Abstract
This study explores the potential of novel Bifidobacterium isolates as targeted probiotic supplements for children with cystic fibrosis (CF), a condition often associated with gut dysbiosis. Five strains of Bifidobacterium genus (B. animalis IATA01, B. pseudocatenulatum IATA35, B. longum IATA02, B. bifidum IATA13, and B. longum IATA05) isolated from maternal–infant fecal samples were assessed in vitro following the FAO/WHO guidelines. Their probiotic potential was evaluated through simulated gastrointestinal digestion in the CF context, their adhesion to mucin, and their carbohydrate fermentation capacity. Additionally, their impact on colonic microbiota modulation was analyzed using static in vitro colonic fermentation with fecal inocula from four pediatric patients with CF to assess the presence of different bacterial groups associated with dysbiosis via qPCR and short-chain fatty acid production by GC-MS. Three strains (IATA01, IATA35, and IATA05) demonstrated survival after gastrointestinal digestion, with IATA01 exhibiting the highest adhesion to mucin but limited carbohydrate fermentation capacity. All strains increased the Bifidobacterium levels after colonic fermentation, while their effects on reducing pathogenic groups and promoting beneficial bacteria such as Akkermansia and Faecalibacterium varied depending on the strain and the individual inoculum. These findings highlight the strain-specific effects of Bifidobacterium and evidence a specific impact on colonic microbiota, depending on the composition of the basal inoculum, highlighting individual-specific responses.
Keywords:
probiotics; Bifidobacterium; gut microbiota; SCFAs; in vitro fermentation; cystic fibrosis 1. Introduction
Dysbiosis, defined as a reduction in microbial diversity along with an imbalance between beneficial and pathogenic bacteria, is a challenge associated with many diseases such as cystic fibrosis (CF) [,]. The consequences of dysbiosis encompass the generation of pro-inflammatory microbial metabolites in the colonic environment, which are behind the origin of several diseases or complications [,]. Several strategies have been proposed to counteract dysbiosis, including specific dietary patterns and nutritional counselling, as well as the use of the biotics family, including prebiotic and probiotic supplements. Among these, probiotics—live microorganisms that, when administered in adequate amounts, confer a health benefit on the host []—have attracted interest for their potential to restore gut microbial balance.
Concerning probiotics, bifidobacteria have been largely used as probiotics, given the fact that they have proven health benefits []. Bifidobacteria are gut commensal bacteria that dominate the intestinal microbiota during early infancy, especially in breast-fed infants; however, they remain consistently present across all age groups []. In this sense, some Bifidobacterium strains have a protective effect against diarrhea, as well as reducing side effects of antibiotic treatment []. Others showed improved inflammatory status in obese children with insulin resistance [], among many other reported health benefits. However, not all Bifidobacterium strains exert the same beneficial effects; therefore, the probiotic potential of specific strains should be tested prior to their application as probiotics [].
CF is characterized as dysbiosis in the gut microbiota as a consequence of combined factors, including lipid maldigestion (unabsorbed fats reaching the colon), an acidic intestinal environment due to reduced pancreatic bicarbonate secretion, and the continuous use of antibiotics to treat lung disease. Thus, children with CF exhibit specific alterations in their gut microbiota in terms of diversity, composition, and functionality. Lower alpha-diversity is reported in infants, as well as increased populations of potential pathogenic bacteria, such as members of Enterobacteriaceae, Streptococcus, and Enterococcus, among others []. In contrast, the relative abundance of various commensal and potentially beneficial bacteria, including Bifidobacterium, Akkermansia, and butyrate-producers such as Faecalibacterium members, was typically decreased [,]. The multifactorial complexity of the disease (pancreatic insufficiency, use of antibiotics, increased respiratory rate and energy expenditure) contributes to the deterioration of nutritional status in affected children []. Current strategies to improve it include probiotic supplementation; however, the limited number of studies that are currently available does not yet support the recommendation of specific strains or patterns for supplementation. Emerging evidence suggests that personalized approaches, tailored to the individual microbiota profiles of patients with CF may be more effective in guiding probiotic interventions []. Therefore, exploring the potential of Bifidobacterium strains as probiotics in the context of CF microbial modulation could be a valid approach to try to improve dysbiosis, but up to date, there is no evidence in CF.
This study aimed to identify novel Bifidobacterium strains with probiotic potential by assessing their functional capabilities relevant to modulating colonic microbiota in paediatric patients with CF.
2. Materials and Methods
2.1. Bifidobacterium Strains and Growth Conditions
The five Bifidobacterium strains used in this study belong to the bacterial collection from the group of Maria Carmen Collado at IATA-CSIC, Spain. The Bifidobacterium strains were isolated from five healthy volunteer mothers and their babies from the MAMI (MAternal MIcrobes) cohort. The MAMI cohort is a prospective mother–infant cohort in the Spanish Mediterranean area, as described elsewhere []. Given our exploratory objective and the pediatric setting, we selected five maternal–infant Bifidobacterium isolates representing phylogenetic and ecological diversity within the genus and known early-life colonisers. This design prioritised breadth of strain behaviours under CF-relevant constraints rather than pre-selection for specific glycan pathways.
All participants received oral and written information about this study and written consent was obtained. This study was approved by the hospital ethics committees (Hospital Clínico Universitario de Valencia) and registered on clinicaltrials.gov with registration number NCT03552939.
The bacteria were isolated as previously described []. For taxonomic identification, a fragment of approximately 898 nt of the 16S rRNA gene was amplified by PCR using the primers 27F (5′-GAGTTTGATCMGGCTCAG-3′) and 924R (5′-CTTGTGCGGGYYCCCGTCAA-3′) to identify the strains taxonomically. PCR mix was prepared with MgCl2 50 mM, DMSO, dNTPs 10 mM, primers 27F 10 µM and 924R 10 µM, Taq polymerase 5 U/µL, reaction buffer for NZY Taq II Pol (Nztech, Auckland, New Zealand), and molecular-grade water. The reaction parameters used were initial denaturation at 94 °C for 4 min and 30 cycles at 92 °C for 30 s, 65 °C for 30 s, 72 °C for 30 s, and 72 °C for 5 min. Amplicons were sent to Eurofins Genomics Europe Shared Services GmbH for Sanger sequencing.
In order to identify the bacterial isolates at the species level, 16S sequence data were analysed by nucleotide BLAST at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov, accessed on 14 May 2023), using the reference RNA sequences database (RefSeq/rRNA). The highest identity (≥99% of identity and ≥98% query cover) was selected to assign a particular species (of note, 16 rRNA supports species-level assignment only, with thresholds used; not subspecies).
Isolated Bifidobacterium strains (conserved in 20% glycerol at −80 °C) were grown on agar o liquid MRS (BD Difco™, Thermo Fisher Scientific, Alcobendas, Spain) media supplemented with 0.05% cysteine. Firstly, bifidobacterial strains were grown on solid media (MRS agar plates supplemented with 0.05% cysteine) and incubated for 48–72 h at 37 °C in anaerobic conditions using anaerobic jars with AnaeroGen sachet (Thermo Fisher Scientific, Alcobendas, Spain). Then, isolated colonies were grown on liquid medium MRS supplemented with 0.05% cysteine an incubated in the same conditions for 16–24 h.
