First Report of Anopheles annularis s.l., An. maculatus s.s., and An. culicifacies s.l. as Malaria Vectors and a New Occurrence Record for An. pseudowillmori and An. sawadwongporni in Alipurduar District Villages, West Bengal, India
Abstract
1. Introduction
2. Materials and Methods
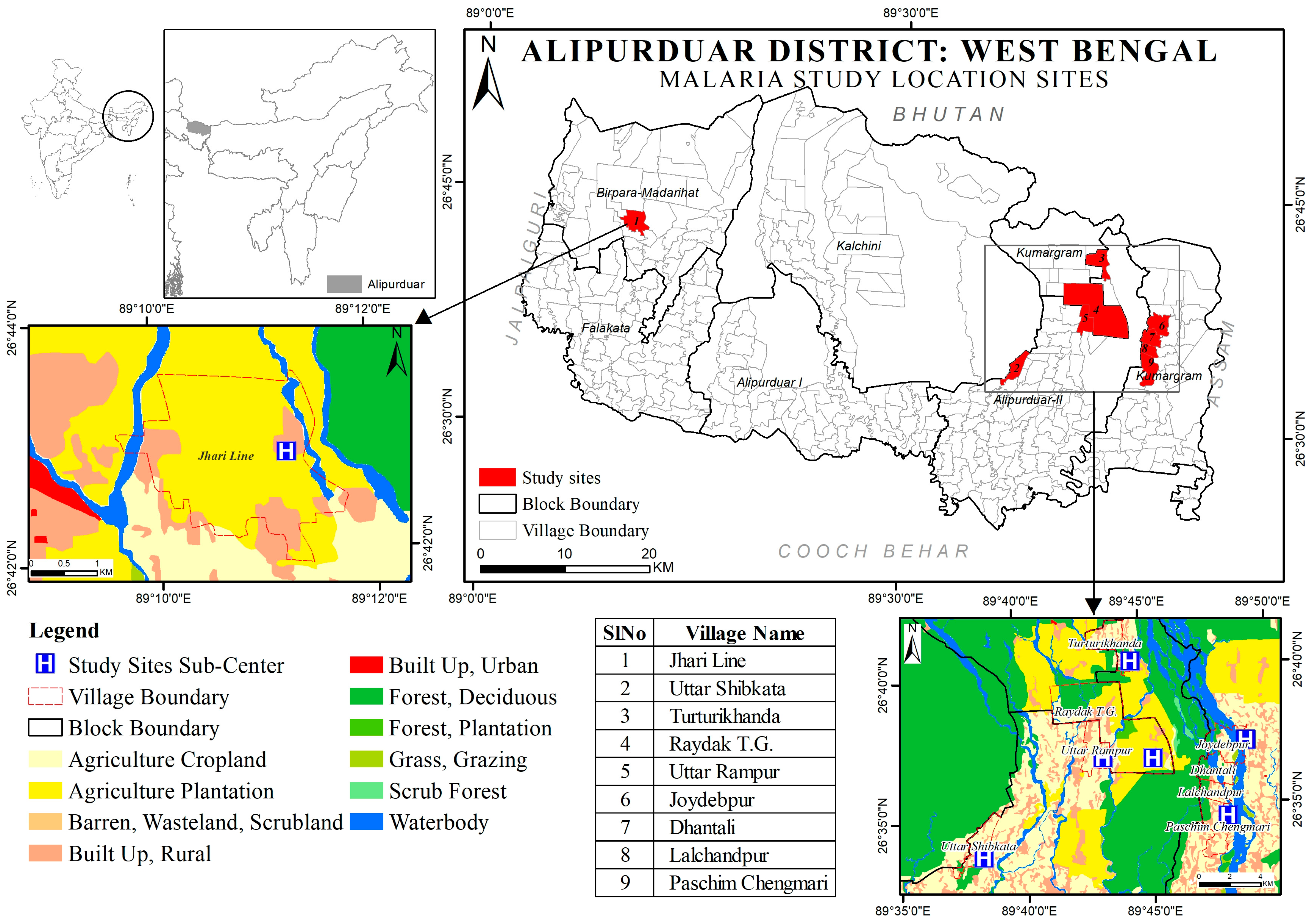
2.1. Study Location
2.2. Collection of Mosquito Samples
2.3. Identification of Mosquito Species
2.4. Identification Based on Morphology
2.5. Mosquito Dissection for Molecular Studies
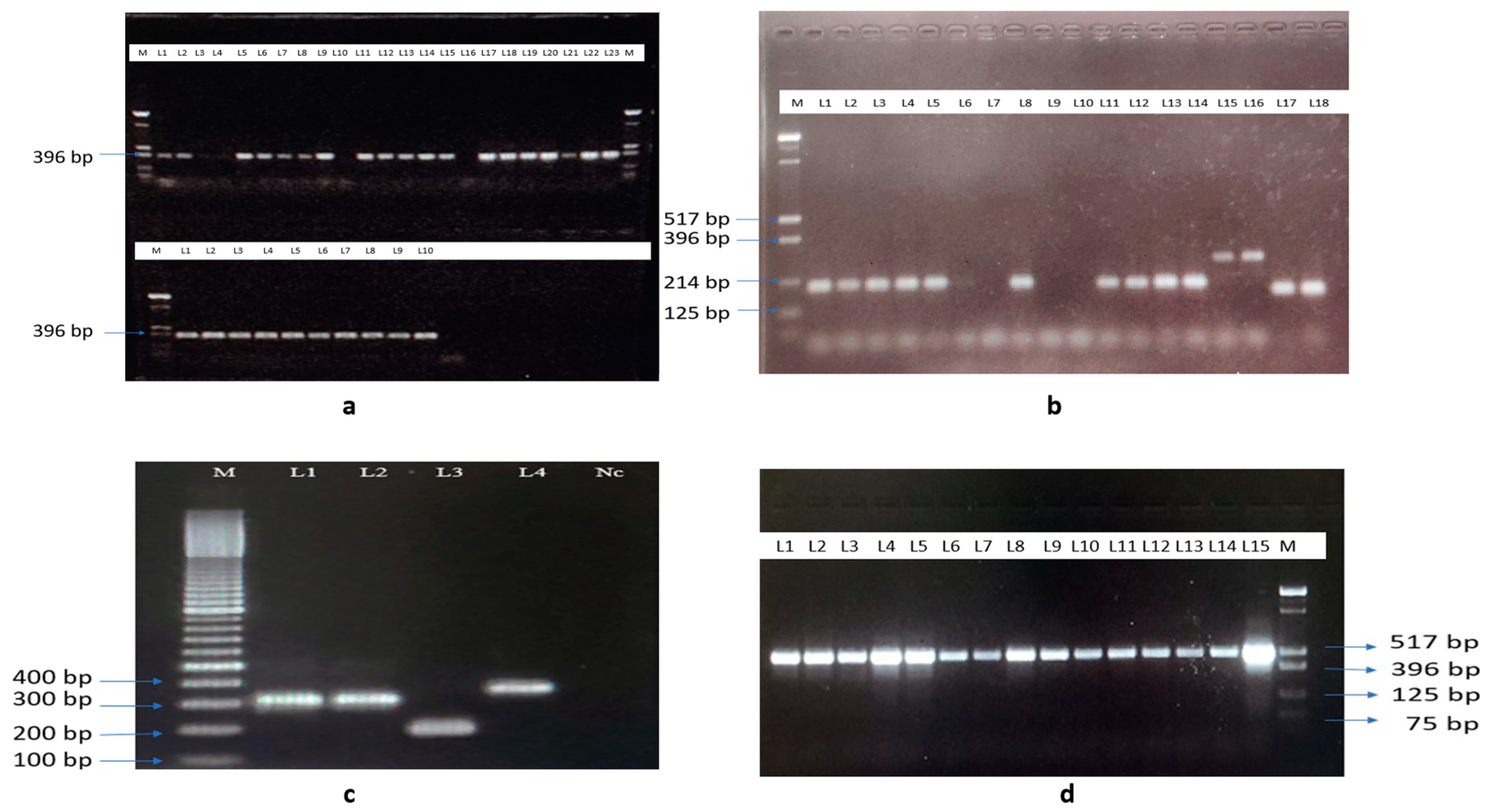
2.6. Polymerase Chain Reaction Assays for Identifying Species
2.7. CO1 Sequencing for Identifying the Species
2.8. Human Blood Meal Detection
2.9. Detection of Plasmodium in Mosquitoes
3. Results
3.1. Study Area Description
Site PHC Wise LULC Pattern
3.2. Identification of Mosquito Species Using Morphological and Molecular Methods
3.3. Blood Meal Analysis
3.4. CO1 Sequencing and Phylogenetic Analysis
3.5. Human Plasmodium Infection in Mosquitoes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, P.K.; Tilak, R. Outbreak prone communicable diseases of public health importance in the northern districts of West Bengal—Current status & the way forward. Indian J. Med. Res. 2021, 153, 358–366. [Google Scholar] [PubMed]
- Sharma, P.K.; Ramanchandran, R.; Hutin, Y.J.; Sharma, R.; Gupte, M.D. A malaria outbreak in Naxalbari, Darjeeling district, West Bengal, India, 2005: Weaknesses in disease control, important risk factors. Malar. J. 2009, 8, 288. [Google Scholar] [CrossRef] [PubMed]
- Penjor, K.; Zangpo, U.; Tshering, D.; Ley, B.; Price, R.N.; Wangdi, K. Imported malaria and its implication to achievement of malaria-free Bhutan. J. Travel Med. 2023, 30, taad044. [Google Scholar] [CrossRef] [PubMed]
- Nandi, J.; Rao, J.S.; Dasgupta, R.K.; Sharma, R.S. Ecological observations on the anopheline mosquitoes of Jalpaiguri Duars, West Bengal. J. Commun. Dis. 1996, 28, 279–286. [Google Scholar] [PubMed]
- Rudra, S.K.; Mukhopadhyay, A. Mosquito species composition of the Dooars of West Bengal, India. Proc. Zool. Soc. 2010, 63, 21–25. [Google Scholar] [CrossRef]
- Iyengar, M.O. Further Observations on Vectors of Malaria in Bengal and Notes on the Seasonal Infectivity of Anopheles. J. Malar. Inst. India 1940, 3, 115–123. [Google Scholar]
