Streptomyces Strains from Amazonian Sediments as Plant Growth Promoters and Biocontrol Agents of Anthracnose in Postharvest Capsicum chinense
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Morphological Characterization
2.3. DNA Extraction
2.4. PCR Amplification
2.5. Sanger Sequencing
2.6. Phylogenetic Analysis
2.7. In Vitro Antifungal Activity
2.8. Postharvest Biocontrol Activity
2.8.1. Fruit Preparation and Pathogen Inoculum
2.8.2. Preparation and Standardization of Streptomyces Spore Suspension
2.8.3. Inoculation and Treatment Procedure
2.8.4. Controls and Evaluation
2.9. Scanning Electron Microscopy
2.10. Evaluating Plant-Growth-Promotion Mechanisms
2.10.1. Phosphate Solubilization and Siderophore Production
2.10.2. Evaluation of Extracellular Enzyme Production
2.10.3. pH and Temperature Tolerance
2.11. Growth Promotion of Capsicum Chinense
2.11.1. Plant Preparation and Cultivation
2.11.2. Bacterial Inoculum Preparation and Application
2.11.3. Experimental Design, Controls, and Growth Evaluation
2.12. Statistical Analysis
3. Results
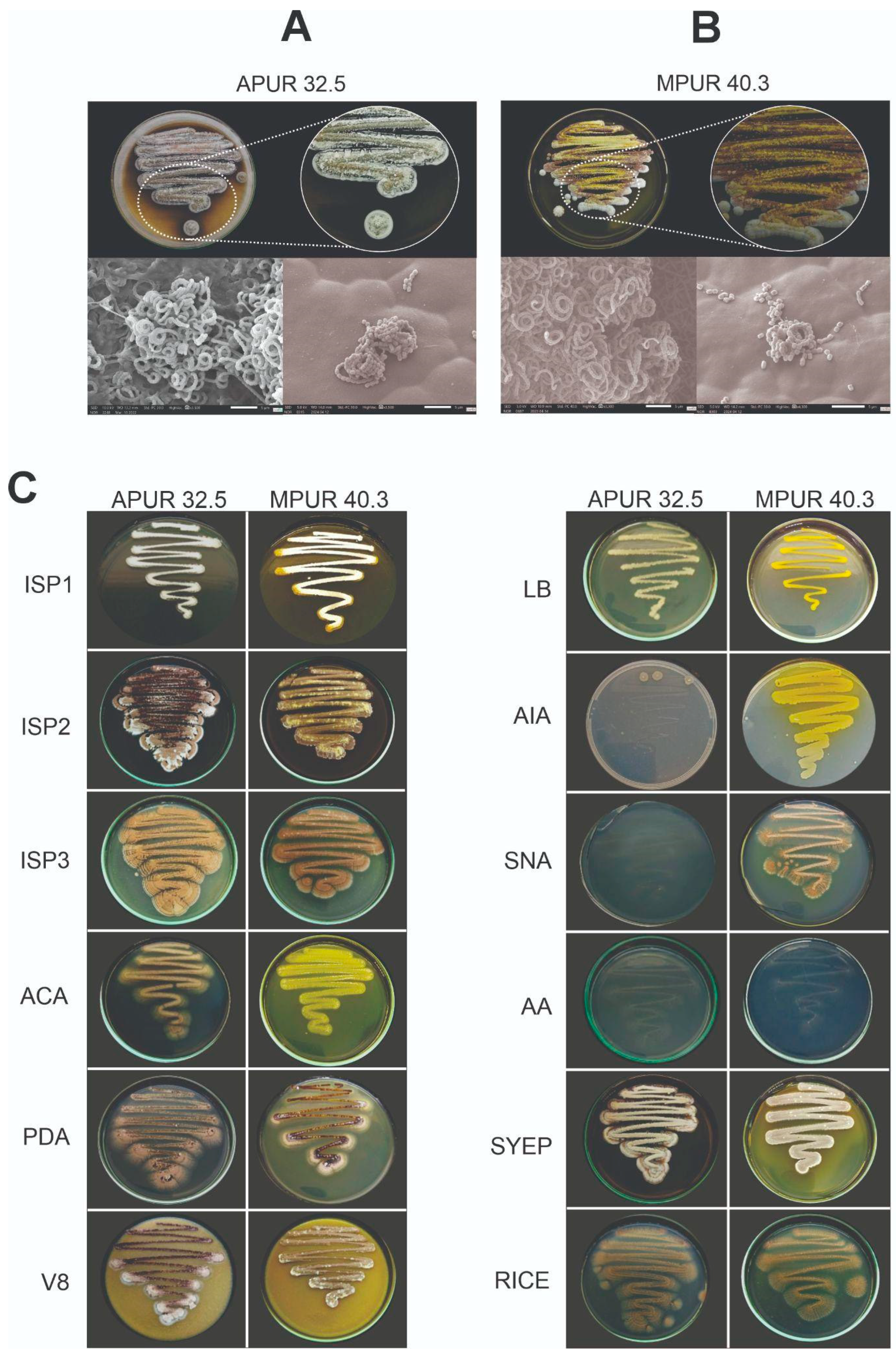
3.1. Morphological Aspects and Molecular Identification
3.1.1. Morphological Characterization
3.1.2. Molecular Identification and Phylogenetic Analysis
3.2. Antifungal Activity
3.3. Effect of Streptomyces Isolates on the Control of Colletotrichum Scovillei in Capsicum Chinense Fruits
3.3.1. Evaluation of the Incidence of Disease
3.3.2. Ultrastructural Analysis of the Pathogen-Antagonist Interactions
3.4. Plant Growth Promotion
3.5. Enzymatic Assays
3.5.1. Hydrolytic Enzymatic Activities
3.5.2. Siderophore Production and Phosphate Solubilization
3.6. pH and Temperature Tolerance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Shahbazi, F.; Golshani, A.; Fattahi, M.; Armin, M. Losses in agricultural produce: A review of causes and solutions, with a specific focus on grain crops. J. Stored Prod. Res. 2025, 111, 102547. [Google Scholar] [CrossRef]
- Nath, B.; Chen, G.; O’sullivan, C.M.; Zare, D. Research and technologies to reduce grain postharvest losses: A review. Foods 2024, 13, 1875. [Google Scholar] [CrossRef]
- Sawicka, B.; Egbuna, C. Pests of agricultural crops and control measures. In Natural Remedies for Pest, Disease and Weed Control; Academic Press: Cambridge, MA, USA, 2020; pp. 1–16. [Google Scholar]
- Zakaria, L. Diversity of Colletotrichum species associated with anthracnose disease in tropical fruit crops—A review. Agriculture 2021, 11, 297. [Google Scholar] [CrossRef]
- Salotti, I.; Baroncelli, R.; Sarrocco, S.; Battilani, P. Development of a model for Colletotrichum diseases with calibration for phylogenetic clades on different host plants. Front. Plant Sci. 2023, 14, 1069092. [Google Scholar] [CrossRef]
