Abstract
Pistachio green hull (PGH) represents the non-edible fraction obtained after the seed is harvested and is an important source of phenolic compounds. Solid-state fermentation (SSF) is a viable biotechnological and economical technique for extracting phenolic compounds. This study aimed to evaluate the SSF with Aspergillus niger GH1 to recover total phenolic compounds (TPC) with antioxidant capacity (AC) from PGH. For this, the time of higher TPC and AC (DPPH [2,2-diphenyl-1-picrylhydrazyl], ABTS [2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonate)], FRAP [ferric reducing antioxidant power]) was selected. Then, moisture, inoculum concentration, and aeration rate were evaluated. A. niger GH1 was able to grow and colonize the PGH, with the higher value of TPC (23.83 mg/g of dry mass (gdm)) obtained after 24 h of culture, which significantly correlated with AC (Pearson’s R = 0.69). Moisture and aeration rate were the main factors influencing TPC. The highest values for both TPC and AC were achieved in treatment 8 (60% moisture, 5 × 106 spores/mL, and 1 L/Kgwm min), resulting in a 129% and 1039% increase, respectively. Gallic acid 4-O-glucoside and geranine were identified in the PGH extracts using high-performance liquid chromatography coupled with mass spectrometry. The SSF provides eco-friendly alternatives for releasing bioactive compounds from PGH, adding value to this waste.
1. Introduction
The pistachio (Pistacia vera L.) is one of the most important dried fruits today, and there are some reports related to its consumption since three hundred thousand years ago [1,2]. Its consumption occurs as snacks, oils, or flavoring for cakes, cookies, and bread [3]. Thanks to its wide demand, by 2023 a global production of 1.1 million tons was estimated, where the main producing countries were the United States, Iran, Turkey, and Syria [4].
Consumption of this product is primarily driven by its culinary properties, but nowadays, consumers also consider its numerous health benefits. It possesses anti-diabetic, cardiovascular, antioxidant, anti-inflammatory, anticancer, and anti-obesogenic properties [3]. Furthermore, it contains 18–23% protein, 18% carbohydrates, and a lower percentage of fiber, minerals, and bioactive compounds, such as TPC [1,5,6].
Pistachio consumption produces two by-product fractions: the first is the edible fraction, which is the commercial fruit, and the second is a residue, known as PGH, which makes up 45 to 60% of the total fruit. Unfortunately, this significant portion is currently not utilized, highlighting the issue of food waste and the need for sustainable practices in the food industry [7,8,9,10]. Finding alternative uses for PGH is essential because its high moisture content promotes the growth of contaminating microorganisms, and its high TPC amount leads to soil alterations in the area where it is deposited, causing environmental issues [9].
Several studies have reported that PGH is a potential source of TPC with antioxidant, antimicrobial, and antimutagenic activities [7,11]. These TPCs are known for the presence of one or more phenol groups, which are aromatic rings with a hydroxyl group [12]. Ersan et al. [13] reported cyanidin, quercetin, and gallic acid in the PGH. Moreno et al. [14] evaluated the Kerman variety, identifying gallic acid and catechin. In contrast, the Bronte variety contained quercetin and eriodictyol, and in the Uzun and Ohadi varieties, chlorogenic acid and luteolin were found [14]. On the other hand, Noorolahi et al. [15] reported gallic acid, quercetin, galloyl-shikimic acid, galloylquinic acid, and galloyl-O-hexoside.
The SSF is a viable biotechnological solution that is not only environmentally friendly but also economically profitable. This innovative approach is becoming increasingly essential for utilizing agro-industrial wastes [16]. There are studies related to the use of Aspergillus niger, considered a GRAS microbe (generally recognized as a safe organism) [17], in SSF with various agricultural by-products such as cascalote pods [18], pomegranate peel [19], Mexican rambutan peel [20], pineapple residue [21], mango seed [22], fig by-products [23], Castilla rose [24], and others. In these studies, the fungus has contributed to the recovery of TPC by producing enzymes that degrade the cell wall of agricultural wastes, thereby enhancing the phenolic content. This adds value to these by-products for developing new products [18,19,20,21,22,24,25,26,27]. This study aims to assess the potential of PGH as a support in SSF with A. niger and its impact on the release of TPC with antioxidant activity and its identification. This study can prove the potential usefulness of PGH in the consumer goods industry.
2. Materials and Methods
2.1. Preparation of the Substrate
The PGH var. Sfax (experimental population) was obtained from anonymous pistachio plots in Meoqui, Chihuahua, Mexico (28°16′04″ N 105°28′56″ O). The material was disinfected with a UV lamp (LES-04, Megaluz, China) for 1 h, sun-dried for 6 h, and then dried again in an incubator (Incubator FE-131, Felisa, Zapopan, Jalisco, Mexico) at 60 °C for 72 h [13]. After that, PGH was ground to reach a particle size of 0.85 mm and stored in jars protected from light until its use.
2.2. Determination of Hydrological Parameters of PGH
The critical humidity point (CHP) was determined according to Orzua et al. [28]. For that, 1 g of PGH was weighed and dried in a thermobalance (MB120, Parsippany, NJ, USA) at 105 °C. The changes in the weight and moisture of the material were recorded, and drying curves were elaborated. The water absorption index (WAI) was obtained following Torres-León et al. [22], where 1.5 g of PGH was placed in a tube (Falcon) of 50 mL with 15 mL of distilled water. The sample was mixed in a vortex (Four E’S Scientific, Guangzhou, China) for 1 min and centrifuged at 3000× g for 10 min. The supernatant was discarded, and the wet sample was recovered, weighed, and dried. According to Cerda-Cejudo et al. [20], the maximum moisture content (MMC) of the material was calculated using the solids balance of the PGH, moisture content, and the values obtained from the WAI.
2.3. Microorganism and Inoculum Production
Aspergillus niger GH1 strain, belonging to the culture collection of the Food Research Department of the Autonomous University of Coahuila and deposited in the Mycoteca of the University of Minho (MUM:23.16), was used. The strain was reactivated on potato-dextrose agar (PDA) (Bioxon®, Mexico City, Mexico) and incubated in a compact microbiological incubator (Heratherm IMC 18, Thermo Scientific, Langenselbold, Hesse, Germany) at 30 °C for 5 d. Once active, it was inoculated in Erlenmeyer flasks (250 mL) with 30 mL of PDA and incubated in a compact microbiological incubator at 30 °C for 5 d. The spores were collected with a sterile 0.01% (v/v) Tween-80 solution and counted in a Neubauer chamber [18].
2.4. Release of Phenolic Compounds via Solid-State Fermentation
Polypropylene-packed bed column bioreactors (PBCB) were used to evaluate the microorganism growth and biological release of phenolic compounds. The bioreactors were packed with 5 gdm of sterile PGH impregnated with 5 mL of Czapek-Dox culture medium ([KCl (1.52 g/L), KH2PO4 (3.04 g/L), MgSO4 (1.52 g/L), and NaNO3 (7.65 g/L)] and inoculated with A. niger GH1 (1 × 106 spores/mL). During the fermentation process (72 h), PBCB were incubated at 30 °C, and forced aeration was supplied (one liter per kilogram of wet mass per minute (L/Kgwm min)). Additionally, experimental units were taken every 12 h to determine the TPC and AC. Experimental units from 0 h were considered as a control. Conversely, microbial growth and substrate consumption were indirectly estimated through CO2 production and O2 consumption [29]. At the end of the culture, the concentration of phenolic compounds and the antioxidant capacity were determined from the fermented material. These analyses require preparing an extract from the fermented material. Therefore, samples of 1 gwm from the culture bed were suspended in 5 mL of ethanol, distilled water, and lactic acid solution (80:19:1 v/v/v). The samples were then sonicated for 20 min and filtered through cotton and 0.45 µm membranes (Millipore™, Darmstadt, Germany). The filtered extracts were placed into 2 mL vials and stored at −18 °C until their analysis.
2.5. Effect of Moisture Content, Inoculum Size, and Aeration on TFC Release
Once the time of the maximum release of TPC and AC was selected, another SSF process was carried out to explore the effect of moisture (40, 50, and 60%), inoculum concentration (1 × 106, 5 × 106, and 1 × 107 spores/mL), and aeration (0.5, 1, and 1.5 L/Kgwm min) (Table 1). The extracts were obtained after 24 h of SSF according to the described protocol in Section 2.4, and it was used to determine the TPC and AC.

Table 1.
Treatment matrix of the Box-Behnken 3k experimental design.
2.6. Analytical Methods
2.6.1. Respirometry Analysis
Respirometry monitoring was implemented to estimate the fungal growth and substrate consumption. A respirometric analyzer described by Méndez-González et al. [30] was used for online measurement of CO2 production, O2 consumption, and airflow. The O2 consumption (OCR) and CO2 production (CPR) rates were obtained from the concentration gradients between the bioreactor exhaust gas and the air supplied and were expressed in mg/gidm h (gidm, gram of initial dry mass). The total O2 consumption (TOC) and CO2 production (TCP) were estimated using the trapezoidal method [29] to determine the area under the OCR and CPR curves (respectively). TOC and TCP were expressed in mg/gidm.
Kinetical parameters linked to CO2 production and O2 consumption were estimated using the Logistic and Pirt models (Equations (1) and (2)) [31].
The specific CO2 production rate (µ), the initial CO2 production (CO2o), and the total CO2 production (CO2max) were estimated by integrating Equation (1) into Equation (3) using the generalized gradient reduction method [30].
The Logistic and Pirt models were coupled as suggested by Méndez-González et al. [30] Equation (4) to describe the TOC curve. Equation (4) was integrated to estimate the O2 consumption as a function of CO2 production in Equation (5). The coefficients associated with the mass yield of CO2 per O2 consumption (YCO2/O2) and maintenance coefficient (mO2) were estimated by multilinear regression.
