Bioinformatic Analysis of Oxalate-Degrading Enzymes in Probiotics: A Systematic Genome-Scale and Structural Survey
Abstract
1. Introduction
2. Materials and Methods
2.1. Genome Retrieval and Quality Control
2.2. Gene Annotation and Phylogenetic Analysis
2.3. Protein Sequence Homology and Catalytic Site Conservation Analysis
2.4. Protein Structure Prediction and Comparison
3. Results
3.1. Quality Control of Genome Data
3.2. Distribution of Oxalate-Degrading Genes Across Species
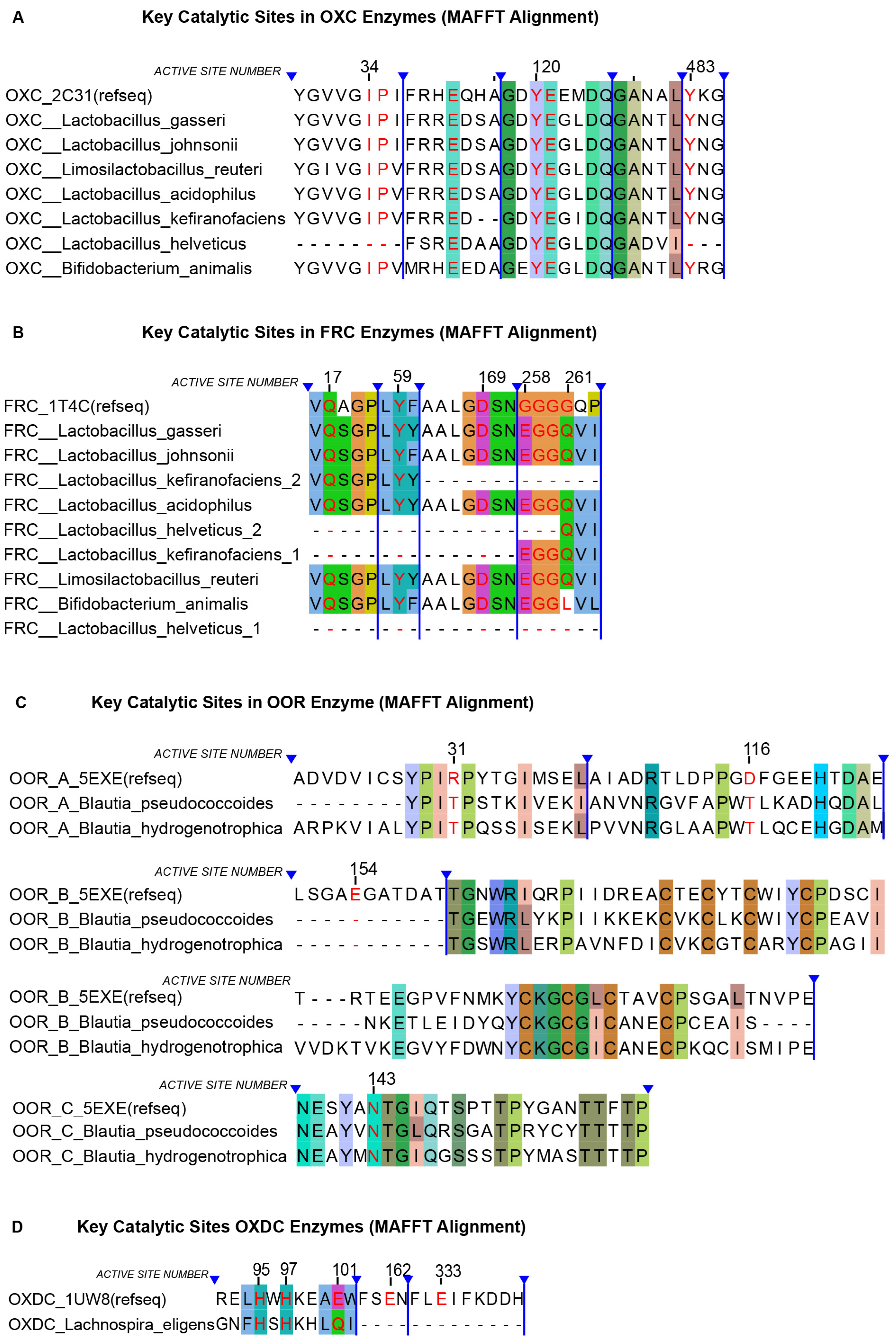
3.3. Protein Homology and Active Site Conservation
3.4. Multiple Sequence Alignment of Catalytic Sites
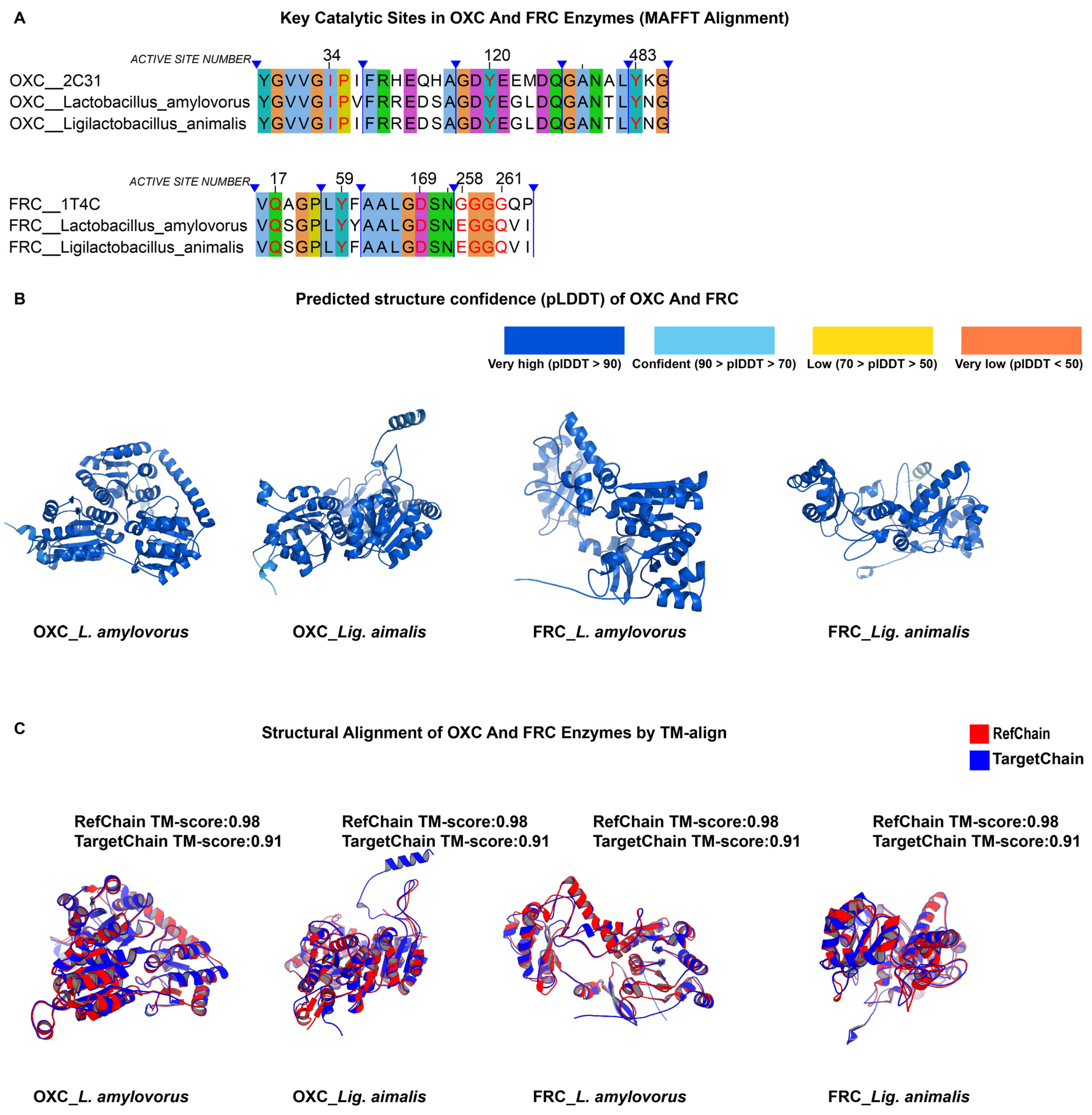
3.5. Structural Prediction and Alignment
3.6. Expansion of Candidate Species Based on International Probiotic Lists
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abufaraj, M.; Xu, T.; Cao, C.; Waldhoer, T.; Seitz, C.; D’Andrea, D.; Siyam, A.; Tarawneh, R.; Fajkovic, H.; Schernhammer, E.; et al. Prevalence and Trends in Kidney Stone Among Adults in the USA: Analyses of National Health and Nutrition Examination Survey 2007–2018 Data. Eur. Urol. Focus 2021, 7, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Jiang, M.; Guan, Y.; Li, S.; Duan, C.; Gong, Y.; Kong, Y.; Shao, Z.; Wu, H.; Yao, X.; et al. Trans-ancestry GWAS identifies 59 loci and improves risk prediction and fine-mapping for kidney stone disease. Nat. Commun. 2025, 16, 3473. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Yang, Y.; Cheng, B.; Yang, S.; Wang, Y. Environmental determinants in the development of kidney stone. Urolithiasis 2025, 53, 43. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.M. Nephrolithiasis: Treatment, causes, and prevention. Cleve Clin. J. Med. 2009, 76, 583–591. [Google Scholar] [CrossRef]
- Lao, M.; Kogan, B.A.; White, M.D.; Feustel, P.J. High recurrence rate at 5-year followup in children after upper urinary tract stone surgery. J. Urol. 2014, 191, 440–444. [Google Scholar] [CrossRef]
- Xia, K.; Xu, Y.; Qi, Q.; Pan, J.; Yao, R.; Huang, Q.; Hao, Z. Ae index is an independent predictor of kidney stone recurrence in overweight and obese patients. BMC Urol. 2023, 23, 151. [Google Scholar] [CrossRef]
