Gut Microbiota Contribute to Heterosis for Growth Trait and Muscle Nutrient Composition in Hybrid Largemouth Bass (Micropterus salmoides)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Populations
2.2. Phenotypic Measurement and Sample Collection
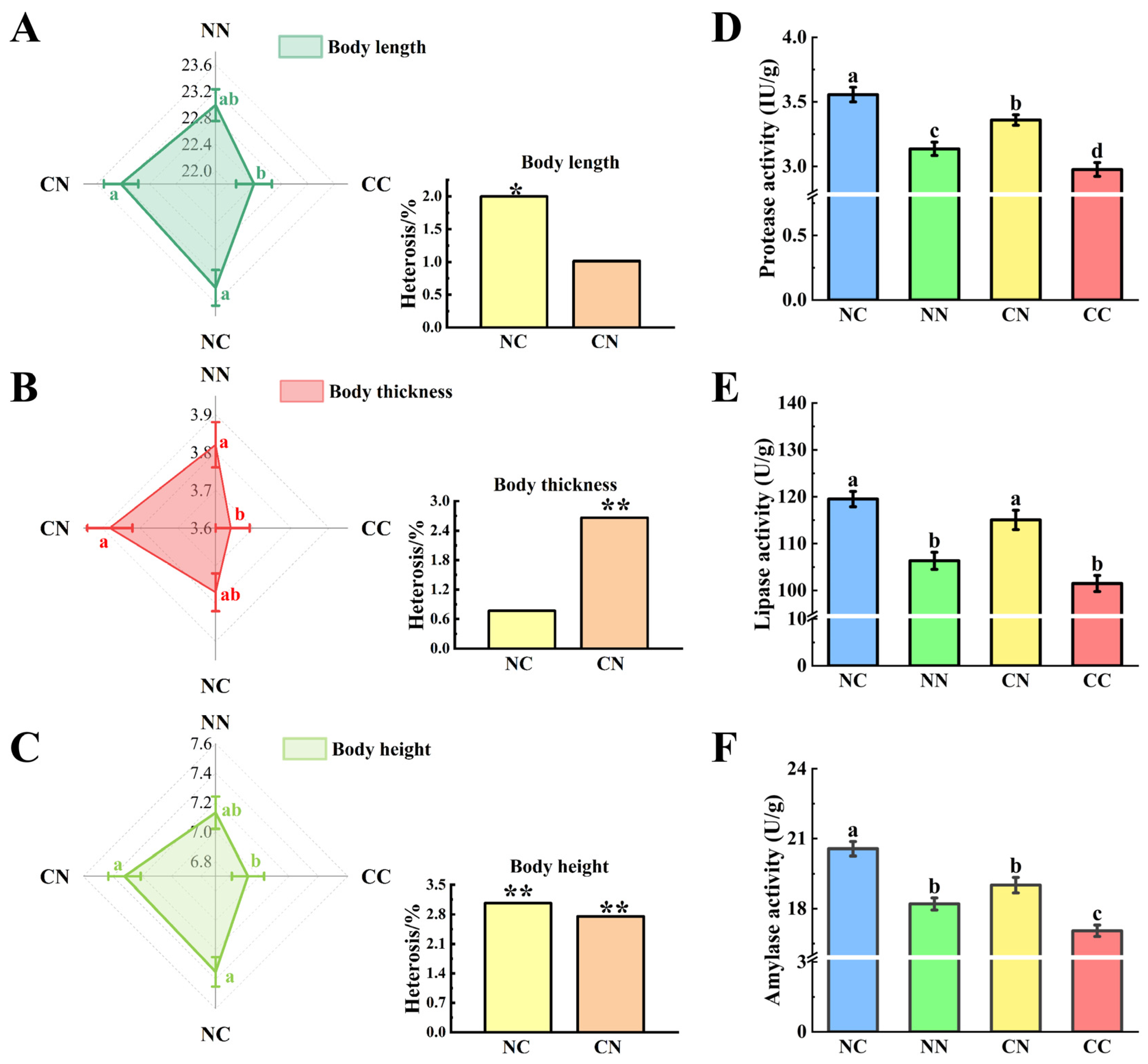
2.3. Determination of Digestive Enzyme Activity
2.4. Proximate Composition of Muscle Samples
2.5. Determination of Muscle Amino Acid and Fatty Acid Composition
2.6. Nucleic Acid Extraction and High-Throughput Sequencing
2.7. Data Preprocessing
2.8. Inheritance Patterns of Microbial Abundance
2.9. Correlation Analysis
3. Results
3.1. Enhancement of Growth Performance
3.2. Differences in Proximate Composition of Muscle
3.3. Differences in Amino Acid and Fatty Acid Composition of Muscle
3.4. Differences in Gut Microbial Community Composition
3.5. Inheritance Patterns of the Microbiome in Hybrid Groups
3.6. Functional Analysis of Microbiome
3.7. Correlations Between Gut Microbiome and Muscle Nutrient Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Q.; Long, Y.; Li, B.; Zhao, L.; Luo, J.; Xu, L.; Luo, W.; Du, Z.; Zhou, J.; Yang, S. Rice-shrimp culture: A better intestinal microbiota, immune enzymatic activities, and muscle relish of crayfish (Procambarus clarkii) in Sichuan Province. Appl. Microbiol. Biotechnol. 2020, 104, 9413–9420. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, Y.; Ni, A.; Li, Y.; Yuan, J.; Ma, H.; Wang, P.; Shi, L.; Zong, Y.; Zhao, J.; et al. Research Note: Heterosis for egg production and oviposition pattern in reciprocal crossbreeds of indigenous and elite laying chickens. Poult. Sci. 2022, 101, 102201. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Li, K.; Miao, N.; Xu, F.; Han, P.; Dai, X.; Abdelkarim, O.F.; Zhu, M.; Zhao, Y. Additive and Dominance Genome-Wide Association Studies Reveal the Genetic Basis of Heterosis Related to Growth Traits of Duhua Hybrid Pigs. Animals 2024, 14, 1944. [Google Scholar] [CrossRef]
- Yu, D.; Gu, X.; Zhang, S.; Dong, S.; Miao, H.; Gebretsadik, K.; Bo, K. Molecular basis of heterosis and related breeding strategies reveal its importance in vegetable breeding. Hortic. Res. 2021, 8, 120. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Sugi, N.; Nojima, Y.; Entani, T.; Shiba, H. Synchronization of Arabidopsis flowering time and vegetative growth stage via FT overexpression can reveal inherent heterosis due to heterozygosity in intraspecific hybrids. Biosci. Biotechnol. Biochem. 2023, 87, 877–882. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, X.; Han, B. Understanding the genetic basis of rice heterosis: Advances and prospects. Crop. J. 2021, 9, 688–692. [Google Scholar] [CrossRef]
- Xiao, Q.; Huang, Z.; Shen, Y.; Gan, Y.; Wang, Y.; Gong, S.; Lu, Y.; Luo, X.; You, W.; Ke, C. Transcriptome analysis reveals the molecular mechanisms of heterosis on thermal resistance in hybrid abalone. BMC Genom. 2021, 22, 650. [Google Scholar] [CrossRef]
