Abstract
The hazardous pest known as rice leaf roller (Marasmia ruralis Wlk.) (Lepidoptera: Pyralidae), which undermines rice (Oryza sativa L.) output globally, folds the leaves of the rice plant. Protein elicitors are thought to be biological elements that causes the rice to become resistant to herbivores. The potential for biocontrol of the emerging elicitor protein evaluated from Verticillium lecanii 2 (PeVL1) was evaluated against M. ruralis. To assess the impact of PeVL1 on immature development, survival, and lifetime, four different PeVL1 concentrations were allocated. Electrical penetration graphs (EPGs) against M. ruralis were used to evaluate adult reproductive efficiency and the interaction between the pest and the pathogen. Furthermore, the characterization of active substances in PeVL1 with multi-acting entomopathogenic effects looked into the direct interactions of PeVL1 with temperature and climatic change in rice (O. sativa) plants. PeVL1 treatments reduced the population increase of second and third generation M. ruralis compared to controls. In a test of host selection, M. ruralis colonized control plants more quickly than PeVL1-treated O. sativa plants. PeVL1 concentrations prolonged the M. ruralis larval stage. Similar to fecundity, PeVL1-treated seedlings produced fewer offspring than control seedlings. On PeVL1-treated leaves, trichomes and wax production created an unfavorable habitat for M. ruralis. PeVL1 changed the surface structure of the leaves, which inhibited colonization and decreased M. ruralis reproduction. The activation of pathways was another aspect of systemic defense activities including jasmonic acid (JA), salicylic acid (SA), and ethylene (ET). Based on these results against M. ruralis, the use of PeVL1 in the agroecosystem with integrated pest management and biocontrol seems appropriate. Our research provides a novel insight into a cutting-edge biocontrol method utilizing V. lecanii 2.
1. Introduction
Successful pathogens must be able to recognize and overcome host plant defense responses. Plants have evolved resistance (R) proteins in response to pathogens that avoid, tolerate, or suppress basal defense, resulting in gene-for-gene resistance. Plants’ ability to resist disease is due to a combination of inducible and constitutive defense mechanisms [1]. To name microbes or pathogens, researchers use conserved, essential compounds. Innate immunity recognizes them via pattern recognition receptors (PRR) [2]. These raise extracellular pH, cause oxidative bursts, make nitric oxide (NO), secondary metabolites, and hypersensitive responses (HR) [1,2]. These resistance responses begin in cells near the infection site. As a result, the plant develops systemic acquired resistance (SAR) against a wide range of pathogens [3]. Plants need two built-in defense mechanisms. For example, flagellin, a pathogen associated molecular pattern (PAMP), helps plants identify microbes and pathogens. PAMP activates the plant’s innate defense system via a number of plant trans-membrane PRRs on the plant’s surface [4]. Gene-for-gene resistance is a type of defense that occurs mostly inside plants. In this case, the pathogen-secreted elicitors are compared to R proteins [5]. As part of effector-triggered immunity, R-proteins trigger hypersensitive responses, oxidative stress, NO production, extracellular pH elevation, cell wall augmentation, and pathogen-related protein expression (ETI) [3,5]. This type of response starts at the infection site and spreads to nearby uninfected cells. This allows the plant to fight pathogens more effectively [6].
Elicitors, in both biotic and abiotic ways, are responsible for stimulating the defense response and mechanism of action in plants [7]. Elicitors help bacteria, viruses, oomycetes, and fungi to grow [8]. Induced proteins, lipids, and carbohydrates are some of the compounds which help plants fight disease [9]. A common link between HR and reactive oxygen species (ROS), such as H2O2 and O2, is ion influx. These molecules act as elicitor signaling molecules [10]. Elicitors are classified as race-specific or universal defense groups that affect both host and non-host plants [11]. In order to ensure food safety, some chemical pesticides may be replaced by elicitors [12,13,14,15,16,17,18]. Aphid defense responses have been examined in many aphid–plant interactions. Aphid resistance in Brassica napus (Brassicaceae) reduced the endurance proportion and population progression of immature Plutella xylostella L. (Lepidoptera: Plutellidae) [19]. Plants that inhale jasmonic acid, salicylic acid, and ethylene strengthen their defenses. Aphids respond to JA and SA [20,21,22], and aphids eating JA–SA increase the expression of defense-related genes [23].
