Abstract
Pelvic inflammatory disease (PID), although traditionally viewed as a sexually transmitted infection (STI), can also result from non-sexually transmitted microorganisms that display distinct epidemiologic and clinical characteristics. Unlike STI-related PID, these infections are less influenced by sexual behavior, often show a bimodal age distribution, and are linked to bacterial vaginosis (BV)-associated dysbiosis, iatrogenic uterine procedures, postpartum states, or inadequate access to timely screening and care. Non-STI-related PID is usually polymicrobial, predominantly involving BV-associated vaginal, enteric, or urinary commensals that ascend into the upper genital tract, while respiratory tract organisms, mycobacteria, and biofilm-associated pathogens may also play a role. Pathophysiological mechanisms include disruption of the endocervical barrier, mucus degradation, biofilm formation, hematogenous or iatrogenic seeding, and chronic cytokine-mediated inflammation and fibrosis. Clinical manifestations range from asymptomatic/subclinical disease to acute pelvic pain and tubo-ovarian abscess (TOA) and can progress to systemic infection and sepsis. Diagnosing non-STI PID is challenging due to nonspecific symptoms, negative STI tests, and inconclusive imaging findings, while management relies on broad-spectrum antimicrobials with surgery as needed. Given these complexities, this review aims to synthesize current knowledge on non-STI-related PID, clarify key considerations for its diagnosis, management, and prevention, and outline future perspectives to improve clinical outcomes.
1. Introduction
Pelvic inflammatory disease (PID) represents a spectrum of infectious and inflammatory conditions affecting the upper female genital tract. It includes endometritis, salpingitis, tubo-ovarian abscess (TOA), and pelvic peritonitis, occurring alone or in combination [1]. Traditionally, PID is considered a sexually transmitted disease (STD) affecting individuals of reproductive age [2]. It is more common among younger women with multiple sexual partners, due to increased prevalence of sexually transmitted pathogens, such as Chlamydia trachomatis and Neisseria gonorrheae and sexual behaviors that increase exposure risk [3]. A study addressing the global burden of PID has reported that, in 2019, the age-standardized prevalence of PID was 53.19 per 100,000 population, emphasizing its continued impact on reproductive-aged women worldwide [4], whereas other studies similarly reported a high prevalence of PID in young individuals [5].
Surprisingly, recent data reveal that, the percentages of Chlamydia trachomatis (C. trachomatis) and Neisseria gonorrhoeae (N. gonorrhoeae) among women with PID are lower than expected in certain patient groups, while other pathogens are more prevalent [6]. This aligns with reports of pediatric and sexually inactive individuals with PID, in whom Escherichia coli and Streptococcus species were the predominant pathogens identified [7], highlighting that PID can occur even in the absence of sexual activity [7,8].
Since PID often leads to long-term complications such as chronic pelvic pain, infertility, and ectopic pregnancy [9,10,11], it is essential for clinicians to recognize and evaluate cases early enough, so as to initiate timely management and reduce the risk of adverse outcomes, especially in cases that are not due to sexually transmitted infections (STIs) as might be expected. This review aims to investigate the pathophysiology and underlying mechanisms of non-STI-related PID, the differences in clinical presentation compared to STI-related cases, the associated diagnostic and management challenges, and potential avenues for future research and improved clinical strategies.
2. Epidemiology
PID is strongly influenced by behavioral risk factors, showing a clear association with non-STI-related PID. Vaginal douching, for example, increases risk by disrupting protective lactobacilli in the vaginal microbiota [12]. In contrast to STI-associated PID, sexual behaviors demonstrate only a weak correlation with non-STI-related cases, a distinction of importance for preventive strategies. Emerging evidence also suggests an association between gastrointestinal health and PID, with higher incidence observed among women with inflammatory bowel disease (IBD) or those with frequent antibiotic use [13]. Major risk factors also include having multiple sexual partners, not using barrier contraception, and being under 25 years of age. The chance of recurrence is increasing following previous PID incidents, due to the preceding tubal damage, maintaining a cycle of ongoing inflammation and infertility [14,15,16,17].
Non-STI PID accounts for approximately 15% of total PID cases, although this proportion may vary across world regions [18]. In high-income countries (Gross National Income per capita > $13,935), studies indicate that 55–62% of PID cases are not attributable to commonly prevalent sexually transmitted pathogens, including C. trachomatis, N. gonorrhoeae, or M. genitalium (UK: 61.8%; Australia: 55%), highlighting a possibly substantial burden of non-STI PID [19,20,21]. In contrast, low-income countries (Gross National Income per capita ≤ $1135) face limited access to diagnostic testing for STIs, likely resulting in underdiagnosis of sexually transmitted PID and a higher observed proportion of non-STI PID [21,22]. Additionally, unsafe gynecological procedures in low-income countries, including an estimated 21.6 million unsafe abortions annually, contribute significantly to PID in these regions, causing approximately 68,000 maternal deaths, 5 million hospital admissions, and 1.7 million cases of secondary infertility each year. These data underscore clear epidemiological disparities in non-STI PID between high- and low-income countries [23].
Geographically, non-STI-related PID prevalence has also been related to bacterial vaginosis (BV) prevalence (higher in Black and Hispanic women in the U.S.) and limited healthcare access in regions with poor STI screening. Approximately 45% of women with BV show subclinical endometritis [14].
Epidemiological patterns specific to age demonstrate significant differences between STI and non-STI-related PID. STI-related PID mainly impacts sexually active women between 15–24 years, whereas non-STI-related PID shows a bimodal age distribution [16,17]. Girls under 15 may sometimes experience PID due to hematogenous spread from gastrointestinal infections such as appendicitis [24], while women aged ≥35 years are more likely to develop PID due to BV, intrauterine device (IUD) use, and postpartum infections [12,16,17]. Ιt is noted that, PID cases requiring hospitalization are more likely to involve enteric bacteria (e.g., E. coli, Bacteroides, etc.) and abscess formation, suggesting non-STI-related etiologies drive severe disease [25,26].
Temporal patterns, however, indicate changing epidemiology. Analysis of PID cases in the U.S. from 2006 to 2016 revealed that, although STI-related PID dropped significantly (6.5% per year in younger women), non-STI PID showed a slower decline (1.4% per year in women aged 30 and older). The shift toward a higher proportion of non-STI PID intensified after 2012, coinciding with increased IUD usage and stable BV prevalence, suggesting that non-STI-related PID may represent a growing proportion of total cases in developed countries [16].
Certain medical procedures, particularly IUD insertion, are associated with a transient increase in PID risk, most notably within the first 21 days post-placement, likely due to the introduction of bacteria during the procedure. An important distinction emerges in etiology: while STIs account for the majority of PID cases among non-IUD users, IUD-associated PID more frequently occurs in women without STIs, suggesting alternative mechanisms, such as ascending vaginal flora or bacteria introduced at the time of insertion. Despite this short-term risk, the overall long-term risk of PID among IUD users remains low. These findings underscore the importance of STI screening prior to IUD placement to avoid introducing an additional risk factor for PID [14,27]. Hence; findings are consistent with current guidelines recommending STI screening prior to IUD insertion to mitigate the risk of PID, yet they also challenge the assumption that IUD-associated PID occurs exclusively in the presence of STIs [28]. It is important to consider that non-STI pathogens, such as bacteria associated with BV, may contribute to PID in IUD users, particularly among those who test negative for common STIs [29].
Surgical interventions, such as hysteroscopy and curettage, may disrupt the cervical mucosal barrier, facilitating the ascent of vaginal flora and subsequent infection. Procedures that breach the endocervical canal, including hysterosalpingography (HSG), sonohysterography, IUD insertion, and endometrial sampling, are classified as clean-contaminated. Despite the absence of routine antimicrobial prophylaxis, the overall risk of infection following these interventions remains low. However, specific circumstances—such as a prior history of PID or abnormal tubal anatomy detected during HSG or laparoscopic chromopertubation—are associated with an elevated risk of postoperative PID or endometritis, warranting perioperative precautions and targeted antimicrobial prophylaxis. Antimicrobial regimens should be selected with consideration for the polymicrobial etiology of these infections. Although uncommon, PID following HSG occurs in approximately 1.4–3.4% of cases and may represent a serious complication [30,31]. Patients with dilated fallopian tubes identified during HSG demonstrate a significantly increased incidence of post-procedural PID, reported at approximately 11% [32].
3. Microbiology
Three primary categories of pathogens linked to PID exist, which can overlap: (1) sexually transmitted pathogens (such as N. gonorrhoeae, C. trachomatis, Mycoplasma genitalium (M. genitalium), Trichomonas vaginalis), (2) bacteria associated with BV (like BVAB3, Prevotella bivia, Atopobium vaginae, Leptotrichia/Sneathia spp.), and (3) bacteria from the gastrointestinal (GI) or respiratory tract (for instance, anaerobes, as well as facultative and aerobic bacteria such as Haemophilus influenzae (H. influenzae), E. coli, Bacteroides [14,15].
Microbiological research indicates that 25–30% of PID cases with non-STI pathogens can be associated with anaerobic bacteria of BV, as well as genital Mycoplasmas and Ureaplasmas, especially in women with vaginal dysbiosis with decreased Lactobacillus predominance [33,34]. Anaerobes associated with BV, such as Prevotella, Atopobium are the most prevalent, found in 25–62% of instances. Moreover, pathogens linked to BV, including respiratory pathogens like H. influenzae, S. pneumoniae, and S. aureus have been associated with acute PID [12,35].
Enteric organisms such as E. coli and Bacteroides fragilis represent 18–22% of incidents, especially after gynecological procedures. E. coli, Enterococcus faecalis, and Bacteroides species are important in around 20–30% of PID instances, especially in cases that are chronic, recurrent, or post-procedural. These organisms probably access the upper genital tract via ascending transmission from the rectal/vaginal region, hematogenous spread (particularly postpartum), or iatrogenic introduction during gynecological procedures [36]. Genital mycoplasmas (M. hominis), Ureaplasma urealyticum are found in 6–12% of instances, whereas new pathogens such as Fusobacterium are involved in chronic PID [15,37].
Other non-STI pathogens show specific epidemiologic patterns: Fusobacterium nucleatum is identified in 28–34% of chronic PID cases and in 42% of STI-negative women, while Actinomyces israelii is strongly linked to prolonged IUD use (>2 years) and associated with granulomatous pelvic disease. In low-resource or endemic regions, genital tuberculosis remains a significant but often overlooked cause, typically affecting women under 40 and disproportionately impacting non-Hispanic Black patients and those with alcohol abuse behaviors [38,39]. Female genital tract tuberculosis (FGTB) due to Mycobacterium Tuberculosis (M. tuberculosis) is a form of genitourinary TB, usually secondary to pulmonary disease via hematogenous spread, accounting for 5% of all pelvic female infections [40]. FGTB should always be considered in low- and middle-income or other high-TB-burden settings when evaluating women with infertility, chronic pelvic pain, abnormal uterine bleeding, or adnexal masses [41].
Notably, available data from cohort studies of non-sexually active adolescent females with PID/TOA reveal a predominance of enteric and urinary pathogens, most commonly E. coli, Bacteroides spp. and Streptococcus spp., with N. gonorrheae and chlamydia typically not detected [42].
Despite increasing recognition of non-STI pathogens in PID, data on their true prevalence remain fragmented, and no systematic review has comprehensively synthesized their epidemiology and clinical patterns. For this purpose, a literature search was conducted by three independent reviewers over a 30-year period (1995–2025) to identify cases or case series of individuals with PID or TOAs associated with non-sexually transmitted organisms. The search was performed in PubMed using the following MeSH terms: pelvic inflammatory disease, tubo-ovarian abscess, endometritis, non-sexually transmitted infections, Actinomyces, Mycobacterium tuberculosis, non-sexually active, oophoritis, salpingitis, Escherichia coli, anaerobes. Because our review focused specifically on individual case reports and case series, PubMed was chosen as the optimal source, as it contains the largest curated collection of peer-reviewed medical case reports globally. Inclusion criteria required that cases presented with clinical symptoms and a diagnosis consistent with non-STI-related PID, and that the causative microorganism was identified in each report. The presence of an abstract was also mandatory for eligibility. Exclusion criteria included PID associated with sexually transmitted pathogens, as well as reports without an abstract or without identification of a specific causative microorganism. The identification, screening, and inclusion of reports were conducted according to the PRISMA framework, as depicted in the Supplementary Materials, Figure S1. All records and full-text reports were screened by three reviewers working independently, with disagreements resolved by consensus. No automation tools were used in any stage of the screening or selection process. Duplicate records were identified and removed using manual review. Articles that were not published in English or did not involve human subjects were excluded from screening to ensure relevance and applicability to clinical practice, whereas an additional 3814 articles did not meet the specific criteria of our search, resulting in 510 reports that were assessed for eligibility. From the 510 reports, studies were further excluded for the following reasons: no abstract available (n = 111), unrelated title (n = 110), no microorganism identified (n = 131), among others, resulting in the final set of 159 reports, case series and cohort studies included in the review. Given the descriptive nature of case reports and the heterogeneity of reported data, meta-analysis was not feasible, and findings were synthesized narratively.
Table 1 summarizes microbiology of non-STI-related PID cases identified in literature since 1995 (Methodology in Supplementary Materials, Figure S1).

Table 1.
Case reports and case series regarding non-STI PID.
When evaluating the proportions as shown in the pie chart (Figure 1), Actinomyces spp. represented the largest share of cases (22.01%), followed by Mycobacterium spp. (9.43%), GAS (8.81%), E. coli (8.18%), and polymicrobial infections (7.55%). Less frequent isolates included Enterobius vermicularis (3.77%), CMV, and S. pneumoniae (each 3.16%), while the remaining 33.96% comprised various microorganisms in smaller proportions.
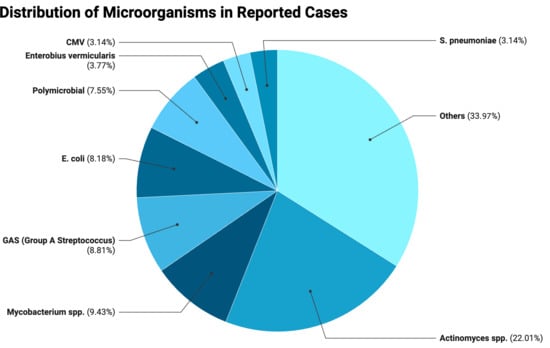
Figure 1.
Distributions of microorganisms in reported cases.
When interpreting these results, certain limitations should be taken into account. The search was restricted to PubMed, which may have led to exclusion of relevant reports indexed in other databases. The evidence base consists predominantly of case reports and small case series, making the findings susceptible to publication bias and selective reporting. Because the presence of an abstract was required for eligibility, some potentially relevant non-indexed reports may have been excluded. Moreover, case reports do not provide denominators, preventing any estimation of true prevalence or incidence. Finally, limiting inclusion to English-language publications may have introduced language bias and underrepresented data from regions where non-STI etiologies, such as tuberculosis, are more common.
Thus, consistent with the findings of our review, Actinomyces species represent a notable non-STI pathogen associated predominantly with chronic PID. Their prevalence is particularly higher among IUD users, with reported rates reaching approximately 2–4% in screened populations and occurring more frequently after prolonged device use [197]. Colonization rates may be even higher, as up to 7% of IUD users can have Actinomyces-like organisms detected on cervical cytology, although this finding has limited predictive value for true infection, making it difficult to determine the real prevalence of clinically significant disease [198]. However, the notably higher proportion of Actinomyces observed in our review should be interpreted with caution, as rare pathogens are often overrepresented in case reports, which tend to emphasize unusual or atypical presentations. In the same line, although FGTB accounts for a substantial proportion of PID cases in endemic regions, often presenting with infertility [199], the proportion observed in our review is higher because it reflects only the distribution among non-STI-related PID cases rather than the prevalence in the general PID population. Further well-designed cohort studies focusing exclusively on non-STI PID are needed to more accurately characterize its epidemiology and clinical burden.
4. Pathophysiology
PID results from disruption of the endocervical barrier, normally protecting the sterile upper genital tract from the polymicrobial vaginal environment [14,15]. Classically, Chlamydia trachomatis and Neisseria gonorrhoeae ascend during menses, damaging the tubal epithelium, destroying cilia, and triggering edema and inflammatory infiltration, which lead to obstruction, infertility, or ectopic pregnancy [200,201,202]. However, non-STI pathogens are increasingly recognized as key drivers of PID, often acting synergistically with or independently of STIs [14,15]. BV-associated anaerobes such as Gardnerella vaginalis, Prevotella, and Atopobium degrade cervical mucus, weaken mucosal defenses, and fuel cytokine-mediated fibrosis [203,204,205], while M. genitalium, Ureaplasma, and M. hominis exploit immune evasion and biofilm formation to sustain chronic inflammation [34,200,201,202,206]. Enteric bacteria including E. coli, Bacteroides, and Enterococcus faecalis reach the adnexa through ruptured appendicitis, diverticulitis, or intra-abdominal abscesses, frequently causing TOAs [207,208]. Other non-STI agents include Fusobacterium nucleatum, which invades via E-cadherin, suppresses immune clearance, and forms resistant biofilms [37,209,210], and Actinomyces israelii, particularly in long-term IUD users, which produces granulomatous inflammation with sulfur granules, fibrosis, and fistulae [71,72,211,212]. In endemic settings, M. tuberculosis disseminates hematogenously to the pelvis, causing granulomatous salpingo-oophoritis and peritonitis. Unlike STI-driven PID, which typically presents acutely with ascending infection, non-STI PID is often polymicrobial, insidious, and characterized by biofilm formation, host–pathogen synergy, and chronic tissue remodeling [213]. Collectively, these mechanisms explain why PID can occur in women without classical STIs and underscore the need to distinguish STI from non-STI-associated disease. Figure 2 summarizes main pathophysiological mechanisms implicated in PID with emphasis in non-STI PID.
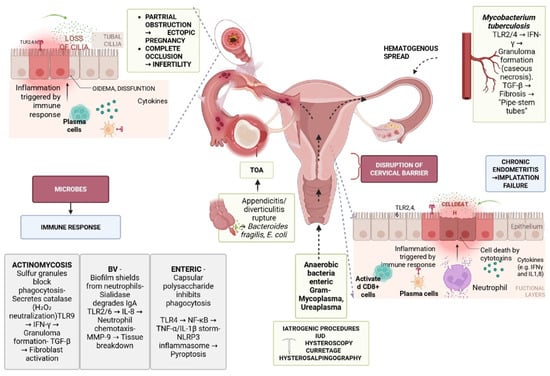
Figure 2.
