Competition-Exclusion for Manganese Is Involved in Antifungal Activity of Two Lactic Acid Bacteria Against Various Dairy Spoilage Fungi
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Conditions
2.2. Impact of Trace Element Supplementation on LAB Antifungal Activity
2.3. Scavenging of Yogurt Trace Elements by Antifungal LAB
2.4. Uptake of Trace Elements by Fungi in Yogurt Cell-Free Whey
2.4.1. Growth Kinetics of Fungal Targets in Yogurt Cell-Free Whey
2.4.2. Statistical Analyses
3. Results
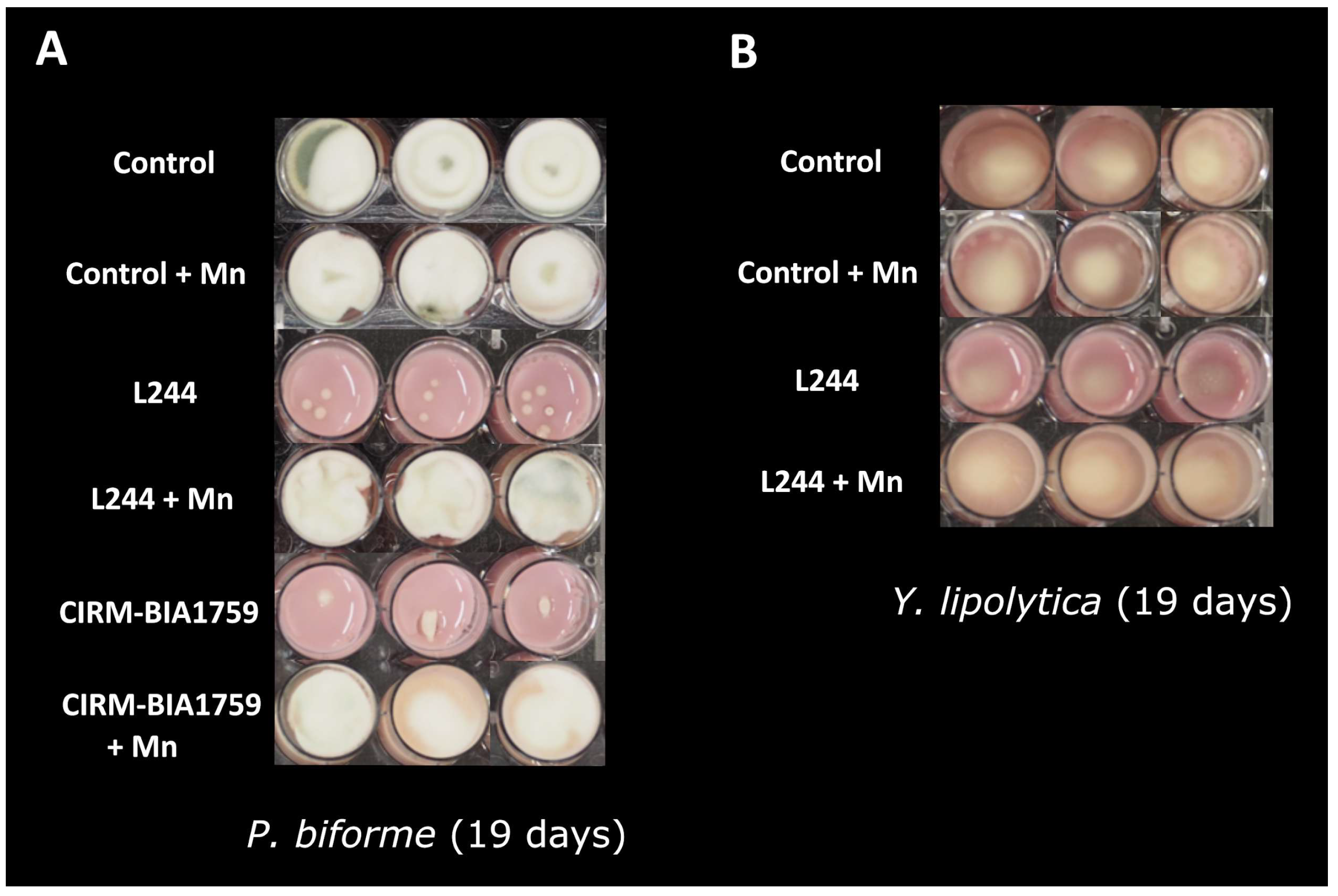
3.1. Impact of Trace Element Supplementation on LAB Antifungal Activity
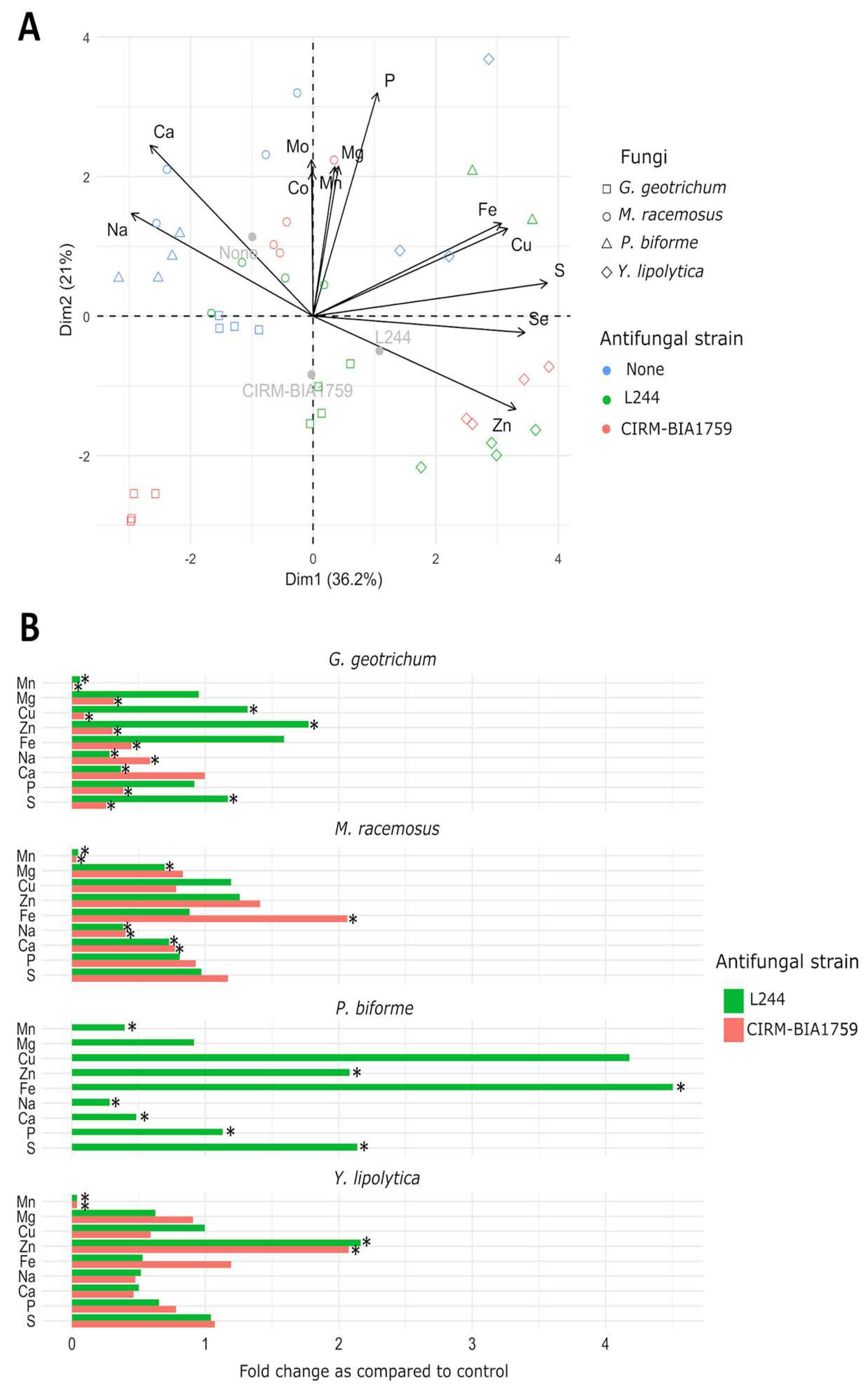
3.2. Scavenging of Trace Elements in Yogurt by Antifungal LAB
3.3. Uptake of Trace Elements by Fungi in Whey
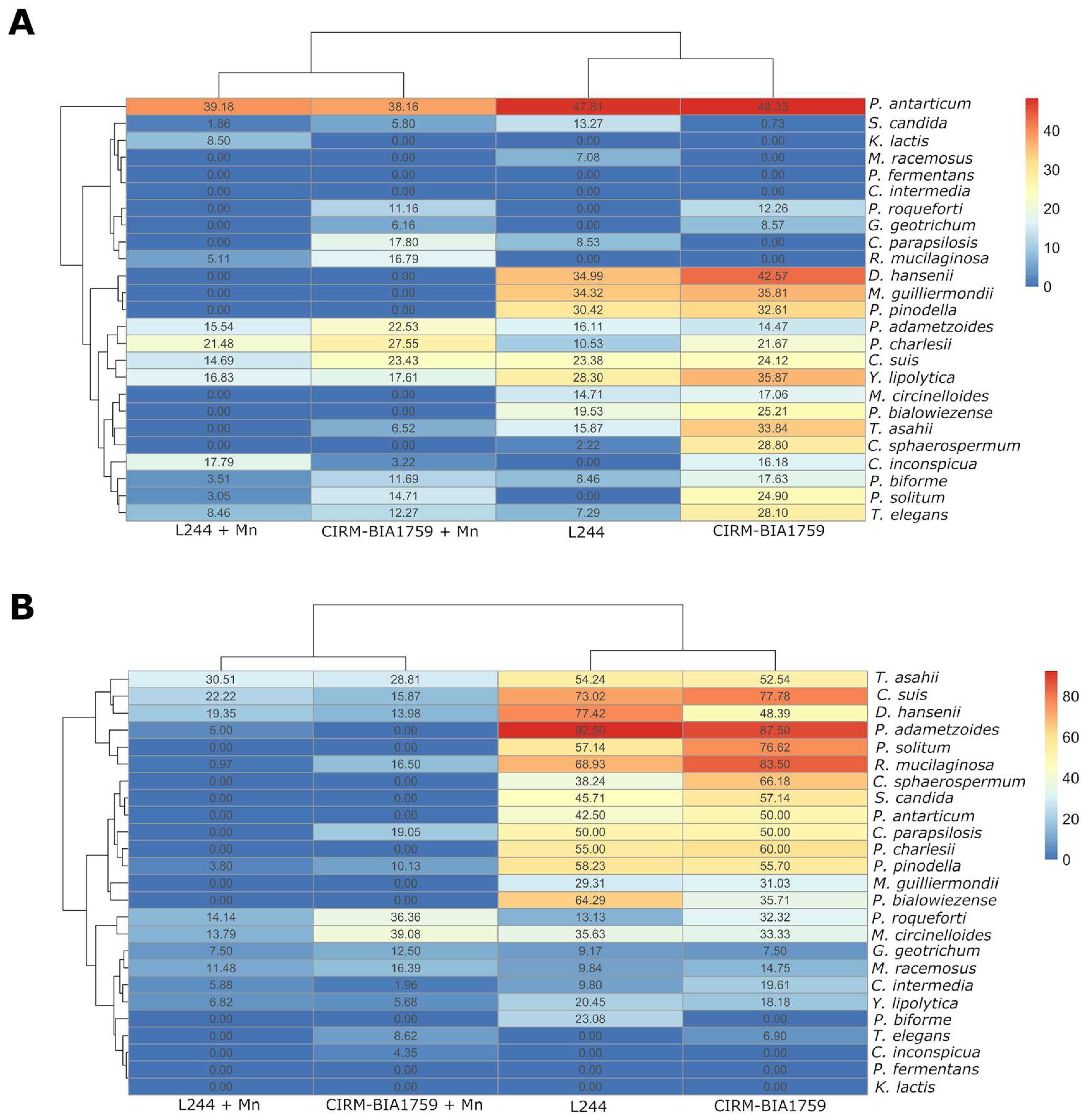
3.4. Growth Kinetics of Fungal Targets in Whey
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | Antifungal |
| ANOVA | Analysis of variance |
| ATP | Adenosine Triphosphate |
| Ca | Calcium |
| Co | Cobalt |
| Cu | Copper |
| Fe | Iron |
| HNO3 | Nitric acid |
| H2O2 | Hydrogen peroxide |
| HR-ICP-MS | High-Resolution Inductively Coupled Plasma Mass Spectrometry |
| LAB | Lactic acid bacteria |
| Lag | Duration of the lag phase |
| µmax | Maximum growth rate |
| MntH1 | Manganese transporter-encoding gene |
| Mg | Magnesium |