- Yang, H.; Li, Q. The DNA methylation level is associated with the superior growth of the hybrid crosses in the Pacific oyster Crassostrea gigas. Aquaculture 2022, 547, 737421. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, G.; Jiang, G.; Hu, Y.; Fang, J.; Chi, Y.; Xu, C.; Liu, W.; Liu, H.; Li, Q. Hybridization between “Haida No. 1” and Orange-shell line of the Pacific oyster reveals high heterosis in survival. Aquaculture 2022, 551, 737945. [Google Scholar] [CrossRef]
- Mai, C.; Wen, C.; Xu, Z.; Xu, G.; Chen, S.; Zheng, J.; Sun, C.; Yang, N. Genetic basis of negative heterosis for growth traits in chickens revealed by genome-wide gene expression pattern analysis. J. Anim. Sci. Biotechnol. 2021, 12, 52. [Google Scholar] [CrossRef]
- Charlesworth, D.; Willis, J.H. The genetics of inbreeding depression. Nat. Rev. Genet. 2009, 10, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.; Lukens, L. An Evaluation of Arabidopsis thaliana Hybrid Traits and Their Genetic Control. G3 Genes|Genomes|Genetics 2011, 1, 571–579. [Google Scholar] [CrossRef] [PubMed]
- McFall-Ngai, M.; Hadfield, M.G.; Bosch, T.C.G.; Carey, H.V.; Domazet-Lošo, T.; Douglas, A.E.; Dubilier, N.; Eberl, G.; Fukami, T.; Gilbert, S.F.; et al. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl. Acad. Sci. USA 2013, 110, 3229–3236. [Google Scholar] [CrossRef] [PubMed]
- Youngblut, N.D.; Reischer, G.H.; Walters, W.; Schuster, N.; Walzer, C.; Stalder, G.; Ley, R.E.; Farnleitner, A.H. Host diet and evolutionary history explain different aspects of gut microbiome diversity among vertebrate clades. Nat. Commun. 2019, 10, 2200. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, Q.; Zhang, Q.; Zhang, Y.; Chen, H.; Liu, G.; Zhu, L. Research Progress of the Gut Microbiome in Hybrid Fish. Microorganisms 2022, 10, 891. [Google Scholar] [CrossRef]
- Hembrom, P.S.; Barik, S.; Deepthi, M.; Kannoth, S.; Grace, T. Influence of gut microbiome on health and development of penaeid shrimps. Aquat. Sci. 2023, 86, 4. [Google Scholar] [CrossRef]
- Brucker, R.M.; Bordenstein, S.R. Speciation by symbiosis. Trends Ecol. Evol. 2012, 27, 443–451. [Google Scholar] [CrossRef]
- Gebiola, M.; Kelly, S.E.; Hammerstein, P.; Giorgini, M.; Hunter, M.S. “Darwin’s corollary” and cytoplasmic incompatibility induced by Cardinium may contribute to speciation in Encarsia wasps (Hymenoptera: Aphelinidae). Evolution 2016, 70, 2447–2458. [Google Scholar] [CrossRef]
- Li, Z.; Wright, A.G.; Si, H.; Wang, X.; Qian, W.; Zhang, Z.; Li, G. Changes in the rumen microbiome and metabolites reveal the effect of host genetics on hybrid crosses. Environ. Microbiol. Rep. 2016, 8, 1016–1023. [Google Scholar] [CrossRef]
- Huang, Q.; Wen, C.; Gu, S.; Jie, Y.; Li, G.; Yan, Y.; Tian, C.; Wu, G.; Yang, N. Synergy of gut microbiota and host genome in driving heterosis expression of chickens. J. Genet. Genom. 2024, 51, 1121–1134. [Google Scholar] [CrossRef]
