Abstract
Aeromonas veronii is widespread in aquatic environments and is capable of infecting various aquatic organisms. A. veronii infection is lethal for Chinese soft-shelled turtles (Trionyx sinensis, CSST). We isolated a gram-negative bacterium from the liver of diseased CSSTs, which was named XC-1908. This isolate was identified as A. veronii based on its morphological and biochemical characteristics, and 16S rRNA gene sequence analysis. A. veronii was pathogenic for CSSTs with an LD50 of 4.17 × 105 CFU/g. The symptoms of CSSTs artificially infected with isolate XC-1908 were consistent with those of the naturally infected CSSTs. The levels of total protein, albumin, and white globule in the serum samples of the diseased turtles were decreased, whereas those of aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase were elevated. Moreover, the diseased CSSTs exhibited the following histopathological changes: the liver contained numerous melanomacrophage centers, renal glomerulus were edematous, intestinal villi were shed, and in oocytes, the number of vacuoles increased and red-rounded particles were observed. Antibiotic sensitivity tests revealed that the bacterium was sensitive to ceftriaxone, doxycycline, florfenicol, cefradine, and gentamicin, and resistant to sulfanilamide, carbenicillin, benzathine, clindamycin, erythromycin, and streptomycin. This study provides control strategies to prevent outbreaks of A. veronii infection in CSSTs.
1. Introduction
The Chinese soft-shelled turtle (CSST; Trionyx sinensis) belongs to the Trionychidae family [1], and more than 30 species have been reported for the genus Trionyx. However, only three species of CSST are found in China, namely Peleochelys bibroni, Palea steindachneri, and T. sinensis [2]. The CSST is one of the most significant aquatic species in China, and it is widely farmed for food production, with a production of 319,081 tons in 2018 [2]. Wild CSST species are mainly distributed in Asia, including China, Japan, eastern Russia, and Korea. However, with advancements in turtle farming, various turtle diseases have been reported, such as red bottom plate disease, putrid skin disease, etc. [3]. In a high-density intensive farming model with high temperature, the mortality rates of infectious turtle diseases are increasing, incurring huge economic losses. Every year during summer, turtle disease outbreaks, especially bacterial diseases [4] caused by Aeromonas hydrophila [5], Bacillus cereus [6], and Edwardsiella tarda. [7], affect the turtle farming industry.
The bacterium Aeromonas veronii, which belongs to the Aeromonadaceae family, was first isolated in 1983 from patients with diarrhea and wound infections by the Centers for Disease Control and Prevention (CDC) [8]. It was named A. veronii in the honor of the French microbiologist Veron [8]. The bacterium can adapt to different types of environments and can infect mammals including humans, especially the elderly, children, and individuals with low immunity [9]. Moreover, it can infect fish, amphibians, and reptiles, causing huge economic losses to the aquaculture industry [10,11]. A. veronii is widely distributed in the aquatic environment. Moreover, it multiplies faster under the following conditions: during summer and autumn, in water with deteriorated quality, and in physically traumatized or immunocompromised aquatic animals [12]. A. veronii infections exhibit high mortality rates, with hemorrhage and ascites being the main pathological features of the affected aquatic animals [13].
In 2022, at a farm in Hubei Province, CSSTs exhibited the symptoms of an infectious disease, such as floating on the surface, slow movement, easily catchable, and white spots on the abdominal surface. Therefore, in the present study, we determined the causal organism of the infection observed in CSSTs at the farm, under aseptic conditions. We identified the bacterium based on morphological, physiological, and biochemical criteria and 16S rRNA gene sequencing. Moreover, the pathogenicity and drug sensitivity of the bacterium were determined using the regression infection test and the agar diffusion method, respectively. The result of this study provides a basis for the development of control strategies for CSST diseases.
2. Materials and Methods
2.1. Trionyx Sinensis
Twelve diseased CSSTs (485 ± 10 g) were obtained from a CSST farm in Hubei Province, China, in July 2022, and were immediately transported to the laboratory for the isolation and identification of the infection-causing microbe. In the experiment, we selected healthy CSSTs that were lively, had intact body surfaces, and had no pathogenic bacteria isolated from their livers. Healthy CSSTs were (480 ± 30 g) purchased from the same CSST farm in Hubei Province, China. The number of healthy CSSTs was 726. On the farm, CSSTs live in an outside pond with an outdoor temperature of (30 ± 1 °C) and a water temperature of (25 ± 1 °C). Food is fed once a day in the morning. In the laboratory, healthy CSSTs were raised in aquariums at a room temperature of (30 ± 1 °C) and a water temperature of (25 ± 1 °C). All animal experiments were performed in accordance with the animal experiment ethics review committee of the Experimental Animal Center of the Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences (ID Number: YFI 2022-zhouyong-28).