2.6.2. Hydrolysable Phenols (HP)
To determine the HP content of the PGH, 20 µL of sample and 20 µL of Folin–Ciocalteu reagent (2 N) were placed in a 96-well microplate. After 5 min, 20 µL of a 0.01 M sodium carbonate solution was added and kept in darkness for 5 min. Finally, 125 µL of distilled water was added, and the absorbance was obtained at 790 nm in a spectrophotometer (Multiskan GO, Thermo Scientific, Vantaa, Uusimaa, Finland) [32]. The results were expressed as mg of gallic acid/g of dry mass (mgGA/gdm). A gallic acid standard curve was used (y = 1.8216x).
2.6.3. Condensed Phenols (CP)
Ferric reagent and HCl–butanol (1:9 v/v) were used to determine the CP content of the PGH. 250 µL of sample, 1500 µL of HCl–butanol, and 50 µL of ferric reagent were placed in a hermetic tube. The mixture obtained was kept boiling for 40 min. Subsequently, the samples were cooled at room temperature, and 200 µL were placed in a 96-well microplate, where the absorbance at 460 nm was measured in a spectrophotometer [20]. The results were expressed as mg of catechin equivalents/g of dry mass (mgCE/gdm). A catechin standard curve was used (y = 0.6359x).
The TPC was calculated as the sum of HP and CP values and expressed as mg/g of dry mass (mg/gdm).
2.6.4. Antioxidant Capacity
The antioxidant capacity of the extracts with DPPH, ABTS, and FRAP assays was evaluated. For the DPPH assay, 60 μM DPPH solution (2,2-diphenyl-1-picrylhydrazyl) (Sigma-Aldrich®, Naucalpan de Juarez, Mexico) in absolute methanol was prepared. 7 µL of sample and 193 µL of DPPH reagent were placed in a 96-well microplate and kept in darkness for 30 min at room temperature before measuring the absorbance at 517 nm [33]. The results were expressed as mg of Trolox equivalents/g of dry mass (mgTE/gdm). A Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) standard curve was used (y = 2.2641x).
For the ABTS assay, 7 mM ABTS solution (2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonate) (Sigma-Aldrich®) in absolute ethanol was prepared and mixed with a 2 mM potassium persulfate solution in a 2:1 v/v ratio. This solution was kept at room temperature in the dark for 12 to 16 h. For the assay, 10 µL of sample and 190 µL of ABTS reagent were placed in a 96-well microplate, and the absorbance was recorded at 734 nm [34]. The results were expressed as mg of Trolox/g of dry mass (mgTE/gdm). A Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) standard curve was used (y = 3.4308x).
To determine the iron-reducing antioxidant power (FRAP) of PGH, a 0.3 M acetate buffer solution at pH 3.6, a 10 Mm TPTZ solution (2,4,6-tri(2-pyridyl)-s-triazine (Sigma-Aldrich®)) in 40 mM HCl and a 20 mM FeCl3*6H2O solution were prepared. Then, the solutions were mixed in a 10:1:1 (v/v/v) ratio, and the FRAP reagent was incubated at 37 °C for 30 min. For the assay, 6 µL of sample, 18 µL of distilled water, and 180 µL of FRAP reagent were placed in a 96-well microplate and incubated at 37 °C for 1 h [18]. The absorbance was determined at 593 nm, and results were expressed as mg of Fe2+/g of dry mass (mgFe2+/gdm). A Fe2SO4 standard curve (Sigma-Aldrich®) was used (y = 7.5781x).
2.6.5. Identification of Phenolic Compounds by RP-HPLC–ESI-MS
The extract obtained was purified by column chromatography using Amberlite XAD-16 (Sigma-Aldrich®, St. Louis, MO, USA), according to the report of Ascacio-Valdés et al. [35]. The solvent was removed by evaporation until a powder was obtained, which was used to determine the profile of TPC present through RP-HPLC–ESI-MS analysis. Reversed-phase high-performance liquid chromatography analysis was performed according to the methodology used by Cerda-Cejudo et al. [20] on a Varian HPLC system that includes an autosampler (Varian ProStar 410, Palo Alto, CA, USA), a ternary bomb (Varian ProStar 230I, Palo Alto, CA, USA), and a photodiode array detector (Varian ProStar 330, Palo Alto, CA, USA). The HPLC system, coupled with an ion trap mass spectrometer (Varian 500-MS IT Mass Spectrometer, Palo Alto, CA, USA) and an electrospray ion source, was also used, operating in negative mode [M-H]−1. MS Workstation software (V 6.9) was used to collect data and process, in full-scan mode, acquired in the m/z range of 50–2000.
2.7. Statistical Analysis
A completely randomized design was established to determine the effect of fermentation time on the TPC content (HP + CP) and AC (DPPH, ABTS, and FRAP) of the fermented PGH. Seven-time levels were evaluated (0, 12, 24, 36, 48, 60, and 72 h), and three repetitions were performed per treatment.
Later, a completely randomized design with a 3k factorial arrangement was used to evaluate the effect of moisture (40, 50, and 60%), inoculum concentration (1 × 106, 5 × 106, and 1 × 107 sp/mL), and aeration rate (0.5, 1, and 1.5 L/Kgwm min) on the AC (DPPH, ABTS, and FRAP) and TPC content. The STATISTICA 7.0 program obtained a Box-Behnken 3k experimental matrix with 15 treatments (Table 1). Three repetitions were carried out per treatment.
The data obtained were analyzed using an analysis of variance (ANOVA, α ≤ 0.05), and subsequently, Fisher’s Least Significant Difference (LSD) test (α ≤ 0.05) was performed. A Pearson correlation analysis was performed between the response variables of TPC and AC (ABTS, DPPH, and FRAP). The results were examined using SAS 9.0 statistical software.
3. Results
3.1. Hydrological Parameters of Pistachio Green Hull
Each organic support presents several physicochemical parameters, so correct characterization is essential to evaluate its potential for use in an SSF process [36]. Table 2 presents the results obtained from WAI, CHP, and MMC of the PGH.

Table 2.
Physicochemical characterization of pistachio green hull.
3.2. Release of Phenolic Compounds via Solid-State Fermentation
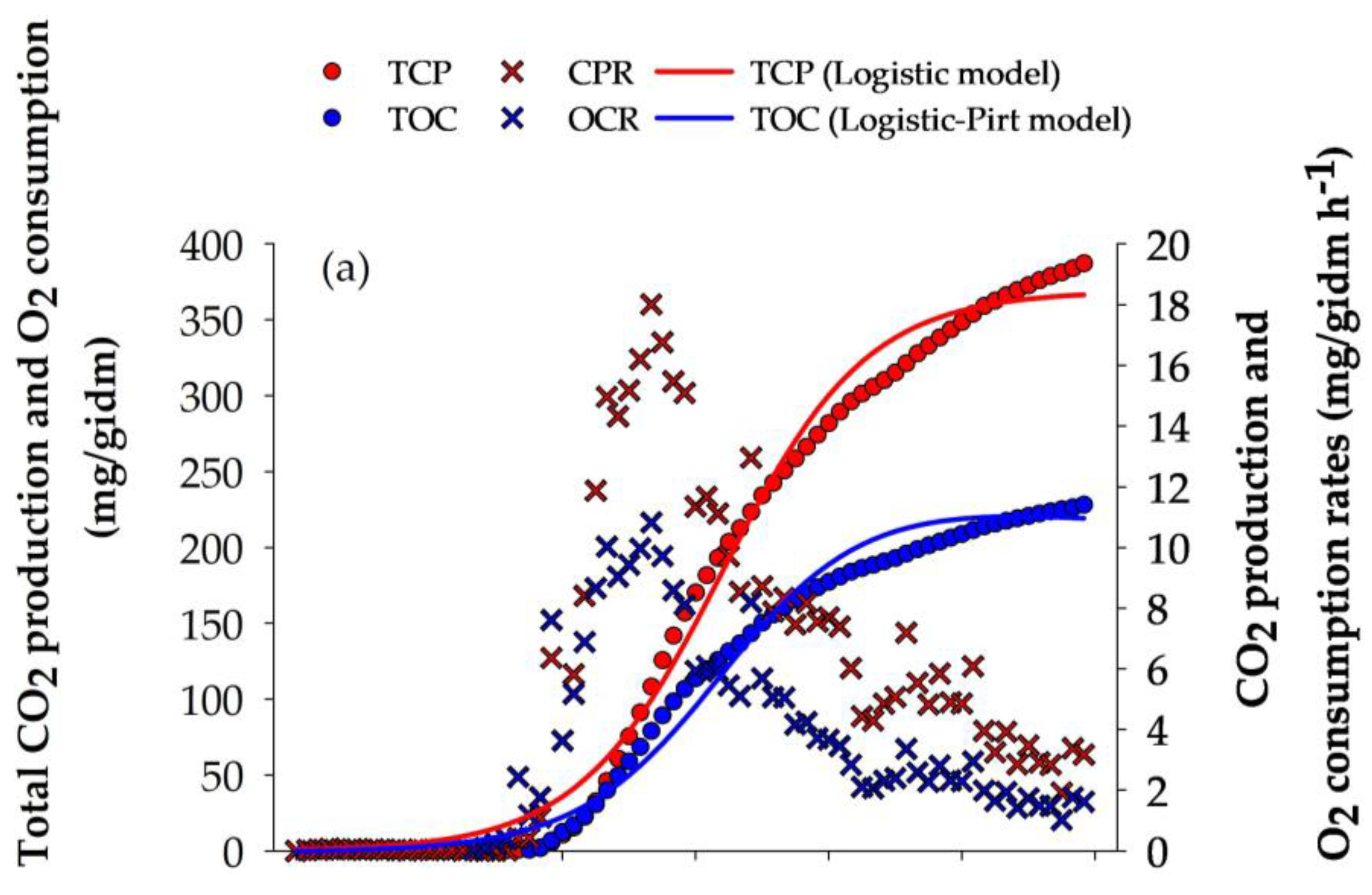
The evolution of the fermentation process was monitored using respirometry analysis. The maximum CPR (18.011 mg/gidm h−1) and OCR (10.825 mg/gidm h−1) were obtained at 32 h of culture (Figure 1a). At the end of the culture, TCP and TOC obtained by A. niger GH1 reached values of 363.08 and 217.55 mg/gidm, respectively. The TCP and TOC curves were described using Equations (3) and (5) (Figure 1a), reaching a high goodness of fit (R2 > 0.98) (Table 3).
