Abstract
The objective of this study was to evaluate the antiviral activity of glucosyl hesperidin (GH), a water-soluble derivative of hesperidin with known antioxidant and anti-inflammatory properties, in order to explore its potential applications. Antiviral activity was assessed using feline calicivirus (FCV), a surrogate model for human norovirus, a major foodborne pathogen. Cytotoxicity testing in Crandell–Rees feline kidney (CRFK) cells demonstrated that GH exhibited high biocompatibility, maintaining 100% cell viability at concentrations up to 8000 μM. Antiviral efficacy assays revealed that GH inhibited FCV replication in a concentration-dependent manner across the range of 250~8000 μM, with a half-maximal inhibitory concentration (IC50) of 3281 μM. Complete viral inhibition, however, was not achieved at the maximum concentration tested. In conclusion, GH was shown to inhibit FCV while maintaining low cytotoxicity, indicating its potential as a natural, water-soluble candidate for the suppression of norovirus.
1. Introduction
Norovirus, a single-stranded RNA virus belonging to the family Caliciviridae, is a leading cause of acute gastroenteritis worldwide [1]. Major outbreaks have been reported across the United States, Europe, and Asia, reflecting its status as a truly global pathogen [2,3,4]. Since its first identification in 1972, norovirus has been recognized as a major pathogen affecting individuals of all age groups, with transmission commonly occurring through contaminated food and water [5]. Infections are particularly frequent in community and institutional settings such as hospitals, nursing homes, schools, and other confined environments, including commercial and cruise ships [6]. In recent years, increasing attention has been given to natural compounds with antiviral properties. Nevertheless, relatively few studies have investigated natural substances with activity against norovirus [7,8]. Research in this area is complicated by the fact that norovirus cannot be readily propagated in conventional cell culture or animal models, as it requires the human intestine as its host [9]. To address this limitation, Feline calicivirus (FCV), a single-stranded RNA virus belonging to the same family, is commonly used as a surrogate model due to its genetic, biochemical, and physicochemical similarities to norovirus [10,11].
Flavonoids, a major class of polyphenolic secondary metabolites, are widely distributed across the plant kingdom [12]. Among them, hesperidin is a prominent flavonoid found abundantly in citrus fruits such as oranges, lemons, and limes. Structurally, it consists of the aglycone hesperetin bound to α-L-rhamnosyl-D-glucose. Extensive research has been conducted on the absorption, bioavailability, and pharmacokinetics of hesperidin and its primary metabolite, hesperetin [13,14]. Hesperidin and its derivatives have been reported to exert diverse pharmacological activities, including anti-inflammatory, antibacterial, antithrombotic, and anticancer effects, as well as inhibitory activity against a range of viruses [15,16,17,18,19,20,21]. However, the poor water solubility of hesperidin limits its practical application in food, cosmetic, and pharmaceutical industries. Various approaches have been explored to enhance its solubility and biological activity. Improved water solubility and antioxidant effects have been reported for hesperidin with chitooligosaccharides [22], while inclusion complexes of hesperidin or hesperetin with hydroxypropyl β-cyclodextrin have been shown to increase solubility and antioxidant potential [23]. Glucosylation of hesperidin has emerged as an efficient and promising approach to increase solubility and bioavailability [24,25].
However, no studies to date have reported on the antiviral effects of glucosyl hesperidin (GH) against FCV or norovirus. In the present study, we investigated the antiviral activity of GH, a water-soluble derivative of hesperidin with reported biological properties [25,26], using FCV as a surrogate model for norovirus. The chemical structure of GH is shown in Figure 1.
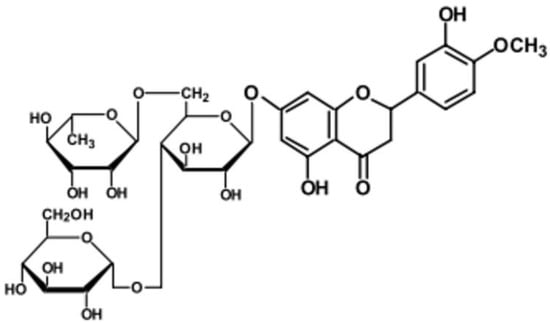
Figure 1.
Structure of glucosyl hesperidin (GH).