| MIC | Minimal inhibitory concentration |
| Mn | Manganese |
| Mo | Molybdenum |
| MRS | Mann–Rogosa–Sharpe |
| Na | Sodium |
| P | Phosphorus |
| PCA | Principal component analysis |
| PP | Polypropylene |
| PTFE | Polytetrafluoroethyle |
| QPS | Qualified Presumption of Safety |
| ROS | Reactive oxygen species |
| S | Sulfur |
| Se | Selenium |
| SOD | Superoxide dismutase |
| Zn | Zinc |
References
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Springer: Boston, MA, USA, 2009; ISBN 978-0-387-92206-5. [Google Scholar] [CrossRef]
- Rohm, H.; Eliskases-Lechner, F.; Bräuer, M. Diversity of Yeasts in Selected Dairy Products. J. Appl. Bacteriol. 1992, 72, 370–376. [Google Scholar] [CrossRef]
- Nelson, C.E. Strategies of Mold Control in Dairy Feeds. J. Dairy Sci. 1993, 76, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Garnier, L.; Valence, F.; Mounier, J. Diversity and Control of Spoilage Fungi in Dairy Products: An Update. Microorganisms 2017, 5, 42. [Google Scholar] [CrossRef]
- Streekstra, H.; Verkennis, A.E.E.; Jacobs, R.; Dekker, A.; Stark, J.; Dijksterhuis, J. Fungal Strains and the Development of Tolerance against Natamycin. Int. J. Food Microbiol. 2016, 238, 15–22. [Google Scholar] [CrossRef]
- Brul, S.; Coote, P. Preservative Agents in Foods: Mode of Action and Microbial Resistance Mechanisms. Int. J. Food Microbiol. 1999, 50, 1–17. [Google Scholar] [CrossRef]