2.2. Functionality Screening
2.2.1. Survival in In Vitro Simulated Gastrointestinal Conditions
The bacterial survival in the gastrointestinal conditions was assessed by subjecting the bifidobacterial strains to the in vitro digestion neonatal model described by Brodkorb et al. (2019) []. The bifidobacterial strains were grown in MRS supplemented with 0.05% cysteine for 16 h. Then, the culture was centrifugated and washed once in 1.8 mL PBS, and the bacterial pellet was resuspended in 1.8 mL PBS. Then, the OD595 was measured and 1 × 108 cfu/mL of bacterial suspension was inoculated in the gastric or intestinal solutions (1 mL, 1/1 v/v). This experiment was performed in duplicate. The gastrointestinal digestion conditions were the following: the simulated gastric fluid (SGF) was added in 1:1 (v/v) ratio to the bacterial suspension, pH was set at 3 with 1M HCl, and the temperature was set at 37 °C. The enzyme concentrations of pepsin and lipase from rabbit gastric fluid in the SGF were 2000 U/mL and 60 U/mL, respectively. After 120 min, the simulated intestinal fluid (SIF) was added to gastric digesta in a 1:1 (v/v) proportion, pH was established at 6.6, and temperature remained at 37 °C for 120 min. The SIF contained pancreatin (trypsin activity = 100 U/mL) and 1 mM bovine bile extract. Lower intestinal pH and bile salts concentration with respect to Brodkorb et al.’s (2019) [] protocol were simulated, so that the intestinal conditions were closer to those present in children with CF [,], as previously done in similar studies [,]. The oral phase was omitted in this model, as the study sample was not a solid food [].
For the survival analysis, aliquots of 1 mL were obtained at each time point (gastric initial, gastric final, intestinal initial, intestinal final) and diluted 100 times in 0.85% NaCl solution. The aliquots (200 µL) were stained with 2 µL Live/Dead BacLight bacterial viability kit (Molecular Probes, Eugene, OR, USA) and incubated for 15 min at room temperature in darkness following the manufacturer’s instructions. Live cells (unstained) and live and dead cells (isopropanol-treated), stained as before, were used to adjust the flow cytometer parameters for an optimal flow rate and events count. Bacterial quantification was carried out in a MACSQuant 16 (Miltenyi Biotec, Bergisch Gladbach, Germany). The data were analysed with FCS express version 5 flow cytometry software.
2.2.2. Carbohydrates Growth Capacity
The growth of Bifidobacterium strains on several carbohydrates, which were the sole carbohydrate source, using glucose as a positive control, were determined in medium minimum MRS (mMRS) prepared from first principles [] and supplemented with 1% (w/v) of each carbohydrate and 0.05% cysteine in 96-well plates (200 µL final volume). The carbohydrates tested were D-(+)-glucose (Sigma-Aldrich, Taufkirchen, Germany), D-(+)-lactose (AppliChem, Darmstadt, Germany), Inulin from chicory (Sigma-Aldrich, Germany), fructooligosaccharides (FOS) from chicory (Sigma-Aldrich, Germany), D-galactose (Boehringer, Germany), D(-)-fructose (Sigma-Aldrich, Germany), and D-(-)-ribose (Sigma-Aldrich, Germany). The carbohydrates were prepared at 10% (w/v) in water and sterilized by 0.2 µm filters. The inclusion of some human milk oligosaccharides was carried out because of recent evidence that they are potential prebiotics for older children and adults [,].
Inulin and FOS were prepared in mMRS at a final concentration of 1% (w/v) and autoclaved at 121 °C for 15 min. The human milk oligosaccharides including lacto-N-tetraose (LNT), lacto-N-neotetraose (LNnT), 2′-fucosyllactose (2FL), 2′-fucosyllactose/difucosyllactose mixture (2FL/DFL), 3′-sialyllactose (3SL), and 6′-sialyllactose (6SL) were kindly provided by DSM, Denmark. The human milk oligosaccharides were added after autoclaving mMRS and filtered using a 0.22 µm filter. For bacterial inoculum, the overnight cultures on MRS were centrifugated at 3385× g for 10 min at 4 °C. The pellets were washed with 1.8 mL of PBS once and centrifugated as before. The pellets were resuspended in 1.8 mL PBS and the OD595 was adjusted to 0.5 in PBS (final OD595 was 0.05). The plates were incubated at 37 °C for 24 h in anaerobic conditions and the final OD595 was measured in a Spectrostar Nano spectrophotometer (BMG Labtech, Ortenberg, Germany).
2.2.3. Adhesion to Mucin
The capability of adhesion to mucin of the bifidobacterial strains was evaluated following the previously described method [] with some modifications. Plates were covered with 500 µg/mL mucin (porcine stomach, Sigma-Aldrich, Germany) in 50 mM carbonate/bicarbonate and left overnight at 4 °C. Wells without mucin were included as the control. Plates were washed with PBS and blocked for 1 h with PBS plus 1% Tween 20. Then, 100 µL of each strain was added to each well in PBS adjusted to an OD595 nm of 1 (1 × 108 cfu/mL) and plates were incubated for 2 h at 37 °C. Non-adhered cells were removed by washing three times with PBS plus 0.05% Tween 20 and the plates were dried at 55 °C. Adhered cells were stained with crystal violet 1 mg/mL for 45 min. After six washes with PBS, the colorant was liberated with 10% acetic acid (100 µL/well) for 45 min and the absorbance at 595 nm was determined in a plate reader. Adherence to mucin was considered as the difference in absorbance at 595 nm between the wells with mucin and those without mucin.
2.3. Simulation of Colonic Fermentation of Infants with Cystic Fibrosis
A static in vitro colonic fermentation model was established to evaluate the potential of the probiotic strains in modifying the basal colonic microbiota and metabolic activity of 4 children with CF. The model consisted of incorporating a fecal inoculum into a glass vial containing a basal culture medium [], adding the bacterial strain, and incubating in anaerobiosis conditions at 37 °C for 24 h. Four different colonic environments were simulated by using the fecal inocula from 4 children with CF, which presented different individual characteristics that could have a different and person-specific impact on their microbiota, such as the use of antibiotics [], pulmonary infections, CFTR modulator therapy [], or age [] (Table 1).

Table 1.
Characteristics of the paediatric patients with cystic fibrosis who donated fecal samples to obtain the inoculum (I) to simulate colonic fermentation, at the moment of sample collection.
The fecal inocula were obtained by mixing 3 g of a fresh fecal sample with PBS 0.9% in a proportion of 1:10 (w/v) and centrifuging (5 min, 1504× g, 4 °C). Then, 0.1 mL of the supernatant was incorporated into a glass vial containing 1.2 mL of a basal culture medium that was previously described []. The resulting mixture was kept overnight in anaerobiosis conditions and at 37 °C to allow for microbiota stabilization in the new in vitro environment. After this time, 0.5 mL of PBS containing the bacterial strain (1 × 108 cells/mL) was added to start the simulation of colonic fermentation over a 24 h period (anaerobiosis, 37 °C). The process was reproduced by placing the glass vials into an anaerobiosis container incorporating anaerobiosis devices (AnaeroGen, Thermo Scientific, Alcobendas, Spain) and then into a thermostatic chamber at 37 °C.
After 24 h of simulated colonic fermentation, final aliquots of 0.75 mL were centrifuged (1504× g, 10 min, 4 °C). The pellet was used to extract DNA and quantify bacterial groups, and the supernatant was kept for SCFA determination.
The changes in the target bacterial groups were expressed as the difference between the number of copies obtained after simulated colonic fermentation and the number of copies identified in the faecal inoculum, both expressed as the logarithm.
The fecal inocula were obtained from 4 pediatric patients with CF in follow-up at the Pediatric Cystic Fibrosis Unit of La Fe University and Polytechnic Hospital, after receiving written informed consent by the parents and the children aged >12 years old. This study was approved by the ethics committee with registration number 2021-111-1, and conducted according to the Declaration of Helsinki.
2.4. Analysis of Colonic Microbiota
To characterize changes in colonic microbiota, different bacterial groups were determined by quantitative PCR (qPCR), following a previously described procedure [], including total bacteria, Bifidobacterium genus, Bacteroides genus, Faecalibacterium prausnitzii, Akkermansia muciniphila, enterobacteria group, and Streptococcus genus (primers indicated in Supplementary Table S1). These bacterial groups are considered key indicators of standard or altered colonic microbiota [].