2. Materials and Methods
2.1. Materials
Feline calicivirus (FCV, VR-782) and its host cell line, Crandell–Rees feline kidney (CRFK, CCL-94), were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Glucosyl hesperidin (GH) was prepared according to the method described by Choi et al. [25]. Nitazoxanide (Sigma-Aldrich, St. Louis, MO, USA) was used as a positive control in the FCV inhibition assay. Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), phosphate-buffered saline (PBS), trypsin/EDTA, and penicillin–streptomycin solution were purchased from Thermo Fisher Scientific Inc. (Waltham, MA, USA). Reagent WST-1 was purchased from DoGenBio (Seoul, Republic of Korea). All other reagents used were of analytical grade or higher.
2.2. Determination of Cytotoxicity
The CRFK cells were cultured in tissue culture flasks using DMEM supplemented with 10% FBS and 1% penicillin/streptomycin at 37 °C in a humidified incubator with 5% CO2. When cultures reached approximately 80% confluency, cells were washed with PBS, detached with trypsin/EDTA, and subcultured following centrifugation and resuspension in fresh growth medium. The medium was replaced every 48 h.
To assess the cytotoxicity of GH, cells were seeded into 96-well plates at approximately 60% confluency and incubated for 24 h at 37 °C with 5% CO2. The test compound was prepared as a 200 mM stock solution in water and serially diluted in growth medium to final concentrations ranging from 31.2 to 8000 μM. Nitazoxanide was prepared as a 20 mM stock solution in DMSO. Cells were exposed to 40 μL of each test solution for 2 h, after which 100 μL of fresh culture medium was added. Following 24 h of incubation, the test solutions were removed, and cell viability was assessed using the WST-1 assay. Reagent WST-1 was added to each well, and plates were incubated for 30–60 min. Absorbance was measured at 450 nm using a microplate reader. Cell viability was calculated using the following equation.
2.3. Determination of Viral Infectivity
Viral infectivity of control and treated samples was determined using a plaque assay as described by Su et al. [27] with minor modifications. Host cells were seeded into 24-well plates at approximately 60% confluency one day prior to infection. Virus stocks were stored at −80 °C, rapidly thawed in a 37 °C water bath, and diluted in virus culture medium to yield an average multiplicity of infection (MOI) of 0.002 per well. After removing the growth medium, cells were inoculated with virus together with GH at final concentrations ranging from 31.2 to 8000 μM (0.2 mL per well). Plates were incubated for 2 h at 37 °C in 5% CO2, with intermittent gentle shaking to facilitate uniform virus adsorption. Following incubation, an overlay medium containing low-melting-point agarose was added, and the plates were cultured for 24 h under standard conditions.
At the end of incubation, cells were fixed with 3.5% formaldehyde for 30 min. The fixative was removed, and monolayers were stained with 1% methylene blue for 30 min. Excess stain was washed off with distilled water, and plates were air-dried at room temperature. Plaques, appearing as unstained areas against the stained background, were quantified using ImageJ software (ver. 1.53t, NIH, Bethesda, MD, USA). Viral infectivity titers were expressed as plaque-forming units (PFU) per well. The percentage inhibition of viral infection was calculated using the following equation.
The selectivity index (SI) was calculated as the ratio of the 50% cytotoxic concentration (CC50) to the 50% inhibitory concentration (IC50).
2.4. Statistical Analyses
Most experimental procedures were performed in triplicate, and results are expressed as mean ± standard deviation (SD). Statistical analyses were conducted using SPSS software (version 22, SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was applied to compare treatment groups, and significant differences were assessed using Duncan’s multiple range test at levels of p < 0.05 and p < 0.01.
3. Results
3.1. Determination of Cytotoxicity
The CRFK cell line, derived from feline kidney epithelium, is highly susceptible to FCV infection and was therefore used as the host cell for antiviral evaluation. The cytotoxicity of GH was assessed by two-fold serial dilutions up to a maximum concentration of 8000 μM and compared with untreated control cells. No significant cytotoxic effects were observed at any concentration tested, with cell viability maintained at 100% even at 8000 μM (Figure 2a). Accordingly, the 50% cytotoxic concentration (CC50) of GH could not be precisely determined and was estimated to be greater than 8000 μM. In contrast, the positive control, nitazoxanide, exhibited marked cytotoxicity, reducing cell viability to 78.2% at 50 μM (Figure 2b).
