Abstract
Prokaryotes play a key role in particulate organic matter’s decomposition and remineralization processes in the vertical scale of seawater, and prokaryotes contribute to more than 70% of the estimated remineralization. However, little is known about the microbial community and metabolic activity of the vertical distribution in the trenches. The composition and distribution of prokaryotes in the water columns and benthic boundary layers of the Kermadec Trench and the Diamantina Trench were investigated using high-throughput sequencing and quantitative PCR, together with the Biolog EcoplateTM microplates culture to analyze the microbial metabolic activity. Microbial communities in both trenches were dominated by Nitrososphaera and Halobacteria in archaea, and by Alphaproteobacteria and Gammaproteobacteria in bacteria, and the microbial community structure was significantly different between the water column and the benthic boundary layer. At the surface water, amino acids and polymers were used preferentially; at the benthic boundary layers, amino acids and amines were used preferentially. Cooperative relationships among different microbial groups and their carbon utilization capabilities could help to make better use of various carbon sources along the water depths, reflected by the predominantly positive relationships based on the co-occurrence network analysis. In addition, the distinct microbial metabolic activity detected at 800 m, which was the lower boundary of the twilight zone, had the lowest salinity and might have had higher proportions of refractory carbon sources than the shallower water depths and benthic boundary layers. This study reflected the initial preference of the carbon source by the natural microbes in the vertical scale of different trenches and should be complemented with stable isotopic tracing experiments in future studies to enhance the understanding of the complex carbon utilization pathways along the vertical scale by prokaryotes among different trenches.
1. Introduction
Prokaryotes play a crucial role in remineralizing and transforming significant amounts of different types of organic matter that exist in seawater, including suspended sediment particles, phytoplankton debris, living plankton, zooplankton fecal materials, aggregates, marine snow, transparent polymeric particles, colloidal particles, and so on [1,2]. This microbially transformed carbon may influence carbon export efficiency by facilitating aggregation/disaggregation activities, depolymerization and degradation [3,4]. Most particulate organic matter (POM) generated from the euphotic zone is degraded and remineralized while sinking through the water column (WC), leading to about 1–40% organic matter transported vertically from the surface ocean to the deep ocean [5,6]. Although the majority of organic matter is consumed in the photic zone, the fate of the sinking particles that reach the deep sea are largely determined by the abundance, diversity, and metabolic activity of microbial communities along the water depth profile [5].
The physicochemical conditions in the ocean WC are not uniform, but varied with increasing depths [7], providing strong selective pressures on microbial communities along the vertical WC [8,9]. These deep-sea communities can differ from the waters above and showed different microbial diversity and metabolic rates [10]. These variations may reflect carbon source availability [11] and dispersal limitation [12]. In addition, the benthic boundary layer (BBL), defined as the bottom layer of the water column directly adjacent to the seabed [13], contains a high concentration of particles resuspended from subsurface sediments [14]. The carbon sources and compositions in the BBL were reported to be distinctly different from the sinking POM and, thus, might affect the diversity and metabolic function of the microbial community. Consequently, molecular ecological studies on microbes from the euphotic layer to the BBL would contribute to understanding the microbially driven organic carbon transformations, as well as linking specific microbial groups involved in the relevant metabolic processes in different water layers.
The Biolog EcoPlatesTM method is a simple and sensitive way to reveal the functional diversity of microbial communities by relying on their microbial capability to use various carbon sources. It has been used to assess the metabolic activity of microbes in different water depths of the South China Sea [15] and surface sediments of the Mariana Trench [16]. But there is still a lack of direct evidence of carbon source utilization by microbial groups from the euphotic zone to abyssal depths. Carbon sources and compositions of organic carbon have been reported to vary with different water depths [17]; therefore, culture-dependent EcoPlates cultivation together with culture-independent molecular study would help to obtain a quick glimpse of the shift in the ecological roles of microbial communities from the euphotic layer to the BBL.
Hadal trenches are unique deep-sea environments with distinct benthic communities, due to their steep topography and periodic disturbance by turbidity flows [18]. The Kermadec Trench is located about 120 km off the northeastern coast of New Zealand in the Southern Hemisphere. It reaches a maximum depth of 10,047 m, making it the fifth deepest trench [19]. It is 1500 km long, with a mean width of 60 km and exhibits the characteristic V-shape cross section common to hadal trenches. The Diamantina Trench is located in the Indian Ocean and has a maximum depth of approximately 8047 m. It is approximately 520 km long and 70 km wide, running in a northeast–southwest direction. It is around 1500 km west of Perth, Australia. As part of the broader coordinated effort to explore the biogeochemistry and ecology of different carbon sources along the vertical WC in hadal trenches, prokaryotes were collected from five water depths, together with four samples from the BBL in the Kermadec and Diamantina Trenches, respectively. The diversity and composition of the microbial communities were studied with high-throughput sequencing and quantitative PCR (qPCR). The Biolog EcoPlatesTM method was also applied to investigate the carbon metabolic capability of microbes. This comparative study will contribute to a better understanding of the shifts and connectivity in the diversity and specific carbon metabolic capabilities of microbial communities from the surface seawater to the BBL in the Kermadec and Diamantina Trenches.
2. Materials and Methods
2.1. Sample Collection and Environmental Factor Measurement
Seawater samples were collected from the Kermadec and Diamantina Trenches during cruise TS29 from November 2022 to March 2023 (Figure 1). Niskin bottles (General Oceanics, Miami, FL, USA) were used to collect WC samples from five different depths (i.e., 0 m, 200 m, 800 m, 2000 m, and 5000 m). BBL samples were collected by the R/V “Fen Dou Zhe” from another eight stations with water depths ranging from 2311 to 9639 m (Table 1). Approximately 2 L of seawater was filtered through 200 μm mesh and was then sequentially filtered through the 3 and 0.22 μm pore size polycarbonate filter (47 mm, EMD Millipore, Billerica, MA, USA). All the filters were then flash frozen and stored at −80 °C until DNA extraction in the laboratory. The in situ environmental parameters (temperature, salinity, and depth) were recorded with a conductivity–temperature–depth (CTD, Sea-Bird Electronics, Bellevue, WA, USA) and the CTD (SBE-49, Seabird Electronics) in the manned submersible the R/V “Fen Dou Zhe”. The concentrations of inorganic nutrients (NO3− + NO2−, NH4+ and PO43−) were analyzed using an auto-analyzer (QuAAtro, Blue Tech Co., Ltd., Tokyo, Japan).
Figure 1.
Map of the sampling stations used in this study.

Table 1.
Sequencing information and diversity parameters of the 16S rRNA gene in this study.
2.2. Microbial Metabolic Activity Analyses
Water samples were filtered through 3 μm pore size polycarbonate filters, and approximately 150 μL of each filtrate was added to each well of the Biolog EcoplateTM microplates. The Ecoplates for samples collected from 0 m were incubated at room temperature (~25 °C), those from 200 m were incubated at room temperature without light (one layer of black plastic bag), and those from the other depths were incubated at ~4 °C without light (two layers of black plastic bag). The absorbance of each well was measured at 590 nm and 750 nm every 24 h for a total of 100 days of continuous cultivation. The difference in absorbance value was used to characterize the color change of the Biolog EcoplateTM microplates by eliminating the change in turbidity caused by fungi at 750 nm. The average well color development (AWCD) was calculated to determine the utilization of carbon sources and metabolism characteristics.
