not all of the added 15N2O was fully converted to 15N2.
In the +15N2 treatment, the concentration of 15N2 decreased during the incubation period, indicating that nitrogen fixation had occurred (Figure 1c).
No detectable gas production was observed in the blank control group during the incubation.
3.3. Quantification of the 16S rRNA Gene in CsCl Gradient Fractions
The abundance of the 16S rRNA gene across the CsCl gradient fractions was compared using qPCR to assess the bacterial incorporation of 15N under different nitrogen source treatments. According to the qPCR results, the maximum relative abundance of the 16S rRNA genes significantly shifted to heavier fractions (BD: 1.6729–1.6801 g/mL) compared to lighter fractions (BD: 1.6678–1.6751 g/mL) in all three treatments (Figure 2), indicating that the DNA of the cultured bacteria had successfully incorporated 15N. The absolute 16S rRNA gene copy numbers across the CsCl gradient fractions for each treatment, as determined by qPCR, are shown in Supplementary Figure S5.
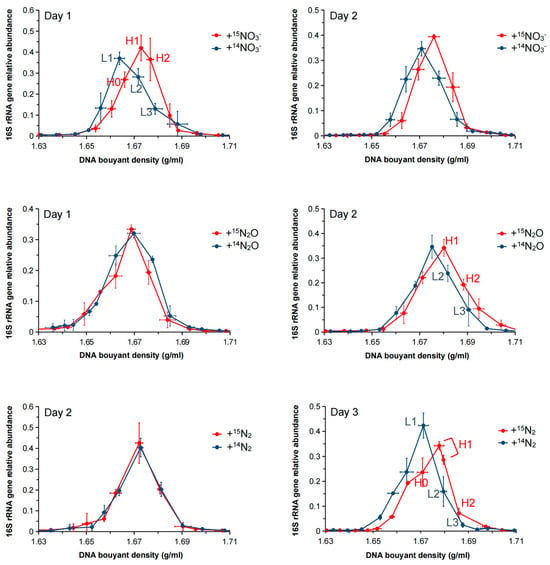
Figure 2.
Relative abundance of the 16S rRNA gene across CsCl gradient fractions from the +15NO3−, +15N2O, and +15N2 treatments after 1–3 days of incubation. The 16S rRNA gene abundance in each fraction was normalized to the total abundance across all fractions. The x-axis indicates buoyant density (g/mL), and the y-axis shows the relative abundance of the 16S rRNA gene. Data are presented as the average of triplicate measurements. Vertical error bars represent the standard error of relative abundance. Horizontal error bars represent the standard error of buoyant density for corresponding fractions.
Interestingly, in the +15N2 treatment, 16S rRNA gene abundance shifted to a heavier fraction owing to N2 fixation after 72 h of culture, whereas in the +15N2O treatment, the shift occurred as early as 48 h. These results suggest that 15N assimilation occurred at a faster rate in the +15N2O treatment (N2O → N2 → NH4+) than in the +15N2 treatment (N2 → NH4+).
3.4. Potential 15N-Assimilating Microorganisms Revealed by DNA-SIP and 16S rRNA Amplicon Sequencing
To identify the microorganisms assimilating 15N, samples were selected at different time points: after 72 h for the +15N2 treatment, after 48 h for the +15N2O treatment, and after 24 h for the +15NO3 treatment. 16S rRNA amplicon sequencing was performed on the CsCl gradient fractions containing the highest concentrations of 16S rRNA genes in the heavy fractions (H0–H2) and the corresponding BD of the light fractions (L1–L3) (Figure 2). LEfSe analysis was used to identify microorganisms that exhibited significant increases after 15N labeling compared with the unlabeled treatments within the same BD. An LDA score threshold was applied to identify taxa with biologically meaningful differences, enabling the robust detection of key microbial taxa actively assimilating 15N under different nitrogen treatments.
In the +15NO3− treatment, the primary 15N-assimilating microorganisms (LDA score ≥ 3.5) were Gemmatimonadaceae, Anaerolineaceae, Bacillus, Geomonas, Xanthomonadaceae, Arenimonas, and Clostridium (Figure 3a). In the +15N2O treatment, the dominant 15N-assimilating bacteria (LDA score > 4.3) were Sulfurospirillum, Geomonas, and Oryzomicrobium (Figure 3b). In the +15N2 treatment, the major N-assimilating microorganisms (LDA score > 4.2) were Clostridium, Geomonas, and Pseudomonadaceae. In addition, Bacillus, Oryzomicrobium, and other bacteria exhibited significant 15N assimilation (LDA score > 3.7) (Figure 3c).
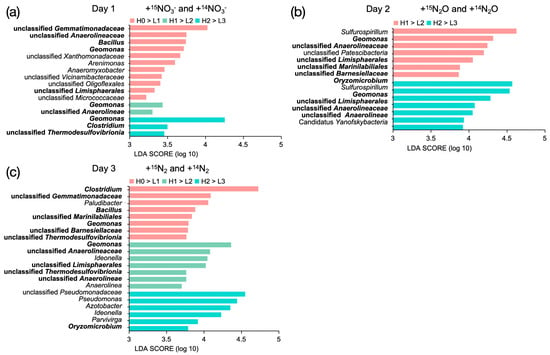
Figure 3.
