Combating Foodborne MRSA: Identification and Silver Nanoparticle-Based Antibacterial Strategies with Antibiotic Synergy and Resistance Evolution Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sampling
2.2. Culture and Preliminary MRSA Screening
2.3. Species Identification by MALDI-TOF MS
2.4. Antimicrobial Susceptibility Testing (AST)
2.5. Broth Microdilution MIC and MBC Testing
2.6. Molecular Confirmation of MRSA
2.7. AgNPs: Source, Preparation, and Characterization
2.8. AgNP–Antibiotic Combination Testing: Checkerboard Assay
2.9. Time–Kill Kinetics
2.10. Biofilm Assays (Subset Analysis)
2.11. Resistance Evolution Experiment (Serial Passage)
2.12. Data Management and Statistical Analysis
2.13. Biosafety and Quality Assurance
3. Results
3.1. Sample Characteristics and Recovery of S. aureus
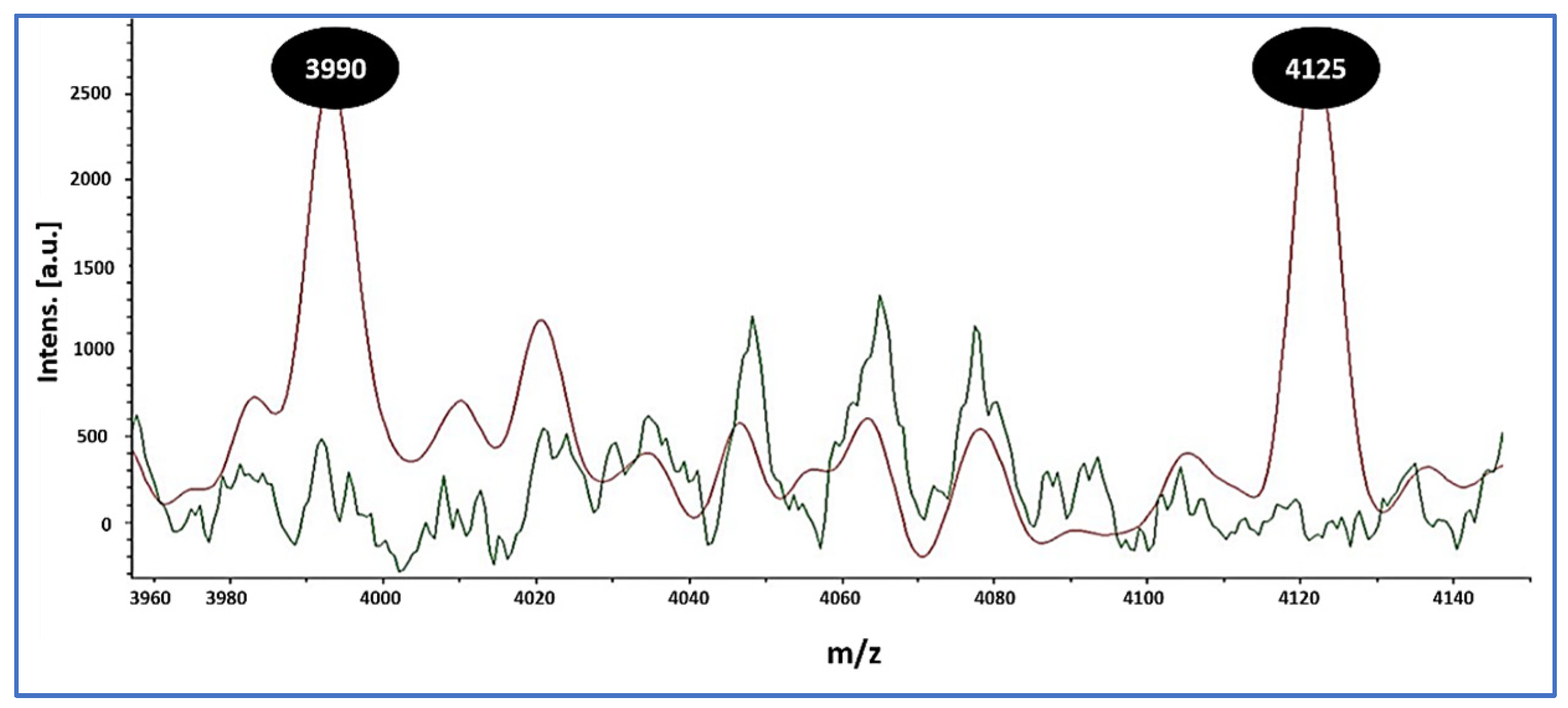