- Malakar, P.; Das, S.; Saha, G.K.; Dasgupta, B.; Hati, A.K. Anophelines of Siliguri-Naxalbari block, Darjeeling, West Bengal. Indian J. Malariol. 1995, 32, 133–139. [Google Scholar] [PubMed]
- Hati, A.K. Vector problem in malaria in West Bengal. Indian J. Public Health 1986, 30, 201–206. [Google Scholar] [PubMed]
- Rattanarithikul, R.; Green, C.A. Formal recognition of the species of the Anopheles maculatus group (Diptera: Culicidae) occurring in Thailand, including the descriptions of two new species and a preliminary key to females. Mosq. Syst. 1986, 18, 4. [Google Scholar]
- Nagpal, B.N.; Sharma, V.P. Indian Anophelines; Science Publishers, Inc.: Enfield, NH, USA, 1995. [Google Scholar]
- Walton, C.; Somboon, V.P.; O’loughlin, S.M.; Zhang, S.; Harbach, R.E.; Linton, Y.-M.; Chen, B.; Nolan, K.; Duong, S.; Fong, M.-Y.; et al. Genetic diversity and molecular identification of mosquito species in the Anopheles maculatus group using the ITS2 region of rDNA. Infect. Genet. Evol. 2007, 7, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Phuc, H.K.; Ball, A.J.; Son, L.; Hanh, N.V.; Tu, N.D.; Lien, N.G.; Verardi, A.; Townson, H. Multiplex PCR assay for malaria vector Anopheles minimus and four related species in the Myzomyia Series from Southeast Asia. Med. Vet. Ѐntomol. 2003, 17, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.; Somboon, P.; Harbach, R.E.; Zhang, S.; Weerasinghe, I.; O’loughlin, S.M.; Phompida, S.; Sochantha, T.; Tun-Lin, W.; Chen, B.; et al. Molecular identification of mosquito species in the Anopheles annularis group in southern Asia. Med. Vet. Ѐntomol. 2007, 21, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.P.; Rajavel, A.R.; Natarajan, R.; Jambulingam, P. DNA barcodes can distinguish species of Indian mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2007, 44, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.; Kar, S.K.; Mohapatra, N.; Mishra, K.; Hazra, R.K.; Kar, P.; Singh, D.V.; Dash, A.P. Multiplex PCR assay for the detection of anopheles fluviatilis species complex, human host preference, and Plasmodium falciparum sporozoite presence, using a unique