Microorganisms significantly enriched in the 15N-labeled treatments compared with the corresponding 14N controls within the same CsCl gradient fractions across the three treatments: (a) +15NO3− vs. +14NO3− after 1 day of incubation; (b) +15N2O vs. +14N2O after 2 days; and (c) +15N2 vs. +14N2 after 3 days. Bacterial taxa that incorporated 15N across multiple treatments are indicated in bold. The horizontal coordinates indicate the LDA scores calculated for the 15N treatment relative to the 14N treatment at corresponding density fractions. Data are presented as the average of triplicate measurements. Significance was assessed by a Kruskal–Wallis test (p < 0.05) followed by a Wilcoxon test (p < 0.05).
Among the 15N-assimilating bacteria identified at the genus level, Geomonas was the only taxon detected in all treatments. Oryzomicrobium was involved in 15N assimilation in the +N2O and +N2 treatments, whereas Clostridium and Bacillus were active in the +15NO3 and +N2 treatments. These bacteria demonstrate a strong capacity for nitrogen incorporation from the environment, with Geomonas, Clostridium, and Bacillus exhibiting a preference for nitrogen assimilation in both liquid and gaseous forms.
3.5. Functional Gene Transcripts and Bacteria Involved in Ammonium Generation Revealed by Metatranscriptomics
In the +15NO3− treatment, expressions of functional genes related to denitrification (narG/napA, nirK/S, norB, and nosZ) were most pronounced (Figure 4b(left)). Among these genes, narG/napA, which catalyzes the reduction of NO3− to NO2−, was predominantly transcribed by Bacillaceae (38.4%) and Paenibacillaceae (27.9%). The expression of nirK/S (NO2− → NO) was attributed primarily to Anaerolineaceae (14.1%), Ardenticatenaceae (11.9%), and Bacillaceae (5.6%). Similarly, norB (NO → N2O) was transcribed mainly by Bacillaceae (51.3%), Anaeromyxobacteraceae (13.1%), Comamonadaceae (3.3%), and Holophagaceae (2.9%), while nosZ (N2O → N2) was expressed predominantly by Bacillaceae (79.4%) (Figure 4c(left)). Although the expression levels of genes related to nitrogen fixation and DNRA were low in this treatment group (Figure 4b(left)), nrfA, a gene involved in DNRA, was transcribed by Bacillaceae (44.4%), Anaeromyxobacteraceae (10.3%), and Holophagaceae (6.7%). Moreover, nitrogenase genes (nifD/K) were expressed by Syntrophobacteraceae (77.3%) and Clostridiaceae (9.2%). In contrast, the genes associated with nitrate and nitrite assimilation were more actively transcribed (Figure 4b(left)). Clostridiaceae and Bacillaceae were implicated in nitrite assimilation (NIT-6/nirA/NasBDE), whereas Bacillaceae was involved in nitrate assimilation (Figure 4c(left)). These findings suggest that, under the +15NO3− treatment, denitrification was the predominant nitrogen transformation process, followed by nitrite and nitrate assimilation. In contrast, nitrogen fixation and ammonium generation via the DNRA were less active. Notably, Bacillaceae dominated the transcription of genes related to denitrification, DNRA, and nitrate or nitrite assimilation, indicating high metabolic activity under nitrate-rich conditions.
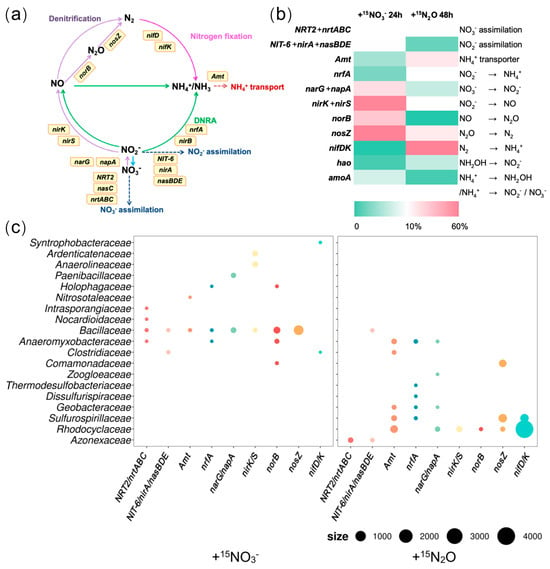
Figure 4.
(a) Nitrogen cycle reactions and related functional genes. (b) Relative expression levels of nitrogen transformation-related genes in the +15N2O and +15NO3− treatments, shown as the proportions TPM within each treatment. (c) Bacterial taxa expressing nitrogen transformation-related functional genes and their expression levels (based on TPM values), size: TPM. Data are presented as the average of triplicate measurements. Arrow colors indicate each nitrogen transformation process (purple: denitrification; green: DNRA; pink: nitrogen fixation).