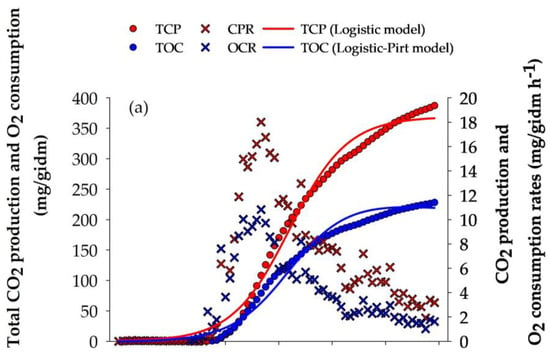
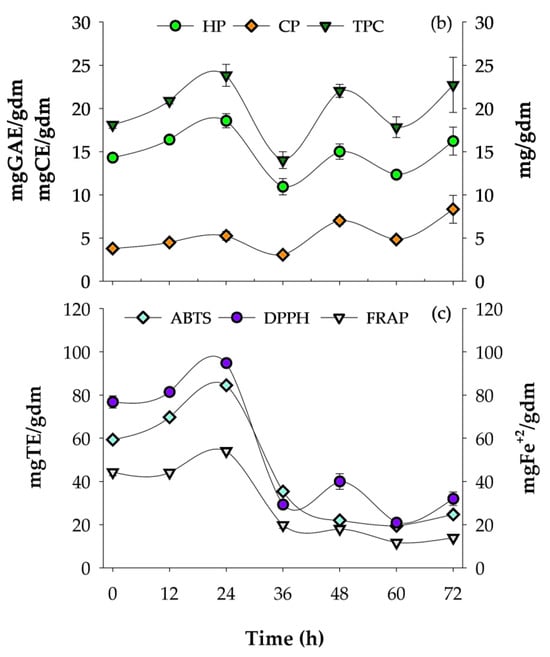
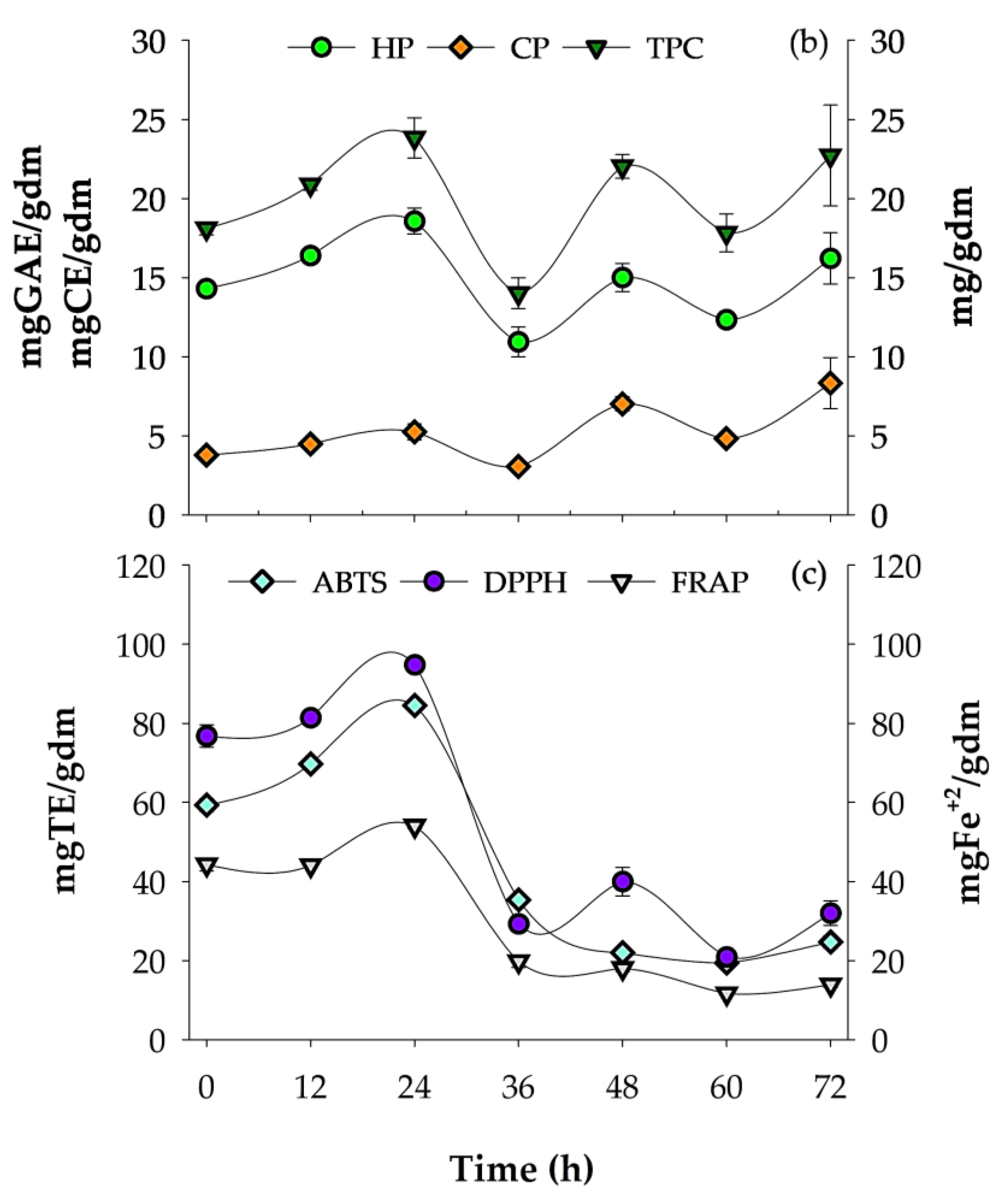
Figure 1.
Respirometry profile of A. niger GH1, kinetics of phenolic compounds, and antioxidant activity during SSF in PGH. (a) O2 consumption rate (OCR) and CO2 production rate (CPR); total O2 consumption (TOC) and total CO2 production (TCP). (b) Hydrolyzable phenols (HP), condensed phenols (CP), and total phenolic content (TPC). (c) Antioxidant assays: ABTS, DPPH, and FRAP. gdm = gram of dry mass; mgGAE = milligram of gallic acid equivalents; mgCE = milligram of catechin equivalents; gdm = gram of dry mass; mgTE = milligrams of Trolox equivalents; mgFe2+ = milligram of ferrous ion; gidm = grams of initial dry mass; results are the mean (n = 3) ± standard deviation.

Table 3.
Respirometric parameters of A. niger GH1 in SSF of PGH.
During the culture, the release of phenolic compounds and antioxidant capacity were determined using HP, CP, TPC, ABTS, DPPH, and FRAP methods (Figure 1b,c). The highest content of HP and TPC was obtained at 24 h of fermentation with values of 18.57 ± 0.83 mgGAE/gdm and 23.83 ± 1.27 mg/gdm, respectively, while the highest CP content was obtained after 72 h of fermentation (8.34 ± 1.61 mgCE/gdm). The HP and TPC content after 24 h of fermentation compared to the unfermented control showed an increase of 29.76% and 38.42%, respectively; additionally, the TPC content at 24 h of cultivation was 4.88% higher than the 72 h time, which was the second value with the highest TPC content. In contrast, the 36 h time was the lowest phenolic content presented, with a percentage of 70% lower than obtained at 24 h.
Regarding the AC of the fermented PGH extracts, the highest result was observed after 24 h of culture, with values of 84.48 ± 0.30 mgTE/gdm, 94.79 ± 0.89 mgTE/gdm, and 54.02 ± 0.62 mgFe2+/gdm for the DPPH, ABTS, and FRAP assays, respectively. Compared to the unfermented treatment, increases of 42.43%, 23.42%, and 21.96% were observed after 24 h of SSF for the ABTS, DPPH, and FRAP assays, respectively.
The Pearson correlations obtained for the different response variables of phenolic content and AC are listed in Table 4. The results show that there is a consistent positive linear relationship between the HP content and AC for the three measurement methods (DPPH, ABTS, and FRAP) (R ≤ 0.6902), and these findings are statistically significant (α ≤ 0.05). This suggests that as the HP content increases, the AC also increases.

Table 4.
Pearson correlation coefficient between the response variables of phenolic content (HP, CP, and TPC) and AC (DPPH, ABTS, and FRAP).