Numerous microorganisms, such as entomopathogenic fungi (EPF), have demonstrated their efficacy against a variety of insect pests [24]. In addition to this, EPF possess the ability to produce endophytes within a variety of plant tissues [25]. EPF promote plant development and create systemic resistance to biotic stressors in several plants, including pathogens and phytoparasites [26]. They also boost yield [27], enhance the nutritional value of plants [28], and encourage the growth of plant roots [29]. In broth cultures, many EPF have been described to produce a wide variety of insecticides, anti-feedants, and bio-actively poisonous compounds [30]. Local and systemic defense responses have been observed in host plants when recombinant PeVL1 from the entomopathogenic fungus V. lecanii 2 was applied. The JA and SA pathways increase plants’ resistance to it. Our study is focused on the purification and characterization of a novel protein elicitor, PeVL1, that was extracted from an entomopathogenic fungus called V. lecanii 2 strain and its impact on M. ruralis management. Additionally, we are interested in the potential bioactivity of this protein elicitor against a pest called M. ruralis, which feeds on O. sativa L. The findings of this study will contribute to and reveal information about the development of a potentially novel strategy for the biological control of M. ruralis.
2. Materials and Methods
2.1. Establishment of M. ruralis Colonies and O. sativa Cultivation
The focus of this research was to grow M. ruralis and O. sativa colonies in a controlled growing season before the tests. Marasmia ruralis was found nearby Triticum aestivum L., and was transferred to seedlings of O. sativa (Gailiangmaofen802F1, Jiaxin Seed Limited Company). During the experiment, a colony of M. ruralis was kept at room temperature for 6 months before the experiment. Marasmia ruralis were reared on O. sativa plants at 26 ± 2 °C and a relative humidity of 70–80% with a photoperiod time of 10 D:14 L. The seeds of O. sativa were cleaned in 75% ethanol for 30 s, before being washed and soaked in water for 2–3 days prior to using.
2.2. PeVL1 Evaluation
PeVL1 was expressed in Escherichia coli BL21-DE3 using the recombinant vector pET30- BamHI and HindIII (Novagen, Darmstadt, Germany), purified using a HisTrap™ HP column (GE Healthcare, Boston, MA, USA), and desalted in a HiTrap desalting column ™ (GE Healthcare, Waukesha, WI, USA) as described by Nam et al., 2020 [31].
2.3. Marasmia ruralis Infestation on the Plant
This experiment was designed to estimate the size of the M. ruralis population that had settled. Oryza sativa were soaked in 89.24 μg mL−1 PeVL1 for one day. Four organic seeds were developed (Flora Guard substrate). After seven days, O. sativa seedlings were sprayed with 89.24 μg mL−1 PeVL1 solution and inoculated with 15 M. ruralis adults. Seedlings were treated weekly. Every five days after inoculation, M. ruralis were counted. The data fractions were used to analyze and comprehend the data. Controls and negative controls were 89.24 μg mL−1 water and 50 mM Tris-HCl at pH 8.0. Plants were caged in transparent, breathable mesh. Each time, four replicates were used.
2.4. Growth Rate of M. ruralis
The purpose of this experiment was to see if feeding PeVL1-treated or control seedlings enhanced the intrinsic growth of M. ruralis. Oryza sativa seeds were managed as in (Section 2.3). Seeds were splattered with 89.24 μg mL−1 of PeVL1 pure protein solution after a day. A glass tube lined with cotton gauze isolated the sprouts, and M. ruralis mobility was restricted on the leaf using a plastic ecological cage (2.7 × 2.7 × 2.7 cm). To prevent mechanical damage to the leaf, the perimeter of the ecological cage was sponge-coated. Every 12 h, the M. ruralis larval instar was checked for larvae production. In order to avoid crowding, newly molted larvae were counted two times every day to govern the total aggregate of time and offspring created. This was done again five days later on seeds and plants. Thirty duplicates of each treatment were used in the experiment. The intrinsic rate was calculated as follows:
rm = 0.738 × (ln Md)/Td
Md counts the set of newborn larvae in a Td development phase (the period of time among an M. ruralis infancy at first reproduction).