- Baum, M.A.; Mandel, M.; Somers, M.J.G. Understanding Rare Kidney Stone Diseases: A Review. Am. J. Kidney Dis. 2025, 86, 236–244. [Google Scholar] [CrossRef]
- Arivoli, K.; Valicevic, A.N.; Oerline, M.K.; Hsi, R.S.; Patel, S.R.; Hollingsworth, J.M.; Shahinian, V.B. Preventive Pharmacological Therapy and Risk of Recurrent Urinary Stone Disease. Clin. J. Am. Soc. Nephrol. 2024, 19, 565–572. [Google Scholar] [CrossRef]
- Zisman, A.L. Effectiveness of Treatment Modalities on Kidney Stone Recurrence. Clin. J. Am. Soc. Nephrol. 2017, 12, 1699–1708. [Google Scholar] [CrossRef]
- Dauw, C.A.; Yi, Y.; Bierlein, M.J.; Yan, P.; Alruwaily, A.F.; Ghani, K.R.; Wolf, J.S., Jr.; Hollenbeck, B.K.; Hollingsworth, J.M. Medication Nonadherence and Effectiveness of Preventive Pharmacological Therapy for Kidney Stones. J. Urol. 2016, 195, 648–652. [Google Scholar] [CrossRef]
- Zeng, G.; Mai, Z.; Xia, S.; Wang, Z.; Zhang, K.; Wang, L.; Long, Y.; Ma, J.; Li, Y.; Wan, S.P.; et al. Prevalence of kidney stones in China: An ultrasonography based cross-sectional study. BJU Int. 2017, 120, 109–116. [Google Scholar] [CrossRef]
- Kim, C.H.; Chung, D.Y.; Rha, K.H.; Lee, J.Y.; Lee, S.H. Effectiveness of Percutaneous Nephrolithotomy, Retrograde Intrarenal Surgery, and Extracorporeal Shock Wave Lithotripsy for Treatment of Renal Stones: A Systematic Review and Meta-Analysis. Medicina 2021, 57, 26. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Han, D.H. Treatment outcomes of retrograde intrarenal surgery for renal stones and predictive factors of stone-free. Korean J. Urol. 2010, 51, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Cho, K.S.; Ham, W.S.; Chung, D.Y.; Kwon, J.K.; Choi, Y.D.; Lee, J.Y. Ureteral stenting can be a negative predictor for successful outcome following shock wave lithotripsy in patients with ureteral stones. Investig. Clin. Urol. 2016, 57, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Tavasoli, S.; Alebouyeh, M.; Naji, M.; Shakiba Majd, G.; Shabani Nashtaei, M.; Broumandnia, N.; Basiri, A. Association of intestinal oxalate-degrading bacteria with recurrent calcium kidney stone formation and hyperoxaluria: A case-control study. BJU Int. 2020, 125, 133–143. [Google Scholar] [CrossRef]
