Abstract
Acute oak decline (AOD) is a multifactorial disease that affects European oaks and represents a growing threat to forests. The disease results from a complex interaction between biotic and abiotic factors: the various environmental stresses, which vary depending on the area in question, and generally increased by climate change, predispose trees to attack by opportunistic pathogens. Among them, we focused on a bacterial consortium associated with AOD, consisting mainly of Brenneria goodwinii, Gibbsiella quercinecans, Rahnella victoriana, and Lonsdalea britannica, which produce degrading enzymes that contribute to phloem necrosis and the development of stem bleeds and bark cracks. However, the role of other pathogens, such as fungi, cannot be ruled out, but instead could be contributory. The potential involvement of xylophagous insects is also being studied, particularly Agrilus biguttatus, which, although, frequently associated with the disease, has not been conclusively demonstrated to act as an active vector of the bacteria. Currently, disease management requires integrated approaches, including monitoring and other forestry strategies to increase forest resilience. Given the phenomenon’s complexity and the risk of the future expansion of that bacterial consortium, further research is necessary to understand the dynamics and to develop effective containment strategies of AOD-associated bacteria.
1. Introduction
Oak forests are particularly recognized worldwide for their economic value and biodiversity [1]. In recent decades, however, such landscapes have been marked by several important tree diseases, including chestnut blight and ash dieback. These diseases are often the result of complex interactions between host, environment, pests, and pathogens [2]. In particular, oak forests have experienced substantial degradation due to the spread of oak decline across Europe [3]. Notably, the incidence of AOD-associated bacteria appears to be progressively expanding further south [4]. Oak decline appears to be becoming a global problem, as it has also been found in Japan [5] and North America [6]. Oak decline, recognized as a complex phenomenon, is one of the most significant forest syndromes worldwide. It is more difficult to understand and manage than the decline of other tree species due to the broad spectrum of oak species affected, including Quercus cerris L., Q. frainetto Ten., Q. petraea (Matt.) Liebl., Q. pubescens Willd., Q. robur L., and Q. suber L., as well as the different environmental conditions within their geographic range that may or may not favor the syndrome [7]. Indeed, the syndrome is considered a multifactorial network [8], with predisposing abiotic factors playing a crucial role. However, biotic factors, including subcortical insects and bacterial and fungal pathogens, also contribute to accelerating the decline process [9].
Two forms of oak decline are currently distinguished: “chronic oak decline,” which progresses gradually, and “acute oak decline,” which manifests itself more rapidly. Trees affected by chronic oak decline often show a gradual progression of symptoms over decades; in contrast, AOD is of greater concern due to its rapid development, causing significant tree mortality [10,11], although some individuals are able to survive [12]. Since 2006, outbreaks of AOD have been reported in England, affecting both native oak species, Q. robur and Q. petraea [13]. The syndrome, identified in Northern and Central Europe [14], has distinct symptoms and poses a significant threat to the world’s forests. However, the full range of causal factors remains poorly understood [15]. As mentioned, AOD is a complex syndrome, as it involves abiotic and biotic factors: abiotic predisposing elements, such as stresses related to variations in temperature and precipitation regimes across different areas, contribute to tree vulnerability, while biotic agents, including the beetle Agrilus biguttatus [16] and a range of bacterial species, mainly Brenneria goodwinii, Gibbsiella quercinecans, Rahnella victoriana, and Lonsdalea britannica, act as predominant factors in the fate of affected oak trees [17]. These different contributing factors can be divided into three categories: “predisposing” agents, which reduce a tree’s resilience in coping with stressors; “inciting” agents, which initiate visible symptoms of decline; and “contributing” agents, which promote disease progression [18]. Among these, stress resulting from a prolonged state of drought is a significant factor in oak decline, often linked to site-specific soil conditions [4]. It cannot be overlooked that this condition is also facilitated by climate change, as progressively warmer years and reduced rainfall contribute to oak susceptibility [19].
Specifically, the crucial involvement in the progression of AOD by different bacterial species is recognizable by specific external symptomatology, including longitudinal fissures and necrotic bark lesions (5–10 cm in size) exuding dark, sticky exudates [20]. As a result, the crown volume is drastically reduced, negatively impacting the tree’s entire fitness, which risks death within four to five years after the onset of symptoms [10]. In addition, characteristic “D”-shaped exit holes, associated with A. biguttatus evasion, are often observed on the stems of affected trees [21]. However, the understanding of the epidemiology of this disease, the routes of spread of the bacteria, and the specific sequence of events leading to the observed symptoms is still limited [22].
2. Role of Bacteria in AOD
The biotic variables that influence the development of AOD continue to be investigated. It is clear from the different necrotic tissues that these are associated with a complex poly-microbial community [23]. As mentioned before, consistently detected bacterial species include G. quercinecans, B. goodwinii, and R. victoriana, with L. britannica occasionally present [17,24] (Table 1). Identifying these species accurately is difficult due to their similarity in colony appearance, phenotypic traits, and 16S rRNA gene sequences. However, the most widespread bacterial families identified in symptomatic tissues include Pseudomonadaceae, Enterobacteriaceae, Halomonadaceae, Shewanellaceae, and Acholeplasmataceae. The diversity within this bacterial community appears to vary depending on the condition of the tissue and the presence of A. biguttatus galleries [22]. G. quercinecans and B. goodwinii are considered to be the two leading bacterial species responsible for AOD, as they are constantly detected in necrotic tissues and their exudates [13]. The genus Brenneria represents a distinct group of Gram-negative bacteria characterized by perithecial flagella [25]. In particular, B. goodwinii is an optional anaerobe [20,26] commonly associated with necrotrophic behavior, killing host tissues and using decomposing material as a substrate. Similarly, the genus Gibbsiella consists of Gram-negative, optional anaerobic bacteria [18]. These cells can exist individually, in pairs, or in groups of four and are characterized by thin fimbriae but not flagella [27]. Both B. goodwinii and G. quercinecans contribute to tissue necrosis and, with A. biguttatus, are responsible for severe cortical symptoms [28]. These bacteria are characterized by virulence genes commonly found in several plant pathogens, which are confirmed in the pathogenesis of AOD [23,29], and that allows them to pass from harmless commensal to aggressive necrotrophic pathogens. This transition is promulgated by the secretion of enzymes that degrade the cell wall polysaccharides of plants, such as pectinases, cellulases, and tannins [30].

Table 1.
Summary table describing the four AOD-associated bacteria.
It has also been verified that G. quercinecans prefers sugar metabolites from phloem tissue, while B. goodwinii tends to use oak sapwood as a carbon source [2]. When several pathogenic bacteria species are present together, the extent of necrosis is even more significant, suggesting a cumulative effect and potential synergistic interactions. In addition, the specific functions of R. victoriana, as well as other members of this microbiome within lesions, remain largely unclear, as do their interactions with the host and possible vectors [31].
L. britannica has shown different levels of pathogenicity, sometimes exhibiting virulence, but it is not always identified in AOD lesions, unlike the bacteria described above [23]. In fact, the degree of pathogenicity of these bacteria is not always predictable since it depends on their concentration and the overall health of the host oak [32]. Gathercole et al. [17] provided evidence of their presence in leaf and foliar tissues, suggesting that these bacteria are ubiquitous. Therefore, their presence in a single tree does not necessarily entail symptoms of AOD. However, the understanding of the ecological and environmental reservoirs that house these agents, both bacterial and non-bacterial, remains limited. Rainwater and forest soils are known to play this role in several plant pathogens. Pettifor et al. [33] describe G. quercinecans as a generalist within forest ecosystems, capable of surviving in both rainwater and soil. Meanwhile, B. goodwinii is a specialized endosymbiont of oaks, which does not survive outside its host.
On the other hand, the influence of fungal sepsis should not be underestimated. Fungi, particularly of the genera Ceratocystis and Ophiostoma, exploit oak trees weakened by environmental stress and insect attack to establish pathogenic relationships, thus triggering further vascular diseases, limiting the tree’s ability to absorb nutrients [34]. The same authors report a case where B. goodwinii was identified as an endophyte in oaks without showing pathogenic behavior [34]. This result suggests that other factors, such as Armillaria, Phytophthora, Erysiphe alphitoides, or abiotic stresses, may have contributed to the observed exudates. The first two species are typically observed on trees infested with beetles [35,36], particularly with the species Q. suber and Q. ilex [37,38]. In particular, Phytophthora citricola, Phytophthora cinnamomi, and Phytophthora cambivora lead to bleeding on the trunk of different oak species, typically occurring 1–2 m above ground level [10]. However, when alone, it does not seem to be able to trigger the decline of the tree directly [39]. Moreover, the severity of its impact varies considerably depending on site-specific environmental conditions [40].
