Abstract
Recently, the presence of melatonin in fermented beverages has been correlated with yeast metabolism during alcoholic fermentation. Melatonin, originally considered a unique product of the pineal gland of vertebrates, has been also identified in a wide range of invertebrates, plants, bacteria, and fungi in the last two decades. These findings bring the challenge of studying the function of melatonin in yeasts and the mechanisms underlying its synthesis. However, the necessary information to improve the selection and production of this interesting molecule in fermented beverages is to disclose the genes involved in the metabolic pathway. So far, only one gene has been proposed as involved in melatonin production in Saccharomyces cerevisiae, PAA1, a polyamine acetyltransferase, a homolog of the vertebrate’s aralkylamine N-acetyltransferase (AANAT). In this study, we assessed the in vivo function of PAA1 by evaluating the bioconversion of the different possible substrates, such as 5-methoxytryptamine, tryptamine, and serotonin, using different protein expression platforms. Moreover, we expanded the search for new N-acetyltransferase candidates by combining a global transcriptome analysis and the use of powerful bioinformatic tools to predict similar domains to AANAT in S. cerevisiae. The AANAT activity of the candidate genes was validated by their overexpression in E. coli because, curiously, this system evidenced higher differences than the overexpression in their own host S. cerevisiae. Our results confirm that PAA1 possesses the ability to acetylate different aralkylamines, but AANAT activity does not seem to be the main acetylation activity. Moreover, we also prove that Paa1p is not the only enzyme with this AANAT activity. Our search of new genes detected HPA2 as a new arylalkylamine N-acetyltransferase in S. cerevisiae. This is the first report that clearly proves the involvement of this enzyme in AANAT activity.
1. Introduction
After the discovery of melatonin outside the animal kingdom, research on melatonin in other clades emerged. Thus, melatonin was found to be a ubiquitous phylogenetically ancient molecule in almost every organism, from primitive photosynthetic bacteria to humans [1]. For melatonin synthesis, the majority of studies have been performed in vertebrates, particularly in mammals, and more recently in plants [2]. The occurrence of melatonin in yeast was described for the first time by Sprenger et al. [3] as a product of the metabolism of precursors such as tryptophan, serotonin, N-acetylserotonin, and 5-methoxytryptamine. These results have been later confirmed and extended by numerous studies demonstrating yeast is responsible for the biosynthesis of this molecule in a fermentative context, bringing the challenge of studying the function of melatonin in yeast and the mechanisms underlying its synthesis [4,5,6,7]. Melatonin exerts multiple physiological roles on different organisms, from regulating biorhythms and aging, modulating immune system response, inhibiting tumor growth, and protecting from UV light, among others [8,9,10]. In the case of Saccharomyces cerevisiae, it has been empirically demonstrated that melatonin has a protective role against oxidizing agents and UV light [11,12,13]. Regarding the melatonin biosynthetic pathway, there is a high degree of conservation of the enzymatic reactions that lead to melatonin synthesis from tryptophan. It is the order of these reactions that characterizes the biosynthetic route in yeasts. S. cerevisiae seems to convert tryptophan to tryptamine in the first decarboxylation step, followed by hydroxylation to form serotonin. Then, melatonin is formed from serotonin by N-acetylation followed by O-methylation of N-acetylserotonin or, alternatively, by an O-methylation of serotonin to form 5-methoxytryptamine followed by its N-acetylation, which is the preferred alternative for S. cerevisiae, although further evidence suggests more branches on the pathway in which the tryptophan as a precursor is [14]. Therefore, the classical melatonin pathway model for vertebrates does not seem to apply to yeast.
Despite all the advances in melatonin biosynthesis in yeast, there is still uncertainty around the specific genes involved in the route. Only one gene has been described and characterized as involved in melatonin production, PAA1, a polyamine acetyltransferase, homolog of the vertebrate’s aralkylamine N-acetyltransferase (AANAT), which can acetylate serotonin to N-acetylserotonin and 5-methoxytryptamine to melatonin [15], while the remaining genes and enzymes of the route are still unknown. Therefore, the search for genes homologous to those described in vertebrates and plants in S. cerevisiae represents a challenging goal and a key point to improve the synthesis of these molecules during fermentation processes in which S. cerevisiae participates.
In this study, we assessed the in vivo function of PAA1 by evaluating the bioconversion of the different possible substrates, such as 5-methoxytryptamine, tryptamine, and serotonin using different protein expression platforms. To that aim, we overexpressed the PAA1 gene, and the aralkylamine N-acetyltransferase of Bos taurus (BtAANAT) as a positive control, in S. cerevisiae and Escherichia coli, and we measured the production of acetylated metabolites after a precursor pulse into the media. As the results evidenced the presence of alternative enzymes with AANAT activity in S. cerevisiae, we expanded the search for N-acetyltransferase candidates by combining a global transcriptional expression analysis (RNAseq), under melatonin synthesis conditions, and the use of powerful bioinformatic tools. This strategy has allowed us to propose new candidates to explain melatonin-related acetylation activity in yeast.