- Ahmed, I.; Jan, K.; Fatma, S.; Dawood, M.A.O. Muscle proximate composition of various food fish species and their nutritional significance: A review. J. Anim. Physiol. Anim. Nutr. 2022, 106, 690–719. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lu, S.; Tao, Y.; Hua, J.; Zhuge, Y.; Chen, W.; Qiang, J. Characteristic Muscle Quality Parameters of Male Largemouth Bass (Micropterus salmoides) Distinguished from Female and Physiological Variations Revealed by Transcriptome Profiling. Biology 2024, 13, 1029. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-N.; Wang, A.-L.; Bao, L.; Wang, J.; Liu, Y.; Sun, R.-Y. Changes of protein-bound and free amino acids in the muscle of the freshwater prawn Macrobrachium nipponense in different salinities. Aquaculture 2004, 233, 561–571. [Google Scholar] [CrossRef]
- Kasiga, T.; White, B.M.; Bruce, T.J.; Brown, M.L. Effect of fish meal replacement with Carinata Brassica carinata in low animal protein diets of rainbow trout Oncorhynchus mykiss (Walbaum) on trypsin activity, protein and amino acid digestibility and bioavailability. Aquac. Res. 2020, 51, 2134–2149. [Google Scholar] [CrossRef]
- Lai, X.; Xiao, Q.; Ji, H.; Huang, Z.; Huang, H.; You, W.; Luo, X.; Ke, C. Comparative analysis of the growth and biochemical composition of backcrosses and their parents in abalone. Aquaculture 2023, 570, 739445. [Google Scholar] [CrossRef]
- Zhang, W.; Song, Q.; Wu, F.; Zhang, J.; Xu, M.; Li, H.; Han, Z.; Gao, H.; Xu, N. Evaluation of the four breeds in synthetic line of Jiaxing Black Pigs and Berkshire for meat quality traits, carcass characteristics, and flavor substances. Anim. Sci. J. 2019, 90, 574–582. [Google Scholar] [CrossRef]
- Slaughter, J.E.; Wright, R.A.; DeVries, D.R. Latitudinal Influence on First-Year Growth and Survival of Largemouth Bass. N. Am. J. Fish. Manag. 2008, 28, 993–1000. [Google Scholar] [CrossRef]
- Du, J.; Li, S.; Shao, J.; Song, H.; Jiang, P.; Lei, C.; Bai, J.; Han, L. Genetic diversity analysis and development of molecular markers for the identification of largemouth bass (Micropterus salmoides L.) based on whole-genome re-sequencing. Front. Genet. 2022, 13, 936610. [Google Scholar] [CrossRef]
- Hargrove, J.S.; Weyl, O.L.F.; Zhao, H.; Peatman, E.; Austin, J.D. Using species-diagnostic SNPs to detail the distribution and dynamics of hybridized black bass populations in southern Africa. Biol. Invasions 2019, 21, 1499–1509. [Google Scholar] [CrossRef]
- Lutz-Carrillo, D.J.; Schlechte, W.; Norman, J.; Bennett, D.L. Lineage and hybridization effects on size potential in the Largemouth Bass complex. Trans. Am. Fish. Soc. 2023, 152, 145–168. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Tan, H.; Yang, C.; Ren, L.; Liu, Q.; Wang, S.; Hu, F.; Xiao, J.; Zhao, R.; et al. Genetic Effects on the Gut Microbiota Assemblages of Hybrid Fish from Parents with Different Feeding Habits. Front. Microbiol. 2018, 9, 2972. [Google Scholar] [CrossRef] [PubMed]
- A Bryden, C.; Heath, J.W.; Heath, D.D. Performance and heterosis in farmed and wild Chinook salmon (Oncorhynchus tshawytscha) hybrid and purebred crosses. Aquaculture 2004, 235, 249–261. [Google Scholar] [CrossRef]
