The Diagnosis, Pathophysiology, and Treatment of Chronic Hepatitis E Virus Infection—A Condition Affecting Immunocompromised Patients
Abstract
1. Introduction
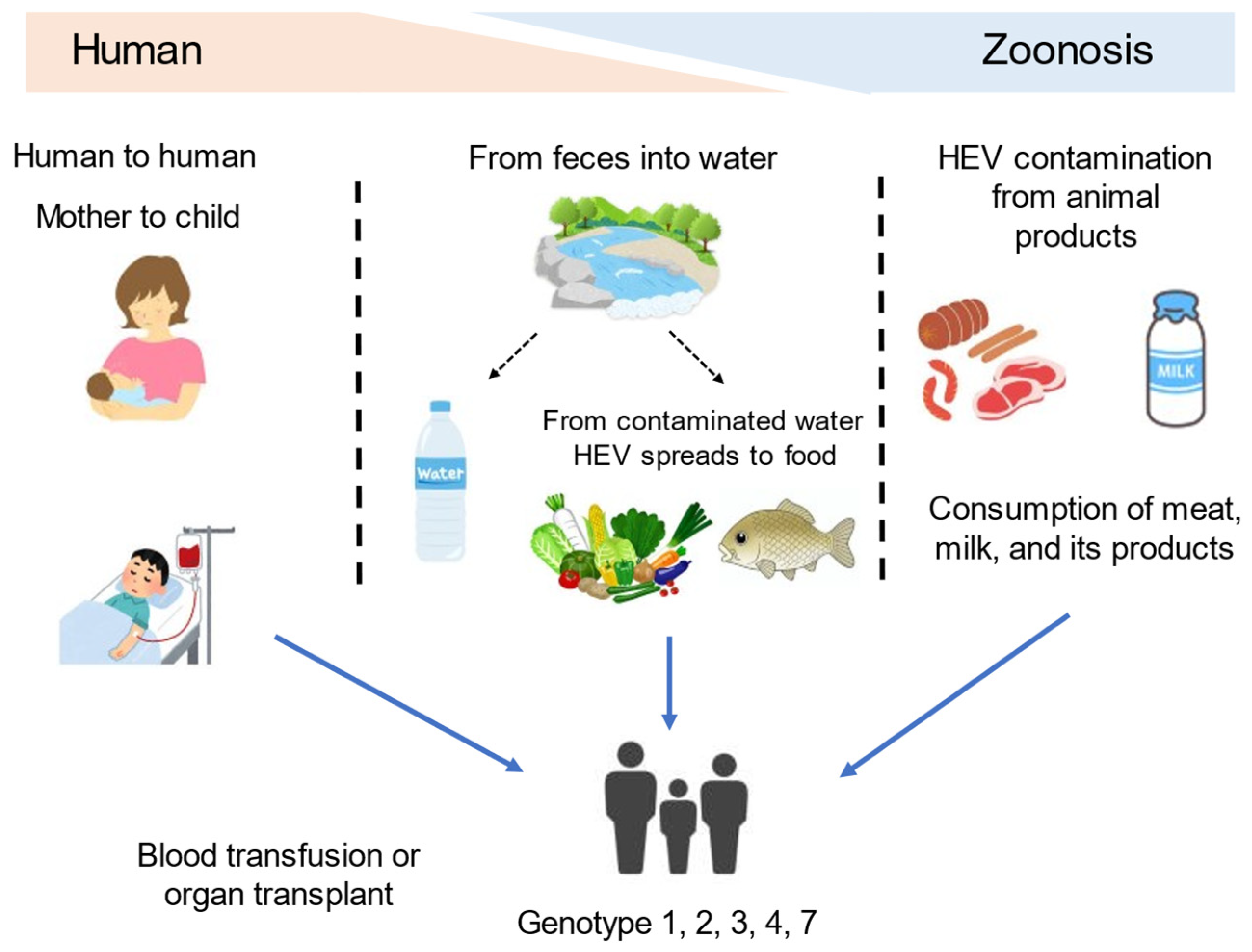
2. Hepatitis E Virus Overview
3. Chronic Hepatitis E (CHE)
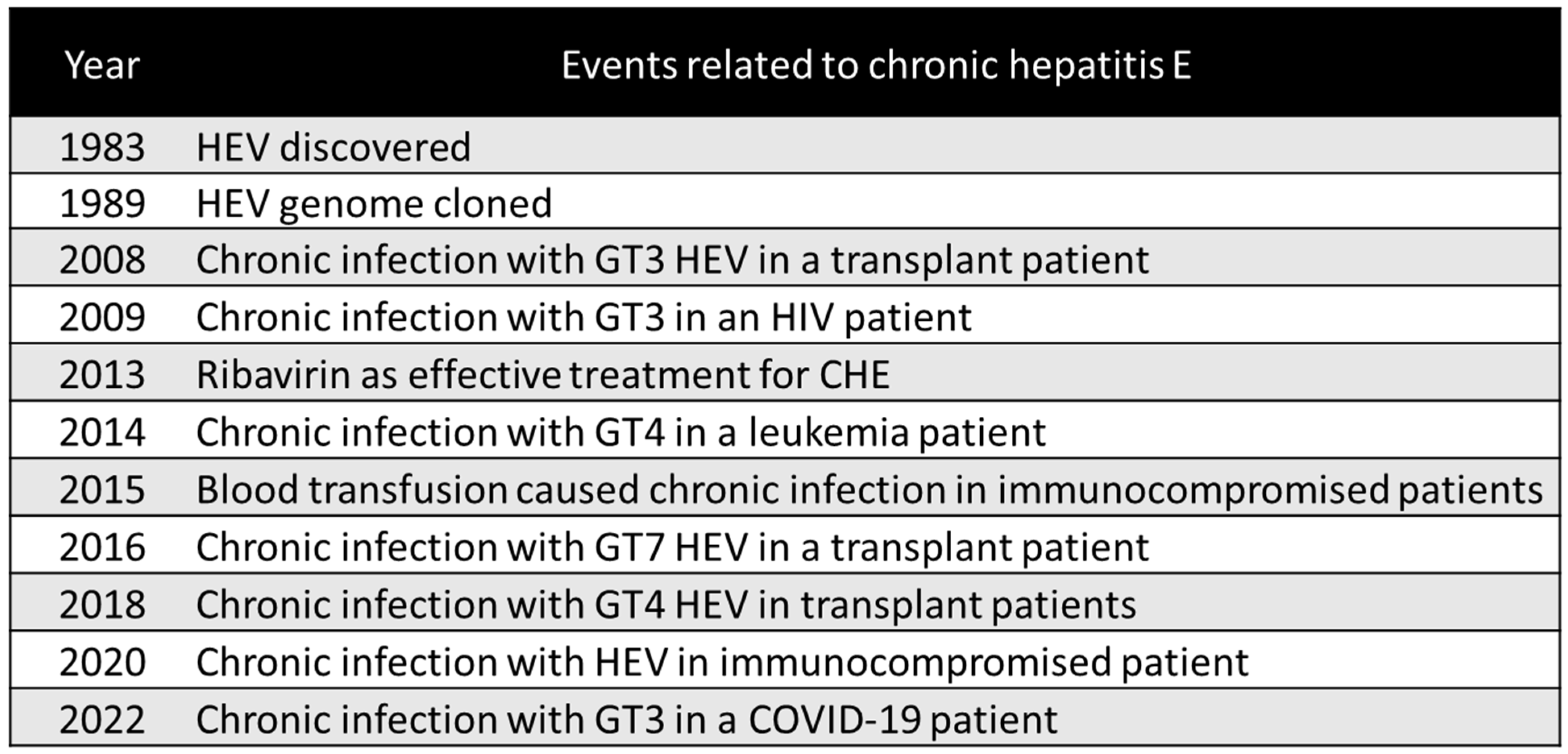
3.1. Definition of CHE and Its Historical Occurrence
3.2. Pathogenesis of CHE
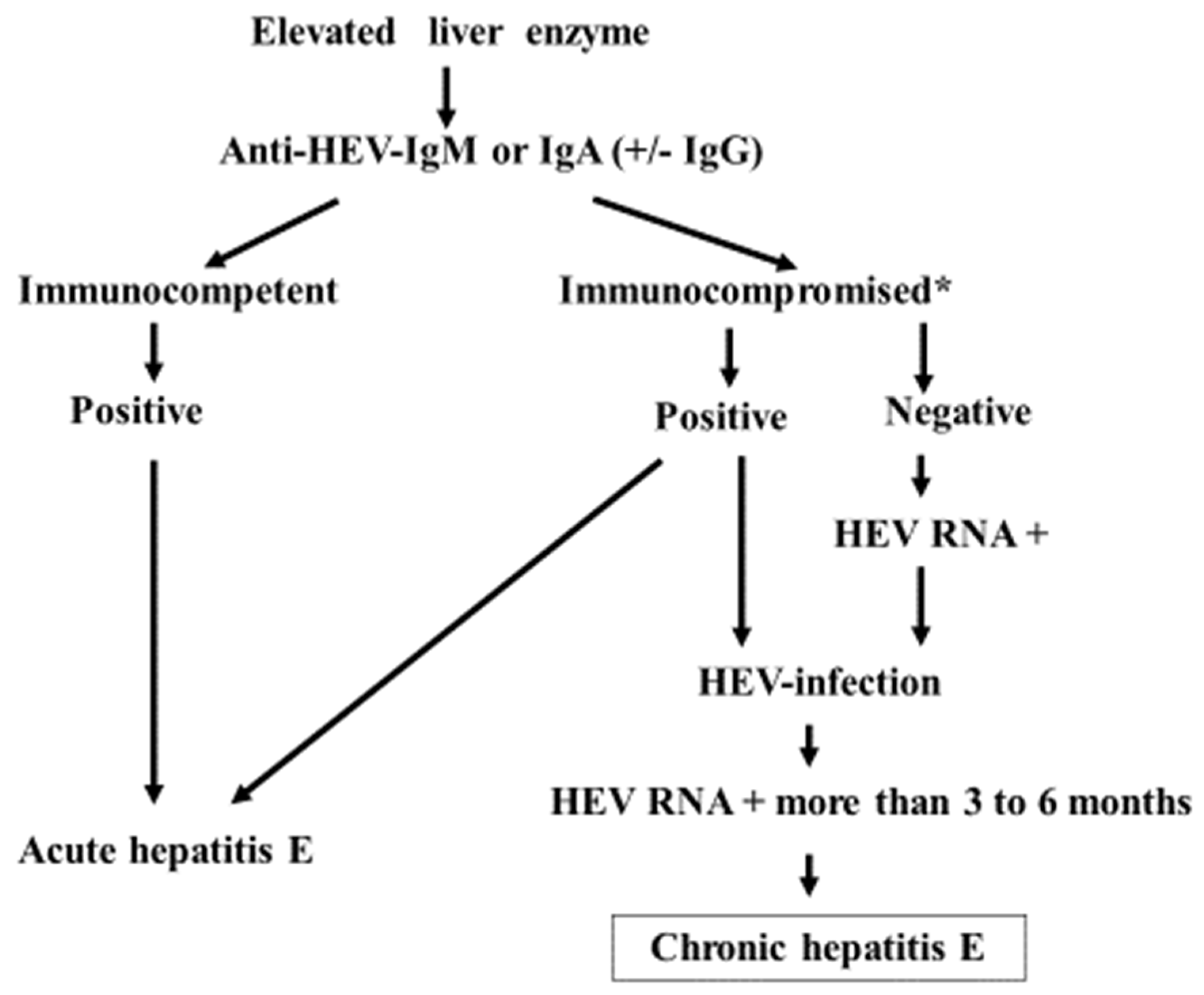
3.3. The Diagnosis and Clinical Course of CHE
3.4. Prevention and Treatment for CHE
3.5. Prognosis of CHE
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pallerla, S.R.; Harms, D.; Johne, R.; Todt, D.; Steinmann, E.; Schemmerer, M.; Wenzel, J.J.; Hofmann, J.; Shih, J.W.K.; Wedemeyer, H.; et al. Hepatitis E virus infection: Circulation, molecular epidemiology, and impact on global health. Pathogens 2020, 9, 856. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Hepatitis, E. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e (accessed on 20 February 2023).
- Nimgaonkar, I.; Ding, Q.; Schwartz, R.E.; Ploss, A. Hepatitis E virus: Advances and challenges. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, P.; Steinmann, E.; Manns, M.P.; Wedemeyer, H. The impact of hepatitis E in the liver transplant setting. J. Hepatol. 2014, 61, 1418–1429. [Google Scholar] [CrossRef] [PubMed]
- Borentain, P.; Colson, P.; Bolon, E.; Gauchez, P.; Coso, D.; Gerolami, R. Hepatocellular carcinoma complicating hepatitis E virus related cirrhosis. Hepatology 2018, 67, 446–448. [Google Scholar] [CrossRef]
- Klöhn, M.; Schrader, J.A.; Brüggemann, Y.; Todt, D.; Steinmann, E. Beyond the usual suspects: Hepatitis E virus and its implications in hepatocellular carcinoma. Cancers 2021, 13, 5867. [Google Scholar] [CrossRef]
- Feldman, M.; Friedman, L.S.; Brandt, L.J. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease e-Book: Pathophysiology, Diagnosis, Management, Expert Consult Premium Edition—Enhanced Online Features; Elsevier Health Sciences: Amsterdam, The Netherlands; p. 1337.
- Tam, A.W.; Smith, M.; Guerra, M.E.; Huang, C.C.; Bradley, D.W.; Fry, K.E.; Reyes, G.R. Hepatitis E virus (HEV): Molecular cloning and sequencing of the full-length viral genome. Virology 1991, 185, 120–131. [Google Scholar] [CrossRef]
- Reyes, G.; Purdy, M.; Kim, J.; Luk, K.C.; Young, L.M.; Fry, K.E.; Bradley, D.W. Isolation of a cDNA from the virus responsible for entericallytransmitted non-A, non-B hepatitis. Science 1990, 247, 1335–1339. [Google Scholar] [CrossRef]
- Takahashi, M.; Kusakai, S.; Mizuo, H.; Suzuki, K.; Fujimura, K.; Masuko, K.; Sugai, Y.; Aikawa, T.; Nishizawa, T.; Okamoto, H. Simultaneous detection of immunoglobulin A (IgA) and IgM antibodies against hepatitis E virus (HEV) Is highly specific for diagnosis of acute HEV infection. J. Clin. Microbiol. 2005, 43, 49–56. [Google Scholar] [CrossRef]
- Song, Y.J. Studies of hepatitis E virus genotypes. Indian J. Med. Res. 2010, 132, 487–488. [Google Scholar]
- Meng, X.J. Hepatitis E virus: Animal reservoirs and zoonotic risk. Vet. Microbiol. 2010, 140, 256–265. [Google Scholar] [CrossRef]
- Kamar, N.; Dalton, H.R.; Abravanel, F.; Izopet, J. Hepatitis E virus infection. Clin. Microbiol. Rev. 2014, 27, 116–138. [Google Scholar] [CrossRef]