2. Materials and Methods
2.1. Strains and Culture Media
Strain E. coli NZYa (NzyTech, Lisboa, Portugal) was used as a cloning host for plasmid construction and amplification. Strain E. coli Rosetta(DE3) competent cells (Novagen, Darmstadt, Germany) were used to enhance the expression of eukaryotic proteins that contain codons rarely used in E. coli. E. coli cells were cultured in LB medium containing 10 g·L−1 of tryptone, 5 g·L−1 of yeast extract, and 5 g·L−1 of NaCl supplemented with 100 µg·L−1 of ampicillin and 34 µg·L−1 chloramphenicol to maintain plasmids at 37 °C. A 2xTY medium consisted of 16 g·L−1 of tryptone, 10 g·L−1 of yeast extract, and 5 g·L−1 of NaCl.
Yeast strain BY4743 without plasmids was maintained and grown in YPD medium (20 g·L−1 glucose, 20 g·L−1 peptone, 10 g·L−1 yeast extract) whereas strains carrying plasmids were maintained and grown in SC without uracil (20 g·L−1 glucose, 1.7 g·L−1 yeast nitrogen base (YNB) without amino acids and ammonium sulfate (BD Difco, Sparks, MD, USA), 5 g·L−1 ammonium sulfate and 1.9 g·L−1 of SC-ura drop-out powder (Formedium, Swaffham, UK)), both supplemented with 16 g·L−1 agar (Condalab, Madrid, Spain) for solid media at 28 °C.
2.2. Plasmid Construction
The plasmids and primers herein used are listed in Table 1 and Table 2, respectively. Genes from Saccharomyces cerevisiae PAA1, ARD1, NAT4, GNA1, YIR042C, HPA2, NAT3, and HAT1 were PCR amplified from genomic DNA of yeast strain BY4743 and BtAANAT was amplified from the plasmid pCfB2628 [16]. For amplification, Phusion DNA polymerase (Thermo Scientific, Waltham, MA, USA) and the primer pairs AANAT F BamHI/AANAT R XhoI, PAA1 F BamHI/PAA1 R XhoI, NAT4 F BamHI/NAT4 R XhoI, GNA1 F BamHI/GNA1 R XhoI, YIR042C F BamHI/YIR042C R XhoI, HPA2 F BamHI/HPA2 R XhoI, and NAT3 F BamHI/NAT3 R XhoI, introducing a BamHI site and an XhoI site, were, respectively, used. In the case of HAT1 and ARD1 genes, HAT1 F EcoRI/HAT1 R XhoI and ARD1 F EcoRI/ARD1 R XhoI, introducing an EcoRI site and an XhoI site, were used. The resulting PCR fragments were digested with BamHI or EcoRI and XhoI and cloned next to the GPD promoter of the opened plasmid p426GPD [17] or pGEX-5X-1. The resulting plasmids (Table 1) were transformed into E. coli and the transformants were screened by colony PCR and sequenced using the pair primers GPDPro-F/CYC1-R and pGEX seq F/ pGEX seq R for p426GPD and pGEX-5X-1, respectively.

Table 1.
Plasmids used in this study.

Table 2.
Primers used in this study.
2.3. Bioconversion Assays
To overexpress heterologous genes in E. coli, the plasmids pGEX-5X-1 constructed with the different candidate genes (Table 1) were transformed into Rosetta™ (DE3) competent cells (Novagen), empty vector was also transformed and used as negative control. Transformants were grown under continuous shaking at 37 °C overnight in 15 mL tubes with 5 mL of LB medium, supplemented with 100 µg·L−1 ampicillin and 34 µg·L−1 chloramphenicol. The next day, 15 µL of grown preculture were inoculated into 1.5 mL of 2xTY medium with 1% glucose, supplemented with ampicillin and chloramphenicol at the same concentrations mentioned above. Cultures were grown at 37 °C until OD600 reached 0.6, after that, 0.25 mM of IPTG and 1 mM of the desired precursor were added to the culture. The culture was further grown for 24 h, at 28 °C under 300 rpm orbital shaking, then samples were taken and stored at −20 °C until extraction and HPLC analysis.
To overexpress the different genes in S. cerevisiae, a vector-based constitutive overexpression system was employed. To overexpress the genes of interest, high-copy number vector p426GPD, which enables strong constitutive expression by using GPD (TDH3) promoter, was used to clone the candidate genes and transformed them into BY4743 yeast strain (EUROSCARF, Oberursel, Germany). Empty vector was also transformed and used as a negative control in the assays. Individual transformants were grown overnight in 1.5 mL tubes containing 0.8 mL of SC without uracil (SC-ura) medium at 28 °C under continuous shaking at 150 rpm, then 30 µL of the grown pre-inoculum was inoculated into 1.5 mL of fresh SC-ura medium in a 24 well microtiter plate with 2 mL well capacity. Plates were incubated at 28 °C under constant shaking in a microplate orbital shaker at 300 rpm and 1 mM of precursor was added to the culture when late exponential phase (0.6 to 0.8 OD600) was reached. Samples were taken after 50 h and stored at −20 °C until extraction and analysis.
2.4. Drop Test
After growth on SC at 28 °C up to the stationary phase, the cells were harvested by centrifugation, washed with sterile water, resuspended in sterile water to an OD600 value of 0.5, and followed by serial dilution. From each dilution, 3.5 µL were spotted onto SC-ura with or without pantothenate agar plates. Plates were incubated at 28 for 2 and 5 days.
