Role of Probiotics in the Management of Patients with Ulcerative Colitis and Pouchitis
Abstract
1. Introduction
2. Pouchitis
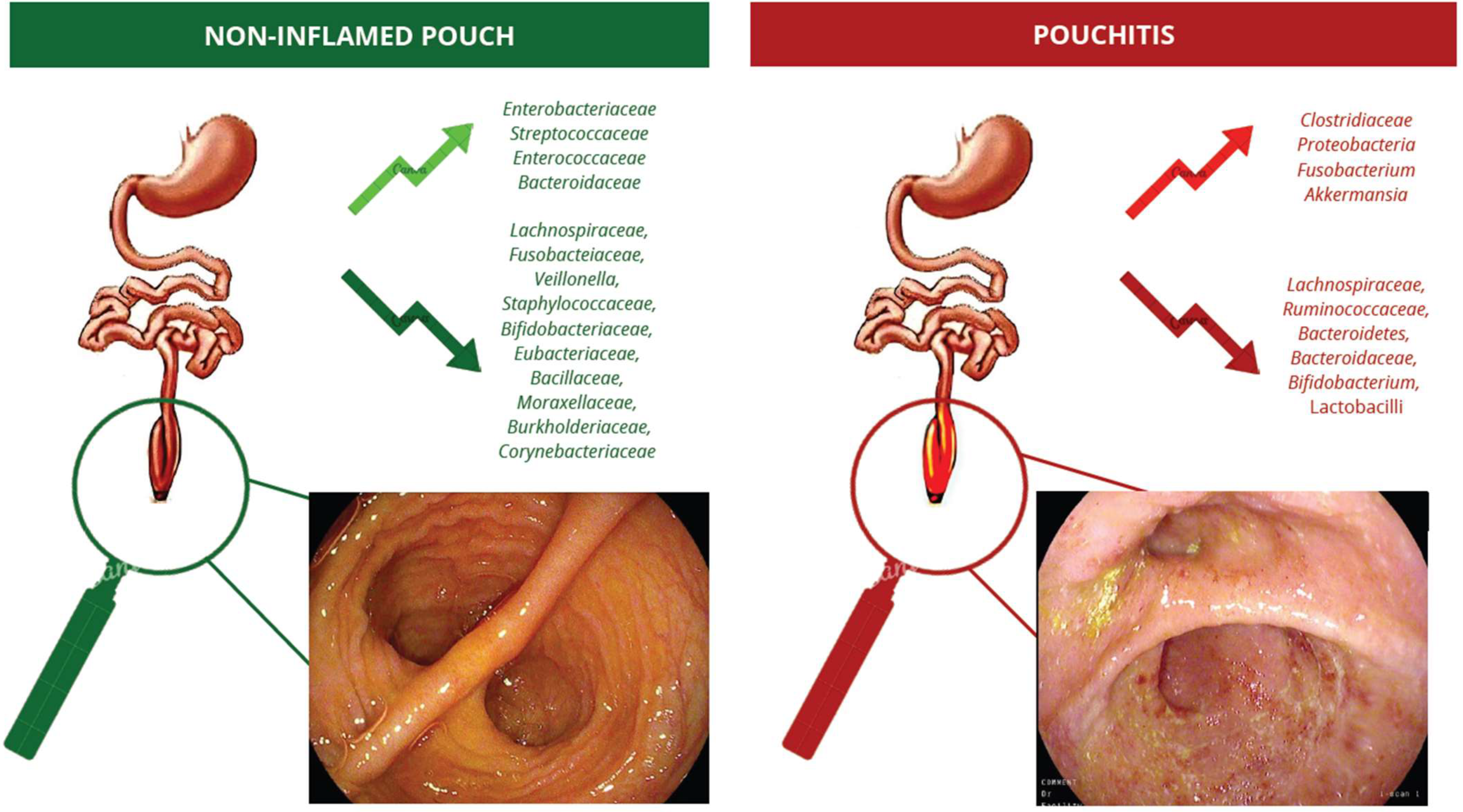
3. The Pouch’s Microbiota in Healthy Patients and Pouchitis
4. Probiotics in Pouchitis
4.1. Prevention of Primary Pouchitis
4.2. Intermittent/Active Pouchitis
4.3. Chronic Recurrent Pouchitis
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-Based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-Intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-Anal Pouch Disorders. J. Crohns Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef]
- Angriman, I.; Scarpa, M.; Castagliuolo, I. Relationship between Pouch Microbiota and Pouchitis Following Restorative Proctocolectomy for Ulcerative Colitis. World J. Gastroenterol. 2014, 20, 9665–9674. [Google Scholar] [CrossRef] [PubMed]
- Barnes, E.L.; Agrawal, M.; Syal, G.; Ananthakrishnan, A.N.; Cohen, B.L.; Haydek, J.P.; Al Kazzi, E.S.; Eisenstein, S.; Hashash, J.G.; Sultan, S.S.; et al. AGA Clinical Practice Guideline on the Management of Pouchitis and Inflammatory Pouch Disorders. Gastroenterology 2024, 166, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Reshef, L.; Kovacs, A.; Ofer, A.; Yahav, L.; Maharshak, N.; Keren, N.; Konikoff, F.M.; Tulchinsky, H.; Gophna, U.; Dotan, I. Pouch Inflammation Is Associated With a Decrease in Specific Bacterial Taxa. Gastroenterology 2015, 149, 718–727. [Google Scholar] [CrossRef]
- Pronio, A.; Montesani, C.; Butteroni, C.; Vecchione, S.; Mumolo, G.; Vestri, A.; Vitolo, D.; Boirivant, M. Probiotic Administration in Patients with Ileal Pouch-Anal Anastomosis for Ulcerative Colitis Is Associated with Expansion of Mucosal Regulatory Cells. Inflamm. Bowel Dis. 2008, 14, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Barnes, E.L.; Herfarth, H.H.; Kappelman, M.D.; Zhang, X.; Lightner, A.; Long, M.D.; Sandler, R.S. Incidence, Risk Factors, and Outcomes of Pouchitis and Pouch-Related Complications in Patients With Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1583–1591.e4. [Google Scholar] [CrossRef]
- Lightner, A.L.; Mathis, K.L.; Dozois, E.J.; Hahnsloser, D.; Loftus, E.V.; Raffals, L.E.; Pemberton, J.H. Results at Up to 30 Years after Ileal Pouch-Anal Anastomosis for Chronic Ulcerative Colitis. Inflamm. Bowel Dis. 2017, 23, 781–790. [Google Scholar] [CrossRef]
- Lohmuller, J.L.; Pemberton, J.H.; Dozois, R.R.; Ilstrup, D.; van Heerden, J. Pouchitis and Extraintestinal Manifestations of Inflammatory Bowel Disease after Ileal Pouch-Anal Anastomosis. Ann. Surg. 1990, 211, 622–627, discussion 627–629. [Google Scholar] [PubMed]
- Pavlides, M.; Cleland, J.; Rahman, M.; Christian, A.; Doyle, J.; Gaunt, R.; Travis, S.; Mortensen, N.; Chapman, R. Outcomes after Ileal Pouch Anal Anastomosis in Patients with Primary Sclerosing Cholangitis. J. Crohns Colitis 2014, 8, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Barnes, E.L.; Kochar, B.; Jessup, H.R.; Herfarth, H.H. The Incidence and Definition of Crohn’s Disease of the Pouch: A Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2019, 25, 1474–1480. [Google Scholar] [CrossRef]