First, DNA was extracted from the pellet fraction obtained after simulated colonic fermentation (approx. 100–200 mg) using the Master-Pure DNA extraction Kit (Epicentre, Madison, WI, USA), applying the manufacturer’s instructions with the following modifications: samples were treated with lysozyme (20 mg/mL), lyticase (2.5 U/mL), and mutanolysin (5 U/mL) for 60 min at 37 °C and then disrupted with 3 µm diameter glass beads, which was followed by 2 cycles of 30 sec at 6 m/s in a FastPrep 24-5G Homogenizer (MP Biomedicals) bead beater []. DNA concentration was measured using Qubit® 2.0 Fluorometer (Life Technology, Carlsbad, CA, USA).
Then, qPCR was conducted to quantify the bacterial groups previously indicated. The qPCR amplification and detection were performed in duplicate on a LightCycler 480 Real-Time PCR System (Roche Technologies, Mannheim, Germany). Each reaction mixture of 10 µL was composed of SYBR Green PCR Master Mix (Roche), 0.5 µL of each primer (concentration 10 mmol/L), and 1 µL of DNA template (Supplementary Table S1). The bacterial concentration in each sample was calculated by comparing the Ct values obtained from standard curves. To create a standard ladder for 16S rRNA gene copy number extrapolation for each bacterial group, genomic DNA were amplified, purified, and quantified as previously described []. Standard curves were obtained by serial 10-fold dilutions of purified standard DNA fragments, corresponding to 16S rRNA gene regions described above, covering the range 10–109 gene copies/mL, which were run in parallel and used to extrapolate the number of 16S rRNA gene copies in the microbiota samples. Each dilution was analysed in triplicate, and Ct values were plotted against the log of the known copy number. The curves showed high linearity (R2 > 0.99), ensuring a robust quantification of the bacterial groups in the microbiota samples.
Data were expressed as total number of copies per mL of sample at baseline and after 24 h of colonic fermentation, and also as the logarithm. Then, changes after 24 h of colonic fermentation with respect to baseline values in each inoculum were expressed as the difference in the logarithm of the number of copies.
2.5. Determination of Short-Chain Fatty Acids (SCFA)
After centrifugation of colonic digesta tubes, the supernatant was used to determine the production of SCFA by gas chromatography-mass spectrometry (GC-MS), following the protocol by Eberhart and collaborators with some modifications []. Briefly, 200 μL aliquots were mixed with 800 μL of the internal standard extraction solution (3-methylvaleric acid) and vortexed. Then, 1 mL of diethyl ether was incorporated along with a spoon tip of Na2SO4 to remove any traces of water in the extract before injection, allowing for 10 min of reaction, and then centrifuged 2000× g for 5 min. The supernatant was, at this point, injected in the Agilent GC7890B-5977B GC-MS with a multipurpose sampler (Gerstel MPS) with an Agilent DB-FATWAX column (30 m × 0.25 mm × 0.25 μm) operated in split mode (20:1). The oven temperature programme was 100 °C for 3 min, with the temperature being ramped to 100 °C at a rate of 5 °C min−1, to 150 °C for 1 min, to 200 °C at a rate of 20 °C min−1, and then finally being held at 200 °C for 5 min. Helium was used as carrier gas at a flow rate of 1 mL min−1; inlet temperature of 250 °C; injection volume: 2 μL. For the quantification of the SCFAs, 3-methylvaleric acid was used as an internal standard at a concentration of 5 mM and standard curves for acetate, propionate, isobutyrate, and butyrate were prepared. Agilent MassHunter Workstation Software version B.09.00 was used for quantitative analysis.
2.6. Statistical Analysis
All the data were summarized by mean and standard deviation. Two-way ANOVA analyses were applied to assess differences between bacterial strains in the different study outcome variables: survival of gastrointestinal digestion (compared to baseline values), carbohydrate fermentation capacity (compared to MRS as control), adhesion to mucin (compared to PBS as control), changes in colonic microbiota (compared to baseline microbiota), and production of SCFA (compared to baseline values). The GraphPad Prism software (version 9.5.1) was used to conduct the statistical analyses.
3. Results
3.1. Identification of Selected Bifidobacterium Isolates
Several Bifidobacterium strains were isolated from mother–infant feces and identified at the species level. Among these, five representatives were selected for further characterization and designated as B. animalis IATA01, B. pseudocatenulatum IATA35, B. longum IATA02, B. bifidum IATA13, and B. longum IATA05 (Table 2). These strains were chosen to represent phylogenetic and potential functional diversity withing the genus. Most of the strains were obtained from maternal faeces, given the fact that the mother is the main source of Bifidobacterium for infant gut microbiota []. While identification was performed at the species level, two strains of B. longum species showed a sequence identity with different subspecies and both were included, although this methodology does not allow sub-species resolution. One B. longum strain from infant faeces (B. longum IATA02) was selected for potential different performance with a B. longum strain from maternal origin (B. longum IATA05).

Table 2.
Taxonomic identification of novel Bifidobacterium strains obtained from the maternal–infant environment.
3.2. Functional Capacities of the Strains
3.2.1. Survival to Gastrointestinal Digestion
The survival of gastrointestinal conditions is an important factor for probiotic characterization. The exposure to gastric and small intestine digestion conditions led to variable effects on bacterial strains survival. According to Figure 1, B. longum IATA05, B. animalis IATA01, and B. pseudocatenulatum IATA35 were able to survive both stages of digestion, at least close to 90%, with this extent being significantly higher than that of the other two strains. In particular, B. animalis IATA01 remained unaltered through the process, but the 2 h duration of small intestine digestion led to a 10% decrease in its survival, while, in the other two strains, the decrease was more evenly distributed. The other two strains, B. bifidum IATA13 and B. longum IATA02, showed different tendencies, with their final extents of survival being below 50%. Focusing on B. longum IATA02, a 5% decrease in viability was observed after 120 min of the gastric stage, and the incorporation of the intestinal fluids (intestinal stage t = 0 min) caused a sharp decrease in survival, which was below 10%. In the case of B. bifidum IATA13, the incorporation of the gastric fluid (gastric stage t = 0 min) was enough to reduce survival to 90%, but this value was maintained until the beginning of the intestinal stage. However, at the end of that stage, survival was reduced to 50%. The strain that showed the worst survival of gastrointestinal digestion was B. longum IATA02, since less than 10% survived in the intestinal conditions.
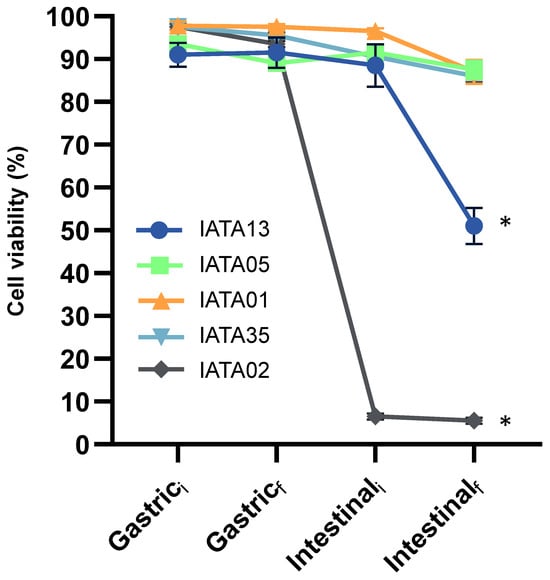
Figure 1.
Percentage of live bacteria along the simulated gastric and intestinal stages of digestion, at time 0 min (i) and time 120 min (f), as measured by MACSQuant 16 and analysed by FCS express v5. Values represent the mean percentage and standard deviations for live populations of two independent replicates. * Statistically significant difference compared to Gastrici. Strain codes: IATA01, Bifidobacterium animalis; IATA35, Bifidobacterium pseudocatenulatum; IATA02, Bifidobacterium longum; IATA13, Bifidobacterium bifidium; IATA05, Bifidobacterium longum.