In Equation (1), R is the absorbance of each well, C is the absorbance of the control well, and n is the number of substrates present in the particular category [20]. Meanwhile, the richness (R), Simpson (D), and Shannon (H) indices were calculated to reflect the functional diversity of the microbial community. The richness index refers to the number of oxidized substrates. It was calculated as the sum of the ODi value of cells which were at least 0.5 after incubation [21], where ODi is the corrected OD value of each individual well, in two consecutive measurements at two different measurement times for tn and tn + 1.
The Simpson index (D) was calculated using the following Equation (2):
where N is the number of substrates and Pi is the relative color development of the well over the total color development of each well of a plate [22].
The Shannon index was calculated according to the following Equation (4):
where Pi and N are the same as in the calculation of the Simpson index [23].
2.3. DNA Extraction, PCR Amplification, and Sequencing
Genomic DNA was extracted from 0.22 μm pore sized polycarbonate filters using a PureLink Genomic DNA Mini Kit (Invitrogen, Thermo Fisher Scientific, Corp., Carlsbad, CA, USA). The DNA was amplified using PCR with universal prokaryotic primers of Pro341F (5′-CCTACGGGNBGCASCAG-3′) and Pro805R (5′-GACTACNVGGGTATCTAATCC-3′) [24], which target the V3–V4 region of the 16S rRNA gene. The primers were tagged with a 6 bp barcode to distinguish amplicons in the pools of all samples multiplexed for Illumina sequencing. PCR amplification was performed in triplicate on a BIO-RAD C1000 TouchTM Thermal Cycler PCR System in a 20 μL PCR reaction mix, containing 2.0 μL 10 × PCR-MgCl2 buffer, 0.7 μL 2.5 mM dNTPs, 0.7 μL MgCl2, 0.8 μL forward primer, 0.8 μL reverse primer, 0.2 μL Platinum® TaqDNA polymerase, 2.5 μL template DNA, and 12.3 μL ddH2O. Thermal cycling was performed at 95 °C for 3 min, followed by 33 cycles at 95 °C for 0.5 min, 55 °C for 45 s, 72 °C for 30 s, and a final extension at 72 °C for 8 min. Double-distilled water was used as a negative control. Amplification and paired-end sequencing of the amplicons were then performed with an Illumina HiSeq PE250 sequencer (Novogene Co., Ltd., Beijing, China, www.novogene.com, accessed on 5 March 2024).
2.4. Quantitative PCR
The abundance of the 16S rRNA gene was quantified using real-time quantitative PCR via a StepOnePlus Real-Time PCR system (Applied Biosystems Inc. Carlsbad, CA, USA). Each qPCR reaction comprised 10 μL 2 × SYBR® Premix Ex TaqTMII (Takara Bio. Inc., Shiga, Japan), 0.3 μM Uni340F (5′-CCTACGGGRBGCASCAG-3′)/Uni806R (5′-GGACTACNNGGGTATCTAAT-3′) primers [25], 2 μL DNA as the template, 0.4 μL ROX reference dye, and water to a total of 20 μL. Quantitative PCR reactions and calibrations were performed as previously reported [25]. Triplicate qPCR reactions were performed for each sample with an efficiency of ~101.7% and the gene copy number was normalized to gene abundance.
2.5. Bioinformatics Analysis
After sequencing, barcoded and low-quality sequences were removed using QIIME 2 with default parameters [26]. Chimeras were detected and removed using UCHIME against the SILVA database release 128 [27] and reads presented as a single copy (i.e., singletons) were manually removed. The remaining reads were then clustered into the Amplicon Sequences Variant (ASV) using DADA2 (Divisive Amplicon Denoising Algorithm) [28]. Taxonomy assignment of ASVs that were not affiliated with prokaryotes, as determined from the SILVA database release 138, were further removed [26]. A filtered ASVs table was generated for each sample with QIIME 2, and the Shannon diversity index and Evenness was calculated. The microbial community structures were visualized by stacked plot using the “ggplot2” package in R (version 3.5.3). Network analysis was conducted to explore the co-occurrence patterns within/between the taxa of prokaryotes. A similarity matrix was first generated by inputting a typical ASV matrix file and then the correlation matrix, r value, and p value were calculated using corr. test in the “psych” package [29] of R version 3.5.3. ASVs, which are strongly and significantly correlated (Spearman’s |r| > 0.6 and FDR-adjusted p < 0.05), were used to construct the networks using Gephi version 0.9.3 [30]. In addition, predicted potential carbon, nitrogen, and sulfur cycle-related pathways based on the 16S rRNA gene were conducted with the open-source R package Tax4Fun [31], with the short reads mode disabled along with SILVA 16S rRNA database (version 138), and were visualized by bubble plots using the “ggplot” package.
2.6. Statistical Analysis and Accession Number
The non-metric multidimensional scaling (nMDS), based on the Bray–Curtis similarity index, was calculated using Paleontological Statistics (PAST) version 3 [32]. An analysis of similarities (ANOSIM), based on the ASVs’ relative abundance was conducted with Paleontological Statistics (PAST) version 3 [32] to test whether there was a significant difference in the microbial community structure and potential metabolic function among the different samples. Values of p < 0.05 and p < 0.01 were considered to indicate different levels of statistical significance. The Statistical Analysis of Metagenomic Profiles (STAMP, version 2.1.3) was performed according to the relative abundance of each ASV [33] and was visualized using R (version 3.5.3). p values were calculated using a two-sided Welch’s t-test with the Benjamin–Hochberg False Discovery Rate (FDR) correction. A redundancy analysis (RDA) was performed with CANOCO V5.0 to identify a possible differentiation of the communities under the constraint of environmental factors. The statistical significance of an explanatory variable added in the course of forward selection was tested with the Monte Carlo permutation test (9999 permutations, p < 0.05). The phylogenetic group data were Hellinger transformed, environmental variables were logarithm transformed, and the effects of collinearity (VIF > 10) were removed.
16S rRNA gene sequences obtained from this study have been deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA), under the accession number PRJNA1059802.
3. Results
3.1. Hydrographic Conditions of Sampling Stations
In both trenches, seawater temperatures decreased sharply from 19.50 °C~20.24 °C in the surface to 1.07 °C~1.12 °C at the depth of 5000 m along the water column, while the average temperatures in the BBL ranged between ~1.79 °C and 1.36 °C, respectively (Figure 2). Salinity was highest for the surface water and decreased with depths to 800 m, and then slightly increased with depth to 5000 m in the WC in the two trenches, while it was generally the same in the BBL of the two trenches. Generally, the concentrations of ambient inorganic nutrients were always higher in the Diamantina Trench than those in the Kermadec Trench, except for NH4+ in the WC. The concentrations of PO43− increased with depth and reached a maximum at 800 m, then decreased with depths in the Kermadec Trench, while those in the Diamantina Trench increased with depth and reached a maximum at 5000 m, except for 2000 m. The NO3− + NO2− concentrations in the WC were both increased with depth in both trenches. The NH4+ concentrations peaked at 0 m in the Kermadec Trench and at 5000 m in the Diamantina Trench and the minimum values were detected at 2000 m in both trenches (Figure 2A,B). All the ambient inorganic nutrients were significantly higher in the BBL than in the WC (p < 0.05, Figure 2).
