Knowledge and Practices of Four Onchocerciasis-Endemic Communities in Cameroon
Simple Summary
Abstract
1. Introduction
2. Methods
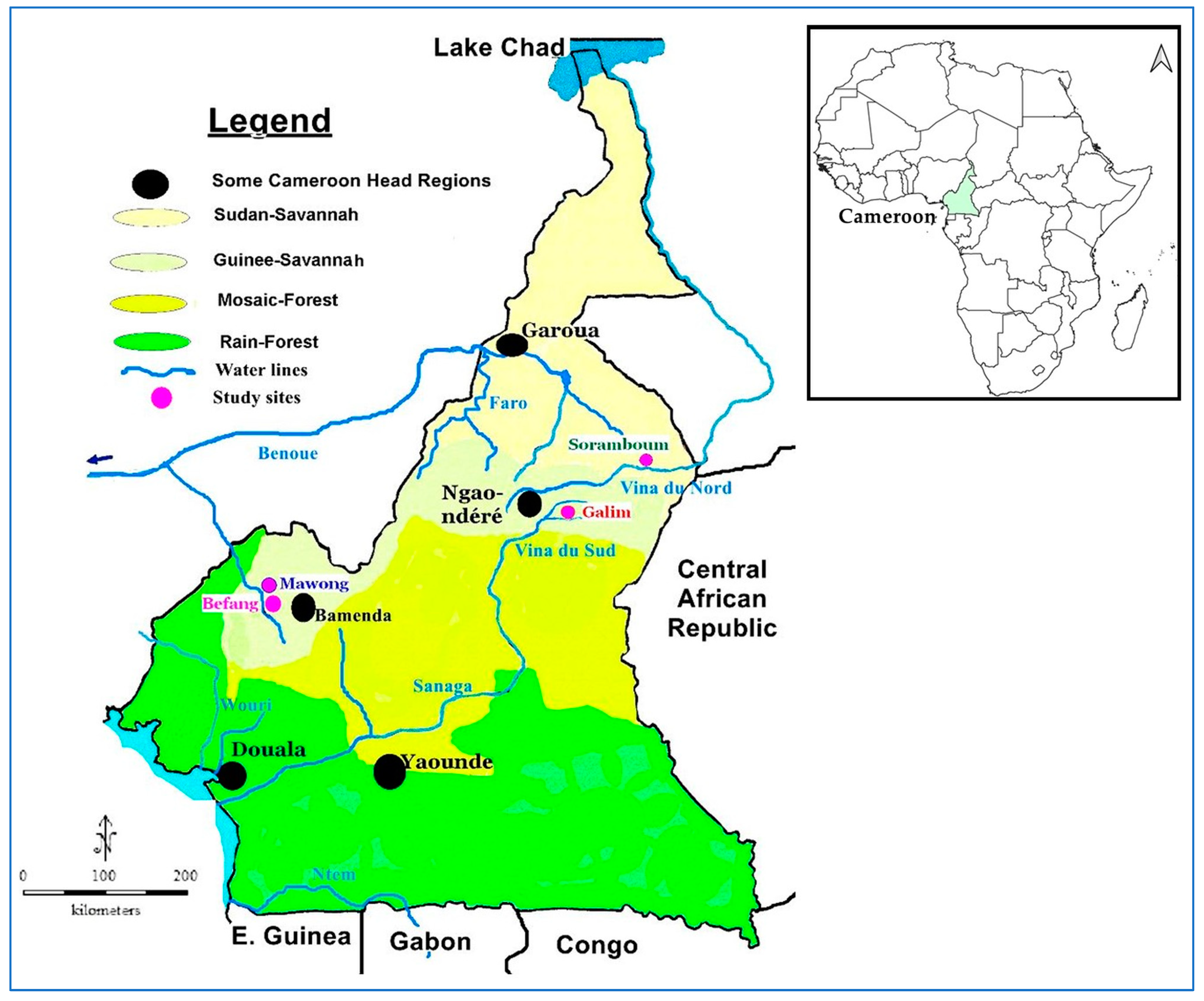
2.1. Description of Study Areas and Populations
2.2. Sampling
2.2.1. Calculation of Sample Size and Selection of Recruitment Sites
2.2.2. Community Mobilization and Data Collection
2.2.3. Ethical Approval
2.2.4. Statistical Analysis
3. Results
3.1. Sociodemographic Characteristics of Study Participants
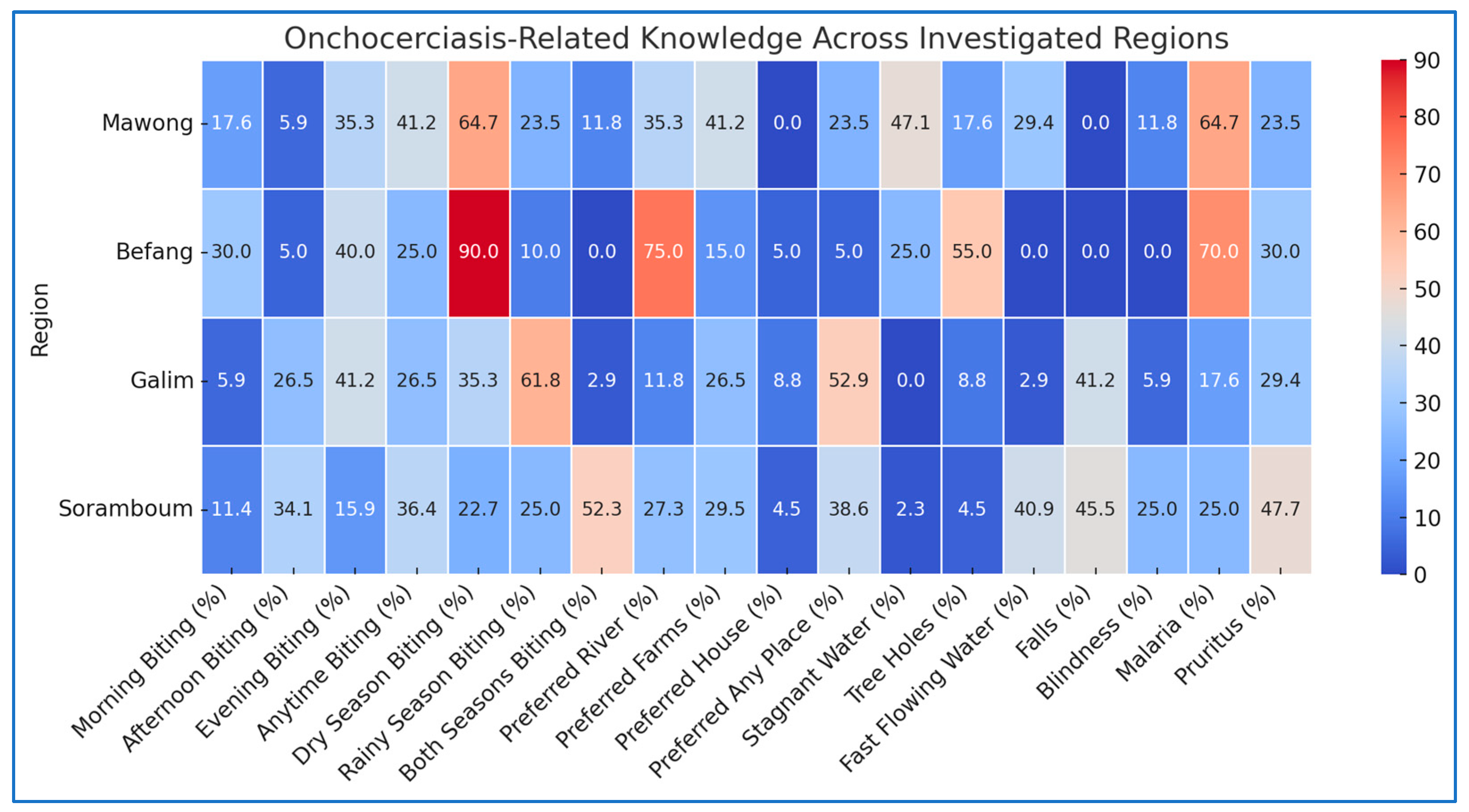
3.2. Knowledge Practice of Community Respondents Regarding Biting Activities of Black Flies
3.3. Knowledge of Community Respondents About Attractants to Black Flies
3.4. Consolidation of All Data from the Investigated Regions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gebrezgabiher, G.; Yirga, D.; Abreha, A. Reaching the Last Mile: Main Challenges Relating to and Recommendations to Accelerate Onchocerciasis Elimination in Africa. Infect. Dis. Poverty 2019, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.R.; Duke, B.O.; Muñoz, B. The Selection of Communities for Treatment of Onchocerciasis with Ivermectin. Trop. Med. Parasitol. 1992, 43, 267–270. [Google Scholar]
- Ozoh, G.A.; Boussinesq, M.; Boatin, B.A.; Remme, J.H.F.; Sékétéli, A. The African Programme for Onchocerciasis Control: Impact on Onchocercal Skin Disease. Trop. Med. Int. Health 2011, 16, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Key, H.; Karam, M.; Awadzi, K.; Albiez, E.J. Efficacy of Ivermectin in the Treatment of Concomitant Mansonella perstans Infections in Onchocerciasis Patients. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Noma, M.; Zouré, H.G.M.; Tekle, A.H.; Enyong, P.; Nwoke, B.E.B.; Remme, J.H.F. The Geographic Distribution of Onchocerciasis in the 20 Participating Countries of the African Programme for Onchocerciasis Control: (1) Priority Areas for Ivermectin Treatment. Parasites Vectors 2014, 7, 325. [Google Scholar] [CrossRef]
- Duke, B.O. The Population Dynamics of Onchocerca volvulus in the Human Host. Trop. Med. Parasitol. 1993, 44, 61–67. [Google Scholar]
- Opara, K.N.; Fagbemi, B.O. Population Dynamics of Onchocerca volvulus Microfilariae in Human Host after Six Years of Drug Control. J. Vector Borne Dis. 2008, 45, 29–37. [Google Scholar]