Pathophysiology of PID (TOA: tubo-ovarian abscess; TLR: Toll-like receptor, IFN-γ; interferon gamma; TGF-β: transforming growth factor beta; IL-8: interleukin-8; IUD: intrauterine device; E. coli: Escherichia coli; MMP-9: matrix metalloproteinase-9; IL-1: interleukin-1; IgA: immunoglobulin A; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells).
5. Clinical Manifestations
PID covers a broad range of clinical manifestations. A substantial proportion of women diagnosed with PID exhibit no clinical signs of genital tract infection, while others become aware of the condition only after a diagnosis of tubal factor infertility. Acute symptomatic PID presents with a broad spectrum of clinical manifestations, ranging from mild, nonspecific pelvic discomfort to severe pain associated with TOAs [214]. The progression of the presentation is generally rapid over a few days, although a slower presentation may occur over weeks to months.
STI-related PID is characterized by acute symptomatic onset, typically presenting as the sudden development of lower abdominal or pelvic pain. Symptoms rarely persist beyond two weeks and are commonly accompanied by pelvic organ tenderness and clinical evidence of genital tract inflammation [1,215,216,217]. Most females with PID experience mild to moderate disease, with only a small percentage developing peritonitis or pelvic abscess, typically presenting with more intense pain, increased tenderness on examination, dyspareunia and systemic symptoms [14,218]. Additional nonspecific symptoms may include increased urinary frequency and abnormal vaginal discharge. When comparing chlamydia-associated PID with M.genitalium–associated PID, patients generally reported similar clinical manifestations; however, cases attributed to M.genitalium demonstrated lower rates of postcoital bleeding but greater lower abdominal tenderness [202].
Of particular note, it is estimated that a large proportion of cases are associated with chronic PID, a slowly progressive form of the condition typically characterized by low-grade fever, weight loss, and persistent abdominal discomfort, and reported predominantly in cases with non-sexually transmitted etiologies [219]. Pelvic actinomycosis manifests as chronic pelvic pain, weight loss, and mass-like lesions that can mimic malignancy or tuberculosis [46,48,51,65]. Similarly, Candida albicans–associated TOAs can develop months to years after IUD placement. Tuberculous and polymicrobial endometritis typically cause secondary amenorrhea, infertility, abnormal bleeding, pelvic pain, and fever [105,115,132,133,138,180].
Certain rare presentations may be misleading or severe. Xanthogranulomatous inflammation can simulate ovarian cancer, presenting with atypical abdominal pain and a pelvic mass. Severe systemic presentations include sepsis or toxic shock syndrome due to Streptococcus pyogenes or Fusobacterium, and in immunocompromised or postmenopausal women, E. coli or CMV-related abscesses may occur [76,84,86,91,92,94,103,108,110,125,130,131,180,181].
Postpartum infections constitute another subgroup. They often present with fever, abdominal pain, or sepsis, commonly caused by Eggerthella lenta, GAS, or CMV, while cesarean-associated cases may involve uterovesical abscesses [89,90,111,122,143,150,182].
6. Diagnosis
Diagnosis of PID is challenging due to the wide variation in clinical presentation, many women have mild, nonspecific, or no symptoms and because it is primarily a clinical diagnosis. Moreover, in cases of non-STI-related PID, the absence of laboratory-confirmed cervical infection with N. gonorrhoeae or C. trachomatis further complicates the diagnostic process [1]. A differential diagnosis for PID should include conditions that can present with similar lower abdominal or pelvic pain and associated symptoms. These include ectopic pregnancy, ruptured ovarian cyst, adnexal torsion, and endometriosis. Urinary tract infections, such as cystitis or pyelonephritis, as well as gastrointestinal conditions like appendicitis, diverticulitis, or irritable bowel syndrome, should also be considered. Additionally, traumatic injury to the pelvic region may mimic the clinical presentation of PID and should be evaluated [220].
6.1. Medical History and Physical Examination
The assessment of medical history in cases of PID frequently emphasizes sexually transmitted risk factors, such as age below 25 years, multiple sexual partners, a partner with a known STI or related symptoms, early initiation of sexual activity, previous STIs, inconsistent use of barrier contraception, and the presence of bacterial vaginosis. However, these parameters may be of limited value in identifying non-STI-related PID [220].
In addition, other risk factors have been reported. The insertion of an IUD, particularly within the first three weeks post-insertion, has been associated with an increased risk of PID [221]. A detailed surgical history is also essential, as actinomycosis unrelated to IUD use is almost invariably linked to prior surgical procedures [222]. Furthermore, previous bowel surgery has been identified as a risk factor for PID in individuals without sexual activity [223].
According to the Centers for Disease Control and Prevention (CDC), the minimum clinical diagnostic criteria for PID include cervical motion tenderness, uterine tenderness, or adnexal tenderness. These criteria primarily apply to sexually active women at risk of STIs [1]. Consequently, their specificity is reduced in cases of non-STI-related PID. Patients with non-STI-related PID commonly present with lower abdominal pain, fever, and gastrointestinal disturbances, and may report a history of urinary tract infections, congenital anomalies, or appendicitis [7]. Additional symptoms may include bilateral lower abdominal pain, dyspareunia, abnormal uterine bleeding, menorrhagia, and abnormal vaginal or cervical discharge, often associated with cervicitis, endometritis, or BV [215].
6.2. Diagnostic Workup
No single physical or laboratory finding demonstrates both high sensitivity and specificity for the accurate diagnosis of acute PID. Although combining multiple diagnostic criteria may improve sensitivity—thereby increasing the detection of true positive cases—or specificity—enhancing the exclusion of false positives—such improvements are typically achieved at the expense of the other parameter. [1].
Serum inflammatory markers such as erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and white blood cell count (WBC) have limited diagnostic specificity and are often normal in mild or moderate cases of PID; thus, they serve only a supportive role in diagnosis [215]. All patients should undergo nucleic acid amplification tests (NAATs) [224], including real-time PCR from endocervical swabs to detect C. trachomatis, N. gonorrhoeae and M. genitalium. Additionally, serological tests for T.pallidum, HIV, as well as a pregnancy test to rule out ectopic pregnancy are recommended [215].
Endometrial cultures may provide useful adjunctive information in patients with clinically suspected acute disease, while laparoscopic evaluation with intra-abdominal bacterial cultures can be valuable in severe cases, both for diagnostic confirmation and for guiding targeted antimicrobial therapy [225,36].
Other laboratory tests, including tumor markers such as Cancer Antigen 125 (CA-125), may also contribute to assessing the extent and potential complications of the disease [226,227].
6.3. Microscopy
Microscopic examination of vaginal and endocervical secretions may reveal WBCs or mucopurulent cervical discharge, findings that can suggest PID but lack diagnostic specificity. In contrast, the absence of pus cells or WBCs on a saline wet mount has a high negative predictive value (approximately 95%), making a diagnosis of PID unlikely [1,215].
6.4. Imaging
6.4.1. Ultrasound
Pelvic ultrasonography, particularly transvaginal ultrasound, is the preferred initial imaging modality for evaluating pelvic pain in nonpregnant women [228,229]. Ultrasound can identify inflammatory changes in the fallopian tubes, with color Doppler imaging demonstrating features such as edema, wall thickening, and increased vascularity. In advanced stages of PID, the inflammation may extend to the ovaries, manifesting as complex adnexal masses with indistinct margins and fluid-filled areas, which can occasionally mimic ovarian malignancy [228].
6.4.2. Computed Tomography (CT)
When ultrasound findings are inconclusive or the procedure cannot be performed due to patient discomfort, CT serves as an important modality [230]. CT scan can identify lesions associated with PID, such as cervicitis, endometritis, acute salpingitis, oophoritis, pyosalpinx, hydrosalpinx, TOA, and pyometra, as well as complications of PID, including tubal damage leading to infertility or ectopic pregnancy, peritonitis from ruptured abscesses, peritoneal adhesions causing bowel obstruction or hydroureteronephrosis. CT is also effective in evaluating conditions that may mimic PID, such as endometriosis, adnexal torsion, ruptured hemorrhagic ovarian cyst, adnexal neoplasms, appendicitis, and diverticulitis [231].
6.4.3. Magnetic Resonance Imaging (MRI)
MRI can aid in narrowing down differential diagnoses and establishing an accurate diagnosis. MRI offers high diagnostic accuracy in detecting PID and TOAs [229]. MRI can identify adnexal lesions that appear T1 hypointense and T2 hyperintense, often demonstrating diffusion restriction, thickened walls, internal septations, and strong post-contrast enhancement, features highly suggestive of TOAs [232].
6.5. Transcervical Endometrial Biopsy
Endometrial biopsy can aid in the diagnosis of PID by identifying histologic signs of endometritis, specifically the presence of neutrophils and plasma cells in the endometrium [200]. It is a minimally invasive procedure that offers an alternative to laparoscopy, particularly when there is diagnostic uncertainty. A negative result has a high negative predictive value for ruling out upper genital tract infection. However, its clinical utility is limited by the need for specialized pathology expertise, delayed turnaround time, and restricted availability [233].
6.6. Laparoscopy
Laparoscopy plays a crucial role in the diagnosis of PID, especially in cases where there is no improvement within 72 h of antibiotic therapy or when imaging shows thickened fallopian tubes, intra-abdominal fluid, or TOAs. It allows for direct visualization of the pelvic organs, helping to confirm the diagnosis, rule out other conditions like appendicitis or endometriosis, and obtain bacterial swabs for microbiological culture [234]. Although considered the gold standard for diagnosing PID, the need for general anesthesia, hospitalization, and surgical expertise significantly limits the routine use of diagnostic laparoscopy [233].
6.7. Biomarkers
Traditionally, CA-125, a glycoprotein produced by coelomic epithelial cells and widely used as a tumor marker, has also been investigated in benign conditions [235]. Elevated levels have been associated with peritoneal involvement in diseases such as PID, endometriosis, uterine fibroids, adenomyosis, and various non-malignant serous effusions, supporting its potential role as a prognostic biomarker and in monitoring treatment response in non-malignant conditions [235].
Other biomarkers, commonly used in the evaluation of infectious diseases [236] such as procalcitonin (PCT) and neutrophil-to-lymphocyte ratio (NLR), have been evaluated in the diagnosis and management of PID and TOA [237]. Elevated levels of these markers have been associated with an increased likelihood of surgical intervention [237]. Similarly, immature granulocytes (IGs) can serve have been proposed as markers of disease severity, as elevated levels have been shown to correlate with more severe clinical presentations of PID [238].
7. Management and Treatment
Current international guidelines do not prioritize pathogen-directed therapy for PID. Although it is recognized that the proportion of PID attributable to classic STIs has decreased and a wider range of microorganisms is now implicated, existing CDC and European guidelines continue to recommend empiric, broad-spectrum regimens rather than tailored antimicrobial approaches based on the causative pathogen [1,215]. Moreover, the low prognostic value of screening tests to rule out upper-genital-tract infection has made tailored, pathogen-directed treatment difficult, as negative results do not reliably exclude involvement of key organisms [1]. Other international guidelines, including those from Australia and Canada, similarly focus on STI-related causes of PID and do not provide pathogen-specific recommendations for non-STI etiologies [239,240]. Table 2 summarizes treatment regimens specific to the pathogens identified in the reported cases above, alongside recommended empiric therapy and alternative regimens for patients with allergies or other contraindications. These pathogen-directed treatments are based on existing guidelines for abdominal or site-specific infections caused by the same organisms and represent therapeutic options that could be applied to PID when these pathogens are implicated.

Table 2.
Regimens for treating non-STI PID.
7.1. Empiric Treatment
Given the risk of long-term complications, such as infertility and ectopic pregnancy, both diagnosis and treatment should be initiated promptly upon clinical suspicion of PID. Early intervention is essential to reduce the risk of irreversible reproductive damage [1]. Several factors may influence therapeutic decision making, including local antimicrobial resistance patterns, regional epidemiology, treatment costs, patient preferences and adherence, and disease severity [215]. The choice between oral and parenteral regimen should be guided by certain criteria such as the severity of the illness, tolerance of oral medications, and the presence of complicating factors, like pregnancy or suspected abscess or other surgical emergencies. For mild to moderate cases, oral and parenteral regimens appear to be equally effective. Hospitalization is indicated for patients with severe clinical presentations, inability to adhere to outpatient therapy, or lack of response to initial oral therapy [1,215,221,241]. Parenteral regimens should be preferred in pregnant patients or in cases of diagnostic uncertainty, ensuring optimal management and minimizing complications. Importantly, adolescents should be managed using the same clinical criteria as adults, as age alone does not necessitate inpatient care [1,215,221,241]. Overall, the antibiotics used for treating PID have high efficacy with favorable safety profiles, most of which are well-tolerated and associated with only mild, manageable side effects [242].
In the management of non-STI-related PID, the therapeutic approach must address two pathogen spectra. In addition to sexually transmitted pathogens such as N. gonorrhoeae, C. trachomatis, M. genitalium, and Trichomonas vaginalis, coverage should extend to BV–associated organisms (e.g., Atopobium vaginae, Sneathia, Megasphaera), as well as enteric and respiratory tract-associated bacteria like Bacteroides, E. coli, Streptococcus species, and H. influenzae [15].
Nonetheless, all treatment regimens for PID should provide coverage for N. gonorrhoeae and C. trachomatis due to the fact that negative endocervical screening does not reliably exclude upper genital tract infection [1,243]. Recommended regimens are presented in Table 2.
7.1.1. Hospitalized Patients
For hospitalized patients, parenteral therapy should include ceftriaxone in combination with doxycycline and metronidazole. Cefoxitin or cefotetan in combination with doxycycline and metronidazole can achieve equivalent therapeutic efficacy [1]. Recent updates in treatment recommendations indicate that metronidazole should be included as a mandatory component of empiric therapy for PID, to ensure adequate coverage of anaerobic organisms [244].
Alternative regimens include combinations such as clindamycin plus gentamicin, or ampicillin–sulbactam combined with doxycycline, both of which provide effective coverage against N. gonorrhoeae, C. trachomatis, and anaerobic bacteria. Other options include azithromycin for a 7-day course combined with metronidazole for 14 days, ampicillin plus clindamycin with gentamicin, levofloxacin plus metronidazole, or imipenem with cilastatin [1]. These regimens may serve as comparably effective alternatives when first-line therapies are contraindicated or unavailable. Due to the discomfort often associated with intravenous infusion, doxycycline is preferably administered orally when tolerated, as its oral formulation offers bioavailability comparable to that of the intravenous form [1].
Transition to oral therapy should be considered after 24 to 48 h of sustained clinical improvement. The recommended oral regimens consist of doxycycline combined with metronidazole to complete a total 14-day course of therapy. For patients unable to tolerate doxycycline, azithromycin may be used as an alternative, while clindamycin can substitute for metronidazole to maintain anaerobic coverage [1]. Despite less robust evidence, European guidelines recommend a 14-day regimen of IV ofloxacin plus IV metronidazole as an alternative treatment option for PID [215]. On the contrary, the CDC does not recommend quinolones for routine PID treatment due to the increasing prevalence of quinolone-resistant N. gonorrhoeae. However, in select cases—such as patients with cephalosporin allergy or low risk of gonorrhea—close follow-up-quinolone-based regimens may be considered. In these circumstances, levofloxacin or moxifloxacin combined with metronidazole for 14 days may be used [1].
7.1.2. Non-Hospitalized Patients
Outpatient therapy is appropriate for females with mild to moderate pelvic in PID who can tolerate oral medications and are likely to adhere to the treatment regimen. The recommended approach includes a single intramuscular (IM) dose of a long-acting cephalosporin, preferably ceftriaxone, combined with doxycycline for 14 days and metronidazole for 14 days. Alternative cephalosporins that may be used include cefoxitin, cefotaxime, or ceftizoxime. For patients who cannot tolerate doxycycline, azithromycin can be substituted for a complete 14-day course, although it should still be combined with a cephalosporin and metronidazole [1].
Emerging data on alternative treatments, including morinidazole in combination with levofloxacin, suggest promising efficacy and safety profiles, particularly in the context of increasing resistance to standard agents such as metronidazole [245].
7.2. Enteric Pathogens
In certain cases, the formation of a TOA arises from localized extension of infection secondary to uncontrolled inflammatory bowel disease, appendicitis, adnexal surgery, or, less commonly, hematogenous spread from distant organs [246,247]. The microbiology profile of TOAs tends to be polymicrobial, involving organisms such as E. coli, aerobic streptococci, B.fragilis, Prevotella, and other anaerobes. Thus; antimicrobial regimens must provide adequate coverage for this broad spectrum of organisms [248]. Although management generally adheres to CDC-recommended guidelines, there is rising concern regarding potential resistance of bowel flora to agents such as cefotetan and cefoxitin [249]. Given that bowel flora are often involved in TOA pathogenesis and the classification of TOA as a serious intra-abdominal infection, broader-spectrum regimens such as ertapenem [250], meropenem, or piperacillin-tazobactam may represent more effective therapeutic options [251,252]. Empiric therapy for Enterobacterales should be stratified by illness severity, patient specific factors (allergies, immunocompromise) [253] and Pseudomonas risk factors which include recent hospitalization, devices, recent IV antibiotics, immunosuppression [254,255]. In stable, immunocompetent patients without healthcare exposures, a broad-spectrum single agent (e.g., a third- or fourth-generation cephalosporin) is appropriate. With healthcare exposures or immunosuppression and in cases of sepsis/septic shock a single antipseudomonal β-lactam (e.g., cefepime/ceftazidime, piperacillin–tazobactam, or a carbapenem) is preferred. For severe β-lactam allergy, aztreonam or a fluoroquinolone may serve as equal alternatives. Severe sepsis/septic shock warrants empiric combination coverage for Gram-negatives (β-lactam plus aminoglycoside rather than fluoroquinolone) [256]. Carbapenem therapy with meropenem, imipenem–cilastatin, or ertapenem is favored for extended spectrum b lactamases (ESBL)-E infections [253]. Ceftolozane–tazobactam, a newer antipseudomonal β-lactam/β-lactamase inhibitor has excellent activity against multidrug-resistant P. aeruginosa, including many strains resistant to piperacillin–tazobactam, ceftazidime, or cefepime though it does not overcome carbapenemase producers [253]. When AmpC β-lactamase production is suspected in organisms with a moderate risk profile, cefepime is an appropriate treatment option [253]. For Class A (KPC) β-lactamases, preferred regimens include meropenem–vaborbactam, ceftazidime–avibactam, or imipenem–cilastatin–relebactam, with cefiderocol as an alternative. For Class B (MBLs such as NDM/VIM/IMP), the preferred approach is ceftazidime–avibactam combined with aztreonam, or cefiderocol as monotherapy. For Class D (OXA-48–like) producers, ceftazidime–avibactam is preferred, with cefiderocol as an alternative [253].