- Schnürer, J.; Magnusson, J. Antifungal Lactic Acid Bacteria as Biopreservatives. Trends Food Sci. Technol. 2005, 16, 70–78. [Google Scholar] [CrossRef]
- Pfeiler, E.A.; Klaenhammer, T.R. The Genomics of Lactic Acid Bacteria. Trends Microbiol. 2007, 15, 546–553. [Google Scholar] [CrossRef]
- Bourdichon, F.; Casaregola, S.; Farrokh, C.; Frisvad, J.C.; Gerds, M.L.; Hammes, W.P.; Harnett, J.; Huys, G.; Laulund, S.; Ouwehand, A.; et al. Food Fermentations: Microorganisms with Technological Beneficial Use. Int. J. Food Microbiol. 2012, 154, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Leyva Salas, M.; Mounier, J.; Valence, F.; Coton, M.; Thierry, A.; Coton, E. Antifungal Microbial Agents for Food Biopreservation-A Review. Microorganisms 2017, 5, 37. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ); Allende, A.; Alvarez-Ordóñez, A.; Bortolaia, V.; Bover-Cid, S.; De Cesare, A.; Dohmen, W.; Guillier, L.; Jacxsens, L.; Nauta, M.; et al. Update of the List of Qualified Presumption of Safety (QPS) Recommended Microbiological Agents Intentionally Added to Food or Feed as Notified to EFSA 22: Suitability of Taxonomic Units Notified to EFSA until March 2025. EFSA J. 2025, 23, e9510. [Google Scholar] [CrossRef]