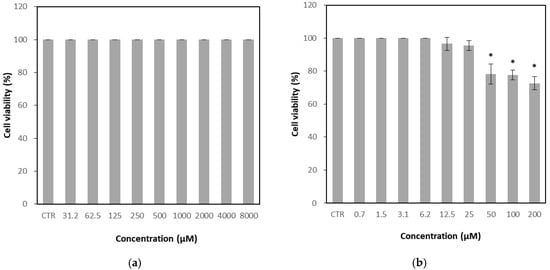
Figure 2.
Cytotoxicity of glucosyl hesperidin (GH) and nitazoxanide on Crandell–Rees feline kidney (CRFK) cells. Cells were treated with (a) GH or (b) nitazoxanide at different concentrations, respectively. Results were represented as mean ± SD (n = 3). Significant differences from the control (* p < 0.05). Note: CTR, Control.
3.2. Determination of Anti-Viral Activity
The infectivity titer of the virus was determined by performing 10-fold serial dilutions of the viral stock, followed by plaque counting after 24 h of infection in host cells. The infectivity titer (PFU/mL) was calculated using the following equation.
Based on the calculated infectivity titer, the viral stock was diluted to achieve a multiplicity of infection (MOI) of approximately 0.002 for the main assay. The results of the plaque reduction assay with GH at this MOI are presented in Figure 3. Untreated cells (without GH treatment) served as a reference, which functionally represented the negative control.
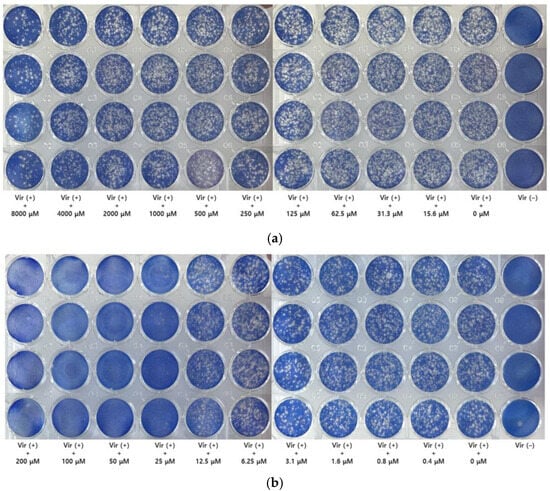
Figure 3.
Plaque assay for glucosyl hesperidin (GH) and nitazoxanide. Plaque assay was performed to evaluate the antiviral activity of GH. The experiment was conducted at a low multiplicity of infection (MOI = 0.002). Cells were treated with (a) GH or (b) nitazoxanide at different concentrations, respectively.
As shown in Figure 4, GH exhibited concentration-dependent antiviral activity against FCV, with an inhibition rate of 4.1% at 250 μM and 77.6% at the maximum tested concentration of 8000 μM. The calculated IC50 value for GH was 3281 μM, whereas that of the positive control, nitazoxanide, was 9.6 μM.
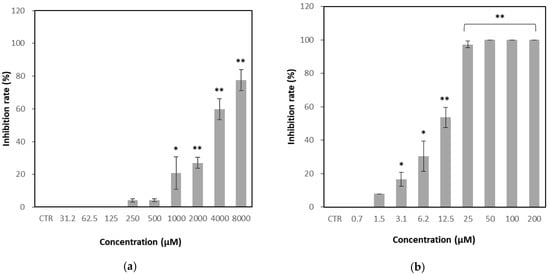
Figure 4.
Effect of glucosyl hesperidin (GH) on viral infection inhibition rate. Crandell–Rees feline kidney (CRFK) cells were treated with (a) GH or (b) nitazoxanide at different concentrations, respectively. Results were represented as mean ± SD (n = 3). Significant differences from the control (* p < 0.05, ** p < 0.01). Note: CTR, Control.
Although a definitive CC50 value could not be established, the dose–response relationship was assessed (Figure 5). For GH, no cytotoxicity was observed in CRFK cells within the tested concentration range, and the CC50 was estimated to be at least 8000 μM. Based on the IC50 value of 3281 μM, the selectivity index (SI) was calculated to be greater than 2.44. In comparison, the positive control nitazoxanide exhibited a CC50 value exceeding 200 μM, an IC50 of 9.6 μM, and a corresponding SI greater than 20.81.