3.2.2. Growth Capacity on Carbohydrates
The colonization capabilities of probiotic strains are influenced by the utilization of certain carbohydrates from the diet or the host. The bifidobacterial strains were tested for their capability to grow on 13 different carbohydrates, including six human milk oligosaccharides (HMOs) (Figure 2). The strains showed differences in carbohydrate utilization. The strain B. pseudocatenulatum IATA35 grew on most of the tested carbohydrates, efficiently utilizing glucose, lactose, LNT, 2′FL, galactose, fructose, and ribose. In contrast, the strain B. animalis IATA01 only grew efficiently on glucose, lactose, and ribose. The strain B. bifidum IATA13 efficiently utilized all the HMOs tested (LNT, LnNT, 2′FL, 3′SL, and 6′SL) for growing, while the B. longum strains B. longum IATA02 and B. longum IATA05 and B. animalis IATA 01 did not grow on most of the carbohydrates, and none of the strains could grow on inulin or FOS.
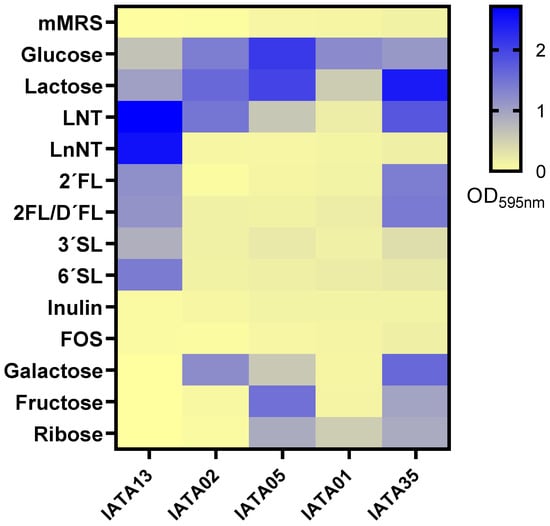
Figure 2.
Carbohydrate utilization of the tested strains of Bifidobacterium on mMRS medium supplemented with 1% (w/v) of different carbohydrates: lacto-N-tetraose (LNT), lacto-N-neotetraose (LnNT), 2′fucosyllactose (2′FL), mix 2′fucosyllactose and difucosyllactose (2′FL/D′FL), 3′-sialyllactose (3′SL), and 6′-sialyllactose (6′SL). The culture of the strain on mMRS was used as a negative control. The figure represents the logarithm of final OD595 after 24 h of incubation at 37 °C in anaerobic conditions. Strain codes: IATA01, Bifidobacterium animalis; IATA35, Bifidobacterium pseudocatenulatum; IATA02, Bifidobacterium longum; IATA13, Bifidobacterium bifidium; IATA05, Bifidobacterium longum.
3.2.3. Mucin Adhesion Capacity
Another key factor in selecting the probiotic functionality of bacteria is their ability to adhere to mucin. The strains B. bifidum IATA13 (OD595 = 0.7) and IATA01 (OD595 = 1.63) were able to efficiently adhere to mucin compared to a PBS control (p < 0.05) (Figure 3). In fact, the strain B. animalis IATA01 showed a high capacity of adhesion. The rest of the strains remained within the response obtained by the PBS control.
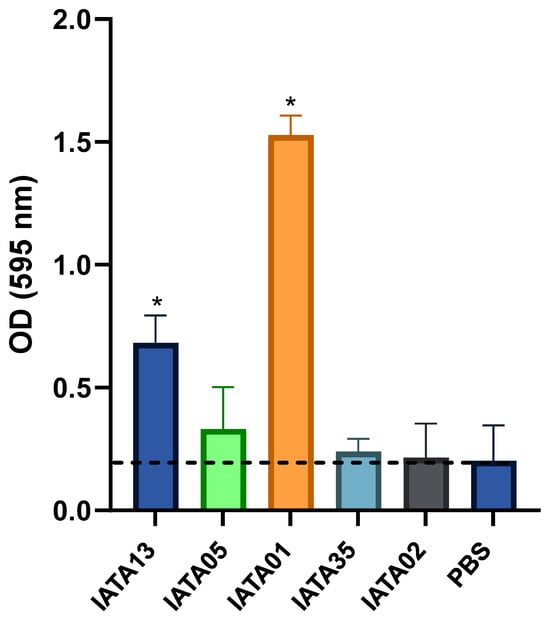
Figure 3.
Adhesion of Bifidobacterium strains to mucin. The strains were incubated with fixed mucin in multi-well plates. PBS without bacteria was used as a control. Data represent means plus standard deviations of three replicates. * Statistically significant differences (p < 0.05) with respect to the control (PBS). The black dashed line represents the mean adhesion value of the negative control (PBS). Strain codes: IATA01, Bifidobacterium animalis; IATA35, Bifidobacterium pseudocatenulatum; IATA02, Bifidobacterium longum; IATA13, Bifidobacterium bifidium; IATA05, Bifidobacterium longum.
3.3. In Vitro Colonic CF Microbiota Modulation
Different effects of the Bifidobacterium strains were accounted depending on the type of fecal inoculum (Figure 4), as compared to the microbiota composition in the original inoculum from each subject (Supplementary Table S2). A common trend, though, was that most of the strains were able to induce a significant increase in the Bifidobacterium genus. This increase was especially relevant in inoculum 3 (I3), as the baseline values for this genus were relatively higher than in the other inocula. Another common observation was that all the strains were successful in reducing the populations of Enterobacteriaceae and Streptococcus in I1 and I3, while, in I2 and I4, the change in these groups was not relevant. Apart from Bifidobacterium, in I1, Bacteroides showed a significant increase in some of the cases. Focusing on I3, a decrease in beneficial groups, e.g., Faecalibacterium prausnitzii and Akkermansia muciniphila, was depicted for most of strains, although it was not detected as statistically significant. In contrast, these species, along with Bacteroides, were increased by most of the strains in I1 and I2, and only by B. animalis IATA01 in I4. Focusing on the exceptions, it is noticeable that B. longum IATA02 and B. bifidum IATA13 were the only strains that induced the growth of Bacteroides and Streptococcus, but this was only noted in I2. Another uncommon result was the growth of the Bacteroides group, which was relatively higher in I2 as induced by the strain B. longum IATA05, as it was the only bacterial group that showed a positive change in I3, apart from Bifidobacterium genus, which is related to B. longum IATA02 and B. pseudocatenulatum IATA35. Of note, B. animalis IATA 01 was the only strain that induced a significant increase in Akkermansia muciniphila, in the context of I2 and I4.
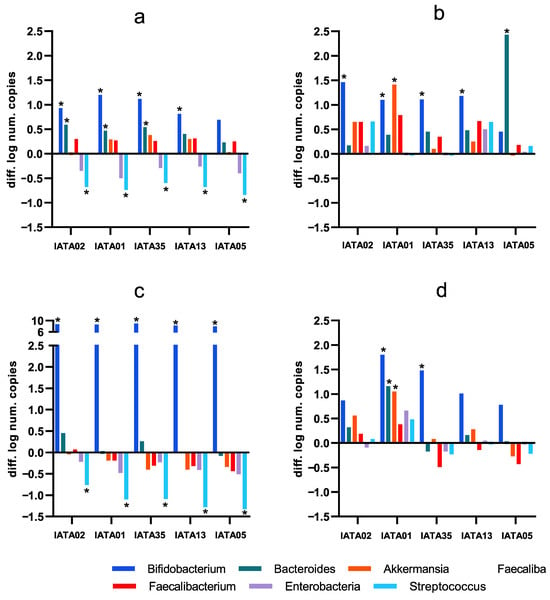
Figure 4.
Changes in colonic microbiota as compared to the baseline fecal inocula composition induced by the simulation of colonic fermentation with the study strains (difference in the number of copies expressed as logarithm: after simulated colonic fermentation minus baseline faecal inoculum values, as determined by qPCR). (a) inoculum 1 (I1); (b) inoculum 2 (I2); (c) inoculum 3 (I3); (d) inoculum 4 (i4). I1–I4 denote the four individual patient inocula. * Statistically significant differences (p < 0.05) compared to baseline values. Strain codes: IATA01, Bifidobacterium animalis; IATA35, Bifidobacterium pseudocatenulatum; IATA02, Bifidobacterium longum; IATA13, Bifidobacterium bifidium; IATA05, Bifidobacterium longum.