2.2. Isolation of Pathogens
Euthanasia was performed in water containing 250 mg/L of ethyl 3-aminobenzoate methane sulfonate (MS-222) (Sigma, St. Louis, MO, USA). Then the animal was removed, placed on ice, and the diseased CSST was dissected after surface disinfection with 70% alcohol. The livers of diseased CSST were sampled in a biosafety cabinet (ESCO, Changi, Singapore) using a sterile inoculation loop. The liver samples were inoculated on agar plates consisting of brain heart infusion medium (BHI, Weymouth, MA, USA) and cultured upside down at 30 °C for 24 h. Single colonies were picked from the plates and re-cultured on BHI agar plates, and the plates were incubated under the same conditions to obtain purified single colonies. The single colonies were separately inoculated in 5 mL of BHI liquid medium and incubated at 30 °C and 200 rpm for 24 h. Glycerol stocks of the bacterial culture obtained from each purified colony were prepared in 1.5 mL sterile Eppendorf tubes, and stored in an ultra-low temperature freezer at −80 °C. The bacterial isolate was named as XC-1908.
2.3. Identification of the Pathogen
2.3.1. Morphological Characterization
We analyzed the morphological characteristics of isolate XC-1908 using a light microscope (Olympus, Tokyo, Japan). The colonies of isolate XC-1908 were resuspended in phosphate buffer solution (PBS, Procell, Wuhan, China); the mixture was smeared on glass slides, air-dried, and subjected to Gram staining (Jiancheng, Nanjing, China) [14]. For further morphological analysis, the bacterium was fixed using 2.5% glutaraldehyde solution(HEAD, Beijing, China), dehydrated, dried [15], and observed using a scanning electron microscope (Hitachi, Tokyo, Japan).
2.3.2. Biochemical Characterization of Isolate XC-1908
Isolate XC-1908 was cultured in BHI solid medium, and a single colony was inoculated in Biolog universal growth agar (BUG, Thinkfar, Wuhan, China) identification plates; the plates were incubated at 28 °C for 16–24 h, and a colony of suitable size was resuspended in IF-A inoculation fluid provided in the Biolog Bacterial Identification Kit (Biolog, Hayward, CA, USA). The turbidity of the solution was measured using the Biolog turbidimeter and adjusted (between 92% T and 98% T; as per Biolog). The inoculation fluid with the resuspended bacterial colony was added to a GEN III plate (100 μL per well) using a pipette, and the plate was incubated in the Biolog system, which recorded the absorbance of each well at different time-points, and identified the isolate.
2.3.3. 16S rRNA Gene Sequencing
Bacteria cultured at BHI for 24 h were harvested by centrifugation at 4000 rpm for 2 min, and genomic DNA of isolate XC-1908 was extracted from the bacterial cell pellet using a Bacterial DNA Kit (Tiangen, Beijing, China) as per the manufacturer’s instructions. PCR to amplify the 16S rRNA gene of the isolate was performed using universal primers (27F: 5′-AGAGTTTGATCATGGCTCAG-3′, 1492R: 5′-TACGGTTACCTTGTTACGACTT-3′) [16] for the 16S rRNA gene. The total volume of the reaction mixture was 25 μL, including 12.5 μL of PCR mix, 1 μL each of 10 μmol/L upstream and downstream primers, 1 μL of template DNA, and 9.5 μL of double-distilled water. The PCR conditions were as follows: pre-denaturation at 94 °C for 3 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and denaturation at 72 °C for 45 s, 35 cycles, and final extension at 72 °C for 10 min. The amplicons were confirmed by 1% agarose gel electrophoresis and sequenced. The sequencing results were placed on NCBI (National Center for Biotechnology Information) (https://www.ncbi.nlm.nih.gov, accessed on 1 March 2023) for sequence homology matching, and then the 16S rRNA gene sequences of A. veronii from different sources were taken to construct a phylogenetic tree by Neighbor-Joining (NJ) in MEGA7.0 (http://www.megasoftware.net/previousVersions.ph, accessed on 1 March 2023) for confidence testing with 1000 bootstrap analysis.
2.4. Biochemical Analysis of Serum Samples of CSSTs
A total of 3 mL of fresh blood was collected from the severed heads of three anesthetized naturally diseased CSSTs. A total of 3 mL of fresh blood was collected from the severed heads of the same three anesthetized healthy CSSTs and placed in separate Eppendorf tubes, which were well labeled. Repeat this experiment three times. The supernatant was obtained after being placed at 4 °C overnight and 4000 rpm for 10 min. The supernatant was transferred to a new Eppendorf tube [17], the levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) activities, total protein (TP), albumin (ALB), and globulin (GLB) in the serum samples were measured using a fully automated biochemical analyzer (Sysmex, Kobe, Japan). Perform three replicate experiments.