- Shen, B.; Achkar, J.-P.; Connor, J.T.; Ormsby, A.H.; Remzi, F.H.; Bevins, C.L.; Brzezinski, A.; Bambrick, M.L.; Fazio, V.W.; Lashner, B.A. Modified Pouchitis Disease Activity Index: A Simplified Approach to the Diagnosis of Pouchitis. Dis. Colon Rectum 2003, 46, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.W.; Maestranzi, S.; Duffy, A.M.; Dewar, D.H.; Forbes, A.; Bjarnason, I.; Sherwood, R.A.; Ciclitira, P.; Nicholls, J.R. Faecal Calprotectin: A Noninvasive Diagnostic Tool and Marker of Severity in Pouchitis. Eur. J. Gastroenterol. Hepatol. 2008, 20, 174–179. [Google Scholar] [CrossRef]
- Yamamoto, T.; Shimoyama, T.; Bamba, T.; Matsumoto, K. Consecutive Monitoring of Fecal Calprotectin and Lactoferrin for the Early Diagnosis and Prediction of Pouchitis after Restorative Proctocolectomy for Ulcerative Colitis. Off. J. Am. Coll. Gastroenterol. 2015, 110, 881. [Google Scholar] [CrossRef] [PubMed]
- Hurst, R.D.; Molinari, M.; Chung, T.P.; Rubin, M.; Michelassi, F. Prospective Study of the Incidence, Timing and Treatment of Pouchitis in 104 Consecutive Patients after Restorative Proctocolectomy. Arch. Surg. 1996, 131, 497–500, discussion 501–502. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Fazio, V.W.; Remzi, F.H.; Lashner, B.A. Clinical Approach to Diseases of Ileal Pouch-Anal Anastomosis. Am. J. Gastroenterol. 2005, 100, 2796–2807. [Google Scholar] [CrossRef] [PubMed]
- Gilliland, A.; Chan, J.J.; De Wolfe, T.J.; Yang, H.; Vallance, B.A. Pathobionts in Inflammatory Bowel Disease: Origins, Underlying Mechanisms, and Implications for Clinical Care. Gastroenterology 2024, 166, 44–58. [Google Scholar] [CrossRef]
- Madden, M.V.; Farthing, M.J.; Nicholls, R.J. Inflammation in Ileal Reservoirs: “Pouchitis”. Gut 1990, 31, 247–249. [Google Scholar] [CrossRef]
- Gosselink, M.P.; Schouten, W.R.; van Lieshout, L.M.C.; Hop, W.C.J.; Laman, J.D.; Ruseler-van Embden, J.G.H. Eradication of Pathogenic Bacteria and Restoration of Normal Pouch Flora: Comparison of Metronidazole and Ciprofloxacin in the Treatment of Pouchitis. Dis. Colon. Rectum. 2004, 47, 1519–1525. [Google Scholar] [CrossRef]
- McLaughlin, S.D.; Clark, S.K.; Tekkis, P.P.; Nicholls, R.J.; Ciclitira, P.J. The Bacterial Pathogenesis and Treatment of Pouchitis. Ther. Adv. Gastroenterol. 2010, 3, 335. [Google Scholar] [CrossRef]
- Tyler, A.D.; Knox, N.; Kabakchiev, B.; Milgrom, R.; Kirsch, R.; Cohen, Z.; McLeod, R.S.; Guttman, D.S.; Krause, D.O.; Silverberg, M.S. Characterization of the Gut-Associated Microbiome in Inflammatory Pouch Complications Following Ileal Pouch-Anal Anastomosis. PLoS ONE 2013, 8, e66934. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, F.; D’Amico, F.; Bencardino, S.; Faggiani, I.; Fanizza, J.; Zilli, A.; Parigi, T.L.; Allocca, M.; Danese, S.; Furfaro, F. Gut Microbiota Metabolites: Unveiling Their Role in Inflammatory Bowel Diseases and Fibrosis. Pharmaceuticals 2024, 17, 347. [Google Scholar] [CrossRef] [PubMed]
- Tannock, G.W.; Lawley, B.; Munro, K.; Lay, C.; Taylor, C.; Daynes, C.; Baladjay, L.; Mcleod, R.; Thompson-Fawcett, M. Comprehensive Analysis of the Bacterial Content of Stool from Patients with Chronic Pouchitis, Normal Pouches, or Familial Adenomatous Polyposis Pouches. Inflamm. Bowel Dis. 2012, 18, 925–934. [Google Scholar] [CrossRef]