- Perriman, B.; Bentzen, P.; Wringe, B.; Duffy, S.; Islam, S.; Fleming, I.; Solberg, M.; Bradbury, I. Morphological consequences of hybridization between farm and wild Atlantic salmon Salmo salar under both wild and experimental conditions. Aquac. Environ. Interactions 2022, 14, 85–96. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, W.; Tao, Y.; Li, Y.; Lu, S.; Xu, P.; Qiang, J. Transport Stress Induces Oxidative Stress and Immune Response in Juvenile Largemouth Bass (Micropterus salmoides): Analysis of Oxidative and Immunological Parameters and the Gut Microbiome. Antioxidants 2023, 12, 157. [Google Scholar] [CrossRef]
- Hou, W.; Yi, Z. Heterosis for biomass yield and quality traits in a reciprocal cross population between Miscanthus sinensis and Miscanthus lutarioriparius. Ind. Crop. Prod. 2023, 205, 117451. [Google Scholar] [CrossRef]
- Hansen, A.-C.; Hemre, G.-I. Effects of replacing fish meal and oil with plant resources in on-growing diets for Atlantic cod Gadus morhua L. Aquac. Nutr. 2013, 19, 641–650. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, Y. Comparative analysis of the nutrient components in the muscle and skin tissues of hybrid sturgeon (Acipenser baerii ♀ × Acipenser schrenckii ♂) of different sizes. Aquac. Res. 2022, 53, 6124–6134. [Google Scholar] [CrossRef]
- Ali, A.; Wang, J.; Khan, I.; Wei, S.; Sun, Q.; Xia, Q.; Wang, Z.; Han, Z.; Liu, S. Physicochemical parameters and nutritional profile of back and abdomen muscle of fresh golden pompano (Trachinotus ovatus) and hybrid grouper (Epinephelus lanceolatus × Epinephelus fuscoguttatus). Food Sci. Nutr. 2023, 11, 1024–1039. [Google Scholar] [CrossRef]
- Lu, X.; Luan, S.; Cao, B.; Sui, J.; Dai, P.; Meng, X.; Luo, K.; Kong, J. Heterosis and heritability estimates for the survival of the Pacific white shrimp (Litopenaeus vannamei) under the commercial scale ponds. Acta Oceanol. Sin. 2016, 36, 62–68. [Google Scholar] [CrossRef]
- Yuan, J.; Zhao, J.; Sun, Y.; Wang, Y.; Li, Y.; Ni, A.; Zong, Y.; Ma, H.; Wang, P.; Shi, L.; et al. The mRNA-lncRNA landscape of multiple tissues uncovers key regulators and molecular pathways that underlie heterosis for feed intake and efficiency in laying chickens. Genet. Sel. Evol. 2023, 55, 69. [Google Scholar] [CrossRef]
- Danckert, N.P.; Wilson, N.; Phan-Thien, K.-Y.; Stone, D.A. The intestinal microbiome of Australian abalone, Haliotis laevigata and Haliotis laevigata × Haliotis rubra, over a 1-year period in aquaculture. Aquaculture 2021, 534, 736245. [Google Scholar] [CrossRef]
- Ziab, M.; Chaganti, S.R.; Heath, D.D. The effects of host quantitative genetic architecture on the gut microbiota composition of Chinook salmon (Oncorhynchus tshawytscha). Heredity 2023, 131, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Sevellec, M.; Laporte, M.; Bernatchez, A.; Derome, N.; Bernatchez, L. Evidence for host effect on the intestinal microbiota of whitefish (Coregonus sp.) species pairs and their hybrids. Ecol. Evol. 2019, 9, 11762–11774. [Google Scholar] [CrossRef] [PubMed]
- Reese, A.T.; Dunn, R.R. Drivers of Microbiome Biodiversity: A Review of General Rules, Feces, and Ignorance. mBio 2018, 9, e01294-18. [Google Scholar] [CrossRef]