3. Role of Other Biotic and Abiotic Factors in AOD Predisposition
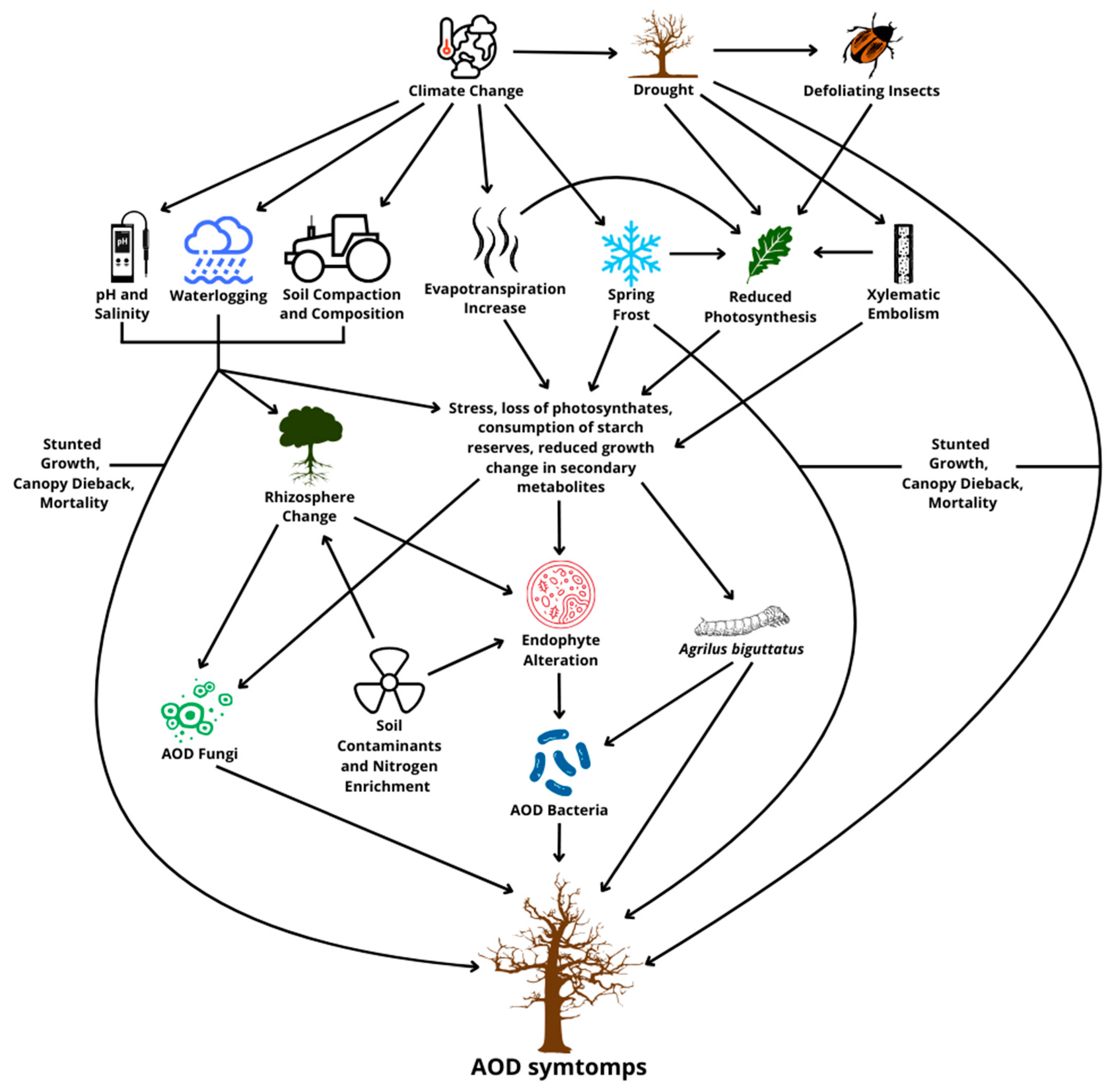
As described above, the decline of oaks is a complex phenomenon shared between several biotic and abiotic factors [41] (Figure 1). These factors may interact more or less directly, also creating cycles of positive effects that lead to the deterioration of the state of the plant in a short amount of time. Among the main predominant factors in the predisposition to decline, we find an increasingly recurring and current problem initiated by abiotic stress, such as drought, which provokes massive defoliation that increases the vulnerability of the oaks, supported by the role of herbivorous insects and attacks by wood-boring insect larvae, fungi, and bacteria [39]. Considered individually, abiotic stresses do not cause, in general, the entire decline and, therefore, the death of the plant; instead, they can induce significant physical, physiological, and chemical alterations. These changes, as mentioned, promote the proliferation of pathogens but also increase the attractiveness of trees for feeding and reproducing insects [42].
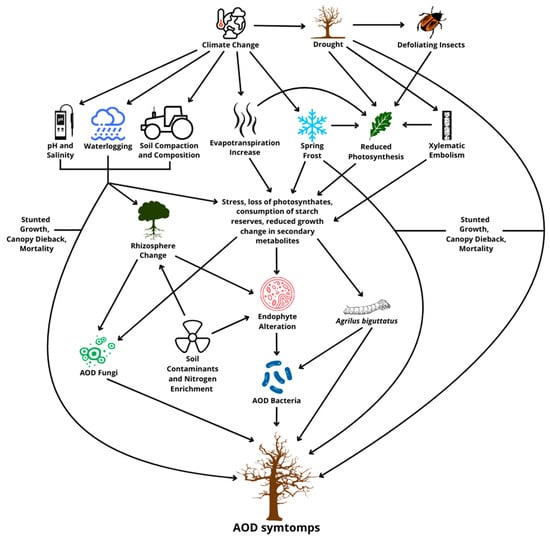
Figure 1.
The biotic and abiotic factors involved in acute oak decline.
Defoliation in particular reduces the leaf surface, limiting the tree’s photosynthetic capacity. Among these insects, standout spring feeding species belonging to different families of Lepidoptera, such as Lymantriidae (Lymantria dispar L., Euproctis chrysorrhoea L.), Geometridae (Operophthera brumata L., Erannis defoliaria Cl.), and Tortricidae (Tortrix viridana L., Archips xylosteana L.) [3]. As a result, the development of new shoots to re-fold the crown is compromised, and the plant is exposed to carbon spills. In fact, the starch reserves of perennial organs remain depleted, reducing the oak’s tolerance to frosts [43] and slower radial growth [44]. As a result, trees particularly weakened by heavy defoliation are then more susceptible to other abiotic stresses [3]. Among the various stressors associated with AOD, the theme of prolonged drought, increasingly frequent due to climate change, has been examined [45]. In this regard, Ref. [46] found that warmer spring and summer temperatures increase evapotranspiration rates and, thus, the vulnerability of oaks [47]. Drought can also cause xylematic embolism, disrupting the tree’s plumbing system and eventually leading to foliage decay and branch mortality [4]. In addition, just like defoliation, drought-induced stress affects carbon allocation, making trees more susceptible to necrotrophic pathogens [48,49].
However, other environmental factors, such as late spring frost, flooding, and particularly cold winters, have been linked to reduced growth of oaks [50]. In parallel, soil composition can also have an impact, such as changes in characteristics such as land management, nitrogen deposition, heavy metal contamination, genetic predisposition, and changes in rhizosphere communities [15,39]. Finally, wood anatomy differences between oak species can influence their stress resilience [4]. Some soil properties, including pH and salinity, are thought to affect the composition of rhizosphere communities, shaping the oak microbiome according to the local pedoclimatic conditions [51]. Furthermore, Ref. [52] observed how the bacterial microbiome may be affected by changes in species based on tissue health, particularly by a higher concentration of Gram-positive bacteria in healthy tissues than of Gram-negative bacteria in diseased tissues [51]. High salinity levels can negatively affect water and nutrient absorption, limiting radial growth in oaks [53], while soils that are too compact and with reduced aeration may decrease root density [54]. It has been suggested that an excess of nitrogen in the soil accelerates growth to such a degree that there is potential for tree structural instability and water shortage if the root system cannot adequately support this expansion [55]. Nitrogen enrichment can also lower the allelochemical concentration of leaves, making trees more prone to insect attack [43]. Soil conservation treatments such as mulching may be effective in increasing the resilience of the oak system but require further investigation [56].
It has been noted that tree mortality rates increase with the increase in typical “D” exit holes in its bark, suggesting that the combined impact of A. biguttatus activity and existing lesions may contribute to the “acute” phase of AOD [57]. However, the specific role of the insect both as an accelerator of decline and as a likely vector for other pathogens remains uncertain. It is hypothesized that it could simply opportunistically exploit the weakened trees and not actively spread the phytopathogenic AOD [35]. In fact, the association between the beetle and bacteria can be random, with both benefiting independently from the stress condition of the oaks. As reported earlier, the coincidence of these two agents can trigger a positive cycle of reinforcement and increased stress, increasing the risk of death of the host [39].