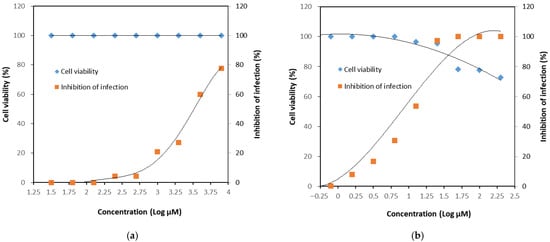
Figure 5.
Dose–response relationship of viral infection inhibition. Crandell–Rees feline kidney (CRFK) cells were treated with (a) glucosyl hesperidin or (b) nitazoxanide at different concentrations, respectively.
4. Discussion
Many natural products, including plant extracts, flavonoids, essential oils, and their derivatives, have been investigated for their anti-norovirus activity using FCV as a surrogate model [28,29,30,31,32]. Flavonoids, in particular, are recognized as promising antiviral agents against both respiratory and enteric viruses [33,34]. Their mechanisms of action include direct inhibition of viral proteases, interference with viral RNA synthesis, and modulation of host immune responses [25]. Hesperidin and its aglycone, hesperetin, have been reported to inhibit SARS-CoV-2 spike-mediated syncytial formation, thereby blocking cell-to-cell viral spread [33]. In addition, hesperetin has been shown to suppress chikungunya virus replication and reduce rotavirus infection, supporting its broad antiviral activity against RNA viruses, including enteric pathogens [34]. Beyond direct antiviral and antibacterial effects, hesperidin-type compounds also promote cellular recovery through antioxidant and anti-inflammatory activities [35,36,37]. Nevertheless, the precise inhibitory concentrations of hesperidin-related compounds against norovirus or FCV have rarely been established, and most studies remain limited in scope [38]. Furthermore, the poor water solubility of hesperidin restricts its broader application.
In the present study, GH, a water-soluble derivative of hesperidin, exhibited measurable antiviral activity against FCV while maintaining minimal cytotoxicity in CRFK cells. Previous studies have reported lower cytotoxicity of GH compared with hesperidin in RAW 264.7 and HaCaT cells [25,26]; the results of this study extend these observations by confirming its safety profile in CRFK cells. Cytotoxicity assays demonstrated that GH maintained 100% cell viability even at the maximum tested concentration of 8000 μM, whereas the reference antiviral nitazoxanide induced cytotoxicity at 50 μM. Antiviral assays further showed that GH inhibited FCV replication in a concentration-dependent manner, with an IC50 of 3281 μM. Although glucosyl hesperidin exhibited a relatively low SI value, its negligible cytotoxicity suggests potential advantages in terms of safety and tolerability. The high CC50 value (>8000 μM) further underscores its safety margin, a key consideration in the development of antivirals for long-term treatment or prophylactic use. Importantly, the balance between solubility, antiviral efficacy, and cytotoxicity is critical for developing effective therapeutic agents. Structural modifications of flavonoids, such as glycosylation or esterification, have been shown to enhance their biological performance [25,26,39,40]. Viral inhibition can occur through mechanisms such as interference with adsorption or penetration, suppression of viral replication, or enhancement of host cell recovery, depending on the specific compound [41,42]. Flavonoids have also been reported to inhibit viral infection through mechanisms that vary according to their structural characteristics [8]. Further studies are required to elucidate the mechanism of action of GH.
While higher SI values are typically regarded as desirable in evaluating antiviral agents, a definitive threshold for this evaluation has not been established. The selectivity index (SI) values of natural products can be influenced by factors such as the viral strain, the chemical structure of the compounds, and the assay conditions used in the experiments [38,43]. The SI value of GH was over 2.44 in this study, which is generally considered insufficiently effective, but for natural products, it may be considered to have potential for development and may require further investigation. It is also suggested that lower SI values may confer certain benefits, where they are often associated with enhanced water solubility and, consequently, improved bioavailability.
Glucosylation of hesperidin enhances hydrophilicity and improves solubility in aqueous media, thereby facilitating the use of higher effective dosages. Considering these properties, further investigation into the structure–activity relationship is warranted to optimize its antiviral efficacy and fully exploit its potential as a safe and effective therapeutic agent.