In the +15N2O treatment, genes related to nitrogen fixation (nifD/K) showed the highest expression and were primarily transcribed by Rhodocyclaceae and Sulfurospirillaceae (82.4% and 10.7%, respectively) (Figure 4c(right)). The second most highly expressed gene was nitrous oxide reductase gene (nosZ), with Sulfurospirillaceae, Comamonadaceae, and Rhodocyclaceae being the primary contributors to its transcription (51.8%, 35.2%, and 7.1%, respectively). Although the expression level of the DNRA-related gene nrfA was relatively low in this study (Figure 4b(right)), it was predominantly transcribed by Anaeromyxobacteraceae (15.1%), Sulfurospirillaceae (6.6%), and Geobacteraceae (2.7%). Additionally, the ammonium assimilation gene (amt) was expressed in Rhodocyclaceae (33.7%), Sulfurospirillaceae (12.3%), Anaeromyxobacteraceae (10.3%), Geobacteraceae (5.2%), and Clostridiaceae (4.1%). A minor expression of genes involved in the early steps of denitrification (NO3− → NO2− → NO) was also detected, suggesting the transient formation of nitrate or nitrite (Figure 4b(right)). However, the lack of expression of hao and amoA expression, which are associated with nitrification, indicated that neither nitrate nor nitrite were produced via this pathway (Figure 4b(right)). These findings indicate that, under the +15N2O treatment, the primary nitrogen transformation processes were N2O reduction to N2 and nitrogen fixation (N2O → N2 → NH4+), followed by ammonium assimilation. Furthermore, under these experimental conditions, the conversion of small amounts of nitrite into ammonium was considered to be driven by bacteria such as Anaeromyxobacteraceae, although the source of nitrite remains unidentified.
4. Discussion
The combined DNA-SIP and metatranscriptomics analyses provided a comprehensive view of inorganic nitrogen transformation in soil under three treatments: +15N2, +15N2O, and +15NO3−. Although DNA-SIP alone may be influenced by potential cross-feeding, its integration with metatranscriptomics helps mitigate this limitation. These analyses reveal the complexity of microbial processes and their critical roles in the nitrogen cycle, particularly in ammonium generation.
In the +15NO3− treatment, metatranscriptomics revealed that denitrification (NO3− → NO2− → NO → N2O → N2) and nitrate or nitrite assimilation were the most active nitrogen-related processes. Ammonium generation appeared to be less prominent, likely because of the low carbon-to-nitrogen (C/N) ratio set for the experiment, which typically suppresses DNRA [36]. However, ammonium accumulation was observed in the soil after prolonged incubation (Figure S3), suggesting that even if microorganisms directly assimilated NO3− or fixed N2, ammonium could be retained in the soil following microbial cell death. In terms of nitrogen assimilation, SIP analysis identified Geomonas belonging to Geobacteraceae, Anaerolineaceae, Gemmatimonadaceae, Bacillaceae, Anaeromyxobacteraceae, and Clostridiaceae as the primary taxa incorporating 15N (Figure 3a). To determine whether these bacteria directly assimilated nitrate or produced ammonium before uptake, a metatranscriptomic analysis was performed. Bacillaceae not only directly assimilated nitrate or nitrite (nrt/nas) but also generated ammonium via DNRA (nrfA), and subsequently incorporated ammonium through ammonium transporters (amt) (Figure 4c(left)). In contrast, Anaeromyxobacteraceae, despite lacking amt expression, assimilated nitrate (nrt), incorporated 15N into DNA, and generated ammonium via DNRA (nrfA). Clostridiaceae incorporated 15N into DNA through nitrite assimilation (nas) and nitrogen fixation (nifD/K). Interestingly, Geomonas, Anaerolineaceae, and Gemmatimonadaceae, which were identified as key 15N-assimilating bacteria in the SIP analysis, did not express nitrogen-related functional genes such as nrt/nas, amt, or nifD/K (Figure 4c(left)). Although Geomonas is a highly active diazotroph in paddy soils [35,37], nifD/K expression was not detected in the present study. Conversely, Syntrophaceae and Syntrophobacteraceae, which transcribed nitrogen fixation genes (nifD/K), were not identified as 15N-assimilating bacteria in the SIP analysis. These discrepancies may reflect a time lag between RNA expression and DNA labeling [38,39,40]. Geomonas, Anaerolineaceae, and Gemmatimonadaceae likely exhibited high RNA expression levels before the SIP detection period (24 h), whereas Syntrophaceae and Syntrophobacteraceae may have incorporated 15N into their DNA after metatranscriptomic sampling. These findings suggest that although denitrification dominated the +15NO3− treatment, ammonium generation via DNRA or denitrification and nitrogen fixation was primarily carried out by Bacillaceae, Anaeromyxobacteraceae, Clostridiaceae, and Geomonas.
In the +15N2O treatment, the metatranscriptomic analysis revealed N2O reduction and nitrogen fixation (N2O → N2 → NH4+) as the dominant processes (Figure 4b(right)). These reactions were driven primarily by Rhodocyclaceae (including Oryzomicrobium) and Sulfurospirillaceae (including Sulfurospirillum), which were also identified as the dominant taxa of 15N-assimilating bacteria based on SIP analysis (Figure 3b and Figure 4c(right)). Additionally, Geobacteraceae, which showed high 15N assimilation in the SIP analysis, exhibited extracellular reduction of nitrate to ammonium (via narG/napA and nrfA), followed by ammonium uptake (via amt), reinforcing the consistency between the SIP results and metatranscriptomic findings (Figure 3b). Thus, in the +15N2O treatment, Rhodocyclaceae, Sulfurospirillaceae, and Geobacteraceae were suggested to generate ammonium via nitrogen fixation and DNRA. The occurrence of DNRA indicated the presence of nitrate in the soil. Interestingly, however, no functional genes associated with nitrate or nitrite production (e.g., hao and amo) were detected in the metatranscriptomic analysis. Additionally, the SIP results showed that 15N assimilation occurred more rapidly in the +15N2O treatment than in the +15N2 treatment (Figure 2). Moreover, the consumption of 15N2O exceeded the production of 15N2 based on GC-MS analysis (Figure 1a,b). These findings suggest that N2O may be consumed through pathways other than its reduction to N2 followed by fixation as NH4+. Frutos et al. proposed that the direct conversion of N2O into nitrate or nitrite is an unknown reaction with thermodynamic advantages [41]. The present findings indicate that it is plausible that if such a pathway exists, the resulting nitrate or nitrite may have been further reduced to ammonium and assimilated into the microbial biomass.