mosquito processing method. Am. J. Trop. Med. Hyg. 2007, 76, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Canier, L.; Khim, N.; Kim, S.; Sluydts, V.; Heng, S.; Dourng, D.; Eam, R.; Chy, S.; Khean, C.; Loch, K.; et al. An innovative tool for moving malaria PCR detection of parasite reservoir into the field. Malar. J. 2013, 12, 405. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, I.P.; Nirmolia, T.; Pandey, A.; Subbarao, S.K.; Nath, A.; Senapati, S.; Tripathy, D.; Pebam, R.; Nag, S.; Roy, R.; et al. Dry Post Wintertime Mass Surveillance Unearths a Huge Burden of P. vivax, and Mixed Infection with P. vivax P. falciparum, a Threat to Malaria Elimination, in Dhalai, Tripura, India. Pathogens 2021, 10, 1259. [Google Scholar] [CrossRef] [PubMed]
- Chandra, G. Age composition of incriminated malaria vector in a rural foothills in West Bengal, India. Indian J. Med. Res. 2008, 127, 607–609. [Google Scholar] [PubMed]
- Viswanathan, D.K. The Assam Medical Research Society, Shillong. A Resume of Its Activities during 1931–41; Calcutta: Kolkata, India, 1941. [Google Scholar]
- Viswanathan, D.K. Experimental Malaria Control in a Hyperendemic Tea Garden in Upper Assam by the Use of Pyrocide 20 as an Insecticidal Spray. J. Malar. Inst. India 1941, 4, 35–55. [Google Scholar]
- Ghosh, K.K.; Chakraborty, S.; Bhattacharya, S.; Palit, A.; Tandon, N.; Hati, A.K. Anopheles annularis as a vector of malaria in rural West Bengal. Indian J. Malariol. 1985, 22, 65–69. [Google Scholar] [PubMed]


| PHC | Kumargram | Madhya Rangalibazna | Turturi | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sub-Centre | Joydebpur | Turturikhanda | West Chengmari | Gopalpur T.G. | Dhowlabasti | Raydak TE-1 | Uttar Shibkata | |||||||
| Village | Joydebpur | Turturikhanda | Dhantali | Lalchandpur | Paschim Chengmari | Jhari Line | Uttar Rampur | Raydak TG | Uttar Shibkata | |||||
| Year (Month) | 2018 (Nov) | 2019 (Feb) | 2018 (Apr, Nov) | 2019 (Feb) | 2019 (Feb) | 2020 (Jul) | 2019 (Feb) | 2018 (Apr) | 2018 (Dec) | 2022 (Nov) | 2018 (Oct) | 2019 (Sep, Oct) | 2018 (Dec) | |
| Total mosquito samples | 1 | 7 | 8 | 3 | 7 | 3 | 3 | 2 | 2 | 28 | 2 | 9 | 3 | 78 |
| An. aconitus (Mor, Mol) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 |