3.3. Effect of Moisture Content, Inoculum Size, and Aeration on TPC Release
The results obtained for phenolic content and AC are presented in Table 5. Results demonstrate an increase in the phenolic content in all treatments compared to the unfermented control. Treatment 8, with conditions of 60% moisture, 1 × 107 sp/mL, and 1 L/Kgwm min of aeration, yielded the best results with values of 34.71 ± 1.04 mgGAE/gdm, 6.77 ± 0.28 mgCE/gdm, and 41.48 ± 0.99 mg/gdm for HP, CP, and TPC, respectively. The TPC of treatment 8 increased by 129% compared to the unfermented control. Treatment 1, which had 60% moisture, 5 × 106 sp/mL, and 0.5 L/Kgwm min of aeration, was the second-best treatment, with a TPC value of 37.03 ± 2.21 mg/gdm. When compared to treatment 8, there was only a 12% difference; in contrast, treatment 7, with 40% moisture, 1x107 sp/mL, and 1 L/Kgwm min of aeration, showed the smallest increase, with a TPC value of 28.58 ± 0.44 mg/gdm, which was 45% lower than treatment 8.

Table 5.
Phenolic content (HP, CP, and TPC) and AC (ABTS, DPPH, and FRAP) in PGH extracts subjected to SSF with A. niger GH1.
Due to the strong correlation between TPC and AC, the highest increase was also observed in treatment 8, with 132.75 ± 4.91 mgTE/gdm and 131.68 ± 2.49 mgTE/gdm for DPPH and ABTS, respectively. In the case of the FRAP assay, no significant differences (p ≤ 0.05) were observed between treatments 1 and 8, with values of 532.96 ± 29.98 and 504.59 ± 8.71 mgFe2+E/gdm, respectively. Therefore, treatment 8 allowed the highest increase of AC in the three methods, with increases of 124, 71, and 1039% (ABTS, DPPH, and FRAP, in that order), compared to the unfermented control.
Table 6 displays the impact of the factors above on the release of TPC, HP, CP, and AC. Moisture content positively influenced all response variables, and this effect was statistically significant (α ≥ 0.05). The inoculum concentration had a significant positive effect on half of the response variables (α ≥ 0.05). Increasing the inoculum concentration was found to favor the content of HP, TPC, and the AC in DPPH radical, while it had no significant effect on the AC in ABTS and FRAP assays, as well as the CP content. Aeration during the SSF process had a significant negative effect (α ≥ 0.05) on all response variables except AC (ABTS). An increase in the aeration rate reduced TPC, HP, CP, and AC (DPPH and FRAP).

Table 6.
Effect of moisture, inoculum, and aeration on the phenolic content (HP, CP, and TPC) and the AC (DPPH, ABTS, and FRAP) of fermented PGH extracts.
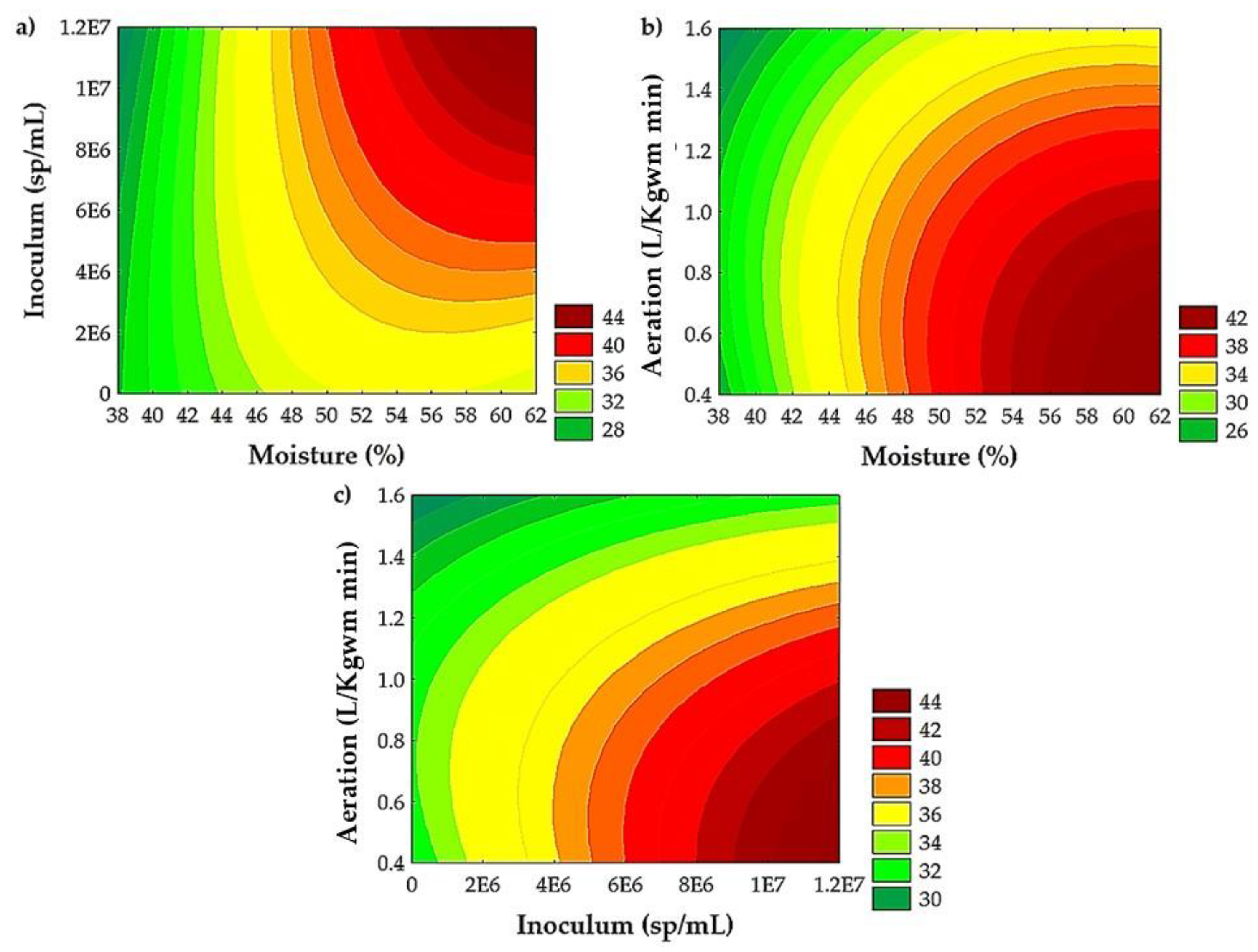
Figure 2 shows the contour plots to analyze the combined effects of factors on the extraction yield of TPC. The contour plots showed an improvement in yield extraction of TPC, ranging from 56 to 62% of moisture (Figure 2a,b), with 0.4 to 0.5 L/Kgwm min of aeration (Figure 2b,c) and an inoculum of 9 × 106 to 1.2 × 107 sp/mL (Figure 2a,c).
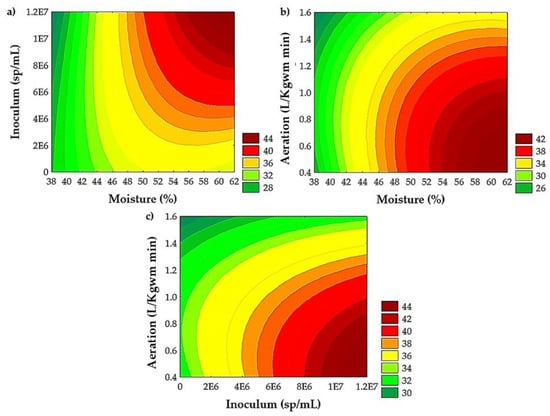
Figure 2.
Interactive effects of (a) moisture and inoculum, (b) moisture and aeration, and (c) inoculum and aeration on yield extraction of TPC from PGH by SSF with A. niger GH1.
3.4. Identification of Phenolic Compounds by RP-HPLC–ESI-MS
A water-soluble fraction and an oily fraction were found. The HPLC–MS analyzed the water-soluble fraction, and two masses (330.9 and 950.5 m/z) corresponding to gallic acid 4-O-glucoside and geraniin were detected (Table 7).

Table 7.
Phenolic compound profile of purified PGH extract.
4. Discussion
Since water is essential for the transport of nutrients from the substrate to the cells, hydrological parameters such as CHP, WAI, and MMC were determined. The CHP indicates the amount of bound water in the solid matrix, which is not available for the microorganism [28,37,38]. Therefore, a high CHP could affect the growth of the microorganism [18,22]. For SSF, values of CHP lower than 40% are recommended for Aspergillus niger [37]. The PGH obtained a CHP of 0.37 ± 0.05%, which indicates that the most water content in the solid substrate is available for the culture. On the other hand, WAI refers to the water absorption capacity of a solid matrix, which is linked to hydrophilic groups in its molecular composition [22]. WAI values from 2.97 to 12.09 g gel/gdm facilitate microbial growth in solid-state substrates [37]. The PGH obtained a WAI value of 5.73 ± 1.05 g gel/gdm, which indicates a proper water absorption capacity. In fact, this value is higher than those obtained in other materials used in SSF processes, such as cascalote pods (2.97 ± 0.07 g gel/gdm) [18], rambutan peel (3.4 ± 0.01 g gel/gdm) [20], Mexican mango seed (3.5 ± 0.04 g gel/gdm) [22], and fig residues (3.74 ± 0.10 g gel/gdm) [23]. The MMC is essential to determine the initial conditions for SSF. Water excess can affect the gas exchange and cause particle agglomeration [20]. The PGH presents a MMC of 82.79 ± 2.77%. The values obtained are higher than those reported for cascalote pods (79.33 ± 2.08%) [18] and rambutan peel (72 ± 0.03%) [20]. García-Zapata [39] indicates that fungi need a moisture content between 40 and 60%, so the PGH provides optimal water conditions to A. niger GH1. Due to the hydrological characteristics of PGH, this waste is suitable for use in SSF to release phenolic compounds.