3.2. Confirmation of S. aureus, MRSA Prevalence, and MALDI Peak Signatures
3.3. Antimicrobial Susceptibility Profiles and Agreement Metrics (Vitek 2 AST-GP71)
3.4. In Vitro Activity of AgNPs: MICs and MBCs
3.5. AgNP–Antibiotic Interactions in MRSA (Checkerboard FICI)
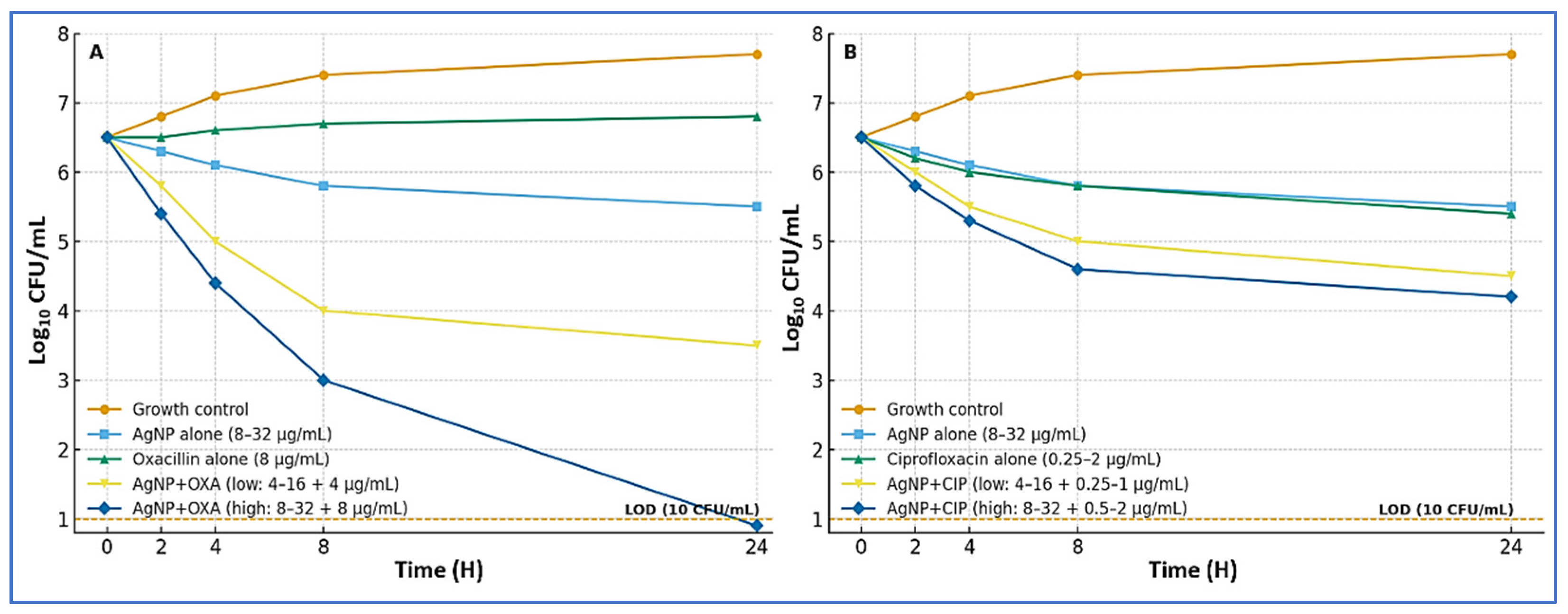
3.6. Time–Kill Kinetics for AgNP Combinations
3.7. Experimental Evolution Under Sub-MIC AgNP Exposure
4. Discussion
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roy, M.C.; Chowdhury, T.; Hossain, M.T.; Hasan, M.M.; Zahran, E.; Rahman, M.M.; Zinnah, K.M.A.; Rahman, M.M.; Hossain, F.M.A. Zoonotic linkage and environmental contamination of Methicillin-resistant Staphylococcus aureus (MRSA) in dairy farms: A one health perspective. One Health 2024, 18, 100680. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024.
