Abstract
Host innate immunity is crucial for orchestrating a protective response against dangerous pathogens. Herein, we demonstrate that interferon-inducible protein (IFI204), a DNA sensor, is implicated in protection against pulmonary pathogenic Mannheimia haemolytica (M. haemolytica) infection by driving inflammasome signaling activation. Ifi204−/− mice are more susceptible to pathogenic M. haemolytica infection compared with their wild-type (WT) counterparts, with decreased survival rates, extensive lung architecture destruction, exacerbated inflammatory cells infiltration, and more bacterial colonization. In vivo and in vitro findings elucidate that Ifi204 deficiency leads to a defect in inflammasome signaling activation, and exogenous recombinant IL-18 is sufficient to rescue the susceptibility of Ifi204−/− mice. Inflammasome signaling downstream of IFI204 facilitates early bacterial killing and clearance. Mechanistically, IFI204 promotes gasdermin D (GSDMD)-dependent inflammasome activation, and GSDMD is required for IFI204-mediated host defense. Notably, IFI204 detects pathogenic M. haemolytica-derived genomic DNA for the inflammasome signaling response. Thus, these data highlight the requirement of IFI204 in host defense response to M. haemolytica infection, and reveal that IFI204 may be a potential therapeutic target for pathogen control.
1. Introduction
Mannheimia haemolytica (M. haemolytica, previously known as Pasteurella haemolytica) is an opportunistic pathogen that causes fibrinonecrotic pneumonia in cattle and is the primary bacterial agent in bovine respiratory disease syndrome (BRD) [1,2,3]. Approximately 30% of BRD-related cattle deaths are attributed to M. haemolytica, causing over $1 billion in annual economic losses in North America [4]. Additionally, M. haemolytica can induce mastitis, pneumonia, acute gastroenteritis, and septicemia in goats and sheep, posing significant global threats to the livestock industry [5,6]. Although traditional antibiotic therapies are widely used, concerns are growing over multidrug-resistant strains [7,8]. While vaccines offer a desirable prophylactic alternative, their efficacy is limited by variable or ineffective cross-immunoprotection across serotypes [9,10,11]. Therefore, it is essential to develop novel strategies for the control of pathogenic M. haemolytica infections.
The innate immune system is the first line of host defense against invading pathogens. Non-self and endogenous danger signals from microbial infection or tissue damage are sensed through interactions with pattern recognition receptors (PRRs) called pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) [12]. It is generally accepted that PRRs in mammals include the following categories: Toll-like receptors (TLRs), RIG-like receptors (RLRs), NOD-like receptors (NLRs), C-type lectin receptors (CLRs), and a range of intracellular DNA sensors [13]. Therefore, it is not surprising that clarification of the host innate sensing mechanisms paves the way in the development of more effective prevention and control strategies to counter M. haemolytica epidemic.
The interferon-inducible protein 204 (IFI204), the murine homolog of human IFI16, functions as an intracellular DNA receptor that can sense invading viruses and bacteria [14,15,16]. Recent research has demonstrated that active IFI204 promotes type I IFN signaling and extracellular traps formation, in response to pathogenic infection [17,18,19]. In addition, although previous work has reported that IFI204 does not recognize intracellular Lipopolysaccharide (LPS) and is dispensable for inflammasome activation [20], there is evidence showing that IFI204 recruits apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) to form an inflammasome, resulting in the production of pro-inflammatory cytokines [21,22]. Indeed, the biological implications of IFI204 have been discussed, although there is little evidence regarding the effect of IFI204 in response to pulmonary M. haemolytica infection.
In our study, we reveal the protective role of IFI204 in host defenses against M. haemolytica infection. Compared with their wild-type counterparts, Ifi204−/− (Ifi204-deficient) mice display increased bacterial loads, reduced survival rates, and more severe organ damage. Importantly, inflammasome signaling downstream of IFI204 facilitates bacterial killing and clearance. In addition, IFI204 detects pathogenic M. haemolytica-derived genomic DNA for the inflammasome signaling response. Thus, our results indicate that IFI204 is critical for host defense against M. haemolytica infection, which provides a reference for the subsequent development of an efficient bovine respiratory disease syndrome vaccine.
2. Materials and Methods
2.1. Mice and Cells
Ifi204-deficient (Ifi204−/−) mice and Gsdmd-deficient (Gsdmd−/−) mice were generously given by Prof. Yong-jun Yang (Jilin University, Changchun, China). All the mice were housed in SPF-grade, independently ventilated cages (IVCs) located at the Laboratory Animal Center of Inner Mongolia University (Hohhot, China). All experimental procedures involving animals reported in this study were granted approval by the Animal Welfare and Research Ethics Committee of Inner Mongolia University ([2022] 072, 1 January 2022). Matured bone-marrow-derived macrophages (BMDMs) were acquired from the femurs of 6–8-week-old mice, and cultured in the RPMI-1640 medium (Gibco, #31800-022, Waltham, MA, USA) containing 10% fetal bovine serum (FBS, Gibco, #A31608-02), 25% L929 cell-conditioned medium, and 100 U/mL penicillin/streptomycin (P/S, Gibco, #15140-122) to differentiate as previously reported [23].
2.2. Phylogenetic Assay
Bacterial genomic DNA was extracted via the EasyPure® Bacteria Genomic DNA Kit (TransGen Biotech, Beijing, China, #EE161-11), and 16S rRNA genes were amplificated and subjected to phylogenetic analysis via the maximum likelihood (ML) method using Mega 11 software [24].
2.3. In Vivo Infection
M. haemolytica strain MH-1, a clinical isolate of pathogenic M. haemolytica (specific 16S rDNA gene sequence data deposited in NCBI GenBank: PQ268926) was cultured at 37 °C in brain–heart infusion (BHI, Qingdao Hi-Tech Industrial Park Haibo Biotechnology Co., Ltd., Qingdao, China, #HB8297-5) medium. To induce pneumonia, six-to-eight-weeks-old sex-matched mice were intranasally infected with 5 × 109 colony-forming units (CFU) of log-phase pathogenic M. haemolytica strain MH-1 (day 0), and the lungs, blood, and bronchoalveolar lavage fluid (BALF) were aseptically collected for the quantification of bacterial loads at 24 h post-infection (hpi, day 1). To administer the exogenous recombinant IL-18 (rIL-18, Novoprotein, Summit, NJ, USA, #CK06), rIL-18 (1.0 µg per mouse) was injected intraperitoneally on day 1 and day 0. For survival experiments, mice were intranasally infected with 8 × 109 CFU of log-phase pathogenic M. haemolytica strain MH-1, and their mortality was monitored over 72 h.