- Duke, B.O.; Anderson, J.; Fuglsang, H. The Onchocerca volvulus Transmission Potentials and Associated Patterns of Onchocerciasis at Four Cameroon Sudan-Savanna Villages. Trop. Med. Parasitol. 1975, 26, 143–154. [Google Scholar]
- Anderson, J.; Fuglsang, H.; Duke, B.O. Studies on Onchocerciasis in the United Cameroon Republic. IV. A Four-Year Follow-Up of Six Rain-Forest and Six Savanna Villages. The Incidence of Ocular Lesions. Trans. R. Soc. Trop. Med. Hyg. 1978, 72, 513–515. [Google Scholar] [CrossRef]
- Cheke, R.A.; Garms, R. Indices of Onchocerciasis Transmission by Different Members of the Simulium damnosum Complex Conflict with the Paradigm of Forest and Savanna Parasite Strains. Acta Trop. 2013, 125, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, M.; Post, R.J.; Krüger, A. The Cytotaxonomy and Morphotaxonomy of Simulium mengense (Diptera: Simuliidae). Ann. Trop. Med. Parasitol. 2004, 98, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Hendy, A.; Slatko, B.E.; Steen, R.G.; Brindley, P.J.; Wanji, S.; Tchioffo, M.T.; Makepeace, B.L.; Kengne-Ouafo, J.A. Onchocerca volvulus Transmission in the Mbam Valley of Cameroon Following 16 Years of Annual Community-Directed Treatment with Ivermectin, and the Description of a New Cytotype of Simulium squamosum. Parasites Vectors 2021, 14, 563. [Google Scholar] [CrossRef]
- Mustapha, M.; Cheke, R.A.; Boakye, D.A.; Post, R.J. A New Cytotype of Simulium squamosum from South-West Cameroon. Med. Vet. Entomol. 2004, 18, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Post, R.J.; Cheke, R.A.; Boakye, D.A. A Guide to the Simulium damnosum Complex (Diptera: Simuliidae) in Nigeria, with a Cytotaxonomic Key for the Identification of the Sibling Species. Ann. Trop. Med. Parasitol. 2011, 105, 277–297. [Google Scholar] [CrossRef]
- Krueger, A.; Hennings, I.C. Molecular Phylogenetics of Blackflies of the Simulium damnosum Complex and Cytophylogenetic Implications. Mol. Phylogenet. Evol. 2006, 39, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, P.O. Simulium spp., Vectors of Onchocerca volvulus: Life Cycle and Control. In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 3380–3382. [Google Scholar]
- Taylor, M.J.; Hoerauf, A.; Bockarie, M. Lymphatic filariasis and onchocerciasis. Lancet 2010, 376, 1175–1185. [Google Scholar] [CrossRef]