- Kohyama, A.; Ogawa, H.; Funayama, Y.; Takahashi, K.-I.; Benno, Y.; Nagasawa, K.; Tomita, S.-I.; Sasaki, I.; Fukushima, K. Bacterial Population Moves toward a Colon-like Community in the Pouch after Total Proctocolectomy. Surgery 2009, 145, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Hinata, M.; Kohyama, A.; Ogawa, H.; Haneda, S.; Watanabe, K.; Suzuki, H.; Shibata, C.; Funayama, Y.; Takahashi, K.-I.; Sasaki, I.; et al. A Shift from Colon- to Ileum-Predominant Bacteria in Ileal-Pouch Feces Following Total Proctocolectomy. Dig. Dis. Sci. 2012, 57, 2965–2974. [Google Scholar] [CrossRef]
- Liu, W.T.; Marsh, T.L.; Cheng, H.; Forney, L.J. Characterization of Microbial Diversity by Determining Terminal Restriction Fragment Length Polymorphisms of Genes Encoding 16S rRNA. Appl. Environ. Microbiol. 1997, 63, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Falk, A.; Olsson, C.; Ahrné, S.; Molin, G.; Adawi, D.; Jeppsson, B. Ileal Pelvic Pouch Microbiota from Two Former Ulcerative Colitis Patients, Analysed by DNA-Based Methods, Were Unstable over Time and Showed the Presence of Clostridium Perfringens. Scand. J. Gastroenterol. 2007, 42, 973–985. [Google Scholar] [CrossRef]
- Abdelrazeq, A.S.; Kandiyil, N.; Botterill, I.D.; Lund, J.N.; Reynolds, J.R.; Holdsworth, P.J.; Leveson, S.H. Predictors for Acute and Chronic Pouchitis Following Restorative Proctocolectomy for Ulcerative Colitis. Color. Dis. 2008, 10, 805–813. [Google Scholar] [CrossRef]
- Madden, M.V.; McIntyre, A.S.; Nicholls, R.J. Double-Blind Crossover Trial of Metronidazole versus Placebo in Chronic Unremitting Pouchitis. Dig. Dis. Sci. 1994, 39, 1193–1196. [Google Scholar] [CrossRef]
- Gionchetti, P.; Rizzello, F.; Venturi, A.; Ugolini, F.; Rossi, M.; Brigidi, P.; Johansson, R.; Ferrieri, A.; Poggioli, G.; Campieri, M. Antibiotic Combination Therapy in Patients with Chronic, Treatment-Resistant Pouchitis. Aliment. Pharmacol. Ther. 1999, 13, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Gionchetti, P.; Rizzello, F.; Helwig, U.; Venturi, A.; Lammers, K.M.; Brigidi, P.; Vitali, B.; Poggioli, G.; Miglioli, M.; Campieri, M. Prophylaxis of Pouchitis Onset with Probiotic Therapy: A Double-Blind, Placebo-Controlled Trial. Gastroenterology 2003, 124, 1202–1209. [Google Scholar] [CrossRef]
- Gionchetti, P.; Rizzello, F.; Venturi, A.; Brigidi, P.; Matteuzzi, D.; Bazzocchi, G.; Poggioli, G.; Miglioli, M.; Campieri, M. Oral Bacteriotherapy as Maintenance Treatment in Patients with Chronic Pouchitis: A Double-Blind, Placebo-Controlled Trial. Gastroenterology 2000, 119, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Okita, Y.; Araki, T.; Tanaka, K.; Hashimoto, K.; Kondo, S.; Kawamura, M.; Koike, Y.; Otake, K.; Fujikawa, H.; Inoue, M.; et al. Predictive Factors for Development of Chronic Pouchitis after Ileal Pouch-Anal Anastomosis in Ulcerative Colitis. Digestion 2013, 88, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium Nucleatum Potentiates Intestinal Tumorigenesis and Modulates the Tumor-Immune Microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef]