2.5. Marasmia ruralis Bioassay
This study’s goal was to examine Marasmia ruralis larval development and fertility. On O. sativa plants, PeVL1 was tested against Marasmia ruralis at 89.24, 53.54, 26.77, and 13.38 μg mL−1. This was calculated using the Bradford assay. Using a separate spray bottle, approximately 3 mL of PeVL1 was sprayed onto the M. ruralis plants at the three-leaf stage. Water and buffer were used to treat the controls (50 mM Tris-HCl, pH 8.0). Between 3-6 Marasmia ruralis with ages of 0–6 h old were given to O. sativa plants which were desiccated instantaneously. The total amount of descendants formed by all M. ruralis larval instars was used to calculate the overall larval development period, while M. ruralis longevity was derived from the number of days lived. The bioassays were performed in triplicate at three different temperatures (23, 25, and 27 °C).
2.6. PeVL1 Impacts on O. sativa Development and Structure
The goal of this study was to see how PeVL1 affected O. sativa growth and structure. Oryza sativa seeds and seedlings were treated as described in Section 2.3. A 3.5 percent glutaraldehyde solution in 0.1 M phosphate solution was used to collect samples for up to two days (pH 7.2). In total, five 2 h submersions in 1 percent osmic acid were performed on all samples. It was used for 15 min with an ethanol gradient of 100% to 95% to 90% to 80% to 70% to 30%. EM critical point drier Leica was used to dry all critical points (CPD030; Leica Bio-systems, Wetzlar, Germany). Samples were then inspected with a Hitachi H-7650 TEM. PeVL1-treated colonies were measured in 10 duplicates.
2.7. HPLC/MS
The goal of this study was to quantify the amount of SA, JA, and ET accumulated in this way; seeds and seedlings were handled as described previously. Seedlings’ aerobatic sections were collected for SA, JA, and ET sampling [32]. This was done using an HPLC/MS (Shimazu Research Instruments, ODS-C18, 3 m, 2.1 per 150 mm, Kyoto, Japan). Methanol mobile phase, 60 percent, and 4 °C sample temperature were used in HPLC. Sim system in negative ion mode was used with the following parameters: solvent 250 °C, heat block 200 °C, gas flow rates 10 L/min, nebulizing gas 1.5 L/min, detector voltage 1.30 kV, and interface 3 kV (SA m/z: 137.00; JA: 209.05).
2.8. Gene Expressions
TransGen Biotech (Beijing, China) kits extract RNA, synthesize cDNA, and perform qRT-PCR (ABI 7500 Real-Time PCR System). The RNA was tested using an NP80 nano-photometer. LOC_Os12g37350.1, LOC_Os11g39220.1, LOC_Os06g23760.1, LOC_Os08g39850.1, LOC_Os11g15040.4, LOC_Os01g56380.1, LOC_Os03g53200.1, LOC_Os05g41210.1, LOC_Os11g08380.1, LOC_Os03g01130.1, LOC_Os01g10940.1, LOC_Os03g37710.1 were tested for the JA, SA and ET pathway. The internal reference was the 18S ribosomal gene [33]. Table 1 lists the primers used. The relative fold expression of genes was assessed using the 2−ΔΔCT method [34].

Table 1.
Primers for plant defense genes JA, SA, and ET.