2.5. Artificial Infection of CSSTs Using Strain XC-1908
Isolate XC-1908 was cultured for 24 h, and the concentration of the bacterium in the broth was determined using the plate counting method. Healthy CSSTs were randomly divided into six groups (one control group and five infection groups) with 30 turtles/group. For artificial infection, five groups of CSSTs were formed based on the concentration of the bacterium used for infection (1 × 104, 1 × 105, 1 × 106, 1 × 107, and 1 × 108 colony-forming unit CFU/g of CSST). The control group was injected with the same volume of PBS. After anesthesia with MS-222, the control group was injected with 0.5 mL of sterile PBS in the peritoneal cavity of the CSSTs, and the test group was injected with 0.5 mL of different concentrations of bacteria in the peritoneal cavity of the CSSTs, respectively. After infection, they were observed continuously for 10 days at room temperature (30 ± 1 °C) in an aquarium with a water temperature of (25 ± 1 °C) and under normal air and light conditions. The number of deaths and symptoms were recorded. The median lethal (LD50) dose of strain XC-1908 to CSST was calculated using the Reed Muench method [18]. Three replicate experiments were performed.
2.6. Histopathological Analysis
Three healthy and three naturally diseased CSSTs were dissected after anesthesia. Small pieces of liver, spleen, ovaries, kidney, and intestine were taken separately and fixed in 4% paraformaldehyde for 24 h. The fixed samples were washed in running water for 12 h and then dehydrated with an ethanol gradient. The dehydrated samples were embedded in paraffin and cut into 5-μm-thick sections. After unfolding and drying on slides, the sections were stained with hematoxylin-eosin (HE) and observed using a light microscope (Olympus, Tokyo, Japan) [19].
2.7. Antibiotic Susceptibility Testing
Isolate XC-1908 was cultured for 24 h, and the concentration of the inoculum was adjusted to 1 × 108 CFU/g using sterile PBS. One hundred microliters of the solution was spread on BHI agar plates, and the plates were undisturbed for 10 min until the solution on the surface of the medium was completely absorbed. The drug-sensitive paper sheets (Hangwei, Hangzhou, China) were placed on the plates, and the plates were incubated in a thermostatic incubator (CIMO, Shanghai, Chain) at 28 °C for 24 h. The size of the inhibition zone was measured, and sensitivity was determined according to the instructions of the drug-sensitive paper sheets.
3. Results
3.1. Clinical Symptoms of Diseased CSSTs
The diseased CSSTs exhibited the following symptoms: floated on the surface of the pond, moved slowly, and exhibited ulcerated skin on their abdomen. Histological analysis revealed thin and sticky blood; large, red, swollen, and bleeding pharyngeal; and enlarged liver, spleen, and kidneys. Moreover, the intestines exhibited bleeding without food (Figure 1).
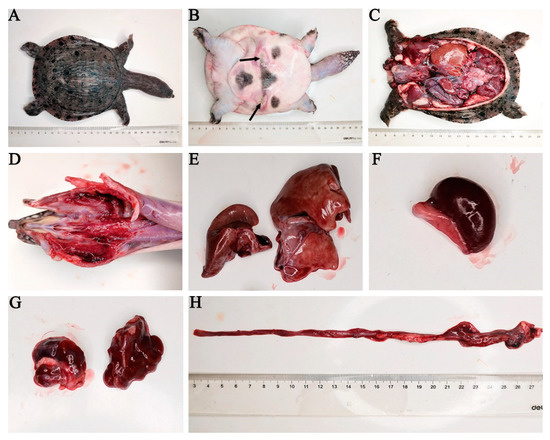
Figure 1.
Clinical symptoms of a diseased CSST. (A) The back of a diseased CSST, (B) abdominal ulceration, (C) anatomical picture of a diseased CSST, (D) redness and bleeding in the gills, (E) enlarged liver, (F) enlarged spleen, (G) kidneys of a diseased CSST, (H) bleeding in the intestine. The black arrow indicates the ulceration point.
3.2. Morphological Characterization of the Isolated Bacterium
The colonies of isolate XC-1908 were yellow in color, had a peculiar odor, and their surface was moist and smooth. They exhibited hemolysis on blood agar. Moreover, the bacterium was gram-negative and rod-shaped, without any budding cells or pods (Figure 2C). Scanning electron microscopy revealed that the bacterium was arc-shaped and approximately 2 μm in length (Figure 2D).
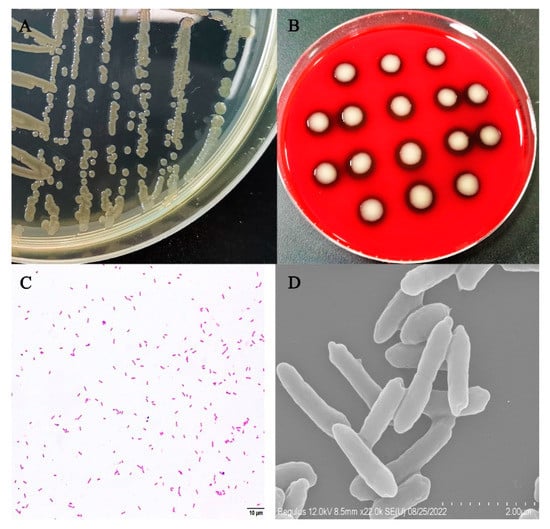
Figure 2.