- Thanh, L.T.H.; Minh, P.T.; Trang, P.T.; Duong, V.T. First report of Colletotrichum siamense and Colletotrichum endophyticum associated with anthracnose on avocado (Persea americana Mill.) in Vietnam. J. Plant Pathol. 2025, 107, 633–647. [Google Scholar] [CrossRef]
- Torres-Calzada, C.; Tapia-Tussell, R.; Higuera-Ciapara, I.; Pérez-Brito, D. Sensitivity of Colletotrichum truncatum to four fungicides and characterization of thiabendazole-resistant isolates. Plant Dis. 2015, 99, 1590–1595. [Google Scholar] [CrossRef]
- Dowling, M.; Peres, N.A.; Villani, S.M.; Schnabel, G. Managing Colletotrichum on fruit crops: A “complex” challenge. Plant Dis. 2020, 104, 2301–2316. [Google Scholar] [CrossRef]
- Ren, L.; Li, G.Q.; Han, Y.C.; Jiang, D.H.; Huang, H.C. Characterisation of sensitivity of Colletotrichum gloeosporioides and Colletotrichum capsici, causing pepper anthracnose, to picoxystrobin. J. Plant Dis. Prot. 2020, 127, 657–666. [Google Scholar] [CrossRef]
- Karim, M.M.; Wu, C.; Zhang, J.; Li, X.; Liu, P.; Sun, G. Fungicide resistance in Colletotrichum fructicola and Colletotrichum siamense causing peach anthracnose in China. Pestic. Biochem. Physiol. 2024, 203, 106006. [Google Scholar] [CrossRef]
- Giacomin, R.M.; Rodrigues, R.; Gonçalves-Vidigal, M.C.; Silva, C.L. Inheritance of anthracnose resistance (Colletotrichum scovillei) in ripe and unripe Capsicum annuum fruits. J. Phytopathol. 2020, 168, 184–192. [Google Scholar] [CrossRef]
- Caires, N.P.; Félix, C.R.; Tessmann, D.J.; Cardoso, J.E.; Maffia, L.A.; Mizubuti, E.S.G. First report of anthracnose on pepper fruit caused by Colletotrichum scovillei in Brazil. Plant Dis. 2014, 98, 1437. [Google Scholar] [CrossRef]
- Giacomin, R.M.; Colletta, G.D.; Gonçalves-Vidigal, M.C.; Rodrigues, R. Post-harvest quality and sensory evaluation of mini sweet peppers. Horticulturae 2021, 7, 287. [Google Scholar] [CrossRef]
- Shi, N.; Zhang, H.; Liu, P.; Sun, G. Resistance risk and resistance-related point mutations in cytochrome b of florylpicoxamid in Colletotrichum scovillei. Pestic. Biochem. Physiol. 2023, 196, 105617. [Google Scholar] [CrossRef]
- Law, C.X.; Lim, L.; Yap, S.K.; Chee, H.Y. A review on anthracnose disease caused by Colletotrichum spp. in fruits and advances in control strategies. Int. J. Food Microbiol. 2025, 394, 111397. [Google Scholar] [CrossRef]
- Chatterjee, K.; Singh, R.; Yadav, A.N. Application of Streptomycetes in medicine. In Bioeconomy of Streptomyces; CRC Press: Boca Raton, FL, USA, 2025; pp. 119–141. [Google Scholar]
- Patel, S.; Gupta, R.; Singh, H.B. Diversity of secondary metabolites from marine Streptomyces with potential anti-tubercular activity: A review. Arch. Microbiol. 2025, 207, 64. [Google Scholar] [CrossRef]