7.3. Anaerobic Pathogen Associated Infections
In case of anaerobic infections, metronidazole is a first-line option. Clindamycin covers many anaerobes but rising resistance in B. fragilis makes it less reliable than metronidazole, β-lactam/β-lactamase inhibitor (BL/BLI) combinations, or carbapenems for empiric therapy. BL/BLI regimens (e.g., amoxicillin–clavulanate, ampicillin–sulbactam, ticarcillin–clavulanate, piperacillin–tazobactam) also provide broad anaerobic coverage. Among cephalosporins, second-generation agents (cefoxitin, cefotetan, cefmetazole) are more active against B. fragilis but, due to increasing resistance, are not recommended for empiric treatment and are used primarily for surgical prophylaxis. Carbapenems (imipenem, meropenem) offer excellent anaerobic and aerobic coverage with meropenem slightly more active against Gram-negatives. Fluoroquinolones (notably levofloxacin, moxifloxacin) have some anaerobic activity and good tissue penetration, but rising resistance limits their role; they are generally reserved for situations such as β-lactam allergy (especially in children) when alternatives are unsuitable [257].
7.4. PID Due to Actinomycosis
The risk of infection by Actinomyces spp. appears to be increased among IUD users, although the exact risk remains unknown. It is important to note that a positive culture or the presence of Actinomyces-like organisms on a Pap test does not necessarily warrant antimicrobial therapy or IUD removal; intervention being recommended only in the presence of clinical evidence of infection [198].
An initial course of intravenous antibiotics for at least two weeks—often extended to 6 weeks—is generally recommended depending on disease severity. Penicillin G remains the recommended IV regimen [258]. IV ampicillin and IV ceftriaxone remain reasonable alternatives [259,260]. In case of penicillin allergy, the selection of alternative agents depends on the type and severity of the allergic reaction. For patients with a non-severe or IgE-mediated allergy, ceftriaxone is generally employed as first line IV therapy [259,261]. However, for patients with severe non-IgE-mediated hypersensitivity reactions doxycycline is preferred, and IV carbapenems such as ertapenem may be considered in consultation with an allergist [262].
Oral regimens include penicillin V or amoxicillin [258,263]. In cases where co-pathogens are suspected amoxicillin-clavulanate is an appropriate option [264]. For penicillin-allergic patients, doxycycline, tetracycline, erythromycin, or azithromycin, have demonstrated in vitro efficacy and have been associated with successful outcomes in limited cases [263,265,266].
The duration of therapy for classic actinomycosis typically ranges from two to six months for mild disease and six to twelve months for severe disease, with treatment continuing for at least one to two months after clinical and/or radiologic resolution of the infection. Longer courses, extending up to 12–18 months, may be required for complicated, invasive infections or in immunocompromised hosts, including those with HIV [267,268].
Shorter durations of therapy for actinomycosis may be considered in select cases, particularly when adequate surgical resection of infected tissue has been performed and bone involvement is absent. Early recognition, rapid clinical response, and smaller, less indurated lesions further support abbreviated therapy. In pelvic actinomycosis associated with IUD use, a reduced treatment course may be sufficient, especially when the IUD is promptly removed and the infection remains localized [269,270,271].
7.5. PID Due to Tuberculosis
Management of FGTB follows the same principles as treatment of drug-susceptible pulmonary tuberculosis, in accordance with WHO and CDC/IDSA guidelines [199]. For drug-susceptible cases, the recommended regimen consists of six months of first-line anti-tuberculous therapy: an intensive phase of two months with isoniazid (H), rifampicin (R), pyrazinamide (Z), and ethambutol (E), followed by a four-month continuation phase with isoniazid and rifampicin (2HRZE/4HR) [15].
7.6. PID Due to CMV
PID due to CMV is rarely suspected in immunocompetent adults, although a few cases have been reported [156]. However, in immunocompromised populations, including individuals with HIV infection, CMV may cause severe and potentially life-threatening disease. In such cases, early recognition and timely initiation of antiviral therapy are critical [182,272].
For mild to moderate disease, in patients able to tolerate oral therapy, valganciclovir is the preferred treatment. For severe or life-threatening cases, intravenous ganciclovir is recommended, with transition to oral valganciclovir once clinical improvement is achieved. In patients who cannot tolerate first-line therapy, second-line options include maribavir or foscarnet, with the choice guided by disease severity, viral load, and drug related toxicity [273].
7.7. PID Due to Parasitic Infection
Infection due to Enterobius vermicularis or other parasites should be considered as a potential cause of PID, particularly in cases with unusual presentations or when common bacterial pathogens are not identified [274,165]. Treatment of Enterobius vermicularis infection includes anti-helminthic therapy with albendazole, mebendazole, or pyrantel pamoate [275]. Treatment for Entamoeba involves systemic therapy with metronidazole for 7–10 days, followed by a luminal agent for 7–20 days depending on the drug. Alternatives to metronidazole include tinidazole, ornidazole, or nitazoxanide [276].
7.8. Surgical Management
Although universally accepted guidelines are lacking, certain clinical scenarios may warrant surgical management in PID. Indications include pelvic peritonitis or TOAs, particularly when abscess size exceeds 3–4 cm, or when severe complications such as rupture or septic shock occur. In these cases, prompt initiation of antimicrobial therapy and abscess drainage—preferably via imaging-guided transvaginal approach—should not be delayed. If severe signs are present, surgical intervention, ideally by laparoscopy, may be required, with drainage preferred over excision [277]. In some cases, inflammatory markers CRP and ESR may help guide management and decisions regarding surgical intervention, as elevated levels have been associated with greater disease severity, larger abscess size, and prolonged hospitalization [278,279,280]. Despite factors such as fever, larger abscess size, and higher inflammatory markers being predictive of treatment failure, scoring systems incorporating these parameters have demonstrated poor discriminatory capacity [281].
When surgical intervention is required, laparoscopic surgery appears to offer advantages over open laparotomy. Reported benefits include shorter operation times, reduced need for blood transfusions, and a shorter hospital stay, without increasing the risk of surgical complications, revision surgery, or in-hospital mortality [282].
8. Complications and Prognosis
PID is strongly associated with long-term complications, including chronic pelvic pain, infertility, and ectopic pregnancy, and these adverse outcomes may occur not only when treatment is delayed but also despite timely and complete therapy, with affected individuals remaining at risk for recurrent episodes and ongoing morbidity [283,284].
TOA is a severe complication of PID and could lead to morbidity and occasional mortality. Clinical presentation of TOA is similar to that of PID, with the addition of a pelvic mass often noted on examination or imaging [207,285]. TOA’s typical symptoms include fever, abdominal pain, adnexal mass, and abnormal vaginal discharge; thus, obtaining a detailed medical history and performing a thorough clinical examination is mandatory. An auxiliary transabdominal or transvaginal ultrasound may be useful, while in case of diagnostic difficulties, a CT and then an MRI may be helpful [286,287].
A history of PID has been identified as an independent predictor of chronic pelvic pain (CPP) in women [288,289]. CPP occurs in 18–36% of cases and can increase up to 67% in women who experience three or more episodes of PID [2,290]. The underlying mechanisms of CPP are not fully understood, though it is generally thought to result from adhesive disease and damage to the fallopian tubes and ovaries caused by the infection, chronic inflammation due to host immunological responses, or recurrent infections as a consequence of repeated exposures and weakened host defenses [291,292]. Approximately one-third of individuals with PID develop CPP within three years, with more than half experiencing highly intense pain, which is often accompanied by impairments in physical function, overall health, vitality, social engagement, and psychological well-being [290,293]. CPP management may include analgesia, hormonal suppression, and physiotherapy of the pelvic floor, whereas peripheral nerve blocks and sacral nerve neuromodulation may be essential in selected cases.
Individuals with PID might experience hydrosalpinx, a condition resulting from inflammation where the injured fallopian tube may get obstructed by surgery, adhesions, accumulate sterile fluid, and increase in size. The hydrosalpinx persists even after the PID is resolved. In individuals receiving in vitro fertilization (IVF), hydrosalpinx adversely affects pregnancy rates, implantation success, early pregnancy loss, preterm delivery, and live births [294].
Infertility should always be recognized as a potential long-term complication of PID, and women with a history of PID should undergo early assessment when planning pregnancy to enable timely discussion of diagnostic and therapeutic options in case spontaneous conception is not achieved [295]. According to the PID Evaluation and Clinical Health (PEACH) trial, the incidence of infertility among participants with PID was 18% after a three-year follow-up [293]. PID seems to be positively associated with infertility and interestingly, women with a history of PID treatment are considerably more likely to experience infertility compared to women without a history of PID [296]. Potential mechanisms leading to infertility following PID include the upward spread of infection from the cervix to the upper reproductive tract. Disruption of the endocervical mucous plug, mid-cycle uterine peristalsis, and retrograde menstrual flow can facilitate pathogen migration into the pelvis, causing tubal damage and impaired fertility [297].
PID has been associated with an increased risk of adverse pregnancy outcomes, including ectopic pregnancy, with studies showing that women with a history of PID have more than twice the risk compared to those without PID [298,299]. Repeated PID episodes are leading to ongoing injury to fallopian tube and pelvic tissues. Every infection increases tubal adhesions and scarring, resulting in progressively greater infertility rates and ectopic pregnancy [241,300]. The likelihood of ectopic pregnancy rises with the frequency and severity of PID episodes [301]. Of note, non-STI-related PID typically leads to milder peri-tubal adhesions and partial tubal blockages, resulting in a moderate risk of infertility and a relatively weaker association with ectopic pregnancy compared to STI-related PID [301,302].
The association between PID and ovarian cancer remains uncertain. It is indicated that, PID correlates with a heightened risk of ovarian cancer and particularly higher risk in patients with five or more PID episodes [303]. Additionally, there was an increase in borderline ovarian tumors and variations in both serous and non-serous types, however non-STI PID is not associated with ovarian cancer risk, suggesting etiological differences [304,305]. However, if PID independently increases ovarian cancer risk, it can also raise the likelihood of low parity and infertility, which are additional risk factors for ovarian cancer. This is related with evidence suggesting that fallopian tubes, not ovaries, are primarily involved in most cases of related carcinomas [205,207].
Perihepatitis (Fitz-Hugh Curtis Syndrome), which is actually inflammation of the liver capsule and the peritoneal surfaces in the anterior right upper quadrant, is typically associated with STIs such as N. gonorrhoeae, C. trachomatis, and possibly M. genitalium, and is generally not observed in cases of non-STI-related PID [306,307].
Overall, the prognosis of non-STI-related PID is closely linked to early recognition, accurate diagnosis, and timely treatment. Most patients have an excellent outcome when appropriate antimicrobial therapy is initiated promptly, whereas delayed or inadequate management increases the risk of complications such as TOAs, infertility, ectopic pregnancy, chronic pelvic pain, and, less commonly, ovarian malignancy. Ultimately, when the index of suspicion for PID is high, the appropriate diagnostic algorithm is applied, and effective therapy is administered, the long-term prognosis is generally favorable, with preservation of reproductive health and overall quality of life [220].
9. Follow-Up and Monitoring
PID is essential to be treated timely and effectively due to the high risk of long-term sequelae [220]. In the absence of strong indications for hospitalization, such as pregnancy, ineffective outpatient treatment, or severe clinical manifestations, mild to moderate PID can be treated on an outpatient basis with a focus on follow-up. More specifically, a clinical reassessment within 48–72 h should be performed, when a substantial improvement in signs and symptoms is expected [215,220,221]. Otherwise, further evaluation should be undertaken with a focus on imaging and repeat clinical assessment, to rule out for potential complications [286].
A high level of suspicion should be maintained for the development of TOAs. Patients with TOAs require IV antibiotic therapy, and significant improvement is expected within 24–48 h, after which a transition to oral antibiotics may be considered. Oral antibiotic therapy continues for at least 14 days. Daily monitoring of the leukocyte count is required to evaluate the process in case of worsening symptoms. In case of no clinical and laboratory improvement, drainage may be necessary. Surgical intervention is needed in case of a ruptured TOA or if the patient is deteriorating [286].
Long-term follow-up is equally important. Infertility may occur due to proximal and/or distal disease of the fallopian tube [295]. When evaluating an individual with a PID history and infertility issues, hysterosalpingography should be performed without delay, and solutions, including surgery, could be assessed [295]. Similarly, high index of suspicion should be raised for ectopic pregnancy or presence of ovarian cancer when evaluating an individual with PID history.
10. Prevention
Prevention of non-STI-related PID requires a multifactorial approach. One of the most critical strategies involves the strict application of aseptic techniques during obstetric and gynecological procedures, as post-procedural infection represents a major contributor to PID of non-sexual origin [308]. It is vital for surgical personnel to wear sterile attire, including sterile gloves, gowns, and other protective gear, to avoid contamination of the surgical field. The sterilization of surgical instruments is critical [308]. Minimally invasive surgical procedures must be preferred, if possible, in terms of minimizing the risk of infection as well as minimizing the risk of long-term consequences, such as infertility, attributed to scar tissue formation [308]. Patient education is essential and among others includes post-surgical scrupulous wound care and prompt recognition of symptoms indicating infection, and seeking medical attention [308].
Antibiotic prophylaxis is also important in terms of prevention, though it is not routinely recommended and is tailored to the procedure and the risk factors [32]. In a meta-analysis regarding perioperative antibiotics to avoid infection following a first-trimester abortion, the utilization of preventive antibiotics decreased infection rates after abortion by 41% [32]. The protective impact of antibiotics was evident irrespective of which subgroup was examined including women with a history of PID, women without a documented history of PID and females who received positive results for chlamydial infection during the procedure [309]. For uterine evacuation following abortion or miscarriage, administration of doxycycline or metronidazole, optionally combined with azithromycin in selected high-risk cases, is recommended [310]. Prophylaxis is also indicated for hysterectomy, typically as a single dose of a first- or second-generation cephalosporin, often combined with metronidazole [310]. In contrast, routine antibiotic prophylaxis is not indicated for hysterosalpingography. However, for patients with a prior history of PID, doxycycline or azithromycin are recommended prior to the procedure. Other gynecologic and obstetric interventions, including intrauterine device insertion, endometrial biopsy, and routine hysteroscopy, generally do not require prophylactic antibiotics [310].
Beyond procedural and pharmacological interventions, public health awareness plays a pivotal role in PID prevention. Educational campaigns targeting both the public and healthcare providers are essential, as they increase recognition of symptoms, improve timely access to care, and reduce the risk of complications such as infertility, chronic pelvic pain, or sepsis [220]. A unique reference should be made to the education of special populations, as prompt recognition and effective management are essential [7,311].
While this review addresses PID of non-sexual origin, raising awareness of the sexually transmitted causes remains equally important. Public health education should emphasize safe sexual practices, including the correct and consistent use of barrier contraceptive methods. The United States Preventive Services Task Force (USPSTF) recommends annual screening for N. gonorrhoeae and C. trachomatis in all sexually active women under 25 years of age, as well as in women aged 25 years and older who possess additional risk factors for infection. [220].
11. Future Directions
Even with advancements in non-STI PID studies, significant challenges remain. Studies depend on data that do not have microbiological validation- examination of 11.7 million cases excluding STI/BV testing [16]. Hospital-centric research tends to emphasize severe clinical image, underestimating milder or subclinical cases. Low-income countries fall behind in consistently monitoring non-STI PID, further complicated by diagnostic inequalities (limited laparoscopy/PCR availability). These limitations hinder prevalence and targeted interventions, particularly in areas with low socioeconomic status, where the incidence of non-STDs may be highest. Enhanced monitoring that includes pathogen identification is urgently required for precise epidemiological evaluation [25,312].
A diagnostic gap persists in PID, as existing methods primarily identify only a restricted subset of causative pathogens, thereby failing to comprehensively characterize the broader vaginal microbiota [313,314]. Traditional diagnostic methods, such as microscopy and culture, commonly present low sensitivity and specificity. In contrast, modern approaches, including multiplex PCR and Next-Generation Sequencing, offer enhanced sensitivity, allowing early and precise identification of both sexually transmitted and polymicrobial contributors. On top of that, AI-based approaches can enhance PID management by integrating microbiome profiling to predict disease risk, guide individualized therapy, and facilitate remote monitoring through mobile health platforms [315].
In this context, the vaginal microbiome is crucial for maintaining reproductive health. Dysbiosis, characterized by decreased Lactobacillus abundance and a shift toward a more diverse, pathogen-rich microbial community, including S. aureus, K. pneumoniae, E. faecalis, and E. coli, has been associated with infertility, particularly in women with PID or idiopathic infertility [316]. Metagenomic analysis of cervical and vaginal microbiota may be useful for detecting changes, such as reduced Lactobacillus abundance and increased bacterial diversity [317]. Deep-sequencing technologies may assist in predicting treatment outcomes and recurrence risk, informing strategies to reduce the recurrence rate of BV and consequent PID [318].
The development of noninvasive, biomarker-based diagnostics represents a promising avenue for improving PID detection. Candidate biomarkers include lipopolysaccharides (LPS), and interleukin-6 (IL-6). Both markers are detectable in endometrial tissue, and could serve as effective diagnostic tools, although upscaling would not be easily implemented [319].
12. Conclusions
Although originally thought to be primarily a sexually transmitted disease, the rising prevalence of non-STI-related PID highlights the importance of recognizing alternative etiologies. Non-STI-related PID is associated with BV, IUD use, postpartum and post-procedural infections, as well as a diverse array of bacterial, viral, and parasitic pathogens. Currently, no specific clinical guidelines exist for the diagnosis or management of non-STI-related PID, presenting significant challenges for clinicians in tailoring therapy. Empiric treatment frequently relies on extrapolation from STI-based protocols, highlighting the urgent need for evidence-based recommendations that address non-STI etiologies. Given the potential for serious complications such as infertility, chronic pelvic pain, and recurrent infections, future research should prioritize strategies for early diagnosis, accurate identification of causative pathogens, and optimization of therapeutic approaches for non-STI PID.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13122813/s1, Figure S1: PRISMA diagram.