- Martínez, R.; Santos, R.; Mascaró, M.; Canseco, L.; Caamal-Monsreal, C.; Rosas, C. Digestive dynamics during chyme formation of Octopus maya (Mollusca, Cephalopoda). Aquac. Res. 2012, 43, 1119–1126. [Google Scholar] [CrossRef]
- Wu, X.; Li, R.; Li, Q.; Bao, H.; Wu, C. Comparative transcriptome analysis among parental inbred and crosses reveals the role of dominance gene expression in heterosis in Drosophila melanogaster. Sci. Rep. 2016, 6, 21124. [Google Scholar] [CrossRef]
- Mai, C.; Wen, C.; Sun, C.; Xu, Z.; Chen, S.; Yang, N. Implications of Gene Inheritance Patterns on the Heterosis of Abdominal Fat Deposition in Chickens. Genes 2019, 10, 824. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, Y.; Yan, T.; Li, Y.; Jiang, N.; Zhou, Y.; Zhou, Q.; Qin, P.; Fu, C.; Lin, H.; et al. Transcriptome profiling of two super hybrid rice provides insights into the genetic basis of heterosis. BMC Plant Biol. 2022, 22, 314. [Google Scholar] [CrossRef]
- Zhang, G.; Li, J.; Zhang, J.; Liang, X.; Zhang, X.; Wang, T.; Yin, S. Integrated Analysis of Transcriptomic, miRNA and Proteomic Changes of a Novel Hybrid Yellow Catfish Uncovers Key Roles for miRNAs in Heterosis. Mol. Cell. Proteom. 2019, 18, 1437–1453. [Google Scholar] [CrossRef]
- Perry, W.B.; Lindsay, E.; Payne, C.J.; Brodie, C.; Kazlauskaite, R. The role of the gut microbiome in sustainable teleost aquaculture. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200184. [Google Scholar] [CrossRef]
- Johny, T.K.; Puthusseri, R.M.; Bhat, S.G. A primer on metagenomics and next-generation sequencing in fish gut microbiome research. Aquac. Res. 2021, 52, 4574–4600. [Google Scholar] [CrossRef]
- Tan, S.; Xu, X.; Cheng, H.; Wang, J.; Wang, X. The alteration of gut microbiome community play an important role in mercury biotransformation in largemouth bass. Environ. Res. 2022, 204, 112026. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Jiang, L.; Hu, S.; Zhu, A. Metabolite features of serum and intestinal microbiota response of largemouth bass (Micropterus salmoides) after Aeromonas hydrophila challenge. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 263. [Google Scholar] [CrossRef]
- Song, Z.; Ye, W.; Tao, Y.; Zheng, T.; Qiang, J.; Li, Y.; Liu, W.; Xu, P. Transcriptome and 16S rRNA Analyses Reveal That Hypoxic Stress Affects the Antioxidant Capacity of Largemouth Bass (Micropterus salmoides), Resulting in Intestinal Tissue Damage and Structural Changes in Microflora. Antioxidants 2022, 12, 1. [Google Scholar] [CrossRef]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Litvak, Y.; Byndloss, M.X.; Bäumler, A.J. Colonocyte metabolism shapes the gut microbiota. Science 2018, 362, eaat9076. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Liu, Q.; Ni, K.; Ding, R.; Wang, J.; Wang, C. High Plasticity of the Gut Microbiome and Muscle Metabolome of Chinese Mitten Crab (Eriocheir sinensis) in Diverse Environments. J. Microbiol. Biotechnol. 2021, 31, 240–249. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Li, Y.; Li, J.; Zhu, S. Gut microbiomes of cyprinid fish exhibit host-species symbiosis along gut trait and diet. Front. Microbiol. 2022, 13, 936601. [Google Scholar] [CrossRef]