- Pacios-Michelena, S.; Ríos-Castro, E.; González, M.; Maldonado-Mendoza, I.E. Application of Streptomyces antimicrobial compounds for the control of phytopathogens. Front. Sustain. Food Syst. 2021, 5, 696518. [Google Scholar] [CrossRef]
- Chouyia, F.E.; Ventorino, V.; Pepe, O. Diversity, mechanisms and beneficial features of phosphate-solubilizing Streptomyces in sustainable agriculture: A review. Front. Plant Sci. 2022, 13, 1035358. [Google Scholar] [CrossRef]
- Beroigui, O.; Errachidi, F. Streptomyces at the heart of several sectors to support practical and sustainable applications: A review. Prog. Microbes Mol. Biol. 2023, 6, a0000345. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Chen, J.; Li, Q.; Liu, G. Streptomyces strains and their metabolites for biocontrol of phytopathogens in agriculture. J. Agric. Food Chem. 2024, 72, 2077–2088. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, V.; Sharma, R. Potential applications of extracellular enzymes from Streptomyces spp. in various industries. Arch. Microbiol. 2020, 202, 1597–1615. [Google Scholar] [CrossRef]
- Khushboo; Singh, S.; Mishra, P. Biotechnological and industrial applications of Streptomyces metabolites. Biofuels Bioprod. Biorefin. 2022, 16, 244–264. [Google Scholar] [CrossRef]
- Pereira, J.O.; Lima, A.L.; Silva, G.C.; Araújo, J.M. Overview on biodiversity, chemistry, and biotechnological potential of microorganisms from the Brazilian Amazon. In Diversity and Benefits of Microorganisms from the Tropics; Moreira, F.M.S., Huising, E.J., Bignell, D.E., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 71–103. [Google Scholar]
- de Oliveira, A.C.F.M.; Santos, E.M.; Silva, C.F.; da Silva e Silva, L.; de Oliveira Veras, A.A.; das Graças, D.A.; Silva, A.; Baraúna, R.A.; Trivella, D.B.B.; Schneider, M.P.C. A metabologenomics approach reveals the unexplored biosynthetic potential of bacteria isolated from an Amazon Conservation Unit. Microbiol. Spectr. 2024, 13, e00996-24. [Google Scholar] [CrossRef]
- Jose, P.A.; Jha, B. Intertidal marine sediment harbours actinobacteria with promising bioactive and biosynthetic potential. Sci. Rep. 2017, 7, 10041. [Google Scholar] [CrossRef]
- Shirling, E.B.; Gottlieb, D. Methods for characterization of Streptomyces species. Int. J. Syst. Evol. Microbiol. 1966, 16, 313–340. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid total DNA preparation procedure for fresh plant tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Labeda, D.P.; Dunlap, C.A.; Rong, X.; Huang, Y.; Doroghazi, J.R.; Ju, K.-S.; Metcalf, W.W. Phylogenetic relationships in the family Streptomycetaceae using multi-locus sequence analysis. Antonie Van Leeuwenhoek 2017, 110, 563–583. [Google Scholar] [CrossRef]