2.4. Histopathology and Immunostaining
For the histology, aseptically excised lungs were fixed in 4% paraformaldehyde (PFA, Macklin, Shanghai, China, #P804536), and the lung sections were stained with hematoxylin and eosin (H&E, Solarbio, Beijing, China, #SL7050-500). For the immunohistochemistry, the lung sections were stained with anti-Gr-1 (Abcam, Cambridge, UK, #ab196436), anti-F4/80 (BioLegend, San Diego, CA, USA, #123119), and anti-IFI204 (Abcam, #ab307201) antibodies.
2.5. Inflammasome Assays
Matured BMDMs were stimulated with 500 ng/mL LPS (Invitrogen, Carlsbad, CA, USA, #tlrl-3pelps) for 4 h prior to their challenge with pathogenic M. haemolytica strain MH-1 (MOI = 50, 5 h) or their transfection with bacterial genomic DNA (50 µg/mL, 8 h). The BMDMs supernatants and extracts were used for immunoblotting, ELISA, and LDH activities analysis.
2.6. Protein Extraction and Immunoblotting
Infected lungs or BMDMs extracts were precipitated by methanol/chloroform for analysis using immunoblot, as previously described [25]. Subsequently, they were incubated with the indicated anti-Caspase-1 (Adipogen, Liestal, Switzerland, #AG-20B-0042-C100), anti-GSDMD (Santa Cruz, Dallas, TX, USA, #sc-393656), anti-IFI204 (Abcam, #ab307201), and anti-GAPDH (Roteintech, Rosemont, IL, USA, #60004-1-Ig) antibodies.
2.7. Cytokine and LDH Activities Detection
Aseptically excised lungs homogenates, BALF, and BMDMs supernatants were measured via an ELISA assay, following the manufacturer’s protocols, using a IL-1β ELISA kit (R&D, Minneapolis, MN, USA, #MLB00C) and TNF-α ELISA kit (R&D, #MTA00B). For LDH activities detection, the BMDMs supernatants were determined using the LDH Cytotoxicity Assay kit® (Beyotime Biotechnology, Shanghai, China, #C0017) in accordance with the manufacturer’s instructions.
2.8. Immunofluorescence
ASC speckles and IFI204 staining were detected using an indirect immunofluorescence method [26]. Stimulated BMDMs were incubated with anti-ASC (Adipogen, #A29151803f), Alexa Fluor 594-conjugated anti-mouse IgG (Invitrogen, #ab150116), anti-IFI204 (Abcam, #ab307201), and Alexa Fluor 488-conjugated anti-rabbit IgG (Invitrogen, #ab150077) antibodies. DAPI (Solarbio, #C0065) was used to stain nuclei.
2.9. Bacterial Killing Analysis
To determine the bacterial killing capacity of BMDMs, LPS-primed BMDMs were incubated with rIL-18 (1000 pg/mL) or PBS for 1 h, before being infected with M. haemolytica strain MH-1 (MOI = 20, 6 h). The cells’ supernatants were collected and plated on BHI agar plates to enumerate the bacteria after their overnight culture.
2.10. Statistical Analysis
The data were expressed as mean ± standard deviation (SD) and performed using Prism software (GraphPad Software, version 8.0.2, La Jolla, CA, USA). A one-way ANOVA (Dunnett’s t-test), unpaired Student’s t-test or log-rank test were used for group comparisons. p-values that were less than 0.05 were regarded as statistically significant (* p < 0.05 and ** p < 0.01).
3. Results
3.1. IFI204 Is Critical for Host Defense Against Pulmonary Pathogenic M. haemolytica Infection
To explore the biological role of IFI204 in pulmonary host protection against pathogenic M. haemolytica infection in vivo, WT and Ifi204−/− mice were intranasally infected with 8 × 109 CFU of log-phase pathogenic M. haemolytica strain MH-1, a clinical isolate of pathogenic M. haemolytica (16S rDNA sequencing analysis, Figure 1A), and the survival rate of animals was monitored for 72 h. Clearly, Ifi204−/− mice displayed significantly higher mortality rates compared with their WT counterparts (Figure 1B). To further characterize the phenotype of Ifi204−/− mice in response to pathogenic M. haemolytica, WT and Ifi204−/− mice were intranasally challenged with 5 × 109 CFU of log-phase pathogenic M. haemolytica for 24 h post-infection (hpi). In accordance with their decreased survival rate, Ifi204−/− mice also tended to acquire extensive pulmonary damage, destroyed pulmonary architecture, and disrupted barrier function (Figure 1C,D). Based on the above results, we speculated that the increased mortality and exacerbated pathology in Ifi204−/− mice during pathogenic M. haemolytica infection might link to increased bacterial load levels. As expected, Ifi204−/− mice harbored significantly elevated loads of pathogenic M. haemolytica in the lungs, blood, and bronchoalveolar lavage fluid (BALF) (Figure 1E). Therefore, these results reveal that IFI204 contributes to host defense against pathogenic M. haemolytica pneumonia.
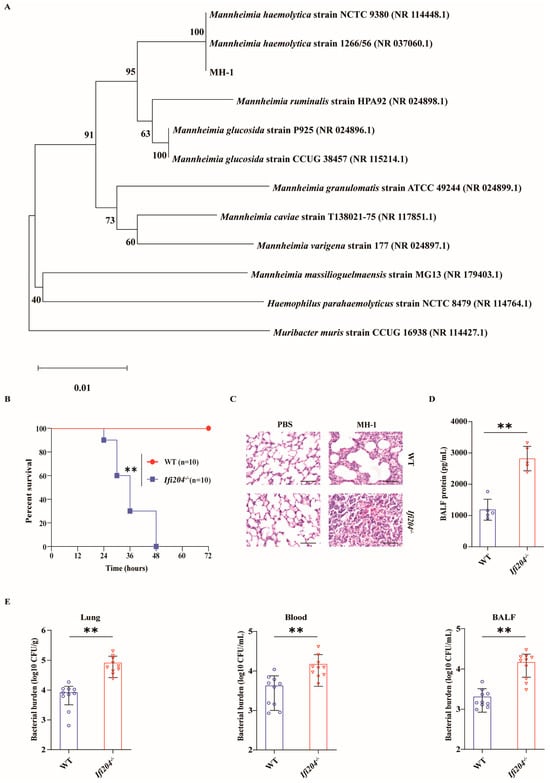
Figure 1.
IFI204 is sufficient to protect against M. haemolytica pulmonary infection. (A) Neighbor-joining phylogenetic tree showing the relative position of the isolated M. haemolytica strain MH-1 based on the 16S rDNA sequences. Age- and sex-matched WT and Ifi204−/− mice (n = 10) were intranasally challenged with log-phase pathogenic M. haemolytica strain MH-1. (B) Survival rate (8 × 109 CFU, p < 0.0001). (C) Representative H&E staining of the lung tissue structures (5 × 109 CFU, at 24 hpi, magnification, ×400). (D) Total protein in BALF was determined (5 × 109 CFU, at 24 hpi, p = 0.0001). (E) Bacterial loads in the lungs (p = 0.0006), blood (p = 0.0079) and BALF (p = 0.0002) were assessed (5 × 109 CFU, at 24 hpi). Graphs are means ± standard deviation (SD) from data pooled from ten (B,E) and five (D) biological replicates. Statistical significance is considered as ** p < 0.01.