In the +15N2 treatment, the SIP analysis revealed Clostridium belonging to Clostridiaceae, Geomonas belonging to Geobacteraceae, and Pseudomonadaceae (including Pseudomonas and Azotobacter) were the primary N-fixing bacteria that generated intracellular ammonium (Figure 3c). Geomonas [35,37], along with Clostridium and Pseudomonadaceae [42,43], have been reported as active diazotrophs in paddy soils.
A notable difference in active nitrogen-fixing bacteria was observed between the +15N2O and +15N2 treatments (Figure 3b,c and Figure 4c(right)). In the +15N2O treatment, Oryzomicrobium and Sulfurospirillum were the dominant nitrogen-fixing bacteria, whereas in the +15N2 treatment, Clostridium, Geomonas, and Pseudomonadaceae (including Pseudomonas and Azotobacter) were dominant. This difference may be attributed to the absence of the nosZ gene in Geomonas, Clostridium, and Azotobacter, which prevents members of these taxa from reducing N2O to N2. Consequently, Rhodocyclaceae (including Oryzomicrobium) and Sulfurospirillaceae (including Sulfurospirillum), which possess nosZ and efficiently utilize N2O for nitrogen fixation, likely outcompete Clostridium, Geomonas, and Pseudomonadaceae.
Overall, this study reveals that denitrifiers, such as Rhodocyclaceae and Bacillaceae, primarily converted nitrate into nitrogen gas; diazotrophs, including Geobacteraceae, Bacillaceae, Pseudomonadaceae, Rhodocyclaceae, Sulfurospirillaceae, and Clostridiaceae, converted nitrogen gas into ammonium; and DNRA bacteria such as Sulfurospirillaceae and Anaeromyxobacteraceae mainly competed with denitrifiers for nitrate or nitrite and reduced these compounds to ammonium. Furthermore, bacterial families, such as Geobacteraceae (under +N2O and +N2 conditions), Bacillaceae (under +NO3− and +N2 conditions), Rhodocyclaceae (under +N2O and +N2 conditions), Anaeromyxobacteraceae (under +NO3− and +N2O conditions), and Clostridiaceae (under +NO3− and +N2 conditions), actively contributed to ammonium generation under multiple nitrogen substrate conditions in the paddy soil environment (Figure 5).
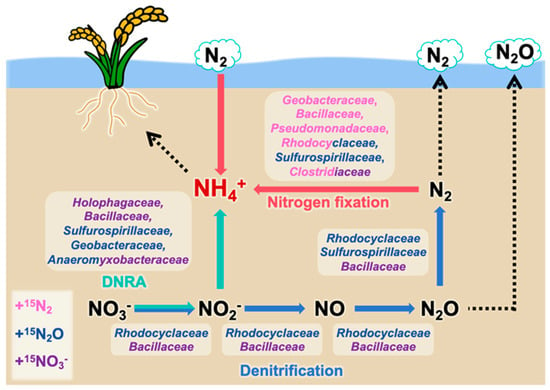
Figure 5.
Overview of ammonium-generating microbial consortia in paddy soils, as proposed by the results of this study. Family names in different colors represent nitrogen-metabolizing microbes identified in different nitrogen source addition groups (purple: +15NO3− treatment; blue: +15N2O treatment; pink: +15N2 treatment). Arrow colors indicate each nitrogen transformation process (blue: denitrification; green: DNRA; red: nitrogen fixation).
Compared with other soils, paddy soils are frequently exposed to anaerobic conditions owing to prolonged flooding and are rich in iron, sulfur, and organic carbon derived from rice plants. These factors provide electron donors and acceptors that support dominant microbial communities [44,45]. This study also confirmed that bacteria already known as Fe(III)-reducing diazotrophs, which include Geobacteraceae, Anaeromyxobacteraceae, Clostridiaceae, and Pseudomonadaceae, generated ammonium through nitrogen fixation [44,46,47,48]. Furthermore, iron has been reported to contribute to ammonium generation through nitrate-dependent iron oxidation (NRFO) and Fe(II)-dependent DNRA (nitrate respiration-associated denitrification), confirming that Anaeromyxobacteraceae play a key role in denitrification, DNRA, and iron reduction [49]. A previous study revealed a strong correlation between DNRA and sulfate reduction rates [50]. This study further demonstrated that Sulfurospirillaceae, a family capable of sulfate reduction, are involved in DNRA. Additionally, organic carbon derived from rice plants, such as rice straw or other residues, is actively decomposed and utilized by Clostridiaceae [51]. The decomposition products generated in this process are subsequently utilized by families including Geobacteraceae and Anaeromyxobacteraceae [37,52,53]. Furthermore, species within the Rhodocyclaceae family, which metabolize recalcitrant organic compounds that include phenolic substances [54], may contribute to lignin degradation from rice straw while simultaneously generating ammonium in paddy soils. Notably, Rhodocyclaceae have also been implicated in the regeneration of rice leaves following damage, whereas Geobacteraceae and Clostridiaceae promote rice recovery from damage or environmental stress, directly supporting rice growth [55].