Morphological characteristics of isolate XC-1908. (A) Isolate XC-1908 cultured on BHI agar, (B) isolate XC-1908 cultured on blood agar (HopeBio, Qingdao, China), (C) image of Gram-stained isolate XC-1908 (scale bar: 10 μm), (D) scanning electron micrograph of isolate XC-1908 strain (scale bar: 2 μm).
3.3. Pathogenicity of Isolate XC-1908
We observed that all concentrations of isolate XC-1908 caused mortalities. The highest mortality rate was observed in the 1 × 108 CFU/g group, with 94% mortality. The control group exhibited no mortality during the experimental period. The LD50 of isolate XC-1908 after intraperitoneal injection in CSSTs was 4.17 × 105 CFU/g. The clinical signs of CSSTs after injection of strain XC-1908 were consistent with those of naturally infected CSSTs. The mortality and survival curves of infected CSSTs are shown in Figure 3.
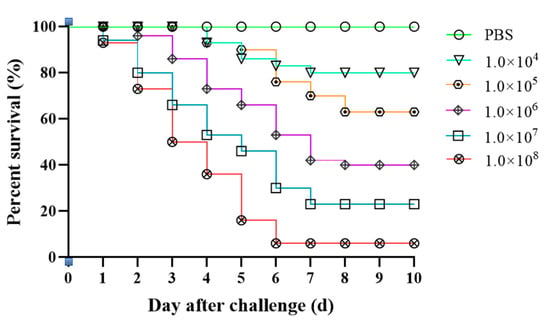
Figure 3.
Regression infection analysis of CSSTs using isolate XC-1908. Survival rate of CSSTs after injection with different concentrations of inoculum (isolate XC-1908). The control group was intraperitoneally injected with 0.5 mL of sterile PBS, and the test group was intraperitoneally injected intraperitoneally with 0.5 mL of different concentrations of bacterial culture (1 × 104, 1 × 105, 1 × 106, 1 × 107, 1 × 108 colony-forming unit CFU/g of CSST); both groups were monitored for 10 days.
3.4. 16S rRNA Gene Sequencing Analysis
As per gel electrophoresis, the length of the 16S rRNA gene amplicon of isolate XC-1908 was 1407 bp. Moreover, 16S rRNA gene sequence analysis revealed that isolate XC-1908 exhibited more than 99% homology with isolates A. veronii (MN220557.1) and A. veronii (MN752428.1) as per the NCBI database. Moreover, phylogenetic analysis revealed that isolate XC-1908 was located on the same branch as A. veronii [20] (Figure 4).
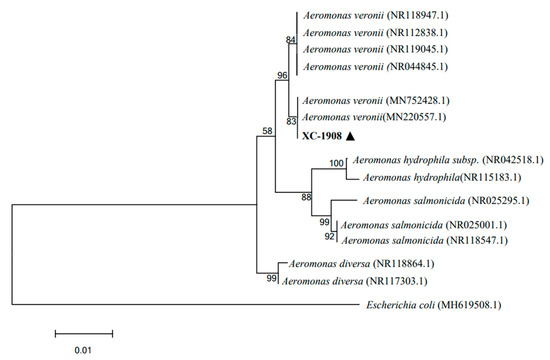
Figure 4.
Phylogenetic analysis of 16S rRNA gene sequence of isolate XC-1908. The phylogenetic tree was constructed using the neighbor-joining method in MEGA 7.0. 16S rRNA gene sequence of isolate XC-1908 was aligned with those of nine members of the genus Aeromonas; the sequences were obtained from the NCBI database. The number at each branch indicates the percentage of bootstrap values for 1000 replicates. The scale bar indicates the number of substitutions per site. The black triangle indicates the isolated pathogenic bacteria.
3.5. Biochemical Characterization of Isolate XC-1908
Isolate XC-1908 was identified as A. veronii using the Biolog fully automated microbial identification system. The biochemical characteristics of the isolate are listed in Table 1.

Table 1.
Biochemical characteristics of isolate XC-1908 as per Biolog.
3.6. Histopathological Changes in Diseased CSSTs
The liver, spleen, kidney, ovary, and intestine of the diseased CSSTs exhibited significant histopathological changes compared with those of the healthy CSSTs. Healthy turtles had intact cell structure in the liver, kidneys, intestines, spleen, and ovaries; color morphology of the tissues was normal, and no congestion was observed. Diseased CSSTs exhibited varying degrees of lesions in the tissues. The kidneys exhibited a large number of erythrocytes in the renal interstitium, and the renal glomerulus was edematous with erythrocyte infiltration (Figure 5B). High erythrocyte infiltration was observed in the infected spleen parenchyma; the red pulp was enlarged, and the necrotic splenocytes exhibited marginated nuclei (Figure 5D). The intestinal villi were disorganized, and the mucosal epithelial cells of the villi shed. Hemorrhaging was observed in the intestinal submucosa, muscle, and serosa (Figure 5F). The liver exhibited a large number of erythrocytes and inflammatory cells and high hemosiderin levels, and the number of vacuoles increased (Figure 5H). Infected oocytes exhibited red and round particles and increase in the number of vacuoles. Blood vessels around the oocytes were dilated and congested (Figure 5J). It was found that the pathological changes in various tissues of the CSSTs after the injection of the XC-1908 bacterium were the same as those of the natural-onset CSSTs.