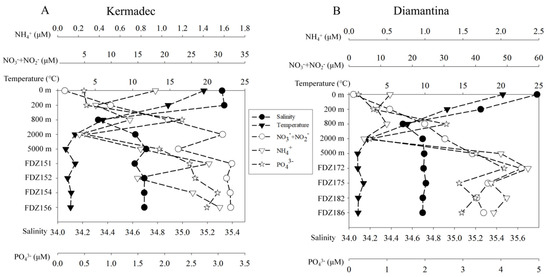
Figure 2.
The temperature, salinity, and inorganic nutrients of ambient water samples of the Kermadec Trench (A) and the Diamantina Trench (B). The black circle and triangle symbols stand for salinity and temperature, while the blank circle, triangle, and star symbols stand for NO2− + NO3−, NH4+, and PO43−, respectively.
3.2. Microbial Community Composition and Diversity
In the Kermadec Trench, 279,116 sequences and 2095 ASVs were generated from the microbial community, with the highest and lowest numbers of ASVs found at 2000 m and Stn. FDZ152, respectively (Table 1). In the Diamantina Trench, 342,737 sequences and 1443 ASVs were generated from the microbial community, with the highest and lowest numbers of ASVs found at 0 m and Stns. FDZ172 and FDZ175, respectively (Table 1). In the Kermadec Trench, the microbial community structure was dominated by Thermoplasmata, Nitrososphaeria, and Halobacteria in archaea, and by Alphaproteobacteria, Gammaproteobacteria, and Actinobacteria in bacteria. The community structures of the euphotic layer were significantly different from those of other layers (ANOSIM, p < 0.05), while those among different BBLs had no significant difference (Figure 3A). In the Diamantina Trench, the microbial community structure was dominated by Nitrososphaeria and Halobacteria in archaea, and Alphaproteobacteria and Gammaproteobacteria in bacteria. The community structure of the 2000 m layer was significantly different from that of the other water layers (ANOSIM, p < 0.05, Figure 3B), while those among different BBLs were basically the same. The lowest Shannon index and Evenness were present at 2000 m in both trenches. The microbial community diversity between WC and BBL was only significantly different in the Kermadec Trench (p < 0.05, Figure 3C), but was not observed in the Diamantina Trench (Figure 3D). An NMDS plot demonstrated a distinct distribution of microbial assemblages between the WC and BBL in both trenches (ANOSIM, p < 0.05, Figure 3E,F). Based on the STAMP analysis, the indicative ASVs leading to significant differences between the WC and BBL in the Kermadec Trench were ASV91 (Alteromonadales, Gammaproteobacteria) and ASV825 (Cellvibrionales, Gammaproteobacteria) (p < 0.05, Figure S1A), while the indicative ASVs leading to significant differences between the WC and BBL in the Diamantina Trench were ASV449 (Propionibacteriales, Actinobacteria) and ASV825 (Cellvibrionales, Gammaproteobacteria) (p < 0.05, Figure S1B).
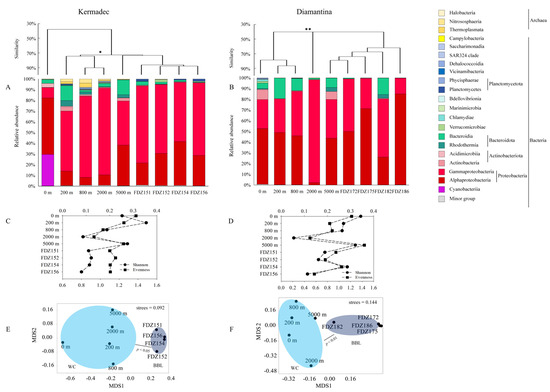
Figure 3.
Microbial community structure in the WC and BBL of the Kermadec Trench (A) and the Diamantina Trench (B). * and ** stand for p < 0.05 and p < 0.01. The Shannon index and Evenness index in the WC and BBL of the Kermadec Trench (C) and the Diamantina Trench (D). The black circle and square symbols stand for the Shannon index and Evenness index, respectively. Non-linear multidimensional scaling (nMDS) analysis of microbial communities in the WC and BBL of the Kermadec Trench (E) and the Diamantina Trench (F) based on Bray–Curtis distances.
3.3. Gene Abundance and Environmental Impacts
For microbial gene abundance based on qPCR, it was significantly higher at the BBL than the water columns in both trenches (p < 0.05), and that in the BBL was significantly higher in the Diamantina Trench than in the Kermadec Trench (p < 0.05, Figure 4A,B). RDA analysis revealed that the first and second axes together contributed 95.05% and 98.56% to the total variance of the whole communities in the Kermadec and the Diamantina Trenches, respectively (Figure 4C,D). Among the tested environmental factors, temperature (p < 0.05), depth (p < 0.05), and NO3− + NO2− (p < 0.01) were statistically significant, contributing to the variation of prokaryotic communities in the Kermadec Trench (Figure 4C), but there was no significant environmental factor detected in the Diamantina Trench (Figure 4D).
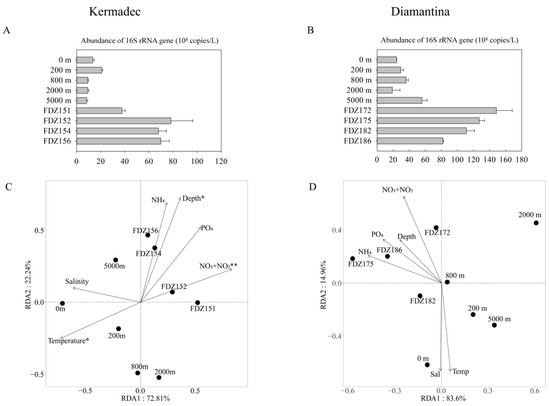
Figure 4.
The abundance of the 16S rRNA gene at the WC and BBL of the Kermadec Trench (A) and the Diamantina Trench (B). Biplot of the redundancy analysis integrating environmental parameters and the microbial communities in the WC and BBL of the Kermadec Trench (C) and the Diamantina Trench (D). * and ** stand for p < 0.05 and p < 0.01.