4. Spatial Distribution of the Main Hosts and Bacteria
The genus Quercus is one of the most significant groups of woody angiosperms in the northern hemisphere, notable for its species diversity, ecological importance, and economic value. It comprises more than 600 species of trees and shrubs, which thrive in many habitats [58]. The predominant broadleaf species in Europe are Q. robur L. (pedunculate oak) and Q. petraea (Matt.) Liebl. (sessile oak). Their range begins in the north, from southern Norway and Sweden, and extends as far south as the northern Iberian Peninsula, southern Italy, the Balkans, and Turkey. Q. robur is a major player in timber production, so much so that it has also been introduced to the United States, where it has now naturalized in some regions [59]. At the same time, Q. suber and Q. ilex are also well adapted to the tropical and subtropical climates of Eurasia and North Africa [60]. This, therefore, highlights the risk of large-scale spread of AOD, finding favorable soil and climatic conditions in different continents and bringing significant environmental, social, and economic implications.
Oak decline has been documented in the UK and various parts of Europe over the past century [11], and bacteria have been frequently associated with different types of decline (Table 2). Ragazzi et al. [7] provide an overview of the earliest records of oak decline across Europe, linking specific oak species to affected countries. Q. petraea has been associated with cases in Germany (1739), Switzerland (1850), France (1921), Poland (1940), Austria (1944), the Czech Republic and Slovakia (1954), Hungary (1877), the former Yugoslavia (1878), Russia (1892), and Romania (1910). Similarly, Q. robur has been connected to decline events in Germany (1739), Switzerland (1850), Hungary (1877), the former Yugoslavia (1878), Romania (1910), Belgium (1921), France (1921), Poland (1940), Austria (1944), the Czech Republic and Slovakia (1954), Italy (1980), and Bulgaria (1982). Meanwhile, Q. suber has been linked to decline in Portugal (1988) and Spain (1989).

Table 2.
Reported occurrences of AOD-associated bacteria and Brenneria quercina on various oak species (Quercus spp.) across different countries.
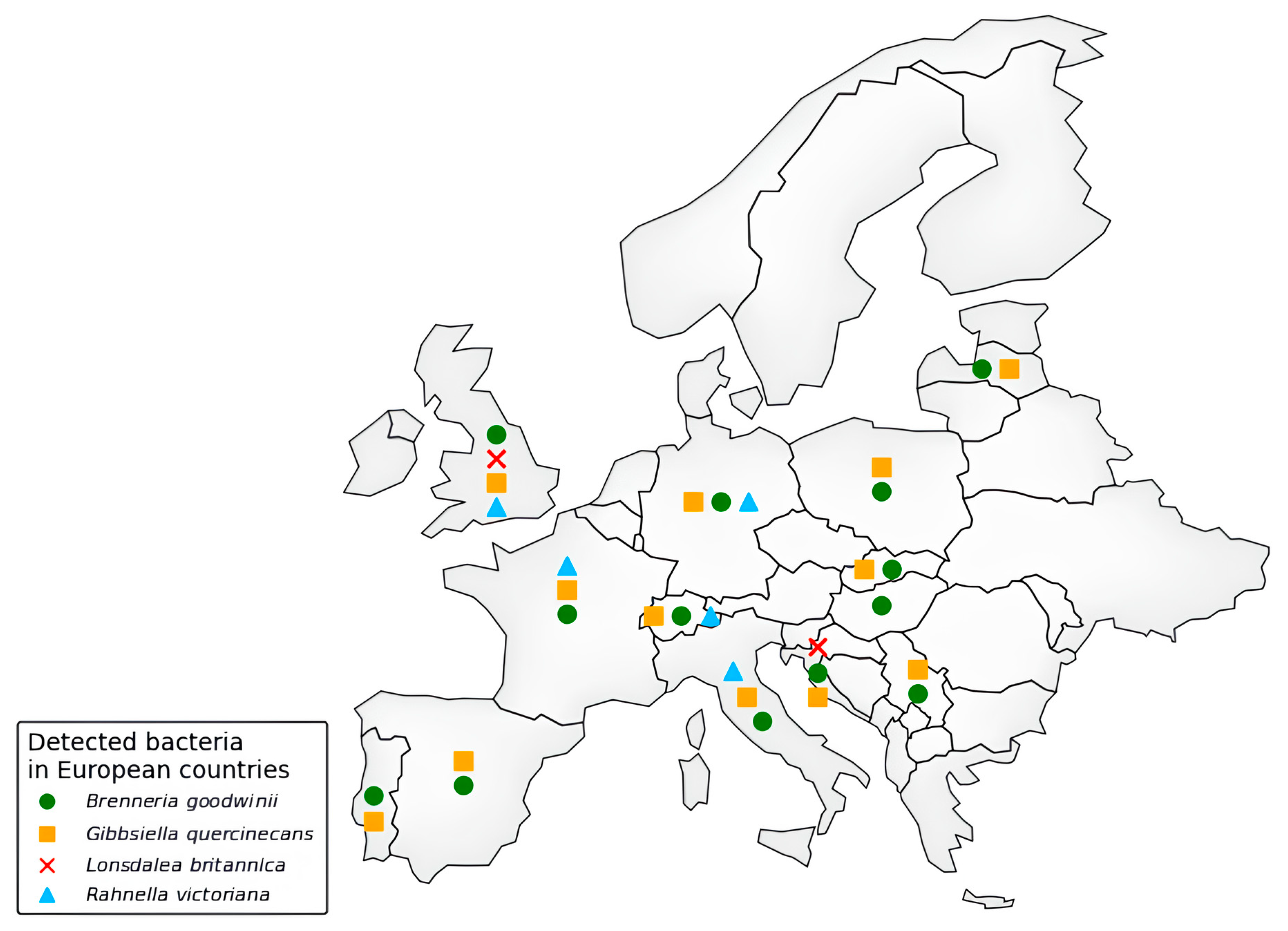
Despite so many reports of the general condition of oak decline, even particularly dated ones, AOD was first formally identified in the UK only in 2014, affecting Q. robur and Q. petraea [21,61]. Indeed, Britain offers an ideal climate pool for the development of, in particular, the bacteria associated with AOD, including B. goodwinii [20], G. quercinecans [27], R. victoriana [52], and L. britannica [70]. In this regard, Ref. [71] points out that temperature is a key factor for bacterial proliferation, indicating a threshold value of 1.19 °C, below which such bacteria and possible vectors are restricted. However, the continuous increase in average global temperatures could widen the range in which these bacteria can thrive. Indeed, recent years have seen an outflow from Great Britain and a gradual expansion into various areas across Europe (Figure 2) and, beyond Europe, into countries such as Iran [19,72]. Samples of symptomatic exudates from oak trunks were collected and analyzed to investigate this decline. Real-time PCR confirmed the presence of the bacterial complex associated with AOD. In Switzerland, B. goodwinii, G. quercinecans, and R. victoriana were identified for the first time in 2017 on Q. petraea, showing stem bleeding symptoms [63]. Similarly, in June 2017, these bacteria were detected on Q. robur in Asturias, Spain [62]. Latvia also recorded its first occurrence of B. goodwinii and G. quercinecans in 2018, affecting multiple Q. robur forest sites [12]. In Portugal, AOD symptoms were observed in March 2018 on Q. suber in Alcácer [67]. Poland reported bacterial presence in 2019 when declining Q. robur trees in the Chojnów Forest District were found to host B. goodwinii and G. quercinecans [64]. AOD has also been observed in Iran’s Caspian Hyrcanian forests, with bacterial infections confirmed in 2020 [19,28]. In Germany, a study conducted by Julius Kühn-Institut [73] confirmed the first detection of B. goodwinii, G. quercinecans, and R. victoriana in the country; the same happened in France in 2024 [16]. In 2021, Pernek et al. [74] detected the presence of B. goodwinii, G. quercinecans, and L. britannica in Croatia. Tkaczyk et al. (2025) [75] detected B. goodwinii and G. quercinecans in Serbia, and these bacteria have also been reported in weakened Q. robur stands in Slovakia [66]. Likewise, in Salento, a coastal Mediterranean region in southern Italy, Q. ilex trees exhibiting AOD-like symptoms have tested positive for AOD-related bacterial infection [14].
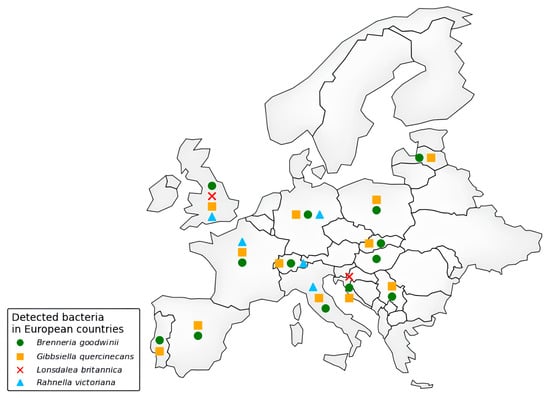
Figure 2.