- Bernardeau, M.; Vernoux, J.P.; Henri-Dubernet, S.; Guéguen, M. Safety Assessment of Dairy Microorganisms: The Lactobacillus Genus. Int. J. Food Microbiol. 2008, 126, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Leyva Salas, M.; Mounier, J.; Maillard, M.-B.; Valence, F.; Coton, E.; Thierry, A. Identification and Quantification of Natural Compounds Produced by Antifungal Bioprotective Cultures in Dairy Products. Food Chem. 2019, 301, 125260. [Google Scholar] [CrossRef]
- Siedler, S.; Rau, M.H.; Bidstrup, S.; Vento, J.M.; Aunsbjerg, S.D.; Bosma, E.F.; McNair, L.M.; Beisel, C.L.; Neves, A.R. Competitive Exclusion Is a Major Bioprotective Mechanism of Lactobacilli against Fungal Spoilage in Fermented Milk Products. Appl. Environ. Microbiol. 2020, 86, e02312-19. [Google Scholar] [CrossRef]
- Archibald, F.S.; Fridovich, I. Manganese, Superoxide Dismutase, and Oxygen Tolerance in Some Lactic Acid Bacteria. J. Bacteriol. 1981, 146, 928–936. [Google Scholar] [CrossRef]
- Wildeman, A.S.; Culotta, V.C. Nutritional Immunity and Fungal Pathogens: A New Role for Manganese. Curr. Clin. Microbiol. Rep. 2024, 11, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Homepage|UBO Culture Collection. Available online: https://www.univ-brest.fr/ubocc/fr (accessed on 9 September 2025).
- CIRM Bacteria. Available online: https://collection-cirmbia.fr/ (accessed on 9 September 2025).