To verify the above, A. niger GH1 was inoculated on pretreated PGH to phenolic compounds via SSF. During the fermentation process, CO2 production and O2 consumption were monitored to estimate the culture evolution. The maximum value of CPR and OCR reached by A. niger (Figure 1a) was obtained at 32 h, which is comparable with those obtained in other substrates [40,41]. Therefore, the substrate composition does not limit the A. niger growth. In fact, the term of Equation (5) associated with YCO2/O2 is higher than that associated with mO2, so biomass production was favored during the culture [30,31,42]. However, the maximal phenolic compound release was obtained at 24 h, near the beginning of the exponential growth phase. In this stage, the energy from substrate consumption is directed equitably to microbial growth and cellular maintenance, favoring enzyme production [43].
During SSF, a wide range of enzymes is produced, leading to structural modifications caused by the biotransformation of the cell wall. This process also results in changes to the organic compounds, such as an increase in protein content and a reduction in carbohydrate and fatty acid content. As a result, this facilitates the release of nutrients and bioactive molecules, including phenolic compounds, which are associated with the structural components of the plant matrix [44].
As mentioned, the SSF process with A. niger GH1 presented the highest phenolic content and AC after 24 h of culture (Figure 1b,c), and it has been reported that this microorganism has a short adaptation phase, reducing the time of maximum growth and the production of some metabolite [28]. The use of A. niger GH1 in SSF processes has been reported to recover TPC with AC from different wastes such as rambutan peel [20], Mexican mango seed [22], Castilla rose [24], and pineapple residues [21], with the maximum release of TPC at 12, 20, 24, and 30 h of culture, respectively. From these data, it is possible to establish a range of 12 to 30 h as the time in which A. niger GH1 releases the highest amount of TPC with AC in SSF processes using agro-industrial wastes as a substrate.
The time with the highest AC coincides with the highest phenolic content, which confirms that these compounds released in PGH increase the AC. As evidence of this, the Pearson correlation coefficient (Table 4) indicates that there is a regular positive linear relationship between the HP content and the AC for the three measured methods (DPPH, ABTS, and FRAP) (r ≤ 0.6902). These values were statistically significant (α ≤ 0.05), which indicates that increasing the HP content (corresponding to the majority fraction observed in Table 3) also increases AC. On the other hand, the Pearson correlation coefficient of CP content and AC showed a weak negative linear relationship (r ≤ −0.3634). This can be explained due to HP stands out for its antioxidant properties, while CP for its bacteriostatic and fungicidal activity thanks to their structure [45], however, Vázquez-Flores et al. [46] mentioned the antioxidant activity of both groups.
The PGH contains a higher content of HP than CP in all treatments (Table 5). This can be explained by the structure of the PGH, which, being an agro-industrial waste, presents a large number of organic molecules such as pectin or structural carbohydrates such as cellulose, hemicelluloses, or lignin [25]. For this reason, there is a higher fraction of TPC bound with these organic compounds than those that create flavonoid polymers such as CP [46].
The SSF process of the PGH showed treatment 8 (60% moisture, 1 × 107 sp/mL, and 1 L/Kgwm min of aeration) with the highest TPC, HP, CP, and AC (Table 5). Compared to the other studies, only two reports of SSF processes use PGH as a substrate. Abbasi et al. [47] performed an SSF at 30 °C and 70% moisture for 20 days, using the fungus Phanerochaete chrysosporium. The maximum TPC was obtained after 16 days [63 mgCAE/gdm (Caffeic acid equivalents)], representing an increase of 29% compared to the control. Although they are not the same units, the value is higher than the 41.48 mgGAE/gdm obtained in this project. However, the authors report the addition of soybean as a nitrogen source in the culture medium, which may overestimate the value of TPC since soybean contains 10.3 to 13.7 mg/g [48]. Karimi et al. [49] reported the SSF of PGH for 10 days at 30 °C and 66% initial moisture, using the fungus A. terreus ATCC74135 (1 × 106 sp/gdm), where a decrease of 81.5% in the TPC was obtained, compared to the unfermented control, attributed to possible hydrolysis when using 6 M HCl during the extraction or to the consumption of the TPC by the fungus due to long-term fermentation. The carbon source is consumed, and the microorganism can secrete enzymes capable of degrading TPC and use them as a carbon source to perform its metabolic actions [22]. On the other hand, studies have reported the extraction of TPC from PGH using various extraction techniques, with values from 22.2 to 127.25 mg/gdm [2,8,11,15,50,51,52]. For these studies, the differences in phenolic content are attributed to the extraction method, the solvent used, time, temperature, solid/solvent ratio, crop, geographical location, harvest season, or the pistachio variety [2,8,50]. However, the results obtained in this project are superior to those reported by Noorolahi et al. [15], Erşan et al. [51], and Neval-Özbek et al. [11], demonstrating the effectiveness of the bioprocess as a strategy to increase the TPC content from PGH.
Several studies have demonstrated the positive effect of SSF with A. niger GH1 on the TPC from various plant substrates. De León-Medina et al. [24] reported a 166% increase in TPC in extracts fermented by A. niger GH1 from rosa Castilla. Torres-León et al. [22] increased the TPC content by 230% in Mexican mango seeds using A. niger GH1. During the SSF process of Mexican rambutan peel with A. niger GH1, Cerda-Cejudo et al. [20] obtained an increase in TPC content of 404%. López-Cárdenas et al. [18] increased the TPC content by 219% in ground cascalote pods using A. niger GH1; Buenrostro-Figueroa et al. [19] achieved an increase of ~526% in pomegranate peel using A. niger GH1; and Ramírez-Esparza et al. [53] increased the TPC content by 83% in corn with the fungus R. oryzae. Using the A. niger HT4 strain in SSF from fig residues, Buenrostro-Figueroa et al. [23] reported an increase in TPC of 548%. In the case of PGH, the TPC of treatment 8 increased by 129% compared to the unfermented control. The increase in phenolic content is because, during the SSF process, the microorganism produces enzymes such as cellulases, lipases, proteases, and amylases responsible for the degradation of cell wall components, thus releasing the bound fraction of TPC, which passes into the free fraction, improving its solubility and extractability [18,19,20,21,22,24,26,27,53,54].
Due to the strong correlation between TPC and AC, the highest increase was also observed in treatment 8, with 132.75 ± 4.91 mgTE/gdm and 131.68 ± 2.49 mgTE/gdm for DPPH and ABTS, respectively. In the case of the AC of the FRAP assay, no significant differences (p ≤ 0.05) were observed between treatment 1 and 8, with values of 532.96 ± 29.98 and 504.59 ± 8.71 mgFe2+/gdm, respectively. Therefore, treatment 8 allowed the highest increase in AC in the three methods, with increases of 124, 71, and 1039% (ABTS, DPPH, and FRAP, in that order), compared to the unfermented control. The increase in AC is attributed to the fact that treatment 8 presented the highest content of TPC (Table 5), which increases said biological properties due to its antioxidant nature [46]. However, as was noted in the present study, a decrease in the AC of fermented extracts of PGH against the DPPH radical has also been reported in another study. During SSF with A. terreus ATCC74135, Karimi et al. [49] observed a 9% decrease after 10 days of culture. On the other hand, Abbasi et al. [47] found that by subjecting the PGH to SSF with P. chrysosporium for 16 days, there was a 22% reduction in AC. This decrease can be attributed to the biodegradation or biotransformation of TPC, which leads to their consumption or modification, thus affecting AC [22].
Studies analyzed the AC of the PGH, with values of 127.65 to 411.98 mgTE/gdm, 117.64 to 466.73 mgTE/gdm, and 122.64 to 2230.8 mgFe2+E/gms for DPPH, ABTS, and FRAP, respectively [15,51]. However, the results obtained in this project are superior to those reported by Erşan et al. [51], demonstrating the effectiveness of the bioprocess as a strategy to increase TPC with AC of the PGH.
Previous studies have reported that SSF increases the AC in extracts obtained from several plant matrices. López-Cárdenas et al. [18] increased the AC by 66.76, 93, and 55.67% for DPPH, ABTS, and FRAP, respectively, in cascalote pods. Buenrostro-Figueroa et al. [19] achieved an increase of 581% for DPPH in pomegranate peel. Recently, Ramírez-Esparza et al. [53] reported an increase of 55, 119, and 125% (ABTS, DPPH, and FRAP, respectively) in corn.
Moisture content showed a positive effect for all response variables, where increasing moisture in the SSF process will also increase TPC, HP, CP, and AC (Table 6). It is known that the different microbial species studied in SSF processes require different moisture contents to achieve their development and a correct release of TPC [19,55]. In SSF processes with fungi, a moisture content of 20 to 70% is recommended [16], while values above 70% cause difficulty in the growth of the microorganism and the production of enzymes, as well as the physical properties of the substrate by higher agglomeration of particles and limitation of oxygen transfer [18,24,28]. On the other hand, moisture values below 20% induce sporulation [36], and this is due to the small amount of available water found in the medium, so the microorganism seeks to perpetuate itself and stop growing until moisture conditions are favorable again to continue with their growth and development [56].
The effect of moisture on the growth of A. niger GH1 and the recovery of bioactive compounds has been reported. López-Cárdenas et al. [18] evaluated the SSF process of cascalote pods at two moisture levels (50 and 60%). A positive effect was observed, with higher achievement of TPC and AC at 60%. On the other hand, Cerda-Cejudo et al. [20] evaluated the effect of moisture (50, 60, and 70%) in an SSF process with A. niger GH1 on Mexican rambutan peel. The authors observed a positive quadratic effect: the increase in moisture content favors the recovery of ellagic acid to a certain extent, reaching the maximum value at 60% moisture, and above this (70%), the value decreases. By evaluating two levels of moisture (50 and 60%), Buenrostro et al. [19] observed an opposite effect during the SSF with A. niger GH1 on pomegranate peels, establishing 50% moisture as the optimal value for the release of the TPC. The studies by López-Cárdenas et al. [18] and Cerda-Cejudo et al. [20] agree with the results obtained in the present study, where a moisture content of 60% was obtained using A. niger GH1 as in the previously mentioned studies. This moisture content of 60% is the value required for the SSF process of the PGH (Figure 2a,b). Because each plant material has a different physicochemical composition, the moisture conditions used for each bioprocess will differ [57].