- Khuroo, M.S.; Khuroo, M.S.; Khuroo, N.S. Transmission of hepatitis E virus in developing countries. Viruses 2016, 8, 253. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mohanty, A.; Joshi, Y.K.; Deka, D.; Mohanty, S.; Panda, S.K. Mother-to-child transmission of hepatitis E virus infection. Indian J. Pediatr. 2003, 70, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, K.; Nagaoka, Y.; Sakata, H.; Sato, S.; Fukai, K.; Kato, T.; Takahashi, K.; Mishiro, S.; Imai, M.; Takeda, N.; et al. Transfusion-transmitted hepatitis E caused by apparently indigenous hepatitis E virus strain in Hokkaido, Japan. Transfusion 2004, 44, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Lhomme, S.; Marion, O.; Abravanel, F.; Izopet, J.; Kamar, N. Clinical manifestations, pathogenesis and treatment of hepatitis E virus infections. J. Clin. Med. 2020, 9, 331. [Google Scholar] [CrossRef] [PubMed]
- Pischke, S.; Wedemeyer, H. Hepatitis E virus infection: Multiple faces of an underestimated problem. J. Hepatol. 2013, 58, 1045–1046. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gracia, M.T.; Suay-García, B.; Mateos-Lindemann, M.L. Hepatitis E and pregnancy: Current state. Rev. Med. Virol. 2017, 27, e1929. [Google Scholar] [CrossRef]
- Bose, P.D.; Das, B.C.; Hazam, R.K.; Kumar, A.; Medhi, S.; Kar, P. Evidence of extrahepatic replication of hepatitis E virus in human placenta. J. Gen. Virol. 2014, 95, 1266–1271. [Google Scholar] [CrossRef]
- Manka, P.; Bechmann, L.P.; Coombes, J.D.; Thodou, V.; Schlattjan, M.; Kahraman, A.; Syn, W.K.; Saner, F.; Gerken, G.; Baba, H.; et al. Hepatitis E virus infection as a possible cause of acute liver failure in Europe. Clin. Gastroenterol. Hepatol. 2015, 13, 1836–1842.e2; quiz e157–158. [Google Scholar] [CrossRef]
- Davern, T.J.; Chalasani, N.; Fontana, R.J.; Hayashi, P.H.; Protiva, P.; Kleiner, D.E.; Engle, R.E.; Nguyen, H.; Emerson, S.U.; Purcell, R.H.; et al. Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology 2011, 141, 1665–1672.e9. [Google Scholar] [CrossRef]
- Tseng, T.C.; Liu, C.J.; Chang, C.T.; Su, T.H.; Yang, W.T.; Tsai, C.H.; Chen, C.L.; Yang, H.C.; Liu, C.H.; Chen, P.J.; et al. HEV superinfection accelerates disease progression in patients with chronic HBV infection and increases mortality in those with cirrhosis. J. Hepatol. 2020, 72, 1105–1111. [Google Scholar] [CrossRef]
- Qiu, L.X.; Huang, Y.; Quan, J.L.; Bi, Z.F.; Zhong, G.H.; Wang, J.Y.; Huang, S.J.; Su, Y.Y.; Wu, T.; Zhang, J.; et al. Prognosis of hepatitis E infection in patients with chronic liver disease: A meta-analysis. J. Viral Hepat. 2023, 30, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Woolson, K.L.; Forbes, A.; Vine, L.; Beynon, L.; McElhinney, L.; Panayi, V.; Hunter, J.G.; Madden, R.G.; Glasgow, T.; Kotecha, A.; et al. Extra-hepatic manifestations of autochthonous hepatitis E infection. Aliment. Pharmacol. Ther. 2014, 40, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Bazerbachi, F.; Haffar, S.; Garg, S.K.; Beynon, L.; McElhinney, L.; Panayi, V.; Hunter, J.G.; Madden, R.G.; Glasgow, T.; Kotecha, A.; et al. Extra-hepatic manifestations associated with hepatitis E virus infection: A comprehensive review of