Distribution of AOD-associated bacteria in European countries (according to Scopus and EPPO databases, last access 17 March 2025).
Beyond Europe, Brenneria species have been isolated from the symptomatic bark of Populus × euramericana cankers in Henan Province, China, establishing no direct link to AOD [25]. In contrast, oak decline has also been attributed worldwide to fungal pathogens such as Ophiostoma spp. and Phytophthora plurivora, which are often associated with bark-boring insect activity in the Czech Republic [9]; Erysiphe alphitoides, which appears to contribute to a reduction in the leaf canopy, along with herbivorous insects and abiotic stresses in Poland [76]; Botryosphaeriaceae species, including Diplodia spp. in California [77]; and Tubakia dryina, which causes several leaf spot diseases [78].
5. Role of Agrilus Biguttatus in AOD
The interior of oak stems is beneficial to a wide range of insects, which vary according to the tree’s stage of decay [57]. Seven insect species, including A. biguttatus, Coraebus florentinus, Coraebus undatus, Cerambyx cerdo, Platypus cylindrus, Scolytus intricatus, and Corythucha arcuata, are frequently identified in AOD diagnostics in Europe and are often associated with different microbial communities [79,80]. Notably, the genus Agrilus comprises around 3000 species and subspecies [81], among which A. biguttatus F., A. sulcicollis Lac., A. angustulus Ill., and A. viridis L. are particularly significant. However, the insect most frequently associated with AOD is A. Biguttatus Fabricius, whose presence is often confirmed following cases of extensive defoliation or severe drought [80].
In Europe, A. biguttatus is considered one of the most significant secondary pests of oak trees [35], particularly those already weakened by abiotic and other stress factors [43,71]. It appears that stressed trees produce volatiles that attract these insects [80], as xylophagous species tend to target trees with weakened defenses, thereby accelerating their decline [39,57]. Mattson et al. [82] hypothesized that this attraction may also be due to the insect’s ability to detect drought-induced ultrasonic emissions caused by the breakage of water columns in the xylem of stressed trees. The beetles seem to prefer attacking areas of the stem beneath the crown, particularly those exposed to sunlight, and they prefer trees with thicker bark, which may provide better protection from parasitoid insects [83]. Its distribution could develop as a function of precisely these microhabitats [84].
Specifically in the context of AOD, A. biguttatus is consistently associated with different species of Gram-negative bacteria [27]. Indeed, where symptoms of this disease are observed, it is common to find the insect’s exit holes, characterized by their distinctive “D” shape [61,82]. Brown and co-workers [85] observed that the disease spread to neighboring trees in a clustered pattern, suggesting a biotic way to spread. However, the role of A. biguttatus as a vector for the bacterial agents of AOD has not yet been confirmed with sufficient evidence [39]. Females can lay up to 82 eggs deep within bark crevices, from which larvae emerge and progress through four instar stages before reaching adulthood [35]. The larvae feed on the inner bark, the cambial layer, and the outer sapwood, creating several inter-crossing galleries up to 1.5 m long, and later form chambers in the outer bark, where they overwinter and pupate [83]. Preferred sites for larval development include cavities, cracks in the wood, areas of bark loss exposing the sapwood, deadwood in the tree crown, and burls [86]. In the initial stages of colonization, the larvae could remain confined by the plant, causing very little damage. The plant’s health also influences its response to the larval attack, as it possesses specific defensive strategies, such as callus formation and gallery flooding [57]. These mechanisms support wound healing by enabling compartmentalization [80]. Consequently, A. biguttatus appears to visit weakened trees for oviposition significantly more often than healthy ones [57]. However, when there is a massive infestation within the wood, bark cracks are produced, the stem becomes deformed, and the plant eventually dies [43].
According to the interpretation of AOD symptomatology, larval excavation of the xylem disrupts the movement of water and mineral nutrients. At the same time, interference with the phloem hampers the transport of assimilates and hormones synthesized in the leaves. This disruption creates nutrient deficiencies and upsets the plant’s normal water status and carbon/nitrogen balance. Nevertheless, tree death only occurs in cases of high colonization (more than 50 larvae) [83]. Adults emerge from the wood two years after oviposition to feed and mate in the canopy [57]. The external symptoms caused by A. biguttatus also attract other insect species, fungi, and bacteria, triggering cellular post-mortem reactions. These reactions lead to bleeding dark exudations and tylosis formation in xylem vessels [83]. Notably, these include B. goodwinii, G. quercinecans, and L. britannica, though their relationship with the insect remains unclear. It is uncertain whether A. biguttatus acts as an accidental vector carrying these bacteria as part of its microbiota, whether the bacteria spread between neighboring trees independently, or whether they are merely endosymbionts that take advantage of plant decay following abiotic stress and subsequent insect attack [2]. Indeed, there is evidence of their co-occurrence in the majority of cases. Still, regarding this role as a vector, there has only been one study in this regard, in which Ref. [87] identified G. quercinecans within the gut of only one A. biguttatus insect out of 20 captures.
On the other hand, no evidence has been found concerning the potential transmission of the bacterium to a plant, so we cannot yet attribute this role to the insect. Regarding fungi, the beetle has been associated with the spread of Collybia fusipes and the presence of Phytophthora spp., for which it may serve as a potential vector [83]. Finally, assessing the influence of temperature on the insect’s development cycle and its potential for spread is particularly interesting [88]. In this regard, Ref. [35] warns of the likely expansion of the insect’s range and, thus, the potential increase of AOD in Europe as climate change progresses. Higher temperatures may facilitate its spread into areas previously unsuitable for its development [52,71].
Various approaches have been implemented to control or contain the insect and mitigate the impact of AOD on oak trees. One strategy involves eliminating the insect through chemical treatments applied to infested or trapped trees [89]. However, no biological control methods have been tested to date. Additionally, preventive strategies focus on enhancing the resilience and vigor of oak trees through targeted forestry practices [80].
6. Diagnostic Tools for AOD-Associated Bacteria
The genera or species of bacteria that can be found in oaks suffering from AOD have been observed. However, identifying them from samples taken in the field is not always easy. Sampling can be complex in terms of the removal of material to be used. Still, it is not always possible to find the presence of the bacteria hypothesized, as it could depend on their concentration, the developmental state of the pathology, or trivially on the portion of material removed from the tree. Generally, the sample consists of small sections picked from the edge of necrotic and healthy bark and sapwood tissues [28]. In any case, the identification of bacteria in plant pathology is carried out using various molecular biology techniques that may consider analysis from nucleic acids, proteins, or even volatile compounds [90]. The best performing technique is that based on polymerase chain reaction (Table 3), and in particular, techniques capable of obtaining amplification and analysis of several DNA or RNA target sequences simultaneously are currently used, such as multiplex PCR [26], HRM, DNA microarray [91], and DNA fingerprinting [13]. A second approach is related to the analysis of specific proteins linked to bacterial activity, studied through enzyme-linked immunosorbent assay, flow cytometry, or immune-fluorescence [92], or even the analysis of volatile organic compounds (VOCs) through gas chromatography–mass spectrometry [93] and electronic-nose devices [94] or through the creation of hyper-spectral images that can indicate changes in the state of the plant even in the primary stages of the disease, but these approaches are still used little and not as effective in the case of AOD.

Table 3.
Primers (5′-3′) employed for qPCR to detect bacteria associated with oak decline.
In conclusion, there are also techniques based on the use of nanotechnology, particularly nanoparticles, such as array-based nano-sensors [95] or gold nanoparticles. Next comes the taxonomic classification of the pathogen identified. In particular, for prokaryotes, the analysis of the gene coding for 16S ribosomal RNA is widely used since it is highly conserved across bacterial species [96]. On the other hand, for specific genera such as Pseudomonas, it is recommended to use protein-coding genes such as gyrase beta subunit (gyrB), RNA polymerase 70 sigma factor (rpoD), and beta subunit of the RNA polymerase (rpoB), as they are less evolutionarily preserved [97]. The next and final step would then be the complete sequencing of the DNA of the specific localized bacterium, a strategy that is becoming increasingly more used and more affordable [90].
7. AOD Management Strategies
AOD is not currently listed in the EC Plant Health Directive nor on the EPPO Alert List, nor are the associated bacteria and their presumed vector, A. biguttatus. Only in the UK has this disease been listed in the Defra Plant Health Risk Register.