3.2. IFI204-Elicited Inflammasome Signaling Confers Protection Against Pulmonary Pathogenic M. haemolytica Infection
To assess the potential immunological role of IFI204 in host protection, we next evaluated the lungs’ inflammatory responses. Although neutrophils and macrophages accumulation dramatically elevated in the lung tissue of infected Ifi204−/− mice relative to the level observed in those of the infected WT controls (Figure 2A), the inflammatory cytokine IL-1β release was markedly suppressed in Ifi204−/− mice (Figure 2B), while TNF-α production was little affected (Figure 2C). Importantly, inflammasome-dependent Caspase-1 cleavage was also dramatically attenuated in the lung tissue of infected Ifi204−/− mice compared with their infected WT counterparts (Figure 2D and Figure S1), indicating that Ifi204 deficiency impaired inflammasome signaling activation during the pulmonary pathogenic M. haemolytica challenge. Subsequently, the study further determined whether aberrant inflammasome signaling accounts for the aggravated susceptibility of Ifi204−/− mice to pathogenic M. haemolytica infection. Exhilaratingly, infected Ifi204−/− mice displayed obviously improved pathological damage in their lung tissue when prophylactically administrated with exogenous recombinant IL-18 (rIL-18) (Figure 2E). Thus, these data showed that inflammasome signaling may be involved in IFI204-mediated host defense against pathogenic M. haemolytica infection.
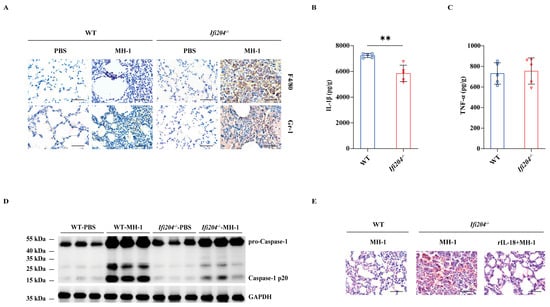
Figure 2.
Involvement of the inflammasome signaling response in IFI204-mediated host defense. Age- and sex-matched WT and Ifi204−/− mice were intranasally stimulated with 5 × 109 CFU log-phase pathogenic M. haemolytica strain MH-1 for 24 h. (A) Representative infected lung sections. Infiltrated inflammatory cells were stained brown (IHC, magnification, ×400). (B,C) Pro-inflammatory cytokines IL-1β (p = 0.0014) and TNF-α (p = 0.7533) production in the homogenate supernatants of infected lung tissue were determined by ELISA. (D) Caspase-1 activation was examined in the homogenate lysate of infected lung tissue by immunoblotting. For one group of Ifi204−/− mice, rIL-18 (1 μg/mouse) was intraperitoneally injected prior to intranasally challenged with 5 × 109 CFU log-phase pathogenic M. haemolytica strain MH-1 for 24 h. (E) Representative H&E staining of the lung tissue structures (magnification, ×400). Graphs are means ± standard deviation (SD) from data pooled from five (B,C) biological replicates. Statistical significance is considered as ** p < 0.01.
3.3. IFI204 Promotes GSDMD-Dependent Inflammasome Activation
To dissect the IFI204-driven host defense signaling mechanisms, the positive expression location of IFI204 was detected in lung sections. Immunohistochemical (IHC) staining revealed that elevated expression of IFI204 was observed in the infiltrated inflammatory cells of the infected tissues (Figure 3A). Correspondingly, the IFI204 protein level was also prominently up-regulated by the pathogenic M. haemolytica challenge in bone marrow-derived macrophages (BMDMs) (Figure 3B,C and Figure S2). Subsequently, we asked if Ifi204 deficiency impairs inflammasome signaling in macrophages. Consistent with the in vivo findings, inflammasome signaling was significantly attenuated in Ifi204−/− BMDMs vs. in WT BMDMs, as shown by the weakened Caspase-1 cleavage, impaired ASC speckles formation, reduced LDH activity, and mature IL-1β secretion (Figure 3D–G,I and Figure S3), while TNF-α secretion was not significantly affected (Figure 3H), suggesting that Ifi204 deficiency leads to a defect in inflammasome signaling activation in response to pathogenic M. haemolytica infection. Especially, gasdermin D (GSDMD), a critical executor of inflammasome activation, was also evidently suppressed in Ifi204−/− BMDMs (Figure 3B). Importantly, IL-1β release and LDH activity were remarkably inhibited in Gsdmd−/− BMDMs following pathogenic M. haemolytica challenge (Figure 3G,I), despite Caspase-1 activation, ASC speckles formation and TNF-α production being little affected (Figure 3D–F,H). These results demonstrated that IFI204 activates inflammasome signaling in a GSDMD-dependent manner.
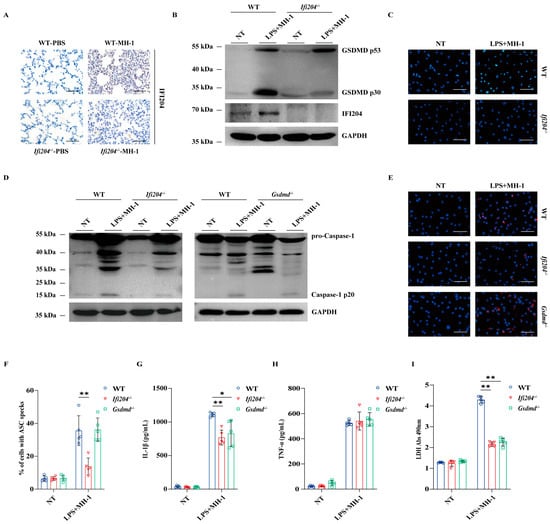
Figure 3.
IFI204 drives inflammasome signaling activation in an GSDMD-dependent manner. Age- and sex-matched WT and Ifi204−/− mice were intranasally challenged with 5 × 109 CFU log-phase pathogenic M. haemolytica strain MH-1 for 24 h. (A) Representative infected lung sections. IFI204 expression was stained brown (IHC, magnification, ×400). LPS-pretreated WT, Ifi204−/− and Gsdmd−/− BMDMs were exposed to log-phase pathogenic M. haemolytica (MOI = 50, 5 h). (B,D) Cleaved GSDMD and IFI204 in cell lysates (Lys.), as well as cleaved Caspase-1 in culture supernatants (Sup.) were detected by immunoblotting. (C,E,F) IFI204 expression, ASC speckles formation (p = 0.0016, p = 0.9842) and quantification were measured by immunofluorescence (magnification, ×400). (G,H) IL-1β (p = 0.00017, p = 0.0014) and TNF-α (p = 0.7583, p = 0.2690) secretion in the BMDMs supernatants were indicated by ELISA. (I) LDH released in the BMDMs supernatants were determined (p < 0.0001, p < 0.0001). Graphs are means ± standard deviation (SD) from data pooled from five (F–I) biological replicates. Statistical significance is considered as * p < 0.05, ** p < 0.01.