the literature. Gastroenterol. Rep. 2016, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Bendall, R.P.; Peron, J.M.; Cintas, P.; Prudhomme, L.; Mansuy, J.M.; Rostaing, L.; Keane, F.; Ijaz, S.; Izopet, J.; et al. Hepatitis E virus and neurologic disorders. Emerg. Infect. Dis. 2011, 17, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Dalton, H.R.; Kamar, N.; van Eijk, J.J.; Mclean, B.N.; Cintas, P.; Bendall, R.P.; Jacobs, B.C. Hepatitis E virus and neurological injury. Nat. Rev. Neurol. 2016, 12, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Weclawiak, H.; Guilbeau-Frugier, C.; Legrand-Abravanel, F.; Cointault, O.; Ribes, D.; Esposito, L.; Cardeau-Desangles, I.; Guitard, J.; Sallusto, F.; et al. Hepatitis E virus and the kidney in solid-organ transplant patients. Transplantation 2012, 93, 617–623. [Google Scholar] [CrossRef]
- Guinault, D.; Ribes, D.; Delas, A.; Milongo, D.; Abravanel, F.; Puissant-Lubrano, B.; Izopet, J.; Kamar, N. Hepatitis E virus-induced cryoglobulinemic glomerulonephritis in a nonimmunocompromised person. Am. J. Kidney Dis. 2016, 67, 660–663. [Google Scholar] [CrossRef]
- Del Bello, A.; Guilbeau-Frugier, C.; Josse, A.G.; Rostaing, L.; Izopet, J.; Kamar, N. Successful treatment of hepatitis E virus-associated cryoglobulinemic membranoproliferative glomerulonephritis with ribavirin. Transpl. Infect. Dis. 2015, 17, 279–283. [Google Scholar] [CrossRef]
- Pischke, S.; Hartl, J.; Pas, S.D.; Lohse, A.W.; Jacobs, B.C.; Van der Eijk, A.A. Hepatitis E virus: Infection beyond the liver? J. Hepatol. 2017, 66, 1082–1095. [Google Scholar] [CrossRef]
- Pavio, N.; Meng, X.J.; Renou, C. Zoonotic hepatitis E: Animal reservoirs and emerging risks. Vet. Res. 2010, 41, 46. [Google Scholar] [CrossRef] [PubMed]
- Satou, K.; Nishiura, H. Transmission dynamics of hepatitis E among swine: Potential impact upon human infection. BMC Vet. Res. 2007, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, T.C.; Chijiwa, K.; Sera, N.; Ishibashi, T.; Etoh, Y.; Shinohara, Y.; Kurata, Y.; Ishida, M.; Sakamoto, S.; Takeda, N.; et al. Hepatitis E virus transmission from wild boar meat. Emerg. Infect. Dis. 2005, 11, 1958–1960. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; de Man, R.A.; Kamar, N.; Pan, Q. Chronic hepatitis E: Advancing research and patient care. J. Hepatol. 2022, 77, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Tan, B.H.; Teo, E.C.; Lim, S.G.; Dan, Y.Y.; Wee, A.; Aw, P.P.; Zhu, Y.; Hibberd, M.L.; Tan, C.K.; et al. Chronic infection with camelid hepatitis E virus in a liver transplant recipient who regularly consumes camel meat and milk. Gastroenterology 2016, 150, 355–357. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL clinical practice guidelines on hepatitis E virus infection. J. Hepatol. 2018, 68, 1256–1271. [Google Scholar] [CrossRef]
- Kamar, N.; Rostaing, L.; Legrand-Abravanel, F.; Izopet, J. How should hepatitis E virus infection be defined in organ-transplant recipients? Am. J. Transplant. 2013, 13, 1935–1936. [Google Scholar] [CrossRef]
- Meisner, S.; Polywka, S.; Memmler, M.; Nashan, B.; Lohse, A.W.; Sterneck, M.; Pischke, S. Definition of chronic hepatitis E after liver transplant conforms to convention. Am. J. Transplant. 2015, 15, 3011–3012. [Google Scholar] [CrossRef]