The findings presented in this study provide a comprehensive overview of the ammonium-generating microbial consortia in paddy soils and suggest that ammonium generation may be closely linked to the reactions of abundant substances such as iron, sulfur, and rice residues in paddy environments. By focusing on the bacterial consortia primarily responsible for ammonium generation and the additional processes they facilitate, it may be possible to enhance microbial activity and consequently promote ammonium generation.
5. Conclusions
This study employed 15N-DNA-SIP and metatranscriptomics to provide the first comprehensive insights into ammonium-generating microbial consortia, focusing specifically on DNRA, denitrification, and nitrogen fixation in paddy soil microcosms amended with 15NO3−, 15N2O, and 15N2 as nitrogen sources. The results indicated that members of the families Holophagaceae, Bacillaceae, Sulfurospirillaceae, Geobacteraceae, and Anaeromyxobacteraceae play a significant role in generating extracellular ammonium via DNRA. Additionally, Geobacteraceae, Bacillaceae, Pseudomonadaceae, Rhodocyclaceae, Sulfurospirillaceae, and Clostridiaceae are identified as key contributors to intracellular ammonium generation through nitrogen fixation. Notably, several taxa, including Geobacteraceae (under +N2O and +N2 conditions), Bacillaceae (under +NO3− and +N2 conditions), Rhodocyclaceae (under +N2O and +N2 conditions), Anaeromyxobacteraceae (under +NO3− and +N2O conditions), and Clostridiaceae (under +NO3− and +N2 conditions), were active under multiple nitrogen substrate conditions, contributing significantly to ammonium generation in the paddy soil environment. Many of these microorganisms, such as those involved in iron or sulfate reduction and rice straw decomposition, are also involved in essential ecological processes characteristic of paddy soils. The study findings revealed ammonium-generating microbial consortia in paddy soils that contain several key bacterial drivers of multiple reductive nitrogen transformations and suggested their diverse functions in paddy soil metabolism. Overall, this study advances our understanding of nitrogen-transforming microbial consortia in paddy soils and may inform sustainable agricultural practices by supporting microbially mediated ammonium generation, improving soil fertility, and reducing the reliance on chemical fertilizers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13071448/s1, Text S1: Detection of N2O content; Text S2: Detection of soil ammonium content; Figure S1: Experimental design (a) nitrate and (b) N2O concentration gradient treatments. The amount of nitrate added was from 2 μmol/g soil to 80 μmol/g soil, and the gas phase of the vials was filled with 90% Ar + 10% C2H2. The N2O concentration was from 10% to 30%, and the negative control received only straw and the gas phase was Ar. The straw concentration was 1.0 g/g mixed soil. Three replicates were set up for each treatment group; Figure S2: Comparison the change of nitrous oxide accumulation in microcosms under different concentrations of nitrate treatment with time. Data are shown as the average of triplicates. The x-axis represents the incubation time; the y-axis indicates the proportion of N2O within the total gas composition in the incubation bottles (a). Comparison of nitrate content remaining in microcosms under different concentrations of nitrate treatment after 0 h and 92 h of incubation. Data are shown as the average of triplicates. The x-axis represents the treatment groups with different concentrations of added nitrate (from 2 μmol NaNO3/g soil to 80 μmol NaNO3/g soil), NC: negative control; the y-axis represents the nitrate content in the incubated soil samples (b); Figure S3: Comparison of the amount of nitrogen in added nitrate converted to nitrogen in ammonium (NH4+) and nitrous oxide (N2O), respectively, in the groups treated with different concentrations of nitrate after 92 h of incubation. The x-axis represents the treatment groups with varying concentrations of added nitrate (from 2 μmol NaNO3/g soil to 80 μmol NaNO3/g soil); the y-axis represents the proportion (%) of nitrogen from nitrate that was converted into nitrous oxide (N2O) and ammonium (NH4+). Data are shown as the average of triplicates; Figure S4: Comparison the change of nitrous oxide concentration in microcosms under different concentrations of nitrate treatment with time. Data are shown as the average of triplicates; Figure S5: Absolute copy numbers of the 16S rRNA gene quantified by qPCR across CsCl gradient fractions from the +15NO3−, +15N2O, and +15N2 treatments after 1–3 days of incubation. The x-axis indicates buoyant density (g/mL), and the y-axis shows the absolute copy number of the 16S rRNA gene determined by qPCR. Data are presented as the average of triplicate measurements. Vertical error bars represent the standard error of relative abundance, while horizontal error bars represent the standard error of buoyant density for corresponding fractions; Table S1: Physicochemical characteristics of the soil; Table S2: PCR reaction for 16S rRNA amplicon sequencing; Table S3: Sequence identity thresholds for 16S rRNA gene used to assign taxonomic ranks; Table S4: Library concentrations and input volumes for metatranscriptomic sequencing; Table S5: Quality assessment of raw metatranscriptomic reads; Table S6: Key nitrogen transformation pathways and the corresponding functional genes.