- Wu, S.; Huang, J.; Zhang, F.; Wu, Q.; Zhang, J.; Pang, R.; Zeng, H.; Yang, X.; Chen, M.; Wang, J. Prevalence and characterization of food-related methicillin-resistant Staphylococcus aureus (MRSA) in China. Front. Microbiol. 2019, 10, 304. [Google Scholar] [CrossRef]
- Esemu, S.N.; Njoh, S.T.; Ndip, L.M.; Keneh, N.K.; Kfusi, J.A.; Njukeng, A.P. Ready-to-Eat Foods: A Potential Vehicle for the Spread of Coagulase-Positive Staphylococci and Antimicrobial-Resistant Staphylococcus aureus in Buea Municipality, South West Cameroon. Can. J. Infect. Dis. Med. Microbiol. 2023, 2023, 9735319. [Google Scholar] [CrossRef] [PubMed]
- Léguillier, V.; Pinamonti, D.; Chang, C.-M.; Mukherjee, R.; Cossetini, A.; Manzano, M.; Anba-Mondoloni, J.; Malet-Villemagne, J.; Vidic, J. A review and meta-analysis of Staphylococcus aureus prevalence in foods. Microbe 2024, 4, 100131. [Google Scholar] [CrossRef]
- Xing, L.; Cheng, M.; Jiang, J.; Li, T.; Zhang, X.; Tian, Y.; Liu, W. Methicillin-resistant Staphylococcus aureus contamination in meat and meat products: A systematic review and meta-analysis. Front. Microbiol. 2025, 16, 1636622. [Google Scholar] [CrossRef]
- Liang, T.; Liang, Z.; Wu, S.; Ding, Y.; Wu, Q.; Gu, B. Global prevalence of Staphylococcus aureus in food products and its relationship with the occurrence and development of diabetes mellitus. Med. Adv. 2023, 1, 53–78. [Google Scholar] [CrossRef]
- EFSA/ECDC. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2022–2023. EFSA J. 2025, 23, e9237. [Google Scholar]
- Sergelidis, D.; Angelidis, A.S. Methicillin-resistant Staphylococcus aureus: A controversial food-borne pathogen. Lett. Appl. Microbiol. 2017, 64, 409–418. [Google Scholar] [CrossRef]
- Larsen, J.; Stegger, M.; Andersen, P.S.; Petersen, A.; Larsen, A.R.; Westh, H.; Agersø, Y.; Fetsch, A.; Kraushaar, B.; Käsbohrer, A. Evidence for human adaptation and foodborne transmission of livestock-associated methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 2016, 63, 1349–1352. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655, Erratum in Lancet 2022, 400, 1102. [Google Scholar] [CrossRef]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Aguilar, G.R.; Mestrovic, T.; Smith, G.; Han, C. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, Y.; Lizou, Y.; Li, J.; Zhang, R. Evaluation of Staphylococcus aureus subtyping module for methicillin-resistant Staphylococcus aureus detection based on matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Front. Microbiol. 2019, 10, 2504. [Google Scholar] [CrossRef]
- Yu, J.; Tien, N.; Liu, Y.-C.; Cho, D.-Y.; Chen, J.-W.; Tsai, Y.-T.; Huang, Y.-C.; Chao, H.-J.; Chen, C.-J. Rapid identification of methicillin-resistant Staphylococcus aureus using MALDI-TOF MS and machine learning from over 20,000 clinical isolates. Microbiol. Spectr. 2022, 10, e00483-22. [Google Scholar] [CrossRef]
- Bobenchik, A.M.; Hindler, J.A.; Giltner, C.L.; Saeki, S.; Humphries, R.M. Performance of Vitek 2 for antimicrobial susceptibility testing of Staphylococcus spp. and Enterococcus spp. J. Clin. Microbiol. 2014, 52, 392–397. [Google Scholar] [CrossRef]
- Elbehiry, A.; Al-Dubaib, M.; Marzouk, E.; Osman, S.; Edrees, H. Performance of MALDI biotyper compared with Vitek™ 2 compact system for fast identification and discrimination of Staphylococcus species isolated from bovine mastitis. MicrobiologyOpen 2016, 5, 1061–1070. [Google Scholar] [CrossRef]
- Lakhundi, S.; Zhang, K. Methicillin-resistant Staphylococcus aureus: Molecular characterization, evolution, and epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef]
- García-Álvarez, L.; Holden, M.T.; Lindsay, H.; Webb, C.R.; Brown, D.F.; Curran, M.D.; Walpole, E.; Brooks, K.; Pickard, D.J.; Teale, C. Meticillin-resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: A descriptive study. Lancet Infect. Dis. 2011, 11, 595–603. [Google Scholar] [CrossRef] [PubMed]
- CDC. Laboratory Testing for Methicillin (Oxacillin)-Resistant Staphylococcus aureus (MRSA). 2025. Available online: https://www.cdc.gov/mrsa/php/laboratories/index.html (accessed on 17 September 2025).