3.4. GSDMD Is Required for IFI204-Mediated Host Defense
To further characterize whether inflammasome-associated GSDMD mediates the protective effect of IFI204, the susceptibility of Gsdmd−/− mice in response to pathogenic M. haemolytica infection was evaluated in vivo. In line with Ifi204−/− mice, Gsdmd−/− mice were more susceptible to pathogenic M. haemolytica infection compared with WT mice. The date show that Gsdmd−/− mice exhibited severe histological damage in their lungs (Figure 4A). Specifically, Gsdmd−/− mice harbored higher bacterial loads in their lungs, blood, and BALF (Figure 4B), indicating that Gsdmd deficiency results in the defect of pathogen clearance in host defense responses. In addition, Gsdmd deficiency also impaired the inflammasome signaling activation following pathogenic M. haemolytica infection (Figure 4C–E and Figure S4), which was consistent with in vitro findings. Altogether, these data showed that inflammasome-activated GSDMD is beneficial to host protection against pulmonary pathogenic M. haemolytica infection.
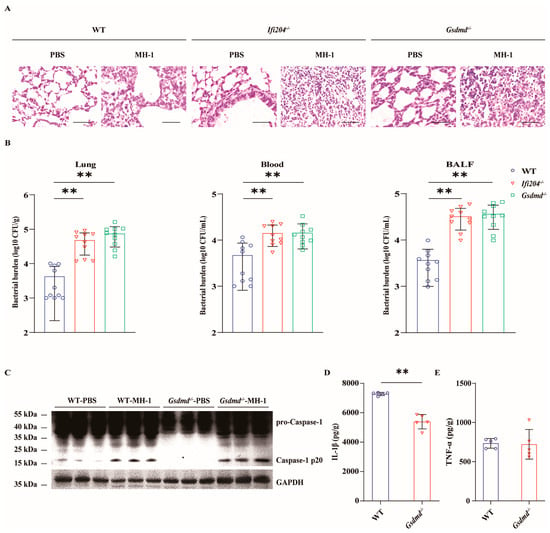
Figure 4.
Gsdmd deficiency impairs host protection against M. haemolytica pulmonary infection. Age- and sex-matched WT, Ifi204−/− and Gsdmd−/− mice (n = 10) were intranasally challenged with 5 × 109 CFU log-phase pathogenic M. haemolytica strain MH-1 for 24 h. (A) Representative H&E staining of the lung tissue structures (magnification, ×400). (B) Bacterial loads in the lungs (p = 0.0003, p < 0.0001), blood (p = 0.0014, p = 0.0028), and BALF (p < 0.0001, p < 0.0001) were enumerated. (C) Caspase-1 activation was examined in the homogenate lysate of infected lung tissue via immunoblotting. (D,E) Pro-inflammatory cytokines IL-1β (p < 0.0001) and TNF-α (p = 0.8934) production in the homogenate supernatants of infected lung tissue were determined via ELISA. Graphs are means ± standard deviation (SD) from data pooled from ten (B) and five (D,E) biological replicates. Statistical significance is considered as ** p < 0.01.
3.5. IFI204-Driven Inflammasome Signaling Facilitates Pathogen Control
Based on the above, Ifi204−/− mice and Gsdmd−/− mice harbored increased tissue bacterial loads, suggesting that there is a defect in pathogen control in the absence of Ifi204 and Gsdmd during pulmonary pathogenic M. haemolytica infection. We set out to characterize the bacterial killing capacity of Ifi204−/− BMDMs and Gsdmd−/− BMDMs in vitro. Similarly, the results revealed that a higher number of recovered viable bacteria were observed in Ifi204−/− BMDMs and Gsdmd−/− BMDMs compared with WT BMDMs following pathogenic M. haemolytica challenge (Figure 5A,B), indicating that Ifi204 and Gsdmd deficiency also impair the bacterial killing capacity of macrophages. Since inflammasome signaling activation is required for host innate immune defenses against pathogenic invasion, we further evaluated the role of IFI204-mediated inflammasome signaling on bacterial growth and proliferation. To our delight, exogenous rIL-18 could strongly decrease the bacterial loads in the lungs, blood, and BALF of Ifi204−/− mice and Gsdmd−/− mice, and could rescue the defect in bacterial killing in Ifi204−/− BMDMs and Gsdmd−/− BMDMs, respectively (Figure 5A–D). These results show that inflammasome signaling downstream of active IFI204 accelerates bacterial killing and clearance. Then, to further illustrate how pathogenic M. haemolytica drives IFI204 signaling, Ifi204−/− BMDMs and WT BMDMs were incubated with pathogenic M. haemolytica-derived genomic DNA. Notably, Ifi204 deficiency attenuated pathogenic M. haemolytica-derived genomic DNA-triggered IL-1β production, while TNF-α release was not significantly affected (Figure 5E,F). Collectively, our findings reported that the DNA sensor IFI204 promotes pathogen control via eliciting GSDMD-dependent inflammasome signaling in response to pulmonary pathogenic M. haemolytica infection.
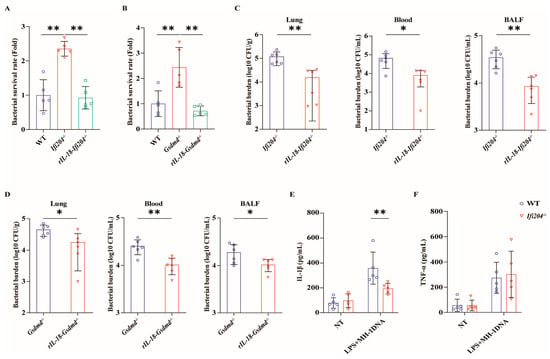
Figure 5.
Inflammasome signaling downstream of IFI204 restricts bacterial invasion. LPS-pretreated WT, Ifi204−/−, and Gsdmd−/− BMDMs were primed with rIL-18 (1 ng/mL, 1 h) or PBS before being stimulated with M. haemolytica (MOI = 20, 6 h). (A,B) The bacterial survival was assessed (p = 0.0003, p < 0.0001). Age- and sex-matched Ifi204−/− and Gsdmd−/− mice were intraperitoneally injected with rIL-18 (1 μg/mouse) or PBS prior to being intranasally challenged with 5 × 109 CFU log-phase pathogenic M. haemolytica strain MH-1 for 24 h (p = 0.0089, p = 0.0014). (C,D) Bacterial loads in the lungs (p = 0.0053, p = 0.0181), blood (p = 0.0413, p = 0.0031), and BALF (p = 0.0019, p = 0.042) were determined. (E,F) LPS-pretreated WT and Ifi204−/− BMDMs were exposed to M. haemolytica-derived genomic DNA (50 µg/mL, 8 h). IL-1β (p = 0.0072) and TNF-α (p = 0.9167) secretion in the BMDMs supernatants were indicated via ELISA. Graphs are means ± standard deviation (SD) from data pooled from five (A,B,E,F) and six (C,D) biological replicates. Statistical significance is considered as * p < 0.05, ** p < 0.01.