3.4. Colonic Metabolic Activity
An analysis of SCFAs was performed as an indicator of modulation of metabolic activity of Bifidobacterium strains on gut microbiota of paediatric patients. An increase in the total SCFA produced by gut microbiota was observed in all inoculum environments with all the Bifidobacterium strains, as compared to baseline (gut microbiota samples without addition of Bifidobacterium strains) (Figure 5). In general, inocula I3 and I4 experienced the highest increase in SCFA production compared to their baseline levels. The most abundant SCFA was acetic acid, followed by propionic acid in all the inocula and for all the strains. However, the increase was only significant for the B. longum strain IATA05 in I1 (p = 0.0349) and I3 (p = 0.001); and in I2 (p = 0.022) and I3 (p = 0.004) for B. longum IATA02. In addition, focusing on I4, which had the overall lowest total SCFA among the four inocula, the most significant increase was achieved by B. longum IATA05 (p = 0.0012), B. pseudocatenulatum IATA35 (p = 0.0262) and B. animalis IATA01 (p = 0.0258).
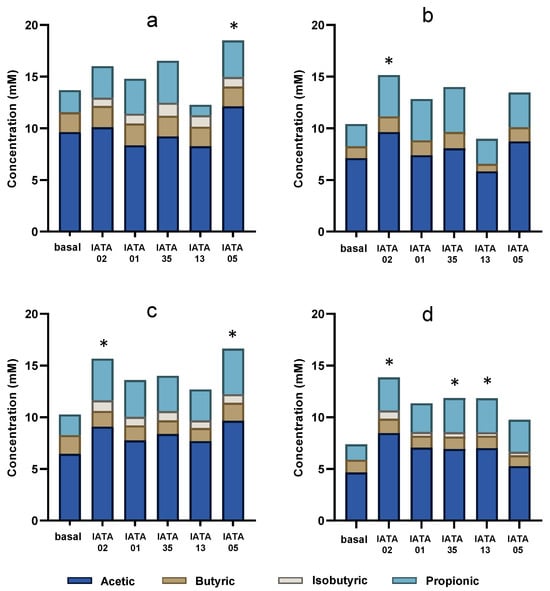
Figure 5.
SCFAs produced after the administration of bifidobacterial strains during colonic fermentation in four faecal inocula. (a) inoculum 1 (I1); (b) inoculum 2 (I2); (c) inoculum 3 (I3); (d) inoculum 4 (i4). * Statistically significant differences (p < 0.05) compared to baseline values. Strain codes: IATA01, Bifidobacterium animalis; IATA35, Bifidobacterium pseudocatenulatum; IATA02, Bifidobacterium longum; IATA13, Bifidobacterium bifidium; IATA05, Bifidobacterium longum.
When assessing the specific detected SCFAs (propionic, isobutyric, butanoic, and acetic acids), a common observation was the absence (or low concentration < 0.005 mM) of isobutyric acid in all the basal inocula, but its presence was detected in I1, I3, and I4, in variable proportions depending on the Bifidobacterium strain. Overall, the strains differentially modulated SCFA production, with the magnitude and profile of changes depending on the specific microbiota inoculum. Specifically, all the strains increased the level of isobutyric acid in all the inocula except I3 and B. longum IATA02 increased the acetic and propionic acids in I2 and the propionic, butyric, and isobutyric acids in I4.
4. Discussion
In the present study, different functional and probiotic capacities of novel isolated representative Bifidobacterium strains were assessed in the context of CF dysbiotic microbiota. Although several Bifidobacterium strains are used as probiotics [], the five isolates from the mother–infant gut microbiota could offer an advantage in colonising the pediatric gut, as vertically transmitted bacteria are more stable colonisers and ecologically better adapted compared with non-maternal strains [,]. Some assessed parameters were related to strain survival in gastrointestinal tract conditions, capabilities of adhesion to the mucus layer (mucin adherence), and functional aspects such as diet carbohydrate utilisation, while others focused on the impact on colonic microbiota. Notably, this is the first study assessing the in vitro effect of Bifidobacterium strains on the colonic microbiota of children with CF. Furthermore, the characterization was designed to reflect key ecological and metabolic challenges regarding the distal colon in patients with CF, such as specific simulated gastrointestinal conditions and limited nutrient availability [,].
The results showed that the strains B. pseudocatenulatum IATA35, B. animalis IATA01, and B. longum IATA02 survived gastrointestinal digestion, but only B. animalis IATA01 adhered to mucin. Despite its lower carbohydrate fermentation capacity, B. animalis IATA01 induced the most beneficial changes in colonic microbiota. Of note, none of the tested strains utilised inulin or FOS in our assay, and we did not genotype β-fructofuranosidase/fructan loci. Inulin is a well-established prebiotic; thus, the absence of fructan utilisation is a limitation of the present strain set, especially considering that other isolates of Bifidobacterium species showed this capacity []. Nevertheless, probiotic effectiveness can arise via complementary mechanisms—mucin adhesion and niche occupation, antagonism of potential pathogens, and cross-feeding that enhances community-level SCFA production. Moreover, in pediatric CF, alternative glycans such as lactose derivatives, HMOs, and GOS may better match both the diet and the glycan capabilities of early-life bifidobacteria. Future work should include whole-genome sequencing to resolve CAZyme repertoires, targeted selection of fructan-utilising strains, and synbiotic designs pairing strains with matched prebiotics.
The study design follows some well-established methods for screening probiotic potential, as indicated by the FAO/WHO guidelines []. Evaluations such as survival in gastrointestinal conditions and mucin adherence are commonly conducted [,,], while others assess antibiotic resistance [] or pathogen inhibition capacity []. However, this study included some particularities for probiotic research. First, the use of a fluorescence staining technique (BacLight Live/Dead kit) to evaluate bacterial survival under simulated gastrointestinal conditions in the context of pediatric cystic fibrosis. This approach enabled the detection of both viable cells and viable but non-culturable (VBNC) cells. Some probiotic strains can exhibit a VBNC state under certain stress conditions; although they are unable to grow on standard culture media—a major limitation for assessing their proliferation—they can still retain key probiotic properties relevant to host interactions [,]. Then, the adhesion to mucin is a relevant test in the context of cystic fibrosis. Alterations in mucus composition and viscosity caused by defective bicarbonate transport in intestinal cells impact the intestinal environment []. Mucins, the structural glycoproteins of mucus, become abnormally concentrated and less hydrated, which leads to a thicker barrier that impairs clearance and facilitates pathogen colonization []. These changes not only contribute to gut dysbiosis and inflammation but also modify the interface where beneficial microbes interact with the host. Thus, a higher ability of adhesion to mucin would increase the colonization capacity of beneficial bacteria more effectively in this altered environment. Furthermore, few studies have examined a probiotic strain’s potential to modify colonic microbiota and metabolite production. Additionally, we assessed this effect in four different individuals within the framework of CF-induced dysbiosis. A comparable study analysed the impact of bacterial strains on colonic microbiota from feecal inocula of obese and normal-weight subjects, finding that probiotic addition did not induce major compositional changes apart from increases in the administered species []. In contrast, our study revealed significant reductions in potential pathogenic bacterial groups and increases in beneficial genera. Notably, the previous study did not assess genera beyond Bifidobacterium and Lactobacillus [].