- Huang, X.; Fang, S.; Yang, H.; Gao, J.; He, M.; Ke, S.; Zhao, Y.; Chen, C.; Huang, L. Evaluating the contribution of gut microbiome to the variance of porcine serum glucose and lipid concentration. Sci. Rep. 2017, 7, 14928. [Google Scholar] [CrossRef]
- Yang, J.H.; Park, J.W.; Kim, H.S.; Lee, S.; Yerke, A.M.; Jaiswal, Y.S.; Williams, L.L.; Hwang, S.; Moon, K.H. Effects of Antibiotic Residues on Fish Gut Microbiome Dysbiosis and Mucosal Barrier-Related Pathogen Susceptibility in Zebrafish Experimental Model. Antibiotics 2024, 13, 82. [Google Scholar] [CrossRef]
- Li, M.; Liang, H.; Yang, H.; Ding, Q.; Xia, R.; Chen, J.; Zhou, W.; Yang, Y.; Zhang, Z.; Yao, Y.; et al. Deciphering the gut microbiome of grass carp through multi-omics approach. Microbiome 2024, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fan, Z.; Yi, M.; Liu, Z.; Ke, X.; Gao, F.; Cao, J.; Wang, M.; Chen, G.; Lu, M. Characterization of the core gut microbiota of Nile tilapia (Oreochromis niloticus): Indication of a putative novel Cetobacterium species and analysis of its potential function on nutrition. Arch. Microbiol. 2022, 204, 690. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Zhang, Z.; Ding, Q.; Yang, Y.; Bindelle, J.; Ran, C.; Zhou, Z. Intestinal Cetobacterium and acetate modify glucose homeostasis via parasympathetic activation in zebrafish. Gut Microbes 2021, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Abraham, T.J.; Paul, P.; Adikesavalu, H.; Patra, A.; Banerjee, S. Stenotrophomonas maltophilia as an opportunistic pathogen in cultured African catfish Clarias gariepinus (Burchell, 1822). Aquaculture 2016, 450, 168–172. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, K.; Zhou, X.; Tang, X.; Yu, X.; Yao, W.; Wu, Z. Histopathology, antioxidant responses, transcriptome and gene expression analysis in triangle sail mussel Hyriopsis cumingii after bacterial infection. Dev. Comp. Immunol. 2021, 124, 104175. [Google Scholar] [CrossRef]
- Zhao, R.; Symonds, J.E.; Walker, S.P.; Steiner, K.; Carter, C.G.; Bowman, J.P.; Nowak, B.F. Relationship between gut microbiota and Chinook salmon (Oncorhynchus tshawytscha) health and growth performance in freshwater recirculating aquaculture systems. Front. Microbiol. 2023, 14, 1065823. [Google Scholar] [CrossRef]
- Mondal, H.K.; Maji, U.J.; Mohanty, S.; Sahoo, P.K.; Maiti, N.K. Alteration of gut microbiota composition and function of Indian major carp, rohu (Labeo rohita) infected with Argulus siamensis. Microb. Pathog. 2022, 164, 105420. [Google Scholar] [CrossRef]
- Wigren, M.A.; Johnson, T.A.; Griffitt, R.J.; Hay, A.G.; Knott, J.A.; Sepúlveda, M.S. Limited impact of weathered residues from the Deepwater Horizon oil spill on the gut-microbiome and foraging behavior of sheepshead minnows (Cyprinodon variegatus). J. Toxicol. Environ. Health Part A 2024, 87, 1–21. [Google Scholar] [CrossRef]