- Zella, G.C.; Hait, E.J.; Glavan, T.; Gevers, D.; Ward, D.V.; Kitts, C.L.; Korzenik, J.R. Distinct Microbiome in Pouchitis Compared to Healthy Pouches in Ulcerative Colitis and Familial Adenomatous Polyposis. Inflamm. Bowel Dis. 2011, 17, 1092–1100. [Google Scholar] [CrossRef]
- Fleshner, P.R.; Vasiliauskas, E.A.; Kam, L.Y.; Fleshner, N.E.; Gaiennie, J.; Abreu-Martin, M.T.; Targan, S.R. High Level Perinuclear Antineutrophil Cytoplasmic Antibody (pANCA) in Ulcerative Colitis Patients before Colectomy Predicts the Development of Chronic Pouchitis after Ileal Pouch-Anal Anastomosis. Gut 2001, 49, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Sagar, P.; Finan, P.; Burke, D.; Schuster, H. Dysbiosis and Pouchitis. Br. J. Surg. 2006, 93, 1325–1334. [Google Scholar] [CrossRef]
- Mimura, T.; Rizzello, F.; Helwig, U.; Poggioli, G.; Schreiber, S.; Talbot, I.C.; Nicholls, R.J.; Gionchetti, P.; Campieri, M.; Kamm, M.A. Four-Week Open-Label Trial of Metronidazole and Ciprofloxacin for the Treatment of Recurrent or Refractory Pouchitis. Aliment. Pharmacol. Ther. 2002, 16, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, M.; Grillo, A.; Pozza, A.; Faggian, D.; Ruffolo, C.; Scarpa, M.; D’Incà, R.; Plebani, M.; Sturniolo, G.C.; Castagliuolo, I.; et al. TLR2 and TLR4 Up-Regulation and Colonization of the Ileal Mucosa by Clostridiaceae Spp. in Chronic/Relapsing Pouchitis. J. Surg. Res. 2011, 169, E145–E154. [Google Scholar] [CrossRef] [PubMed]
- Nasmyth, D.G.; Godwin, P.G.; Dixon, M.F.; Williams, N.S.; Johnston, D. Ileal Ecology after Pouch-Anal Anastomosis or Ileostomy. A Study of Mucosal Morphology, Fecal Bacteriology, Fecal Volatile Fatty Acids, and Their Interrelationship. Gastroenterology 1989, 96, 817–824. [Google Scholar] [CrossRef]
- Onderdonk, A.B.; Dvorak, A.M.; Cisneros, R.L.; McLeod, R.S.; Antionoli, D.; Silen, W.; Blair, J.E.; Monahan-Earley, R.A.; Cullen, J.; Cohen, Z. Microbiologic Assessment of Tissue Biopsy Samples from Ileal Pouch Patients. J. Clin. Microbiol. 1992, 30, 312–317. [Google Scholar] [CrossRef]
- Komanduri, S.; Gillevet, P.M.; Sikaroodi, M.; Mutlu, E.; Keshavarzian, A. Dysbiosis in Pouchitis: Evidence of Unique Microfloral Patterns in Pouch Inflammation. Clin. Gastroenterol. Hepatol. 2007, 5, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; St. Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-Phylogenetic Characterization of Microbial Community Imbalances in Human Inflammatory Bowel Diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [PubMed]
- Gophna, U.; Sommerfeld, K.; Gophna, S.; Doolittle, W.F.; Veldhuyzen van Zanten, S.J.O. Differences between Tissue-Associated Intestinal Microfloras of Patients with Crohn’s Disease and Ulcerative Colitis. J. Clin. Microbiol. 2006, 44, 4136–4141. [Google Scholar] [CrossRef]
- Scarpa, M.; Grillo, A.; Faggian, D.; Ruffolo, C.; Bonello, E.; D’Incà, R.; Scarpa, M.; Castagliuolo, I.; Angriman, I. Relationship between Mucosa-Associated Microbiota and Inflammatory Parameters in the Ileal Pouch after Restorative Proctocolectomy for Ulcerative Colitis. Surgery 2011, 150, 56–67. [Google Scholar] [CrossRef]