The specific impact on colonic microbiota varied by inoculum, highlighting individual-specific responses. In I1 and I3, all five Bifidobacterium strains reduced potentially pathogenic groups (enterobacteria and Streptococcus). However, no shared characteristics between I1 and I3 were identified apart from them being the oldest participants. Additionally, these two individuals exhibited the highest overall SCFA production. In I1, I2, and I4, at least one Bifidobacterium strain promoted the growth of bacterial groups beyond Bifidobacterium, whereas, in I3, only Bifidobacterium growth was observed, with the highest detected abundance among all cases. In I2, only positive or neutral growth effects were detected, and this inoculum exhibited the highest growth of Bacteroides and Faecalibacterium. Notably, I2 was the only individual without pancreatic insufficiency, a common CF condition that alters gastrointestinal parameters such as pH, nutrient absorption, and motility [,,,]. In I1, all strains enhanced beneficial bacterial groups while reducing potential pathogens. This participant was the only one undergoing CFTR modulator therapy, which can reverse the genetic defect underlying CF, improving gastrointestinal complications and potentially colonic microbiota dysbiosis []. These findings suggest that individual characteristics influence colonic microbiota modulation by probiotics, and that specific strains may be more effective depending on the basal microbiota profile.
Among the study strains, B. animalis IATA01 emerged as the most promising candidate for microbiota improvement and future probiotic applications. In I1, its effects were comparable to those of other strains; in I2, it induced the highest Akkermansia and Faecalibacterium growth; in I3, its impact was similar to that of the other strains; and in I4, it promoted the highest growth of Bacteroides, Bifidobacterium, Akkermansia, and Faecalibacterium.
B. animalis IATA01 exhibited high mucin adhesion, a key characteristic for probiotic bacteria that enhances persistence in the gastrointestinal tract, triggers host responses, and prevents pathogen colonisation by competing for binding sites [,,]. A previous study identified a B. breve strain with the highest digestion survival and mucin adherence, but these properties were not assessed in B. animalis []. Harata et al. (2021) similarly found that B. animalis strains exhibited the highest mucin adherence []. Most probiotic screening studies focus on B. infantis, B. breve, B. adolescentis, or B. longum, which limits direct comparisons with B. animalis []. Likewise, B. pseudocatenulatum, which also demonstrated promising results, has been scarcely studied in this context. Mucin adhesion in Bifidobacterium is strain-specific, with higher adherence observed in B. longum [,] and B. pseudolongum subsp. pseudolongum []. Various adhesins contribute to this trait, including DnaK in B. animalis subsp. lactis Bi-0744 and exopolysaccharides in B. animalis IPLA-R145. B. animalis IATA01′s strong mucin-binding ability may be particularly relevant in CF, where antibiotic use increases the risk of pathogenic colonisation [,]. Additionally, probiotic adhesion facilitates host interactions. B. animalis IATA01 metabolised glucose, lactose, and ribose, which suggests potential for cross-feeding with other colonic bacteria, enhancing SCFA production [,].
Regarding SCFAs, this study demonstrated that the selected Bifidobacterium strains modulated the production of SCFAs, particularly acetate, propionate, and isobutyric acids, while changes in butyric acid were minor during colonic fermentation. Although acetic acid is the main SCFA produced by Bifidobacterium strains, these results highlight the modulatory effect of these strains on composition and functionality, suggesting cross-feeding interactions that enhance SCFA output. These metabolites are central in host interactions since they have been associated with improved mucosal integrity, reduced inflammation, and immune and metabolic modulations []. These findings are relevant in the CF context, as it has been described that children without CF had higher levels of acetate, butyrate, and propionate compared to a similar cohort of children with CF [,].
This exploratory framework (strain functional characterization including in vitro modulation of gut microbiota), applied to a larger cohort, could serve as a clinical tool for identifying suitable probiotics based on individual characteristics. Despite its relevance, this study has several limitations. The colonic fermentation medium contained only basal nutrients, which may have constrained bacterial growth compared to a medium supplemented with specific substrates. Other limitations include the small number and heterogenicity of faecal inocula, the static in vitro fermentation model, and the use of qPCR rather than 16S sequencing for microbiota analysis. Nonetheless, qPCR was deemed appropriate given this study’s screening purpose, allowing reliable and fast quantification of relevant bacterial groups. Another limitation is that qPCR does not distinguish between culturable, viable but nonculturable (VBNC), or dead cells. Of note, the VBCN state is characterized by the loss of culturability of microbial cells on/in nutrient media that normally support their growth, while maintaining metabolic activity []. Moreover, the ability of probiotics to impact VBNC pathogens may still be ecologically relevant, since VBNC cells can resuscitate under favourable conditions and contribute to infection, and future studies using complementary approaches such as viability-qPCR or biocide efficiency that apply culture-based methods could further resolve this issue []. Whole-genome sequencing and further analyses are necessary for deeper strain identification, safety evaluation, and elucidation of probiotic–host interaction mechanisms.
Conversely, this study presents notable strengths. Bacterial viability and gastrointestinal survival assessments were conducted stepwise using an appropriate in vitro digestion model (enzyme combination, duration, temperature, agitation, pH values), in contrast to previous studies that evaluated only gastric pH and bile salt concentrations []. Additionally, this screening specifically examined microbial modulation in children with CF, supporting probiotic-based treatments tailored to paediatric patients with CF. Another key contribution is the assessment of Bifidobacterium strains derived from the maternal–infant environment, a crucial source for this genus, with potential applications in probiotic development. Finally, by focusing on CF, a condition characterised by gastrointestinal dysfunction and altered microbiota with significant reduction in the Bifidobacterium genus, this study highlights opportunities for probiotic interventions to support gastrointestinal health. Thus, this study analysed the carbohydrate capability of Bifidobacterium strains to assess their bifidogenic properties and to allow their establishment and functionality in the cystic fibrosis environment, supporting their use in targeted synbiotic interventions.
In conclusion, this study suggests the strain-dependent activity of Bifidobacterium and evidence that the potential probiotic effects vary according to the initial microbial community, emphasizing the importance of individual-specific responses.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13112523/s1, Table S1. Primers used in this study; Table S2. Bacterial groups in the original inoculum samples of each patient (I1–I4) expressed as number of copies/mL.
Author Contributions
Conceptualization: M.E.-T., A.A.-G. and J.C.-L.; Methodology: M.E.-T., A.A.-G., N.R. and M.B.; Investigation: M.E.-T., I.B., M.B. and J.C.-L.; Data curation: M.E.-T. and J.C.-L.; Formal analysis: M.E.-T. and J.C.-L.; Writing original draft: M.E.-T. and J.C.-L.; Writing—review & editing: all the authors. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Generalitat Valenciana with the Emerging Research Groups project (CIGE/2022/122) awarded to J. Calvo-Lerma, A. Asensio-Grau and D. Hervás.