- Wang, H.; Wang, M.; Xu, X.; Gao, P.; Xu, Z.; Zhang, Q.; Li, H.; Yan, A.; Kao, R.Y.-T.; Sun, H. Multi-target mode of action of silver against Staphylococcus aureus endows it with capability to combat antibiotic resistance. Nat. Commun. 2021, 12, 3331, Erratum in Nat. Commun. 2021, 12, 4140. [Google Scholar] [CrossRef] [PubMed]
- Rahim, K.A.A.A.; Mohamed, A.M.A. Bactericidal and antibiotic synergistic effect of nanosilver against methicillin-resistant Staphylococcus aureus. Jundishapur J. Microbiol. 2015, 8, e25867. [Google Scholar] [CrossRef] [PubMed]
- Casals, E.; Gusta, M.F.; Bastus, N.; Rello, J.; Puntes, V. Silver Nanoparticles and Antibiotics: A Promising Synergistic Approach to Multidrug-Resistant Infections. Microorganisms 2025, 13, 952. [Google Scholar] [CrossRef]
- Akram, F.E.; El-Tayeb, T.; Abou-Aisha, K.; El-Azizi, M. A combination of silver nanoparticles and visible blue light enhances the antibacterial efficacy of ineffective antibiotics against methicillin-resistant Staphylococcus aureus (MRSA). Ann. Clin. Microbiol. Antimicrob. 2016, 15, 48. [Google Scholar] [CrossRef]
- Castro, I.M.d.; Antunes, C.; Valentim, C.C.; Spoladori, L.F.d.A.; Suzukawa, H.T.; Correia, G.F.; Silva-Rodrigues, G.; Borges, P.H.G.; Bartolomeu-Gonçalves, G.; Silva, M.L. Synergistic antibacterial interaction of geraniol and biogenic silver nanoparticles on methicillin-resistant Staphylococcus aureus. Plants 2025, 14, 1059. [Google Scholar] [CrossRef]
- Hochvaldová, L.; Panáček, D.; Válková, L.; Večeřová, R.; Kolář, M.; Prucek, R.; Kvítek, L.; Panáček, A.E. coli and S. aureus resist silver nanoparticles via an identical mechanism, but through different pathways. Commun. Biol. 2024, 7, 1552. [Google Scholar] [CrossRef]
- Panáček, D.; Hochvaldová, L.; Bakandritsos, A.; Malina, T.; Langer, M.; Belza, J.; Martincová, J.; Večeřová, R.; Lazar, P.; Poláková, K. Silver covalently bound to cyanographene overcomes bacterial resistance to silver nanoparticles and antibiotics. Adv. Sci. 2021, 8, 2003090. [Google Scholar] [CrossRef]
- Gillieatt, B.F.; Coleman, N.V. Unravelling the mechanisms of antibiotic and heavy metal resistance co-selection in environmental bacteria. FEMS Microbiol. Rev. 2024, 48, fuae017. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; CLSI: Malvern, PA, USA, 2025. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters; EUCAST: Växjö, Sweden, 2025. [Google Scholar]
- Stegger, á.; Andersen, P.; Kearns, A.; Pichon, B.; Holmes, M.; Edwards, G.; Laurent, F.; Teale, C.; Skov, R.; Larsen, A. Rapid detection, differentiation and typing of methicillin-resistant Staphylococcus aureus harbouring either mecA or the new mecA homologue mecALGA251. Clin. Microbiol. Infect. 2012, 18, 395–400. [Google Scholar] [CrossRef] [PubMed]
- DTU Food, National Food Institute (DTU/NFI). MRSA Multiplex PCR-2 Protocol; Technical University of Denmark: Kongens Lyngby, Denmark, 2024. [Google Scholar]
- Strommenger, B.; Braulke, C.; Heuck, D.; Schmidt, C.; Pasemann, B.; Nubel, U.; Witte, W. spa typing of Staphylococcus aureus as a frontline tool in epidemiological typing. J. Clin. Microbiol. 2008, 46, 574–581. [Google Scholar] [CrossRef] [PubMed]
- ISO 22412; Particle Size Analysis—Dynamic Light Scattering (DLS). ISO: Geneva, Switzerland, 2025.
- ISO 13099-1; Colloidal Systems—Methods for Zeta-Potential Determination. Part 1: Electroacoustic and Electrokinetic Phenomena. ISO: Geneva, Switzerland, 2012.
- ISO 17294-2; Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Part 2: Determination of Selected Elements Including Uranium Isotopes. ISO: Geneva, Switzerland, 2023.