2.9. Data Analysis
Using Statistix software version 8.1, ANOVA and Leven’s tests were used to compare two treatments, while LSD and ANOVA compared three or more treatments (Tallahassee, FL, USA). These data were first square-root transformed before analysis. To eliminate disparities, we used a 95 percent probability LSD test on treatment variables such as PeVL1 elicitor concentrations and temperature regimes. For gene expressions, the comparative CT (2−∆∆CT) method was used; the fold changes having protein elicitor and buffer applied were compared at (α = 0.05)
3. Results
3.1. Marasmia ruralis Indoors
PeVL1 induced M. ruralis resistance in two ways. Marasmia ruralis-treated O. sativa seedlings had significantly reduced M. ruralis populations. Figure 1 compares the population declines in the PeVL1 treatment to the buffer and control. When M. ruralis fed on PeVL1-treated seedlings, their everyday reproductive potential was reduced; all generations showed lower growth rates, according to the findings (Figure 2).
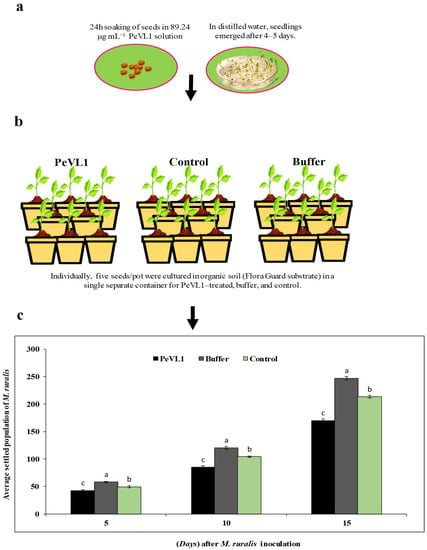
Figure 1.
Marasmia ruralis population differences were observed in PeVL1-, control-, and buffer-treated O. sativa seedlings. Using one-way ANOVA, Levene’s test with SPSS 18.0, the LSD at p = 0.05. (a,b) Seeds and seedling treatment. (c) Seedlings treated with PeVL1 saw a substantial M. ruralis population loss (mean ± SD). (a–c) small letters on each bar are significant differences among treatments and control.
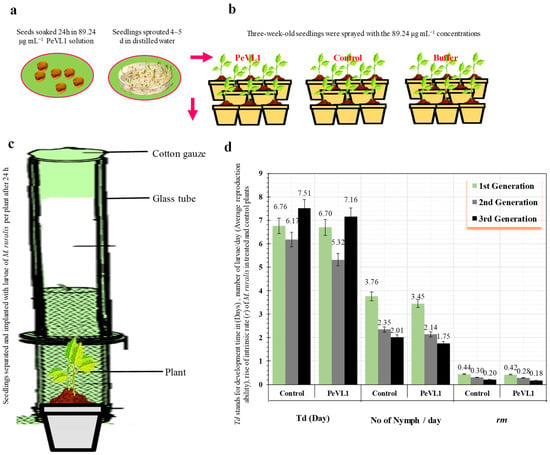
Figure 2.
Marasmia ruralis development time in O. sativa seedlings treated with PeVL1 and control, (a,b) Seeds and seedling treatment (Mean ± SD), (c) seedlings treated with PeVL1 and control in glass tube (d) Seedlings treated with PeVL1 saw a substantial change of generation in M. ruralis population (mean ± SD) the study used SPSS 18.0 using LSD and one-way ANOVA at p = 0.05.
3.2. PeVL1 Influenced M. ruralis Larval Development and Fecundity
The interaction of different PeVL1 concentrations with three temperature regimes influenced the overall development period of M. ruralis. As PeVL1 concentrations increased, so did larval instar development time (Figure 3). Fourth larval instar development time was 3.7 days at 89.24 µg ml−1 and 23 °C; a concentration of 13.38 µg ml−1 at 27 °C produced a minimum of 1.5 days larval growth. Marasmia ruralis fecundity influenced PeVL1 concentrations and temperatures (Figure 4). The experiment found that fecundity was lowest at 27 °C and highest at 23 °C.