3.4. Co-Occurrence Network and Potential Metabolic Functions of Prokaryotes
To elucidate the interactions between different microbial groups in the WC and the BBL, network analyses were conducted based on the top 200 ASVs (Figure 5). The modularity index was 0.94 and 0.81 of the WC and the BBL in the Kermadec Trench, while it was 0.92 and 0.68 of the WC and the BBL in the Diamantina Trench. The index was larger than 0.4, suggesting that the network had a modular structure (Newman, 2006). Mainly positive correlations were shown among the archaeal groups, while more negative correlations were found among different bacterial groups. The correlations between different groups of archaea and bacteria were mainly positive, with the proportions of positive correlations accounting for 84.4% and 77.5% of the WC and the BBL in the Kermadec Trench (Figure 5A,B), respectively; and accounting for 90.4% and 95.4% of the WC and the BBL in the Diamantina Trench, respectively (Figure 5C,D). The detected negative correlations were mainly between Proteobacteria and Cyanobacteria of the WC and Proteobacteria and Actinobacterio of the BBL in the Kermadec Trench (Figure 5A,B), and between Proteobacteria, Bacteroidota, and Actinobacterio of the WC and Proteobacteria and Actinobacterio of the BBL in the Diamantina Trench (Figure 5C,D).
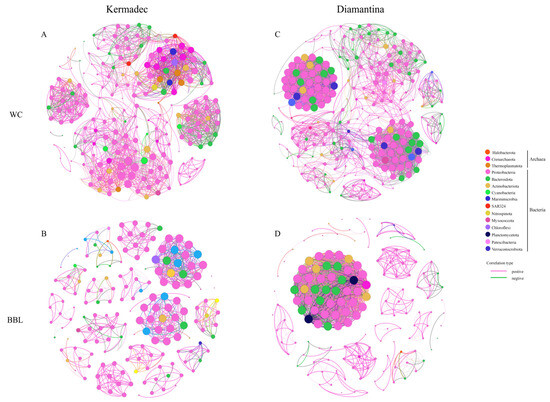
Figure 5.
The networks analysis among archaeal and bacterial groups in the WC and BBL of the Kermadec Trench (A,B) and the Diamantina Trench (C,D). The network represents relationships between co-occurring ecosystems, the edges represent co-occurrence relationships consistent at the 0.6 correlation level, and the nodes represent archaeal and bacterial taxa.
Potential functions related to carbon, nitrogen, and sulfur metabolism were predicted based on the 16S rRNA genes by Tax4Fun (Figure S2). Generally, the abundance of carbon metabolism was higher than the nitrogen and sulfur metabolism. Hydrocarbon degradation and aerobic chemoheterotrophy were the major carbon metabolic processes in the two trenches. The functions related to dark oxidation of sulfur compounds and sulfide/sulfite oxidation metabolism were only found in the BBL and were significantly higher in the Diamantina Trench than in the Kermadec Trench (p < 0.05).
3.5. Prokaryotic Metabolic Activity
Biolog EcoplateTM microplates contained 31 different carbon sources belonging to 6 carbon categories, which were carbohydrates, polymers, phenolic, carboxylic, amino acids, and amines. The average well color development (AWCD) was calculated to determine the utilization of carbon sources and metabolism characteristics. Based on the AWCD, compared to the Kermadec Trench, carbon source utilization was more consistent across the Diamantina Trench, except for at 800 m. The utilization of different carbon categories by microbes was significantly higher at 0 m in the WC of the Kermadec Trench (p < 0.01) (Figure 6A). Metabolic diversity (AWCD, Richness, Simpson, and Shannon) was significantly lower at 800 m than at the other depths in the WC of the two trenches (p < 0.05; Figure 6). Metabolic activity reflected by the AWCD demonstrated that microbes first entered the exponential growth period and then the stable period during cultivation. The microbial community at 0 m entered the stable period (34 days) prior to other samples (40 days) in the Kermadec Trench. Meanwhile, microbial communities at 0 m and 800 m depths entered the stable period (20 days) prior to other samples (40 days) in the Diamantina Trench. In the surface water, amino acids and polymers were preferentially used; in the benthic boundary layers, amino acids and amines were preferentially used in the two trenches (Figure 7). The utilization of carboxylic acids was significantly higher in the BBL of Stns. FDZ154 and FDZ156 in the Kermadec Trench (p < 0.01, Figure 7G). In the Diamantina Trench, the utilization of carboxylic acids was significantly higher in samples taken from 0 m and 200 m, compared to the other samples (p < 0.01, Figure 7H). The utilization of phenolic acids was significantly higher in the BBL of Stns. FDZ154 and FDZ156 of the Kermadec Trench (p < 0.01, Figure 7G) than other samples. In the Diamantina Trench, phenolic acids were the least used and there were no significant differences among all the depths (Figure 7H). The utilization of amines was significantly higher in the BBL than in the WC, except at 0 m in the Kermadec Trench (p < 0.05, Figure 7K), while no significant differences between the WC and the BBL were observed in the Diamantina Trench (Figure 7L).
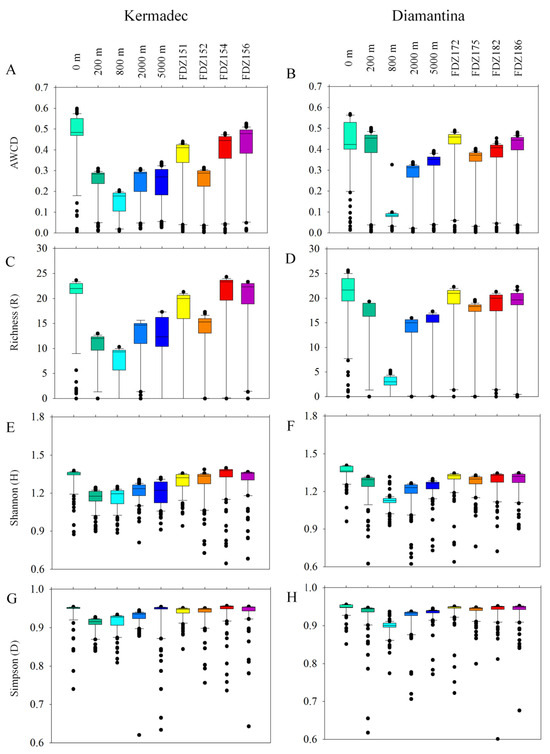
Figure 6.
Box plots of the microbial metabolic activity of AWCD (A,B), Richness index (C,D), Shannon index (E,F), and Simpson index (G,H) in the WC and BBL of the Kermadec Trench and the Diamantina Trench.
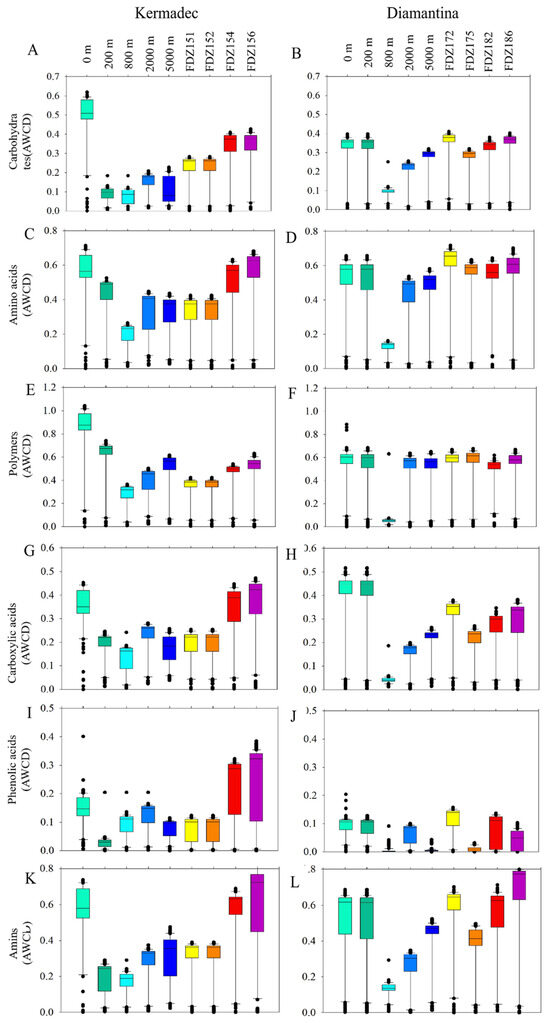
Figure 7.