- Wan Mat Khalir, W.K.A.; Shameli, K.; Jazayeri, S.D.; Othman, N.A.; Che Jusoh, N.W.; Hassan, N.M. Biosynthesized silver nanoparticles by aqueous stem extract of Entada spiralis and screening of their biomedical activity. Front. Chem. 2020, 8, 620. [Google Scholar] [CrossRef]
- Wang, W.; Baloch, Z.; Jiang, T.; Zhang, C.; Peng, Z.; Li, F.; Fanning, S.; Ma, A.; Xu, J. Enterotoxigenicity and antimicrobial resistance of Staphylococcus aureus isolated from retail food in China. Front. Microbiol. 2017, 8, 2256. [Google Scholar] [CrossRef] [PubMed]
- Basanisi, M.; La Bella, G.; Nobili, G.; Tola, S.; Cafiero, M.; La Salandra, G. Prevalence and characterization of methicillin-resistant Staphylococcus aureus (MRSA) isolates from retail meat in south Italy. Ital. J. Food Sci. 2020, 32, 410–419. [Google Scholar]
- Normanno, G.; Corrente, M.; La Salandra, G.; Dambrosio, A.; Quaglia, N.C.; Parisi, A.; Greco, G.; Bellacicco, A.; Virgilio, S.; Celano, G.V. Methicillin-resistant Staphylococcus aureus (MRSA) in foods of animal origin product in Italy. Int. J. Food Microbiol. 2007, 117, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Huber, H. Prevalence and Characteristics of Meticillin-Resistant Staphylococcus aureus in Humans in Contact with Farm Animals, in Livestock, and in Food of Animal Origin, Switzerland, 2009; University of Zurich: Zurich, Switzerland, 2010. [Google Scholar]
- Weese, J.; Avery, B.; Reid-Smith, R. Detection and quantification of methicillin-resistant Staphylococcus aureus (MRSA) clones in retail meat products. Lett. Appl. Microbiol. 2010, 51, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, M.A.; Banasser, T.M.; El-Bali, M.; Bulkhi, R.A.; Qamash, R.A.; Trenganno, A.; Khayyat, M.; Kurdi, M.A.; Al Majrashi, A.; Bahewareth, F. Assessment of microbiological quality of food preparation process in some restaurants of Makkah city. Saudi J. Biol. Sci. 2021, 28, 5993–5997. [Google Scholar] [CrossRef] [PubMed]
- Moges, M.; Rodland, E.K.; Ambelu, A. Health risk assessment of Staphylococcus aureus and Salmonella from the consumption of street foods in Ethiopia. BMC Infect. Dis. 2025, 25, 576. [Google Scholar] [CrossRef]
- Beshiru, A.; Isichei-Ukah, B.O.; Uwhuba, K.E.; Igere, B.E.; Igbinosa, E.O. Prevalence, characterization, and implications of methicillin-resistant Staphylococcus aureus (MRSA) in ready-to-eat foods from Delta, Nigeria: A concern for consumer safety. Sustain. Microbiol. 2024, 1, qvae007. [Google Scholar] [CrossRef]
- Fusaro, C.; Miranda-Madera, V.; Serrano-Silva, N.; Bernal, J.E.; Ríos-Montes, K.; González-Jiménez, F.E.; Ojeda-Juárez, D.; Sarria-Guzmán, Y. Antibiotic-resistant bacteria isolated from street foods: A systematic review. Antibiotics 2024, 13, 481. [Google Scholar] [CrossRef] [PubMed]
- Asiegbu, C.; Lebelo, S.; Tabit, F. Microbial quality of ready-to-eat street vended food groups sold in the Johannesburg Metropolis, South Africa. J. Food Qual. Hazards Control 2020, 1, qvae007. [Google Scholar] [CrossRef]
- Aung, K.T.; Hsu, L.Y.; Koh, T.H.; Hapuarachchi, H.C.; Chau, M.L.; Gutiérrez, R.A.; Ng, L.C. Prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in retail food in Singapore. Antimicrob. Resist. Infect. Control 2017, 6, 94. [Google Scholar] [CrossRef]
- Zwe, Y.H.; Mohan, R.D.; Lim, S.J.; Lai, W.C.; Sim, K.H.; Leyau, Y.L.; Lew, K.; Chua, J.M.C.; Aung, K.T.; Chng, K.R. Occurrence & characterization of Staphylococcus aureus from ready-to-eat (RTE), and cooked food in Singapore: A retrospective analysis. Int. J. Food Microbiol. 2025, 436, 111213. [Google Scholar]