- Kamar, N.; Garrouste, C.; Haagsma, E.B.; Garrigue, V.; Pischke, S.; Chauvet, C.; Dumortier, J.; Cannesson, A.; Cassuto-Viguier, E.; Thervet, E.; et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology 2011, 140, 1481–1489. [Google Scholar] [CrossRef]
- Suneetha, P.V.; Pischke, S.; Schlaphoff, V.; Grabowski, J.; Fytili, P.; Gronert, A.; Bremer, B.; Markova, A.; Jaroszewicz, J.; Bara, C.; et al. Hepatitis E virus (HEV)-specific T-cell responses are associated with control of HEV infection. Hepatology 2012, 55, 695–708. [Google Scholar] [CrossRef]
- Bremer, W.; Blasczyk, H.; Yin, X.; Salinas, E.; Grakoui, A.; Feng, Z.; Walker, C. Resolution of hepatitis E virus infection in CD8+ T cell-depleted rhesus macaques. J. Hepatol. 2021, 75, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qu, C.; Yu, P.; Ou, X.; Pan, Q.; Wang, W. The interplay between host innate immunity and hepatitis E Virus. Viruses 2019, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Selves, J.; Mansuy, J.M.; Ouezzani, L.; Péron, J.M.; Guitard, J.; Cointault, O.; Esposito, L.; Abravanel, F.; Danjoux, M.; et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N. Engl. J. Med. 2008, 358, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Dalton, H.R.; Bendall, R.P.; Keane, F.E.; Tedder, R.S.; Ijaz, S. Persistent carriage of hepatitis E virus in patients with HIV infection. N. Engl. J. Med. 2009, 361, 1025–1027. [Google Scholar] [CrossRef] [PubMed]
- Keane, F.; Gompels, M.; Bendall, R.; Drayton, R.; Jennings, L.; Black, J.; Baragwanath, G.; Lin, N.; Henley, W.; Ngui, S.L.; et al. Hepatitis E virus coinfection in patients with HIV infection. HIV Med. 2012, 13, 83–88. [Google Scholar] [CrossRef]
- Von Felden, J.; Alric, L.; Pischke, S.; Aitken, C.; Schlabe, S.; Spengler, U.; Giordani, M.T.; Schnitzler, P.; Bettinger, D.; Thimme, R.; et al. The burden of hepatitis E among patients with haematological malignancies: A retrospective European cohort study. J. Hepatol. 2019, 71, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolomeo, S.; Carubbi, F.; Cipriani, P. Hepatitis E virus and rheumatic diseases: What do rheumatologists need to know? BMC Rheumatol. 2020, 4, 51. [Google Scholar] [CrossRef]
- Grewal, P.; Kamili, S.; Motamed, D. Chronic hepatitis E in an immunocompetent patient: A case report. Hepatology 2014, 59, 347–348. [Google Scholar] [CrossRef]
- Höner zu Siederdissen, C.; Pischke, S.; Schlue, J.; Deterding, K.; Hellms, T.; Schuler-Lüttmann, S.; Schwarz, A.; Manns, M.P.; Cornberg, M.; Wedemeyer, H. Chronic hepatitis E virus infection beyond transplantation or human immunodeficiency virus infection. Hepatology 2014, 60, 1112–1113. [Google Scholar] [CrossRef]
- Colson, P.; Schleinitz, N.; Vely, F.; Poveda, J.D.; Jacomo, V.; Demerle, C.; Borentain, P.; Gerolami, R. Chronic hepatitis E in absence of severe immune deficiency. Clin. Res. Hepatol. Gastroenterol. 2020, 44, e1–e4. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Pan, Q.; Zhao, J. Chronic hepatitis E in an immunocompetent patient. Clin. Res. Hepatol. Gastroenterol. 2020, 44, e66–e68. [Google Scholar] [CrossRef] [PubMed]