4. Discussion
M. haemolytica pneumonia is one of the most economically important infectious diseases of ruminants, with a wide prevalence throughout the continents [27]. What is worse, with the emergence of multidrug-resistant M. haemolytica strains [28,29], the development of new prophylaxis and treatment strategies has become more urgent. Correspondingly, a M. haemolytica wild-type isolate MH-1 has previously been isolated and confirmed. Detailed understanding of M. haemolytica–host interactions is central in controlling this infection. In the current study, we find that IFI204 promotes bacterial killing and clearance by driving GSDMD-dependent inflammasome signaling, and we indicate that therapeutic interventions targeting IFI204 may show a clinical benefit in combating M. haemolytica infections.
Indeed, Ifi16, the homolog of murine Ifi204 expression, is reported in various ruminants [30,31]. To investigate the possible involvement of IFI204 in the host response to M. haemolytica infection in vivo, using a M. haemolytica pneumonia model, this study observed that Ifi204−/− mice exhibited increased bacterial loads, decreased survival, and severe destruction of lung architecture, as expected, indicating that IFI204 contributes to host protection against M. haemolytica pulmonary infection. In line with this, IFI204/IFI16 has also been implicated in response to intracellular and extracellular bacterial infections including Francisella novicida [14], Listeria monocytogenes [32], Mycobacterium bovis [15], and Staphylococcus aureus [18,19]. Altogether, this finding extends and highlights the potential immunological and biological role of IFI204 in pathogens infections.
IFI204/IFI16 is an intracellular innate immune receptor that functions as a DNA sensor, recognizing pathogen-derived double-stranded DNA to activate inflammasome signaling [33,34] and interacting with STING to trigger type I IFN signaling upon cytosolic DNA detection [35,36]. To elucidate IFI204-mediated host defense mechanisms, we assessed its role in inflammatory responses. Notably, M. haemolytica pulmonary infection induced a significantly higher release of IL-1β and Caspase-1 cleavage in WT mice compared to Ifi204−/− mice, indicating IFI204’s involvement in M. haemolytica-induced inflammasome activation—a pathway responsible for IL-1β and IL-18 processing [37,38]. Further investigation into inflammasome-dependent IL-1 family members revealed their critical role in resistance. Crucially, exogenous rIL-18 administration strongly protected infected Ifi204−/− mice, evidenced by reduced bacterial burden and attenuated lung damage. Collectively, these results show that inflammasome signaling downstream of IFI204 confers resistance to M. haemolytica infection.
Given the prominent polymorphonuclear neutrophils (PMNs) infiltration and predominant IFI204 localization within recruited inflammatory cells following M. haemolytica infection, we employed BMDMs to investigate the molecular mechanisms of IFI204-mediated inflammasome activation. Evidence shows IFI16/IFI204-mediated inflammasome activation via bacterial infections, like Campylobacter concisus [39,40], and via viral pathogens, such as KSHV and HIV [34,41,42,43]. p204 (IFI204) recruits ASC, via its N-terminal PYD domain, upon cytosolic dsDNA sensing, to form an inflammasome [21,44]. However, IFI204-mediated anti-infective signaling in M. haemolytica infection remains poorly characterized. We found that M. haemolytica induces IFI204 expression in BMDMs, and Ifi204 deficiency impairs inflammasome signaling, as evidenced by reduced Caspase-1 cleavage, ASC speckle formation, mature IL-1β production, and LDH activity. GSDMD, an executor of cytokine release and pyroptosis [45], was inhibited in the absence of Ifi204 after the M. haemolytica challenge. Hence, our in vivo and in vitro findings demonstrate that GSDMD is involved in M. haemolytica-initiated IFI204-related inflammasome signaling.
Appropriate inflammasome activation is crucial for host defense against bacterial, viral, fungal, and protozoan pathogens [40,46,47,48], promoting immune responses that restrict invasion by pathogens such as Salmonella, Bacillus anthracis, influenza, Candida albicans, Clostridium tyzzeri, and Francisella tularensis [49,50,51,52,53,54]. Inflammasome-activated gasdermin D (N-terminal cleavage product, GSDMD-NT) directly kills both Gram-negative (e.g., E. coli) and Gram-positive (e.g., S. aureus, L. monocytogenes) bacteria [55]. Similarly to Ifi204−/− mice, we then utilized Gsdmd−/− mice to further demonstrate that GSDMD facilitates host survival and bacterial control during M. haemolytica pulmonary infection. Exogenous rIL-18 rescues the susceptibility of Gsdmd−/− mice to M. haemolytica infection in vivo and the defect in bacteria clearance of Ifi204−/− and Gsdmd−/− BMDMs in vitro, highlighting the importance of IFI204-modulated inflammasome signaling in response to M. haemolytica invasion. Given that IFI204/IFI16 functions as an innate immune sensor of pathogens and host DNA in the cytoplasm and nucleus [56,57,58,59], we investigated if M. haemolytica genomic DNA triggers IFI204 signaling. Notably, Ifi204 deficiency significantly suppressed bacterial genomic DNA-induced mature IL-1β release, whereas TNF-α level was unchanged, indicating that M. haemolytica genomic DNA potentially promotes IFI204-modulated inflammasome signaling. While the mechanisms underlying IFI204 activation by M. haemolytica require further investigation, our findings enhance understandings of IFI204’s immunological role in host defense.
In conclusion, we demonstrate here that IFI204/IFI16 plays a nonredundant role in restricting M. haemolytica pneumonia, through activating the GSDMD-dependent inflammasome signaling. We also show that bacterial genomic DNA may be a critical factor that induces IFI204/IFI16-elicited inflammasome signaling activation. Therefore, it is of future interest to evaluate whether pharmacological target of IFI204/IFI16 signaling confers protection in pathogenic infectious diseases.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13112557/s1, Figure S1: Quatification of Caspase-1 p20 determined by densitometry of protein bands from three experiments.The GAPDH served as a loading control (p < 0.0001).Graphs are means ± standard deviation (SD), Statistical significance is considered as ** p < 0.01. Figure S2: Quatification of Caspase-1 p20 and IFI204 determined by densitometry of protein bands from three experiments.The GAPDH served as a loading control (p < 0.0001).Graphs are means ± standard deviation(SD), Statistical significance is considered as ** p < 0.01. Figure S3: Quatification of Caspase-1 p20 determined by densitometry of protein bands from three experiments. The GAPDH served as a loading control (p < 0.0001, p = 0.1205). Graphs are means ± standard deviation (SD), Statistical significance is considered as ** p < 0.01. Figure S4: Quatification of Caspase-1 p20 determined by densitometry of protein bands from three experiments. The GAPDH served as a loading control (p = 0.5604). Graphs are means ± standard deviation (SD), Statistical significance is considered as ** p < 0.01.