- Alvarez-Moreno, C.; Pardo-Turriago, R.; Rodríguez-Morales, A.J. Long-Term Consequences of the Misuse of Ivermectin Data. Lancet Infect. Dis. 2021, 21, 1624–1626. [Google Scholar] [CrossRef] [PubMed]
- Ōmura, S.; Crump, A. The Life and Times of Ivermectin—A Success Story. Nat. Rev. Microbiol. 2004, 2, 984–989. [Google Scholar]
- Domche, A.; Pion, S.D.S.; Kamga, G.R.; Nana-Djeunga, H.C.; Ntep, M.M.; Mba, R.M.; Epee, E.; Wanji, S.; Boussinesq, M. Knowledge/Perception and Attitude/Practices of Populations of Two First-Line Communities of the Centre Region of Cameroon Regarding Onchocerciasis and Black Fly Nuisance and Bio-Ecology. Parasites Vectors 2021, 14, 546. [Google Scholar] [CrossRef]
- Eisenbarth, A.; Achukwi, M.D.; Renz, A. Ongoing Transmission of Onchocerca volvulus after 25 Years of Annual Ivermectin Mass Treatments in the Vina du Nord River Valley, in North Cameroon. PLoS Negl. Trop. Dis. 2016, 10, e0004392. [Google Scholar] [CrossRef]
- Duke, B.O. Studies on Factors Influencing the Transmission of Onchocerciasis. IV. The Biting-Cycles, Infective Biting Density and Transmission Potential of Forest Simulium damnosum. Ann. Trop. Med. Parasitol. 1968, 62, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Tekle, A.H.; Elhassan, E.; Isiyaku, S.; Amazigo, U.V.; Bush, S.; Noma, M.; Cousens, S.; Abiose, A.; Remme, J.H. Impact of Long-Term Treatment of Onchocerciasis with Ivermectin in Kaduna State, Nigeria: First Evidence of the Potential for Elimination in the Operational Area of the African Programme for Onchocerciasis Control. Parasites Vectors 2012, 5, 28. [Google Scholar] [CrossRef]
- Weldegebreal, F.; Medhin, G.; Weldegebreal, B.; Legesse, M. Assessment of Community’s Knowledge, Attitude and Practice about Onchocerciasis and Community-Directed Treatment with Ivermectin in Quara District, North-Western Ethiopia. Parasites Vectors 2014, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Economy. Cameroon, Benakuma Council Development Plan Agenda of Municipality; Ministry of Economy: Yaoundé, Cameroon, 2012; Volume 1, p. 165.
- Seidenfaden, R.; Fischer, A.; Bonow, I.; Ekale, D.; Tanya, V.; Renz, A. Combined Benefits of Annual Mass Treatment with Ivermectin and Cattle Zooprophylaxis on the Severity of Human Onchocerciasis in Northern Cameroon. Trop. Med. Int. Health 2001, 6, 715–725. [Google Scholar] [CrossRef]