The eradication of AOD can be considered only in sites with a few impacted oaks. In this regard, good silvicultural practices are recommended to reduce the spread and impact of the disease. Management focuses on finding the causes and considering coordinated management actions, with no statutory measures against detected cases [98]. Silvicultural practices to limit predisposing factors such as drought or defoliation are necessary choices so that the disease does not spread and so recovery can be favored in already-affected trees. The removal and destruction of damaged bark or dead oaks due to AOD, preferably during scheduled selective thinning and not during rainy conditions, will limit the future emergence of damaging insects and their possible role as vectors of AOD-associated bacteria [61,84]. On the other hand, this choice is hotly debated. To date, it is preferable to cut down trees only if there are only a few affected individuals within a forest; otherwise, it is preferred to leave them. Pruning trees that are already infected is, on the other hand, not recommended at all [27]. Again, control of material that is moved from an infected to a non-infected area, such as firewood, should be organized and enforced [99].
Finally, another not insignificant possibility is to make the forest attractive to woodpeckers or parasitoid insects, favoring their population growth to prey on A. biguttatus, thus limiting the damage done to the bark of oak trees [100,101]. The application of insecticides is discussed, but they are only partially effective. In fact, it is noted that the application of pyrethroids can only control the insect in the outermost layer of the bark (in pupal chambers) but not the larvae, which feed deep down into the sapwood [102]. The use of systemic products has yielded more results, but their use is debated due to their impact on other species that inhabit oak wood, with related damage to the eco-systemic balance of forests [103].
Reforestation by planting new oak trees in areas affected by AOD can be considered, but with the caveat of increasing the biodiversity and thus the resilience of the affected species, rather than using only those that are already present in the area and particularly susceptible [104].
Certainly at the basis of controlling the spread of the disease is the construction of a database of the current prevalence of symptoms, bacteria, and insects associated with AOD for each country involved, and consequently, the possibility of creating predictive models of its future spread; monitoring is therefore fundamental [27]. Concerning places where AOD is not yet present, the option of stricter control on timber that is imported, especially from countries already harboring the disease, or the possible imposition of timber treatments with temperatures higher than 60 °C to eliminate the bacteria before distribution, should not be underestimated [27,96]. The possible active participation of ordinary citizens in helping to identify trees affected by AOD symptoms in forests close to urban centers should not be underestimated either, so it is good to inform and educate on the issue.
8. Conclusions
AOD is a relatively new phenomenon that is particularly complex, but its exact nature is unclear at present due to the co-participation of numerous abiotic and biotic factors. It has been pointed out that there are still several gaps in the general understanding of the disease, which increases the risk of AOD spreading undisturbed and control strategies being applied too late. In this sense, it is crucial to emphasize the importance of additional study in this field to better understand the disease’s causes and interactions, as well as the possibilities for control. In this regard, an interdisciplinary approach integrating ecology, microbiology, entomology, and forestry could prove crucial in addressing the complexity of AOD and identifying more effective solutions.
Furthermore, constant monitoring using advanced technologies, such as remote sensing, machine learning, and GIS systems, could significantly improve the ability to detect the disease early and develop predictive models that can anticipate its spread. In parallel, sustainable forest management, through tree species diversification, soil care, and the use of beneficial bio-inoculation, could contribute to increasing forest resilience and limiting the impact of AOD. Therefore, future studies should focus on analyzing the pathogenicity of the bacteria and their interaction with other species, the role of A. biguttatus in the transmission of AOD-associated bacteria, and the possible active spread of the disease, as well as the responsibility of fungal agents participating in the decline. In addition, strategies to increase the resilience of forests, which are still not considered, will need to be investigated.
Author Contributions
Conceptualization, A.B., M.V. and A.L.; bibliographic resources, A.B., L.P. and G.C.; writing—original draft preparation, A.B. and M.V.; writing—review and editing, A.G.D.D., L.D.B. and A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AOD | Acute oak decline |
| PCR | Polymerase chain reaction |
| HRM | High-resolution melting analysis |
| EPPO | European and Mediterranean Plant Protection Organization |
| VOCs | Volatile organic compounds |
References
- Mölder, A.; Meyer, P.; Nagel, R.V. Integrative Management to Sustain Biodiversity and Ecological Continuity in Central European Temperate Oak (Quercus robur, Q. petraea) Forests: An Overview. For. Ecol. Manag. 2019, 437, 324–339. [Google Scholar] [CrossRef]
- Doonan, J.M.; Broberg, M.; Denman, S.; McDonald, J.E. Host-Microbiota-Insect Interactions Drive Emergent Virulence in a Complex Tree Disease. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200956. [Google Scholar] [CrossRef]
- Mcmanus, M.L.; Liebhold, A.M.; Führer, E. Proceedings: Population Dynamics, Impacts, and Integrated Management of Forest Defoliating Insects; McManus, M.L., Liebhold, A.M., Eds.; US Department of Agriculture, Forest Service, Northeastern Research Station: Radnor, PA, USA, 1998; pp. 222–229. [Google Scholar]
- Gentilesca, T.; Camarero, J.J.; Colangelo, M.; Nolè, A.; Ripullone, F. Drought-Induced Oak Decline in the Western Mediterranean Region: An Overview on Current Evidences, Mechanisms and Management Options to Improve Forest Resilience. iForest 2017, 10, 796–806. [Google Scholar] [CrossRef]
- Nakajima, H.; Ishida, M. Decline of Quercus crispula in Abandoned Coppice Forests Caused by Secondary Succession and Japanese Oak Wilt Disease: Stand Dynamics over Twenty Years. For. Ecol. Manag. 2014, 334, 18–27. [Google Scholar] [CrossRef]
- Elliott, K.J.; Swank, W.T. Impacts of Drought on Tree Mortality and Growth in a Mixed Hardwood Forest. J. Veg. Sci. 1994, 5, 229–236. [Google Scholar] [CrossRef]
- Ragazzi, A.; Vagniluca, S.; Moricca, S. European Expansion of Oak Decline: Involved Microorganisms and Methodological Approaches. Phytopathol. Mediterr. 1995, 34, 207–226. [Google Scholar]
- Kowsari, M.; Karimi, E. A review on oak decline: The global situation, causative factors, and new research approaches. For. Syst. 2023, 32, 3. [Google Scholar] [CrossRef]
- Macháčová, M.; Nakládal, O.; Samek, M.; Baťa, D.; Zumr, V.; Pešková, V. Oak Decline Caused by Biotic and Abiotic Factors in Central Europe: A Case Study from the Czech Republic. Forests 2022, 13, 1223. [Google Scholar] [CrossRef]
- Denman, S.; Kirk, S.; Webber, J. Managing Acute Oak Decline; Forestry Commission: Farnham, UK, 2010; pp. 1–6. [Google Scholar]
- Barnes, C. Remote of Sensing Larch Disease and Acute Oak Decline in Britain. Ph.D. Thesis, University of Leicester, Leicester, UK, 2018. [Google Scholar]
- Celma, L.; Zaļkalns, O.; Šmits, A.; Legzdiņa, L.; Silbauma, L.; Ozols, J.; Kļaviņa, D.; Bokuma, G.; Ruņģis, D. Assessment of Acute Oak Decline in Latvia. Balt. For. 2024, 30, 745. [Google Scholar] [CrossRef]
- Sapp, M.; Lewis, E.; Moss, S.; Barrett, B.; Kirk, S.; Elphinstone, J.G.; Denman, S. Metabarcoding of Bacteria Associated with the Acute Oak Decline Syndrome in England. Forests 2016, 7, 95. [Google Scholar] [CrossRef]