Author Contributions
K.A. conceived idea; E.P., E.N. and M.G.: performed literature search and wrote manuscript; E.P. and E.N.: drew figures and tables; K.A. critically corrected manuscript; K.A.: oversaw study. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AI | Artificial Intelligence |
| AIDS | Acquired Immunodeficiency Syndrome |
| BL/BLI | Beta-lactam/Beta-lactamase inhibitor |
| BV | Bacterial Vaginosis |
| CA-125 | Cancer Antigen 125 |
| CDC | Centers for Disease Control and Prevention |
| CMV | Cytomegalovirus |
| CPP | Chronic Pelvic Pain |
| CRP | C-reactive Protein |
| CRAB | Carbapenem Resistant Acinetobacter baumanii |
| CRE | Carbapenem resistant Enterobacteriales |
| CRPA | Carbapenem resistant Pseudomonas aeruginosa |
| CT | Computed Tomography |
| DNA | Deoxyribonucleic Acid |
| E. coli | Escherichia coli |
| ESBL | Extended-Spectrum β-Lactamase |
| ESR | Erythrocyte Sedimentation Rate |
| FGTB | Female Genital Tract Tuberculosis |
| GI | Gastrointestinal |
| GAS | Group A Streptococcus |
| H. influenzae | Haemophilus influenzae |
| HSG | Hysterosalpingography |
| HIV | Human Immunodeficiency Virus |
| HR | Rifampicin + Isoniazid regimen |
| HRZE | Isoniazid, Rifampicin, Pyrazinamide, and Ethambutol regimen |
| IBD | Inflammatory Bowel Disease |
| IDSA | Infectious Diseases Society of America |
| IFN-γ | Interferon Gamma |
| IgA | Immunoglobulin A |
| IgE | Immunoglobulin E |
| IL-1 | Interleukin-1 |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IM | Intramuscular |
| IUD | Intrauterine Device |
| IV | Intravenous |
| K. pneumoniae | Klebsiella pneumoniae |
| LPS | Lipopolysaccharide |
| MBL | Metallo-β-lactamase |
| M. genitalium | Mycoplasma genitalium |
| M. hominis | Mycoplasma hominis |
| M. tuberculosis | Mycobacterium tuberculosis |
| MDR | Multidrug-Resistant |
| MMP-9 | Matrix Metalloproteinase-9 |
| MRI | Magnetic Resonance Imaging |
| NAAT | Nucleic Acid Amplification Test |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NLR | Neutrophil-to-Lymphocyte Ratio |
| N. gonorrhoeae | Neisseria gonorrhoeae |
| N. meningitidis | Neisseria meningitidis |
| P. aeruginosa | Pseudomonas aeruginosa |
| PCR | Polymerase Chain Reaction |
| PID | Pelvic Inflammatory Disease |
| PCT | Procalcitonin |
| RNA | Ribonucleic Acid |
| S. aureus | Staphylococcus aureus |
| S. lugdunensis | Staphylococcus lugdunensis |
| S. pneumoniae | Streptococcus pneumoniae |
| S. viridans | Viridans Group Streptococci |
| STD | Sexually Transmitted Disease |
| STI | Sexually Transmitted Infection |
| TB | Tuberculosis |
| TGF-β | Transforming Growth Factor Beta |
| TLR | Toll-Like Receptor |
| TOA | Tubo-Ovarian Abscess |
| USPSTF | United States Preventive Services Task Force |
| WHO | World Health Organization |
| WBC | White Blood Cell |
References
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm. Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef] [PubMed]
- Weström, L. Incidence, prevalence, and trends of acute pelvic inflammatory disease and its consequences in industrialized countries. Am. J. Obstet. Gynecol. 1980, 138 Pt 2, 880–892. [Google Scholar] [CrossRef]
- Brunham, R.C.; Gottlieb, S.L.; Paavonen, J. Pelvic inflammatory disease. N. Engl. J. Med. 2015, 372, 2039–2048. [Google Scholar] [CrossRef]
- He, D.; Wang, T.; Ren, W. Global burden of pelvic inflammatory disease and ectopic pregnancy from 1990 to 2019. BMC Public Health 2023, 23, 1894. [Google Scholar] [CrossRef] [PubMed]
- Papy, P.; Emmanuel, N.; Agwu, E.; Extension, K.P. Prevalence of Pelvic Inflammatory Disease among Women Attending the the Gynecology Clinic at Kampala International University Teaching Hospital, Uganda. IDOSR J. Sci. Technol. 2022, 9, 1–9. [Google Scholar]
- Yagur, Y.; Weitzner, O.; Barchilon Tiosano, L.; Paitan, Y.; Katzir, M.; Schonman, R.; Klein, Z.; Miller, N. Characteristics of pelvic inflammatory disease caused by sexually transmitted disease—An epidemiologic study. J. Gynecol. Obstet. Human. Reprod. 2021, 50, 102176. [Google Scholar] [CrossRef]
- Surd, A.; Mureșan, R.; Oprea, A.; Snakovszki, K.; Sur, L.M.; Usatiuc, L.-O.; Ciongradi, C.-I.; Sârbu, I. Diagnostic Challenges and Management Strategies of Pelvic Inflammatory Disease in Sexually Inactive Pediatric and Adolescent Patients: A Systematic Review of Case Reports. J. Clin. Med. 2025, 14, 3971. [Google Scholar] [CrossRef]
- Cho, H.-W.; Koo, Y.-J.; Min, K.-J.; Hong, J.-H.; Lee, J.-K. Pelvic Inflammatory Disease in Virgin Women With Tubo-ovarian Abscess: A Single-Center Experience and Literature Review. J. Pediatr. Adolesc. Gynecol. 2017, 30, 203–208. [Google Scholar] [CrossRef]
- Haggerty, C.L.; Peipert, J.F.; Weitzen, S.; Hendrix, S.L.; Holley, R.L.; Nelson, D.B.; Randall, H.; Soper, D.E.; Wiesenfeld, H.C.; Ness, R.B. Predictors of chronic pelvic pain in an urban population of women with symptoms and signs of pelvic inflammatory disease. Sex. Transm. Dis. 2005, 32, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Weström, L.; Joesoef, R.; Reynolds, G.; Hagdu, A.; Thompson, S.E. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex. Transm. Dis. 1992, 19, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Abatangelo, L.; Okereke, L.; Parham-Foster, C.; Parrish, C.; Scaglione, L.; Zotte, D.; Taub, L.F. If pelvic inflammatory disease is suspected empiric treatment should be initiated. J. Am. Acad. Nurse Pract. 2010, 22, 117–122. [Google Scholar] [CrossRef]
- Ness, R.B.; Hillier, S.L.; Kip, K.E.; Soper, D.E.; Stamm, C.A.; McGregor, J.A.; Bass, D.C.; Sweet, R.L.; Rice, P.; Richter, H.E. Bacterial vaginosis and risk of pelvic inflammatory disease. Obstet. Gynecol. 2004, 104, 761–769. [Google Scholar] [CrossRef]
- Cohen, C.R.; Lingappa, J.R.; Baeten, J.M.; Ngayo, M.O.; Spiegel, C.A.; Hong, T.; Donnell, D.; Celum, C.; Kapiga, S.; Delany, S.; et al. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: A prospective cohort analysis among African couples. PLoS Med. 2012, 9, e1001251. [Google Scholar] [CrossRef]
- Wiesenfeld, H.C.; Sweet, R.L.; Ness, R.B.; Krohn, M.A.; Amortegui, A.J.; Hillier, S.L. Comparison of acute and subclinical pelvic inflammatory disease. Sex. Transm. Dis. 2005, 32, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.M.; Anyalechi, G.E.; Cohen, C.R.; Haggerty, C.L.; Manhart, L.E.; Hillier, S.L. Etiology and Diagnosis of Pelvic Inflammatory Disease: Looking Beyond Gonorrhea and Chlamydia. J. Infect. Dis. 2021, 224 (Suppl. 2), S29–S35. [Google Scholar] [CrossRef] [PubMed]
- Kreisel, K.M.; Llata, E.; Haderxhanaj, L.; Pearson, W.S.; Tao, G.; Wiesenfeld, H.C.; Torrone, E.A. The Burden of and Trends in Pelvic Inflammatory Disease in the United States, 2006-2016. J. Infect. Dis. 2021, 224 (Suppl. 2), S103–S112. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.S.; Horner, P.J.; Price, M.J.; Mitchell, H.D.; Soldan, K. What Do Diagnoses of Pelvic Inflammatory Disease in Specialist Sexual Health Services in England Tell Us About Chlamydia Control? J. Infect. Dis. 2021, 224 (Suppl. 2), S113–S120. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuran, O.A.; Al-Mehaisen, L.; Al-Karablieh, M.; Abu Ajamieh, M.; Flefil, S.; Al-Mashaqbeh, S.; Albustanji, Y.; Al-Kuran, L. Gynecologists and pelvic inflammatory disease: Do we actually know what to do?: A cross-sectional study in Jordan. Medicine 2023, 102, e35014. [Google Scholar] [CrossRef] [PubMed]
- Goller, J.L.; De Livera, A.M.; Fairley, C.K.; Guy, R.J.; Bradshaw, C.S.; Chen, M.Y.; Hocking, J.S. Characteristics of pelvic inflammatory disease where no sexually transmitted infection is identified: A cross-sectional analysis of routinely collected sexual health clinic data. Sex. Transm. Infect. 2017, 93, 68–70. [Google Scholar] [CrossRef]
- Sweeney, S.; Bateson, D.; Fleming, K.; Huston, W. Factors associated with pelvic inflammatory disease: A case series analysis of family planning clinic data. Womens Health 2022, 18, 17455057221112263. [Google Scholar] [CrossRef]
- Metreau, E.; Elizabeth, K.; Shwetha, Y.; Eapen, G. World Bank Country Classifications by Income Level for 2024–2025. Available online: https://blogs.worldbank.org/en/opendata/world-bank-country-classifications-by-income-level-for-2024-2025 (accessed on 22 November 2025).
- World Health Organization. Sexually Transmitted Infections (STIs). Available online: https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis) (accessed on 20 November 2025).
- Gravett, C.A.; Gravett, M.G.; Martin, E.T.; Bernson, J.D.; Khan, S.; Boyle, D.S.; Lannon, S.M.; Patterson, J.; Rubens, C.E.; Steele, M.S. Serious and life-threatening pregnancy-related infections: Opportunities to reduce the global burden. PLoS Med. 2012, 9, e1001324. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.; Sarowa, B.K.; Abdessalam, S.F. Ruptured Appendicitis Leading to Development of a Tubo-Ovarian Abscess in a Non-sexually Active Adolescent Patient. Cureus 2023, 15, e41226. [Google Scholar] [CrossRef] [PubMed]
- Center of Disease Control and Prevention. Sexually Transmitted Infections Surveillance. 2021. Available online: https://www.cdc.gov/sti-statistics/media/pdfs/2024/07/2021-STD-Surveillance-Report-PDF_ARCHIVED-2-16-24.pdf (accessed on 22 August 2025).
- Reekie, J.; Donovan, B.; Guy, R.; Hocking, J.S.; Kaldor, J.M.; Mak, D.B.; Pearson, S.; Preen, D.; Stewart, L.; Ward, J.; et al. Risk of Pelvic Inflammatory Disease in Relation to Chlamydia and Gonorrhea Testing, Repeat Testing, and Positivity: A Population-Based Cohort Study. Clin. Infect. Dis. 2018, 66, 437–443. [Google Scholar] [CrossRef]
- Mohllajee, A.P.; Curtis, K.M.; Peterson, H.B. Does insertion and use of an intrauterine device increase the risk of pelvic inflammatory disease among women with sexually transmitted infection? A systematic review. Contraception 2006, 73, 145–153. [Google Scholar] [CrossRef]
- Curtis, K.M.; Jatlaoui, T.C.; Tepper, N.K.; Zapata, L.B.; Horton, L.G.; Jamieson, D.J.; Whiteman, M.K. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm. Rep. 2016, 65, 1–66. [Google Scholar] [CrossRef]
- Hubacher, D. Intrauterine devices & infection: Review of the literature. Indian J. Med. Res. 2014, 140 (Suppl. 1), S53–S57. [Google Scholar]
- Pittaway, D.E.; Winfield, A.C.; Maxson, W.; Daniell, J.; Herbert, C.; Wentz, A.C. Prevention of acute pelvic inflammatory disease after hysterosalpingography: Efficacy of doxycycline prophylaxis. Am. J. Obstet. Gynecol. 1983, 147, 623–626. [Google Scholar] [CrossRef]
- Møller, B.R.; Allen, J.; Toft, B.; Hansen, K.B.; Taylor-Robinson, D. Pelvic inflammatory disease after hysterosalpingography associated with Chlamydia trachomatis and Mycoplasma hominis. Br. J. Obstet. Gynaecol. 1984, 91, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- ACOG Practice Bulletin No. 195: Prevention of Infection After Gynecologic Procedures. Obstet. Gynecol. 2018, 131, e172–e189. [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Lee, Y.S.; Smith, A.; Marchesi, J.R.; Lehne, B.; Bhatia, R.; Lyons, D.; Paraskevaidis, E.; Li, J.V.; et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci. Rep. 2015, 5, 16865. [Google Scholar] [CrossRef]
- Haggerty, C.L.; Totten, P.A.; Tang, G.; Astete, S.G.; Ferris, M.J.; Norori, J.; Bass, D.C.; Martin, D.H.; Taylor, B.D.; Ness, R.B. Identification of novel microbes associated with pelvic inflammatory disease and infertility. Sex. Transm. Infect. 2016, 92, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Hebb, J.K.; Cohen, C.R.; Astete, S.G.; Bukusi, E.A.; Totten, P.A. Detection of novel organisms associated with salpingitis, by use of 16S rDNA polymerase chain reaction. J. Infect. Dis. 2004, 190, 2109–2120. [Google Scholar] [CrossRef]
- Schindlbeck, C.; Dziura, D.; Mylonas, I. Diagnosis of pelvic inflammatory disease (PID): Intra-operative findings and comparison of vaginal and intra-abdominal cultures. Arch. Gynecol. Obstet. 2014, 289, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Greydanus, D.; Bacopoulou, F. Acute pelvic inflammatory disease. Pediatr. Med. 2019, 2, 36. [Google Scholar] [CrossRef]
- Hunter, R.L. Pathology of post primary tuberculosis of the lung: An illustrated critical review. Tuberculosis 2011, 91, 497–509. [Google Scholar] [CrossRef]
- Desai, R.M.; Kumar, S.; Brindini, U. Female genital tuberculosis: A clinicopathological study. Int. J. Reprod. Contracept. Obstet. Gynecol. 2017, 5, 2780–2783. [Google Scholar] [CrossRef][Green Version]
- Namavar Jahromi, B.; Parsanezhad, M.E.; Ghane-Shirazi, R. Female genital tuberculosis and infertility. Int. J. Gynaecol. Obstet. 2001, 75, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, H.; Mohammad, S.; Pathak, P. Tuberculosis in the Female Genital Tract. Cureus 2022, 14, e28708. [Google Scholar] [CrossRef]
- Pradhan, S.; Kyrillos, A.; Hayes, K.; Gromet, E.; Vash-Margita, A.; Pennesi, C.; O’Flynn O’Brien, K.; Gomez-lobo, V. 9. Pelvic Inflammatory Disease in Non-sexually active Pediatric and Adolescent Patients. J. Pediatr. Adolesc. Gynecol. 2020, 33, 242. [Google Scholar] [CrossRef]
- Asemota, O.A.; Girda, E.; Dueñas, O.; Neal-Perry, G.; Pollack, S.E. Actinomycosis pelvic abscess after in vitro fertilization. Fertil. Steril. 2013, 100, 408–411. [Google Scholar] [CrossRef]
- Sawtelle, A.L.; Chappell, N.P.; Miller, C.R. Actinomyces-Related Tubo-Ovarian Abscess in a Poorly Controlled Type II Diabetic With a Copper Intrauterine Device. Mil. Med. 2017, 182, e1874–e1876. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nwanguma, A.; Arora, K. Pseudoactinomycotic Radiate Granules in the Gynecological Tract: A Case Report. Cureus 2024, 16, e69062. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, H.; Zemni, I.; Souissi, M.; Henchiri, H.; Boukhris, S.; Ayadi, M.A.; Achouri, L. Pseudo tumor pelvic actinomycosis revealed by colonic obstruction with hydronephrosis: Can extensive surgery be avoided? A case report. Womens Health 2023, 19, 17455057231181009. [Google Scholar] [CrossRef] [PubMed]
- Saramago, S.M.; Cominho, J.C.; Proença, S.S.M.; Conde, P.J.C.; Nunes, F.M.P. Pelvic Actinomycosis Mimicking Pelvic Malignancy. Rev. Bras. Ginecol. Obstet. 2019, 41, 463–466. [Google Scholar] [CrossRef]
- Bartoš, V.; Doboszová, J.; Sudek, M. Actinomycotic Endomyometritis Associated with a Long-Term Use of Intrauterine Device Lasting for 42 Years. Acta Medica 2019, 62, 35–38. [Google Scholar] [CrossRef]
- González-García, S.M.; Pastrana-Arroyo, M.J.; Medina-Parrilla, E.; González, A.; Martín, J. A Rare Case of Non-IUD-Related Chronic Endometritis caused by Actinomyces Bacteria in a Postmenopausal Woman: A Case Report. P. R. Health Sci. J. 2022, 41, 165–167. [Google Scholar]
- Shunmugam, D.; Shanmugasundaram, S.; Gandhi, A. Rare cause of ovarian mass. BMJ Case Rep. 2018, 2018, bcr-2018-225564. [Google Scholar] [CrossRef]
- Dhillon, A.K.; Fairlie, N.; Finch, G. Pelvic Actinomyces israelii abscess: A differential diagnosis of a pelvic mass. BMJ Case Rep. 2015, 2015, bcr-2015-211595. [Google Scholar] [CrossRef]
- Desteli, G.A.; Gürsu, T.; Bircan, H.Y.; Kızılkılıç, E.; Demiralay, E.; Timurkaynak, F. Thrombocytosis and small bowel perforation: Unusual presentation of abdominopelvic actinomycosis. J. Infect. Dev. Ctries. 2013, 7, 1012–1015. [Google Scholar] [CrossRef][Green Version]