| An. annularis s.l. (Mor, Mol) | 1 | 2 | 2 | 0 | 3 | 1 | 0 | 0 | 0 | 18 | 0 | 6 | 1 | 34 |
| An. barbirostris s.l. (Mor) | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 |
| An. culicifacies s.l. (Mor, Mol) | 0 | 0 | 5 | 0 | 0 | 1 | 2 | 1 | 2 | 2 | 0 | 0 | 0 | 13 |
| An. fluviatilis (Mol) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| An. hyrcanus s.l. (Mor) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| An. maculatus s.s. (Mor, Mol) | 0 | 4 | 0 | 2 | 4 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 13 |
| An. minimus (Mor, Mol) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| An. pseudowillmori (Mol) | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 |
| An. sawadwongporni (Mol) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 |
| An. splendidus (Mor) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| An. vagus (Mor) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 4 |
| An. varuna (Mor, Mol) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Mosquito Species | Total Samples Tested with PCR | Pf (%) | Pv (%) | Mixed (Pf + Pv) (%) | Total Plasmodium-Positive (%) | Human Blood Meal Positivity Using Exclusive PCR for Human Blood |
|---|---|---|---|---|---|---|
| An. aconitus | 2 | 0 (0) | 1 (50) | 1 (50) | 2 (100) | 1.0 |
| An. annularis s.l. | 34 | 7 (20.59) | 5 (14.71) | 9 (26.47) | 21 (61.76) | 0.76 |
| An. barbirostris s.l. | 3 | 0 (0) | 1 (33.33) | 0 (0) | 1 (33.33) | 1.0 |
| An. culicifacies s.l. | 13 | 1 (7.69) | 1(7.69) | 0 (0) | 2 (15.38) | 0.92 |
| An. fluviatilis | 1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.0 |
| An. hyrcanus s.l. | 1 | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 1.0 |
| An. maculatus s.s. | 13 | 2 (15.38) | 2 (15.38) | 2 (15.38) | 6 (46.15) | 0.92 |
| An. minimus | 1 | 0 (0) | 1 (100) | 0 (0) | 1 (100) | 1.0 |
| An. pseudowillmori | 2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.5 |
| An. sawadwongporni | 2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.5 |
| An. splendidus | 1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 |
| An. vagus | 4 | 1 (25) | 0 (0) | 0 (0) | 1 (25) | 0.5 |
| An. varuna | 1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.0 |