- Fleshner, P.; Ippoliti, A.; Dubinsky, M.; Vasiliauskas, E.; Mei, L.; Papadakis, K.A.; Rotter, J.I.; Landers, C.; Targan, S. Both Preoperative Perinuclear Antineutrophil Cytoplasmic Antibody and Anti-CBir1 Expression in Ulcerative Colitis Patients Influence Pouchitis Development after Ileal Pouch-Anal Anastomosis. Clin. Gastroenterol. Hepatol. 2008, 6, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Targan, S.R.; Landers, C.J.; Yang, H.; Lodes, M.J.; Cong, Y.; Papadakis, K.A.; Vasiliauskas, E.; Elson, C.O.; Hershberg, R.M. Antibodies to CBir1 Flagellin Define a Unique Response That Is Associated Independently with Complicated Crohn’s Disease. Gastroenterology 2005, 128, 2020–2028. [Google Scholar] [CrossRef]
- Barrett, J.C.; Hansoul, S.; Nicolae, D.L.; Cho, J.H.; Duerr, R.H.; Rioux, J.D.; Brant, S.R.; Silverberg, M.S.; Taylor, K.D.; Barmada, M.M.; et al. Genome-Wide Association Defines More than 30 Distinct Susceptibility Loci for Crohn’s Disease. Nat. Genet. 2008, 40, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Einerhand, A.W.C.; Renes, I.B.; Makkink, M.K.; van der Sluis, M.; Büller, H.A.; Dekker, J. Role of Mucins in Inflammatory Bowel Disease: Important Lessons from Experimental Models. Eur. J. Gastroenterol. Hepatol. 2002, 14, 757–765. [Google Scholar] [CrossRef]
- Kyo, K.; Parkes, M.; Takei, Y.; Nishimori, H.; Vyas, P.; Satsangi, J.; Simmons, J.; Nagawa, H.; Baba, S.; Jewell, D.; et al. Association of Ulcerative Colitis with Rare VNTR Alleles of the Human Intestinal Mucin Gene, MUC3. Hum. Mol. Genet. 1999, 8, 307–311. [Google Scholar] [CrossRef]
- Parker, N.; Tsai, H.H.; Ryder, S.D.; Raouf, A.H.; Rhodes, J.M. Increased Rate of Sialylation of Colonic Mucin by Cultured Ulcerative Colitis Mucosal Explants. Digestion 1995, 56, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Tytgat, K.M.; van der Wal, J.W.; Einerhand, A.W.; Büller, H.A.; Dekker, J. Quantitative Analysis of MUC2 Synthesis in Ulcerative Colitis. Biochem. Biophys. Res. Commun. 1996, 224, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeir, J.; Vrese, M. de Probiotics, Prebiotics, and Synbiotics—Approaching a Definition123. Am. J. Clin. Nutr. 2001, 73, 361s–364s. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-K.; Salminen, S. The Coming of Age of Probiotics. Trends Food Sci. Technol. 1995, 6, 241–245. [Google Scholar] [CrossRef]
- Ng, S.C.; Hart, A.L.; Kamm, M.A.; Stagg, A.J.; Knight, S.C. Mechanisms of Action of Probiotics: Recent Advances. Inflamm. Bowel Dis. 2009, 15, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Yasueda, A.; Mizushima, T.; Nezu, R.; Sumi, R.; Tanaka, M.; Nishimura, J.; Kai, Y.; Hirota, M.; Osawa, H.; Nakajima, K.; et al. The Effect of Clostridium Butyricum MIYAIRI on the Prevention of Pouchitis and Alteration of the Microbiota Profile in Patients with Ulcerative Colitis. Surg. Today 2016, 46, 939–949. [Google Scholar] [CrossRef]
- Bifidobacterium Longum BB-536 and Prevention of Acute Pouchitis Cochrane Library. Available online: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01262758/full (accessed on 12 August 2024).