- Lyttle, B.; Johnson, J.V. Chronic Actinomyces Infection Caused by Retained Cervical Cerclage: A Case Report. J. Reprod. Med. 2016, 61, 179–181. [Google Scholar]
- Flynn, A.N.; Lyndon, C.A.; Church, D.L. Identification by 16S rRNA gene sequencing of an Actinomyces hongkongensis isolate recovered from a patient with pelvic actinomycosis. J. Clin. Microbiol. 2013, 51, 2721–2723. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yilmaz, M.; Akbulut, S.; Samdanci, E.T.; Yilmaz, S. Abdominopelvic actinomycosis associated with an intrauterine device and presenting with a rectal mass and hydronephrosis: A troublesome condition for the clinician. Int. Surg. 2012, 97, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Hong, J.H.; Lee, J.K. Ovarian and vesical actinomycosis: A case report and literature review. Arch. Gynecol. Obstet. 2009, 279, 591–593. [Google Scholar] [CrossRef]
- Pusiol, T.; Morichetti, D.; Pedrazzani, C.; Ricci, F. Abdominal-pelvic actinomycosis mimicking malignant neoplasm. Infect. Dis. Obstet. Gynecol. 2011, 2011, 747059. [Google Scholar] [CrossRef] [PubMed]
- Maxová, K.; Menzlová, E.; Kolařík, D.; Dundr, P.; Halaška, M. Case report: Pelvic actinomycosis. Prague Med. Rep. 2012, 113, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Devendra, K.; Chen, C.M. Pelvic actinomycosis masquerading as an acute abdomen from a small bowel perforation. Singap. Med. J. 2008, 49, 158–159. [Google Scholar]
- Dunn, T.S.; Cothren, C.; Klein, L.; Krammer, T. Pelvic actinomycosis: A case report. J. Reprod. Med. 2006, 51, 435–437. [Google Scholar]
- Iwasaki, M.; Nishikawa, A.; Akutagawa, N.; Fujimoto, T.; Teramoto, M.; Kudo, R. A case of ovarian actinomycosis. Infect. Dis. Obstet. Gynecol. 2003, 11, 171–173. [Google Scholar] [CrossRef]
- Jonas, L.; Baguhl, F.; Wilken, H.P.; Haas, H.J.; Nizze, H. Copper accumulation in actinomyces druses during endometritis after long-term use of an intrauterine contraceptive device. Ultrastruct. Pathol. 2002, 26, 323–329. [Google Scholar] [CrossRef]
- Cobellis, L.; Messalli, E.M.; Pierno, G. Pelvic actinomycosis in menopause: A case report. Maturitas 2001, 39, 79–81. [Google Scholar] [CrossRef]
- Hawnaur, J.M.; Reynolds, K.; McGettigan, C. Magnetic resonance imaging of actinomycosis presenting as pelvic malignancy. Br. J. Radiol. 1999, 72, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, D.; Kustrup, J.F., Jr. Large bowel obstruction due to intrauterine device: Associated pelvic inflammatory disease. Am. Surg. 1999, 65, 1165–1166. [Google Scholar] [CrossRef]
- Lo, T.S.; Chen, F.P.; Chu, K.K.; Soong, Y.K. Advanced actinomycosis involving urogenital organs simulating malignancy: A case report. Chang. Yi Xue Za Zhi 1997, 20, 313–317. [Google Scholar]
- Hinnie, J.; Jaques, B.C.; Bell, E.; Hansell, D.T.; Milroy, R. Actinomycosis presenting as carcinoma. Postgrad. Med. J. 1995, 71, 749–750. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Woo, P.C.; Fung, A.M.; Lau, S.K.; Teng, J.L.; Wong, B.H.; Wong, M.K.; Hon, E.; Tang, G.W.; Yuen, K.Y. Actinomyces hongkongensis sp. nov. a novel Actinomyces species isolated from a patient with pelvic actinomycosis. Syst. Appl. Microbiol. 2003, 26, 518–522. [Google Scholar] [CrossRef]
- Elsayed, S.; George, A.; Zhang, K. Intrauterine contraceptive device-associated pelvic actinomycosis caused by Actinomyces urogenitalis. Anaerobe 2006, 12, 67–70. [Google Scholar] [CrossRef]
- Ferjaoui, M.A.; Arfaoui, R.; Khedhri, S.; Hannechi, M.A.; Abdessamia, K.; Samaali, K.; Fezai, W.; Salhi, M.; Malek, M.; Neji, K. Pelvic actinomycosis: A confusing diagnosis. Int. J. Surg. Case Rep. 2021, 86, 106387. [Google Scholar] [CrossRef]
- Laranjo, M.; Varejão, A.M.; Costa, P.; Peixinho, C. Pelvic actinomycosis: Abdominal mass caused by a forgotten IUD. BMJ Case Rep. 2022, 15, bcr-2022-251392. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Cao, Y.; Zhang, Y.; Niu, L.; Wang, S.; Sang, C. A Case Report of Pelvic Actinomycosis and a Literature Review. Am. J. Case Rep. 2020, 21, e922601. [Google Scholar] [CrossRef] [PubMed]
- Restaino, S.; Gomba, B.; Zero, C.; Stabile, G.; Ronsini, C.; Della Corte, L.; Cianci, S.; Perelli, F.; Piacenti, I.; Driul, L.; et al. Pelvic Actinomycosis and Diagnostic Complexity: Case Report with Literature Review. Healthcare 2025, 13, 485. [Google Scholar] [CrossRef]
- Ayoub, F.; Asour, A.; Miah, A. Case Series of Abdominal Actinomycosis: An Old Diagnostic Conundrum. Cureus 2024, 16, e69763. [Google Scholar] [CrossRef] [PubMed]
- Müller-Holzner, E.; Ruth, N.R.; Abfalter, E.; Schröcksnadel, H.; Dapunt, O.; Martin-Sances, L.; Nogales, F.F. IUD-associated pelvic actinomycosis: A report of five cases. Int. J. Gynecol. Pathol. 1995, 14, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Akhan, S.E.; Dogan, Y.; Akhan, S.; Iyibozkurt, A.C.; Topuz, S.; Yalcin, O. Pelvic actinomycosis mimicking ovarian malignancy: Three cases. Eur. J. Gynaecol. Oncol. 2008, 29, 294–297. [Google Scholar] [PubMed]
- Kayikcioglu, F.; Akif Akgul, M.; Haberal, A.; Faruk Demir, O. Actinomyces infection in female genital tract. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 118, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Chudacoff, R.M.; Taylor, R.R. Streptococcus pneumoniae pelvic inflammatory disease. A case report. J. Reprod. Med. 1995, 40, 649–651. [Google Scholar] [PubMed]
- Pasticci, M.B.; Donnini, A.; Mencacci, A.; Lapalorcia, L.M.; Cavazzoni, E.; Baldelli, F. A diagnosis of pneumococcal peritonitis secondary to pyo-salpinx in a young healthy female by culturing peritoneal pus. New Microbiol. 2008, 31, 295–298. [Google Scholar] [PubMed]
- Mack, E.; Wee, H.Y. Pelvic inflammatory disease caused by Streptococcus pneumoniae in a heavy smoker after laparoscopic surgery. Ann. Acad. Med. Singap. 2012, 41, 309–310. [Google Scholar] [CrossRef]
- Sirotnak, A.P.; Eppes, S.C.; Klein, J.D. Tuboovarian abscess and peritonitis caused by Streptococcus pneumoniae serotype 1 in young girls. Clin. Infect. Dis. 1996, 22, 993–996. [Google Scholar] [CrossRef]
- Bucher, A.; Müller, F. Spectrum of abdominal and pelvic infections caused by pneumococci in previously healthy adult women. Eur. J. Clin. Microbiol. Infect. Dis. 2002, 21, 474–477. [Google Scholar] [CrossRef]
- Makhijani, N.; Sondheim, S.E.; Saul, T.; Yetter, E. Loculated Fluid Visualized in Hepatorenal Space with Point-of-care Ultrasound in Patient with Pelvic Inflammatory Disease Caused by Group A Streptococcus: Case Report. Clin. Pract. Cases Emerg. Med. 2024, 8, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Dharia, S.; Shah, S.; Kissinger, M.; Sanders, A.; Singh, G. Group A Streptococcal Endometritis and Toxic Shock causing Septic Pelvic Thrombophlebitis and Septic Pulmonary Emboli. BMJ Case Rep. 2023, 16, bcr-2023-255455. [Google Scholar] [CrossRef] [PubMed]
- Wolfenden, E.; Mittal, M.; Sussman, R. Complex clinical management of group A Streptococcal pelvic inflammatory disease after bilateral tubal ligation in a small community hospital. BMJ Case Rep. 2020, 13, bcr-2020-236326. [Google Scholar] [CrossRef]
- Lusby, H.; Brooks, A.; Hamayoun, E.; Finley, A. Uncommon cause of pelvic inflammatory disease leading to toxic shock syndrome. BMJ Case Rep. 2018, 2018, bcr-2018-224955. [Google Scholar] [CrossRef]
- Lamb, E.K.; Anasti, J.N.; Leonetti, H.B. Group A Streptococcus causing PID from an initial pharyngeal infection. A case report. J. Reprod. Med. 1999, 44, 639–641. [Google Scholar]
- Horii, T.; Izumida, S.; Takeuchi, K.; Tada, T.; Ishikawa, J.; Tsuboi, K. Acute peritonitis and salpingitis associated with streptococcal toxic shock syndrome caused by Lancefield group G alpha-haemolytic Streptococcus dysgalactiae subsp. equisimilis. J. Med. Microbiol. 2006, 55, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Reaves, S.; Mehta, V.; Baxter, J.K.; Ross, R. Postpartum Group A strep sepsis after third trimester uterine prolapse: Case report and literature review. Arch. Gynecol. Obstet. 2022, 306, 1949–1952. [Google Scholar] [CrossRef] [PubMed]
- Riad, M.; Thottacherry, E.; Crawley, C.; Phillip-Abraham, N.; Ibrahim, F. Invasive Group A streptococcal postpartum endometritis associated with multi-organ infarctions: An uncommon case presentation and literature review. Postgrad. Med. 2020, 132, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Blot, M.; de Curraize, C.; Salmon-Rousseau, A.; Gehin, S.; Bador, J.; Chavanet, P.; Neuwirth, C.; Piroth, L.; Amoureux, L. Streptococcus pyogenes: An unusual cause of salpingitis. Case report and review of the literature. Infection 2017, 45, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Solt, I.; Ioffe, Y.; Elmore, R.G.; Solnik, M.J. Group A streptococcal peritonitis and ruptured tubo-ovarian abscess three years after Essure® insertion: A case report. J Womens Health 2011, 20, 781–783. [Google Scholar] [CrossRef] [PubMed]
- Kouijzer, I.J.; Polderman, F.N.; Bekers, E.M.; Bloks, P.H.; Schneeberger, P.M.; de Jager, C.P. Initially unrecognised group A streptococcal pelvic inflammatory disease in a postmenopausal woman. Neth. J. Med. 2014, 72, 494–496. [Google Scholar]
- Snyder, A.; Schmalzle, S.A. Spontaneous Streptococcus pyogenes pelvic inflammatory disease; Case report and review of the literature. IDCases 2020, 20, e00785. [Google Scholar] [CrossRef]
- Gendron, N.; Joubrel, C.; Nedellec, S.; Campagna, J.; Agostini, A.; Doucet-Populaire, F.; Casetta, A.; Raymond, J.; Poyart, C.; Kernéis, S. Group A Streptococcus endometritis following medical abortion. J. Clin. Microbiol. 2014, 52, 2733–2735. [Google Scholar] [CrossRef] [PubMed]
- Garvey, P.; Ledger, W.J. Group a streptococcus in the gynecologic patient. Infect. Dis. Obstet. Gynecol. 1997, 5, 391–394. [Google Scholar] [CrossRef]
- Simpson-Camp, L.; Richardson, E.J.; Alaish, S.M. Streptococcus viridans tubo-ovarian abscess in an adolescent virgin. Pediatr. Int. 2012, 54, 706–709. [Google Scholar] [CrossRef]
- Alenazi, H. Ruptured Appendiceal Diverticulum Leading to Tubo-Ovarian Abscess in a Non-Sexually Active Woman: A Case Study. Am. J. Case Rep. 2024, 25, e945366. [Google Scholar] [CrossRef] [PubMed]
- Mills, D.; Sharon, B.; Schneider, K. Streptococcus constellatus Tubo-ovarian Abscess in a Non-Sexually Active Adolescent Female. Pediatr. Emerg. Care 2018, 34, e100–e101. [Google Scholar] [CrossRef]
- Inglot, M.; Szymczak, A.; Fleischer-Stepniewska, K.; Fleischer, M.; Staszek-Zurowska, B.; Gladysz, A. Tubo-ovarian abscess during therapy of chronic hepatitis C with pegylated interferon and ribavirin. Neuro Endocrinol. Lett. 2011, 32, 1–3. [Google Scholar] [PubMed]
- Algren, S.D.; Strickland, J.L. Beta hemolytic streptococcus group f causing pelvic inflammatory disease in a 14-year-old girl. J. Pediatr. Adolesc. Gynecol. 2005, 18, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Endo, Y.; Furukawa, S.; Ono, A.; Kiko, Y.; Soeda, S.; Watanabe, T.; Takahashi, T.; Fujimori, K. Successful laparoscopic resection of ovarian abscess caused by Staphylococcus aureus in a 13-year-old girl: A case report and review of literature. BMC Womens Health 2021, 21, 198. [Google Scholar] [CrossRef] [PubMed]
- Punia, R.S.; Aggarwal, R.; Amanjit; Mohan, H. Xanthogranulomatous oophoritis and salpingitis: Late sequelae of inadequately treated staphylococcal PID. Indian. J. Pathol. Microbiol. 2003, 46, 80–81. [Google Scholar] [PubMed]
- Buitrago, M.I.; Crompton, J.A.; Bertolami, S.; North, D.S.; Nathan, R.A. Extremely low excretion of daptomycin into breast milk of a nursing mother with methicillin-resistant Staphylococcus aureus pelvic inflammatory disease. Pharmacotherapy 2009, 29, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Bello, C.; Eskandar, M.; El, G.R.; Sobande, A.; Nour, H.; Shafiq, H. Staphylococcus lugdunensis endometritis: A case report. West. Afr. J. Med. 2007, 26, 243–245. [Google Scholar]
- Ntioudi, M.; Vasiliadou, K.; Charalampidou-Keremidou, P. Idiopathic pyometra and tubo-ovarian abscess in a postmenopausal patient treated conservatively. Ger. Med. Sci. 2022, 20, Doc09. [Google Scholar] [CrossRef]
- Bravender, T.; Matson, S.C. Adolescents, IUDs, PID, and Enterococcus: A report of two cases. J. Pediatr. Adolesc. Gynecol. 2012, 25, e73–e74. [Google Scholar] [CrossRef]
- Covin, B.D.; Chapa, H.; Pham, N. Clostridium perfringens of unclear origin causing pelvic inflammatory disease and toxic shock syndrome in a previously healthy young woman. BMJ Case Rep. 2021, 14, bcr-2021-242492. [Google Scholar] [CrossRef]
- Yavuzcan, A.; Cağlar, M.; Dilbaz, S.; Kumru, S.; Avcioğlu, F.; Ustün, Y. Identification of Clostridium septicum in a tubo-ovarian abscess: A rare case and review of the literature. Vojnosanit. Pregl. 2014, 71, 884–888. [Google Scholar] [CrossRef]
- Fischer, M.; Bhatnagar, J.; Guarner, J.; Reagan, S.; Hacker, J.K.; Van Meter, S.H.; Poukens, V.; Whiteman, D.B.; Iton, A.; Cheung, M.; et al. Fatal toxic shock syndrome associated with Clostridium sordellii after medical abortion. N. Engl. J. Med. 2005, 353, 2352–2360. [Google Scholar] [CrossRef]
- Priputnevich, T.; Lyubasovskaya, L.; Muravieva, V.; Kondrakhin, A.; Ignateva, A.; Gordeev, A.; Shmakov, R.; Sukhikh, G.; Yarotskaya, E. Postpartum endometritis and obstetrical sepsis associated with Eggerthella lenta. Case report and review of the literature. J. Matern. Fetal Neonatal Med. 2021, 34, 313–317. [Google Scholar] [CrossRef]
- Veale, R.; Hughes, C.; Woolley, I. A novel case of bilateral tubo-ovarian abscesses attributed to Ruminococcus gnavus without gastrointestinal involvement. Anaerobe 2021, 67, 102312. [Google Scholar] [CrossRef] [PubMed]
- Gensheimer, W.G.; Reddy, S.Y.; Mulconry, M.; Greves, C. Abiotrophia/Granulicatella tubo-ovarian abscess in an adolescent virginal female. J. Pediatr. Adolesc. Gynecol. 2010, 23, e9–e12. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Muraoka, Y.; Toda, Y.; Kiyomoto, C.; Okubo, Y.; Nagashima, T.; Furukawa, S.; Fujiwara, M.; Mochizuki, M.; Kobayashi, Y.; et al. Finegoldia magna myometritis with uterine necrosis after uterine artery embolisation. J. Obstet. Gynaecol. 2017, 37, 688–689. [Google Scholar] [CrossRef]
- Yamagishi, Y.; Mikamo, H.; Tanaka, K.; Watanabe, K. A case of uterine endometritis caused by Atopobium vaginae. J. Infect. Chemother. 2011, 17, 119–121. [Google Scholar] [CrossRef]
- Carrillo-Ávila, J.A.; Bonilla-García, L.; Navarro-Marí, J.M.; Gutiérrez-Fernández, J. The first reported case of pelvic inflammatory disease caused by Actinobaculum massiliense. Anaerobe 2019, 55, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Naha, K.; Dasari, S.; Vivek, G.; Prabhu, M. Primary abdominal nocardiosis masquerading as tubercular pelvic inflammatory disease in an immunocompetent individual. BMJ Case Rep. 2013, 2013, bcr-2012-008076. [Google Scholar] [CrossRef] [PubMed]
- Bonifaz, A.; Espinosa-Díaz, S.; Argáez, J.; Hernández-Castro, R.; Xicohtencatl-Cortes, J.; Tirado-Sánchez, A. Actinomycetoma due to Nocardia brasiliensis with extension to the ovaries. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 211, 224–225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goodwin, K.; Fleming, N.; Dumont, T. Tubo-ovarian abscess in virginal adolescent females: A case report and review of the literature. J. Pediatr. Adolesc. Gynecol. 2013, 26, e99–e102. [Google Scholar] [CrossRef]