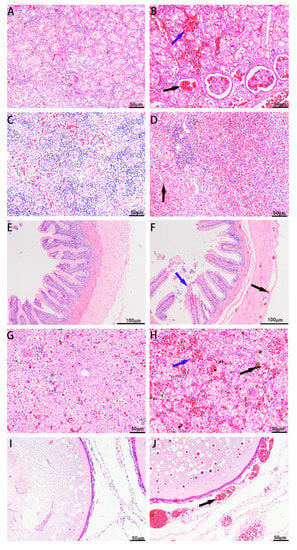
Figure 5.
Histopathological observations from healthy and diseased CSSTs. (A) Kidneys of a healthy CSST, (B) kidneys of a diseased CSST infiltrated with a large number of inflammatory cells, glomerulus atrophy; blue arrows indicate erythrocytes, and black arrow indicated renal glomerulus, (C) spleen of a healthy CSST, (D) diseased CSST spleen with a large number of red blood cells and inflammatory cells; black arrow indicates erythrocytes, (E) intestine of a healthy CSST, (F) intestinal villi shedding in a diseased CSST; blue arrow indicates epithelial cells, and black arrow indicates bleeding point, (G) liver of a healthy CSST, (H) liver of diseased CSST with high hemosiderin levels; blue arrow indicates erythrocytes, and black arrow indicates hemosiderin, (I) eggs of a healthy CSST, (J) increase in number of vacuoles in egg cells of a diseased CSST; blood vessels dilated and congested; black arrow indicates blood vessels. Scar bar = 50 μm for (A–D,G–I); for (E,F), scale bar = 100 μm.
3.7. Biochemical Analysis of Serum Samples
Biochemical analysis of the serum samples of affected CSSTs revealed that the levels of total protein, albumin, and globulin were 25.6 g/L, 7.6 g/L, and 18 g/L, respectively, which were significantly lower than those of healthy CSSTs. The levels of aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase were 594 g/L, 559 g/L, and 23.5 g/L, respectively, in the affected CSSTs, which were significantly higher than those of the healthy CSSTs (Figure 6).
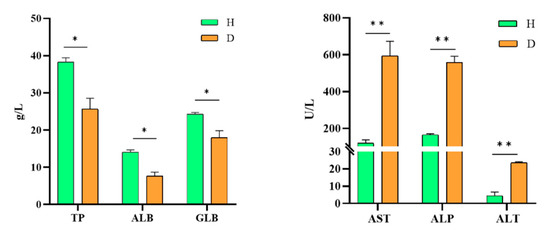
Figure 6.
Biochemical analyses of serum samples of isolate XC-1908-infected CSSTs. TP: total protein, ALB: albumin, GLB: globulin, AST: aspartate aminotransferase, AKP: alkaline phosphatase, ALT: alanine aminotransferase, H: healthy CSST, D: diseased CSST. (* p > 0.05,** p < 0.01).
3.8. Antibiotic Susceptibility Analysis
It was observed that the isolated strain A. veronii XC-1908 was sensitive to ceftriaxone, doxycycline, flupenthixol, cefradine, and gentamicin; moderately sensitive to enrofloxacin, neomycin, ampicillin, meldimycin, and polymyxin; and resistant to sulfonamide, carbenicillin, benzocillin, clindamycin, erythromycin, and streptomycin (Table 2).

Table 2.
Drug sensitivity analysis of isolate XC-1908.
4. Discussion
Recently, A. veronii infections have increased in several varieties of fish [21], such as Lateolabrax maculatus [22], Loach misgurnus anguillicaudatus [23], and Poecilia reticulata [24]. Different species of fish infected by A. veronii exhibit different symptoms, but the main symptoms were skin ulcers, organ bleeding, and severe ascites. When Cilurus asotus, Gadus, Cyprinus carpio, and Caridina cantonensis were infected with A. veronii, the main symptoms are scale shedding and ulceration in severe cases [25]. A. veronii can also infect amphibians and can cause skin decay in giant salamanders [26]. In the present study, the CSSTs infected with A. veronii exhibited ulceration of the abdomin, extensive redness, and bleeding of the pharyngeal, enlarged liver, enlarged kidneys, and bleeding of the intestine without food. Infection of CSSTs by A. veronii has been reported, and virulence genes, antibiotic susceptibility, and PCR have been described in diseased CSSTs [27]. In addition, we performed morphological analysis and bacterial physiological and biochemical identification of this bacterium. The isolated XC-1908 was Identified as A. veronii.
The LD50 of A. veronii varies for different aquatic animals [28]. The LD50 for longsnout catfish and largemouth bass was 3.47 × 104 CFU/g [29] and 3.72 × 104 CFU/g [30], respectively. In the present study, the LD50 of A. veronii XC-1908 for CSST was 4.17 × 105 CFU/g, which is higher than that for longsnout catfish and largemouth bass. A. veronii XC-1908 was peritoneally injected into CSSTs, and the clinical symptoms were consistent with those observed in case of natural infection.