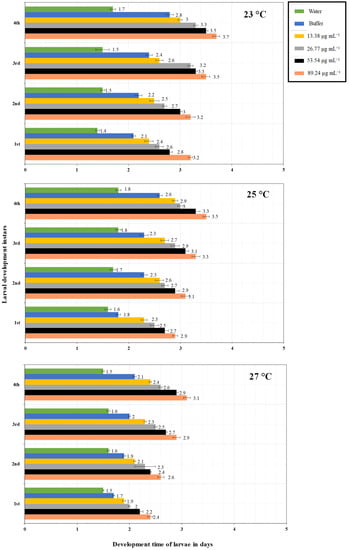
Figure 3.
On O. sativa plants, the PeVL1 elicitor protein elicited the development of M. ruralis larval instars at various doses and temperatures (23, 25, 27 °C) (n = 10; one-way ANOVA with factorial analysis; LSD at α = 0.05).
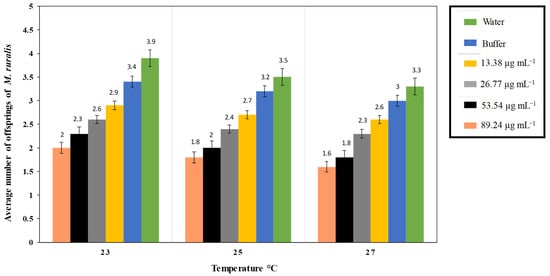
Figure 4.
Marasmia ruralis average fecundity (n = 10), fecundity lessened in O. sativa seedlings treated with PeVL1, (one-way ANOVA with factorial analysis; LSD at α = 0.05).
3.3. PeVL1 Influenced the Development and Structure of O. sativa
PeVL1 significantly influenced the plant height and surface structure of O. sativa seedlings compared to control seedlings (Figure 5). PeVL1-treated seedlings had significantly more trichomes than control seedlings, with (96.10 ± 0.58 mm−2 in PeVL1-treated seedlings versus 34.15 ± 0.31 mm−2 in control seedlings; p = 0.05). With a better surface environment and a more complex wax structure, M. ruralis colonization should be more difficult.
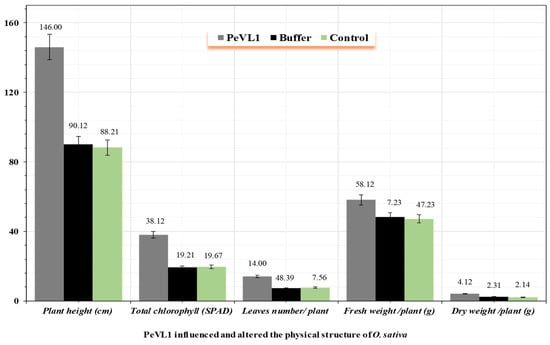
Figure 5.
The effect of PeVL1 on the growth of treated and untreated seedlings. (n = 10) PeVL1 and buffer seedling (mean ±SD). The data were compared with LSD and one-way ANOVA with Levene’s test (p = 0.05) in SPSS 18.0.
3.4. SA, JA, and ET Quantities
We examined links between JA, SA, and ET and cuticular wax deposition, trichome number, and M. ruralis infestation on PeVL1. Content of JA, SA, and ET was found to be higher in PeVL1-treated seedlings (Figure 6). The development of M. ruralis resistance in O. sativa requires all three signaling pathways. The protein elicitor elicited an innate immunological or defensive response in O. sativa plants, according to the findings.
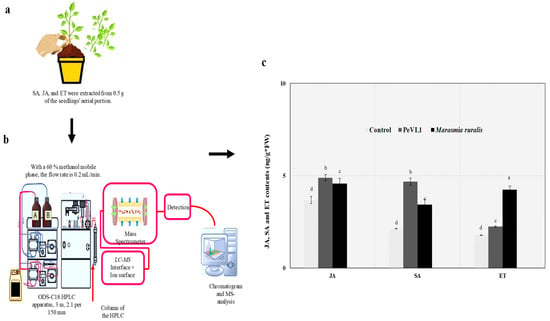
Figure 6.