- Verta, S.; Brambs, C.E.; Christmann, C. Large Douglas Abscess with Distinctive Bilateral Salpingitis in a Young Virginal Woman 6 Months Following Small Bowel Perforation at the Level of the Jejunojejunostomy After Roux-en-Y Gastric Bypass: A Case Report. Int. J. Womens Health 2024, 16, 2343–2354. [Google Scholar] [CrossRef]
- Linck, J.; Torres, W.; Dayal, S. Post-hysteroscopy Ruptured Tubo-Ovarian Abscess With Atypical Bacteremia: A Case Report. Cureus 2023, 15, e45618. [Google Scholar] [CrossRef]
- Chen, K.Y.; Tseng, J.Y.; Yang, C.Y. Tubo-ovarian abscess with sepsis in a nonagenarian woman: A case report and literature review. BMC Womens Health 2019, 19, 81. [Google Scholar] [CrossRef]
- Bernick, J.; Beliavsky, A.; Bogoch, I.I. Endometritis and Bacteremia With a New Delhi Metallo-Beta-Lactamase 1 (NDM-1)-containing Organism in a Remote Traveler. J. Obstet. Gynaecol. Can. 2019, 41, 753–754. [Google Scholar] [CrossRef] [PubMed]
- He, X.F.; Du, X.P.; Qiao, C.F. Successful laparoscopic resection of fallopian tube abscess caused by Escherichia coli in a 12-year-old adolescent virgin:a case report and review of the literature. BMC Pediatr. 2023, 23, 282. [Google Scholar] [CrossRef]
- Boleken, M.E.; Günendi, T.; Yol, C.; Kaya, V.; Kocaman, O.H.; Dörterler, M.E. Xanthogranulomatous Salpingitis Presenting as Pyosalpinx in a Non-Sexually Active Adolescent Girl. J. Pediatr. Adolesc. Gynecol. 2023, 36, 324–327. [Google Scholar] [CrossRef]
- Sabzanov, S.; Ganz, M.; Adout, B.; Farahmandpour, N.; Colarusso, J.; Yusupov, D.; Riznyk, N.; Mishail, B.; Miller, D. Recurrent Tubo-Ovarian Abscesses in a Non-sexually Active Adolescent: A Case Report and Review of Atypical Risk Factors. Cureus 2025, 17, e84671. [Google Scholar] [CrossRef]
- Hornemann, A.; Koschitzky, H.; Bohlmann, M.; Hornung, D.; Diedrich, K.; Tafazzoli, K. Isolated pyosalpinx in a 13-year-old virgin. Fertil. Steril. 2009, 91, e2732.e9–e2732.e10. [Google Scholar] [CrossRef]
- Liberty, G.; Hyman, J.H.; Margalioth, E.J. Peri-implantation pelvic inflammatory disease with normal pregnancy outcome. Fertil. Steril. 2007, 88, 969.e1–969.e2. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Hsieh, Y.Y.; Tsai, H.D.; Lin, C.C. Tubo-ovarian abscess presenting as pneumoperitoneum. J. Assist. Reprod. Genet. 2002, 19, 42–43. [Google Scholar] [CrossRef] [PubMed]
- Gray, Y.; Libbey, N.P. Xanthogranulomatous salpingitis and oophoritis: A case report and review of the literature. Arch. Pathol. Lab. Med. 2001, 125, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Raj, J.A.; Jagadeesha, M.; Naveen, S.; Ramachandra, U. Xanthogranulomatous oophoritis: Pathologic findings with clinical correlation. J. Indian. Med. Assoc. 2012, 110, 653–654. [Google Scholar] [PubMed]
- Martin, D.; Dbouk, R.H.; Deleon-Carnes, M.; del Rio, C.; Guarner, J. Haemophilus influenzae acute endometritis with bacteremia: Case report and literature review. Diagn. Microbiol. Infect. Dis. 2013, 76, 235–236. [Google Scholar] [CrossRef]
- Nishimura, Y.; Hagiya, H.; Kawano, K.; Yokota, Y.; Oka, K.; Iio, K.; Hasegawa, K.; Obika, M.; Haruma, T.; Ono, S.; et al. Invasive non-typeable Haemophilus influenzae infection due to endometritis associated with adenomyosis. BMC Infect. Dis. 2020, 20, 521. [Google Scholar] [CrossRef]
- Valayatham, V. Salmonella: The pelvic masquerader. Int. J. Infect. Dis. 2009, 13, e53–e55. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.H.; Jeng, C.J.; Su, S.C.; Wang, K.G. Pelvic abscess caused by Salmonella: A case report. Zhonghua Yi Xue Za Zhi (Taipei) 1996, 57, 457–459. [Google Scholar]
- Sharma, P.; Bhuju, A.; Tuladhar, R.; Parry, C.M.; Basnyat, B. Tubo-ovarian abscess infected by Salmonella typhi. BMJ Case Rep. 2017, 2017, bcr-2017-221213. [Google Scholar] [CrossRef]
- Kudesia, R.; Gupta, D. Pelvic Salmonella infection masquerading as gynecologic malignancy. Obstet. Gynecol. 2011, 118, 475–477. [Google Scholar] [CrossRef]
- Anandathirtha, K.; Shabnam, Z.; Malempati, L.; Ramesh, N. Xanthogranulomatous endometritis with unilateral salpingo-oophoritis in a postmenopausal woman masquerading as a malignancy. BMJ Case Rep. 2023, 16, e247341. [Google Scholar] [CrossRef]
- King, J.A.; Olsen, T.G.; Lim, R.; Nycum, L.R. Pseudomonas aeruginosa-infected IUD associated with pelvic inflammatory disease. A case report. J. Reprod. Med. 2002, 47, 1035–1037. [Google Scholar]
- Alajeel, A.A.; Garland, S.M. An unusual cause of pelvic inflammatory disease due to Neisseria meningitidis. Sex. Health 2004, 1, 157–160. [Google Scholar] [CrossRef]
- AlHabil, Y.; Owda, A.N.; Zaid, B.J.; Hameedi, S.; Saadeddin, L.; Awad, M.A.A. Concurrent acute cystitis, pancolitis, and tubo-ovarian abscess following laparoscopic ovarian cystectomy: A case report. BMC Womens Health 2024, 24, 489. [Google Scholar] [CrossRef] [PubMed]
- Nernsai, P.; Sophonsritsuk, A.; Lertvikool, S.; Jinawath, A.; Chitasombat, M.N. A case report of Tubo-ovarian abscess caused by Burkholderia pseudomallei. BMC Infect. Dis. 2018, 18, 73. [Google Scholar] [CrossRef] [PubMed]
- Shittu, S.; Athar, S.; Khyatt, O.; Chaponda, M.; Thodi, V.; Al-Maslamani, K.; Alansari, L. Maternal sepsis due to Bacteroides fragilis: A case report and review of the literature. J. Med. Case Rep. 2025, 19, 347. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, A.; Black, A.Y.; Lortie, K.; Fleming, N.A. A case of adolescent pelvic inflammatory disease caused by a rare bacterium: Fusobacterium nucleatum. J. Pediatr. Adolesc. Gynecol. 2013, 26, e113–e115. [Google Scholar] [CrossRef]
- Chayachinda, C.; Leelaporn, A.; Ruangvutilert, P.; Thamkhantho, M. Post-partum, post-sterilization tubo-ovarian abscess caused by Fusobacterium necrophorum: A case report. J. Med. Case Rep. 2012, 6, 330. [Google Scholar] [CrossRef]
- Koshy, K.M.; Malik, W.; Roberts, S.C. Myometritis with pelvic septic vein thrombophlebitis secondary to Fusobacterium necrophorum sepsis. BMJ Case Rep. 2022, 15, bcr-2022-250097. [Google Scholar] [CrossRef]
- Tamura, S.; Jwa, S.C.; Tarumoto, N.; Ishihara, O. Septic Shock Caused by Fusobacterium Necrophorum after Sexual Intercourse during Recovery from Infectious Mononucleosis in an Adolescent: A Case Report. J. Pediatr. Adolesc. Gynecol. 2020, 33, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.H.; Kuo, S.F.; Lee, C.H. Concurrency of splenomegaly and numerous enlarged mesenteric and retroperitoneal lymph nodes in a patient with pelvic inflammatory disease caused by Edwardsiella tarda: Mimicking lymphoma. Kaohsiung J. Med. Sci. 2019, 35, 446–447. [Google Scholar] [CrossRef] [PubMed]
- Mora-Palma, J.C.; Rodríguez-Oliver, A.J.; Navarro-Marí, J.M.; Gutiérrez-Fernández, J. Emergent genital infection by Leptotrichia trevisanii. Infect. 2019, 47, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Masschaele, T.; Steyaert, S.; Goethals, R. Leptrotrichia Amnionii, an emerging pathogen of postpartum endometritis. Acta Clin. Belg. 2018, 73, 368–371. [Google Scholar] [CrossRef]
- Tanabe, S.; Uehara, E.; Ichida, K.; Kan, T.; Morishima, S. A case of periodic abdominal pain and fever due to left ovarian abscess. Radiol. Case Rep. 2024, 19, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Loïez, C.; Wallet, F.; Husson, M.O.; Courcol, R.J. Pasteurella multocida and intrauterine device: A woman and her pets. Scand. J. Infect. Dis. 2002, 34, 473. [Google Scholar] [CrossRef]
- Roth, T.; Hentsch, C.; Erard, P.; Tschantz, P. Pyosalpinx: Not always a sexual transmitted disease? Pyosalpinx caused by Plesiomonas shigelloides in an immunocompetent host. Clin. Microbiol. Infect. 2002, 8, 803–805. [Google Scholar] [CrossRef][Green Version]
- Kitamura, S.; Matsumura, N.; Ohtake, N.; Kita, M.; Konishi, I. Tubo-ovarian abscess with endometrial cyst probably infected by Campylobacter fetus: Two cases. J. Obstet. Gynaecol. Res. 2016, 42, 1052–1057. [Google Scholar] [CrossRef]
- Walder, G.; Meusburger, H.; Hotzel, H.; Oehme, A.; Neunteufel, W.; Dierich, M.P.; Würzner, R. Chlamydophila abortus pelvic inflammatory disease. Emerg. Infect. Dis. 2003, 9, 1642–1644. [Google Scholar] [CrossRef]
- Nitta, Y.; Shibata, T.; Kato, H.; Nakago, S. Pelvic inflammatory disease associated with cytomegalovirus infection in an immunocompetent adult: Case report and literature review. Clin. Case Rep. 2024, 12, e9323. [Google Scholar] [CrossRef]
- Takagi, I.; Akiyama, H.; Matsuba, H.; Rikitake, J.; Kozuki, Y.; Miyata, Y.; Nakanishi, M.; Inaba, M.; Iwata, N.; Kakiuchi, S. Cytomegalovirus Oophoritis Mimicking Burkitt’s Lymphoma Recurrence: A Case Report and Literature Review. Intern. Med. 2023, 62, 1861–1866. [Google Scholar] [CrossRef] [PubMed]
- Fpathfu, W.A.; Nausheen, Y.; Fpathkfu, R.A.; Khudairi, A.A.; Nemenqani, D. Histopathological features of an incidental case of cytomegalovirus salpingitis in a patient with inflammatory bowel disease. J. Pak. Med. Assoc. 2013, 63, 780–783. [Google Scholar]
- Manfredi, R.; Alampi, G.; Talò, S.; Calza, L.; Tadolini, M.; Martinelli, G.N.; Chiodo, F. Silent oophoritis due to cytomegalovirus in a patient with advanced HIV disease. Int. J. STD AIDS 2000, 11, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Nieto, Y.; Ross, M.; Gianani, R.; Shpall, E.J.; Cagnoni, P.J.; Bearman, S.I.; Jones, R.B. Post-mortem incidental finding of cytomegalovirus oophoritis after an allogeneic stem cell transplant. Bone Marrow Transplant. 1999, 23, 1323–1324. [Google Scholar] [CrossRef][Green Version]
- Hsu, W.C.; Lee, Y.H.; Chang, D.Y. Tuboovarian abscess caused by Candida in a woman with an intrauterine device. Gynecol. Obstet. Invest. 2007, 64, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Calore, E.E.; Calore, N.M.; Cavaliere, M.J. Salpingitis due to Entamoeba histolytica. Braz. J. Infect. Dis. 2002, 6, 97–99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wadhwa, N.; Raoot, A. An unusual case of adult filarial oophoritis. Int. J. Gynecol. Pathol. 2011, 30, 549–552. [Google Scholar] [CrossRef]
- Chan, R.M.; Lee, P.; Wroblewski, J. Deep-seated trichosporonosis in an immunocompetent patient: A case report of uterine trichosporonosis. Clin. Infect. Dis. 2000, 31, 621. [Google Scholar] [CrossRef]
- Mentessidou, A.; Theocharides, C.; Patoulias, I.; Panteli, C. Enterobius vermicularis-Associated Pelvic Inflammatory Disease in a Child. J. Pediatr. Adolesc. Gynecol. 2016, 29, e25–e27. [Google Scholar] [CrossRef]
- Saleem, F.; Malik, F.; Fatima, S. Enterobius vermicularis in tubo-ovarian abscess: A rare and interesting incidental finding—A case Report. J. Pak. Med. Assoc. 2017, 67, 630–633. [Google Scholar] [PubMed]
- Ngui, R.; Ravindran, S.; Ong, D.B.; Chow, T.K.; Low, K.P.; Nureena, Z.S.; Rajoo, Y.; Chin, Y.T.; Amir, A.; Ahmad, A.F.; et al. Enterobius vermicularis salpingitis seen in the setting of ectopic pregnancy in a Malaysian patient. J. Clin. Microbiol. 2014, 52, 3468–3470. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tandan, T.; Pollard, A.J.; Money, D.M.; Scheifele, D.W. Pelvic inflammatory disease associated with Enterobius vermicularis. Arch. Dis. Child. 2002, 86, 439–440. [Google Scholar] [CrossRef]
- Das, D.K.; Pathan, S.K.; Hira, P.R.; Madda, J.P.; Hasaniah, W.F.; Juma, T.H. Pelvic abscess from enterobius vermicularis. Report of a case with cytologic detection of eggs and worms. Acta Cytol. 2001, 45, 425–429. [Google Scholar] [CrossRef]
- Erhan, Y.; Zekioğlu, O.; Ozdemir, N.; Sen, S. Unilateral salpingitis due to enterobius vermicularis. Int. J. Gynecol. Pathol. 2000, 19, 188–189. [Google Scholar] [CrossRef] [PubMed]
- de Otazu, R.D.; García-Nieto, L.; Izaguirre-Gondra, E.; Mayayo, E.; Ciani, S.; Nogales, F.F. Endometrial coccidiosis. J. Clin. Pathol. 2004, 57, 1104–1105. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, J.H.; Kephart, G.M.; Frankson, J.L. Eosinophilic oophoritis: Association with positive Strongyloides stercoralis serology and clinical response to ivermectin. J. Pediatr. Adolesc. Gynecol. 2006, 19, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Lechner, A.; Bogner, G.; Hasenöhrl, G. Postpartal endomyometritis in a case of unknown tertian malaria. Infection 1997, 25, 185–186. [Google Scholar] [CrossRef]
- Egbe, T.O.; Kobenge, F.M.; Arlette, M.M.J.; Belley-Priso, E. Pyosalpinges after hysterosalpingography in a patient with lower genital tract infection and managed by laparoscopic surgery in a resource low tertiary hospital case report and literature review. Fertil. Res. Pract. 2018, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Andrews, T.J.; Hicar, M.; Islam, S. The Role of a Spinning Top Urethra in the Development of Pyosalpinx in a Precoital Female. Cureus 2022, 14, e27099. [Google Scholar] [CrossRef]
- Colussi, M.; Horwood, G.; McCall, J.; Gale, J.; Singh, S. Septic shock after a saline infusion hysterosalpingosonogram in a woman with stage IV endometriosis and infertility: A case report. Case Rep. Womens Health 2024, 44, e00663. [Google Scholar] [CrossRef]
- Maraqa, T.; Mohamed, M.; Coffey, D.; Sachwani-Daswani, G.R.; Alvarez, C.; Mercer, L. Bilateral recurrent pyosalpinx in a sexually inactive 12-year-old girl secondary to rare variant of Mullerian duct anomaly. BMJ Case Rep. 2017, 2017, bcr-2016-218924. [Google Scholar] [CrossRef]
- King, A.L.; Stamatopoulos, N. Concurrent Escherichia coli tubo-ovarian abscess and Campylobacter jejuni gastroenteritis: A case report. Case Rep. Womens Health 2020, 26, e00192. [Google Scholar] [CrossRef] [PubMed]
- Floyd, R.; Anglim, B. Tubo-ovarian abscess after vaginal delivery: A case report and review of current literature. Case Rep. Womens Health 2023, 39, e00526. [Google Scholar] [CrossRef]
- Noack, F.; Briese, J.; Stellmacher, F.; Hornung, D.; Horny, H.P. Lethal outcome in xanthogranulomatous endometritis. Apmis 2006, 114, 386–388. [Google Scholar] [CrossRef]
- Batool, R.; Abdul Wahab, N.A.; Selvamani, S.; Hennessy, G. Recurrent pyometra coupled with xanthogranulomatous endometritis mimicking pyelonephritis and malignancy: Unravelling clinical complexities. BMJ Case Rep. 2025, 18, bcr-2023-259441. [Google Scholar] [CrossRef]
- Giraldo-Isaza, M.A.; Jaspan, D.; Cohen, A.W. Postpartum endometritis caused by herpes and cytomegaloviruses. Obstet. Gynecol. 2011, 117, 466–467. [Google Scholar] [CrossRef]
- Wu, C.M.; Noska, A. Intrauterine device infection causing concomitant streptococcal toxic shock syndrome and pelvic abscess with Actinomyces odontolyticus bacteraemia. BMJ Case Rep. 2016, 2016, bcr-2015-213236. [Google Scholar] [CrossRef]
- Yu, T. Minimally invasive treatment of uterine necrosis with favorable outcomes: An uncommon case presentation and literature review. BMC Women’s Health 2024, 24, 267. [Google Scholar] [CrossRef] [PubMed]
- Perniola, G.; Di Tucci, C.; Derme, M.; Muzii, L.; Lecce, F.; Benedetti Panici, P. Tuberculous endometritis in woman with abnormal uterine bleeding: A case report and literature review. J. Obstet. Gynaecol. 2021, 41, 671–672. [Google Scholar] [CrossRef] [PubMed]
- Gazos, E.; Tsonis, O.; Gkrozou, F.; Paschopoulos, M. “Silent” post-menopausal genital tuberculosis with lethal outcome. Indian. J. Tuberc. 2020, 67, 357–359. [Google Scholar] [CrossRef]