- Carluccio, G.; Vergine, M.; Vita, F.; Sabella, E.; Delle Donne, A.; De Bellis, L.; Luvisi, A. Long-Distance Finding of AOD-Related Bacteria in the Natural Environment: Risks to Quercus ilex (L.) in Italy. Forests 2024, 15, 2055. [Google Scholar] [CrossRef]
- Barsoum, N.; A’Hara, S.W.; Cottrell, J.E.; Forster, J.; Garcia, M.S.J.; Schonrogge, K.; Shaw, L. Root Ectomycorrhizal Status of Oak Trees Symptomatic and Asymptomatic for Acute Oak Decline in Southern Britain. For. Ecol. Manag. 2021, 482, 118800. [Google Scholar] [CrossRef]
- Eichenlaub, L.; Denman, S.; Brady, C.; Maddock, D.; Robledo-Garcia, F.; Aubert, A.; Husson, C.; Robin, C. First Report of Brenneria goodwinii, Gibbsiella quercinecans and Rahnella victoriana in Declining Oaks in France. New Dis. Rep. 2024, 49, 2023–2024. [Google Scholar] [CrossRef]
- Gathercole, L.A.P.; Nocchi, G.; Brown, N.; Coker, T.L.R.; Plumb, W.J.; Stocks, J.J.; Nichols, R.A.; Denman, S.; Buggs, R.J.A. Evidence for the Widespread Occurrence of Bacteria Implicated in Acute Oak Decline from Incidental Genetic Sampling. Forests 2021, 12, 1683. [Google Scholar] [CrossRef]
- Bonham, E. Pathways of Two Bacteria Associated with Acute Oak Decline Brenneria Goodwinii and Gibsiella Quercinecans. Ph.D.Thesis, Harper Adams University, Newport, UK, 2021. [Google Scholar]
- Araeinejhad, M.H.; Falahi Chrakhabi, N.; Rahimian, H.; Brady, C. Association of Dryocola boscaweniae, Gibbsiella greigii and Gibbsiella quercinecans with Oak Decline in Iran. Eur. J. For. Res. 2024, 143, 803–811. [Google Scholar] [CrossRef]
- Denman, S.; Brady, C.; Kirk, S.; Cleenwerck, I.; Venter, S.; Coutinho, T.; De Vos, P. Brenneria goodwinii Sp. Nov., Associated with Acute Oak Decline in the UK. Int. J. Syst. Evol. Microbiol. 2012, 62, 2451–2456. [Google Scholar] [CrossRef]
- Brown, N.; Vanguelova, E.; Parnell, S.; Broadmeadow, S.; Denman, S. Predisposition of Forests to Biotic Disturbance: Predicting the Distribution of Acute Oak Decline Using Environmental Factors. For. Ecol. Manag. 2018, 407, 145–154. [Google Scholar] [CrossRef]
- Brady, C.; Arnold, D.; McDonald, J.; Denman, S. Taxonomy and Identification of Bacteria Associated with Acute Oak Decline. World J. Microbiol. Biotechnol. 2017, 33, 143. [Google Scholar] [CrossRef]
- Denman, S.; Doonan, J.; Ransom-Jones, E.; Broberg, M.; Plummer, S.; Kirk, S.; Scarlett, K.; Griffiths, A.R.; Kaczmarek, M.; Forster, J.; et al. Microbiome and Infectivity Studies Reveal Complex Polyspecies Tree Disease in Acute Oak Decline. ISME J. 2018, 12, 386–399. [Google Scholar] [CrossRef]
- Brady, C.; Orsi, M.; Doonan, J.M.; Denman, S.; Arnold, D. Brenneria goodwinii Growth in Vitro Is Improved by Competitive Interactions with Other Bacterial Species Associated with Acute Oak Decline. Curr. Res. Microb. Sci. 2022, 3, 100102. [Google Scholar] [CrossRef]
- Li, Y.; Fang, W.; Xue, H.; Liang, W.X.; Wang, L.F.; Tian, G.Z.; Wang, X.Z.; Lin, C.L.; Li, X.; Piao, C.G. Brenneria populi sp. Nov., Isolated from Symptomatic Bark of Populus X Euramericana Canker. Int. J. Syst. Evol. Microbiol. 2015, 65, 432–437. [Google Scholar] [CrossRef]
- Crampton, B.G.; Plummer, S.J.; Kaczmarek, M.; McDonald, J.E.; Denman, S. A Multiplex Real-Time PCR Assay Enables Simultaneous Rapid Detection and Quantification of Bacteria Associated with Acute Oak Decline. Plant Pathol. 2020, 69, 1301–1310. [Google Scholar] [CrossRef]
- Brady, C.; Denman, S.; Kirk, S.; Venter, S.; Rodríguez-Palenzuela, P.; Coutinho, T. Description of Gibbsiella quercinecans Gen. Nov., Sp. Nov., Associated with Acute Oak Decline. Syst. Appl. Microbiol. 2010, 33, 444–450. [Google Scholar] [CrossRef]
- Bakhshi ganje, M.; Shams-Bakhsh, M.; Mackay, J.; Rahimian, H. Identification and Characterization of Bacterial Strains Associated with Diseased Oak Trees in Northern Iran. For. Pathol. 2020, 50, 12571. [Google Scholar] [CrossRef]
- Basavand, E.; Khodaygan, P.; Doonan, J.M.; Rahimian, H. Gibbsiella Quercinecans as New Pathogen Involved in Bacterial Canker of Russian Olive. 3 Biotech 2021, 11, 286. [Google Scholar] [CrossRef]
- Araeinejhad, M.H.; Charkhabi, N.F.; Brady, C.; Rahimian, H. Reliable and Specific Detection and Identification of Brenneria goodwinii, the Causal Agent of Oak and Oriental Beech Decline. Front. For. Glob. Change 2024, 7, 1325897. [Google Scholar] [CrossRef]
- Broberg, M.; Doonan, J.; Mundt, F.; Denman, S.; McDonald, J.E. Integrated Multi-Omic Analysis of Hostmicrobiota Interactions in Acute Oak Decline. Microbiome 2018, 6, 21. [Google Scholar] [CrossRef]
- Doonan, J.; Denman, S.; Pachebat, J.A.; McDonald, J.E. Genomic Analysis of Bacteria in the Acute Oak Decline Pathobiome. Microb. Genom. 2019, 5. [Google Scholar] [CrossRef]
- Pettifor, B.J.; Doonan, J.; Denman, S.; McDonald, J.E. Survival of Brenneria goodwinii and Gibbsiella quercinecans, Associated with Acute Oak Decline, in Rainwater and Forest Soil. Syst. Appl. Microbiol. 2020, 43, 126052. [Google Scholar] [CrossRef]
- Tkaczyk, M.; Sikora, K. The Role of Bacteria in Acute Oak Decline in South-West Poland. Microorganisms 2024, 12, 993. [Google Scholar] [CrossRef]
- Reed, K.; Denman, S.; Leather, S.R.; Forster, J.; Inward, D.J.G. The Lifecycle of Agrilus biguttatus: The Role of Temperature in Its Development and Distribution, and Implications for Acute Oak Decline. Agric. For. Entomol. 2018, 20, 334–346. [Google Scholar] [CrossRef]
- Colangelo, M.; Camarero, J.J.; Borghetti, M.; Gentilesca, T.; Oliva, J.; Redondo, M.A.; Ripullone, F. Drought and Phytophthora are Associated with the Decline of Oak Species in Southern Italy. Front. Plant Sci. 2018, 871, 1595. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, U. A Conceptual Model for the Development of Phytophthora Disease in Quercus Robur. New Phytol. 2006, 171, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.S.; Romero, M.Á.; Homet, P.; Gómez-Aparicio, L. Climate Change Impact on the Population Dynamics of Exotic Pathogens: The Case of the Worldwide Pathogen Phytophthora cinnamomi. Agric. For. Meteorol. 2022, 322, 109002. [Google Scholar] [CrossRef]
- Gosling, R.H.; Jackson, R.W.; Elliot, M.; Nichols, C.P. Oak Declines: Reviewing the Evidence for Causes, Management Implications and Research Gaps. Ecol. Solut. Evid. 2024, 5, 12395. [Google Scholar] [CrossRef]
- Bashiri, S.; Abdollahzadeh, J. Taxonomy and Pathogenicity of Fungi Associated with Oak Decline in Northern and Central Zagros Forests of Iran with Emphasis on Coelomycetous Species. Front. Plant Sci. 2024, 15, 1377441. [Google Scholar] [CrossRef]
- Moradi-Amirabad, Y.; Rahimian, H.; Babaeizad, V.; Denman, S. Brenneria Spp. and Rahnella victoriana Associated with Acute Oak Decline Symptoms on Oak and Hornbeam in Iran. For. Pathol. 2019, 49, 12535. [Google Scholar] [CrossRef]
- Wargo, P.M. Consequences of Environmental Stress on Oak: Predisposition to Pathogens. Ann. Des Sci. For. 1996, 53, 359–368. [Google Scholar] [CrossRef]
- Thomas, F.M.; Blank, R.; Hartmann, G. Abiotic and Biotic Factors and Their Interactions as Causes of Oak Decline in Central Europe. For. Pathol. 2002, 32, 277–307. [Google Scholar] [CrossRef]
- Losseau, J.; Jonard, M.; Vincke, C. Pedunculate Oak Decline in Southern Belgium: A Long-Term Process Highlighting the Complex Interplay among Drought, Winter Frost, Biotic Attacks, and Masting. Can. J. For. Res. 2020, 50, 380–389. [Google Scholar] [CrossRef]
- Prado, I.; Gauli, A.; Kohl, M. Influence of climate on the growth of oaks in forest and urban sites: A dendro climatological analysis. SSRN 2022, 24, 5–7. [Google Scholar]
- Petritan, A.M.; Petritan, I.C.; Hevia, A.; Walentowski, H.; Bouriaud, O.; Sánchez-Salguero, R. Climate Warming Predispose Sessile Oak Forests to Drought-Induced Tree Mortality Regardless of Management Legacies. For. Ecol. Manag. 2021, 491, 119097. [Google Scholar] [CrossRef]
- Sohar, K.; Helama, S.; Läänelaid, A.; Raisio, J.; Tuomenvirta, H. Oak Decline in a Southern Finnish Forest as Affected by a Drought Sequence. Geochronometria 2014, 41, 92–103. [Google Scholar] [CrossRef]