- Gosselink, M.P.; Schouten, W.R.; van Lieshout, L.M.C.; Hop, W.C.J.; Laman, J.D.; Ruseler-van Embden, J.G.H. Delay of the First Onset of Pouchitis by Oral Intake of the Probiotic Strain Lactobacillus Rhamnosus GG. Dis. Colon Rectum 2004, 47, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Gionchetti, P.; Rizzello, F.; Morselli, C.; Poggioli, G.; Tambasco, R.; Calabrese, C.; Brigidi, P.; Vitali, B.; Straforini, G.; Campieri, M. High-Dose Probiotics for the Treatment of Active Pouchitis. Dis. Colon Rectum 2007, 50, 2075–2082, 2082–2084. [Google Scholar] [CrossRef] [PubMed]
- Laake, K.O.; Line, P.D.; Aabakken, L.; Løtveit, T.; Bakka, A.; Eide, J.; Røsetti, A.; Grzyb, K.; Bjørneklett, A.; Vatn, M.H. Assessment of Mucosal Inflammation and Circulation in Response to Probiotics in Patients Operated with Ileal Pouch Anal Anastomosis for Ulcerative Colitis. Scand. J. Gastroenterol. 2003, 38, 409–414. [Google Scholar] [CrossRef]
- Kuisma, J.; Mentula, S.; Jarvinen, H.; Kahri, A.; Saxelin, M.; Farkkila, M. Effect of Lactobacillus Rhamnosus GG on Ileal Pouch Inflammation and Microbial Flora. Aliment. Pharmacol. Ther. 2003, 17, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Mimura, T.; Rizzello, F.; Helwig, U.; Poggioli, G.; Schreiber, S.; Talbot, I.C.; Nicholls, R.J.; Gionchetti, P.; Campieri, M.; Kamm, M.A. Once Daily High Dose Probiotic Therapy (VSL#3) for Maintaining Remission in Recurrent or Refractory Pouchitis. Gut 2004, 53, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Ulisse, S.; Gionchetti, P.; D’Alò, S.; Russo, F.P.; Pesce, I.; Ricci, G.; Rizzello, F.; Helwig, U.; Cifone, M.G.; Campieri, M.; et al. Expression of Cytokines, Inducible Nitric Oxide Synthase, and Matrix Metalloproteinases in Pouchitis: Effects of Probiotic Treatment. Am. J. Gastroenterol. 2001, 96, 2691–2699. [Google Scholar] [CrossRef]
- Herfarth, H.; Barnes, E.L.; Long, M.D.; Isaacs, K.L.; Leith, T.; Silverstein, M.; Gerardin, Y.; Kassam, Z. Combined Endoscopic and Oral Fecal Microbiota Transplantation in Patients with Antibiotic-Dependent Pouchitis: Low Clinical Efficacy Due to Low Donor Microbial Engraftment. Inflamm. Intest. Dis. 2019, 4, 1–6. [Google Scholar] [CrossRef]
- Karjalainen, E.K.; Renkonen-Sinisalo, L.; Satokari, R.; Mustonen, H.; Ristimäki, A.; Arkkila, P.; Lepistö, A.H. Fecal Microbiota Transplantation in Chronic Pouchitis: A Randomized, Parallel, Double-Blinded Clinical Trial. Inflamm. Bowel Dis. 2021, 27, 1766–1772. [Google Scholar] [CrossRef]
- Palmieri, O.; Castellana, S.; Biscaglia, G.; Panza, A.; Latiano, A.; Fontana, R.; Guerra, M.; Corritore, G.; Latiano, T.; Martino, G.; et al. Microbiome Analysis of Mucosal Ileoanal Pouch in Ulcerative Colitis Patients Revealed Impairment of the Pouches Immunometabolites. Cells 2021, 10, 3243. [Google Scholar] [CrossRef] [PubMed]
- de Silva, H.J.; Millard, P.R.; Kettlewell, M.; Mortensen, N.J.; Prince, C.; Jewell, D.P. Mucosal Characteristics of Pelvic Ileal Pouches. Gut 1991, 32, 61–65. [Google Scholar] [CrossRef]
- Moutsoglou, D.; Vaughn, B.P. Rethinking Faecal Microbiota Transplant for Pouchitis. J. Crohns Colitis 2024, 18, 1739–1740. [Google Scholar] [CrossRef]