Pathological diagnosis is an important clinical diagnostic tool. It is also used for the pathological analysis of aquatic animals [31]. In A. veronii-infected crucian carp, cell necrosis in several tissues and organs, internal congestion of blood vessels, and the infiltration of inflammatory cells in the kidneys was observed [21]. After being infected by A. veronii, the liver samples of Nile tilapia [32] exhibited histopathological changes with accumulation of iron-containing heme, and the spleen of Danio rerio exhibited infiltration of erythrocytes [33]. In the present study, histopathological analysis elucidated that the intestinal villi were shed, large number of erythrocytes and inflammatory cells infiltrated the spleen and kidney interstitium, and the number of inflammatory cells and the levels of hemosiderin increased in the liver. Similar histopathological features were observed in Cyprinus carpio, Tilapia nilotica, and Danio rerio infected by A. veronii in previous studies.
The serum samples of animals are important indicators of their physiological and biochemical condition [34]. In the serum biochemical subanalysis of Pseudosciaena crocea infected by Vibrio harveyi [35], the levels of TP, ALB, and GLB decreased, whereas those of AST and AKP increased. In the present study, TP, ALB, and GLB levels decreased (p < 0.05), suggesting that the CSSTs were infected and their immune system was compromised. Moreover, the levels of AST, AKP, and ALT were remarkably elevated, indicating that the liver and heart muscle of the diseased CSSTs were inflamed. These results are in accordance with the results of the biochemical analyses of the serum samples of diseased Pseudosciaena crocea [35].
The K-B paper diffusion method for testing drug sensitivity is a useful technique in the field of bacteriology and clinical pharmacology [36]. In reported reports of A. veronii infection in CSSTs, the results of drug susceptibility tests indicated that A. veronii was sensitive to streptomycin. However, in the present study, the pathogenic bacterium A. veronii XC-1908 was sensitive to ceftriaxone, doxycycline, florfenicol, cefradine, and gentamicin; moderately sensitive to enrofloxacin, neomycin, ampicillin, methicillin, and polymyxin; and resistant to sulfonamide, carbenicillin, benzocillin, clindamycin, erythromycin, and streptomycin. The sensitivity results obtained in this study differ from those already reported for drug sensitivity [27]. This may be because resistance against antibiotics varies with the genotype of the isolate. According to the results of the drug sensitivity test in this experiment and the comparison of previous literature. We found that even the same pathogenic bacteria can have different drug resistance because of their different sources. Therefore, we suggest that for the prevention and control of bacterial diseases in CSST culture, daily monitoring of bacterial drug resistance should be done so that farmers have effective antibiotic species. In the event of a disease outbreak, the correct drugs should be used to control the disease in the first instance. In the process of breeding, it is important to do a good drug sensitivity test, targeted drug use, and keep good records when using drugs. We should try to use a combination of narrow-spectrum antibiotics, combination, and rotation of drugs.
5. Conclusions
In this study, bacterium A. veronii XC-1908 was isolated from infected CSSTs. The LD50 of the bacterium was 4.17 × 105 CFU/g for CSSTs. It was highly pathogenic to the CSSTs and caused internal bleeding. The findings of this study will provide new insights into the diagnosis and treatment of infections caused by A. veronii in aquatic animals and will elucidate the pathogenesis of A. veronii infections in CSSTs.
Author Contributions
X.H. and Z.X. conceived and designed the study, performed the data collection, analysis, statistical analysis, and wrote the manuscript. B.L. and M.X. conducted the software analysis and literature review. P.C. and N.J. conducted the animal management and sample collections. F.Q. and Y.F. performed the microbial analysis, pathological analysis, and literature review. X.K. and Y.Z. contributed to acquisition of funding, conceptualization, writing—review & editing, and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Central Public-interest Scientific Institution Basal Research Fund, grant number 2020TD44 and the National Freshwater Aquatic Germplasm Resource Center, grant number FGRC18537.