Author Contributions
Conceptualization, Y.M.; methodology, C.-N.W., Y.M. and K.S.; laboratory work, C.-N.W.; formal analysis, C.-N.W. and Y.M.; data curation, C.-N.W. and Y.M.; writing—original draft preparation, C.-N.W.; writing—review and editing, Y.M. and K.S.; visualization, C.-N.W.; supervision, Y.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI (Grant Numbers JP23K04976 and JP20H05679).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The nucleotide sequences obtained by amplicon sequencing and metatranscriptomics in this study have been deposited in the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/) (Bioproject: PRJNA1250227, PRJNA1250229, PRJNA1250230, PRJNA1250231, PRJNA1250232 and PRJNA1250233, and Bioproject: PRJNA1251509 and PRJNA1251506).
Acknowledgments
We greatly appreciate the technical staff at the Niigata Agricultural Research Institute working on field management. We would like to also thank the reviewers for their valuable comments during the preparation of the manuscript. Chao-Nan Wang thanks the financial support by the China Scholarship Council (CSC).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the result.
Abbreviations
The following abbreviations are used in this manuscript:
| LEfSe | Linear discriminant analysis effect size |
| DNRA | Dissimilatory nitrate reduction to ammonium |
| GC-MS | Gas chromatography-mass spectrometry |
| qPCR | Quantitative PCR |
| LDA | Linear discriminant analysis |
| SIP | Stable isotope probing |
References
- Tilman, D.; Clark, M. Global diets link environmental sustainability and human health. Nature 2014, 515, 518–522. [Google Scholar] [CrossRef]
- Mohapatra, P.K.; Sahu, B.B. Importance of rice as human food. In Panicle Architecture of Rice and Its Relationship with Grain Filling; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–25. [Google Scholar] [CrossRef]
- Bolin, B.; Arrhenius, E. Nitrogen: An essential life factor and a growing environmental hazard report from nobel symposium no. 38. Ambio 1977, 6, 96–105. Available online: http://www.jstor.org/stable/4312254 (accessed on 20 September 2024).
- Muhammad, I.; Yang, L.; Ahmad, S.; Farooq, S.; Al-Ghamdi, A.A.; Khan, A.; .Khan, A.; Zeeshan, M.; Elshikh, M.S.; Abbasi, A.M.; et al. Nitrogen fertilizer modulates plant growth, chlorophyll pigments and enzymatic activities under different irrigation regimes. Agronomy 2022, 12, 845. [Google Scholar] [CrossRef]
- Bu, Y.; Takano, T.; Nemoto, K.; Liu, S. Research progress of ammonium transporter in rice plants. Genom. Appl. Biol. 2011, 2, 19–23. [Google Scholar] [CrossRef]
- Li, B.Z.; Merrick, M.; Li, S.M.; Li, H.Y.; Zhu, S.W.; Shi, W.M.; Su, Y.H. Molecular basis and regulation of ammonium transporter in rice. Rice Sci. 2009, 16, 314–322. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Searchinger, T.D. A “more ammonium solution” to mitigate nitrogen pollution and boost crop yields. Proc. Natl. Acad. Sci. USA 2021, 118, e2107576118. [Google Scholar] [CrossRef]
- de Vries, W. Impacts of nitrogen emissions on ecosystems and human health: A mini review. Curr. Opin. Environ. Sci. Health 2021, 21, 100249. [Google Scholar] [CrossRef]
- Kraft, B.; Tegetmeyer, H.E.; Sharma, R.; Klotz, M.G.; Ferdelman, T.G.; Hettich, R.L.; Geelhoed, J.S.; Strous, M. The environmental controls that govern the end product of bacterial nitrate respiration. Science 2014, 345, 676–679. [Google Scholar] [CrossRef]
- Kuypers, M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Li, X.; Sardans, J.; Gargallo-Garriga, A.; Asensio, D.; Vallicrosa, H.; Penuelas, J. Nitrogen reduction processes in paddy soils across climatic gradients: Key controlling factors and environmental implications. Geoderma 2020, 368, 114275. [Google Scholar] [CrossRef]
- Luo, X.; Fu, X.; Yang, Y.; Cai, P.; Peng, S.; Chen, W.; Huang, Q. Microbial communities play important roles in modulating paddy soil fertility. Sci. Rep. 2016, 6, 20326. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.Y.; Klotz, M.G. The nitrogen cycle. Curr. Biol. 2016, 26, R94–R98. [Google Scholar] [CrossRef]
- Shan, J.; Zhao, X.; Sheng, R.; Xia, Y.; Ti, C.; Quan, X.; Wang, S.; Wei, W.; Yan, X. Dissimilatory nitrate reduction processes in typical Chinese paddy soils: Rates, relative contributions, and influencing factors. Environ. Sci. Technol. 2016, 50, 9972–9980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lan, T.; Müller, C.; Cai, Z. Dissimilatory nitrate reduction to ammonium (DNRA) plays an important role in soil nitrogen conservation in neutral and alkaline but not acidic rice soil. J. Soils Sediments 2015, 15, 523–531. [Google Scholar] [CrossRef]