- Thampi, A.; Bhai, R.S. Rhizosphere actinobacteria for combating Phytophthora capsici and Sclerotium rolfsii, the major soil borne pathogens of black pepper (Piper nigrum L.). Biol. Control 2017, 109, 1–13. [Google Scholar] [CrossRef]
- Falguera, J.V.T.; Stratton, K.J.; Bush, M.J.; Jani, C.; Findlay, K.C.; Schlimpert, S.; Nodwell, J.R. DNA damage-induced block of sporulation in Streptomyces venezuelae involves downregulation of ssgB. Microbiology 2022, 168, 001198. [Google Scholar] [CrossRef]
- Ratul, N.; Sharma, G.D.; Madhumita, B. Phosphate solubilization, siderophore production and extracellular enzyme production activities of endophytic fungi isolated from tea (Camellia sinensis) bushes of Assam, India. Res. J. Biotechnol. 2023, 18, 49–57. [Google Scholar] [CrossRef]
- Liotti, R.G.; da Silva Figueiredo, M.I.; Soares, M.A. Streptomyces griseocarneus R132 controls phytopathogens and promotes growth of pepper (Capsicum annuum). Biol. Control 2019, 138, 104065. [Google Scholar] [CrossRef]
- Komaki, H. Reclassification of Streptomyces costaricanus and Streptomyces phaeogriseichromatogenes as later heterotypic synonyms of Streptomyces murinus. Int. J. Syst. Evol. Microbiol. 2021, 71, 004638. [Google Scholar] [CrossRef]
- Gren, T.; Pahl, A.; Nieselt, K.; Heide, L.; Luzhetskyy, A. Characterization and engineering of Streptomyces griseofuscus DSM 40191 as a potential host for heterologous expression of biosynthetic gene clusters. Sci. Rep. 2021, 11, 18301. [Google Scholar] [CrossRef]
- Das, V.; Thomas, L.; Mathew, J.; Joseph, B. Exploration of natural product repository by combined genomics and metabolomics profiling of mangrove-derived Streptomyces murinus THV12 strain. Fermentation 2023, 9, 576. [Google Scholar] [CrossRef]
- Esnard, J.; Potter, T.L.; Zuckerman, B.M. Streptomyces costaricanus sp. nov., isolated from nematode-suppressive soil. Int. J. Syst. Evol. Microbiol. 1995, 45, 775–779. [Google Scholar] [CrossRef]
- Cerna-Chávez, E.; López-Méndez, B.; Rivera-Morales, J.; Martínez, A. Potential of Streptomyces avermitilis: A review on avermectin production and its biocidal effect. Metabolites 2024, 14, 374. [Google Scholar] [CrossRef]
- Evangelista-Martínez, Z.; Torres, M.; Hernández, L.; Reyes, A. Potential of Streptomyces sp. strain AGS-58 in controlling anthracnose-causing Colletotrichum siamense from post-harvest mango fruits. J. Plant Pathol. 2022, 104, 553–563. [Google Scholar] [CrossRef]
- Alam, K.; Tian, J.; Wu, J.; Zhang, L.; Zhang, C. Streptomyces: The biofactory of secondary metabolites. Front. Microbiol. 2022, 13, 968053. [Google Scholar] [CrossRef]