Box plots of the utilization capability of the six major carbon groups, carbohydrates (A,B), amino acids (C,D), polymers (E,F), carboxylic acids (G,H), phenolic acids (I,J), and amines (K,L) by microbes in the WC and the BBL of the Kermadec Trench (left panel) and Diamantina Trench (right panel).
4. Discussion
4.1. Geographical Distribution and Environmental Effects
In the two trenches, the significantly different community structures between the WC and the BBL might be attributed to the in situ concentration and availability of organic matter. Generally, the BBL contains a high concentration of particles resuspended from subsurface sediments [14,34], which could explain the significantly higher gene abundance of prokaryotes in the BBL than in the WC. A higher proportion of Gammaproteobacteria in the BBL was found in the Kermadec Trench than in the Diamantina Trench and this microbial group could attach to particles to avoid the nutrient-depleted conditions in the surrounding waters [35] and easily assimilated organic carbon sources [36]. Proteobacteria and Nitrososphaeria predominate in the WC and this was generally consistent with previous studies conducted in the Kermadec Trench [37]. Halobacteria as a class of Euryarchaeota are extremely halophilic archaea that can adapt to a wide range of salt concentrations [38] and could catalyze the terminal step in the degradation of organic matter in anoxic environments where light was limiting [39]. The community structure of the euphotic layer was distinct from that of other layers in the Kermadec Trench, this might be due to the higher relative proportions of Cyanobacteria, which play an important role in the uptake and conversion of CO2 to bioproducts through their photosynthetic system [40].
The most important impacting factors in the Kermadec Trench were the sampling depth, temperature, and NO3− + NO2− concentrations. Sampling depth has been reported as a significant driver of community composition in the Mariana Trenches [37]. Temperature could affect the community composition and metabolic activity involved in the remineralization of POC in the WC [15,41]. In addition, NO3− was reported as the primary chemical parameter affecting the microbial community composition [42]. While no significant impacting factors were identified for microbial communities in the Diamantina Trench, this was possibly due to other important factors that were not measured, such as dissolved organic/inorganic carbon, POC, and that should be included in future studies.
4.2. Carbon Source Utilization and Potential Metabolic Activity
Heterotrophic microorganisms are crucial in the decomposition of POM and the subsequent remineralization processes in the seawater. Although natural carbon sources should be more complex than those contained in the Biolog EcoplateTM microplates, substrates which were promptly and preferentially utilized during the microplate incubation might provide important information on the carbon source in natural environments [43]. At the surface water, amino acids and polymers were used preferentially; at the BBL, amino acids and amines were used preferentially. The latter might be attributed to the presence of a high proportion of Alphaproteobacteria. This bacterial group can utilize a range of organic compounds, mainly including amino acids, nucleic acids, fatty acids, and other low molecular weight compounds, including organic and aromatic hydrocarbons produced by algae [44,45]. Amino acids were preferentially used in both the WC and BBL in the two trenches, which could be due to the fact that they were most used in cooperative communities, which was found in previous studies [46,47]. In this study, the microbial metabolic activity, reflected by the AWCD values, at 800 m was significantly different from those at the other depths. The enzyme active genes of carbohydrate esterases and auxiliary activities was reported to be positively related to the salinity [48] and this might explain the lowest microbial metabolic activities at 800 m, which always showed the lowest salinity in the WC in both trenches.
Microorganisms commonly metabolize simple carbohydrates, such as glucose, due to their easy digestibility and prevalence [49]. Phytoplankton exudates were the main source of dissolved carbohydrates in marine systems [50] and this might explain the higher metabolic activity involving carbohydrates in the surface layer of the Kermadec Trench, where a higher relative abundance of Cyanobacteria is harbored. Amino acids were primarily derived from marine biodegradation, protein hydrolysis, and extracellular excretion and were important components of marine organic nitrogen and organic carbon. Some amines, such as putrescine, are produced during the degradation of amino acids. The degradation of complex polymers requires a complex metabolic capability and the biogenic amines which are produced by bacteria were controlled by the high hydrostatic pressure and low temperature [51,52]. In addition, annotated functional genes could provide an assessment of microbial metabolic activities, allowing for the elucidation of various processes associated with substance and energy metabolism. In this study, the high abundance of aerobic chemoheterotrophy in the BBL were significantly different from those in the WC (p < 0.05) in the Diamantina Trench. This might be due to the fact that the BBL contained a high concentration of resuspended particles from subsurface sediments as extra carbon sources, and heterotrophic prokaryotes transform the organic matter to obtain energy and carbon substances via an aerobic process [53].
4.3. Microbial Interaction and Ecological Significance
Microbial interactions could lead to a series of competitive or collaborative relationships and have been suggested as biotic drivers that affect microbial community composition [54,55]. More positive relationships were detected in the WC and BBL of the two trenches, indicating the possibility of cross-feeding, co-colonization, and niche overlap [56]. Different microbial groups and their carbon utilization capabilities could help to make better use of various carbon sources along the water depths. A previous study showed that the heterotrophic archaea preferentially used heavy carbon sources, such as algal carbohydrates [57], and Crenarchaeota had genes for using carbohydrates as an organic carbon source and genes for transporting amino acids from the environment [58]. Proteobacteria contain a diverse functional repertoire including their chemolithotrophic ability to utilize sulfur and C1 compounds and their chemo-organotrophic ability to utilize environment-derived fatty acids, aromatics, carbohydrates, and peptides [59]. Some groups of Proteobacteria could rapidly utilize D-glucose and produced refractory dissolved organic matter that persisted for more than a year [60]. These organic moieties from resistant polymers could be utilized by deep-sea microorganisms due to their high ectoenzymatic activity [61,62]. For example, Chloroflexi harbored pathways for the complete hydrolytic or oxidative degradation of various recalcitrant organic matters [15], and Bacteroidota were possibly responsible for the decomposition of polymers [63]. In addition, Actinobacteria have evolved with numerous biosynthetic gene clusters to produce diverse bioactive secondary metabolites [64]. Those microbial groups were very likely worked together with diverse organic matter accessing strategies, contributing to the degradation of marine organic matter in the WC [65].