2.5. Gene Expression Analysis
To study global gene expression under melatonin synthesis conditions, preinocula of BY4743 were grown overnight in SC medium and inoculated in 250 mL shake flasks containing 100 mL of SC medium to an initial OD600 of 0.15 and incubated at 28 °C with 150 rpm orbital shaking. When cells reached OD600 of 0.8, a supplementation of 5-methoxytryptamine was added to a final concentration of 1 mM. Non-supplemented cultures were used as negative controls and 12.5 mL of each culture were taken as samples 15 and 45 min after supplementation. For each sample, cells were pelleted and snap-frozen with liquid nitrogen and stored at −80 °C for further RNA extraction. Supernatant was also collected to determine melatonin production under these experimental conditions by HPLC-MS/MS.
To assess the overexpression of PAA1 in yeast we performed quantitative PCR (qPCR) on cells bearing plasmid p426GPD PAA1 and the wild-type control. Cells were grown overnight in SC-ura and SC media, respectively, then inoculated in shake flasks as described above. When they reached an OD600 of 0.8 cells were pelleted, snap-frozen, and stored at −80 °C until RNA extraction.
To extract RNA, cell pellets were resuspended in 0.4 mL of LETS buffer [0.1 M LiCl, 0.01 M EDTA, pH 8.0, 0.01 MTris-HCl, pH 7.4, and 0.2% (w/v) SDS] and added to 2 mL screw tubes containing 0.4 mL of phenol (pH 4.5)-chloroform (5:1) and 0.3 mL of glass beads. Then, cells were ruptured with a Tehtnica MillMix 20 homogenizer (Tehtnica, Zelezniki, Slovenia). Supernatants were extracted with phenol–chloroform (5:1) and chloroform-isoamyl alcohol (24:1). RNA was precipitated twice overnight at −20 °C, first by adding 2.5 volumes of 96% ethanol and 0.1 volume of 5 M LiCl, and secondly by adding 2.5 volumes of 96% ethanol and 0.1 volume of 3 M sodium acetate. RNA was finally resuspended in RNase-free MilliQ water, and the concentration was determined in a NanoDrop spectrophotometer (Thermo Scientific, USA).
RNA sequencing was performed by SCSIE (University of Valencia, Valencia, Spain). Briefly, RNA quality was determined with a Bioanalyzer 2100 (Agilent, Santa Clara, CA, USA), and concentration was measured with a Qubit®2.0 (Life Technologies, Carlsbad, CA, USA), yielding RNA integrity numbers (RIN) between 9.4 and 9.7, thus indicating nearly intact RNA, and concentrations ranged between 400 and 900 ng/mL across all samples. Libraries for RNA-Seq were prepared with TruSeq® stranded mRNA Kit (Illumina, San Diego, CA, USA) following the manufacturer’s protocol and subsequently sequenced on an Illumina HiSeq 2500 with 2 × 75 bp paired-end reads. Adapters from raw reads were trimmed with trimmomatic. Adapter content and read quality were assessed with FastQC, then reads were mapped to the S288c genome with Bowtie2, and count tables were obtained with htseq-count. The gene expression abundance was normalized by log2-CPM (counts per million, corrected for the different library sizes, expressed in a log2 scale) using edgeR (v3.36.0). limma package (v3.50.3) was used to estimate log2-CPM mean variance, and establish the different contrasts of hypothesis, and an empirical Bayes moderation of the standard errors was applied to increase statistical power of differentially expressed genes (DEGs). Significance (p < 0.05) was accounted for on the corrected p-value obtained from the tests applied by eBayes module (limma package). Whole statistical computing was run on R software (v4.1.3).
Enrichment of GO terms and gene clustering was analyzed based on the identified DEGs. Specific gene functions and biological pathways were annotated according to SGD http://www.yeastgenome.org (accessed on 7 February 2023) and UniProt http://www.uniprot.org/ (accessed on 7 February 2023). The interaction networks of DEGs were obtained using the STRING v11.5 database http://string-db.org/ (accessed on 7 February 2023).
RNA used in qPCR analysis was treated for 15 min at 25 °C with DNase I RNase-free (Roche, Basil, Switzerland) according to the manufacturer’s recommendations using 1 µg of total RNA from each sample. NZY First-Strand cDNA Synthesis kit (NZTtech, Lisboa, Portugal) was used to synthesize cDNA from the DNase I-treated RNA following the manufacturer’s protocol. Quantitative real-time PCR was performed in a Light Cycler 480 II (Roche) using the SYBR Premix Ex Taq kit (TaKaRa, Shiga, Japan) for fluorescent labeling. For this purpose, 2.5 µL cDNA was added to each reaction at a final volume of 10 µL. The real-time PCRs were performed using 0.2 µM of the corresponding oligonucleotides under the following conditions: 95 °C for 10 s, followed by 40 cycles of 10 s at 95 °C and 15 s at 55 °C. At the end of the amplification cycles, a melting curve analysis was conducted to verify the specificity of the reaction. A standard curve was made with serial dilutions of the cDNA sample (2 × 10−1, 1 × 10−1, 2 × 10−2, 1 × 10−2, 2 × 10−3, 1 × 10−3). The primers used to determine the transcript levels are represented in Table 2.