- Garnier, L.; Valence, F.; Pawtowski, A.; Auhustsinava-Galerne, L.; Frotté, N.; Baroncelli, R.; Deniel, F.; Coton, E.; Mounier, J. Diversity of Spoilage Fungi Associated with Various French Dairy Products. Int. J. Food Microbiol. 2017, 241, 191–197. [Google Scholar] [CrossRef]
- Delavenne, E.; Mounier, J.; Déniel, F.; Barbier, G.; Le Blay, G. Biodiversity of Antifungal Lactic Acid Bacteria Isolated from Raw Milk Samples from Cow, Ewe and Goat over One-Year Period. Int. J. Food Microbiol. 2012, 155, 185–190. [Google Scholar] [CrossRef]
- Garnier, L.; Salas, M.L.; Pinon, N.; Wiernasz, N.; Pawtowski, A.; Coton, E.; Mounier, J.; Valence, F. Technical Note: High-Throughput Method for Antifungal Activity Screening in a Cheese-Mimicking Model. J. Dairy Sci. 2018, 101, 4971–4976. [Google Scholar] [CrossRef]
- Leyva Salas, M.; Thierry, A.; Lemaître, M.; Garric, G.; Harel-Oger, M.; Chatel, M.; Lê, S.; Mounier, J.; Valence, F.; Coton, E. Antifungal Activity of Lactic Acid Bacteria Combinations in Dairy Mimicking Models and Their Potential as Bioprotective Cultures in Pilot Scale Applications. Front. Microbiol. 2018, 9, 1787. [Google Scholar] [CrossRef] [PubMed]
- Anses Table de Composition Nutritionnelle Des Aliments Ciqual. Available online: https://ciqual.anses.fr/ (accessed on 5 August 2025).