- Olivry, J.C. Rivers and Streams of Cameroon; ORSTOM Hydrological Monographs; Mesres-Orstom: Paris, France, 1986; Volume 9, 781p. [Google Scholar]
- Renz, A.; Wenk, P.; Anderson, J.; Fuglsang, H. Studies on the Dynamics of Transmission of Onchocerciasis in a Sudan-Savanna Area of North Cameroon. V. What Is a Tolerable Level of Annual Transmission Potential? Ann. Trop. Med. Parasitol. 1987, 81, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Wahl, G.D.; Ekale, D.; Schmitz, A. Onchocerca ochengi: Assessment of the Simulium Vectors in North Cameroon. Parasitology 1998, 116, 327–336. [Google Scholar] [CrossRef]
- Brieger, W.R.; Otusanya, S.A.; Oke, G.A.; Oshiname, F.O.; Adeniyi, J.D. Factors associated with coverage in community-directed treatment with ivermectin for onchocerciasis control in Oyo State, Nigeria. Trop. Med. Int. Health 2001, 6, 802–810. [Google Scholar]
- Yahaya, A.; Oyeyi, T. Current Status of Onchocerciasis in Tudun Wada and Doguwa Local Government Areas of Kano State. Niger. J. Parasitol. 2007, 24, 77–88. [Google Scholar]
- Yirga, D.; Negussu, N.; Dagne, H.; Taye, H. Knowledge and Belief about Cause and Prevention of Onchocerciasis in Bebeka, Southwest Ethiopia. Ethiop. J. Health Sci. 2008, 18, 3. [Google Scholar]
- Adewale, B.; Mafe, M.A.; Oyerinde, J.P. Infectivity and Transmission Dynamics of Simulium damnosum s. around Owena Dam (Ondo State). West Afr. J. Med. 1999, 18, 257–260. [Google Scholar]
- Dozie, I.N.; Onwuliri, C.O.; Nwoke, B.E. Onchocerciasis in Imo State, Nigeria. Community Knowledge and Beliefs about Transmission, Treatment and Prevention. Public Health 2004, 118, 128–158. [Google Scholar] [CrossRef] [PubMed]
- Tadele, A. Knowledge, Attitude and Practices Towards Onchocerciasis Prevention and Control in Bench Maji Zone, South Western Ethiopia. Jpn. J. Ophthalmol. 2019, 7, 8. [Google Scholar] [CrossRef]
- Renz, A.; Wenk, P. Studies on the Dynamics of Transmission of Onchocerciasis in a Sudan-Savanna Area of North Cameroon. II. Seasonal and Diurnal Changes in the Biting Densities and in the Age-Composition of the Vector Population. Ann. Trop. Med. Parasitol. 1987, 81, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Ayisi, F.; Takougang, I.; Njiokou, F.; Simo, G.; Nana-Djeunga, H.C. Onchocerciasis in the Cameroon-Chad Border Area after More than 20 Years of Annual Mass Ivermectin Distribution. Parasites Vectors 2024, 17, 219. [Google Scholar] [CrossRef]