5. Conclusions
To evaluate the applicability of GH, a highly water-soluble derivative of hesperidin, its antiviral activity against FCV was assessed. It exhibited no detectable cytotoxicity in CRFK cells at concentrations up to 8000 μM and inhibited FCV replication in a concentration-dependent manner, with an IC50 value of 3281 μM. To our knowledge, this is the first study to demonstrate the antiviral activity of GH against FCV, a surrogate for human norovirus. These findings suggest that GH has potential for broader applications as a safe, naturally derived antiviral agent.
Author Contributions
Conceptualization, S.-S.C. and K.-A.L.; methodology, S.-S.C. and K.-A.L.; software, S.-S.C. and S.-H.L.; validation, S.-S.C. and K.-A.L.; formal analysis, S.-H.L.; investigation, S.-S.C. and S.-H.L.; resources, S.-S.C. and K.-A.L.; data curation, S.-S.C. and S.-H.L.; writing—original draft preparation, S.-S.C.; writing—review and editing, S.-S.C. and K.-A.L.; visualization, S.-H.L.; supervision, K.-A.L.; project administration, K.-A.L.; funding acquisition, S.-S.C. and K.-A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GH | Glucosyl hesperidin |
| FCV | Feline calicivirus |
| CRFK | Crandell–Rees feline kidney |
| PFU | Plaque-forming units |
| SI | Selectivity index |
| CC50 | 50% cytotoxic concentration |
| IC50 | 50% inhibitory concentration |
| MOI | Multiplicity of infection |
References
- Widdowson, M.A.; Sulka, A.; Bulens, S.N.; Beard, S.R.; Chaves, S.S. Norovirus and foodborne disease, United States, 1991–2000. Emerg. Infect. Dis. 2005, 11, 95–102. [Google Scholar] [CrossRef]
- Bull, R.A.; Tu, E.T.V.; McIver, C.J.; Rawlinson, W.D.; White, P.A. Emergence of a new norovirus genotype II.4 variant associated with global outbreaks of gastroenteritis. J. Clin. Microbiol. 2006, 44, 327–333. [Google Scholar] [CrossRef]
- Lopman, B.; Vennema, H.; Kohli, E.; Pothier, P.; Sanchez, A.; Negredo, A.; Buesa, J.; Schreier, E.; Reacher, M.; Brown, D.; et al. Increase in viral gastroenteritis outbreaks in Europe and epidemic spread of new norovirus variant. Lancet 2004, 363, 682–688. [Google Scholar] [CrossRef]
- Lee, S.G.; Cho, H.G.; Paik, S.Y. Molecular epidemiology of norovirus in South Korea. BMB Rep. 2015, 48, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Begall, L.F.; Mauroy, A.; Thiry, E. Noroviruses—The State of the Art, Nearly Fifty Years after Their Initial Discovery. Viruses 2021, 13, 1541. [Google Scholar] [CrossRef] [PubMed]
- Robilotti, E.; Deresinski, S.; Pinsky, B.A. Norovirus. Clin. Microbiol. Rev. 2015, 28, 134–164. [Google Scholar] [CrossRef]
- Tian, W.J.; Wang, X.J. Broad-Spectrum Antivirals Derived from Natural Products. Viruses 2023, 15, 1100. [Google Scholar] [CrossRef]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef]
- Duizer, E.; Schwab, K.J.; Neill, F.H.; Atmar, R.L.; Koopman, M.P.G.; Estes, M.K. Laboratory efforts to cultivate noroviruses. J. Gen. Virol. 2004, 85, 79–87. [Google Scholar] [CrossRef]
- Gehrke, C.; Steinmann, J.; Goroncy-Bermes, P. Inactivation of feline calicivirus, a surrogate of norovirus (formerly Norwalk-like viruses), by different types of alcohol in vitro and in vivo. J. Hosp. Infect. 2004, 56, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, J. Surrogate viruses for testing virucidal efficacy of chemical disinfectants. J. Hosp. Infect. 2004, 56, 49–54. [Google Scholar] [CrossRef]
- Beecher, G.R. Overview of dietary flavonoids: Nomenclature, occurrence and intake. J. Nutr. 2003, 133, 3248S–3254S. [Google Scholar] [CrossRef]