2.6. Statistical Analysis
All the experiments were carried out at least in triplicate. Data were expressed as the mean values ± standard deviation. Experimental results were analyzed and compared by statistical analyses such as ANOVA, Tukey’s honestly significant difference (HSD), and t-tests using GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA) software (v7.00). A confidence level of at least 95% was considered. Significance was graphically shown either as p < 0.05 (*), p < 0.005 (**), and p < 0.001 (***) or using letters “a”, “b”, and “c” to reflect significant differences. All statistical program packages involved in the RNAseq analysis were run on R software (v4.1.3) as described above.
3. Results and Discussion
Ganguly et al. [15] cloned and overexpressed the S. cerevisiae PAA1 gene in E. coli and, after its purification, characterized its enzymatic activity in vitro. These authors concluded that this enzyme has activity generally typical for AANAT family members, although the substrate preference pattern was somewhat broader, the specific activity was lower, and the pH optimum was higher than the reference mammalian AANAT. Complementary to this previous study, we aim to characterize the in vivo function of PAA1 by overexpressing this gene in S. cerevisiae and determine the impact of this overexpression on the acetylated products of the melatonin biosynthesis pathway.
3.1. Overexpression of PAA1 in S. cerevisiae
Conversely to our expectations, the overexpression of PAA1 in S. cerevisiae, in a medium supplemented with 5-methoxytryptamine, as this precursor has been described as the best amine substrate for PAA1 [15], did not show significant differences in melatonin production in comparison to the wild-type strain transformed with the empty vector (control strain). Nonetheless, BtAANAT overexpression produced a 25-fold higher melatonin concentration than the control and the PAA1 overexpressing strain (PAA1) (Figure 1A). In view of this unexpected result, we also tested the acetylase capacity of PAA1 on other substrates (tryptamine and serotonin). In this assay, we also included the null mutant strain (paa1) to evaluate a possible loss of acetylating function for any of the substrates. Again, no significant differences in acetylated products from any substrate were detected among these strains. Null mutants still showed the same levels of acetylated products as the control or the overexpressed strains (Figure 1B). This result contradicts the previous Ganguly et al. [15] study, which reported a reduced arylalkylamine acetylation in crude homogenates of the paa1 mutant strain in comparison with the wild type.
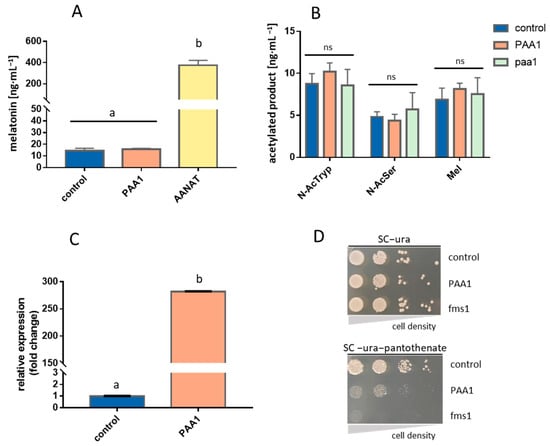
Figure 1.
In vivo testing of PAA1 function in yeast strain BY4743. (A) Bioconversion assay for melatonin production on overexpressing strains. (B) Yeast in vivo production of N-acetyltryptamine (N-AcTryp), N-acetylserotonin (N-AcSer), and melatonin (Mel) was achieved by supplementing precursors tryptamine, serotonin, and 5-methoxytryptamine, respectively, either in overexpressing (PAA1) or null mutant (paa1) strain. (C) An effective overexpression of PAA1 resulted in a great increase in mRNA levels when compared to control strain. (D) PAA1 is functional as it increases the consumption of polyamines as substrates and provokes a growth defect when no pantothenate is available. This growth defect reflects depletion of polyamine precursors of pantothenate due to the increased action of Paa1p. p426GPD backbone with no cloned gene was used as a control in all cases. Different letters “a, b” indicate groups that are significantly different (p < 0.05). “ns” reflects no significant difference when comparing the mean values below.
In order to assure that the induction of PAA1 in the overexpressing strain had been achieved, we determined the gene transcriptional activity of both the control and overexpressing PAA1 strain by qPCR. PAA1 was found to be expressed over 270 time-fold in the PAA1 strain compared to the control strain, which indicates that overexpression had been correctly achieved (Figure 1C). As the lack of increases in acetylation activity was not connected with the transcriptional activity, we tested if functional overexpressed proteins were also obtained. Liu et al. [18] reported that PAA1 overexpression caused partial growth inhibition in a medium without pantothenate, but not in a rich medium. An increase in intracellular Paa1p led to an excess of acetylated polyamines, such as putrescine, spermidine, and spermine, which are the immediate precursors in the synthesis of pantothenate (Figure S1). A shortage in these polyamines turned out in a reduction of intracellular pantothenate and a growth defect in the absence of this vitamin in the growth medium. In turn, the FMS1 gene encodes a polyamine oxidase that converts spermine into 3-aminopropanal, which is then converted to β-alanine. The β-alanine is a precursor of pantothenate, which is in turn a precursor of coenzyme A (Figure S1). Based on these previous metabolic data, we performed a drop test in an SC medium with and without pantothenate to indirectly verify if we had functionally overexpressed PAA1. We also tested the FMS1 null mutant strain (fms1) which is unable to grow in the absence of pantothenate. These drop tests showed a growth defect in the PAA1 strain and a total inhibition in the fms1 strain, as previously reported [18]. This result evidenced that PAA1 was not only overexpressed but also translated, into functional proteins (Figure 1D).