- Staggs, C.G.; Sealey, W.M.; McCabe, B.J.; Teague, A.M.; Mock, D.M. Determination of the Biotin Content of Select Foods Using Accurate and Sensitive HPLC/Avidin Binding. J. Food Compos. Anal. 2004, 17, 767–776. [Google Scholar] [CrossRef]
- Zekai, T.; Dağ, B. Mineral and Heavy Metal by Inductively Coupled Plasma Optical Emission Spectrometer in Traditional Turkish Yogurts. Int. J. Phys. Sci. 2013, 8, 963–966. [Google Scholar] [CrossRef]
- Wehmeier, S.; Morrison, E.; Plato, A.; Raab, A.; Feldmann, J.; Bedekovic, T.; Wilson, D.; Brand, A.C. Multi Trace Element Profiling in Pathogenic and Non-Pathogenic Fungi. Fungal Biol. 2020, 124, 516–524. [Google Scholar] [CrossRef]
- Wirth, N.; Funk, J.; Donati, S.; Nikel, P.I. QurvE: User-Friendly Software for the Analysis of Biological Growth and Fluorescence Data. Nat. Protoc. 2023, 18, 2401–2403. [Google Scholar] [CrossRef]
- Delavenne, E.; Ismail, R.; Pawtowski, A.; Mounier, J.; Barbier, G.; Le Blay, G. Assessment of Lactobacilli Strains as Yogurt Bioprotective Cultures. Food Control 2013, 30, 206–213. [Google Scholar] [CrossRef]
- Walker, G.M.; White, N.A. Introduction to Fungal Physiology. In Fungi: Biology and Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 1–35. ISBN 978-1-119-37431-2. [Google Scholar] [CrossRef]
- Hébert, E.M.; Raya, R.R.; Savoy De Giori, G. Evaluation of Minimal Nutritional Requirements of Lactic Acid Bacteria Used in Functional Foods. In Environmental Microbiology; Walker, J.M., Spencer, J.F.T., Ragout De Spencer, A.L., Eds.; Humana Press: Totowa, NJ, USA, 2004; pp. 139–148. ISBN 978-1-58829-116-5. [Google Scholar] [CrossRef]
- Shi, C.; Knøchel, S. Sensitivity of Molds from Spoiled Dairy Products Towards Bioprotective Lactic Acid Bacteria Cultures. Front. Microbiol. 2021, 12, 631730. [Google Scholar] [CrossRef]
- Shi, C.; Knøchel, S. Bioprotection Potential of Lacticaseibacillus rhamnosus LRH01 and Lactiplantibacillus plantarum LP01 against Spoilage-Associated Penicillium Strains in Yoghurt. Molecules 2023, 28, 7397. [Google Scholar] [CrossRef] [PubMed]
- Crittenden, R.G.; Martinez, N.R.; Playne, M.J. Synthesis and Utilisation of Folate by Yoghurt Starter Cultures and Probiotic Bacteria. Int. J. Food Microbiol. 2003, 80, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Kneifel, W.; Kaufmann, M.; Fleischer, A.; Ulberth, F. Screening of Commercially Available Mesophilic Dairy Starter Cultures: Biochemical, Sensory, and Microbiological Properties. J. Dairy Sci. 1992, 75, 3158–3166. [Google Scholar] [CrossRef]
- Schut, S.; Zauner, S.; Hampel, G.; König, H.; Claus, H. Biosorption of Copper by Wine-Relevant Lactobacilli. Int. J. Food Microbiol. 2011, 145, 126–131. [Google Scholar] [CrossRef]
- Mrvčić, J.; Stanzer, D.; Bacun-Druzina, V.; Stehlik-Tomas, V. Copper Binding by Lactic Acid Bacteria (LAB). Biosci. Microflora 2009, 28, 1–6. [Google Scholar] [CrossRef][Green Version]
- Fridovich, I. Superoxide Radical and Superoxide Dismutases. In Proceedings of the Oxygen and Living Processes; Gilbert, D.L., Ed.; Springer: New York, NY, USA, 1981; pp. 250–272. [Google Scholar] [CrossRef]