The inoculum concentration showed a positive effect where increasing the inoculum concentration favors the content of HP, TPC, and AC (the DPPH radical) (Table 6). Being an important parameter during an SSF process, the inoculum has been widely studied since the success of the process depends on it. The adequate concentration of inoculum allows the reduction of the time of the adaptation phase of the microorganism, accelerating microbial growth and the production of secondary metabolites and reducing fermentation times to obtain products of interest [36]. The use of filamentous fungi is attributed to their ability to adapt to a wide variety of substrates and carbon sources, thanks to the hydrolytic enzymes that they produce, and additionally contribute to the release or bioconversion of TPC [18].
The increase in inoculum concentration promotes a higher release of TPC through SSF with A. niger GH1 on rambutan peel, Castilla rose, and cascalote pods [18,20,24]. The results shown for the A. niger GH1 strain demonstrate higher efficiency in the release of TPC using high concentrations of inoculum (Figure 2a,c), which translates into greater generation or synthesis of enzymes that release these compounds [27].
Aeration during the SSF process had a significant negative effect on all response variables except for ABTS, whereby a reduction in TPC, HP, CP, and AC (DPPH and FRAP assays) was observed when increasing aeration (Table 6). Aeration plays an important role in the removal of heat and CO2 from the medium, which are products of microbial metabolism. Likewise, it supplies the oxygen required by the microorganism for its growth and development [58,59,60]. There are no reports of the effect of aeration in an SSF process on TPC release. However, its effect on biomass production and various enzymes has been evaluated. Méndez-González et al. [29] evaluated five aeration levels (0.16, 0.33, 0.66, 0.96, and 1.28 L/Kgwm min) on the production of Metarhizium robertsii conidia, obtaining the highest value using the lowest level (0.33 L/Kgwm min). Ridder et al. [61] reported the production of xylanases from Trichoderma longibrachiatum at three aeration levels (0, 2.9, and 5.7 L/Kgwm min), with the highest enzymatic activity at 2.9 L/Kgwm min. On the other hand, Zhou et al. [60] conclude that the glycoprotein production of Streptomyces kanasenisi ZX01 increases as aeration increases, with the highest value at an aeration rate of 2.0 vvm (volume of air per liter per minute, similar to L/Kgwm min). According to the above, each SSF process is unique, and therefore, the aeration rate will depend on the product obtained, the substrate, and the microorganism used. In the case of PGH, this decrease in TPC (Figure 2b,c) and AC observed in SSF can be explained by the possible capture of free radicals given by the TPC generated by the SSF process [62].
Based on the results obtained, treatment 8 presents the best performance in terms of TPC recovery from PGH by SSF with A. niger GH1 compared to the unfermented control, where an increase of 129, 142, and 78% is reflected for the response variables TPC, HP, and CP, respectively. Regarding the AC obtained after the SSF process, an increase of 72, 124, and 1.039% was obtained for the response variables DPPH, ABTS, and FRAP, respectively. In general, the SSF process of PGH with A. niger GH1 requires high moisture conditions (60%), a high inoculum concentration (1 × 107 sp/mL), and a reduced aeration rate (0.5 L/Kgwm min) to enhance the performance of TPC with AC in the PGH.
The TPC profile showed gallic acid 4-O-glucoside, which has already been widely reported, since Moreno-Rojas et al. [14], Arjeh et al. [9], Ghandahari et al. [63], and Grace et al. [52] report it as one of the majority TPC in the PGH. This compound has the lowest molecular weight of TPC and presents antioxidant activity (by being a donor of electrons or hydrogen atoms) and antibacterial activity by increasing the permeability of membranes in bacteria [9,63] and the changes it causes in pH [8]. The other compound shown by the profile was geraniin, which also presents antioxidant activity thanks to its ability to capture radicals; anticancer potential since it prevents the proliferation of cancer cells; cytoprotective activity by generating a strong barrier that can protect the different tissues of the human; and antibacterial activity [64]. These compounds obtained are responsible for the AC observed in the PGH extracts. However, an oily fraction was also obtained, and Arjeh et al. [9], Grace et al. [52], and Schulze et al. [65] reported the presence of phenolic lipids in PGH, which have an aromatic ring with a long aliphatic chain, which gives them biological properties. These phenolic lipids are known as anacardic acids, and it is probable that these compounds were the oily fraction obtained during chromatography but were not analyzed. These compounds are classified within phenolic acids and are a taxonomic character since they are only present in plant species belonging to the Anacardiaceae family, such as pistachio [9].
Phenolic compounds have a range of applications across different industries. In the pharmaceutical sector, these compounds are widely used as antioxidants due to their ability to donate hydrogen atoms or electrons to free radicals, thereby halting oxidation reactions and potentially inhibiting health issues such as cancer, hypertension, and diabetes. In the cosmetic industry, phenolic compounds are incorporated into various products as bioactive ingredients. Their structure enables them to capture ultraviolet radiation through chromophores, protecting the skin from damage caused by solar exposure. In the textile industry, phenolic compounds serve as natural dyes, helping to reduce water pollution associated with synthetic dyes while also providing solar protection for cotton garments. In the food industry, these compounds are utilized as colorants and additives, enhancing food preservation thanks to their antioxidant and antimicrobial properties, which can extend shelf life. However, it is essential to examine the bioavailability and absorption of these compounds in vivo, as these factors can influence their bioactive properties [66].
5. Conclusions
This study showed that PGH could be used as a substrate in SSF due to its high moisture content (82.29%) and water absorption capacity (5.73 g gel/gdm).
Aspergillus niger GH1 was able to grow and invade the PGH, showing the highest release of TPC and AC at 24 h of fermentation with the process conditions of 60% moisture, an inoculum concentration of 1 × 107 sp/mL, and 1 L/Kgwm min of aeration.
The SSF process significantly increased the TPC, ABTS, DPPH, and FRAP in PGH by 129, 71, 124, and 1039%, respectively.
The purified PGH extract contains gallic acid 4-O-glucoside and geranine, an important bioactive compound with antioxidant and antimicrobial properties. This study demonstrates the antioxidant potential of PGH after SSF, which constitutes a biotechnological strategy to valorize agroindustry residues in order to recover molecules with potential applications in the consumer goods industry, such as the food industry, cosmetics, or pharmaceuticals.
Author Contributions
Conceptualization, J.J.B.-F., L.S.-T. and L.A.P.-B.; methodology, A.J.O.-C., U.R.-E. and F.M.-G.; software, A.J.O.-C. and F.M.-G.; validation, M.A.-G., L.S.-T. and R.B.-J.; formal analysis, A.J.O.-C. and U.R.-E.; investigation, A.J.O.-C. and J.J.B.-F.; resources, J.J.B.-F. and L.A.P.-B.; data curation, A.J.O.-C. and F.M.-G.; writing-original draft preparation, A.J.O.-C.; writing-review and editing, all authors; supervision, M.A.-G., R.B.-J., L.S.-T., J.J.B.-F. and L.A.P.-B.; project administration, J.J.B.-F.; funding acquisition, J.J.B.-F. and L.A.P.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
A.J.O.-C. thanks CONAHCyT for the postgraduate scholarship provided during his postgraduate studies.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mandalari, G.; Barreca, D.; Gervasi, T.; Roussell, M.A.; Klein, B.; Feeney, M.J.; Carughi, A. Pistachio Nuts (Pistacia vera L.): Production, Nutrients, Bioactives and Novel Health Effects. Plants 2022, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Pakdaman, N.; Dargahi, R.; Nadi, M.; Javanshah, A.; Shakerardekani, A.; Saberi, N. Optimizing the Extraction of Phenolic Compounds from Pistachio Hulls. J. Nuts 2021, 4, 361–370. [Google Scholar] [CrossRef]
- Mateos, R.; Salvador, M.D.; Fregapane, G.; Goya, L. Why Should Pistachio Be a Regular Food in Our Diet? Nutrients 2022, 14, 3207. [Google Scholar] [CrossRef]
- USDA. Tree Nuts: World Markets and Trade; United States Department of Agriculture: Washington, DC, USA, 2024; p. 8.