In light of these results, the absence of significant arylalkylamine acetylation in the PAA1 overexpressing strain could be explained because arylalkylamines are not the main in vivo substrate of Paa1p, although it conserves a lower specific activity than that of the mammalian enzyme [15]. Liu et al. [18] also provided strong evidence that spermine was the main in vivo substrate of Paa1p. However, the fact that we also detected acetylated activity in the null paa1 mutant suggests that there may be other N-acetyltransferases in S. cerevisiae. Another explanation of the obtained results is that, in spite of the overexpression, this higher transcriptional activity in PAA1 is not enough to increase the concentration of acetylated arylalkylamines. As it is well known that E. coli produces higher expression levels of recombinant proteins than S. cerevisiae, and several previously uncharacterized members of the yeast N-acetyl transferases were expressed in E. coli, and the recombinant proteins were purified and assayed for their acetylation activity [15,19], we decided to overexpress PAA1 in E. coli and determine the acetylation activity in different arylalkylamines, in a similar in vivo bioconversion assay to the one performed in S. cerevisiae.
3.2. Overexpression of PAA1 in E. coli
In order to assess PAA1 activity in E. coli, an in vivo bioconversion of 5-methoxytryptamine into melatonin was performed in an inducible overexpression system and, conversely to the overexpression in S. cerevisiae, a significant increase in melatonin production was detected in comparison to the wild-type strain (Figure 2A). As previously, the BtAANAT was also overexpressed as a positive control and this system yielded much higher titers of melatonin than the strain overexpressing PAA1 (more than 20-folds), evidencing a much higher specific activity of the mammalian enzyme. Once the overexpression of PAA1 showed positive bioconversion results, we tested Paa1p acetylase activity for other possible substrates related to the melatonin pathway, namely tryptamine and serotonin, to test PAA1 specificity and preference of substrate by measuring the corresponding acetylated products N-acetyltryptamine and N-acetylserotonin. Interestingly, we observed in vivo acetylase activity of PAA1 using tryptamine, 5-methoxytryptamine, and serotonin as a substrate, being tryptamine the preferred substrate that yielded approximately 1 µg·mL−1 of N-acetyltryptamine, while serotonin produced the lowest amount of acetylated product with 50 ng·mL−1, which was still significant when compared to the wild-type strain (Figure 2B).
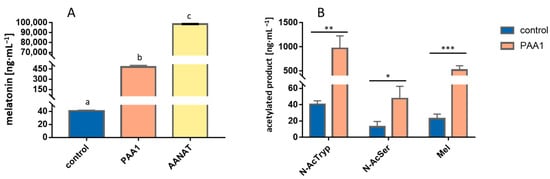
Figure 2.
In vivo testing of PAA1 function in E. coli by bioconversion assays showing a significant acetylation activity for melatonin production when supplemented with 5-methoxytryptamine (A), but also for N-AcTryp and N-AcSer when supplemented with tryptamine and serotonin, respectively, demonstrating a broad substrate scope of this gene (B). pGEX-5X-1 backbone without any cloned gene was transformed into E. coli and used as a control in these assays. Different letters “a–c“ indicate variables that are significantly different from each other (p < 0.05). Asterisks show significant differences in relation to their control (p < 0.05 (*), p < 0.005 (**), and p < 0.001 (***)).
With all the evidence obtained with the overexpression of PAA1 in both host organisms, we reasoned PAA1 might be involved in the melatonin biosynthetic pathway in yeast, but it is not essential for an in vivo significant production of this compound. This makes clear there are still other candidates to consider as responsible for the acetylase step in the melatonin pathway, and probably, as well as for PAA1, they may have other main functions. The fact melatonin is produced in such small amounts in yeast, and the enzymes responsible for the different necessary reactions are not exclusive of this pathway, highlights the difficulty of the search for candidates using yeast cells for the bioconversion assays. Thus, we concluded bioconversion assays for testing possible candidates should be carried out using the bacterial expression system because they produce an outsized effect of the tested protein, revealing any possible acetylating activity, even when it happens in a residual way in yeast.
3.3. Search for New N-Acetyltransferases in S. cerevisiae
Melatonin biosynthesis in yeast is not a conspicuous trait in terms of the amount of generated product, but its importance relies on melatonin’s free radical scavenging activity and its modulation of gene expression, even when it is present at low concentrations [20,21]. All gathered evidence around the PAA1 gene, together with the usual low concentrations of melatonin detected as a result of spontaneous biosynthesis, led us to infer that enzymes involved in this biosynthetic route, and especially PAA1, may not be exclusive for this route but they rather have other main functions instead, even though they can eventually contribute to melatonin biosynthesis in a leaky and inefficient manner. The search for these possible gene candidates involved in melatonin synthesis becomes a complicated and subtle labor in which expression levels of specific genes can bring out their relevance in this process. As we understand, a global transcriptome analysis during melatonin production can provide an interesting starting point in the search for candidate genes, especially those involved in the acetyltransferase activity, if a melatonin synthesis situation triggers a differential expression of the genes involved in it. Despite multiple pieces of evidence of melatonin function in S. cerevisiae, the external conditions that are capable of inducing its production are difficult to establish as no media and growth conditions have been unequivocally associated with a spontaneous increase in detectable melatonin levels. For this reason, following our previous approach for a reproducible melatonin synthesis [14], we directly induced this synthesis by supplementing the growth media with an immediate precursor such as 5-methoxytryptamine to observe melatonin production (Figure 3A).