- Kitai, S.; Shimizu, A.; Kawano, J.; Sato, E.; Nakano, C.; Uji, T.; Kitagawa, H. Characterization of methicillin-resistant Staphylococcus aureus isolated from retail raw chicken meat in Japan. J. Vet. Med. Sci. 2005, 67, 107–110. [Google Scholar] [CrossRef]
- Sato, T.; Usui, M.; Konishi, N.; Kai, A.; Matsui, H.; Hanaki, H.; Tamura, Y. Closely related methicillin-resistant Staphylococcus aureus isolates from retail meat, cows with mastitis, and humans in Japan. PLoS ONE 2017, 12, e0187319. [Google Scholar] [CrossRef]
- Somda, N.S.; Traoré, A.M.E.; Hien, D.F.d.S.; Bockarie, Y.; Tankoano, A.; Kaboré, D.; Bonkoungou, O.J.I.; Sawadogo-Lingani, H.; Savadogo, A. Molecular characterization of Methicillin-resistant Staphylococcus aureus isolated in ready-to-eat food sold in supermarkets in Bobo-Dioulasso: Case of charcuterie products. BMC Infect. Dis. 2024, 24, 722. [Google Scholar] [CrossRef]
- Islam, M.A.; Parveen, S.; Rahman, M.; Huq, M.; Nabi, A.; Khan, Z.U.M.; Ahmed, N.; Wagenaar, J.A. Occurrence and characterization of methicillin resistant Staphylococcus aureus in processed raw foods and ready-to-eat foods in an urban setting of a developing country. Front. Microbiol. 2019, 10, 503. [Google Scholar] [CrossRef]
- Saber, T.; Samir, M.; El-Mekkawy, R.M.; Ariny, E.; El-Sayed, S.R.; Enan, G.; Abdelatif, S.H.; Askora, A.; Merwad, A.M.; Tartor, Y.H. Methicillin-and vancomycin-resistant Staphylococcus aureus from humans and ready-to-eat meat: Characterization of antimicrobial resistance and biofilm formation ability. Front. Microbiol. 2022, 12, 735494. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Kim, I.; Chung, S.H.; Chung, Y.; Han, M.; Kim, J.-S. Rapid discrimination of methicillin-resistant Staphylococcus aureus by MALDI-TOF MS. Pathogens 2019, 8, 214. [Google Scholar] [CrossRef]
- Wang, Y.R.; Qian, C.; Cui, S.H.; Li, F.Q. Characterization of Staphylococcus aureus isolated from clinical specimens by matrix assisted laser desorption/ionization time-of-flight mass spectrometry. Biomed. Environ. Sci. 2013, 26, 430–436. [Google Scholar] [PubMed]
- Jeon, K.; Kim, J.-M.; Rho, K.; Jung, S.H.; Park, H.S.; Kim, J.-S. Performance of a machine learning-based methicillin resistance of Staphylococcus aureus identification system using MALDI-TOF MS and comparison of the accuracy according to SCC mec types. Microorganisms 2022, 10, 1903. [Google Scholar] [CrossRef] [PubMed]
- Alkuraythi, D.M.; Alkhulaifi, M.M. Methicillin-resistant Staphylococcus aureus prevalence in food-producing animals and food products in Saudi Arabia: A review. Vet. World 2024, 17, 1753. [Google Scholar] [CrossRef]
- Thapa, D.; Pyakurel, S.; Thapa, S.; Lamsal, S.; Chaudhari, M.; Adhikari, N.; Shrestha, D. Staphylococcus aureus with inducible clindamycin resistance and methicillin resistance in a tertiary hospital in Nepal. Trop. Med. Health 2021, 49, 99. [Google Scholar] [CrossRef]
- Islam, S.; Nasrin, N.; Tithi, N.S.; Khatun, F.; Asaduzzaman, M.; Topa, A.F.; Kabir, M.F.; Haque, F.K.M.; Jubair, M.; Rahman, M. Antimicrobial Susceptibility and Genomic Profiles of Multidrug-Resistant Staphylococcus aureus from Nasopharynx of Asymptomatic Children in Dhaka, Bangladesh. Life 2024, 14, 971. [Google Scholar] [CrossRef]
- Raji, M.A.; Garaween, G.; Ehricht, R.; Monecke, S.; Shibl, A.M.; Senok, A. Genetic characterization of Staphylococcus aureus isolated from retail meat in Riyadh, Saudi Arabia. Front. Microbiol. 2016, 7, 911. [Google Scholar] [CrossRef]
- Majhi, S.; Dash, M.; Mohapatra, D.; Mohapatra, A.; Chayani, N. Detection of inducible and constitutive clindamycin resistance among Staphylococcus aureus isolates in a tertiary care hospital, Eastern India. Avicenna J. Med. 2016, 6, 75–80. [Google Scholar] [CrossRef]