- Lan, T.; Han, Y.; Roelcke, M.; Nieder, R.; Car, Z. Sources of nitrous and nitric oxides in paddy soils: Nitrification and denitrification. J. Environ. Sci. 2014, 26, 581–592. [Google Scholar] [CrossRef]
- Wada, H.; Panichsakpatana, S.; Kimura, M.; Takai, Y. Nitrogen fixation in paddy soils: I. Factors affecting N2 fixation. Soil Sci. Plant Nutr. 1978, 24, 357–365. [Google Scholar] [CrossRef]
- Mise, K.; Masuda, Y.; Senoo, K.; Itoh, H. Undervalued pseudo-nifH sequences in public databases distort metagenomic insights into biological nitrogen fixers. Msphere 2021, 6, e00785-21. [Google Scholar] [CrossRef]
- Pandey, C.B.; Kumar, U.; Kaviraj, M.; Minick, K.J.; Mishra, A.K.; Singh, J.S. DNRA: A short-circuit in biological N-cycling to conserve nitrogen in terrestrial ecosystems. Sci. Total Environ. 2020, 738, 139710. [Google Scholar] [CrossRef]
- Lewis, K.; Epstein, S.; D’onofrio, A.; Ling, L.L. Uncultured microorganisms as a source of secondary metabolites. J. Antibiot. 2010, 63, 468–476. [Google Scholar] [CrossRef]
- Hahn, M.W.; Koll, U.; Schmidt, J. Isolation and cultivation of bacteria. In The Structure and Function of Aquatic Microbial Communities; Springer International Publishing: Cham, Switzerland, 2019; pp. 313–351. [Google Scholar] [CrossRef]
- Uhlik, O.; Leewis, M.C.; Strejcek, M.; Musilova, L.; Mackova, M.; Leigh, M.B.; Macek, T. Stable isotope probing in the metagenomics era: A bridge towards improved bioremediation. Biotechnol. Adv. 2013, 31, 154–165. [Google Scholar] [CrossRef]
- Cadisch, G.; Espana, M.; Causey, R.; Richter, M.; Shaw, E.; Morgan, J.A.W.; Rahn, C.; Bending, G.D. Technical considerations for the use of 15N-DNA stable-isotope probing for functional microbial activity in soils. Rapid Commun. Mass Spectrom. Int. J. Devoted Rapid Dissem. Up-Minute Res. Mass Spectrom. 2005, 19, 1424–1428. [Google Scholar] [CrossRef] [PubMed]
- Reuter, J.A.; Spacek, D.V.; Snyder, M.P. High-throughput sequencing technologies. Mol. Cell 2015, 58, 586–597. [Google Scholar] [CrossRef]
- Masuda, Y.; Shiratori, Y.; Ohba, H.; Ishida, T.; Takano, R.; Satoh, S.; Shen, W.; Gao, N.; .Itoh, H.; Senoo, K. Enhancement of the nitrogen-fixing activity of paddy soils owing to iron application. Soil Sci. Plant Nutr. 2021, 67, 243–247. [Google Scholar] [CrossRef]
- Soper, F.M.; Simon, C.; Jauss, V. Measuring nitrogen fixation by the acetylene reduction assay (ARA): Is 3 the magic ratio? Biogeochemistry 2021, 152, 345–351. [Google Scholar] [CrossRef]
- Isobe, K.; Koba, K.; Ueda, S.; Senoo, K.; Harayama, S.; Suwa, Y. A simple and rapid GC/MS method for the simultaneous determination of gaseous metabolites. J. Microbiol. Methods 2011, 84, 46–51. [Google Scholar] [CrossRef]
- Angel, R.; Panhölzl, C.; Gabriel, R.; Herbold, C.; Wanek, W.; Richter, A.; Eichorst, S.A.; Woebken, D. Application of stable-isotope labelling techniques for the detection of active diazotrophs. Environ. Microbiol. 2018, 20, 44–61. [Google Scholar] [CrossRef] [PubMed]
- Dunford, E.A.; Neufeld, J.D. DNA stable-isotope probing (DNA-SIP). J. Vis. Exp. JoVE 2010, 42, 2027. [Google Scholar] [CrossRef]
- Yarza, P.; Yilmaz, P.; Pruesse, E.; Glöckner, F.O.; Ludwig, W.; Schleifer, K.H.; Whitman, W.B.; Euzéby, J.; Amann, R.; Rosselló-Móra, R. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat. Rev. Microbiol. 2014, 12, 635–645. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Cd-hit, W.L. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Lyu, F.; Han, F.; Ge, C.; Mao, W.; Chen, L.; Hu, H.; Chen, G.; Lang, Q.; Fang, C. OmicStudio: A composable bioinformatics cloud platform with real-time feedback that can generate high-quality graphs for publication. iMeta 2023, 2, e85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Masuda, Y.; Xu, Z.; Shiratori, Y.; Ohba, H.; Senoo, K. Active nitrogen fixation by iron-reducing bacteria in rice paddy soil and its further enhancement by iron application. Appl. Sci. 2023, 13, 8156. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, G.; Wu, M.; Wang, D.; Liu, Q. Straw return and low N addition modify the partitioning of dissimilatory nitrate reduction by increasing conversion to ammonium in paddy fields. Soil Biol. Biochem. 2021, 162, 108425. [Google Scholar] [CrossRef]
- Masuda, Y.; Mise, K.; Xu, Z.; Zhang, Z.; Shiratori, Y.; Senoo, K.; Itoh, H. Global soil metagenomics reveals distribution and predominance of Deltaproteobacteria in nitrogen-fixing microbiome. Microbiome 2024, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Bremer, H.D.P.P.; Dennis, P.P. Modulation of chemical composition and other parameters of the cell by growth rate. EcoSal Plus 2008, 3, 1553–1569. [Google Scholar] [CrossRef]