- Renz, A.; Wenk, P. The Distribution of the Microfilariae of Onchocerca volvulus in the Different Body Regions in Relation to the Attacking Behaviour of Simulium damnosum s.l. in the Sudan Savanna of Northern Cameroon. Trans. R. Soc. Trop. Med. Hyg. 1983, 77, 748–752. [Google Scholar] [CrossRef]
- Afolabi, O.; Okaka, C.; Oke, I.; Oniya, M. Knowledge, Attitude and Perception of Onchocerciasis and Ivermectin Treatment in Idogun Community, Ondo State, Nigeria. Br. J. Med. Med. Res. 2016, 13, 1–7. [Google Scholar] [CrossRef]
- Nandha, B.; Krishnamoorthy, K. Impact of Education Campaign on Community-Based Vector Control in Hastening the Process of Elimination of Lymphatic Filariasis in Tamil Nadu, South India. Health Educ. Res. 2011, 27, 585–594. [Google Scholar] [CrossRef][Green Version]
- Okanlawon, F.A.; Osanyintolu, G.D. Pilot Training of Community Mobilizers as Health Educators to Prevent Onchocerciasis in Bugai, Kaduna State, Nigeria. Int. Q. Community Health Educ. 2011, 32, 171–176. [Google Scholar] [CrossRef]

| Capture Site | Number of Parous Flies Dissected (%) | Number of Flies Carrying Onchocerca Larvae (% of Parous Flies Infected) | |||
|---|---|---|---|---|---|
| L1 | L2 | L3 | |||
| O. v. | O. o. | ||||
| Befang | 520 (61.3) | 31 (5.96) | 9 (1.73) | 6 (1.15) | 1 (0.19) |
| Mawong | 38 (16.5) | 2 (5.26) | 0 (0.0) | 4 (10.53) | 0 (0.0) |
| Total | 558 (51.8) | 33 (5.91) | 9 (1.61) | 10 (1.79) | 1 (0.18) |
| Guinea Savannah | Sudan Savannah | p-Value | ||||
|---|---|---|---|---|---|---|
| Variables | Mawong N (%) | Befang N (%) | Galim N (%) | Soramboum N (%) | ||
| Sex | Male | 80 (58.8) | 136 (85.0) | 44 (64.7) | 46 (52.3) | |
| Female | 56 (41.2) | 24 (15.0) | 24 (35.3) | 42 (47.7) | <0.001 | |
| Age group | Less than 20 | 16 (11.8) | 104 (65.0) | 6 (8.8) | 14 (15.9) | |
| 20 to 30 | 40 (29.4) | 32 (20.0) | 28 (41.2) | 30 (34.1) | ||
| 30 to 40 | 48 (35.3) | 16 (10.0) | 10 (14.7) | 12 (13.6) | 0.002 | |
| 40 to 50 | 32 (23.5) | 8 (5.0) | 24 (35.3) | 32 (36.4) | ||
| Occupation | School children | 30 (22.1) | 16 (10) | 0 (0.0) | 0 (0.0) | |
| Sand dredging | 2 (1.5) | 94 (58.8) | 0 (0.0) | 0 (0.0) | ||
| Students | 4 (2.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Farmers | 56 (41.2) | 32 (20.0) | 20 (29.4) | 80 (90.9) | ||