- Ali, E.M.; Rajendran, P.; Abdallah, B.M. Mycosynthesis of silver nanoparticles from endophytic Aspergillus parasiticus and their antibacterial activity against methicillin-resistant Staphylococcus aureus in vitro and in vivo. Front. Microbiol. 2024, 15, 1483637. [Google Scholar] [CrossRef]
- Do, B.L.; Bui, T.H.; Ho, T.G.-T.; Duong, N.L.; Nguyen, V.M.; Dang-Bao, T.; Nguyen, T.; Phuong, P.H. Green synthesis of nano-silver and its antibacterial activity against methicillin-resistant Staphylococcus aureus. J. Saudi Chem. Soc. 2023, 27, 101722. [Google Scholar]
- Elghazaly, E.M.; Torky, H.A.; Tawfik, R.G. Effect of silver nanoparticles and REP-PCR typing of Staphylococcus aureus isolated from various sources. Sci. Rep. 2024, 14, 21997. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Hwang, H.-M. Nanotechnology in food science: Functionality, applicability, and safety assessment. J. Food Drug Anal. 2016, 24, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Sultan, A.; Azam, A.; Shahid, M.; Shujatullah, F. Antibacterial activity of silver nanoparticles dispersion against MSSA and MRSA isolated from wounds in a tertiary care hospital of north India. Int. J. Appl. Biol. Pharm. Technol. 2011, 2, 34–42. [Google Scholar]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Lin, X. Metal-regulated antibiotic resistance and its implications for antibiotic therapy. Microb. Biotechnol. 2024, 17, e14537. [Google Scholar] [CrossRef] [PubMed]


| Category→/Outlet | Restaurants | Supermarkets | Street Vendors | Total |
|---|---|---|---|---|
| Meat-based RTE | 18 | 14 | 8 | 40 |
| Poultry-based RTE | 14 | 11 | 7 | 32 |
| Salads/vegetables RTE | 10 | 10 | 8 | 28 |
| Dairy-based RTE | 8 | 11 | 6 | 25 |
| Bakery/sweets | 10 | 8 | 6 | 24 |
| Column total | 60 | 54 | 35 | 149 |
| By Food Category | |||
| Stratum | Positive/Total | Positive (%) | 95% CI (%) |
| Meat-based RTE | 11/40 | 27.5 | 14.6–43.9 |
| Poultry-based RTE | 9/32 | 28.1 | 13.7–46.7 |
| Salads/vegetables RTE | 6/28 | 21.4 | 8.3–41.0 |
| Dairy-based RTE | 7/25 | 28.0 | 12.1–49.4 |
| Bakery/sweets | 3/24 | 12.5 | 2.7–32.4 |
| By Outlet Type | |||
| Stratum | Positive/Total | Positive (%) | 95% CI (%) |
| Restaurants/takeaways | 13/60 | 21.7 | 12.1–34.2 |
| Supermarkets/delis | 11/54 | 20.4 | 10.6–33.5 |
| Street vendors/markets | 12/35 | 34.3 | 19.1–52.2 |
| By Food Category | |||
| Stratum | MRSA/Total | MRSA (%) | 95% CI (%) |
| Meat-based RTE | 4/40 | 10.0 | 2.8–23.7 |
| Poultry-based RTE | 3/32 | 9.4 | 2.0–25.0 |
| Salads/vegetables RTE | 1/28 | 3.6 | 0.1–18.3 |
| Dairy-based RTE | 1/25 | 4.0 | 0.1–20.4 |
| Bakery/sweets | 1/24 | 4.2 | 0.1–21.1 |
| By Outlet Type | |||
| Stratum | MRSA/Total | MRSA (%) | 95% CI (%) |
| Restaurants/takeaways | 4/60 | 6.7 | 1.8–16.2 |
| Supermarkets/delis | 3/54 | 5.6 | 1.2–15.4 |
| Street vendors/markets | 3/35 | 8.6 | 1.8–23.1 |
| Antimicrobial Agent | MRSA | MSSA | Total | CA | VME | ME | mE | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | R | S | R | S | R | |||||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Cefoxitin (screen) | 0 | 0.0 | 10 | 100.0 | 23 | 95.8 | 1 | 4.2 | 23 | 67.6 | 11 | 32.4 | 33 | 97.1 | 0/10 | 0.0 | 1 | 4.2 | 0 | 0.0 |
| Ciprofloxacin | 1 | 10.0 | 9 | 90.0 | 22 | 91.7 | 2 | 8.3 | 23 | 67.6 | 11 | 32.4 | 34 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Clindamycin | 7 | 70.0 | 3 | 30.0 | 22 | 91.7 | 2 | 8.3 | 29 | 85.3 | 5 | 14.7 | 33 | 97.1 | 0 | 0.0 | 0 | 0.0 | 1 | 2.94 |
| Daptomycin | 10 | 100.0 | 0 | 0.0 | 24 | 100.0 | 0 | 0.0 | 34 | 100.0 | 0 | 0.0 | 34 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Erythromycin | 3 | 30.0 | 7 | 70.0 | 19 | 79.2 | 5 | 20.8 | 22 | 64.7 | 12 | 35.3 | 34 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Gentamicin | 9 | 90.0 | 1 | 10.0 | 23 | 95.8 | 1 | 4.2 | 32 | 94.1 | 2 | 5.9 | 33 | 97.1 | 0 | 0.0 | 0 | 0.0 | 1 | 2.94 |