- Ciofini, A.; Di Mattia, T.; Rossi, M.; Santini, C. Management of post-harvest anthracnose: Current approaches and future perspectives. Plants 2022, 11, 1856. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Pham, L.D.; Tran, Q.N.; Le, T.A. Biological control of Streptomyces murinus against Colletotrichum causing anthracnose disease on tomato fruits. J. Pure Appl. Microbiol. 2025, 19, 542–557. [Google Scholar] [CrossRef]
- Song, W.; Yu, X.; Yu, X.; Zhang, H.; Zhang, K.; Guo, L.; Wang, J.-D.; Tian, D.-L.; Yu, Q.; Wang, X.; et al. Antifungal Activity and Potential Mechanisms of Two Bafilomycin Analogues Isolated from Streptomyces sp. NEAU-Y11 against Colletotrichum orbiculare. J. Agric. Food Chem. 2025, 73, 11814–11828. [Google Scholar] [CrossRef]
- Shahid, M.; Singh, B.N.; Verma, S.; Choudhary, P.; Das, S.; Chakdar, H.; Murugan, K.; Goswami, S.K.; Saxena, A.K. Bioactive antifungal metabolites produced by Streptomyces amritsarensis V31 help to control diverse phytopathogenic fungi. Braz. J. Microbiol. 2021, 52, 1687–1699. [Google Scholar] [CrossRef]
- Mishra, P.; Sharma, A.; Singh, V. Microbial enzymes in biocontrol of phytopathogens. In Microbial Enzymes: Roles and Applications in Industries; Academic Press: Cambridge, MA, USA, 2020; pp. 259–285. [Google Scholar]
- Riseh, R.S.; Akbarzadeh, A.; Mousavi, S.M.; Hassanzadeh, S. Unveiling the role of hydrolytic enzymes from soil biocontrol bacteria in sustainable phytopathogen management. Front. Biosci. 2024, 29, 105–125. [Google Scholar] [CrossRef]
- Daunoras, J.; Kačergius, A.; Gudiukaitė, R. Role of soil microbiota enzymes in soil health and activity changes depending on climate change and the type of soil ecosystem. Biology 2024, 13, 85. [Google Scholar] [CrossRef]
- Huang, Q.; Li, Y.; Wang, X.; Chen, H.; Zhou, L. Production of extracellular amylase contributes to the colonization of Bacillus cereus 0–9 in wheat roots. BMC Microbiol. 2022, 22, 205. [Google Scholar] [CrossRef]
- Sousa, T.F.; Oliveira, R.; Lima, J.C.; Gomes, L.F.; Costa, P.R. Trichoderma agriamazonicum sp. nov. (Hypocreaceae), a new ally in the control of phytopathogens. Microbiol. Res. 2023, 275, 127469. [Google Scholar] [CrossRef]
- Renuka, R.; Sathiyabama, M.; Kumar, P. Exploring the potentiality of native actinobacteria to combat the chilli fruit rot pathogens under post-harvest pathosystem. Life 2023, 13, 426. [Google Scholar] [CrossRef]
- Zhong, J.; Bai, X.Y.; Zhang, Z.; Li, X.G.; Zhu, J.Z. Biocontrol potential of Streptomyces lactacystinicus producing volatile organic compounds against postharvest anthracnose of chili pepper. Postharvest Biol. Technol. 2025, 230, 113758. [Google Scholar] [CrossRef]
- Zhong, J.; Bai, X.Y.; Li, X.G.; Zhu, J.Z. Streptomyces olivoreticuli ZZ-21 act as a potential biocontrol strain against pepper anthracnose caused by Colletotrichum scovillei. Int. J. Food Microbiol. 2025, 441, 111319. [Google Scholar] [CrossRef]