| Housekeeper | 44 (32.3) | 18 (11.3) | 18 (26.5) | 2 (2.3) | <0.001 | |
| Carpenter | 0 (0.0) | 0 (0.0) | 2 (2.9) | 0 (0.0) | ||
| Motorcycle cab | 0 (0.0) | 0 (0.0) | 8 (11.8) | 0 (0.0) | ||
| Builder | 0 (0.0) | 0 (0.0) | 2 (2.9) | 0 (0.0) | ||
| Shepherd | 0 (0.0) | 0 (0.0) | 10 (14.7) | 3 (3.4) | ||
| Seamstress | 0 (0.0) | 0 (0.0) | 2 (2.9) | 0 (0.0) | ||
| Shopkeeper | 0 (0.0) | 0 (0.0) | 2 (2.9) | 0 (0.0) | ||
| Retired | 0 (0.0) | 0 (0.0) | 2 (2.9) | 3 (3.4) | ||
| Health worker | 0 (0.0) | 0 (0.0) | 2 (2.9) | 0 (0.0) | ||
| Educational Status | Primary school | 72 (52.9) | 80 (50.0) | 28 (41.2) | 46 (52.3) | |
| Secondary school | 48 (35.3) | 80 (50.0) | 2 (2.9) | 14 (15.9) | ||
| University | 16 (11.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | <0.0001 | |
| Did not attend school | 0 (0.0) | 0 (0.0) | 38 (55.9) | 28 (31.8) | ||
| Guinea Savannah | Sudan Savannah | |||||
|---|---|---|---|---|---|---|
| Variables | Mawong N (%) | Befang N (%) | Galim N (%) | Soramboum N (%) | p-Value | |
| Biting period | Morning | 24 (17.6) | 48 (30.0) | 4 (5.9) | 10 (11.4) | <0.001 |
| Afternoon | 8 (5.9) | 8 (5.0) | 18 (26.5) | 30 (34.1) | ||
| Evening | 48 (35.3) | 64 (40.0) | 28 (41.2) | 14 (15.9) | ||
| Anytime | 56 (41.2) | 40 (25.0) | 18 (26.5) | 32 (36.4) | ||
| No idea | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (2.3) | ||
| Biting season | Dry season | 88 (64.7) | 144 (90.0) | 24 (35.3) | 20 (22.7) | <0.001 |
| Rainy season | 32 (23.5) | 16 (10.0) | 42 (61.8) | 22 (25.0) | ||
| Both | 16 (11.8) | 0 (0.0) | 2 (2.9) | 46 (52.3) | ||
| Preferred biting site | River | 48 (35.3) | 120 (75.0) | 8 (11.8) | 24 (27.3) | <0.001 |
| Farms | 56 (41.2) | 24 (15.0) | 18 (26.5) | 26 (29.5) | ||
| House | 0 (0.0) | 8 (5.0) | 6 (8.8) | 4 (4.5) | ||
| Any place | 32 (23.5) | 8 (5.0) | 36 (52.9) | 34 (38.6) | ||
| Breeding sites | Stagnant water | 64 (47.1) | 40 (25.0) | 0 (0.0) | 2 (2.3) | <0.001 |
| Tree holes | 24 (17.6) | 88 (55.0) | 6 (8.8) | 4 (4.5) | ||
| fast flowing water | 40 (29.4) | 0 (0.0) | 2 (2.9) | 36 (40.9) | ||
| Grass | 0 (0.0) | 0 (0.0) | 2 (2.9) | 0 (0.0) | ||
| Not known | 8 (5.9) | 32 (20.0) | 30 (44.1) | 6 (6.8) | ||
| Falls | 0 (0.0) | 0 (0.0) | 28 (41.2) | 40 (45.5) | ||
| Preferred biting parts | Leg | 48 (35.3) | 152 (95.0) | 0 (0.0) | 4 (4.5) | <0.001 |
| Face | 0 (0.0) | 0 (0.0) | 24 (35.3) | 0(0.0) | ||