The two trenches exhibited different fluxes of organic matter. The annual rates of primary production in the overlying waters of the Kermadec Trench, which was the first trench in the Pacific Ocean to receive Lower Circumpolar Deep Water [66], have been estimated as 87 g C m−2 yr−1. In contrast, a higher surface chlorophyll in the euphotic layer of the Diamantina Trench of the Indian Ocean [67,68] might affect the sinking carbon sources and microbial community composition as well [69]. Significant differences in microbial metabolic activity (AWCD) existed between the WC and BBL in the Kermadec Trench, consistent with the NMDS plot. This might be due to the vertical shifts of community composition leading to the utilization shift of the organic detritus, which might be due to different accessing strategies of organic matter by heterogeneous microorganisms [65,69]. The microbial metabolic activity (AWCD) at 800 m was significantly lower from those at the other depths found in both trenches (p < 0.05), which was also found in the South China Sea [15]. The water depth at 800 m is usually defined as the lower boundary of the twilight zone [70] and the majority of sinking organic matter in the WC was in the form of marine snow, fecal pellets, and other particles of detritus and might have been previously ingested and reworked multiple times by zooplankton with selective absorbance of the most labile and nutritious dietary compounds above this layer [71], leading to an increase in the relative proportions of refractory polysaccharides in detritus at this depth. It should be noted that the microplate used in this study had limited types of carbon sources and the incubation results only provide a quick look at the carbon source preference and might not accurately reflect the actual carbon source compositions found in natural seawater. Therefore, the mineralization rate and the biochemical fate of different carbon sources into inorganic carbon via respiration and microbial assimilation at different depths needs to be further studied using stable isotopic tracing experiments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms12040708/s1, Figure S1: Extended error plot between the WC and BBL for the Kermadec Trench (A) and the Diamantina Trench (B) visualized through STAMP software. Mean proportions in different categories were displayed in the left bar graph. The colored circles (brown and blue) showed the 95% confidence intervals calculated using Welch’s t-test. Figure S2: Summary of the mean abundance of pathways associated with sulfur, nitrogen, and carbon metabolize in the WC and the BBL of the Kermadec Trench and the Diamantina Trench.
Author Contributions
Conceived, designed the experiment, contributed reagents/materials, revised manuscript and funding acquisition, H.J.; performed the experiment, analyzed the data, and wrote the manuscript, H.L. All the authors provided critical feedback and helped to shape the research, analysis, and manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Hainan Provincial Natural Science Foundation of China (424MS115), the National Key R&D Program of China (2022YFC2805505; 2022YFC2805304), the Hainan Province Science and Technology Special Fund (ZDKJ2021036), and the International Partnership Program of Chinese Academy of Sciences for Big Science (183446KYSB20210002).
Data Availability Statement
16S rRNA gene sequences obtained from this study have been deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) under the accession number PRJNA1059802.
Acknowledgments
We thank the crew of the R/V “Fen Dou Zhe” and the crew of the R/V “Tan Suo Yi Hao” for their professional service during the cruise of TS14 for their professional service during the cruise of TS29 from October 2022 to March 2023. We would like to thank the Institutional Center for Shared Technologies and Facilities of IDSSE, CAS for Shuai Tang, and Jie Chen.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the Funding statement. This change does not affect the scientific content of the article.
References
- Azam, F.; Malfatti, F. Microbial structuring of marine ecosystems. Nat. Rev. Microbiol. 2007, 5, 782–791. [Google Scholar] [CrossRef]
- Dang, H.; Lovell, C.R. Microbial surface colonization and biofilm development in marine environments. Microbiol. Mol. Biol. Rev. 2016, 80, 91–138. [Google Scholar] [CrossRef]
- Steinberg, D.K.; Van Mooy, B.A.S.; Buesseler, K.O.; Boyd, P.W.; Kobari, T.; Karl, D.M. Bacterial vs. zooplankton control of sinking particle flux in the ocean’s twilight zone. Limnol. Oceanogr. 2008, 53, 1327–1338. [Google Scholar] [CrossRef]
- Grabowski, E.; Letelier, R.M.; Laws, E.A.; Karl, D.M. Coupling carbon and energy fluxes in the North Pacific Subtropical Gyre. Nat. Commun. 2019, 10, 1895. [Google Scholar] [CrossRef] [PubMed]
- Herndl, G.J.; Reinthaler, T. Microbial control of the dark end of the biological pump. Nat. Geosci. 2013, 6, 718–724. [Google Scholar] [CrossRef]
- Mestre, M.; Ruiz-González, C.; Logares, R.; Duarte, C.M.; Gasol, J.M.; Sala, M.M. Sinking particles promote vertical connectivity in the ocean microbiome. Proc. Natl. Acad. Sci. USA 2018, 115, E6799–E6807. [Google Scholar] [CrossRef]
- Jamieson, A.J.; Fujii, T.; Mayor, D.J.; Solan, M.; Priede, I.G. Hadal trenches: The ecology of the deepest places on Earth. Trends Ecol. Evol. 2010, 25, 190–197. [Google Scholar] [CrossRef]
- Walsh, E.A.; Kirkpatrick, J.B.; Rutherford, S.D.; Smith, D.C.; Sogin, M.; D’hondt, S. Bacterial diversity and community composition from seasurface to subseafloor. ISME J. 2016, 10, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wei, X.; Song, W.; Wang, L.; Cao, J.; Wu, J.; Thomas, T.; Jin, T.; Wang, Z.; Wei, W.; et al. Novel Chloroflexi genomes from the deepest ocean reveal metabolic strategies for the adaptation to deep-sea habitats. Microbiome 2022, 10, 75. [Google Scholar] [CrossRef]
- Nagata, T.; Tamburini, C.; Arístegui, J.; Baltar, F.; Bochdansky, A.B.; Fonda-Umani, S.; Fukuda, H.; Gogou, A.; Hansell, D.A.; Hansman, R.L.; et al. Emerging concepts on microbial processes in the bathypelagic ocean—Ecology, biogeochemistry, and genomics. Deep. Sea Res. Part II 2010, 57, 1519–1536. [Google Scholar] [CrossRef]
- Hansell, D.A.; Ducklow, H.W. Bacterioplankton distribution and production in the bathypelagic ocean: Directly coupled to particulate organic carbon export? Limnol. Oceanogr. 2003, 48, 150–156. [Google Scholar] [CrossRef]