- Vittori, M.; Bove, P.; Signoretti, M.; Cipriani, C.; Gasparoli, C.; Antonucci, M.; Carilli, M.; Maiorino, F.; Iacovelli, V.; Petta, F.; et al. Oral supplementation with probiotics, potassium citrate, and magnesium in reducing crystalluria in stone formers: A phase II study. Urologia 2024, 91, 681–686. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, L.; Li, J. Prediction of calcium oxalate kidney stones: A comprehensive analysis of clinical and gut microbiome characteristics. Medicine 2025, 104, e43103. [Google Scholar] [CrossRef]
- Abratt, V.R.; Reid, S.J. Oxalate-degrading bacteria of the human gut as probiotics in the management of kidney stone disease. Adv. Appl. Microbiol. 2010, 72, 63–87. [Google Scholar] [CrossRef]
- Lieske, J.C.; Goldfarb, D.S.; De Simone, C.; Regnier, C. Use of a probiotic to decrease enteric hyperoxaluria. Kidney Int. 2005, 68, 1244–1249. [Google Scholar] [CrossRef]
- Siener, R.; Ebert, D.; Nicolay, C.; Hesse, A. Dietary risk factors for hyperoxaluria in calcium oxalate stone formers. Kidney Int. 2003, 63, 1037–1043. [Google Scholar] [CrossRef]
- Pei, X.; Liu, M.; Yu, S. How is the human microbiome linked to kidney stones? Front. Cell. Infect. Microbiol. 2025, 15, 1602413. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, D.; Li, T.; Jia, B. Probiotics in the prevention and treatment of calcium oxalate kidney stones: Mechanisms and therapeutic potential. Front. Microbiol. 2025, 16, 1663138. [Google Scholar] [CrossRef] [PubMed]
- Mani, R.R.; Ranganathan, V.; Panneerselvam, J.; Begam, S.; Chinnappan, S.; Anbalagan, M. Therapeutic Applications of Oxalate-degrading Bacteria in Kidney Stone Prevention. Nat. Prod. J. 2025, 16, e22103155352643. [Google Scholar] [CrossRef]
- Al-Kabe, S.H.; Niamah, A.K. Current Trends and Technological Advancements in the Use of Oxalate-Degrading Bacteria as Starters in Fermented Foods-A Review. Life 2024, 14, 1338. [Google Scholar] [CrossRef]
- Alelign, T.; Petros, B. Kidney Stone Disease: An Update on Current Concepts. Adv. Urol. 2018, 2018, 3068365. [Google Scholar] [CrossRef]
- Bianco, J.; Chu, F.; Bergsland, K.; Coe, F.; Worcester, E.; Prochaska, M. What treatments reduce kidney stone risk in patients with bowel disease? Urolithiasis 2022, 50, 557–565. [Google Scholar] [CrossRef]