- Olvera-Bautista, M.R. Caracterización Química y Nutracéutica del Pistache (Pistacia vera L.) Tostado, así Como la Evaluación de la Bioaccesibilidad in Vitro, Permeabilidad ex Vivo y Análisis Quimioinformático in Silico. Bachelor’s Thesis, Universidad Autónoma de Querétaro, Queretaro, Mexico, 2022; p. 89. [Google Scholar]
- Martínez-Ruíz, N.R.; Rodrigo-García, J.; Corral-Díaz, B. Efecto del Secado Controlado Sobre la Calidad Nutrimental del Pistache (Pistacia vera L.) y Subproductos Producido en el Valle de Juárez, Chihuahua, México; Universidad Autónoma de Ciudad Juárez: Ciudad Juárez, Mexico, 2019; p. 22. [Google Scholar]
- Cardullo, N.; Leanza, M.; Muccilli, V.; Tringali, C. Valorization of Agri-Food Waste from Pistachio Hard Shells: Extraction of Polyphenols as Natural Antioxidants. Resources 2021, 10, 45. [Google Scholar] [CrossRef]
- Elhadef, K.; Akermi, S.; Ben Hlima, H.; Ennouri, K.; Fourati, M.; Ben Braïek, O.; Mellouli, L.; Smaoui, S. Tunisian Pistachio Hull Extracts: Phytochemical Content, Antioxidant Activity, and Foodborne Pathogen Inhibition. J. Food Qual. 2021, 2021, 9953545. [Google Scholar] [CrossRef]
- Arjeh, E.; Akhavan, H.-R.; Barzegar, M.; Carbonell-Barrachina, Á.A. Bio-active compounds and functional properties of pistachio hull: A review. Trends Food Sci. Technol. 2020, 97, 55–64. [Google Scholar] [CrossRef]
- Hamed, M.; Bougatef, H.; Karoud, W.; Krichen, F.; Haddar, A.; Bougatef, A.; Sila, A. Polysaccharides extracted from pistachio external hull: Characterization, antioxidant activity and potential application on meat as preservative. Ind. Crops Prod. 2020, 148, 112315. [Google Scholar] [CrossRef]
- Özbek, H.N.; Halahlih, F.; Göğüş, F.; Koçak Yanık, D.; Azaizeh, H. Pistachio (Pistacia vera L.) Hull as a Potential Source of Phenolic Compounds: Evaluation of Ethanol–Water Binary Solvent Extraction on Antioxidant Activity and Phenolic Content of Pistachio Hull Extracts. Waste Biomass Valoriz. 2018, 11, 2101–2110. [Google Scholar] [CrossRef]
- Martín-Gordo, D.A. Los Compuestos Fenólicos, Un Acercamiento A Su Biosíntesis, Síntesis Y Actividad Biológica. Rev. De Investig. Agrar. Y Ambient. 2018, 9, 81–104. [Google Scholar] [CrossRef]
- Erşan, S.; Güçlü Üstündağ, Ö.; Carle, R.; Schweiggert, R.M. Identification of Phenolic Compounds in Red and Green Pistachio (Pistacia vera L.) Hulls (Exo- and Mesocarp) by HPLC-DAD-ESI-(HR)-MSn. J. Agric. Food Chem. 2016, 64, 5334–5344. [Google Scholar] [CrossRef]
- Moreno-Rojas, J.M.; Velasco-Ruiz, I.; Lovera, M.; Ordoñez-Díaz, J.L.; Ortiz-Somovilla, V.; De Santiago, E.; Arquero, O.; Pereira-Caro, G. Evaluation of Phenolic Profile and Antioxidant Activity of Eleven Pistachio Cultivars (Pistacia vera L.) Cultivated in Andalusia. Antioxidants 2022, 11, 609. [Google Scholar] [CrossRef] [PubMed]
- Noorolahi, Z.; Sahari, M.A.; Barzegar, M.; Ahmadi Gavlighi, H. Tannin fraction of pistachio green hull extract with pancreatic lipase inhibitory and antioxidant activity. J. Food Biochem. 2020, 44, e13208. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Beltrán, G.M.; Salazar-Garcés, D.M. Fermentación Sólida en la Industria Alimentaria. Bachelor’s Thesis, Universidad Técnica de Ambato, Ambato, Ecuador, 2021. [Google Scholar]
- Thegarathah, P.; Jewaratnam, J.; Simarani, K. Turbidity reduction in palm oil mill effluent (POME) by submerged fermentation with immobilized Aspergillus niger spores using coconut husk. IOP Conf. Ser. Earth Environ. Sci. 2022, 1074, 012027. [Google Scholar] [CrossRef]
- López-Cárdenas, F.; Ochoa-Reyes, E.; Baeza-Jiménez, R.; Tafolla-Arellano, J.C.; Ascacio-Valdés, J.A.; Buenrostro-Figueroa, J.J. Solid-State Fermentation as a Sustainable Tool for Extracting Phenolic Compounds from Cascalote Pods. Fermentation 2023, 9, 823. [Google Scholar] [CrossRef]
- Buenrostro-Figueroa, J.J.; Nevárez-Moorillón, G.V.; Chávez-González, M.L.; Sepúlveda, L.; Ascacio-Valdés, J.A.; Aguilar, C.N.; Pedroza-Islas, R.; Huerta-Ochoa, S.; Prado-Barragán, L.A. Improved Extraction of High Value-Added Polyphenols from Pomegranate Peel by Solid-State Fermentation. Fermentation 2023, 9, 530. [Google Scholar] [CrossRef]
- Cerda-Cejudo, N.D.; Buenrostro-Figueroa, J.J.; Sepúlveda, L.; Torres-Leon, C.; Chávez-González, M.L.; Ascacio-Valdés, J.A.; Aguilar, C.N. Recovery of ellagic acid from mexican rambutan peel by solid-state fermentation-assisted extraction. Food Bioprod. Process. 2022, 134, 86–94. [Google Scholar] [CrossRef]
- Paz-Arteaga, S.L.; Ascacio-Valdés, J.A.; Aguilar, C.N.; Cadena-Chamorro, E.; Serna-Cock, L.; Aguilar-González, M.A.; Ramírez-Guzmán, N.; Torres-León, C. Bioprocessing of pineapple waste for sustainable production of bioactive compounds using solid-state fermentation. Innov. Food Sci. Emerg. Technol. 2023, 85, 103313. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzmán, N.; Ascacio-Valdés, J.; Serna-Cock, L.; dos Santos Correia, M.T.; Contreras-Esquivel, J.C.; Aguilar, C.N. Solid-state fermentation with Aspergillus niger to enhance the phenolic contents and antioxidative activity of Mexican mango seed: A promising source of natural antioxidants. LWT 2019, 112, 108236. [Google Scholar] [CrossRef]
- Buenrostro-Figueroa, J.J.; Velázquez, M.; Flores-Ortega, O.; Ascacio-Valdés, J.A.; Huerta-Ochoa, S.; Aguilar, C.N.; Prado-Barragán, L.A. Solid state fermentation of fig (Ficus carica L.) by-products using fungi to obtain phenolic compounds with antioxidant activity and qualitative evaluation of phenolics obtained. Process Biochem. 2017, 62, 16–23. [Google Scholar] [CrossRef]
- De León-Medina, J.C.; Sepúlveda, L.; Morlett-Chávez, J.; Meléndez-Renteria, P.; Zugasti-Cruz, A.; Ascacio-Valdés, J.; Aguilar, C.N. Solid-State Fermentation with Aspergillus niger GH1 to Enhance Polyphenolic Content and Antioxidative Activity of Castilla Rose (Purshia plicata). Plants 2020, 9, 1518. [Google Scholar] [CrossRef]
- Cano y Postigo, L.O.; Jacobo-Velázquez, D.A.; Guajardo-Flores, D.; Garcia Amezquita, L.E.; García-Cayuela, T. Solid-state fermentation for enhancing the nutraceutical content of agrifood by-products: Recent advances and its industrial feasibility. Food Biosci. 2021, 41, 100926. [Google Scholar] [CrossRef]
- Gómez Rojas, M.P.; Arboleda Valencia, J.W.; Mosquera Martínez, O.M. Género Aspergillus: Fuente potencial de péptidos bioactivos. Rev. Fac. De Cienc. Básicas 2021, 17, 73–89. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Treichel, H.; Kumar, V.; Pandey, A. Chapter 1—Advances in Solid-State Fermentation. In Current Developments in Biotechnology and Bioengineering; Pandey, A., Larroche, C., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–17. [Google Scholar]
- Orzua, M.C.; Mussatto, S.I.; Contreras-Esquivel, J.C.; Rodriguez, R.; de la Garza, H.; Teixeira, J.A.; Aguilar, C.N. Exploitation of agro industrial wastes as immobilization carrier for solid-state fermentation. Ind. Crops Prod. 2009, 30, 24–27. [Google Scholar] [CrossRef]
- Méndez-González, F.; Figueroa-Montero, A.; Saucedo-Castañeda, G.; Loera, O.; Favela-Torres, E. Addition of spherical-style packing improves the production of conidia by Metarhizium robertsii in packed column bioreactors. J. Chem. Technol. Biotechnol. 2022, 97, 1517–1525. [Google Scholar] [CrossRef]
- Méndez-González, F.; Loera, O.; Saucedo-Castañeda, G.; Buenrostro-Figueroa, J.J.; Favela-Torres, E. Improved packed bed column bioreactor to produce fungal conidia for biological control. Syst. Microbiol. Biomanuf. 2024. [Google Scholar] [CrossRef]
- Martínez-Ramírez, C.; Esquivel-Cote, R.; Ferrera-Cerrato, R.; Martínez-Ruiz, J.A.; Rodríguez-Serrano, G.; Saucedo-Castañeda, G. Solid-state culture of Azospirillum brasilense: A reliable technology for biofertilizer production from laboratory to pilot scale. Bioprocess Biosyst. Eng. 2021, 44, 1525–1538. [Google Scholar] [CrossRef]
- Wong-Paz, J.E.; Muñiz-Márquez, D.B.; Aguilar-Zárate, P.; Rodríguez-Herrera, R.; Aguilar, C.N. Microplate Quantification of Total Phenolic Content from Plant Extracts Obtained by Conventional and Ultrasound Methods. Phytochem. Anal. 2014, 25, 439–444. [Google Scholar] [CrossRef]