- van der Lelie, D.; Oka, A.; Taghavi, S.; Umeno, J.; Fan, T.-J.; Merrell, K.E.; Watson, S.D.; Ouellette, L.; Liu, B.; Awoniyi, M.; et al. Rationally Designed Bacterial Consortia to Treat Chronic Immune-Mediated Colitis and Restore Intestinal Homeostasis. Nat. Commun. 2021, 12, 3105. [Google Scholar] [CrossRef]
- Timmis, K.; Timmis, J.K.; Brüssow, H.; Fernández, L.Á. Synthetic Consortia of Nanobody-Coupled and Formatted Bacteria for Prophylaxis and Therapy Interventions Targeting Microbiome Dysbiosis-Associated Diseases and Co-Morbidities. Microb. Biotechnol. 2019, 12, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Walana, W.; Ye, Y.; Li, M.; Wang, J.; Wang, B.; Cheng, J.-W.; Gordon, J.R.; Li, F. IL-8 Antagonist, CXCL8(3-72)K11R/G31P Coupled with Probiotic Exhibit Variably Enhanced Therapeutic Potential in Ameliorating Ulcerative Colitis. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 103, 253–261. [Google Scholar] [CrossRef] [PubMed]
| Authors | Sample Size | Study Design and Endpoints | Main Outcomes |
|---|---|---|---|
| Nasmyth et al. (1989) [39] | 29 UC patients (15 with pouch and 14 with ileostomy) | Comparison between ileal ecology after pouch–anal anastomosis or ileostomy | Pouch vs. ileostomy: 8.3 vs. 9 Jog10 cfu/mL of total aerobes (p < 0.05) Pouch vs. ileostomy: 10.6 vs. 9.6 Jog10 cfu/mL of total anaerobes (p < 0.05) Pouch vs. ileostomy: 9.8 vs. 5.7 Jog10 cfu/mL of Bacteroides (p < 0.01) Pouch vs. ileostomy: 8.7 vs. 0 Jog10 cfu/mL of Bifidobacteria (p < 0.05) |
| Onderdonk et al. (1992) [40] | 78 patients with pouch or ileostomy | Comparison in microbiologic cultures from normal pouch, pouchitis, indeterminate, normal, and ileostomy patients | OA negative: 4/23 normal pouch vs. 6/12 pouchitis (p < 0.043) FA negative: 6/23 normal pouch (p < 0.006) vs. 0/12 pouchitis (p < 0.05) vs. 5/14 indeterminate (p < 0.07) vs. 5/9 normal (p < 0.00) vs. 8/20 ileostomy (p < 0.03) |
| Komanduri et al. (2007) [41] | 20 UC patients with pouch | Microfloral patterns characterization in pouchitis | Clostridium paraputrificum % composition in pouch control: 0.08 Streptococcus sp % composition in pouch control: 0.34 Fusobacterium varium % composition in pouchitis: 0.12 |
| Zella et al. (2011) [34] | 19 patients (9 UCP, 3 HUC, 7 FAP) | Cross-sectional study: comparison of mucosal and luminal flora in UCP, HUC, and FAP pouches using TRFLP and DNA sanger sequencing | Lactobacillus and Streptococcus TRFLP in FAP vs. UCP at a ratio of 5:1 in mucosa, 3:1 in stool Clostridium, Eubacterium, and Roseburia in HUC vs. UCP at a ratio of 1:15 and in UCP vs. FAP at a ratio of 5:1 Escherichia, Streptococcus, and various sulfur-oxidizing bacteria in HUC vs. UCP at a ratio of 1:2 UCP vs. FAP: Firmicutes (51.2% vs. 21.2%) and Verrucomicrobia (20.2% vs. 3.2%), Bacteroidetes (17.9% vs. 60.5%) and Proteobacteria (9.8% vs. 14.7%) |