| Total | 78 | 12 (15.38) | 11(14.10) | 12 (15.38) | 35 (44.87) | 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajkonwar, J.; Shende, V.; Maji, A.K.; Pandey, A.; Sharma, P.K.; Gunasekaran, K.; Subbarao, S.K.; Bhattacharyya, D.R.; Raghavendra, K.; Pebam, R.; et al. First Report of Anopheles annularis s.l., An. maculatus s.s., and An. culicifacies s.l. as Malaria Vectors and a New Occurrence Record for An. pseudowillmori and An. sawadwongporni in Alipurduar District Villages, West Bengal, India. Microorganisms 2024, 12, 95. https://doi.org/10.3390/microorganisms12010095
Rajkonwar J, Shende V, Maji AK, Pandey A, Sharma PK, Gunasekaran K, Subbarao SK, Bhattacharyya DR, Raghavendra K, Pebam R, et al. First Report of Anopheles annularis s.l., An. maculatus s.s., and An. culicifacies s.l. as Malaria Vectors and a New Occurrence Record for An. pseudowillmori and An. sawadwongporni in Alipurduar District Villages, West Bengal, India. Microorganisms. 2024; 12(1):95. https://doi.org/10.3390/microorganisms12010095
Chicago/Turabian StyleRajkonwar, Jadab, Varun Shende, Ananta Kumar Maji, Apoorva Pandey, Puran K. Sharma, Kasinathan Gunasekaran, Sarala K. Subbarao, Dibya Ranjan Bhattacharyya, Kamaraju Raghavendra, Rocky Pebam, and et al. 2024. "First Report of Anopheles annularis s.l., An. maculatus s.s., and An. culicifacies s.l. as Malaria Vectors and a New Occurrence Record for An. pseudowillmori and An. sawadwongporni in Alipurduar District Villages, West Bengal, India" Microorganisms 12, no. 1: 95. https://doi.org/10.3390/microorganisms12010095
APA StyleRajkonwar, J., Shende, V., Maji, A. K., Pandey, A., Sharma, P. K., Gunasekaran, K., Subbarao, S. K., Bhattacharyya, D. R., Raghavendra, K., Pebam, R., Mayakrishnan, V., Gogoi, P., Senapati, S., Sarkar, P., Biswas, S., Debbarma, D., Nirmolia, T., Jena, S. R., Bayan, B., ... Bhowmick, I. P. (2024). First Report of Anopheles annularis s.l., An. maculatus s.s., and An. culicifacies s.l. as Malaria Vectors and a New Occurrence Record for An. pseudowillmori and An. sawadwongporni in Alipurduar District Villages, West Bengal, India. Microorganisms, 12(1), 95. https://doi.org/10.3390/microorganisms12010095