- Malinen, A.M.; Turtola, M.; Parthiban, M.; Vainonen, L.; Johnson, M.S.; Belogurov, G.A. Active site opening and closure control translocation of multisubunit RNA polymerase. Nucleic Acids Res. 2012, 40, 7442–7451. [Google Scholar] [CrossRef][Green Version]
- Pham, T.M.; Tan, K.W.; Sakumura, Y.; Okumura, K.; Maki, H.; Akiyama, M.T. A single-molecule approach to DNA replication in Escherichia coli cells demonstrated that DNA polymerase III is a major determinant of fork speed. Mol. Microbiol. 2013, 90, 584–596. [Google Scholar] [CrossRef]
- Frutos, O.D.; Arvelo, I.A.; Pérez, R.; Quijano, G.; Muñoz, R. Continuous nitrous oxide abatement in a novel denitrifying off-gas bioscrubber. Appl. Microbiol. Biotechnol. 2015, 99, 3695–3706. [Google Scholar] [CrossRef]
- Rosenblum, E.D.; Wilson, P.W. Fixation of isotopic nitrogen by Clostridium. J. Bacteriol. 1949, 57, 413–414. [Google Scholar] [CrossRef]
- Desnoues, N.; Lin, M.; Guo, X.; Ma, L.; Carreño-Lopez, R.; Elmerich, C. Nitrogen fixation genetics and regulation in a Pseudomonas stutzeri strain associated with rice. Microbiology 2003, 149, 2251–2262. [Google Scholar] [CrossRef]
- Masuda, Y.; Itoh, H.; Shiratori, Y.; Isobe, K.; Otsuka, S.; Senoo, K. Predominant but previously-overlooked prokaryotic drivers of reductive nitrogen transformation in paddy soils, revealed by metatranscriptomics. Microbes Environ. 2017, 32, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Freney, J.R.; Jacq, V.A.; Baldensperger, J.F. The significance of the biological sulfur cycle in rice production. In Microbiology of Tropical Soils and Plant Productivity; Springer: Dordrecht, The Netherlands, 1982; pp. 271–317. [Google Scholar] [CrossRef]
- Li, L.; Qu, Z.; Jia, R.; Wang, B.; Wang, Y.; Qu, D. Excessive input of phosphorus significantly affects microbial Fe (III) reduction in flooded paddy soils by changing the abundances and community structures of Clostridium and Geobacteraceae. Sci. Total Environ. 2017, 607, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jia, R.; Qu, Z.; Li, T.; Shen, W.; Qu, D. Coupling between nitrogen-fixing and iron (III)-reducing bacteria as revealed by the metabolically active bacterial community in flooded paddy soils amended with glucose. Sci. Total Environ. 2020, 716, 137056. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Wang, K.; Li, L.; Qu, Z.; Shen, W.; Qu, D. Abundance and community succession of nitrogen-fixing bacteria in ferrihydrite enriched cultures of paddy soils is closely related to Fe (III)-reduction. Sci. Total Environ. 2020, 720, 137633. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, N.; Yu, Y.; Zheng, Z.; Yao, H. Soil carbon and nitrogen cycles driven by iron redox: A review. Sci. Total Environ. 2024, 918, 170660. [Google Scholar] [CrossRef]
- Kaviraj, M.; Kumar, U.; Chatterjee, S.; Parija, S.; Padbhushan, R.; Nayak, A.K.; Gupta, V.V. Dissimilatory nitrate reduction to ammonium (DNRA): A unique biogeochemical cycle to improve nitrogen (N) use efficiency and reduce N-loss in rice paddy. Rhizosphere 2024, 30, 100875. [Google Scholar] [CrossRef]
- Weber, S.; Stubner, S.; Conrad, R. Bacterial populations colonizing and degrading rice straw in anoxic paddy soil. Appl. Environ. Microbiol. 2001, 67, 1318–1327. [Google Scholar] [CrossRef]
- Masuda, Y.; Yamanaka, H.; Xu, Z.X.; Shiratori, Y.; Aono, T.; Amachi, S.; Senoo, K.; Itoh, H. Diazotrophic Anaeromyxobacter isolates from soils. Appl. Environ. Microbiol. 2020, 86, e00956-20. [Google Scholar] [CrossRef]
- Xu, Z.; Masuda, Y.; Itoh, H.; Ushijima, N.; Shiratori, Y.; Senoo, K. Geomonas oryzae gen. nov., sp. nov., Geomonas edaphica sp. nov., Geomonas ferrireducens sp. nov., Geomonas terrae sp. nov., Four Ferric-Reducing Bacteria Isolated From Paddy Soil, and Reclassification of Three Species of the Genus Geobacter as Members of the Genus Geomonas gen. nov. Front. Microbiol. 2019, 10, 2201. [Google Scholar] [CrossRef]
- Liu, C.T.; Lin, S.Y.; Hameed, A.; Liu, Y.C.; Hsu, Y.H.; Wong, W.T.; Tseng, C.H.; Lur, H.S.; Young, C.C. Oryzomicrobium terrae gen. nov., sp. nov., of the family Rhodocyclaceae isolated from paddy soil. Int. J. Syst. Evol. Microbiol. 2017, 67, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, F.; Tian, J.; Zhang, W.; Xie, K. Two rice cultivars recruit different rhizospheric bacteria to promote aboveground regrowth after mechanical defoliation. Microbiol. Spectr. 2025, 13, e01254-24. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).