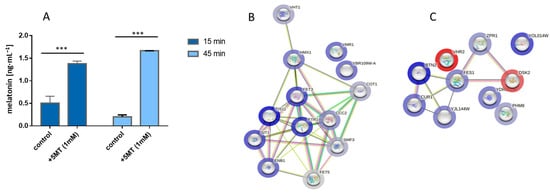
Figure 3.
Melatonin production modulated yeast transcriptional response. (A) Melatonin production achieved by supplementing the media with precursor 5-methoxytryptamine (5MT). Samples were taken at 15 and 45 min after the 5MT addition are shown. Transcriptional response is depicted as a network of predicted interaction between the different genes (nodes) showing whether they are overexpressed (blue rim) or repressed (red rim) relative to control at time 15 min (B) or 45 min (C). Asterisks show significant differences in relation to their control (p < 0.001 (***)).
We explored transcriptional response after 15 and 45 min of the precursor supplementation and, under our strict criteria for significance, only 13 and 8 genes were significantly upregulated at times of 15 and 45 min, respectively. We observed an intense activation of genes related to iron and copper homeostasis and specific genes related to transmembrane transporter activity, especially after 15 min of the precursor addition, when we compared supplemented culture with its non-supplemented control (Figure 3B). A reasonable explanation for this result is that melatonin and some precursors such as 5-methoxytryptamine can act as iron and copper chelators, as previously pointed out [20,22,23]. Therefore, a change in metal ion availability in the growth medium may occur when using 5-methoxytryptamine as a supplement to produce melatonin. Differences in media composition regarding heavy metals between our supplemented and control cultures need to be taken into account when analyzing expression patterns as a certain grade of the transcriptional response to metal deficiency is inevitably expected. For a time of 45 min, the overexpressed genes belong to the “protein folding, and protein targeting to ER” network cluster (STRING), indicating the response to chelation occurred in a short period of time, and the main overexpression at 45 min is related to replicative and translational stress response (Figure 3C).
We believe the response to the chelating effect of the studied compounds has apparently masked any less conspicuous gene expression and hindered their significance. Therefore, in order to be able to detect other activated genes in response to 5-methoxytryptamine supplementation or melatonin production, we looked further down in the list of overexpressed genes which are structurally related to AANAT and cautiously considered them as possible candidates. We used RNAseq information as a guide and crossed it with the InterPro database [24] to search for protein candidates among S. cerevisiae with a functional homology with the GNAT domain (IPR000182) of the reference gene AANAT. We found eight coincidences between the family domain search results and overexpressed genes from the RNAseq results, one of them expectedly being PAA1, so we considered seven new gene candidates with a GNAT domain for testing for melatonin production (Table 1).
3.4. Detection of AANAT Activity in the New Gene Candidates by Overexpression in E. coli
The seven selected candidates were overexpressed in E. coli as described above. Six out of these seven genes did not show any significant difference in the melatonin yielded in comparison with the wild type. Thus, the involvement of these genes in the putative production of melatonin or in the acetylation of other compounds of the route can be ruled out. Nonetheless, we detected that the overexpression of the gene HPA2 resulted in significantly higher titers of melatonin, in an amount similar to the previously reported for PAA1 and, again, in lower concentration than the production of the overexpression of BtAANAT (Figure 4). The new positive candidate HPA2 was early overexpressed at a time of 15 min, while PAA1 overexpression was detected only in the next time point analyzed at 45 min (Figure 5). The sequential transcription of different genes involved in melatonin synthesis can explain the lack of a strong transcriptional response of one single responsible gene, reinforcing the theory of a common function from multiple genes when it comes to melatonin biosynthesis. HPA2 is a tetrameric histone acetyltransferase, a member of the Gcn5 acetyltransferase family, which acetylates histones H3 and H4 in vitro and also acetylates polyamines. However, this is the first report that clearly proves the involvement of this enzyme in the acetylation of arylalkylamines, such as 5-methoxytryptamine, and, therefore, in the synthesis of melatonin in yeasts. As was the case for PAA1, the substrate specificity and the specific activity was significantly lower than the mammalian enzyme, but, likely, as a moonlighting protein, Hpa2 was able to significantly convert 5-methoxytryptamine into melatonin.