- Takakusagi, S.; Takagi, H.; Yamazaki, Y.; Kosone, T.; Nagashima, S.; Takahashi, M.; Murata, K.; Okamoto, H. Chronic hepatitis E in an elderly immunocompetent patient who achieved a sustained virologic response with ribavirin treatment. Clin. J. Gastroenterol. 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of undernourished—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Lesourd, B.M. Nutrition and immunity in the elderly: Modification of immune responses with nutritional treatments. Am. J. Clin. Nutr. 1997, 66, 478S–484S. [Google Scholar] [CrossRef]
- Quiroga, J.A.; Llorente, S.; Castillo, I.; Rodríguez-Iñigo, E.; López-Alcorocho, J.M.; Pardo, M.; Carreño, V. Virus-specific T-cell responses associated with hepatitis C virus (HCV) persistence in the liver after apparent recovery from HCV infection. J. Med. Virol. 2006, 78, 1190–1197. [Google Scholar] [CrossRef]
- Grüner, N.H.; Gerlach, T.J.; Jung, M.C.; Diepolder, H.M.; Schirren, C.A.; Schraut, W.W.; Hoffmann, R.; Zachoval, R.; Santantonio, T.; Cucchiarini, M.; et al. Association of hepatitis C virus–specific CD8+ T cells with viral clearance in acute hepatitis C. J. Infect. Dis. 2000, 181, 1528–1536. [Google Scholar] [CrossRef]
- Kemming, J.; Gundlach, S.; Panning, M.; Huzly, D.; Huang, J.; Lütgehetmann, M.; Pischke, S.; Schulze Zur Wiesch, J.; Emmerich, F.; Llewellyn-Lacey, S.; et al. Mechanisms of CD8+ T-cell failure in chronic hepatitis E virus infection. J. Hepatol. 2022, 77, 978–990. [Google Scholar] [CrossRef]
- Lampejo, T.; Curtis, C.; Ijaz, S.; Haywood, B.; Flores, A.; Sudhanva, M.; El Bouzidi, K.; Patel, S.; Dowling, M.; Zuckerman, M. Nosocomial transmission of hepatitis E virus and development of chronic infection: The wider impact of COVID-19. J. Clin. Virol. 2022, 148, 105083. [Google Scholar] [CrossRef]
- Owada, Y.; Oshiro, Y.; Inagaki, Y.; Harada, H.; Fujiyama, N.; Kawagishi, N.; Yagisawa, T.; Usui, J.; Akutsu, N.; Itabashi, Y.; et al. A nationwide survey of hepatitis E virus infection and chronic hepatitis in heart and kidney transplant recipients in Japan. Transplantation 2020, 104, 437–444. [Google Scholar] [CrossRef]
- Kurihara, T.; Yoshizumi, T.; Itoh, S.; Harimoto, N.; Harada, N.; Ikegami, T.; Inagaki, Y.; Oshiro, Y.; Ohkohchi, N.; Okamoto, H.; et al. Chronic hepatitis E virus infection after living donor liver transplantation via blood transfusion: A case report. Surg. Case Rep. 2016, 2, 32. [Google Scholar] [CrossRef]
- Miyoshi, M.; Kakinuma, S.; Tanabe, Y.; Ishii, K.; Li, T.C.; Wakita, T.; Tsuura, Y.; Watanabe, H.; Asahina, Y.; Watanabe, M.; et al. Chronic hepatitis E infection in a persistently immunosuppressed patient unable to be eliminated after ribavirin therapy. Intern. Med. 2016, 55, 2811–2817. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Akamatsu, N.; Sakamoto, Y.; Inagaki, Y.; Oshiro, Y.; Ohkohchi, N.; Okamoto, H.; Kokudo, N. Treatment with ribavirin for chronic hepatitis E following living donor liver transplantation: A case report. Hepatol. Res. 2016, 46, 1058–1059. [Google Scholar] [CrossRef] [PubMed]
- Ankcorn, M.J.; Ijaz, S.; Poh, J.; Elsharkawy, A.M.; Smit, E.; Cramb, R.; Ravi, S.; Martin, K.; Tedder, R.; Neuberger, J. Toward systematic screening for persistent hepatitis E virus infections in transplant patients. Transplantation 2018, 102, 1139–1147. [Google Scholar] [CrossRef]