- Salazar, G.; Cornejo-Castillo, F.M.; Benítez-Barrios, V.; Fraile-Nuez, E.; Álvarez-Salgado, X.A.; Duarte, C.M.; Gasol, J.M.; Acinas, S.G. Global diversity and biogeography of deep-sea pelagic prokaryotes. ISME J. 2015, 10, 596–608. [Google Scholar] [CrossRef]
- Turley, C. Bacteria in the cold deep-sea benthic boundary layer and sediment-water interface of the NE Atlantic. FEMS Microbiol. Ecol. 2000, 33, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Tarn, J.; Peoples, L.M.; Hardy, K.; Cameron, J.; Bartlett, D.H. Identification of free-living and particle-associated microbial communities present in Hadal regions of the Mariana Trench. Front. Microbiol. 2016, 7, 665. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, F.; Liu, H.; Jing, H. Metabolic activity and community structure of prokaryotes associated with particles in the twilight zone of the South China Sea. Front. Microbiol. 2022, 13, 1056860. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Y.; Jing, H.; Liu, H. Spatial variation and metabolic diversity of microbial communities in the surface sediments of the Mariana Trench. Front. Microbiol. 2022, 13, 1051999. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Grossart, H.; Schweitzer, B.; Ploug, H. Microbial ecology of organic aggregates in aquatic ecosystems. Aquat. Microb. Ecol. 2002, 28, 175–211. [Google Scholar] [CrossRef]
- Leduc, D.; Rowden, A.A. Nematode communities in sediments of the Kermadec Trench, Southwest Pacific Ocean. Deep. Sea Res. Part I 2018, 134, 23–31. [Google Scholar] [CrossRef]
- Angel, M.V. Ocean trench conservation. Environmentalist 1982, 2, 1–17. [Google Scholar] [CrossRef]
- Feigl, V.; Ujaczki, É.; Vaszita, E.; Molnár, M. Influence of red mud on soil microbial communities: Application and comprehensive evaluation of the Biolog EcoPlate approach as a tool in soil microbiological studies. Sci. Total Environ. 2017, 595, 903–911. [Google Scholar] [CrossRef]
- Farkas, É.; Feigl, V.; Gruiz, K.; Vaszita, E.; Fekete-Kertész, I.; Tolner, M.; Kerekes, I.; Pusztai, É.; Kari, A.; Uzinger, N.; et al. Long-term effects of grain husk and paper fibre sludge biochar on acidic and calcareous sandy soils—A scale-up field experiment applying a complex monitoring toolkit. Sci. Total Environ. 2020, 731, 138988. [Google Scholar] [CrossRef]
- Tam, L.; Derry, A.; Kevan, P.; Trevors, J. Functional diversity and community structure of microorganisms in rhizosphere and non-rhizosphere Canadian arctic soils. Biodivers. Conserv. 2001, 10, 1933–1947. [Google Scholar] [CrossRef]
- Moore, J.C. “Diversity, Taxonomic Versus Functional” in Encyclopedia of Biodiversity, 2nd ed.; Levin, S., Ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2013; Volume 2, pp. 648–656. [Google Scholar]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of bacteria and Archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef] [PubMed]
- Takai, K.; Horikoshi, K. Rapid detection and quantification of members of the archaeal community by quantitative PCR using fluorogenic probes. Appl. Environ. Microbiol. 2000, 66, 5066–5072. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Peña, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Revelle, W. Package ‘Psych’. The Comprehensive R Archive Network. 30 August 2015. Available online: https://cran.r-project.org/web/packages/psych/psych.pdf (accessed on 1 December 2023).
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An open source software for exploring and manipulating networks. Proc. Int. AAAI Conf. Web Soc. Media 2009, 3, 361–362. [Google Scholar] [CrossRef]
- Aßhauer, K.P.; Wemheuer, B.; Daniel, R.; Meinicke, P. Tax4Fun: Predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics 2015, 31, 2882–2884. [Google Scholar] [CrossRef]
- Hammer, Y.; Harper, D.A.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol Electron 2001, 4, 1. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef] [PubMed]
- Glud, R.N.; Wenzhöfer, F.; Middelboe, M.; Oguri, K.; Turnewitsch, R.; Canfield, D.E.; Kitazato, H. High rates of microbial carbon turnover in sediments in the deepest oceanic trench on Earth. Nat. Geosci. 2013, 6, 284–288. [Google Scholar] [CrossRef]
- Acinas, S.G.; Antón, J.; Rodríguez-Valera, F. Diversity of free-living and attached bacteria in offshore western Mediterranean waters as depicted by analysis of genes encoding 16S rRNA. Appl. Environ. Microbiol. 1999, 65, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Pinhassi, J.; Berman, T. Differential growth response of colony-forming alpha and gamma-proteobacteria in dilution culture and nutrient addition experiments from Lake Kinneret (Israel), the eastern Mediterranean Sea, and the Gulf of Eilat. Appl. Environ. Microbiol. 2003, 69, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Peoples, L.M.; Donaldson, S.; Osuntokun, O.; Xia, Q.; Nelson, A.; Blanton, J.; Allen, E.E.; Church, M.J.; Bartlett, D.H. Vertically distinct microbial communities in the Mariana and Kermadec trenches. PLoS ONE 2018, 13, e0195102. [Google Scholar] [CrossRef]
- Gaba, S.; Kumari, A.; Medema, M.; Kaushik, R. Pan-genome analysis and ancestral state reconstruction of class halobacteria: Probability of a new super-order. Sci. Rep. 2020, 10, 21205. [Google Scholar] [CrossRef]
- Oren, A.; Oremland, R.S. Diversity of anaerobic halophilic microorganisms. In Instruments, Methods, and Missions for Astrobiology III; SPIE: San Diego, CA, USA, 2000; Volume 4137, pp. 96–105. [Google Scholar] [CrossRef]
- Zhang, A.; Carroll, A.L.; Atsumi, S. Carbon recycling by cyanobacteria: Improving CO2 fixation through chemical pro-duction. FEMS Microbiol. Lett. 2017, 364, fnx165. [Google Scholar] [CrossRef]
- Kong, L.-F.; He, Y.-B.; Xie, Z.-X.; Luo, X.; Zhang, H.; Yi, S.-H.; Lin, Z.-L.; Zhang, S.-F.; Yan, K.-Q.; Xu, H.-K.; et al. Illuminating key microbial players and metabolic processes involved in the remineralization of particulate organic carbon in the ocean’s twilight zone by metaproteomics. Appl. Environ. Microbiol. 2021, 87, AEM0098621. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, Y.; Fang, W.; Liu, J.; Hong, J.; Zou, C.; Zhang, J. Microbial community structural response to variations in physicochemical features of different aquifers. Front. Microbiol. 2023, 14, 1025964. [Google Scholar] [CrossRef]
- Melita, M.; Amalfitano, S.; Preziosi, E.; Ghergo, S.; Frollini, E.; Parrone, D.; Zoppini, A. Physiological profiling and functional diversity of groundwater microbial communities in a municipal solid waste landfill area. Water 2019, 11, 2624. [Google Scholar] [CrossRef]