Institutional Review Board Statement
All animal experiments were approved by the Animal Experimental Ethical Inspection of Laboratory Animal Centre, Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences (ID Number: YFI 2022-zhouyong-28).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lyu, S.; Yuan, X.; Liu, L.; Zhang, H.; Yu, Z.; Hang, X.; Shi, W.; Wu, Y. Application of a recombinant replicase to localize the Trionyx sinensis hemorrhagic syndrome virus and evaluate its effects on antiviral genes of T. sinensis. J. Zhejiang Univ. Sci. B 2021, 22, 295–304. [Google Scholar] [CrossRef]
- Chu, D.; Zhu, D.; Wu, H.; Li, C.; Zhang, H.; Chen, Y.; Han, X.; Liu, N.; He, Y.; Li, Y.; et al. Development of the embryonic liver and pancreas of the Chinese softshell turtleb Trionyx sinensis. J. Histotechnol. 2021, 44, 2–11. [Google Scholar] [CrossRef]
- Tang, Z.H.; Chen, B.J.; Niu, C.J. Antioxidant defense response during hibernation and arousal in Chinese soft-shelled turtle Pelodiscus sinensis juveniles. Cryobiology 2021, 99, 46–54. [Google Scholar] [CrossRef]
- Fan, N.N.; Hu, K.; Yang, Y.B.; Chen, X.X.; Yang, X.L. Isolation, identification and pathological study of sarcoidosis in Trionyx sinensis. Microbiol. China 2019, 46, 798–810. [Google Scholar]
- Lv, Z.; Hu, Y.; Tan, J.; Wang, X.; Liu, X.; Zeng, C. Comparative Transcriptome Analysis Reveals the Molecular Immunopathogenesis of Chinese Soft-Shelled Turtle (Trionyx sinensis) Infected with Aeromonas hydrophila. Biology 2021, 10, 1218. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, X.; Chen, X.; Wang, H.; Liu, J.; Liang, X.; Gu, Y.; Fang, C.; Yang, Y. Bacillus cereus causes fatal disease in soft-shelled turtle (Trionyx sinensis). Aquaculture 2022, 547, 737473. [Google Scholar] [CrossRef]
- Shen, J.Y.; Pan, X.Y.; Yu, X.P.; Yin, W.L.; Cao, Z.; Wu, Y.L. Pathogen in white abdominal shell disease of soft-shelled turtle (Trionyx sinensis). J. Fish. Sci. China 2007, 5, 815–822. [Google Scholar]
- Liu, G.X.; Li, J.; Jiang, Z.; Zhu, X.; Gao, X.; Jiang, Q.; Wang, J.; Wei, W.; Zhang, X. Pathogenicity of Aeromonas veronii causing mass mortalities of Odontobutis potamophila and its induced host immune response. Fish Shellfish Immunol. 2022, 125, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Parte, A.C.; Sarda Carbasse, J.; Meier-Kolthoff, J.P.; Reimer, L.C.; Goker, M. List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef]
- Tekedar, H.C.; Kumru, S.; Blom, J.; Perkins, A.D.; Griffin, M.J.; Abdelhamed, H.; Karsi, A.; Lawrence, M.L. Comparative genomics of Aeromonas veronii: Identification of a pathotype impacting aquaculture globally. PLoS ONE 2019, 14, 0221018. [Google Scholar] [CrossRef]
- Wu, C.J.; Wu, J.J.; Yan, J.J.; Lee, H.C.; Lee, N.Y.; Chang, C.M.; Shih, H.I.; Wu, H.M.; Wang, L.R.; Ko, W.C. Clinical significance and distribution of putative virulence markers of consecutive clinical Aeromonas isolates in southern Taiwan. J. Infect. 2007, 54, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zu, S.; Zhang, D.; Zhao, Z.; Ji, Y.; Xi, H.; Shan, X.; Qian, A.; Han, W.; Gu, J. Oral vaccination with recombinant Lactobacillus casei expressing Aha1 fused with CTB as an adjuvant against Aeromonas veronii in common carp (Cyprinus carpio). Microb. Cell. Fact. 2022, 21, 114. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.L.; Shan, X.F.; Meng, Q.F.; Guo, S.; Wang, L.; Qian, A.D. Advances in Aeromonas veronii. Chin. J. Vet. Drug 2011, 45, 41–44. [Google Scholar]
- Moyes, R.B.; Reynolds, J.; Breakwell, D.P. Differential staining of bacteria: Gram stain. Curr. Protoc. Microbiol. 2009, 15, A-3C. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, E.; Enríquez, L.; Sánchez, A.; Ovalle, M.; Olivas, A. Scanning electron microscopy of bacteria Tetrasphaera duodecadis. Scanning 2014, 36, 547–550. [Google Scholar] [CrossRef]
- Loy, A.; Lehner, A.; Lee, N.; Adamczyk, J.; Meier, H.; Ernst, J.; Schleifer, K.H.; Wagner, M. Oligonucleotide Microarray for 16S rRNA Gene-Based Detection of All Recognized Lineages of Sulfate-Reducing Prokaryotes in the Environment. Appl. Environ. Microb. 2002, 68, 5064–5081. [Google Scholar] [CrossRef]
- Lin, X.X.; Ye, Y.T.; Wu, P.; Teng, F.; Wang, M.; Jiang, R.; Duan, R.H.; Chen, G.; Gao, L.S.; Liu, J.C.; et al. The Injury Effect of Infection by Cyprinid Herpesvirus 2 (CyHV-2) on tissues and Organs of Gibel Carp (Carassiua Auratus Gibelio). Chin. J. Fish 2016, 29, 16–23. [Google Scholar]