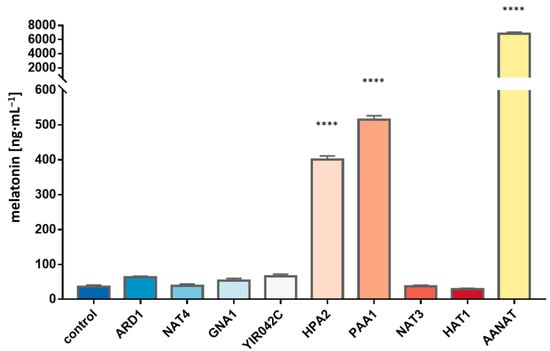
Figure 4.
Melatonin production from 5-methoxytryptamine of the different gene candidates extracted from RNAseq results and homology domain search results. Genes were overexpressed in E. coli and pGEX-5X-1 backbone with no cloned gene was used as a control. Asterisks show significant differences in relation to the control (p < 0.0001 (****)).
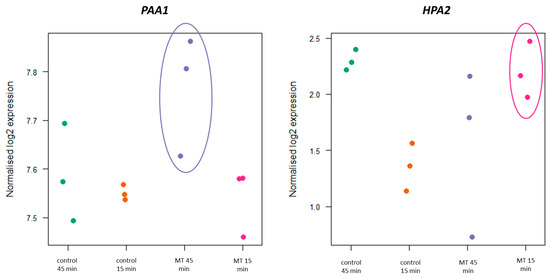
Figure 5.
Sequential overexpression of HPA2 and PAA1 under melatonin synthesis conditions. Different samples are depicted as colored dots where each color belongs to a combination of treatment (control or supplemented) and sampling time (15 or 45 min). RNAseq results showed expression levels of the 5-methoxytryptamine supplemented samples (MT) for HPA2 were higher than control at time 15 min (p-value: 0.0185), while PAA1 showed higher transcript levels at time 45 min (p-value: 0.0319).
4. Conclusions
So far, only the PAA1 gene has been correlated with the unknown pathway of melatonin biosynthesis in yeasts. We aimed to characterize the enzymatic activity of this gene by whole-cell biotransformation. To this end, we overexpressed PAA1 in its own host, S. cerevisiae, and in E. coli. However, we did not detect significant acetylation activity in S. cerevisiae whereas this higher activity was evident in E. coli. Therefore, a clear conclusion for the future search for new enzymes of the route is that the overexpression in E. coli reveals higher differences in the enzymatic activity. Our results also evidenced that PAA1 was not the only enzyme with AANAT activity in S. cerevisiae. The combination of criteria from transcriptomics and structure prediction to find similar domains let us narrow down the list of gene candidates to test in an in vivo assay that resulted in the proposal of a new gene candidate. We can conclude that HPA2, a histone acetyltransferase also related to polyamine acetylation, was able to significantly convert 5-methoxytryptamine to melatonin. Therefore, together with Paa1, Hpa2 should be also considered an arylalkylamine N-acetyltransferase in S. cerevisiae. However, both enzymes should be considered moonlighting proteins, and taking into account the yield of acetylated arylalkylamine in comparison with the mammalian enzyme, this AANAT activity does not seem to be the main acetylation activity. Therefore, the presence of an AANAT enzyme with a higher arylalkylamine substrate specificity in S. cerevisiae cannot be ruled out. We are currently applying new and powerful bioinformatic tools for the search for the most specific AANAT enzymes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms11051115/s1, Figure S1: Biosynthetic pathway for the synthesis of coenzyme A in yeast. Adapted from Liu et al. 2005 [18]; Table S1: Yeast strains used in this study; Additional data: RNAseq files.rar.
Author Contributions
Conceptualization, R.B., A.P.-C., S.M.-C. and J.M.G.; methodology, R.B., S.M.-C., J.A.-d.-R. and J.M.G.; formal analysis, R.B. and J.A.-d.-R.; investigation, R.B., A.P.-C. and S.M.-C.; resources, J.M.G.; data curation, R.B. and J.A.-d.-R.; writing—original draft preparation, R.B., A.P.-C., S.M.-C. and J.M.G.; writing—review and editing, R.B. and J.M.G.; visualization, R.B.; supervision, S.M.-C. and J.M.G.; project administration, J.M.G.; funding acquisition, J.M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MCIN/AEI/10.13039/501100011033, grant number PID2019-108722RB-C31. RB and APC thank their respective BES-2017-079640 and FPU19/02060 grants funded by MCIN/AEI/10.13039/501100011033 and by “ESF Investing in your future”.
Data Availability Statement
The data presented in this study are available in Supplementary Materials.