- Luan, P.; Li, H.; Zhang, J.; Chen, R.; Wang, S. Biocontrol potential and action mechanism of Bacillus amyloliquefaciens DB2 on Bipolaris sorokiniana. Front. Microbiol. 2023, 14, 1149363. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Zhao, J.; Tang, Y. Biocontrol potential of volatile organic compounds produced by Streptomyces corchorusii CG-G2 to strawberry anthracnose caused by Colletotrichum gloeosporioides. Food Chem. 2024, 437, 137938. [Google Scholar] [CrossRef]
- Kawicha, P.; Somwang, P.; Runguphan, W.; Supapvanich, S. Evaluation of soil Streptomyces spp. for the biological control of Fusarium wilt disease and growth promotion in tomato and banana. Plant Pathol. J. 2023, 39, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Al-Quwaie, D.A. The role of Streptomyces species in controlling plant diseases: A comprehensive review. Australas. Plant Pathol. 2024, 53, 1–14. [Google Scholar] [CrossRef]
- Nazari, M.T.; Fakhari, M.; Hemmati, R. Using Streptomyces spp. as plant growth promoters and biocontrol agents. Rhizosphere 2023, 27, 100741. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, I.J.S.; Sousa, T.F.; de Souza, T.M.; Gomes, B.M.; de Lima Procópio, R.E.; Muniz, A.W.; Hanada, R.E.; Koolen, H.H.F.; da Silva, G.F. Streptomyces Strains from Amazonian Sediments as Plant Growth Promoters and Biocontrol Agents of Anthracnose in Postharvest Capsicum chinense. Microorganisms 2025, 13, 2713. https://doi.org/10.3390/microorganisms13122713
da Silva IJS, Sousa TF, de Souza TM, Gomes BM, de Lima Procópio RE, Muniz AW, Hanada RE, Koolen HHF, da Silva GF. Streptomyces Strains from Amazonian Sediments as Plant Growth Promoters and Biocontrol Agents of Anthracnose in Postharvest Capsicum chinense. Microorganisms. 2025; 13(12):2713. https://doi.org/10.3390/microorganisms13122713
Chicago/Turabian Styleda Silva, Ingride Jarline Santos, Thiago Fernandes Sousa, Thayná Marães de Souza, Beatriz Miranda Gomes, Rudi Emerson de Lima Procópio, Aleksander Westphal Muniz, Rogério Eiji Hanada, Hector Henrique Ferreira Koolen, and Gilvan Ferreira da Silva. 2025. "Streptomyces Strains from Amazonian Sediments as Plant Growth Promoters and Biocontrol Agents of Anthracnose in Postharvest Capsicum chinense" Microorganisms 13, no. 12: 2713. https://doi.org/10.3390/microorganisms13122713
APA Styleda Silva, I. J. S., Sousa, T. F., de Souza, T. M., Gomes, B. M., de Lima Procópio, R. E., Muniz, A. W., Hanada, R. E., Koolen, H. H. F., & da Silva, G. F. (2025). Streptomyces Strains from Amazonian Sediments as Plant Growth Promoters and Biocontrol Agents of Anthracnose in Postharvest Capsicum chinense. Microorganisms, 13(12), 2713. https://doi.org/10.3390/microorganisms13122713