- Lenggenhager, D.; Pawel, S.; Honcharova-Biletska, H.; Evert, K.; Wenzel, J.J.; Montani, M.; Furrer, E.; Fraga, M.; Moradpour, D.; Sempoux, C.; et al. The histologic presentation of hepatitis E reflects patients’ immune status and pre-existing liver condition. Mod. Pathol. 2021, 34, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Lin, X.; Lin, Q.X.; Pu, X.; Liu, J.; Li, X.F.; Hou, J.; Liu, X.; Chen, R. Association between hepatitis B and E virus infection and hepatocellular carcinoma risk. Int. J. Cancer 2021, 148, 2974–2981. [Google Scholar] [CrossRef] [PubMed]
- Amougou Atsama, M.; Atangana, P.J.A.; Noah Noah, D.; Moundipa, P.F.; Pineau, P.; Njouom, R. Hepatitis E virus infection as a promoting factor for hepatocellular carcinoma in Cameroon: Preliminary observations. Int. J. Infect. Dis. 2017, 64, 4–8. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Friedman, S.L. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology 2012, 56, 769–775. [Google Scholar] [CrossRef]
- Ghouri, Y.A.; Mian, I.; Rowe, J.H. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J. Carcinog. 2017, 16, 1. [Google Scholar]
- Zhu, F.C.; Zhang, J.; Zhang, X.F.; Zhou, C.; Wang, Z.Z.; Huang, S.J.; Wang, H.; Yang, C.L.; Jiang, H.M.; Cai, J.P.; et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: A large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet 2010, 376, 895–902. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.F.; Huang, S.J.; Wu, T.; Hu, Y.M.; Wang, Z.Z.; Wang, H.; Jiang, H.M.; Wang, Y.J.; Yan, Q.; et al. Long-term efficacy of a hepatitis E vaccine. N. Engl. J. Med. 2015, 372, 914–922. [Google Scholar] [CrossRef]
- Li, M.; Li, S.; He, Q.; Liang, Z.; Wang, L.; Wang, Q.; Wang, L. Hepatitis E-related adverse pregnancy outcomes and their prevention by hepatitis E vaccine in a rabbit model. Emerg. Microbes Infect. 2019, 8, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Zaman, K.; Dudman, S.; Stene-Johansen, K.; Qadri, F.; Yunus, M.; Sandbu, S.; Gurley, E.S.; Overbo, J.; Julin, C.H.; Dembinski, J.L.; et al. HEV study protocol: Design of a cluster-randomised, blinded trial to assess the safety, immunogenicity and effectiveness of the hepatitis E vaccine HEV 239 (Hecolin) in women of childbearing age in rural Bangladesh. BMJ Open 2020, 10, e033702. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhang, F.; Shu, J.; Li, S.; Liang, Z.; Du, M.; Liu, X.; Liu, T.; Li, M.; Yin, X.; et al. Immunocompromised rabbit model of chronic HEV reveals liver fibrosis and distinct efficacy of different vaccination strategies. Hepatology 2022, 76, 788–802. [Google Scholar] [CrossRef]
- Kamar, N.; Lhomme, S.; Abravanel, F.; Marion, O.; Peron, J.M.; Alric, L.; Izopet, J. Treatment of HEV infection in patients with a solid-organ transplant and chronic hepatitis. Viruses 2016, 8, 222. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Izopet, J.; Tripon, S.; Bismuth, M.; Hillaire, S.; Dumortier, J.; Radenne, S.; Coilly, A.; Garrigue, V.; D’Alteroche, L.; et al. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N. Engl. J. Med. 2014, 370, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Markakis, G.E.; Papatheodoridis, G.V.; Cholongitas, E. Epidemiology and treatment of hepatitis E in the liver transplantation setting: A literature review. J. Viral Hepat. 2022, 29, 698–718. [Google Scholar] [CrossRef]