- Sumithra, T.; Gayathri, S.; Sharma, S.K.; Ebeneezar, S.; Anikuttan, K.; Sajina, K.; Narasimapallavan, G.I.; Reshma, K.; Vishnu, R.; Tamilmani, G.; et al. Metagenomic signatures of transportation stress in the early life stages of cobia (Rachycentron canadum) to aid in mitigation strategies. Aquaculture 2022, 559, 738407. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, X.; Wang, Y.; Peng, L.; Li, W. Nitrogen removal characteristics of Pseudomonas stutzeri F11 and its application in grass carp culture. Fish. Sci. 2016, 83, 89–98. [Google Scholar] [CrossRef]
- Wen, C.; Wang, Q.; Gu, S.; Jin, J.; Yang, N. Emerging perspectives in the gut–muscle axis: The gut microbiota and its metabolites as important modulators of meat quality. Microb. Biotechnol. 2024, 17, e14361. [Google Scholar] [CrossRef]




| Items | NC | NN | CN | CC |
|---|---|---|---|---|
| Moisture (g/100 g) | 77.15 ± 0.42 | 76.73 ± 0.19 | 77.2 ± 0.43 | 77.38 ± 0.23 |
| Ash (g/100 g) | 1.23 ± 0.05 a | 1.2 ± 0.04 ab | 1.1 ± 0.00 b | 1.15 ± 0.03 ab |
| Lipid (g/100 g) | 1.35 ± 0.09 a | 0.88 ± 0.09 b | 1.2 ± 0.15 ab | 0.93 ± 0.10 b |
| Protein (g/100 g) | 19.85 ± 0.33 | 20.63 ± 0.17 | 19.93 ± 0.23 | 20.1 ± 0.30 |
| Amino Acid (g/100 g) | NC | NN | CN | CC |
|---|---|---|---|---|
| Aspartate (Asp) | 1.73 ± 0.02 a | 1.74 ± 0.01 a | 1.66 ± 0.02 b | 1.57 ± 0.01 c |
| * Threonine (Thr) | 0.77 ± 0.02 a | 0.77 ± 0.01 a | 0.74 ± 0.01 b | 0.70 ± 0.00 c |
| Serine (Ser) | 0.62 ± 0.01 a | 0.61 ± 0.01 a | 0.60 ± 0.01 a | 0.56 ± 0.00 b |
| Glutamate (Glu) | 2.46 ± 0.05 a | 2.45 ± 0.03 a | 2.34 ± 0.04 a | 2.20 ± 0.02 b |
| * Isoleucine (IIe) | 0.80 ± 0.01 b | 0.84 ± 0.00 a | 0.77 ± 0.01 c | 0.74 ± 0.01 c |
| * Leucine (Leu) | 1.41 ± 0.02 a | 1.42 ± 0.01 a | 1.35 ± 0.02 b | 1.27 ± 0.00 c |
| Tyrosine (Tyr) | 0.57 ± 0.01 a | 0.57 ± 0.01 a | 0.54 ± 0.01 a | 0.50 ± 0.00 b |
| * Phenylalanine (Phe) | 0.74 ± 0.01 a | 0.75 ± 0.01 a | 0.71 ± 0.01 b | 0.68 ± 0.00 c |
| * Lysine (Lys) | 1.65 ± 0.03 a | 1.66 ± 0.01 a | 1.57 ± 0.02 b | 1.49 ± 0.00 c |
| * Histidine (His) | 0.39 ± 0.01 ab | 0.40 ± 0.01 a | 0.37 ± 0.01 bc | 0.35 ± 0.01 c |
| Arginine (Arg) | 1.04 ± 0.02 a | 1.05 ± 0.02 a | 1.01 ± 0.01 a | 0.94 ± 0.01 b |
| Proline (Pro) | 0.57 ± 0.00 a | 0.58 ± 0.01 a | 0.56 ± 0.01 ab | 0.54 ± 0.01 b |
| Glycine (Gly) | 0.85 ± 0.01 | 0.84 ± 0.02 | 0.83 ± 0.00 | 0.81 ± 0.02 |
| Alanine (Ala) | 1.07 ± 0.01 a | 1.07 ± 0.01 a | 1.02 ± 0.01 b | 0.98 ± 0.01 c |
| Cystine (Cys) | 0.22 ± 0.01 a | 0.20 ± 0.01 ab | 0.20 ± 0.01 ab | 0.19 ± 0.01 b |
| * Valine (Val) | 0.87 ± 0.01 b | 0.91 ± 0.00 a | 0.84 ± 0.01 c | 0.80 ± 0.00 d |
| * Methionine (Met) | 0.48 ± 0.01 a | 0.47 ± 0.02 a | 0.46 ± 0.02 a | 0.40 ± 0.00 b |
| Total essential amino acid (EAA) | 7.11 ± 0.07 a | 7.22 ± 0.06 a | 6.79 ± 0.09 b | 6.44 ± 0.03 c |
| Total non-essential amino acids (NEAA) | 9.12 ± 0.12 a | 9.12 ± 0.12 a | 8.75 ± 0.09 b | 8.30 ± 0.08 c |
| Delicious amino acid (DAA) | 6.11 ± 0.08 a | 6.10 ± 0.07 a | 5.85 ± 0.07 b | 5.57 ± 0.05 c |
| Total amino acids (TAA) | 16.23 ± 0.2 a | 16.33 ± 0.18 a | 15.53 ± 0.18 b | 14.73 ± 0.09 c |
| Essential amino acids/total amino acids (EAA/TAA) | 0.44 ± 0.00 | 0.44 ± 0.00 | 0.44 ± 0.00 | 0.44 ± 0.00 |