- Sturtz, L.A.; Diekert, K.; Jensen, L.T.; Lill, R.; Culotta, V.C. A Fraction of Yeast Cu,Zn-Superoxide Dismutase and Its Metallochaperone, CCS, Localize to the Intermembrane Space of Mitochondria. A Physiological Role for SOD1 in Guarding against Mitochondrial Oxidative Damage. J. Biol. Chem. 2001, 276, 38084–38089. [Google Scholar] [CrossRef]
- Fréalle, E.; Noël, C.; Viscogliosi, E.; Camus, D.; Dei-Cas, E.; Delhaes, L. Manganese Superoxide Dismutase in Pathogenic Fungi: An Issue with Pathophysiological and Phylogenetic Involvements. FEMS Immunol. Med. Microbiol. 2005, 45, 411–422. [Google Scholar] [CrossRef]
- Squizani, E.; Reuwsaat, J.; Motta, H.; Tavanti, A.; Kmetzsch, L. Calcium: A Central Player in Cryptococcus Biology. Fungal Biol. Rev. 2021, 36, 27–41. [Google Scholar] [CrossRef]
- Jackson, S.L.; Heath, I.B. Roles of Calcium Ions in Hyphal Tip Growth. Microbiol. Rev. 1993, 57, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Kisser, M.; Kubicek, C.P.; Röhr, M. Influence of Manganese on Morphology and Cell Wall Composition of Aspergillus niger during Citric Acid Fermentation. Arch. Microbiol. 1980, 128, 26–33. [Google Scholar] [CrossRef]
- Aunsbjerg, S.D.; Andersen, K.R.; Knøchel, S. Real-Time Monitoring of Fungal Inhibition and Morphological Changes. J. Microbiol. Methods 2015, 119, 196–202. [Google Scholar] [CrossRef]
- Piper, P.W. Yeast Superoxide Dismutase Mutants Reveal a Pro-Oxidant Action of Weak Organic Acid Food Preservatives. Free Radic. Biol. Med. 1999, 27, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Knøchel, S. Susceptibility of Dairy Associated Molds towards Microbial Metabolites with Focus on the Response to Diacetyl. Food Control 2021, 121, 107573. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boulet, C.; Coton, E.; Rouget, M.-L.; Valence, F.; Mounier, J. Competition-Exclusion for Manganese Is Involved in Antifungal Activity of Two Lactic Acid Bacteria Against Various Dairy Spoilage Fungi. Microorganisms 2025, 13, 2543. https://doi.org/10.3390/microorganisms13112543
Boulet C, Coton E, Rouget M-L, Valence F, Mounier J. Competition-Exclusion for Manganese Is Involved in Antifungal Activity of Two Lactic Acid Bacteria Against Various Dairy Spoilage Fungi. Microorganisms. 2025; 13(11):2543. https://doi.org/10.3390/microorganisms13112543
Chicago/Turabian StyleBoulet, Charlène, Emmanuel Coton, Marie-Laure Rouget, Florence Valence, and Jérôme Mounier. 2025. "Competition-Exclusion for Manganese Is Involved in Antifungal Activity of Two Lactic Acid Bacteria Against Various Dairy Spoilage Fungi" Microorganisms 13, no. 11: 2543. https://doi.org/10.3390/microorganisms13112543
APA StyleBoulet, C., Coton, E., Rouget, M.-L., Valence, F., & Mounier, J. (2025). Competition-Exclusion for Manganese Is Involved in Antifungal Activity of Two Lactic Acid Bacteria Against Various Dairy Spoilage Fungi. Microorganisms, 13(11), 2543. https://doi.org/10.3390/microorganisms13112543