| Reshef et al. (2015) [4] | 140 pouch patients (131 UC and 9 FAP) | Prospective study: correlations of microbiota to the PDAI with 16S rRNA gene amplicon pyrosequencing | (*) Coriobacteriaceae, Collinsella R = −0.611, p = 0.001; Lachnospiraceae, Roseburia R = −0.539, p = 0.002 Bacteroidaceae, Bacteroides R = −0.487, p = 0.006 Ruminococcaceae R = −0.466, p = 0.008 Lachnospiraceae, Eubacterium R = −0.457, p = 0.008; Lachnospiraceae, Coprococcus R = −0.414, p = 0.016; Lachnospiraceae, Clostridium R = −0.414, p = 0.016; Veillonellaceae, Megamonas R = −0.401, p = 0.018; Lachnospiraceae, R = −0.367, p = 0.033; Fusobacteriaceae, Fusobacterium R = 0.352, p = 0.039; Ruminococcacea, Faecalibacterium R = −0.345, p = 0.041; Lachnospiraceae, Ruminococcus R = −0.334, p = 0.046 |
| Author | Duration | Probiotic | Control | Remission (Probiotic; Placebo) | p |
|---|---|---|---|---|---|
| Prevention of pouchitis | |||||
| Yasueda et al. [55] | 12 months | Clostridium butyricum | Placebo | 1/9; 4/8 | 0.07 |
| Gionchetti et al. [30] | 12 months | De Simone formulation | Placebo | 2/20; 8/20 | <0.05 |
| Brown et al. [56] | 6 months | Bifidobacterium longum BB-536 | Placebo | 1/7; 2/5 | NA |
| Gosselink et al. [57] | >36 months | Lactobacillus rhamnosus GG | Placebo | 3/39; 27/78 | NA |
| Pronio et al. [5] | 12 months | De Simone formulation | No treatment | 12, ↓ PDAI; 7, =PDAI | <0.01 |
| Intermittent/Active pouchitis | |||||
| Gionchetti et al. [58] | 1 month | De Simone formulation | Open-label | 16/23 | <0.01 |
| Laake et al. [59] | 1 month | Lactobacillus (La-5) and Bifidobacterium (Bb-12) | Open-label | 5/10 ↓ endoscopic score | NA |
| Kuisma et al. [60] | 3 months | Lactobacillus rhamnosus GG | Placebo | 1/10; 0/10 | NA |
| Chronic recurrent pouchitis | |||||
| Gionchetti et al. [31] | 9 months | De Simone formulation | Placebo | 17/20; 20/20 | <0.01 |
| Mimura et al. [61] | 12 months | De Simone formulation | Placebo | 17/20; 15/16 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardi, F.; Fanizzi, F.; Parigi, T.L.; Zilli, A.; Allocca, M.; Furfaro, F.; Peyrin-Biroulet, L.; Danese, S.; D’Amico, F. Role of Probiotics in the Management of Patients with Ulcerative Colitis and Pouchitis. Microorganisms 2025, 13, 19. https://doi.org/10.3390/microorganisms13010019
Bernardi F, Fanizzi F, Parigi TL, Zilli A, Allocca M, Furfaro F, Peyrin-Biroulet L, Danese S, D’Amico F. Role of Probiotics in the Management of Patients with Ulcerative Colitis and Pouchitis. Microorganisms. 2025; 13(1):19. https://doi.org/10.3390/microorganisms13010019
Chicago/Turabian StyleBernardi, Francesca, Fabrizio Fanizzi, Tommaso Lorenzo Parigi, Alessandra Zilli, Mariangela Allocca, Federica Furfaro, Laurent Peyrin-Biroulet, Silvio Danese, and Ferdinando D’Amico. 2025. "Role of Probiotics in the Management of Patients with Ulcerative Colitis and Pouchitis" Microorganisms 13, no. 1: 19. https://doi.org/10.3390/microorganisms13010019
APA StyleBernardi, F., Fanizzi, F., Parigi, T. L., Zilli, A., Allocca, M., Furfaro, F., Peyrin-Biroulet, L., Danese, S., & D’Amico, F. (2025). Role of Probiotics in the Management of Patients with Ulcerative Colitis and Pouchitis. Microorganisms, 13(1), 19. https://doi.org/10.3390/microorganisms13010019