- Ingec, M.; Erdogan, F.; Kumtepe, Y.; Isaoglu, U.; Gundogdu, C.; Kadanali, S. Management of bilateral fallopian tube carcinoma coexistent with tuberculous salpingitis. J. Obstet. Gynaecol. Res. 2005, 31, 65–67. [Google Scholar] [CrossRef]
- Onuigbo, W.; Esimai, B.; Nwaekpe, C.; Chijioke, G. Tubercular endometritis detected through Pap smear campaign in Enugu, Nigeria. Pan Afr. Med. J. 2012, 11, 47. [Google Scholar]
- Semfke, A.; Wackernagel, C.; Vier, H.; Schütz, A.; Wiechmann, V.; Gillissen, A. Histologically proven isoniazid hepatoxicity in complicated tuberculous salpingitis. Ther. Adv. Respir. Dis. 2009, 3, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Dadhwal, V.; Gupta, N.; Bahadur, A.; Mittal, S. Flare-up of genital tuberculosis following endometrial aspiration in a patient of generalized miliary tuberculosis. Arch. Gynecol. Obstet. 2009, 280, 503–504. [Google Scholar] [CrossRef]
- Cicinelli, E.; Tinelli, R.; Colafiglio, G.; Saliani, N.; Pastore, A. Tubercular endometritis: A rare condition reliably detectable with fluid hysteroscopy. J. Minim. Invasive Gynecol. 2008, 15, 752–754. [Google Scholar] [CrossRef]
- Güngördük, K.; Ulker, V.; Sahbaz, A.; Ark, C.; Tekirdag, A.I. Postmenopausal tuberculosis endometritis. Infect. Dis. Obstet. Gynecol. 2007, 2007, 27028. [Google Scholar] [CrossRef]
- Narayan, R.L.; Patel, A.; McDonald, R.J. Tuberculous peritonitis and tuberculous endometritis diagnosed in the same patient by high clinical suspicion and a minimally invasive approach. Mt. Sinai J. Med. 2006, 73, 1112–1114. [Google Scholar] [PubMed]
- Gascón, J.; Acién, P. Large bilateral tubercular pyosalpinx in a young woman with genitourinary malformation: A case report. J. Med. Case Rep. 2014, 8, 176. [Google Scholar] [CrossRef]
- Dwivedi, K.; Prasad, M. Tuberculous endometritis—An unusual cause of postmenopausal bleeding in the United Kingdom. J. Obstet. Gynaecol. 2016, 36, 124–125. [Google Scholar] [CrossRef]
- Perdhana, R.; Sutrisno, S.; Sugiri, Y.J.; Baktiyani, S.C.; Wiyasa, A. Patients with secondary amenorrhea due to tuberculosis endometritis towards the induced anti-tuberculosis drug category 1. Pan Afr. Med. J. 2016, 24, 121. [Google Scholar] [CrossRef]
- Nayar, M.; Chandra, M.; Chitraratha, K.; Kumari Das, S.; Rai Chowdhary, G. Incidence of actinomycetes infection in women using intrauterine contraceptive devices. Acta Cytol. 1985, 29, 111–116. [Google Scholar]
- Westhoff, C. IUDs and colonization or infection with Actinomyces. Contraception 2007, 75, S48–S50. [Google Scholar] [CrossRef]
- Tzelios, C.; Neuhausser, W.M.; Ryley, D.; Vo, N.; Hurtado, R.M.; Nathavitharana, R.R. Female Genital Tuberculosis. Open Forum Infect. Dis. 2022, 9, ofac543. [Google Scholar] [CrossRef]
- Cohen, C.R.; Mugo, N.R.; Astete, S.G.; Odondo, R.; Manhart, L.E.; Kiehlbauch, J.A.; Stamm, W.E.; Waiyaki, P.G.; Totten, P.A. Detection of Mycoplasma genitalium in women with laparoscopically diagnosed acute salpingitis. Sex. Transm. Infect. 2005, 81, 463–466. [Google Scholar] [CrossRef]
- Lis, R.; Rowhani-Rahbar, A.; Manhart, L.E. Mycoplasma genitalium infection and female reproductive tract disease: A meta-analysis. Clin. Infect. Dis. 2015, 61, 418–426. [Google Scholar] [CrossRef]
- Short, V.L.; Totten, P.A.; Ness, R.B.; Astete, S.G.; Kelsey, S.F.; Haggerty, C.L. Clinical presentation of Mycoplasma genitalium Infection versus Neisseria gonorrhoeae infection among women with pelvic inflammatory disease. Clin. Infect. Dis. 2009, 48, 41–47. [Google Scholar] [CrossRef][Green Version]
- Haggerty, C.L.; Ness, R.B.; Totten, P.A.; Farooq, F.; Tang, G.; Ko, D.; Hou, X.; Fiedler, T.L.; Srinivasan, S.; Astete, S.G.; et al. Presence and Concentrations of Select Bacterial Vaginosis-Associated Bacteria Are Associated With Increased Risk of Pelvic Inflammatory Disease. Sex. Transm. Dis. 2020, 47, 344–346. [Google Scholar] [CrossRef]
- Srinivasan, S.; Hoffman, N.G.; Morgan, M.T.; Matsen, F.A.; Fiedler, T.L.; Hall, R.W.; Ross, F.J.; McCoy, C.O.; Bumgarner, R.; Marrazzo, J.M.; et al. Bacterial communities in women with bacterial vaginosis: High resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS ONE 2012, 7, e37818. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Rycroft, A.N.; Dogan, B.; Craven, M.; Bromfield, J.J.; Chandler, A.; Roberts, M.H.; Price, S.B.; Gilbert, R.O.; Simpson, K.W. Specific strains of Escherichia coli are pathogenic for the endometrium of cattle and cause pelvic inflammatory disease in cattle and mice. PLoS ONE 2010, 5, e9192. [Google Scholar] [CrossRef] [PubMed]
- Wiesenfeld, H.C.; Hillier, S.L.; Meyn, L.A.; Amortegui, A.J.; Sweet, R.L. Subclinical pelvic inflammatory disease and infertility. Obstet. Gynecol. 2012, 120, 37–43. [Google Scholar] [CrossRef]
- Nishida, N.; Shono, T.; Shono, K.; Hashimoto, Y.; Kawakami, K. Late Occurrence of the Tubo-Ovarian Abscess after Appendectomy for Perforated Appendicitis in a Virginal Adolescent Girl. J. Pediatr. Adolesc. Gynecol. 2022, 35, 509–511. [Google Scholar] [CrossRef]
- Margaux Becker, V.; Silver, S.; Seufert, R.; Muensterer, O.J. The Association of Appendectomy, Adhesions, Tubal Pathology, and Female Infertility. J. Soc. Laparoendosc. Surg. 2019, 23, e2018.00099. [Google Scholar] [CrossRef]
- Meng, Q.; Gao, Q.; Mehrazarin, S.; Tangwanichgapong, K.; Wang, Y.; Huang, Y.; Pan, Y.; Robinson, S.; Liu, Z.; Zangiabadi, A.; et al. Fusobacterium nucleatum secretes amyloid-like FadA to enhance pathogenicity. EMBO Rep. 2021, 22, e52891. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, T.S.; Noh, E.J.; Jang, A.R.; Ahn, J.H.; Kim, D.Y.; Jung, D.H.; Song, E.J.; Lee, Y.J.; Lee, Y.J.; et al. Receptor-interacting protein kinase 2 contributes to host innate immune responses against Fusobacterium nucleatum in macrophages and decidual stromal cells. Am. J. Reprod. Immunol. 2021, 86, e13403. [Google Scholar] [CrossRef]
- Sehnal, B.; Beneš, J.; Kolářová, Z.; Mojhová, M.; Zikán, M. Pelvic actinomycosis and IUD. Ceska Gynekol. 2018, 83, 386–390. [Google Scholar] [PubMed]
- Wong, V.K.; Turmezei, T.D.; Weston, V.C. Actinomycosis. BMJ 2011, 343, d6099. [Google Scholar] [CrossRef]
- Akbulut, S.; Arikanoglu, Z.; Basbug, M. Tubercular tubo-ovarian cystic mass mimicking acute appendicitis: A case report. J. Med. Case Rep. 2011, 5, 363. [Google Scholar] [CrossRef][Green Version]
- Hillier, S.L.; Bernstein, K.T.; Aral, S. A Review of the Challenges and Complexities in the Diagnosis, Etiology, Epidemiology, and Pathogenesis of Pelvic Inflammatory Disease. J. Infect. Dis. 2021, 224, S23–S28. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.; Guaschino, S.; Cusini, M.; Jensen, J. 2017 European guideline for the management of pelvic inflammatory disease. Int. J. STD AIDS 2018, 29, 108–114. [Google Scholar] [CrossRef]
- Ross, J.; Cole, M.; Evans, C.; Lyons, D.; Dean, G.; Cousins, D.; PPI Representative; British Association for Sexual Health and HIV. United Kingdom National Guideline for the Management of Pelvic Inflammatory Disease (2019 Interim Update). Available online: https://www.bashh.org/_userfiles/pages/files/resources/pidupdate2019.pdf (accessed on 22 August 2025).
- Korn, A.P. Pelvic inflammatory disease in women infected with HIV. AIDS Patient Care STDS 1998, 12, 431–434. [Google Scholar] [CrossRef]
- Peipert, J.F.; Ness, R.B.; Blume, J.; Soper, D.E.; Holley, R.; Randall, H.; Sweet, R.L.; Sondheimer, S.J.; Hendrix, S.L.; Amortegui, A.; et al. Clinical predictors of endometritis in women with symptoms and signs of pelvic inflammatory disease. Am. J. Obstet. Gynecol. 2001, 184, 856–864. [Google Scholar] [CrossRef]
- Ness, R.B.; Soper, D.E.; Richter, H.E.; Randall, H.; Peipert, J.F.; Nelson, D.B.; Schubeck, D.; McNeeley, S.G.; Trout, W.; Bass, D.C.; et al. Chlamydia antibodies, chlamydia heat shock protein, and adverse sequelae after pelvic inflammatory disease: The PID Evaluation and Clinical Health (PEACH) Study. Sex. Transm. Dis. 2008, 35, 129–135. [Google Scholar] [CrossRef]
- Jenkins, S.M.; Vadakekut, E.S. Pelvic Inflammatory Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Curry, A.; Williams, T.; Penny, M.L. Pelvic Inflammatory Disease: Diagnosis, Management, and Prevention. Am. Fam. Physician 2019, 100, 357–364. [Google Scholar]
- Liu, Y. Actinomycosis-induced adnexal and uterine masses mimicking malignancy on FDG PET/CT. Am. J. Obstet. Gynecol. 2019, 220, 281. [Google Scholar] [CrossRef] [PubMed]
- Vincent, S.; Lowe, S.; Sumida, M.; Dietrich, J. 89. Pelvic Inflammatory Disease in the Sexually Active and Non-sexually Active Pediatric and Adolescent Population. J. Pediatr. Adolesc. Gynecol. 2025, 38, 273–274. [Google Scholar] [CrossRef]
- Cazanave, C.; de Barbeyrac, B. Pelvic inflammatory diseases: Microbiologic diagnosis—CNGOF and SPILF Pelvic Inflammatory Diseases Guidelines. Gynecol. Obstet. Fertil. Senol. 2019, 47, 409–417. [Google Scholar] [CrossRef]
- Amin-Hanjani, S.; Chatwani, A. Endometrial Cultures in Acute Pelvic Inflammatory Disease. Infect. Dis. Obstet. Gynecol. 1995, 3, 56–59. [Google Scholar] [CrossRef]
- Moore, E.; Soper, D.E. Clinical utility of CA125 levels in predicting laparoscopically confirmed salpingitis in patients with clinically diagnosed pelvic inflammatory disease. Infect. Dis. Obstet. Gynecol. 1998, 6, 182–185. [Google Scholar] [CrossRef]
- Duk, J.M.; Kauer, F.M.; Fleuren, G.J.; de Bruijn, H.W. Serum CA 125 levels in patients with a provisional diagnosis of pelvic inflammatory disease. Clinical and theoretical implications. Acta Obstet. Gynecol. Scand. 1989, 68, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.D.; Young, S.W.; Dahiya, N. Ultrasound of Pelvic Pain in the Nonpregnant Woman. Radiol. Clin. N. Am. 2019, 57, 601–616. [Google Scholar] [CrossRef]
- Dick, E.A.; Blanco, A.; De La Hoz Polo, M.; Basilico, R. ESR Essentials: Gynaecological causes of acute pelvic pain in women: A primer for emergent evaluation-practice recommendations by the European Society of Emergency Radiology. Eur. Radiol. 2025, 35, 6682–6695. [Google Scholar] [CrossRef]
- Franco, P.N.; García-Baizán, A.; Aymerich, M.; Maino, C.; Frade-Santos, S.; Ippolito, D.; Otero-García, M. Gynaecological Causes of Acute Pelvic Pain: Common and Not-So-Common Imaging Findings. Life 2023, 13, 2025. [Google Scholar] [CrossRef]
- Revzin, M.V.; Mathur, M.; Dave, H.B.; Macer, M.L.; Spektor, M. Pelvic Inflammatory Disease: Multimodality Imaging Approach with Clinical-Pathologic Correlation. Radio Graphics 2016, 36, 1579–1596. [Google Scholar] [CrossRef] [PubMed]
- Singla, V.; Dua, A.; Singh, T.; Jain, V. Multimodality imaging of acute gynecological emergencies—A pictorial essay. Abdom. Radiol. 2024, 49, 4042–4056. [Google Scholar] [CrossRef]
- Soper, D.E.; Wiesenfeld, H.C. The Continued Challenges in the Diagnosis of Acute Pelvic Inflammatory Disease: Focus on Clinically Mild Disease. J. Infect. Dis. 2021, 224, S75–S79. [Google Scholar] [CrossRef]
- Schindlbeck, C.; Mylonas, I. Pelvic inflammatory disease: Indication for laparoscopy? Gynakol. Prax. 2012, 36, 663–675. [Google Scholar]
- Bălăceanu, L.A.; Grigore, C.; Dina, I.; Gurău, C.D.; Mihai, M.M.; Bălăceanu-Gurău, B. CA125 as a Potential Biomarker in Non-Malignant Serous Effusions: Diagnostic and Prognostic Considerations. J. Clin. Med. 2025, 14, 4152. [Google Scholar] [CrossRef] [PubMed]
- Bourika, V.; Rekoumi, E.A.; Giamarellos-Bourboulis, E.J. Biomarkers to guide sepsis management. Ann. Intensive Care 2025, 15, 103. [Google Scholar] [CrossRef]
- Genç, S.; Toplu, M.I.; Salman, T.; Halk, E.; Özalp, M.; Çaltek, N.; Mihmanlı, V. Procalcitonin and inflammatory biomarkers in tubo-ovarian abscess: Predicting surgical intervention. Ulus. Travma Acil Cerrahi Derg. 2025, 31, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Aytan, P.; Gökulu, S.G.; Durukan, H.; Bozkurt-Babus, S.; Tasin, C.; Aslan, E.S.; Aytan, H. A New Marker for the Diagnosis of Acute Pelvic Inflammatory Disease: Immature Granulocyte. Clin. Lab. 2024, 70. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, N.; Wong, T. Canadian guidelines on sexually transmitted infections, 2006. Cmaj 2007, 176, 175–176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ong, J.; Bourne, C.; Dean, J.; Ryder, N.; Cornelisse, V.; Murray, S.; Kenchington, P.; Moten, A.; Gibbs, C.; Maunsell, S.; et al. Australian sexually transmitted infection (STI) management guidelines for use in primary care 2022 update. Sex. Health 2022, 20, 1–8. [Google Scholar] [CrossRef]
- Yusuf, H.; Trent, M. Management of Pelvic Inflammatory Disease in Clinical Practice. Ther. Clin. Risk Manag. 2023, 19, 183–192. [Google Scholar] [CrossRef]
- Ferrero, S.; Leone Roberti Maggiore, U.; Paudice, M.; Vellone, V.G.; Perrone, U.; Barra, F. Safety and efficacy of pharmacotherapies for pelvic inflammatory disease and endometriosis. Expert. Opin. Drug Saf. 2025, 24, 273–286. [Google Scholar] [CrossRef]
- Verdon, R. Treatment of uncomplicated pelvic inflammatory disease: CNGOF and SPILF Pelvic Inflammatory Diseases Guidelines. Gynecol. Obstet. Fertil. Senol. 2019, 47, 418–430. [Google Scholar] [CrossRef]
- Dalby, J.; Stoner, B.P. Sexually Transmitted Infections: Updates from the 2021 CDC Guidelines. Am. Fam. Physician 2022, 105, 514–520. [Google Scholar]
- Zhou, T.; Yuan, M.; Cui, P.; Li, J.; Jia, F.; Wang, S.; Liu, R. Effectiveness and safety of morinidazole in the treatment of pelvic inflammatory disease: A multicenter, prospective, open-label phase IV trial. Front Med. 2022, 9, 888186. [Google Scholar] [CrossRef]
- Mohammed, H.; Bokhary, R.; Nassif, M.; Mosli, M. Ovarian Crohn’s Disease: A Case Report and Review of the Literature. Case Rep. Gastrointest. Med. 2020, 2020, 1826469. [Google Scholar] [CrossRef] [PubMed]
- Canas, A.M.; Holloran-Schwartz, B.; Myles, T. Tuboovarian abscess 12 years after total abdominal hysterectomy. Obstet. Gynecol. 2004, 104, 1039–1041. [Google Scholar] [CrossRef]
- Landers, D.V.; Sweet, R.L. Tubo-ovarian abscess: Contemporary approach to management. Rev. Infect. Dis. 1983, 5, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Itani, K.M.; Wilson, S.E.; Awad, S.S.; Jensen, E.H.; Finn, T.S.; Abramson, M.A. Ertapenem versus cefotetan prophylaxis in elective colorectal surgery. N. Engl. J. Med. 2006, 355, 2640–2651. [Google Scholar] [CrossRef] [PubMed]
- Pelak, B.A.; Citron, D.M.; Motyl, M.; Goldstein, E.J.; Woods, G.L.; Teppler, H. Comparative in vitro activities of ertapenem against bacterial pathogens from patients with acute pelvic infection. J. Antimicrob. Chemother. 2002, 50, 735–741. [Google Scholar] [CrossRef][Green Version]
- Sartelli, M.; Chichom-Mefire, A.; Labricciosa, F.M.; Hardcastle, T.; Abu-Zidan, F.M.; Adesunkanmi, A.K.; Ansaloni, L.; Bala, M.; Balogh, Z.J.; Beltrán, M.A.; et al. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J. Emerg. Surg. 2017, 12, 29. [Google Scholar] [CrossRef]
- Solomkin, J.S.; Mazuski, J.E.; Bradley, J.S.; Rodvold, K.A.; Goldstein, E.J.C.; Baron, E.J.; O’Neill, P.J.; Chow, A.W.; Dellinger, E.P.; Eachempati, S.R.; et al. Diagnosis and Management of Complicated Intra-abdominal Infection in Adults and Children: Guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 133–164. [Google Scholar] [CrossRef]
- Tamma, P.D.; Heil, E.L.; Justo, J.A.; Mathers, A.J.; Satlin, M.J.; Bonomo, R.A. Infectious Diseases Society of America 2024 Guidance on the Treatment of Antimicrobial-Resistant Gram-Negative Infections. Clin. Infect. Dis. 2024, ciae403. [Google Scholar] [CrossRef]