- Sadaf, H.; Raza, S.I.; Hassan, S.W. Role of gut microbiota against calcium oxalate. Microb. Pathog. 2017, 109, 287–291. [Google Scholar] [CrossRef]
- Zhao, E.; Zhang, W.; Geng, B.; You, B.; Wang, W.; Li, X. Intestinal dysbacteriosis leads to kidney stone disease. Mol. Med. Rep. 2021, 23, 180. [Google Scholar] [CrossRef]
- Denburg, M.R.; Koepsell, K.; Lee, J.J.; Gerber, J.; Bittinger, K.; Tasian, G.E. Perturbations of the Gut Microbiome and Metabolome in Children with Calcium Oxalate Kidney Stone Disease. J. Am. Soc. Nephrol. 2020, 31, 1358–1369. [Google Scholar] [CrossRef]
- Svedruzić, D.; Jónsson, S.; Toyota, C.G.; Reinhardt, L.A.; Ricagno, S.; Lindqvist, Y.; Richards, N.G. The enzymes of oxalate metabolism: Unexpected structures and mechanisms. Arch. Biochem. Biophys. 2005, 433, 176–192. [Google Scholar] [CrossRef]
- Tanner, A.; Bornemann, S. Bacillus subtilis YvrK is an acid-induced oxalate decarboxylase. J. Bacteriol. 2000, 182, 5271–5273. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Ostell, J.; Pruitt, K.D.; Sayers, E.W. GenBank. Nucleic Acids Res. 2018, 46, D41–D47. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Chaumeil, P.-A.; Mussig, A.J.; Hugenholtz, P.; Parks, D.H. GTDB-Tk: A toolkit to classify genomes with the Genome Taxonomy Database. Bioinformatics 2019, 36, 1925–1927. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chen, L.; Crichlow, G.V.; Christie, C.H.; Dalenberg, K.; Di Costanzo, L.; Duarte, J.M.; et al. RCSB Protein Data Bank: Powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 2021, 49, D437–D451. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Madeira, F.; Pearce, M.; Tivey, A.R.N.; Basutkar, P.; Lee, J.; Edbali, O.; Madhusoodanan, N.; Kolesnikov, A.; Lopez, R. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res. 2022, 50, W276–W279. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Zhang, Y.; Skolnick, J. TM-align: A protein structure alignment algorithm based on the TM-score. Nucleic Acids Res. 2005, 33, 2302–2309. [Google Scholar] [CrossRef]
- Schrödinger, LLC. The PyMOL Molecular Graphics System, Version 3.1.0a0 (Open-Source); 2023. Available online: https://pymol.org/support.html?#citing (accessed on 25 July 2025).