- Németh, K.; Plumb, G.W.; Berrin, J.-G.; Juge, N.; Jacob, R.; Naim, H.Y.; Williamson, G.; Swallow, D.M.; Kroon, P.A. Deglycosylation by small intestinal epithelial cell β-glycosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003, 42, 29–42. [Google Scholar] [CrossRef]
- Matsumoto, H.; Ikoma, Y.; Sugiura, M.; Yano, M.; Hasegawa, Y. Identification and quantification of the conjugated metabolites derived from orally administered hesperidin in rat plasma. J. Agric. Food Chem. 2004, 52, 6653–6659. [Google Scholar] [CrossRef]
- Haidari, F.; Keshavarz, S.A.; Rashidi, M.R.; Shahi, M.M. Orange juice and hesperetin supplementation to hyperuricemic rats alter oxidative stress markers and xanthine oxidoreductase activity. J. Clin. Biochem. Nutr. 2009, 45, 285–291. [Google Scholar] [CrossRef]
- Hirata, A.; Murakami, Y.; Shoji, M.; Kadoma, Y.; Fujisawa, S. Kinetics of radical-scavenging activity of hesperetin and hesperidin and their inhibitory activity on COX-2 expression. Anticancer Res. 2005, 25, 3367–3374. [Google Scholar]
- Hao, Y.; Wei, Z.; Wang, Z.; Li, G.; Yao, Y.; Dun, B. Biotransformation of flavonoids improves antimicrobial and anti-breast cancer activities in vitro. Foods 2021, 10, 2367. [Google Scholar] [CrossRef]
- Olas, B. A review of in vitro studies of the anti-platelet potential of citrus fruit flavonoids. Food Chem. Toxicol. 2021, 150, 112090. [Google Scholar] [CrossRef]
- Paredes, A.; Aluzuru, M.; Mendez, J.; Rodriguez-Ortega, M. Anti-Sindbis activity of flavanones hesperetin and naringenin. Biol. Pharm. Bull. 2003, 26, 108–109. [Google Scholar] [CrossRef]
- Pyrzynska, K. Hesperidin: A review on extraction methods, stability and biological activities. Nutrients 2022, 14, 2387. [Google Scholar] [CrossRef]
- Cheng, F.J.; Huynh, T.K.; Yang, C.S.; Hu, D.W.; Shen, Y.C.; Tu, C.Y.; Wu, Y.C.; Tang, C.H.; Huang, W.C.; Chen, Y. Hesperidin is a potential inhibitor against SARS-CoV-2 infection. Nutrients 2021, 13, 2800. [Google Scholar] [CrossRef]
- Cao, R.; Zhao, Y.; Zhou, Z.; Zhao, X. Enhancement of the water solubility and antioxidant activity of hesperidin by chitooligosaccharide. J. Sci. Food Agric. 2018, 98, 2422–2427. [Google Scholar] [CrossRef]
- Wdowiak, K.; Rosiak, N.; Tykarska, E.; Zarowski, M.; Płazińska, A.; Płaziński, W.; Cielecka-Piontek, J. Amorphous Inclusion Complexes: Molecular Interactions of Hesperidin and Hesperetin with HP-B-CD and Their Biological Effects. Int. J. Mol. Sci. 2022, 23, 4000. [Google Scholar] [CrossRef]
- Yamamoto, M.; Suzuki, A.; Hase, T. Short-term effects of glucosyl hesperidin and hesperetin on blood pressure and vascular endothelial function in spontaneously hypertensive rats. J. Nutr. Sci. Vitaminol. 2008, 54, 95–98. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, S.H.; Lee, K.A. A Comparative Study of Hesperetin, Hesperidin and Glucosyl hesperidin: Antioxidant, Anti-Inflammatory, and Antibacterial Activities In Vitro. Antioxidants 2022, 11, 1618. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, S.H.; Hong, S.K.; Gil, B.I.; Lee, K.A. In vitro biological activities of hesperidin-related compounds with different solubility. Antioxidants 2024, 13, 727. [Google Scholar] [CrossRef]
- Su, X.; Zivanovic, S.; D’Souza, D.H. Effect of chitosan on the infectivity of murine norovirus, feline calicivirus, and bacteriophage MS2. J. Food Prot. 2009, 72, 2623–2628. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Dice, L.; Ailavadi, S.; D’Souza, D.H. Antiviral effects of Quillaja saponaria extracts against human noroviral surrogates. Food Environ. Virol. 2023, 15, 167–175. [Google Scholar] [CrossRef]