| Hands | 0 (0.0) | 0 (0.0) | 4 (5.9) | 4 (4.5) | ||
| Any exposed part | 88 (64.7) | 8 (5.0) | 40 (58.8) | 80 (91.0) | ||
| Effect of fly bite | Blindness | 16 (11.8) | 0 (0.0) | 4 (5.9) | 22 (25.0) | <0.001 |
| Malaria | 88 (64.7) | 112 (70.0) | 12 (17.6) | 22 (25.0) | ||
| Pruritus | 32 (23.5) | 48 (30.0) | 20 (29.4) | 42 (47.7) | ||
| Tuberculosis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (2.3) | ||
| No idea | 0 (0.0) | 0 (0.0) | 32 (47.1) | 0 (0.0) | ||
| Guinea Savannah | Sudan Savannah | |||||
|---|---|---|---|---|---|---|
| Variables | Mawong N (%) | Befang N (%) | Galim N (%) | Soramboum N (%) | p-Value | |
| Dress colour | White | 32 (23.5) | 24 (15.0) | 12 (17.6) | 14 (15.9) | <0.001 |
| Black | 24 (17.6) | 24 (15.0) | 34 (50.0) | 42 (47.7) | ||
| Red | 0 (0.0) | 64 (40.0) | 4 (5.9) | 6 (6.8) | ||
| All colours | 80 (58.8) | 48 (30.0) | 18 (26.5) | 26 (29.5) | ||
| Body size | Fat | 16 (11.8) | 16 (10.0) | 17 (25.0) | 24 (27.3) | <0.001 |
| Slim | 8 (5.9) | 8 (5.0) | 2 (2.9) | 12 (13.6) | ||
| All | 88 (64.7) | 136 (85.0) | 49 (72.1) | 48 (54.5) | ||
| Not known | 24 (17.6) | 0 (0.0) | 0 (0.0) | 4 (4.5) | ||
| Height | Short | 0 (0.0) | 0 (0.0) | 108 (14.7) | 10 (11.4) | <0.001 |
| Tall | 8 (5.9) | 16 (10.0) | 6 (8.8) | 14 (15.9) | ||
| All | 104 (76.5) | 144 (90.0) | 38 (55.9) | 62 (70.5) | ||
| Not known | 24 (17.6) | 0 (0.0) | 14 (20.6) | 2 (2.3) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierre, K.; Flore, N.N.; Archile, P.; Alfons, R. Knowledge and Practices of Four Onchocerciasis-Endemic Communities in Cameroon. Microorganisms 2025, 13, 736. https://doi.org/10.3390/microorganisms13040736
Pierre K, Flore NN, Archile P, Alfons R. Knowledge and Practices of Four Onchocerciasis-Endemic Communities in Cameroon. Microorganisms. 2025; 13(4):736. https://doi.org/10.3390/microorganisms13040736
Chicago/Turabian StylePierre, Kamtsap, Nguemaïm Ngoufo Flore, Paguem Archile, and Renz Alfons. 2025. "Knowledge and Practices of Four Onchocerciasis-Endemic Communities in Cameroon" Microorganisms 13, no. 4: 736. https://doi.org/10.3390/microorganisms13040736
APA StylePierre, K., Flore, N. N., Archile, P., & Alfons, R. (2025). Knowledge and Practices of Four Onchocerciasis-Endemic Communities in Cameroon. Microorganisms, 13(4), 736. https://doi.org/10.3390/microorganisms13040736