Author Contributions
Conceptualization, J.-Q.L. and S.-X.Y.; methodology, Y.Z. and Z.-Y.L.; software, Y.-J.W.; validation, J.-Q.L., Y.Z., and Z.-Y.L.; formal analysis, X.C. and S.-X.Z.; investigation, M.-Y.Z. and A.-B.H.; resources, S.-X.Y.; data curation, Z.-Y.L. and P.S.; writing—original draft preparation, J.-Q.L., Y.Z., and Z.-Y.L.; writing—review and editing, J.-Q.L., Y.Z., Z.-Y.L., and S.-X.Y.; visualization, Z.-J.Z. and Q.X.; supervision, S.-X.Y.; project administration, J.-Q.L. and Y.Z.; funding acquisition, S.-X.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China [No. 32302886, No. 32160832], Natural Science Foundation of Inner Mongolia Autonomous Region of China [No. 2025YQ030], Program for Young Talents of Science and Technology in Universities of Inner Mongolia Autonomous Region of China [No. NJYT22104], Science and Technology Leading Talent Team in Inner Mongolia Autonomous Region of China [No. 2022LJRC0009], and National Key Laboratory of Reproductive Regulation and Breeding of Grassland Livestock (Jointly Built by the Province and Ministry)—Identification of Specific Target Points for Important Pathogens in Cattle and Sheep and Development of Novel Diagnostic Technologies [No. 2025KYPT0066].
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Animal Welfare and Research Ethics Committee of Inner Mongolia University ([2022] 072 and 1 January 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Highlander, S.K. Molecular genetic analysis of virulence in Mannheimia (pasteurella) haemolytica. Front Biosci. 2001, 6, D1128-50. [Google Scholar] [CrossRef]
- Klima, C.L.; Alexander, T.W.; Selinger, L.B.; Read, R.R.; Shewan, P.E.; Gow, S.P.; Booker, C.W.; McAllister, T.A. Comparison of repetitive PCR and pulsed-field gel electrophoresis for the genotyping of Mannheimia haemolytica. J. Microbiol. Methods. 2010, 81, 39–47. [Google Scholar] [CrossRef]
- Prysliak, T.; Vulikh, K.; Caswell, J.L.; Perez-Casal, J. Mannheimia haemolytica increases Mycoplasma bovis disease in a bovine experimental model of BRD. Vet. Microbiol. 2023, 283, 109793. [Google Scholar] [CrossRef]
- Noyes, N.R.; Benedict, K.M.; Gow, S.P.; Booker, C.W.; Hannon, S.J.; McAllister, T.A.; Morley, P.S. Mannheimia haemolytica in feedlot cattle, Prevalence of recovery and associations with antimicrobial use, resistance, and health outcomes. J. Vet. Intern. Med. 2015, 29, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Zhang, B.H.; Luo, Z.D.; Yao, X.P.; Wang, Y.; Yang, Z.X.; Luo, Y.; Cao, S.Z. Isolation, Identification and Whole Genome Sequence Analysis of a Goat-derived Type 2 Mannheimia haemolytica. J. YUNNAN Agric. Univ. (Nat. Sci.) 2021, 36, 623–630, 699. [Google Scholar] [CrossRef]
- Santos-Rivera, M.; Woolums, A.; Thoresen, M.; Blair, E.; Jefferson, V.; Meyer, F.; Vance, C.K. Profiling Mannheimia haemolytica infection in dairy calves using near infrared spectroscopy (NIRS) and multivariate analysis (MVA). Sci. Rep. 2021, 11, 1392. [Google Scholar] [CrossRef]
- Sorin-Dupont, B.; Poyard, A.; Assié, S.; Picault, S.; Ezanno, P. Individual or collective treatments, How to target antimicrobial use to limit the spread of Mannheimia haemolytica among beef cattle? arXiv 2024, arXiv:2408.16269. [Google Scholar] [CrossRef]
- Bahr, A.D.; Salib, F.A.; Soliman, Y.A.; Amin, M.A. Multi-drug resistant Pasteurella multocida and Mannheimia haemolytica strains isolated from different hosts affected by pneumonic pasteurellosis in Egypt. Adv. Anim. Vet. Sci. 2021, 9, 356–364. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kaldis, A.; Menassa, R.; Ortiz Guluarte, J.; Barreda, D.R.; Guan, L.L.; Alexander, T.W. Mucosal Immunization with Spore-Based Vaccines against Mannheimia haemolytica Enhances Antigen-Specific Immunity. Vaccines 2024, 12, 375. [Google Scholar] [CrossRef]
- Confer, A.W.; Ayalew, S. Mannheimia haemolytica in bovine respiratory disease, Immunogens, potential immunogens, and vaccines. Anim. Health Res. Rev. 2018, 19, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Bkiri, D.; Elmejdoub, S.; Bamouh, Z.; Fihri, O.F.; El-Harrak, M. Comparative protection of small ruminants against Mannheimia haemolytica infection by inactivated bacterin and toxoid vaccines. Vet. World. 2023, 16, 68–75. [Google Scholar] [CrossRef]
- Zindel, J.; Kubes, P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu. Rev. Pathol. 2020, 15, 493–518. [Google Scholar] [CrossRef]
- Walsh, D.; McCarthy, J.; O’Driscoll, C.; Melgar, S. Pattern recognition receptors—Molecular orchestrators of inflammation in inflammatory bowel disease. Cytokine & growth factor reviews. 2013, 24, 91–104. [Google Scholar] [CrossRef]
- Storek, K.M.; Gertsvolf, N.A.; Ohlson, M.B.; Monack, D.M. cGAS and Ifi204 cooperate to produce type I IFNs in response to Francisella infection. J. Immunol. 2015, 194, 3236–3245. [Google Scholar] [CrossRef] [PubMed]
- Chunfa, L.; Xin, S.; Qiang, L.; Sreevatsan, S.; Yang, L.; Zhao, D.; Zhou, X. The Central Role of IFI204 in IFN-β Release and Autophagy Activation during Mycobacterium bovis Infection. Front. Cell Infect. Microbiol. 2017, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.N.; Roy, M.; Ong, S.E.; Mertins, P.; Villani, A.C.; Li, W.; Dotiwala, F.; Sen, J.; Doench, J.G.; Orzalli, M.H.; et al. Identification of regulators of the innate immune response to cytosolic DNA and retroviral infection by an integrative approach. Nat. Immunol. 2013, 14, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Ji, Y.; Zeng, L.; Liu, Q.; Zhang, Z.; Guo, S.; Guo, X.; Tong, Y.; Zhao, X.; Li, C.M.; et al. P200 family protein IFI204 negatively regulates type I interferon responses by targeting IRF7 in nucleus. PLoS Pathog. 2019, 15, e1008079. [Google Scholar] [CrossRef]