- Al-Hasan, M.N.; Eckel-Passow, J.E.; Baddour, L.M. Impact of healthcare-associated acquisition on community-onset Gram-negative bloodstream infection: A population-based study: Healthcare-associated Gram-negative BSI. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1163–1171. [Google Scholar] [CrossRef]
- Schechner, V.; Nobre, V.; Kaye, K.S.; Leshno, M.; Giladi, M.; Rohner, P.; Harbarth, S.; Anderson, D.J.; Karchmer, A.W.; Schwaber, M.J.; et al. Gram-negative bacteremia upon hospital admission: When should Pseudomonas aeruginosa be suspected? Clin. Infect. Dis. 2009, 48, 580–586. [Google Scholar] [CrossRef]
- Micek, S.T.; Welch, E.C.; Khan, J.; Pervez, M.; Doherty, J.A.; Reichley, R.M.; Kollef, M.H. Empiric combination antibiotic therapy is associated with improved outcome against sepsis due to Gram-negative bacteria: A retrospective analysis. Antimicrob. Agents Chemother. 2010, 54, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.; Khetarpal, S. Anaerobic Infections. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Valour, F.; Sénéchal, A.; Dupieux, C.; Karsenty, J.; Lustig, S.; Breton, P.; Gleizal, A.; Boussel, L.; Laurent, F.; Braun, E.; et al. Actinomycosis: Etiology, clinical features, diagnosis, treatment, and management. Infect. Drug Resist. 2014, 7, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Skoutelis, A.; Petrochilos, J.; Bassaris, H. Successful treatment of thoracic actinomycosis with ceftriaxone. Clin. Infect. Dis. 1994, 19, 161–162. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Hall, V.; Thakker, B.; Gemmell, C.G. Antimicrobial susceptibility testing of Actinomyces species with 12 antimicrobial agents. J. Antimicrob. Chemother. 2005, 56, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Stone, B.; Patel, T.G.; De Silva, T.I.; Green, S.T.; Chapman, A.L.N. Successful treatment of actinomyces brain abscesses using once daily parenteral ceftriaxone in an outpatient setting: A case series. J. Infect. 2008, 57, 426–427. [Google Scholar] [CrossRef]
- Fuchs, P.C.; Barry, A.L.; Brown, S.D. In vitro activities of ertapenem (MK-0826) against clinical bacterial isolates from 11 North American medical centers. Antimicrob. Agents Chemother. 2001, 45, 1915–1918. [Google Scholar] [CrossRef][Green Version]
- Martin, M.V. The use of oral amoxycillin for the treatment of actinomycosis. A clinical and in vitro study. Br. Dent. J. 1984, 156, 252–254. [Google Scholar] [CrossRef]
- Bonifaz, A.; Tirado-Sánchez, A.; Calderón, L.; Montes de Oca, G.; Torres-Camacho, P.; Ponce, R.M. Treatment of cutaneous actinomycosis with amoxicillin/clavulanic acid. J. Dermatol. Treat. 2017, 28, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, K.; Nord, C.E.; Dornbusch, K. Antimicrobial in vitro susceptibility of Actinomyces israelii and arachnia propionica. Scand. J. Infect. Dis. 1977, 9, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Lerner, P.I. Susceptibility of pathogenic actinomycetes to antimicrobial compounds. Antimicrob. Agents Chemother. 1974, 5, 302–309. [Google Scholar] [CrossRef][Green Version]
- Choi, J.; Koh, W.J.; Kim, T.S.; Lee, K.S.; Han, J.; Kim, H.; Kwon, O.J. Optimal duration of IV and oral antibiotics in the treatment of thoracic actinomycosis. Chest 2005, 128, 2211–2217. [Google Scholar] [CrossRef]
- Hamid, D.; Baldauf, J.J.; Cuenin, C.; Ritter, J. Treatment strategy for pelvic actinomycosis: Case report and review of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 89, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, S.S.; Ross, J.J. Short-term treatment of actinomycosis: Two cases and a review. Clin. Infect. Dis. 2004, 38, 444–447. [Google Scholar] [CrossRef]
- Trutnovsky, G.; Tamussino, K.; Reich, O. Short-term antibiotic treatment of pelvic actinomycosis. Int. J. Gynaecol. Obstet. 2008, 101, 203–204. [Google Scholar] [CrossRef]
- Hirshberg, A.; Tsesis, I.; Metzger, Z.; Kaplan, I. Periapical actinomycosis: A clinicopathologic study. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2003, 95, 614–620. [Google Scholar] [CrossRef]
- Shree, K.K.; Somashekar, S.; Loganathan, E. Cytomegalovirus induced genital ulcer in human immunodeficiency virus positive patient. Indian. J. Sex. Transm. Dis. AIDS 2022, 43, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Kotton, C.N.; Kumar, D.; Manuel, O.; Chou, S.; Hayden, R.T.; Danziger-Isakov, L.; Asberg, A.; Tedesco-Silva, H.; Humar, A.; on behalf of The Transplantation Society International CMV Consensus Group. The Fourth International Consensus Guidelines on the Management of Cytomegalovirus in Solid Organ Transplantation. Transplantation 2025, 109, 1066–1110. [Google Scholar] [CrossRef] [PubMed]
- Smolyakov, R.; Talalay, B.; Yanai-Inbar, I.; Pak, I.; Alkan, M. Enterobius vermicularis infection of female genital tract: A report of three cases and review of literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 107, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sharma, S. Enterobius Vermicularis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Chou, A.; Austin, R.L. Entamoeba histolytica Infection. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Brun, J.-L.; Castan, B.; de Barbeyrac, B.; Cazanave, C.; Charvériat, A.; Faure, K.; Mignot, S.; Verdon, R.; Fritel, X.; Graesslin, O. Pelvic inflammatory diseases: Updated French guidelines. J. Gynecol. Obstet. Human. Reprod. 2020, 49, 101714. [Google Scholar] [CrossRef]
- Demirtas, O.; Akman, L.; Demirtas, G.S.; Hursitoglu, B.S.; Yilmaz, H. The role of the serum inflammatory markers for predicting the tubo-ovarian abscess in acute pelvic inflammatory disease: A single-center 5-year experience. Arch. Gynecol. Obstet. 2013, 287, 519–523. [Google Scholar] [CrossRef]
- Ribak, R.; Schonman, R.; Sharvit, M.; Schreiber, H.; Raviv, O.; Klein, Z. Can the Need for Invasive Intervention in Tubo-ovarian Abscess Be Predicted? The Implication of C-reactive Protein Measurements. J. Minim. Invasive Gynecol. 2020, 27, 541–547. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kim, B.W.; Kim, S.R.; Kim, J.H. The prediction of surgical intervention in patients with tubo-ovarian abscess. J. Obstet. Gynaecol. 2022, 42, 97–102. [Google Scholar] [CrossRef]
- Marshall, A.; Wimsett, J.; Handforth, C.; Unsworth, L.; Wilson, J.; Van Der Merwe, A.M.; Oyston, C. The Tubo-ovarian abscess study (TOAST): A single-center retrospective review of predictors of failed medical management. Int. J. Gynaecol. Obstet. 2025, 170, 927–935. [Google Scholar] [CrossRef]
- Shigemi, D.; Matsui, H.; Fushimi, K.; Yasunaga, H. Laparoscopic Compared With Open Surgery for Severe Pelvic Inflammatory Disease and Tubo-Ovarian Abscess. Obstet. Gynecol. 2019, 133, 1224–1230. [Google Scholar] [CrossRef]
- Witkin, S.S.; Minis, E.; Athanasiou, A.; Leizer, J.; Linhares, I.M. Chlamydia trachomatis: The Persistent Pathogen. Clin. Vaccine Immunol. 2017, 24, 10. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Shin, A.; Gibson, P.R. AGA Clinical Practice Update on Functional Gastrointestinal Symptoms in Patients With Inflammatory Bowel Disease: Expert Review. Clin. Gastroenterol. Hepatol. 2019, 17, 380–390.e1. [Google Scholar] [CrossRef] [PubMed]
- Hakim, J.; Childress, K.J.; Hernandez, A.M.; Bercaw-Pratt, J.L. Tubo-Ovarian Abscesses in Nonsexually Active Adolescent Females: A Large Case Series. J. Adolesc. Health 2019, 65, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Kairys, N.; Roepke, C. Tubo-Ovarian Abscess. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Lareau, S.M.; Beigi, R.H. Pelvic inflammatory disease and tubo-ovarian abscess. Infect. Dis. Clin. N. Am. 2008, 22, 693–708. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, F.; Xu, J.; Chen, W.; Ding, B.; Shen, Y. Risk factors and nomogram construction for predicting women with chronic pelvic pain:a cross-sectional population study. Heliyon 2024, 10, e34534. [Google Scholar] [CrossRef]
- Howard, F.M. Chronic Pelvic Pain, Pelvic Inflammatory Disease and Adhesions. In Encyclopedia of Pain; Gebhart, G.F., Schmidt, R.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 652–655. [Google Scholar]
- Haggerty, C.L.; Schulz, R.; Ness, R.B. Lower quality of life among women with chronic pelvic pain after pelvic inflammatory disease. Obstet. Gynecol. 2003, 102, 934–939. [Google Scholar] [CrossRef]
- Weström, L. Pelvic inflammatory disease. JAMA 1991, 266, 2612. [Google Scholar] [CrossRef]
- Møller, B.R.; Kristiansen, F.V.; Thorsen, P.; Frost, L.; Mogensen, S.C. Sterility of the uterine cavity. Acta Obstet. Gynecol. Scand. 1995, 74, 216–219. [Google Scholar] [CrossRef]
- Ness, R.B.; Soper, D.E.; Holley, R.L.; Peipert, J.; Randall, H.; Sweet, R.L.; Sondheimer, S.J.; Hendrix, S.L.; Amortegui, A.; Trucco, G.; et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: Results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) Randomized Trial. Am. J. Obstet. Gynecol. 2002, 186, 929–937. [Google Scholar] [CrossRef]
- Kawwass, J.F.; Crawford, S.; Kissin, D.M.; Session, D.R.; Boulet, S.; Jamieson, D.J. Tubal factor infertility and perinatal risk after assisted reproductive technology. Obstet. Gynecol. 2013, 121, 1263–1271. [Google Scholar] [CrossRef]
- Hunt, S.; Vollenhoven, B. Pelvic inflammatory disease and infertility. Aust. J. General. Pract. 2023, 52, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tan, X.; Lv, A. Association between pelvic inflammatory disease and infertility in American women: Results from the National Health and Nutrition Survey 2013 to 2018. BMC Women’s Health 2025, 25, 578. [Google Scholar]
- Haggerty, C.L.; Ness, R.B. Diagnosis and treatment of pelvic inflammatory disease. Womens Health 2008, 4, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Huang, C.C.; Lin, S.Y.; Chang, C.Y.; Lin, W.C.; Chung, C.H.; Lin, F.H.; Tsao, C.H.; Lo, C.M.; Chien, W.C. Association of pelvic inflammatory disease (PID) with ectopic pregnancy and preterm labor in Taiwan: A nationwide population-based retrospective cohort study. PLoS ONE 2019, 14, e0219351. [Google Scholar] [CrossRef] [PubMed]
- Brim, A.C.S.; Barretto, V.R.D.; Reis-Oliveira, J.G.; da Silveira de Araújo, R.B.; Romeo, A. Risk factors for ectopic pregnancy occurrence: Systematic review and meta-analysis. Int. J. Gynaecol. Obstet. 2025, 168, 919–932. [Google Scholar] [CrossRef]
- Quintar, A.A.; Mukdsi, J.H.; del Valle Bonaterra, M.; Aoki, A.; Maldonado, C.A.; Pérez Alzaa, J. Increased expression of uteroglobin associated with tubal inflammation and ectopic pregnancy. Fertil. Steril. 2008, 89, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, W.H.; Zhu, Q.; Cao, S.J.; Ping, H.; Xi, X.; Qin, G.J.; Yan, M.X.; Zhang, D.; Qiu, J.; et al. Risk factors for ectopic pregnancy: A multi-center case-control study. BMC Pregnancy Childbirth 2015, 15, 187. [Google Scholar] [CrossRef]
- Hufstetler, K.; Llata, E.; Miele, K.; Quilter, L.A.S. Clinical Updates in Sexually Transmitted Infections, 2024. J. Womens Health 2024, 33, 827–837. [Google Scholar] [CrossRef]
- Lehtoranta, L.; Ala-Jaakkola, R.; Laitila, A.; Maukonen, J. Healthy Vaginal Microbiota and Influence of Probiotics Across the Female Life Span. Front. Microbiol. 2022, 13, 819958. [Google Scholar] [CrossRef]
- Jonsson, S.; Jonsson, H.; Lundin, E.; Häggström, C.; Idahl, A. Pelvic inflammatory disease and risk of epithelial ovarian cancer: A national population-based case-control study in Sweden. Am. J. Obstet. Gynecol. 2024, 230, e71–e75. [Google Scholar] [CrossRef]
- Stewart, L.M.; Stewart, C.J.R.; Spilsbury, K.; Cohen, P.A.; Jordan, S. Association between pelvic inflammatory disease, infertility, ectopic pregnancy and the development of ovarian serous borderline tumor, mucinous borderline tumor and low-grade serous carcinoma. Gynecol. Oncol. 2020, 156, 611–615. [Google Scholar] [CrossRef]
- Towns, J.M.; Williamson, D.A.; Bradshaw, C.S. Case of Mycoplasma genitalium pelvic inflammatory disease with perihepatitis. Sex. Transm. Infect. 2021, 97, 628. [Google Scholar] [CrossRef]
- Loehr, S.; Bitter, C. Fitz Hugh Curtis Case Report. J. Educ. Teach. Emerg. Med. 2020, 5, V19–V21. [Google Scholar] [CrossRef] [PubMed]
- Ronghe, V.; Modak, A.; Gomase, K.; Mahakalkar, M.G. From Prevention to Management: Understanding Postoperative Infections in Gynaecology. Cureus 2023, 15, e46319. [Google Scholar] [CrossRef] [PubMed]
- Low, N.; Mueller, M.; Van Vliet, H.A.; Kapp, N. Perioperative antibiotics to prevent infection after first-trimester abortion. Cochrane Database Syst. Rev. 2012, 2012, Cd005217. [Google Scholar] [CrossRef]
- Petousis, S.; Angelou, P.; Almperis, A.; Laganà, A.S.; Titilas, G.; Margioula-Siarkou, C.; Dinas, K. Prophylactic Antibiotics before Gynecologic Surgery: A Comprehensive Review of Guidelines. J. Pers. Med. 2024, 14, 327. [Google Scholar] [CrossRef]
- Marcinkowski, K.A.; Mehta, V.; Mercier, R.; Berghella, V. Pelvic inflammatory disease in pregnancy: A systematic review focusing on perinatal outcomes. Am. J. Obstet. Gynecol. MFM 2022, 4, 100643. [Google Scholar] [CrossRef]
- Center of Disease Control and Prevention. Sexually Transmitted Infections Surveillance. 2023. Available online: https://www.cdc.gov/sti-statistics/annual/index.html (accessed on 22 August 2025).
- Li, T.; Liu, Z.H.; Li, K.; Bai, H.H. Evaluation of the vaginal microbiome in clinical diagnosis and management of vaginal infectious diseases. Chin. Med. J. 2019, 132, 1100–1103. [Google Scholar] [CrossRef]
- Graspeuntner, S.; Bohlmann, M.K.; Gillmann, K.; Speer, R.; Kuenzel, S.; Mark, H.; Hoellen, F.; Lettau, R.; Griesinger, G.; König, I.R.; et al. Microbiota-based analysis reveals specific bacterial traits and a novel strategy for the diagnosis of infectious infertility. PLoS ONE 2018, 13, e0191047. [Google Scholar] [CrossRef]
- Rana, S.; Panjwal, P.; Malik, N.; Bhatia, V.; Kushawaha, S.K.; Ashawat, M.S. The silent threat: Pelvic inflammatory disease and long-term health outcomes with management. J. Endometr. Pelvic Pain. Disord. 2025, 17, 22840265251350207. [Google Scholar] [CrossRef]
- Hasan, Z.; Begum, N.; Ahmed, S.; Yasmin, M. Association of opportunistic bacterial pathogens with female infertility: A case-control study. J. Obstet. Gynaecol. Res. 2025, 51, e16243. [Google Scholar] [CrossRef]
- Filardo, S.; Di Pietro, M.; Porpora, M.G.; Recine, N.; Farcomeni, A.; Latino, M.A.; Sessa, R. Diversity of Cervical Microbiota in Asymptomatic Chlamydia trachomatis Genital Infection: A Pilot Study. Front. Cell Infect. Microbiol. 2017, 7, 321. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Niu, X.; Han, N.; Wang, B.; Du, P.; Na, R.; Chen, C.; Liao, Q. Predictive value of the composition of the vaginal microbiota in bacterial vaginosis, a dynamic study to identify recurrence-related flora. Sci. Rep. 2016, 6, 26674. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, E.; Kim, S.; Tomita, K.; Minase, T.; Kayano, M.; Watanabe, H.; Tetsuka, M.; Sasaki, M.; Iwayama, H.; Sanai, H.; et al. Evaluation of Lipopolysaccharide and Interleukin-6 as Useful Screening Tool for Chronic Endometritis. Int. J. Mol. Sci. 2024, 25, 2017. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).