- Kupke, P.; Adenugba, A.; Schemmerer, M.; Bitterer, F.; Schlitt, H.J.; Geissler, E.K.; Wenzel, J.J.; Werner, J.M. Immunomodulation of natural killer cell function by ribavirin involves TYK-2 activation and subsequent increased IFN-γ secretion in the context of in vitro hepatitis E virus infection. Cells 2023, 12, 453. [Google Scholar] [CrossRef]
- Kamar, N.; Abravanel, F.; Behrendt, P.; Hofmann, J.; Pageaux, G.P.; Barbet, C.; Moal, V.; Couzi, L.; Horvatits, T.; De Man, R.A.; et al. Ribavirin for hepatitis E virus infection after organ transplantation: A large European retrospective multicenter study. Clin. Infect. Dis. 2020, 71, 1204–1211. [Google Scholar] [CrossRef]
- Debing, Y.; Emerson, S.U.; Wang, Y.; Pan, Q.; Balzarini, J.; Dallmeier, K.; Neyts, J. Ribavirin inhibits in vitro hepatitis E virus replication through depletion of cellular GTP pools and is moderately synergistic with alpha interferon. Antimicrob. Agents Chemother. 2014, 58, 267–273. [Google Scholar] [CrossRef]
- Todt, D.; Gisa, A.; Radonic, A.; Nitsche, A.; Behrendt, P.; Suneetha, P.V.; Pischke, S.; Bremer, B.; Brown, R.J.; Manns, M.P.; et al. In vivo evidence for ribavirin-induced mutagenesis of the hepatitis E virus genome. Gut 2016, 65, 1733–1743. [Google Scholar] [CrossRef]
- Wang, B.; Mahsoub, H.M.; Li, W.; Heffron, C.L.; Tian, D.; Hassebroek, A.M.; LeRoith, T.; Meng, X.J. Ribavirin treatment failure-associated mutation, Y1320H, in the RNA-dependent RNA polymerase of genotype 3 hepatitis E virus (HEV) enhances virus replication in a rabbit HEV infection model. mBio 2023, e0337222, online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Rostaing, L.; Abravanel, F.; Garrouste, C.; Esposito, L.; Cardeau-Desangles, I.; Mansuy, J.M.; Selves, J.; Peron, J.M.; Otal, P.; et al. Pegylated interferon-alpha for treating chronic hepatitis E virus infection after liver transplantation. Clin. Infect. Dis. 2010, 50, e30–e33. [Google Scholar] [CrossRef] [PubMed]
- Haagsma, E.B.; Riezebos-Brilman, A.; van den Berg, A.P.; Porte, R.J.; Niesters, H.G. Treatment of chronic hepatitis E in liver transplant recipients with pegylated interferon alpha-2b. Liver Transpl. 2010, 16, 474–477. [Google Scholar] [CrossRef]
- Rostaing, L.; Modesto, A.; Baron, E.; Cisterne, J.M.; Chabannier, M.H.; Durand, D. Acute renal failure in kidney transplant patients treated with interferon alpha 2b for chronic hepatitis C. Nephron 1996, 74, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Cornberg, M.; Pischke, S.; Müller, T.; Behrendt, P.; Piecha, F.; Benckert, J.; Todt, D.; Steinmann, E.; Papkalla, A.; von Karpowitz, M.; et al. Sofosbuvir monotherapy fails to achieve HEV RNA elimination in patients with chronic hepatitis E—The HepNet SofE pilot study. J. Hepatol. 2020, 73, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, T.; Kobayashi, T.; Jirintai, S.; Nagashima, S.; Primadharsini, P.P.; Nishizawa, T.; Okamoto, H. Antiviral candidates against the hepatitis E virus (HEV) and their combinations inhibit HEV growth in in vitro. Antiviral. Res. 2019, 170, 104570. [Google Scholar] [CrossRef] [PubMed]