- Chen, W.; Yu, S.X.; Zhou, F.H.; Zhang, X.J.; Gao, W.Y.; Li, K.Y.; Liu, Z.Z.; Han, W.Y.; Yang, Y.J. DNA Sensor IFI204 Contributes to Host Defense Against Staphylococcus aureus Infection in Mice. Front. Immunol. 2019, 10, 474. [Google Scholar] [CrossRef]
- Zhang, J.G.; Chen, W.; Zhou, C.K.; Ma, K.; Liu, Z.Z.; Gao, Y.; Lin, X.Q.; Yang, Y.J. IFI204 protects host defense against Staphylococcus aureus-induced pneumonia by promoting extracellular traps formation. Exp. Cell Res. 2023, 422, 113415. [Google Scholar] [CrossRef]
- Yi, Y.S.; Jian, J.; Gonzalez-Gugel, E.; Shi, Y.X.; Tian, Q.; Fu, W.; Hettinghouse, A.; Song, W.; Liu, R.; He, M.; et al. p204 Is Required for Canonical Lipopolysaccharide-induced TLR4 Signaling in Mice. EBioMedicine. 2018, 29, 78–91. [Google Scholar] [CrossRef]
- Veeranki, S.; Choubey, D. Interferon-inducible p200-family protein IFI16, an innate immune sensor for cytosolic and nuclear double-stranded DNA, Regulation of subcellular localization. Mol. Immunol. 2012, 49, 567–571. [Google Scholar] [CrossRef]
- Guo, T.; Lai, Y.; Wu, S.; Lin, C.; Zhou, X.; Lin, P.; Zheng, M.; Chen, J.; Lin, F. IFI204 in microglia mediates traumatic brain injury-induced mitochondrial dysfunction and pyroptosis via SENP7 interaction. Cell Biol. Toxicol. 2025, 41, 89. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lei, Y.X.; Li, J.W.; Ma, Y.Z.; Wang, X.Y.; Meng, F.H.; Wu, Y.J.; Wang, N.; Liang, J.; Zhao, C.Q.; et al. G Protein-Coupled Receptor 120 Mediates Host Defense against Clostridium perfringens Infection through Regulating NOD-like Receptor Family Pyrin Domain-Containing 3 Inflammasome Activation. J. Agric. Food Chem. 2023, 71, 7119–7130. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.Y.; Hu, C.M.; Yin, Q.; Zhang, X.M.; Liu, Z.Z.; Zhou, C.K.; Zhang, J.G.; Chen, W.; Yang, Y.J. Dual-Mechanism Peptide SR25 has Broad Antimicrobial Activity and Potential Application for Healing Bacteria-infected Diabetic Wounds. Adv Sci. 2024, 11, e2401793. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xing, L.H.; Li, F.X.; Wang, N.; Ma, Y.Z.; Li, J.W.; Wu, Y.J.; Liang, J.; Lei, Y.X.; Wang, X.Y.; et al. Erratum, Mixed lineage kinase-like protein protects against Clostridium perfringens infection by enhancing NLRP3 inflammasome-extracellular traps axis. iScience. 2023, 26, 106149. [Google Scholar] [CrossRef]
- Yu, S.X.; Du, C.T.; Chen, W.; Lei, Q.Q.; Li, N.; Qi, S.; Zhang, X.J.; Hu, G.Q.; Deng, X.M.; Han, W.Y.; et al. Genipin inhibits NLRP3 and NLRC4 inflammasome activation via autophagy suppression. Sci. Rep. 2015, 5, 17935. [Google Scholar] [CrossRef]
- Boorei, M.A.; Paul, B.T.; Abdullah Jesse, F.F.; Teik Chung, E.L.; Mohd Lila, M.A. Responses of selected biomarkers, female reproductive hormones and tissue changes in non-pregnant does challenged with Mannheimia haemolytica serotype A2 and its outer membrane protein (OMP) immunogen. Microb. Pathog. 2022, 169, 105674. [Google Scholar] [CrossRef]
- Credille, B. Antimicrobial resistance in Mannheimia haemolytica, Prevalence and impact. Anim. Health Res. Rev. 2020, 21, 196–199. [Google Scholar] [CrossRef]
- Woolums, A.R.; Karisch, B.B.; Frye, J.G.; Epperson, W.; Smith, D.R.; Blanton, J., Jr.; Austin, F.; Kaplan, R.; Hiott, L.; Woodley, T.; et al. Multidrug resistant Mannheimia haemolytica isolated from high-risk beef stocker cattle after antimicrobial metaphylaxis and treatment for bovine respiratory disease. Vet. Microbiol. 2018, 221, 143–152. [Google Scholar] [CrossRef]
- Kumar, S.; Chera, J.S.; Vats, A.; De, S. Nature of selection varies on different domains of IFI16-like PYHIN genes in ruminants. BMC Evol. Biol. 2019, 19, 26. [Google Scholar] [CrossRef]
- Manjari, P.; Reddi, S.; Alhussien, M.; Mohammed, S.; De, S.; Mohanty, A.K.; Sivalingam, J.; Dang, A.K. Neutrophil gene dynamics and plasma cytokine levels in dairy cattle during peri-implantation period. Vet. Immunol. Immunopathol. 2016, 173, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.; Prabakaran, T.; Laustsen, A.; Jørgensen, S.E.; Rahbæk, S.H.; Jensen, S.B.; Nielsen, R.; Leber, J.H.; Decker, T.; Horan, K.A.; et al. Listeria monocytogenes induces IFNβ expression through an IFI16-, cGAS- and STING-dependent pathway. EMBO J. 2014, 33, 1654–1666. [Google Scholar] [CrossRef]
- Monroe, K.M.; Yang, Z.; Johnson, J.R.; Geng, X.; Doitsh, G.; Krogan, N.J.; Greene, W.C. IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science 2014, 343, 428–432. [Google Scholar] [CrossRef]
- Yan, Q.; Zhou, J.; Wang, Z.; Ding, X.; Ma, X.; Li, W.; Jia, X.; Gao, S.J.; Lu, C. NAT10-dependent N4-acetylcytidine modification mediates PAN RNA stability, KSHV reactivation, and IFI16-related inflammasome activation. Nat. Commun. 2023, 14, 6327. [Google Scholar] [CrossRef]