Institutional Review Board Statement
This study was approved by the Ethics committees of University Hospital La Fe (Valencia, Spain) (Ref. 2021-111-1; Approval date: 20 December 2021) and Universitat Politècnica de València (Valencia, Spain) (Ref. P09_24_11_2021; Approval date 26 November 2021), and performed in accordance with de Declaration of Helinski. Inform consent was obtained from the participants older than 12 years’ old and from the individuals’ guardians.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
Authors thank the families of the children with CF who participated in the study, and the healthcare professionals working in the participating CF units. M. Bernabeu would like to acknowledge the award of the Spanish government MCIN/AEI to the IATA-CSIC as Center of Excellence Accreditation Severo Ochoa (CEX2021-001189-S/MCIN/AEI/10.13039/501100011033). Maria Esteban-Torres thanks the support received from the European Commission MSCA-IF postdoctoral grant (MicroMi-898088) and Ramón y Cajal grant (RYC 2023-045839-I) funded by MCIU/AEI/10.13039/501100011033 and the European Social Fund Plus (FSE+). A. Asensio-Grau acknowledges the post-doctoral “Juan de la Cierva” grant (ref. JDC2022-048768-I) MCIU/AEI and European Union “Next Generation EU”/PRTR grant. M. Bernabeu is grateful to have received a post-doctoral “Juan de la Cierva” grant (ref. JDC2022- 050269-I) MCIU/AEI and European Union “Next Generation EU”/PRTR grant.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Caley, L.R.; White, H.; de Goffau, M.C.; Floto, R.A.; Parkhill, J.; Marsland, B.; Peckham, D.G. Cystic fibrosis-related gut dysbiosis: A systematic review. Dig. Dis. Sci. 2023, 68, 1797–1814. [Google Scholar] [CrossRef]
- Zeng, M.E.; Inohara, M.Y.; Nuñex, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Sharma, M.; Wasan, A.; Sharma, R.K. Recent developments in probiotics: An emphasis on Bifidobacterium. Food Biosci. 2021, 41, 100993. [Google Scholar] [CrossRef]
- Saturio, S.; Nogacka, A.M.; Suárez, M.; Fernández, N.; Mantecón, L.; Mancabelli, L.; Gueimonde, M. Early-life development of the bifidobacterial community in the infant gut. Int. J. Mol. Sci. 2021, 22, 3382. [Google Scholar] [CrossRef]
- Jungersen, M.; Wind, A.; Johansen, E.; Christensen, J.E.; Stuer-Lauridsen, B.; Eskesen, D. The science behind the probiotic strain Bifidobacterium animalis subsp. lactis BB-12®. Microorganisms 2014, 2, 92–110. [Google Scholar] [CrossRef]
- Sanchis-Chordà, J.; Del Pulgar, E.M.G.; Carrasco-Luna, J.; Benítez-Páez, A.; Sanz, Y.; Codoñer-Franch, P. Bifidobacterium pseudocatenulatum CECT 7765 supplementation improves inflammatory status in insulin-resistant obese children. Eur. J. Nutr. 2019, 58, 2789–2800. [Google Scholar] [CrossRef] [PubMed]
- Thavamani, A.; Salem, I.; Sferra, T.J.; Sankararaman, S. Impact of altered gut microbiota and its metabolites in cystic fibrosis. Metabolites 2021, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, M.; Nathan, N.; Sarouk, I.; Aluma, B.B.; Dagan, A.; Bezalel, Y.; Efrati, O. Nutritional status in childhood as a prognostic factor in patients with cystic fibrosis. Lung 2019, 197, 371–376. [Google Scholar] [CrossRef] [PubMed]
- van Dorst, J.M.; Tam, R.Y.; Ooi, C.Y. What do we know about the microbiome in cystic fibrosis? Is there a role for probiotics and prebiotics? Nutrients 2022, 14, 480. [Google Scholar] [CrossRef]
- García-Mantrana, I.; Alcántara, C.; Selma-Royo, M.; Boix-Amorós, A.; Dzidic, M.; Gimeno-Alcañiz, J.; Úbeda-Sansano, I.; Sorribes-Monrabal, I.; Escuriet, R.; Collado, M.C. MAMI: A birth cohort focused on maternal-infant microbiota during early life. BMC Pediatr. 2019, 19, 140. [Google Scholar] [CrossRef] [PubMed]
- Samarra, A.; Renwick, S.; Arzamasov, A.A.; Rodionov, D.A.; Spann, K.; Cabrera-Rubio, R.; Collado, M. Human milk oligosaccharide metabolism and antibiotic resistance in early gut colonizers: Insights from bifidobacteria and lactobacilli in the maternal-infant microbiome. Gut Microbes 2025, 17, 2501192. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Balance, S.; Recio, I. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Gelfond, D.; Ma, C.; Semler, J.; Borowitz, D. Intestinal pH and gastrointestinal transit profiles in cystic fibrosis patients measured by wireless motility capsule. Dig. Dis. Sci. 2013, 58, 2275–2281. [Google Scholar] [CrossRef]
- Humbert, L.; Rainteau, D.; Tuvignon, N.; Wolf, C.; Seksik, P.; Laugier, R.; Carrière, F. Postprandial bile acid levels in intestine and plasma reveal altered biliary circulation in chronic pancreatitis patients. J. Lipid Res. 2018, 59, 2202–2213. [Google Scholar] [CrossRef]
- Asensio-Grau, A.; Calvo-Lerma, J.; Heredia, A.; Andrés, A. In vitro digestion of salmon: Influence of processing and intestinal conditions on macronutrients digestibility. Food Chem. 2021, 342, 128387. [Google Scholar] [CrossRef]
- Calvo-Lerma, J.; Fornés-Ferrer, V.; Heredia, A.; Andrés, A. In vitro digestion models to assess lipolysis: The impact of the simulated conditions of gastric and intestinal pH, bile salts and digestive fluids. Food Res. Int. 2019, 125, 108511. [Google Scholar] [CrossRef]
- Arboleya, S.; Bottacini, F.; O’Connell-Motherway, M.; Ryan, C.A.; Ross, P.; van Sinderen, D.; Stanton, C. Gene-trait matching across the Bifidobacterium longum pan-genome reveals considerable diversity in carbohydrate catabolism among human infant strains. BMC Genom. 2018, 19, 33. [Google Scholar] [CrossRef]
- De Bruyn, F.; James, K.; Cottenet, G.; Dominick, M.; Katja, J. Combining Bifidobacterium longum subsp. infantis and human milk oligosaccharides synergistically increases short chain fatty acid production ex vivo. Commun. Biol. 2024, 7, 943. [Google Scholar] [CrossRef]
- Jacobs, J.P.; Lee, M.L.; Rechtman, D.J.; Sun, A.K.; Autran, C.; Niklas, V. Human milk oligosaccharides modulate the intestinal microbiome of healthy adults. Sci. Rep. 2023, 13, 14308. [Google Scholar] [CrossRef]
- Muñoz-Provencio, D.; Llopis, M.; Antolín, M.; de Torres, I.; Guarner, F.; Pérez-Martínez, G.; Monedero, V. Adhesion properties of Lactobacillus casei strains to resected intestinal fragments and components of the extracellular matrix. Arch. Microbiol. 2009, 191, 153–161. [Google Scholar] [CrossRef]
- Calvo-Lerma, J.; Bueno-Llamoga, P.; Bäuerl, C.; Cortés-Macias, E.; Selma-Royo, M.; Pérez-Cano, F.; Collado, M.C. Persistence of anti-SARS-CoV-2 antibodies in breast milk from infected and vaccinated women after in vitro-simulated gastrointestinal digestion. Nutrients 2022, 14, 2117. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.; Prevaes, S.M.; Kalkman, G.; Tramper-Stranders, G.A.; Hasrat, R.; de Winter-de Groot, K.M.; Bogaert, D. Development of the gut microbiota in early life: The impact of cystic fibrosis and antibiotic treatment. J. Cyst. Fibros. 2020, 19, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Needham, B.; Leach, S.T.; Day, A.S.; Jaffe, A.; Thomas, T.; Ooi, C.Y. Disrupted progression of the intestinal microbiota with age in children with cystic fibrosis. Sci. Rep. 2016, 6, 24857. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Selma-Royo, M.; Arboleya, S.; Martínez-Costa, C.; Solís, G.; Suárez, M.; Gueimonde, M. Levels of predominant intestinal microorganisms in 1-month-old full-term babies and weight gain during the first year of life. Nutrients 2021, 13, 2412. [Google Scholar] [CrossRef]