- Melendez, N.P.; Nevárez-Moorillón, V.; Rodríguez-Herrera, R.; Espinoza, J.C.; Aguilar, C.N.; Obal, N. A microassay for quantification of 2, 2-diphenyl-1-picrylhydracyl (DPPH) free radical scavenging. Afr. J. Biochem. Res. 2014, 8, 14–18. [Google Scholar]
- Rojo-Gutiérrez, E.; Carrasco-Molinar, O.; Tirado-Gallegos, J.M.; Levario-Gómez, A.; Chávez-González, M.L.; Baeza-Jiménez, R.; Buenrostro-Figueroa, J.J. Evaluation of green extraction processes, lipid composition and antioxidant activity of pomegranate seed oil. J. Food Meas. Charact. 2021, 15, 2098–2107. [Google Scholar] [CrossRef]
- Ascacio-Valdés, J.A.; Aguilera-Carbó, A.; Martínez-Hernández, J.L.; Rodríguez-Herrera, R.; Aguilar, C.N. Euphorbia antisyphilitica residues as a new source of ellagic acid. Chem. Pap. 2010, 64, 528–532. [Google Scholar] [CrossRef]
- Kumar, V.; Ahluwalia, V.; Saran, S.; Kumar, J.; Patel, A.K.; Singhania, R.R. Recent developments on solid-state fermentation for production of microbial secondary metabolites: Challenges and solutions. Bioresour. Technol. 2021, 323, 124566. [Google Scholar] [CrossRef]
- Buenrostro-Figueroa, J.; Ascacio-Valdés, A.; Sepúlveda, L.; De la Cruz, R.; Prado-Barragán, A.; Aguilar-González, M.A.; Rodríguez, R.; Aguilar, C.N. Potential use of different agroindustrial by-products as supports for fungal ellagitannase production under solid-state fermentation. Food Bioprod. Process. 2014, 92, 376–382. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Aguilar, C.N.; Rodrigues, L.R.; Teixeira, J.A. Colonization of Aspergillus japonicus on synthetic materials and application to the production of fructooligosaccharides. Carbohydr. Res. 2009, 344, 795–800. [Google Scholar] [CrossRef]
- García-Zapata, A.M. Marco Conceptual Sobre la Influencia de la Temperatura y la Humedad Relativa en la Fermentación Sólida del Grano de Café Arábica Sobre el Contenido del Ácido 5-O-Cafeoilquínico. Doctoral Dissertation, Universidad Pontificia Bolivariana, Medellin, Colombia, 2016. [Google Scholar]
- Méndez-Alvarez, J.A. Producción de Ácido Glucónico Aplicando Cinética de Crecimiento Microbiano a Partir de Aspergillus niger y Como Medio de Cultivo, Ducle de Atado. Doctoral Dissertation, Universidad de El Salvador, San Salvador, El Salvador, 2013. [Google Scholar]
- Figueroa-Montero, A.A. Modelamiento de la Transferencia de Calor y Masa (Agua) en un Biorreactor de Charolas para Fermentación en Medio Sólido. Doctoral Dissertation, Universidad Autónoma Metropolitana, Mexico City, Mexico, 2011. [Google Scholar]
- Pirt, S.J. Maintenance energy: A general model for energy-limited and energy-sufficient growth. Arch. Microbiol. 1982, 133, 300–302. [Google Scholar] [CrossRef]
- Viniegra-González, G.; Favela-Torres, E.; Aguilar, C.N.; Rómero-Gomez, S.d.J.; Díaz-Godínez, G.; Augur, C. Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochem. Eng. J. 2003, 13, 157–167. [Google Scholar] [CrossRef]
- Puspitasari, C.; Pinsirodom, P.; Wattanachaisaereekul, S. Effect of solid-state fermentation using Aspergillus oryzae and Aspergillus niger on bitter and bioactive compounds of Moringa oleifera seed flour. LWT—Food Sci. Technol. 2024, 207, 116616. [Google Scholar] [CrossRef]
- Alvarez-Agüero, C.M.; Lock de Ugaz, O. Taninos. Rev. De Química 1992, 6, 47–63. [Google Scholar]
- Vázquez-Flores, A.A.; ’Álvarez-Parrilla, E.; López-Díaz, J.A.; Wall-Medrano, A.; De la Rosa, L.A. Taninos hidrolizables y condensados: Naturaleza química, ventajas y desventajas de su consumo: Hydrolyzable and condensed tannins: Chemistry, advantages and disadvantages of their intake. Tecnociencia Chihuah. 2012, 6, 84–93. [Google Scholar] [CrossRef]
- Abbasi, S.; Vahabzadeh, F.; Mehranian, M. Profiles of Phenolics and Antioxidant Activity of Pistachio Hulls During Solid-State Fermentation by Phanerochaete chrysosporium- Involvement of Lignin Peroxidase and Manganese Peroxidase. Sci. Iran. 2007, 14, 373–378. [Google Scholar]
- Król-Grzymała, A.; Amarowicz, R. Phenolic Compounds of Soybean Seeds from Two European Countries and Their Antioxidant Properties. Molecules 2020, 25, 2075. [Google Scholar] [CrossRef]
- Karimi, E.; Oskoueian, E.; Hendra, R.; Hze, J. Solid state fermentation effects on pistachio hulls antioxidant activities. Asia-Pac. J. Sci. Technol. 2010, 15, 360–366. [Google Scholar]
- Özbek, H.N.; Yanık, D.K.; Fadıloğlu, S.; Göğüş, F. Optimization of microwave-assisted extraction of bioactive compounds from pistachio (Pistacia vera L.) hull. Sep. Sci. Technol. 2019, 55, 289–299. [Google Scholar] [CrossRef]
- Erşan, S.; Güçlü Üstündağ, Ö.; Carle, R.; Schweiggert, R.M. Subcritical water extraction of phenolic and antioxidant constituents from pistachio (Pistacia vera L.) hulls. Food Chem. 2018, 253, 46–54. [Google Scholar] [CrossRef]
- Grace, M.H.; Esposito, D.; Timmers, M.A.; Xiong, J.; Yousef, G.; Komarnytsky, S.; Lila, M.A. Chemical composition, antioxidant and anti-inflammatory properties of pistachio hull extracts. Food Chem. 2016, 210, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Esparza, U.; Ochoa-Reyes, E.; Baeza-Jiménez, R.; Buenrostro-Figueroa, J.J. Efecto de la fermentación en medio sólido sobre el contenido de fenoles totales y la capacidad antioxidante del maíz. CienciaUAT 2023, 18, 136–144. [Google Scholar] [CrossRef]
- Brück, W.M.; Díaz Escobar, V.D.; Droz-dit-Busset, L.; Baudin, M.; Nicolet, N.; Andlauer, W. Fermentative Liberation of Ellagic Acid from Walnut Press Cake Ellagitannins. Foods 2022, 11, 3102. [Google Scholar] [CrossRef]
- Cerda-Cejudo, N.D.; Buenrostro-Figueroa, J.J.; Sepúlveda-Torre, L.; Torres-León, C.; Chávez-González, M.L.; Ascacio-Valdés, J.A.; Aguilar, C.N. Solid-State Fermentation for the Recovery of Phenolic Compounds from Agro-Wastes. Resources 2023, 12, 36. [Google Scholar] [CrossRef]
- Cañedo, V.; Ames, T. Manual de laboratorio para el manejo de hongos entomopatgenos; International Potato Center (CIP): Lima, Perú, 2004. [Google Scholar]
- Martínez-Ávila, G.; Ascacio-Valdés, J.; Sepúlveda, L.; Rodriguez, R.; Aguilera-Carbo, A.; Aguilar, C. Extracción Asistida por Fermentación Fúngica de Antioxidantes Fenólicos. Acta Química Mex. 2013, 5, 16–24. [Google Scholar]
- Jachimowicz, P.; Cydzik-Kwiatkowska, A.; Szklarz, P. Effect of Aeration Mode on Microbial Structure and Efficiency of Treatment of TSS-Rich Wastewater from Meat Processing. Appl. Sci. 2020, 10, 7414. [Google Scholar] [CrossRef]
- Casciatori, F.P.; Thoméo, J.C. Heat transfer in packed-beds of agricultural waste with low rates of air flow applicable to solid-state fermentation. Chem. Eng. Sci. 2018, 188, 97–111. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, L.-R.; He, H.-W.; Sang, B.; Yu, D.-L.; Feng, J.-T.; Zhang, X. Effects of Agitation, Aeration and Temperature on Production of a Novel Glycoprotein GP-1 by Streptomyces kanasenisi ZX01 and Scale-Up Based on Volumetric Oxygen Transfer Coefficient. Molecules 2018, 23, 125. [Google Scholar] [CrossRef] [PubMed]
- Ridder, E.R.; Nokes, S.E.; Knutson, B.L. Optimization of Solid-State Fermentation parameters for the production of xylanase by Trichoderma longibrachiatum on wheat bran in a forced aeration system. Trans. ASAE 1999, 42, 1785–1790. [Google Scholar] [CrossRef]
- Venereo-Gutiérrez, J.R. Daño oxidativo, radicales libres y antioxidantes. Rev. Cuba. De Med. Mil. 2002, 31, 126–133. [Google Scholar]
- Ghandahari-Yazdi, A.P.; Barzegar, M.; Sahari, M.A.; Ahmadi Gavlighi, H. Optimization of the enzyme-assisted aqueous extraction of phenolic compounds from pistachio green hull. Food Sci. Nutr. 2019, 7, 356–366. [Google Scholar] [CrossRef]
- Cheng, H.S.; Ton, S.H.; Abdul Kadir, K. Ellagitannin geraniin: A review of the natural sources, biosynthesis, pharmacokinetics and biological effects. Phytochem. Rev. 2017, 16, 159–193. [Google Scholar] [CrossRef]
- Schulze-Kaysers, N.; Feuereisen, M.M.; Schieber, A. Phenolic compounds in edible species of the Anacardiaceae family—A review. RSC Adv. 2015, 5, 73301–73314. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).