- Taton, B.; Moreau, K.; Lepreux, S.; Bachelet, T.; Trimoulet, P.; De Ledinghen, V.; Pommereau, A.; Ronco, P.; Kamar, N.; Merville, P.; et al. Hepatitis E virus infection as a new probable cause of de novo membranous nephropathy after kidney transplantation. Transpl. Infect. Dis. 2013, 15, E211–E215. [Google Scholar] [CrossRef]
- Van Ton, A.M.P.; Gevers, T.J.G.; Drenth, J.P.H. Antiviral therapy in chronic hepatitis E: A systematic review. J. Viral Hepat. 2015, 22, 965–973. [Google Scholar] [CrossRef]
| Case | Age (Years) | Sex | Underlying Disease | Immunosuppressant | HEV Sub-Genotype | Anti-HEV IgG | Anti-HEV IgM | Anti-HEV IgA | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 30 | M | DCM, after heart transplantation | PSL, CyA, MMF | 3b | − | − | + | [61] |
| 2 | 33 | M | CRF, after kidney transplantation | PSL, CyA, MMF | 3a | + | − | + | |
| 3 | 25 | M | RN, after kidney transplantation | PSL, CyA, MMF | 3a | + | + | + | |
| 4 | 41 | M | HCC, LC-NASH, after liver transplantation | PSL, tacrolimus, MMF | 3b | + | + | − | [62] |
| 5 | 37 | F | Complete remission of Burkitt lymphoma | Rit, CPA, VCR, DXR, Ara-C, DEX | 3 | N.D. | N.D. | − | [63] |
| 6 | 60 | F | PBC, after liver transplantation | tacrolimus, MMF | 3 | + | N.D. | N.D. | [64] |
| 7 | 76 | F | Hypertension, dyslipidemia | PSL, AZP | 3a | + | + | − | [54] |
| Patients/Recipients | Recommended Treatment | |
|---|---|---|
| Patients on the waiting list for organ transplantation Pregnant women living in endemic regions | Vaccination | |
| Immunocompromised CHE patients | Reduction in immunosuppressive therapy RBV | |
| Immunocompetent CHE patients | RBV | |
| Extrahepatic manifestations | Neurological disorders | Intravenous immunoglobulin, corticosteroids, RBV |
| Glomerulonephritis associated with CHE | RBV, corticosteroids | |
| Acute pancreatitis | Supportive treatments | |
| Thrombocytopenia | No any specific treatments | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takakusagi, S.; Kakizaki, S.; Takagi, H. The Diagnosis, Pathophysiology, and Treatment of Chronic Hepatitis E Virus Infection—A Condition Affecting Immunocompromised Patients. Microorganisms 2023, 11, 1303. https://doi.org/10.3390/microorganisms11051303
Takakusagi S, Kakizaki S, Takagi H. The Diagnosis, Pathophysiology, and Treatment of Chronic Hepatitis E Virus Infection—A Condition Affecting Immunocompromised Patients. Microorganisms. 2023; 11(5):1303. https://doi.org/10.3390/microorganisms11051303
Chicago/Turabian StyleTakakusagi, Satoshi, Satoru Kakizaki, and Hitoshi Takagi. 2023. "The Diagnosis, Pathophysiology, and Treatment of Chronic Hepatitis E Virus Infection—A Condition Affecting Immunocompromised Patients" Microorganisms 11, no. 5: 1303. https://doi.org/10.3390/microorganisms11051303
APA StyleTakakusagi, S., Kakizaki, S., & Takagi, H. (2023). The Diagnosis, Pathophysiology, and Treatment of Chronic Hepatitis E Virus Infection—A Condition Affecting Immunocompromised Patients. Microorganisms, 11(5), 1303. https://doi.org/10.3390/microorganisms11051303