| Fatty Acid (%) | NC | NN | CN | CC |
|---|---|---|---|---|
| C14:0 | 0.83 ± 0.05 b | 1.04 ± 0.02 a | 1.19 ± 0.04 a | 1.21 ± 0.08 a |
| C16:0 | 20.17 ± 0.88 a | 17.24 ± 0.24 b | 18.92 ± 0.50 ab | 17.91 ± 0.33 b |
| C18:0 | 10.08 ± 0.45 a | 7.28 ± 0.40 b | 6.25 ± 0.28 b | 6.33 ± 0.62 b |
| SFA | 31.09 ± 1.25 a | 25.56 ± 0.16 b | 26.37 ± 0.68 b | 25.45 ± 0.60 b |
| C16:1 | 1.89 ± 0.05 b | 2.11 ± 0.08 b | 2.87 ± 0.11 a | 2.72 ± 0.03 a |
| C18:1n9c | 19.73 ± 0.21 c | 20.79 ± 0.84 bc | 25.12 ± 0.77 a | 22.18 ± 0.17 b |
| C20:1 | 0.82 ± 0.00 b | 0.80 ± 0.01 b | 0.94 ± 0.04 a | 0.90 ± 0.02 a |
| C22:1n9 | 2.18 ± 0.10 ab | 2.26 ± 0.17 a | 1.81 ± 0.08 b | 1.94 ± 0.09 ab |
| C24:1 | 1.82 ± 0.04 a | 1.17 ± 0.13 b | 1.15 ± 0.18 b | 0.88 ± 0.08 b |
| MUFA | 26.45 ± 0.32 c | 27.13 ± 0.63 bc | 31.88 ± 0.87 a | 28.62 ± 0.17 b |
| C18:2n6c | 19.08 ± 0.37 b | 22.91 ± 0.64 a | 22.19 ± 0.73 a | 23.40 ± 0.42 a |
| C18:3n3 | 0.81 ± 0.03 b | 1.43 ± 0.03 a | 1.41 ± 0.06 a | 1.44 ± 0.07 a |
| C20:2 | 0.91 ± 0.03 a | 0.79 ± 0.04 ab | 0.75 ± 0.06 b | 0.79 ± 0.04 ab |
| C20:3n6 | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.35 ± 0.03 a |
| C20:4n6 | 1.88 ± 0.05 a | 0.78 ± 0.05 b | 0.96 ± 0.2 b | 1.07 ± 0.19 b |
| C20:5n3 (EPA) | 1.33 ± 0.02 b | 1.92 ± 0.06 a | 1.28 ± 0.04 b | 1.35 ± 0.09 b |
| C22:6n3 (DHA) | 18.46 ± 0.56 a | 19.48 ± 0.89 a | 15.16 ± 0.72 b | 17.54 ± 0.27 a |
| PUFA | 42.47 ± 0.93 b | 47.31 ± 0.6 a | 41.75 ± 0.87 b | 45.93 ± 0.51 a |
| PUFA n-3 | 20.59 ± 0.60 b | 22.83 ± 0.91 a | 17.86 ± 0.69 c | 20.33 ± 0.35 b |
| PUFA n-6 | 20.96 ± 0.36 c | 23.69 ± 0.62 b | 23.14 ± 0.54 b | 24.82 ± 0.24 a |
| ∑n-6/∑n-3 | 1.02 ± 0.02 b | 1.04 ± 0.06 b | 1.30 ± 0.06 a | 1.22 ± 0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, J.; Wang, Q.; Tao, Y.; Sun, H.; Lu, S.; Zhuge, Y.; Chen, W.; Liu, K.; He, J.; Qiang, J. Gut Microbiota Contribute to Heterosis for Growth Trait and Muscle Nutrient Composition in Hybrid Largemouth Bass (Micropterus salmoides). Microorganisms 2025, 13, 1449. https://doi.org/10.3390/microorganisms13071449
Hua J, Wang Q, Tao Y, Sun H, Lu S, Zhuge Y, Chen W, Liu K, He J, Qiang J. Gut Microbiota Contribute to Heterosis for Growth Trait and Muscle Nutrient Composition in Hybrid Largemouth Bass (Micropterus salmoides). Microorganisms. 2025; 13(7):1449. https://doi.org/10.3390/microorganisms13071449
Chicago/Turabian StyleHua, Jixiang, Qingchun Wang, Yifan Tao, Hui Sun, Siqi Lu, Yan Zhuge, Wenhua Chen, Kai Liu, Jie He, and Jun Qiang. 2025. "Gut Microbiota Contribute to Heterosis for Growth Trait and Muscle Nutrient Composition in Hybrid Largemouth Bass (Micropterus salmoides)" Microorganisms 13, no. 7: 1449. https://doi.org/10.3390/microorganisms13071449
APA StyleHua, J., Wang, Q., Tao, Y., Sun, H., Lu, S., Zhuge, Y., Chen, W., Liu, K., He, J., & Qiang, J. (2025). Gut Microbiota Contribute to Heterosis for Growth Trait and Muscle Nutrient Composition in Hybrid Largemouth Bass (Micropterus salmoides). Microorganisms, 13(7), 1449. https://doi.org/10.3390/microorganisms13071449