- Reed, L.J. A simple method of estimating fifty percent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, X.; Xue, M.; Zhang, M.; Liu, W.; Fan, Y.; Chen, X.; Chu, Z.; Gong, F.; Zeng, L.; et al. Vibrio metschnikovii, a Potential Pathogen in Freshwater-Cultured Hybrid Sturgeon. Animals 2022, 12, 1011. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Chen, F.; Sun, J.; Han, Z.; Yang, X.; Xian, J.A.; Lv, A.; Hu, X.; Shi, H. Isolation, Identification and Characteristics of Aeromonas veronii From Diseased Crucian Carp (Carassius auratus gibelio). Front. Microbiol. 2019, 10, 2742. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hu, J.; Feng, J.; Zhang, Y.; Sun, Y.; Jiang, B.; Li, W.; Liu, C.; Huang, Y.; Su, Y. Acute septicemia and immune response of spotted sea bass (Lateolabrax maculatus) to Aeromonas veronii infection. Fish Shellfish Immunol. 2022, 124, 47–55. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, X.R.; Li, J.; Li, G.Y.; Liu, Z.P.; Mo, Z.L. Identification and virulence properties of Aeromonas veronii bv. sobria isolates causing an ulcerative syndrome of loach Misgurnus anguillicaudatus. J. Fish. Dis. 2016, 39, 777–781. [Google Scholar] [CrossRef]
- Lazado, C.C.; Zilberg, D. Pathogenic characteristics of Aeromonas veronii isolated from the liver of a diseased guppy (Poecilia reticulata). Lett. Appl. Microbiol. 2018, 67, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.L.; Cheung, W.K.; Janda, J.M. The genus Aeromonas: Biochemical characteristics, atypical reactions, and phenotypic identification schemes. J. Clin. Microbiol 2003, 41, 2348–2357. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Gui, Y.Q.; Yan, L.; Lan, Z.; Hui, Z. Isolation and identification on pathogenic bacteria of “rotten skin” disease in Chinese giant salamander (Andrias davidianus). Chin. J. Zoonoses 2010, 26, 944–948. [Google Scholar]
- Ye, Y.; Jiang, Y.; Fan, T.; Jiang, Q.; Cheng, Y.; Lu, J.; Lin, L. Resistance characterization, virulence factors, and ERIC-PCR fingerprinting of Aeromonas veronii strains isolated from diseased Trionyx sinensis. Foodborne Pathog. Dis. 2012, 11, 1053–1055. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Zhao, X.; Adam, F.E.A.; Xie, Q.; Feng, H.; Ding, J.; Bai, X.; Wang, J.; Yang, Z. Isolation and Identification of Aeromonas veronii in Sheep with Fatal Infection in China: A Case Report. Microorganisms 2023, 11, 333. [Google Scholar] [CrossRef]
- Cai, S.H.; Wu, Z.H.; Jian, J.C.; Lu, Y.S.; Tang, J.F. Characterization of pathogenic Aeromonas veronii bv. veronii associated with ulcerative syndrome from chinese longsnout catfish (Leiocassis longirostris Günther). Braz. J. Microbiol. 2012, 43, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Pei, C.; Song, H.; Zhu, L.; Qiao, D.; Yan, Y.; Li, L.; Zhao, X.; Zhang, J.; Jiang, X.; Kong, X. Identification of Aeromonas veronii isolated from largemouth bass (Micropterus salmoides) and histopathological analysis. Aquaculture 2021, 540, 736707. [Google Scholar] [CrossRef]
- Xiao, Z.; Xue, M.; Wu, X.; Zeng, L.; Zhu, Y.; Jiang, N.; Fan, Y.; Zhou, Y. Isolation and identification of Staphylococcus warneri from diseased Coreius guichenoti. Aquac. Rep. 2022, 22, 100988. [Google Scholar] [CrossRef]
- Dong, H.T.; Techatanakitarnan, C.; Jindakittikul, P.; Thaiprayoon, A.; Taengphu, S.; Charoensapsri, W.; Khunrae, P.; Rattanarojpong, T.; Senapin, S. Aeromonas jandaei and Aeromonas veronii caused disease and mortality in Nile tilapia, Oreochromis niloticus (L.). J. Fish Dis. 2017, 40, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Qamar, A.; Waheed, J.; Zhang, Q.; Namula, Z.; Chen, Z.; Chen, J.J. Immunotoxicological effects of dioxin-like polychlorinated biphenyls extracted from Zhanjiang Bay sediments in zebrafish. Environ. Monit. Assess. 2020, 192, 479. [Google Scholar] [CrossRef]
- Wang, Y.W.; Field, C.J.; Sim, J.S. Dietary polyunsaturated fatty acids alter lymphocyte subset proportion and proliferation, serum immunoglobulin G concentration, and immune tissue development in chicks. Poult. Sci. 2000, 79, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.J.; Xu, B.; Wang, J.; Su, Y.Q.; Zhang, Z.W.; Chen, X. Studies on blood chemistry indices and histopathology of Pseudosciaena crocea artificially challenged with Vibrio harveyi. J. Fish. China 2010, 34, 618–625. [Google Scholar] [CrossRef]
- Tan, Y.; Zhao, Q.; Shu, W.Q.; Chen, H. K-B Paper Diffusion Method Drug Sensitivity Test. Clin. Lab. Med. 2010, 7, 2290–2291. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).