| Linezolid | 10 | 100.0 | 0 | 0.0 | 24 | 100.0 | 0 | 0.0 | 34 | 100.0 | 0 | 0.0 | 34 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Nitrofurantoin | 10 | 100.0 | 0 | 0.0 | 24 | 100.0 | 0 | 0.0 | 34 | 100.0 | 0 | 0.0 | 34 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Oxacillin | 0 | 0.0 | 10 | 100.0 | 24 | 100.0 | 0 | 0.0 | 24 | 70.6 | 10 | 29.4 | 34 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Benzylpenicillin | 0 | 0.0 | 10 | 100.0 | 2 | 8.3 | 22 | 91.7 | 2 | 5.9 | 32 | 94.1 | 32 | 94.12 | 0 | 0.0 | 0 | 0.0 | 2 | 5.88 |
| Quinupristin–dalfopristin | 10 | 100.0 | 0 | 0.0 | 24 | 100.0 | 0 | 0.0 | 34 | 100.0 | 0 | 0.0 | 34 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Rifampin | 8 | 80.0 | 2 | 20.0 | 22 | 91.7 | 2 | 8.3 | 30 | 88.2 | 4 | 11.8 | 34 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Tigecycline | 10 | 100.0 | 0 | 0.0 | 24 | 100.0 | 0 | 0.0 | 34 | 100.0 | 0 | 0.0 | 34 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Trimethoprim–sulfamethoxazole | 10 | 100.0 | 0 | 0.0 | 23 | 95.8 | 1 | 4.2 | 33 | 97.1 | 1 | 2.9 | 34 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Vancomycin | 10 | 100.0 | 0 | 0.0 | 24 | 100.0 | 0 | 0.0 | 34 | 100.0 | 0 | 0.0 | 34 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Isolate ID | Food Category | Isolate Type | AgNP MIC (µg/mL) | Fold-Change vs. MIC50 (16 µg/mL) | AgNP MBC (µg/mL) | MBC/MIC | Killing Classification | Baseline MICs (µg/L): OXA; CIP |
|---|---|---|---|---|---|---|---|---|
| MRSA-01 | Meat-based | Restaurant/Takeaways | 16 | 1.0 | 32 | 2 | Bactericidal | 8; 2 |
| MRSA-02 | Meat-based | Supermarket/Deli | 16 | 1.0 | 32 | 2 | Bactericidal | 8; 0.5 |
| MRSA-03 | Meat-based | Street vendor/Market | 32 | 2.0 | 64 | 2 | Bactericidal | 8; 2 |
| MRSA-04 | Meat-based | Restaurant/Takeaways | 8 | 0.5 | 16 | 2 | Bactericidal | 8; 1 |
| MRSA-05 | Poultry-based | Supermarket/Deli | 16 | 1.0 | 32 | 2 | Bactericidal | 8; 2 |
| MRSA-06 | Poultry-based | Restaurant/Takeaways | 8 | 0.5 | 16 | 2 | Bactericidal | 8; 2 |
| MRSA-07 | Poultry-based | Street vendor/Market | 16 | 1.0 | 64 | 4 | Bactericidal | 8; 1 |
| MRSA-08 | Salads/vegetables | Street vendor/Market | 32 | 2.0 | 64 | 2 | Bactericidal | 8; 2 |
| MRSA-09 | Dairy-based | Supermarket/Deli | 16 | 1.0 | 32 | 2 | Bactericidal | 8; 2 |
| MRSA-10 | Bakery/sweets | Restaurant/Takeaways | 16 | 1.0 | 128 | 8 | Non-bactericidal (isolate) | 8; 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abalkhail, A.; Marzouk, E. Combating Foodborne MRSA: Identification and Silver Nanoparticle-Based Antibacterial Strategies with Antibiotic Synergy and Resistance Evolution Assessment. Microorganisms 2025, 13, 2393. https://doi.org/10.3390/microorganisms13102393
Abalkhail A, Marzouk E. Combating Foodborne MRSA: Identification and Silver Nanoparticle-Based Antibacterial Strategies with Antibiotic Synergy and Resistance Evolution Assessment. Microorganisms. 2025; 13(10):2393. https://doi.org/10.3390/microorganisms13102393
Chicago/Turabian StyleAbalkhail, Adil, and Eman Marzouk. 2025. "Combating Foodborne MRSA: Identification and Silver Nanoparticle-Based Antibacterial Strategies with Antibiotic Synergy and Resistance Evolution Assessment" Microorganisms 13, no. 10: 2393. https://doi.org/10.3390/microorganisms13102393
APA StyleAbalkhail, A., & Marzouk, E. (2025). Combating Foodborne MRSA: Identification and Silver Nanoparticle-Based Antibacterial Strategies with Antibiotic Synergy and Resistance Evolution Assessment. Microorganisms, 13(10), 2393. https://doi.org/10.3390/microorganisms13102393