- Oliva, J.; Stenlid, J.; Martínez-Vilalta, J. The Effect of Fungal Pathogens on the Water and Carbon Economy of Trees: Implications for Drought-Induced Mortality. New Phytol. 2014, 203, 1028–1035. [Google Scholar] [CrossRef]
- Haavik, L.J.; Billings, S.A.; Guldin, J.M.; Stephen, F.M. Emergent Insects, Pathogens and Drought Shape Changing Patterns in Oak Decline in North America and Europe. For. Ecol. Manag. 2015, 354, 190–205. [Google Scholar] [CrossRef]
- Camarero, J.J.; Colangelo, M.; Gazol, A.; Azorín-Molina, C. Drought and Cold Spells Trigger Dieback of Temperate Oak and Beech Forests in Northern Spain. Dendrochronologia 2021, 66, 125812. [Google Scholar] [CrossRef]
- Ahmadi, E.; Kowsari, M.; Azadfar, D.; Jouzani, G.S. Cultivable Bacteriome Dynamics in Different Persian Oak Tissues and Soil during Oak Decline Syndrome Development in Iran. Authorea 2020, 1–22. [Google Scholar] [CrossRef]
- Denman, S.; Plummer, S.; Kirk, S.; Peace, A.; McDonald, J.E. Isolation Studies Reveal a Shift in the Cultivable Microbiome of Oak Affected with Acute Oak Decline. Syst. Appl. Microbiol. 2016, 39, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Kostić, S.; Kesić, L.; Matović, B.; Orlović, S.; Stojnić, S.; Stojanović, D.B. Soil Properties Are Significant Modifiers of Pedunculate Oak (Quercus robur L.) Radial Increment Variations and Their Sensitivity to Drought. Dendrochronologia 2021, 67, 125838. [Google Scholar] [CrossRef]
- Gaertig, T.; Schack-Kirchner, H.; Hildebrand, E.E.; Wilpert, K.V. The Impact of Soil Aeration on Oak Decline in Southwestern Germany. For. Ecol. Manag. 2002, 159, 15–25. [Google Scholar] [CrossRef]
- Quine, C.P.; Atkinson, N.; Denman, S.; Desprez-Loustau, M.-L.; Jackson, R.; Kirby, K. Action Oak Knowledge Review: An Assessment of the Current Evidence on Oak Health in the UK, Identification of Evidence Gaps and Prioritization of Research Needs; Action Oak: Haslemere, UK, 2019; ISBN 9781527241930. [Google Scholar]
- Gagen, M.; Matthews, N.; Denman, S.; Bridge, M.; Peace, A.; Pike, R.; Young, G. The Tree Ring Growth Histories of UK Native Oaks as a Tool for Investigating Chronic Oak Decline: An Example from the Forest of Dean. Dendrochronologia 2019, 55, 50–59. [Google Scholar] [CrossRef]
- Brown, N.; Jeger, M.; Kirk, S.; Williams, D.; Xu, X.; Pautasso, M.; Denman, S. Acute Oak Decline and Agrilus biguttatus: The Co-Occurrence of Stem Bleeding and D-Shaped Emergence Holes in Great Britain. Forests 2017, 8, 87. [Google Scholar] [CrossRef]
- Nixon, K.C. Global and Neotropical Distribution and Diversity of Oak (Genus Quercus) and Oak Forests. Ecol. Conserv. Neotrop. Mont. Oak For. 2006, 185, 3–13. [Google Scholar] [CrossRef]
- Eaton, E.; Caudullo, G.; Oliveira, S.; de Rigo, D. Quercus robur and Quercus petraea in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; Publication Office of the European Union: Luxembourg, 2016; pp. 160–163. [Google Scholar]
- Uzun, A.; Uzun, S.P. World Oak Trees (Quercus): Silent Guardians of the Forest Ecosystems in International Studies and Evaluations in the Field of Agriculture, Forestry and Aquaculture Sciences; Özrenk, K., Bolat, A., Eds.; Serüven Yayin Evi: Ankara, Turkey, 2024; Volume 11, ISBN 9788578110796. [Google Scholar]
- Denman, S.; Brown, N.; Kirk, S.; Jeger, M.; Webber, J. A Description of the Symptoms of Acute Oak Decline in Britain and a Comparative Review on Causes of Similar Disorders on Oak in Europe. Forestry 2014, 87, 535–551. [Google Scholar] [CrossRef]
- González, A.J.; Ciordia, M. Brenneria goodwinii and Gibbsiella quercinecans Isolated from Weeping Cankers on Quercus robur L. in Spain. Eur. J. Plant Pathol. 2020, 156, 965–969. [Google Scholar] [CrossRef]
- Ruffner, B.; Schneider, S.; Meyer, J.; Queloz, V.; Rigling, D. First Report of Acute Oak Decline Disease of Native and Non-native Oaks in Switzerland. New Dis. Rep. 2020, 41, 18. [Google Scholar] [CrossRef]
- Tkaczyk, M.; Celma, L.; Ruņģis, D.E.; Bokuma, G. First Report of Brenneria goodwinii and Gibbsiella quercinecans Bacteria, Detected on Weaken Oak Trees in Poland. Balt. For. 2021, 27, 563. [Google Scholar] [CrossRef]
- Zalkalns, O.; Celma, L. The Distribution of Bacteria Gibbsiella quercinecans and Brenneria goodwinii in Oak (Quercus robur L.) Stands in Latvia. IOP Conf. Ser. Earth Environ. Sci. 2021, 875, 012033. [Google Scholar] [CrossRef]
- Tkaczyk, M.; Sikora, K.; Galko, J. First Report of Bacteria Causing Acute Oak Decline on Quercus Robur in Slovakia. Eur. J. Plant Pathol. 2024, 169, 113–120. [Google Scholar] [CrossRef]
- Fernandes, C.; Duarte, L.; Naves, P.; Sousa, E.; Cruz, L. First Report of Brenneria goodwinii causing Acute Oak Decline on Quercus suber in Portugal. J. Plant Pathol. 2022, 104, 837–838. [Google Scholar] [CrossRef]
- Carluccio, G.; Sabella, E.; Greco, D.; Vergine, M.; Delle Donne, A.G.; Nutricati, E.; Aprile, A.; De Bellis, L.; Luvisi, A. Acute and Chronic Oak Decline in Urban and Forest Ecosystems in Southern Italy. For. An Int. J. For. Res. 2024, 97, 739–749. [Google Scholar] [CrossRef]
- Biosca, E.G.; González, R.; López-López, M.J.; Soria, S.; Montón, C.; Pérez-Laorga, E.; López, M.M. Isolation and Characterization of Brenneria quercina, Causal Agent for Bark Canker and Drippy Nut of Quercus spp. in Spain. Phytopathology 2003, 93, 485–492. [Google Scholar] [CrossRef]
- Brady, C.L.; Cleenwerck, I.; Denman, S.; Venter, S.N.; Rodríguez-Palenzuela, P.; Coutinho, T.A.; De Vos, P. Proposal to reclassify Brenneria quercina (Hildebrand and Schroth 1967) Hauben et al. 1999 into a new genus, Lonsdalea gen. nov., as Lonsdalea quercina comb. nov., descriptions of Lonsdalea quercina subsp. quercina comb. nov., Lonsdalea quercina subsp. iberica subsp. nov. and Lonsdalea quercina subsp. britannica subsp. nov., emendation of the description of the genus Brenneria, reclassification of Dickeya dieffenbachiae as Dickeya dadantii subsp. dieffenbachiae comb. nov., and emendation of the description of Dickeya dadantii. Int. J. Syst. Evol. Microbiol. 2012, 62, 1592–1602. [Google Scholar] [CrossRef]
- Tkaczyk, M. Bioclimatic Variables and Their Impact on the Potential Distribution of Brenneria goodwinii in Europe. For. Pathol. 2023, 53, 12820. [Google Scholar] [CrossRef]
- Maddock, D.; Brady, C.; Denman, S.; Arnold, D. Bacteria Associated with Acute Oak Decline: Where Did They Come From? We Know Where They Go. Microorganisms 2023, 11, 2789. [Google Scholar] [CrossRef]
- Julius Kühn-Institut (JKI). Institut Für Nationale Und Internationale Angelegenheiten Der Pflanzengesundheit Notification of the Presence of a Harmful Organism–Closing Note; Julius Kühn-Institut (JKI): Quedlinburg, Germany, 2023; pp. 1–4. [Google Scholar]
- Pernek, M.; Kovač, M.; Jukić, A.; Dubravac, T.; Lacković, N.; Brady, C. Acute oak decline (AOD) new complex disease on holm oak (Quercus ilex L.) and possibilities of spread on other oak species in Croatia. Šumarski list. 2022, 146, 446. [Google Scholar] [CrossRef]
- Tkaczyk, M.; Sikora, K.; Milenković, I. First Report of Bacteria Associated With Bleeding Cankers on Oak Trees in Serbia. For. Pathol. 2025, 55, 70010. [Google Scholar] [CrossRef]
- Szewczyk, W.; Kuźmiński, R.; Mańka, M.; Kwaśna, H.; Łakomy, P.; Baranowska-Wasilewska, M.; Behnke-Borowczyk, J. Occurrence of Erysiphe alphitoides in oak stands affected by flood disaster. For. Res. Papers 2015, 76, 73–77. [Google Scholar] [CrossRef][Green Version]
- Lynch, S.C.; Eskalen, A.; Zambino, P.J.; Mayorquin, J.S.; Wang, D.H. Identification and pathogenicity of Botryosphaeriaceae species associated with coast live oak (Quercus agrifolia) decline in southern California. Mycologia 2013, 105, 125–140. [Google Scholar] [CrossRef]
- Pearce, M.; Williams-Woodward, J. Key to diseases of oaks in the landscape. In Learning for Life; University of Georgia: Washington, DC, USA, 2009. [Google Scholar]
- Hrašovec, B.; Posarić, D.; Lukić, I.; Pernek, M. First record of oak lace bug (Corythucha arcuata) in Croatia. Šumarski list. 2013, 137, 499–503. Available online: https://hrcak.srce.hr/111641 (accessed on 2 April 2025).