- Buchan, A.; LeCleir, G.R.; Gulvik, C.A.; Gonzalez, J.M. Master recyclers: Features and functions of bacteria associated with phytoplankton blooms. Nat. Rev. Microbiol. 2014, 12, 686–698. [Google Scholar] [CrossRef]
- Mishamandani, S.; Gutierrez, T.; Berry, D.; Aitken, M.D. Response of the bacterial community associated with a cosmopolitan marine diatom to crude oil shows a preference for the biodegradation of aromatic hydrocarbons. Environ. Microbiol. 2016, 18, 1817–1833. [Google Scholar] [CrossRef]
- Pacheco, A.R.; Moel, M.; Segrè, D. Costless metabolic secretions as drivers of interspecies interactions in microbial ecosystems. Nat. Commun. 2019, 10, 103. [Google Scholar] [CrossRef]
- Machado, D.; Maistrenko, O.M.; Andrejev, S.; Kim, Y.; Bork, P.; Patil, K.R.; Patil, K.R. Polarization of microbial communities between competitive and cooperative metabolism. Nat. Ecol. Evol. 2021, 5, 195–203. [Google Scholar] [CrossRef]
- Yang, C.; Lv, D.; Jiang, S.; Lin, H.; Sun, J.; Li, K.; Sun, J. Soil salinity regulation of soil microbial carbon metabolic function in the Yellow River Delta, China. Sci. Total Environ. 2021, 790, 148258. [Google Scholar] [CrossRef] [PubMed]
- Picazo, A.; Villaescusa, J.A.; Rochera, C.; Miralles-Lorenzo, J.; Quesada, A.; Camacho, A. Functional metabolic diversity of bacterioplankton in Maritime Antarctic Lakes. Microorganisms 2021, 9, 2077. [Google Scholar] [CrossRef]
- Urbani, R.; Magaletti, E.; Sist, P.; Cicero, A.M. Extracellular carbohydrates released by the marine diatoms Cylindrotheca closterium, Thalassiosira pseudonana and Skeletonema costatum: Effect of P-depletion and growth status. Sci. Total Environ. 2005, 353, 300–306. [Google Scholar] [CrossRef]
- Emborg, J.; Dalgaard, P. Formation of histamine and biogenic amines in cold-smoked tuna: An investigation of psychrotolerant bacteria from samples implicated in cases of histamine fish poisoning. J. Food Prot. 2006, 69, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Naila, A.; Flint, S.; Fletcher, G.; Bremer, P.; Meerdink, G. Control of biogenic amines in food—Existing and emerging approaches. J. Food Sci. 2010, 75, R139–R150. [Google Scholar] [CrossRef]
- López-González, J.; Suárez-Estrella, F.; Vargas-García, M.; López, M.; Jurado, M.; Moreno, J. Dynamics of bacterial microbiota during lignocellulosic waste composting: Studies upon its structure, functionality and biodiversity. Bioresour. Technol. 2015, 175, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Calcagno, V.; Jarne, P.; Loreau, M.; Mouquet, N.; David, P. Diversity spurs diversification in ecological communities. Nat. Commun. 2017, 8, 15810. [Google Scholar] [CrossRef] [PubMed]
- Hunt, D.E.; Ward, C.S. A network-based approach to disturbance transmission through microbial interactions. Front. Microbiol. 2015, 6, 1182. [Google Scholar] [CrossRef]
- Faust, K.; Raes, J. Microbial interactions: From networks to models. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef]
- Hoefs, M.; Schouten, S.; De Leeuw, J.W.; King, L.L.; Wakeham, S.G.; Damste, J. Ether lipids of planktonic archaea in the marine water column. Appl. Environ. Microbiol. 1997, 63, 3090–3095. [Google Scholar] [CrossRef]
- Parada, A.E.; Weber, P.K.; Mayali, X.; Fuhrman, J.A.; Pett-Ridge, J.; Dekas, A.E. Metatranscriptomic analysis of marine Thaumarchaea suggests intra-phylum variability in in situ heterotrophic carbon and nitrogen metabolim. In Proceedings of the AGU Fall Meeting Abstracts, San Francisco, CA, USA, 12–16 December 2016; p. B13J-03. [Google Scholar]
- Zhou, Z.; Tran, P.Q.; Kieft, K.; Anantharaman, K. Genome diversification in globally distributed novel marine Proteobacteria is linked to environmental adaptation. ISME J. 2020, 14, 2060–2077. [Google Scholar] [CrossRef]
- Gruber, D.F.; Simjouw, J.-P.; Seitzinger, S.P.; Taghon, G.L. Dynamics and characterization of refractory dissolved organic matter produced by a pure bacterial culture in an experimental predator-prey system. Appl. Environ. Microbiol. 2006, 72, 4184–4191. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, H.-G.; Ullrich, S. Profiles of ectoenzymes in the Indian Ocean: Phenomena of phosphatase activity in the mesopelagic zone. Aquat. Microb. Ecol. 1999, 19, 139–148. [Google Scholar] [CrossRef]
- Tamburini, C.; Garcin, J.; Ragot, M.; Bianchi, A. Biopolymer hydrolysis and bacterial production under ambient hydrostatic pressure through a 2000 m water column in the NW Mediterranean. Deep. Sea Res. Part II 2002, 49, 2109–2123. [Google Scholar] [CrossRef]
- Huang, J.; Gao, K.; Yang, L.; Lu, Y. Successional action of Bacteroidota and Firmicutes in decomposing straw polymers in a paddy soil. Environ. Microbiome 2023, 18, 76. [Google Scholar] [CrossRef]
- van Bergeijk, D.A.; Terlouw, B.R.; Medema, M.H.; van Wezel, G.P. Ecology and genomics of Actinobacteria: New concepts for natural product discovery. Nat. Rev. Microbiol. 2020, 18, 546–558. [Google Scholar] [CrossRef]
- Teske, A.; Durbin, A.; Ziervogel, K.; Cox, C.; Arnosti, C. Microbial community composition and function in permanently cold seawater and sediments from an Arctic fjord of Svalbard. Appl. Environ. Microbiol. 2011, 77, 2008–2018. [Google Scholar] [CrossRef] [PubMed]
- Belyaev, G.M. Deep-Sea Ocean Trenches and Their Fauna; Nauka Publishing House: Moscow, Russia, 1989. [Google Scholar]
- Jamieson, A.J. The Hadal Zone: Life in the Deepest Oceans; Cambridge University Press: London, UK, 2015. [Google Scholar]
- Pandey, S.; Bhagawati, C.; Dandapat, S.; Chakraborty, A. Surface chlorophyll anomalies associated with Indian Ocean Dipole and El Niño Southern Oscillation in North Indian Ocean: A case study of 2006–2007 event. Environ. Monit. Assess. 2020, 191 (Suppl. S3), 807. [Google Scholar] [CrossRef] [PubMed]
- Galand, P.E.; Potvin, M.; Casamayor, E.O.; Lovejoy, C. Hydrography shapes bacterial biogeography of the deep Arctic Ocean. ISME J. 2009, 4, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Buesseler, K.O.; Lamborg, C.H.; Boyd, P.W.; Lam, P.J.; Trull, T.W.; Bidigare, R.R.; Bishop, J.K.B.; Casciotti, K.L.; Dehairs, F.; Elskens, M.; et al. Revisiting Carbon Flux Through the Ocean’s Twilight Zone. Science 2007, 316, 567–570. [Google Scholar] [CrossRef]
- Mayor, D.J.; Cook, K.; Thornton, B.; Walsham, P.; Witte, U.F.M.; Zuur, A.F.; Anderson, T.R. Absorption efficiencies and basal turnover of C, N and fatty acids in a marine Calanoid copepod. Funct. Ecol. 2011, 25, 509–518. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).