- Prasad, J.; Gill, H.; Smart, J.; Gopal, P.K. Selection and characterisation of Lactobacillus and Bifidobacterium strains for use as probiotics. Int. Dairy J. 1998, 8, 993–1002. [Google Scholar] [CrossRef]
- Eberhart, B.L.; Wilson, A.S.; O’Keefe, S.J.D.; Ramaboli, M.C.; Nesengani, L.T. A simplified method for the quantitation of short-chain fatty acids in human stool. Anal. Biochem. 2021, 612, 114016. [Google Scholar] [CrossRef]
- Feehily, C.; O’Neill, I.J.; Walsh, C.J.; Moore, R.L.; Killeen, S.L.; Geraghty, A.A.; Lawton, E.M.; Byrne, D.; Sanchez-Gallardo, R.; Nori, S.R.C.; et al. Detailed mapping of Bifidobacterium strain transmission from mother to infant via a dual culture-based and metagenomic approach. Nat. Commun. 2023, 14, 3015. [Google Scholar] [CrossRef]
- Rutter, J.W.; Dekker, L.; Owen, K.A.; Barnes, C.P. Microbiome engineering: Engineered live biotherapeutic products for treating human disease. Front. Bioeng. Biotechnol. 2022, 10, 1000873. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Blanco-Míguez, A.; Manghi, P.; Asnicar, F.; Dubois, L.; Golzato, D.; Segata, N. The person-to-person transmission landscape of the gut and oral microbiomes. Nature 2023, 614, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, P.; Pasolli, E.; Tett, A.; Asnicar, F.; Gorfer, V.; Fedi, S.; Segata, N. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe 2018, 24, 133–145. [Google Scholar] [CrossRef]
- Falony, G.; Lazidou, K.; Verschaeren, A.; Weckx, S.; Maes, D.; De Vuyst, L. In vitro kinetic analysis of fermentation of prebiotic inulin-type fructans by Bifidobacterium species reveals four different phenotypes. Appl. Environ. Microbiol. 2009, 75, 454–461. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO Working Group. Guidelines for the Evaluation of Probiotics in Food; FAO/WHO Working Group: London, ON, Canada, 2011; pp. 1–11. [Google Scholar]
- Liu, Z.; Jiang, Z.; Zhou, K.; Li, P.; Liu, G.; Zhang, B. Screening of bifidobacteria with acquired tolerance to human gastrointestinal tract. Anaerobe 2007, 13, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Lemos Junior, W.J.F.; Guerra, A.F.; Tarrah, A.; da Silva Duarte, V.; Giacomini, A.; Luchese, R.H.; Corich, V. Safety and stability of two potentially probiotic Lactobacillus strains after in vitro gastrointestinal transit. Probiotics Antimicrob. Proteins 2020, 12, 657–666. [Google Scholar] [CrossRef]
- Presti, I.; D’Orazio, G.; Labra, M.; La Ferla, B.; Mezzasalma, V.; Bizzaro, G.; Di Gennaro, P. Evaluation of the probiotic properties of new Lactobacillus and Bifidobacterium strains and their in vitro effect. Appl. Microbiol. Biotechnol. 2015, 99, 5613–5626. [Google Scholar] [CrossRef]
- Singh, S.; Bhatia, R.; Khare, P.; Sharma, S.; Rajarammohan, S.; Bishnoi, M.; Kondepudi, K.K. Anti-inflammatory Bifidobacterium strains prevent dextran sodium sulfate-induced colitis and associated gut microbial dysbiosis in mice. Sci. Rep. 2020, 10, 18597. [Google Scholar] [CrossRef]
- Boyte, M.E.; Benkowski, A.; Pane, M.; Shehata, H.R. Probiotic and postbiotic analytical methods: A perspective of available enumeration techniques. Front. Microbiol. 2023, 14, 1304621. [Google Scholar] [CrossRef]
- Pazos-Rojas, L.A.; Cuellar-Sánchez, A.; Romero-Cerón, A.L.; Rivera-Urbalejo, A.; Van Dillewijn, F.; Luna-Vital, D.A.; Muñoz-Rojas, J.; Morales-García, Y.E.; Bustillos-Cristales, M.d.R. The viable but non-culturable (VBNC) state, a poorly explored aspect of beneficial bacteria. Microorganisms 2024, 12, 39. [Google Scholar] [CrossRef]
- Dorsey, J.; Gonska, T. Bacterial overgrowth, dysbiosis, inflammation, and dysmotility in the cystic fibrosis intestine. J. Cyst. Fibros. 2017, 16, S14–S23. [Google Scholar] [CrossRef]
- Nogacka, A.M.; de Los Reyes-Gavilán, C.G.; Arboleya, S.; Ruas-Madiedo, P.; Martínez-Faedo, C.; Suarez, A.; Gueimonde, M. In vitro selection of probiotics for microbiota modulation in normal-weight and severely obese individuals: Focus on gas production and interaction with intestinal epithelial cells. Front. Microbiol. 2021, 12, 630572. [Google Scholar] [CrossRef]
- Ooi, C.Y.; Syed, S.A.; Rossi, L.; Garg, M.; Needham, B.; Avolio, J.; Gonska, T. Impact of CFTR modulation with ivacaftor on gut microbiota and intestinal inflammation. Sci. Rep. 2018, 8, 17834. [Google Scholar] [CrossRef]
- Collado, M.C.; Gueimonde, M.; Hernández, M.; Sanz, Y.; Salminen, S. Adhesion of selected Bifidobacterium strains to human intestinal mucus and the role of adhesion in enteropathogen exclusion. J. Food Prot. 2005, 68, 2672–2678. [Google Scholar] [CrossRef] [PubMed]
- Gorreja, F.; Walker, W.A. The potential role of adherence factors in probiotic function in the gastrointestinal tract of adults and pediatrics: A narrative review of experimental and human studies. Gut Microbes 2022, 14, 2149214. [Google Scholar] [CrossRef] [PubMed]
- Westermann, C.; Gleinser, M.; Corr, S.C.; Riedel, C.U. A critical evaluation of bifidobacterial adhesion to the host tissue. Front. Microbiol. 2016, 7, 1220. [Google Scholar] [CrossRef]
- Harata, G.; Yoda, K.; Wang, R.; Miyazawa, K.; Sato, M.; He, F.; Endo, A. Species- and age/generation-dependent adherence of Bifidobacterium bifidum to human intestinal mucus in vitro. Microorganisms 2021, 9, 542. [Google Scholar] [CrossRef]
- Kim, H.J.; Shin, S.I.; Lee, S.J.; Moon, T.W.; Lee, J.C. Screening and selection of Bifidobacterium strains isolated from human feces capable of utilizing resistant starch. J. Sci. Food Agric. 2018, 98, 5901–5907. [Google Scholar] [CrossRef]
- Astó, E.; Huedo, P.; Altadill, T.; Aguiló García, M.; Sticco, M.; Perez, M.; Espadaler-Mazo, J. Probiotic properties of Bifidobacterium longum KABP042 and Pediococcus pentosaceus KABP041 show potential to counteract functional gastrointestinal disorders in an observational pilot trial in infants. Front. Microbiol. 2022, 12, 741391. [Google Scholar] [CrossRef]
- Fernández Juliá, P.J.; Commane, D.; van Sinderen, D.; Munoz, J. Cross-feeding interactions between human gut commensals belonging to the Bacteroides and Bifidobacterium genera when grown on dietary glycans. Microb. Res. Rep. 2022, 1, 12. [Google Scholar] [CrossRef]
- Rios-Covian, D.; Gueimonde, M.; Duncan, S.H.; Flint, H.J.; de Los Reyes-Gavilan, C.G. Enhanced butyrate formation by cross-feeding between Faecalibacterium prausnitzii and Bifidobacterium adolescentis. FEMS Microbiol. Lett. 2015, 362, fnv176. [Google Scholar] [CrossRef]
- Liu, M.; Lu, Y.; Xue, G.; Han, L.; Jia, H.; Wang, Z.; Zhou, Y. Role of short-chain fatty acids in host physiology. Anim. Models Exp. Med. 2024, 7, 641–652. [Google Scholar] [CrossRef]
- Vernocchi, P.; Del Chierico, F.; Russo, A.; Majo, F.; Rossitto, M.; Valerio, M.; Putignani, L. Gut microbiota signatures in cystic fibrosis: Loss of host CFTR function drives the microbiota enterophenotype. PLoS ONE 2018, 13, e0208171. [Google Scholar] [CrossRef]
- Liu, J.; Yang, L.; Kjellerup, B.V.; Xu, Z. Viable but nonculturable (VBNC) state, an underestimated and controversial microbial survival strategy. Trends Microbiol. 2023, 31, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).