- Unterholzner, L.; Keating, S.E.; Baran, M.; Horan, K.A.; Jensen, S.B.; Sharma, S.; Sirois, C.M.; Jin, T.; Latz, E.; Xiao, T.S.; et al. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 2010, 11, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Bustos, M.A.; Hayashi, Y.; Yu, Q.; Hoon, D. Interferon-induced factor 16 is essential in metastatic melanoma to maintain STING levels and the immune responses upon IFN-γ response pathway activation. J. Immunother. Cancer. 2024, 12, e009590. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Netea, M.G.; Dinarello, C.A.; Joosten, L.A. Inflammasome activation and IL-1β and IL-18 processing during infection. Trends Immunol. 2011, 32, 110–116. [Google Scholar] [CrossRef]
- Rathinam, V.A.; Vanaja, S.K.; Fitzgerald, K.A. Regulation of inflammasome signaling. Nat. Immunol. 2012, 13, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Kaakoush, N.O.; Deshpande, N.P.; Man, S.M.; Burgos-Portugal, J.A.; Khattak, F.A.; Raftery, M.J.; Wilkins, M.R.; Mitchell, H.M. Transcriptomic and proteomic analyses reveal key innate immune signatures in the host response to the gastrointestinal pathogen Campylobacter concisus. Infect. Immun. 2015, 83, 832–845. [Google Scholar] [CrossRef]
- Man, S.M.; Karki, R.; Kanneganti, T.D. DNA-sensing inflammasomes, Regulation of bacterial host defense and the gut microbiota. Pathog Dis. 2016, 74, ftw028. [Google Scholar] [CrossRef]
- Kerur, N.; Veettil, M.V.; Sharma-Walia, N.; Bottero, V.; Sadagopan, S.; Otageri, P.; Chandran, B. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe. 2011, 9, 363–375. [Google Scholar] [CrossRef]
- Doitsh, G.; Cavrois, M.; Lassen, K.G.; Zepeda, O.; Yang, Z.; Santiago, M.L.; Hebbeler, A.M.; Greene, W.C. Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell. 2010, 143, 789–801. [Google Scholar] [CrossRef]
- Church, J.A. Cell Death by Pyroptosis Drives CD4 T-Cell Depletion in HIV-1 Infection. Pediatrics 2014, 134 (Suppl. S3), S184. [Google Scholar] [CrossRef]
- Loeven, N.A.; Medici, N.P.; Bliska, J.B. The pyrin inflammasome in host-microbe interactions. Curr. Opin. Microbiol. 2020, 54, 77–86. [Google Scholar] [CrossRef]
- He, W.T.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.H.; Zhong, C.Q.; Han, J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef]
- Vladimer, G.I.; Marty-Roix, R.; Ghosh, S.; Weng, D.; Lien, E. Inflammasomes and host defenses against bacterial infections. Curr. Opin. Microbiol. 2013, 16, 23–31. [Google Scholar] [CrossRef]
- Ulland, T.K.; Ferguson, P.J.; Sutterwala, F.S. Evasion of inflammasome activation by microbial pathogens. J. Clin. Invest. 2015, 125, 469–477. [Google Scholar] [CrossRef]
- Franchi, L.; Muñoz-Planillo, R.; Núñez, G. Sensing and reacting to microbes through the inflammasomes. Nat. Immunol. 2012, 13, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.X.; Chen, W.; Liu, Z.Z.; Zhou, F.H.; Yan, S.Q.; Hu, G.Q.; Qin, X.X.; Zhang, J.; Ma, K.; Du, C.T.; et al. Non-Hematopoietic MLKL Protects Against Salmonella Mucosal Infection by Enhancing Inflammasome Activation. Front. Immunol. 2018, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Terra, J.K.; Cote, C.K.; France, B.; Jenkins, A.L.; Bozue, J.A.; Welkos, S.L.; LeVine, S.M.; Bradley, K.A. Cutting edge, Resistance to Bacillus anthracis infection mediated by a lethal toxin sensitive allele of Nalp1b/Nlrp1b. J. Immunol. 2010, 184, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.G.; Dash, P.; Aldridge, J.R., Jr.; Ellebedy, A.H.; Reynolds, C.; Funk, A.J.; Martin, W.J.; Lamkanfi, M.; Webby, R.J.; Boyd, K.L.; et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 2009, 30, 566–575. [Google Scholar] [CrossRef]
- Hise, A.G.; Tomalka, J.; Ganesan, S.; Patel, K.; Hall, B.A.; Brown, G.D.; Fitzgerald, K.A. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009, 5, 487–497. [Google Scholar] [CrossRef]
- Sateriale, A.; Gullicksrud, J.A.; Engiles, J.B.; McLeod, B.I.; Kugler, E.M.; Henao-Mejia, J.; Zhou, T.; Ring, A.M.; Brodsky, I.E.; Hunter, C.A.; et al. The intestinal parasite Cryptosporidium is controlled by an enterocyte intrinsic inflammasome that depends on NLRP6. Proc. Natl. Acad. Sci. USA 2021, 118, e2007807118. [Google Scholar] [CrossRef]
- Rathinam, V.A.; Jiang, Z.; Waggoner, S.N.; Sharma, S.; Cole, L.E.; Waggoner, L.; Vanaja, S.K.; Monks, B.G.; Ganesan, S.; Latz, E.; et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 2010, 11, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef]
- Li, T.; Diner, B.A.; Chen, J.; Cristea, I.M. Acetylation modulates cellular distribution and DNA sensing ability of interferon-inducible protein IFI16. Proc. Natl. Acad. Sci. USA 2012, 109, 10558–10563. [Google Scholar] [CrossRef] [PubMed]
- Jønsson, K.L.; Laustsen, A.; Krapp, C.; Skipper, K.A.; Thavachelvam, K.; Hotter, D.; Egedal, J.H.; Kjolby, M.; Mohammadi, P.; Prabakaran, T.; et al. IFI16 is required for DNA sensing in human macrophages by promoting production and function of cGAMP. Nat. Commun. 2017, 8, 14391. [Google Scholar] [CrossRef] [PubMed]
- Dunphy, G.; Flannery, S.M.; Almine, J.F.; Connolly, D.J.; Paulus, C.; Jønsson, K.L.; Jakobsen, M.R.; Nevels, M.M.; Bowie, A.G.; Unterholzner, L. Non-canonical Activation of the DNA Sensing Adaptor STING by ATM and IFI16 Mediates NF-κB Signaling after Nuclear DNA Damage. Mol. Cell. 2018, 71, 745–760.e5. [Google Scholar] [CrossRef]
- Choubey, D.; Panchanathan, R. IFI16, an amplifier of DNA-damage response, Role in cellular senescence and aging-associated inflammatory diseases. Ageing Res. Rev. 2016, 28, 27–36. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).