Acknowledgments
The authors are grateful for the technical support of the genomics department from SCSIE (University of Valencia) and thank Simo Abdessamad Baallal Jacobsen of the Technical University of Denmark, for kindly providing plasmid pCfB2628.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Tan, D.X.; Hardeland, R.; Back, K.; Manchester, L.C.; Alatorre-Jimenez, M.A.; Reiter, R.J. On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: Comparisons across species. J. Pineal Res. 2016, 61, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a potent and inducible endogenous antioxidant: Synthesis and metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef]
- Sprenger, J.; Hardeland, R.; Fuhrberg, B. Melatonin and Other 5-Methoxylated Indoles in Yeast: Presence in High Concentrations and Dependence on Tryptophan Availability. Cytologia 1999, 64, 209–213. [Google Scholar] [CrossRef]
- Fernández-Pachón, M.S.; Medina, S.; Herrero-Martín, G.; Cerrillo, I.; Berná, G.; Escudero-Lõpez, B.; Ferreres, F.; Martín, F.; García-Parrilla, M.C.; Gil-Izquierdo, A. Alcoholic fermentation induces melatonin synthesis in orange juice. J. Pineal Res. 2014, 56, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Moreno, H.; Calvo, J.R.; Maldonado, M.D. High levels of melatonin generated during the brewing process. J. Pineal Res. 2013, 55, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Naranjo, M.I.; Gil-Izquierdo, A.; Troncoso, A.M.; Cantos-Villar, E.; García-Parrilla, M.C. Melatonin is synthesised by yeast during alcoholic fermentation in wines. Food Chem. 2011, 126, 1608–1613. [Google Scholar] [CrossRef]
- Vigentini, I.; Gardana, C.; Fracassetti, D.; Gabrielli, M.; Foschino, R.; Simonetti, P.; Tirelli, A.; Iriti, M. Yeast contribution to melatonin, melatonin isomers and tryptophan ethyl ester during alcoholic fermentation of grape musts. J. Pineal Res. 2015, 58, 388–396. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central organelles for melatonins antioxidant and anti-Aging actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef]
- Rosales-Corral, S.A.; Acuña-Castroviejo, D.; Coto-Montes, A.; Boga, J.A.; Manchester, L.C.; Fuentes-Broto, L.; Korkmaz, A.; Ma, S.; Tan, D.X.; Reiter, R.J. Alzheimer’s disease: Pathological mechanisms and the beneficial role of melatonin. J. Pineal Res. 2012, 52, 167–202. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Paredes, S.D.; Korkmaz, A.; Sainz, R.M.; Mayo, J.C.; Fuentes-Broto, L.; Reiter, R.J. The changing biological roles of melatonin during evolution: From an antioxidant to signals of darkness, sexual selection and fitness. Biol. Rev. 2010, 85, 607–623. [Google Scholar] [CrossRef]
- Bisquert, R.; Muñiz-Calvo, S.; Guillamón, J.M. Protective role of intracellular Melatonin against oxidative stress and UV radiation in Saccharomyces cerevisiae. Front. Microbiol. 2018, 9, 318. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.; González, B.; Sempere, V.; Mas, A.; Torija, M.J.; Beltran, G. Melatonin reduces oxidative stress damage induced by hydrogen peroxide in Saccharomyces cerevisiae. Front. Microbiol. 2017, 8, 1066. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.; Grillitsch, K.; Daum, G.; Mas, A.; Torija, M.J.; Beltran, G. Melatonin minimizes the impact of oxidative stress induced by hydrogen peroxide in Saccharomyces and Non-conventional yeast. Front. Microbiol. 2018, 9, 1933. [Google Scholar] [CrossRef]
- Muñiz-Calvo, S.; Bisquert, R.; Fernández-Cruz, E.; García-Parrilla, M.C.; Guillamón, J.M. Deciphering the melatonin metabolism in Saccharomyces cerevisiae by the bioconversion of related metabolites. J. Pineal Res. 2019, 66, e12554. [Google Scholar] [CrossRef]
- Ganguly, S.; Mummaneni, P.; Steinbach, P.J.; Klein, D.C.; Coon, S.L. Characterization of the Saccharomyces cerevisiae Homolog of the Melatonin Rhythm Enzyme Arylalkylamine N-Acetyltransferase (EC 2.3.1.87). J. Biol. Chem. 2001, 276, 47239–47247. [Google Scholar] [CrossRef] [PubMed]
- Germann, S.M.; Baallal Jacobsen, S.A.; Schneider, K.; Harrison, S.J.; Jensen, N.B.; Chen, X.; Stahlhut, S.G.; Borodina, I.; Luo, H.; Zhu, J.; et al. Glucose-based microbial production of the hormone melatonin in yeast Saccharomyces cerevisiae. Biotechnol. J. 2016, 11, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Mumberg, D.; Müller, R.; Funk, M. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 1995, 156, 119–122. [Google Scholar] [CrossRef]
- Liu, B.; Sutton, A.; Sternglanz, R. A yeast polyamine acetyltransferase. J. Biol. Chem. 2005, 280, 16659–16664. [Google Scholar] [CrossRef]
- Neuwald, A.F.; Landsman, D. GCN5-related histone N-acetyltransferases belong to a diverse superfamily that includes the yeast SPT10 protein. Trends Biochem. Sci. 1997, 22, 154–155. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 253–278. [Google Scholar] [CrossRef]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Medina, M.E.; Tan, D.X.; Reiter, R.J. Melatonin and its metabolites as copper chelating agents and their role in inhibiting oxidative stress: A physicochemical analysis. J. Pineal Res. 2015, 58, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lv, Y.; Shi, Y.; Li, T.; Chen, Y.; Zhao, D.; Zhao, Z. The role of phyto-melatonin and related metabolites in response to stress. Molecules 2018, 23, 1887. [Google Scholar] [CrossRef] [PubMed]
- Mulder, N.J.; Kersey, P.; Pruess, M.; Apweiler, R. In silico characterization of proteins: UniProt, InterPro and Integr8. Mol. Biotechnol. 2008, 38, 165–177. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).