- Sallé, A.; Nageleisen, L.M.; Lieutier, F. Bark and Wood Boring Insects Involved in Oak Declines in Europe: Current Knowledge and Future Prospects in a Context of Climate Change. For. Ecol. Manag. 2014, 328, 79–93. [Google Scholar] [CrossRef]
- Flø, D.; Krokene, P.; Økland, B. Invasion Potential of Agrilus Planipennis and Other Agrilus Beetles in Europe: Import Pathways of Deciduous Wood Chips and MaxEnt Analyses of Potential Distribution Areas. EPPO Bull. 2015, 45, 259–268. [Google Scholar] [CrossRef]
- Mattson, W.J.; Haack, R.A. The Role of Drought in Outbreaks of Plant-Eating Insects. Bioscience 1987, 37, 110–118. [Google Scholar] [CrossRef]
- Hedde, M.; Aubert, M.; Bureau, F.; Margerie, P.; Decaëns, T. Soil detritivore macro-invertebrate assemblages throughout a managed beech rotation. Ann. For. Sci. 2007, 64, 219–228. [Google Scholar] [CrossRef]
- Vermunt, B.; Cuddington, K.; Sobek-Swant, S.; Crosthwaite, J. Cold Temperature and Emerald Ash Borer: Modelling the Minimum under-Bark Temperature of Ash Trees in Canada. Ecol. Modell. 2012, 235–236, 19–25. [Google Scholar] [CrossRef]
- Brown, N.; Jeger, M.; Kirk, S.; Xu, X.; Denman, S. Spatial and Temporal Patterns in Symptom Expression within Eight Woodlands Affected by Acute Oak Decline. For. Ecol. Manag. 2016, 360, 97–109. [Google Scholar] [CrossRef]
- Parmain, G.; Bouget, C. Large Solitary Oaks as Keystone Structures for Saproxylic Beetles in European Agricultural Landscapes. Insect Conserv. Divers. 2018, 11, 100–115. [Google Scholar] [CrossRef]
- Brown, N.; Inward, D.J.G.; Jeger, M.; Denman, S. A Review of Agrilus biguttatus in UK Forests and Its Relationship with Acute Oak Decline. Forestry 2014, 88, 53–63. [Google Scholar] [CrossRef]
- Bale, J.S.; Gerday, C.; Parker, A.; Marahiel, M.A.; Shanks, I.A.; Davies, P.L.; Warren, G. Insects and Low Temperatures: From Molecular Biology to Distributions and Abundance. Philos. Trans. R. Soc. B Biol. Sci. 2002, 357, 849–862. [Google Scholar] [CrossRef]
- Gallardo, A.; Jiménez, A.; Antonietty, C.A.; Villagrán, M.; Ocete, M.E.; Soria, F.J. Forecasting Infestation by Coraebus undatus (Coleoptera, Buprestidae) in Cork Oak Forests. Int. J. Pest Manag. 2012, 58, 275–280. [Google Scholar] [CrossRef]
- Bueno-Gonzalez, V. Towards a Rapid Diagnostic Method to Identify Bacteria Associated with AOD. Ph.D. Thesis, University of the West of England, Bristol, UK, 2022. [Google Scholar]
- López, M.M.; Llop, P.; Olmos, A.; Marco-Noales, E.; Cambra, M.; Bertolini, E. Are Molecular Tools Solving the Challenges Posed by Detection of Plant Pathogenic Bacteria and Viruses? Curr. Issues Mol. Biol. 2009, 11, 13–46. [Google Scholar] [CrossRef]
- Caruso, P.; Gorris, M.T.; Cambra, M.; Palomo, J.L.; Collar, J.; López, M.M. Enrichment Double-Antibody Sandwich Indirect Enzyme-Linked Immunosorbent Assay That Uses a Specific Monoclonal Antibody for Sensitive Detection of Ralstonia Solanacearum in Asymptomatic Potato Tubers. Appl. Environ. Microbiol. 2002, 68, 3634–3638. [Google Scholar] [CrossRef]
- Kushalappa, A.C.; Lui, L.H.; Chen, C.R.; Lee, B. Volatile Fingerprinting (SPME-GC-FID) to Detect and Discriminate Diseases of Potato Tubers. Plant Dis. 2002, 86, 131–137. [Google Scholar] [CrossRef]
- Laothawornkitkul, J.; Moore, J.P.; Taylor, J.E.; Possell, M.; Gibson, T.D.; Hewitt, C.N.; Paul, N.D. Discrimination of Plant Volatile Signatures by an Electronic Nose: A Potential Technology for Plant Pest and Disease Monitoring. Environ. Sci. Technol. 2008, 42, 8433–8439. [Google Scholar] [CrossRef]
- Li, Z.; Yu, T.; Paul, R.; Fan, J.; Yang, Y.; Wei, Q. Agricultural Nanodiagnostics for Plant Diseases: Recent Advances and Challenges. Nanoscale Adv. 2020, 2, 3083–3094. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.Y.; Lau, S.K.P.; Teng, J.L.L.; Tse, H.; Yuen, K.Y. Then and Now: Use of 16S RDNA Gene Sequencing for Bacterial Identification and Discovery of Novel Bacteria in Clinical Microbiology Laboratories. Clin. Microbiol. Infect. 2008, 14, 908–934. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Rosen, G.; Hershberg, R. Marker Genes That Are Less Conserved in Their Sequences Are Useful for Predicting Genome-Wide Similarity Levels between Closely Related Prokaryotic Strains. Microbiome 2016, 4, 18. [Google Scholar] [CrossRef]
- Denman, S.; Webber, J. Rapid PRA for Acute Oak Decline. For. Res. 2014, 1, 1–27. [Google Scholar]
- Hacck, R.A.; Petrice, T.R.; Wiedenhoeft, A.C. Incidence of Bark- and Wood-Boring Insects in Firewood: A Survey at Michigan’s Mackinac Bridge. J. Econ. Entomol. 2010, 103, 1682–1692. [Google Scholar] [CrossRef]
- Kenis, M.; Hilszczanski, J. Natural Enemies of Cerambycidae and Buprestidae Infesting Living Trees. In Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis 2007; Springer: Dordrecht, The Netherlands, 2004; ISBN 9781402022401. [Google Scholar]
- Duan, J.J.; Ulyshen, M.D.; Bauer, L.S.; Gould, J.; Driesche, R. Van Measuring the Impact of Biotic Factors on Populations of Immature Emerald Ash Borers (Coleoptera: Buprestidae). Environ. Entomol. 2010, 39, 1513–1522. [Google Scholar] [CrossRef]
- Habermann, M.; Preller, J. Studies on the biology and control of two-spotted lichen buprestid (Agrilus biguttatus Fabr.). Forst und Holz. 2003, 58, 215–220. [Google Scholar]
- Herms, D.A.; Mccullough, D.G.; Smitley, D.R.; Sadof, C.S.; Cranshaw, W. Insecticide Options for Protecting Ash Trees from Emerald Ash Borer, 3rd ed.; North Central IPM Center Bulletin, U.S. Department of Agriculture’s National Institute of Food and Agriculture: Ames, IA, USA, 2019; 16p. [Google Scholar]
- Mitchell, J.R.; Bellamy, P.E.; Ellis, C.J.; Hewison, R.L.; Hodgetts, N.G.; Iason, G.R.; Littlewood, N.A.; Newey, S.; Stockan, J.A.; Taylor, A.F.S. Collapsing foundations: The ecology of the British oak, implications of its decline and mitigation options. Biol. Conserv. 2019, 223, 316–327. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).