- Lanave, G.; Pellegrini, F.; Catella, C.; Mateos, H.; Palazzo, G.; Gentile, A.; Diakoudi, G.; Burgio, M.; Tempesta, M.; Martella, V. Virucidal activity of lemon juice against feline calicivirus, surrogate of norovirus. Antibiotics 2025, 14, 273. [Google Scholar] [CrossRef]
- Triratapiban, C.; Lueangaramkul, V.; Phecharat, N.; Pantanam, A.; Lekcharoensuk, P.; Theerawatanasirikul, S. First study on in vitro antiviral and virucidal effects of flavonoids against feline infectious peritonitis virus at the early stage of infection. Vet. World 2023, 16, 618–630. [Google Scholar] [CrossRef]
- Su, X.; D’Souza, D.H. Naturally occurring flavonoids against human norovirus surrogates. Food Environ. Virol. 2013, 5, 97–102. [Google Scholar] [CrossRef]
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising natural compounds against viral infections. Arch. Virol. 2017, 162, 2539–2551. [Google Scholar] [CrossRef]
- Kumara, D.; Harsan, H.S.; Septisetyani, E.P.; Prasetyaningrum, P.W.; Paramitasari, K.A.; Syaifudin, M.; Astirin, O.P.; Ikawati, M.; Meiyanto, E. Inhibitory effects of citrus-derived flavonoids hesperidin and hesperetin on SARS-CoV-2 spike-mediated syncytia formation using in vitro cell model. Adv. Pharm. Bull. 2025, 15, 416–427. [Google Scholar] [CrossRef]
- Eberle, R.J.; Olivier, D.S.; Pacca, C.C.; Avilla, C.M.S.; Nogueira, M.L.; Amaral, M.S.; Willbold, D.; Arni, R.K.; Coronado, M.A. In vitro study of hesperetin and hesperidin as inhibitors of zika and chikungunya virus proteases. PLoS ONE 2021, 16, e0246319. [Google Scholar] [CrossRef]
- Lee, K.A. Cell recovery, anti-inflammatory, and melanogenesis inhibitory activity of water soluble hesperidin in vitro. J. Korean Appl. Sci. Technol. 2023, 40, 1278–1288. [Google Scholar]
- Man, M.Q.; Yang, B.; Elias, P.M. Benefits of hesperidin for cutaneous functions. Evid. Based Complement. Altern. Med. 2019, 2019, 266307. [Google Scholar] [CrossRef]
- Park, C.; Yoo, J.H.; Park, S.U. Recent insights into the biological functions of hesperidin. EXCLI J. 2025, 24, 8730. [Google Scholar]
- McDonagh, P.; Sheehy, P.A.; Fawcett, A.; Norris, J.M. Antiviral effect of mefloquine on feline calicivirus in vitro. Vet. Microbiol. 2015, 176, 370–377. [Google Scholar] [CrossRef]
- Mellou, F.; Loutrari, H.; Stamatis, H.; Roussos, C.; Kolisis, F.N. Enzymatic esterification of flavonoids with unsaturated fatty acids: Effect of the novel esters on vascular endothelial growth factor release from K562 cells. Process Biochem. 2006, 41, 2029–2034. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Lai, X.; Nong, J.; Zhao, G.; Xiao, X. One-pot biocatalytic synthesis and antioxidant activities of highly lipophilic naringin derivatives by using bi-functional whole-cells. Food Res. Int. 2020, 136, 109291. [Google Scholar] [CrossRef]
- Schnitzler, P.; Schuhmacher, A.; Astani, A.; Reichling, J. Melissa officinalis oil affects infectivity of enveloped herpesviruses. Phytomedicine 2008, 15, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, D.; Sarkar, M.C.; Chatterjee, T.; Sharma Dey, R.; Bag, P.; Chakraborti, S.; Khan, M.T. Recent advancements for the evaluation of anti-viral activities of natural products. New Biotechnol. 2009, 25, 347–368. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Meng, S.; Li, Z.R.; Peng, Z.G.; Han, Y.X.; Guo, S.S.; Cui, X.L.; Li, Y.H.; Jiang, J.D. The antiviral effect of 7-hydroxyisoflavone against Enterovirus 71 in vitro. J. Asian Nat. Prod. Res. 2013, 15, 382–389. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).