- Karamad, D.; Khosravi-Darani, K.; Khaneghah, A.M.; Miller, A.W. Probiotic Oxalate-Degrading Bacteria: New Insight of Environmental Variables and Expression of the oxc and frc Genes on Oxalate Degradation Activity. Foods 2022, 11, 2876. [Google Scholar] [CrossRef]
- Klimesova, K.; Whittamore, J.M.; Hatch, M. Bifidobacterium animalis subsp. lactis decreases urinary oxalate excretion in a mouse model of primary hyperoxaluria. Urolithiasis 2015, 43, 107–117. [Google Scholar] [CrossRef]
- Karamad, D.; Khosravi-Darani, K.; Hosseini, H.; Tavasoli, S.; Miller, A.W. Assessment of the Process Variables for Degradation of Oxalate by Lactobacillus acidophilus ATCC 4356 Using Simulated Rumen Fluid Media and Tea. Appl. Food Biotechnol. 2020, 7, 195–204. [Google Scholar] [CrossRef]
- Taheri, H.; Feizabadi, M.M.; Keikha, R.; Afkari, R. Therapeutic effects of probiotics and herbal medications on oxalate nephrolithiasis: A mini systematic review. Iran. J. Microbiol. 2024, 16, 4–18. [Google Scholar] [CrossRef]
- Cole, C.G.; Zhang, Z.J.; Dommaraju, S.R.; Dong, Q.; Pope, R.L.; Son, S.S.; McSpadden, E.J.; Woodson, C.K.; Lin, H.; Dylla, N.P.; et al. Lantibiotic-producing bacteria impact microbiome resilience and colonization resistance. bioRxiv 2025. [Google Scholar] [CrossRef]
- van de Velde, C.; Joseph, C.; Simoens, K.; Raes, J.; Bernaerts, K.; Faust, K. Technical versus biological variability in a synthetic human gut community. Gut Microbes 2023, 15, 2155019. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, S.; Sun, K.; Xiao, B.; Liu, Z. Bioinformatic Analysis of Oxalate-Degrading Enzymes in Probiotics: A Systematic Genome-Scale and Structural Survey. Microorganisms 2025, 13, 2553. https://doi.org/10.3390/microorganisms13112553
Du S, Sun K, Xiao B, Liu Z. Bioinformatic Analysis of Oxalate-Degrading Enzymes in Probiotics: A Systematic Genome-Scale and Structural Survey. Microorganisms. 2025; 13(11):2553. https://doi.org/10.3390/microorganisms13112553
Chicago/Turabian StyleDu, Shengda, Ke Sun, Bo Xiao, and Zhihua Liu. 2025. "Bioinformatic Analysis of Oxalate-Degrading Enzymes in Probiotics: A Systematic Genome-Scale and Structural Survey" Microorganisms 13, no. 11: 2553. https://doi.org/10.3390/microorganisms13112553
APA StyleDu, S., Sun, K., Xiao, B., & Liu, Z. (2025). Bioinformatic Analysis of Oxalate-Degrading Enzymes in Probiotics: A Systematic Genome-Scale and Structural Survey. Microorganisms, 13(11), 2553. https://doi.org/10.3390/microorganisms13112553