Oryza Sativa seedling JA, SA, and ET levels (mean ± SD). (a,b) An additional day after spraying, PeVL1 data were collected. (c) Marisma ruralis were infected in both treatments. The LSD, ANOVA, and Leven’s test were used to compare data. Lower-case letters indicate significant JA, SA, or ET treatment differences. (p = 0.05).
3.5. Defense-Related Gene Expression Fold Change
PeVL1 boosted defenses in O. sativa seedlings. PeVL1 treatment slightly upregulated all JA, SA, and ET pathway test genes, respectively (Figure 7a–c). The Log2 of all test genes was calculated using fold-change expression values, indicating that transcription triggered M. ruralis confrontation.
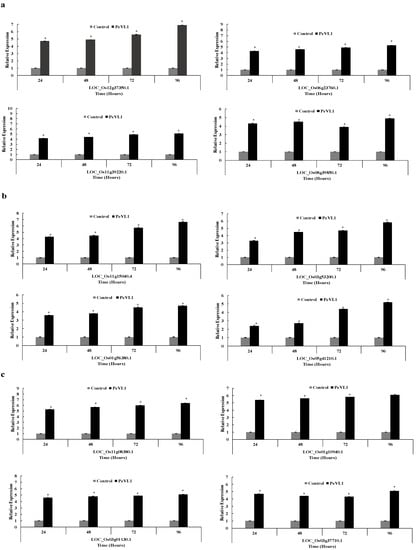
Figure 7.
After PeVL1 elicitor treatment and M. ruralis infestation, the relative expression of plant defense from the JA, SA and ET pathway, i.e., (a–c), respectively, was detected. An asterisk next to each gene indicates a significant difference from the buffer control, as determined using Student’s t-test (p < 0.05).
4. Discussion
A new biological tool for pest control, elicitors share a role in signaling systems and plant defense [13,14,15,16,17,35]. PAMPs and MAMPs are abundant in both necrotrophic or biotrophic pathogenic bacteria and fungi [36]. PeVL1, a protein elicitor, has antimicrobial and biocontrol potential against M. ruralis. In O. sativa crops, chemical elicitors such as methyl-jasmonate and benzo-thiadiazole, as well as proteinase inhibitors, have been shown to significantly reduce herbivore pest activity. According to previous research, methyl salicylate reduces the population of Aphis glycines Matsumura (Hemiptera: Aphididae) by up to 40% [36,37]. In this study, PeVL1 inhibited herbivores by altering plant physical characteristics. Trichomes are the first step in building physical resistance to pathogens and herbivores. These affect herbivore shape and trichome density in Solanum spp. Seven trichomes with two major defense-related effects were evaluated [38]. Therefore, for example, thick matte hair on a plant’s surface provides energy while limiting feeding and preventing insect access. Tomato Solanum hirsutum, for example, is avoided by M. ruralis. The plant’s “pubescence” provides resistance to pests by covering the plant’s surface with epidermal cell appendages of unicellular or multicellular hairs. Soybeans with thick trichomes had lower Leptinotarsa decemlineata (Chrysomelidae: Coleoptera) settlement than those with thin trichomes [39]. PeVL1 reduced disease severity by initiating a photosynthetic process [40], and increased induced resistance in S. lycopersicum seedlings treated with PeVL1. The physical barrier aids by means of a ration of cell wall expansion and is responsible for plant resilience [41]. The lignin concentration of Chrysanthemum indicum increased aphid tolerance [42]. In response to biotic and abiotic stress, plants produce trichomes and wax [43]. A number of exogenous phyto-hormones have been shown to influence trichome density and cuticular wax deposition in Arabidopsis and tomatoes [44]. Brassica napus has waxy leaves that require SA [45]. SA and JA accumulation increased trichome density and cuticular wax deposition in O. sativa plants treated with PeVL1. The PeVL1 elicitor had a negative influence on M. ruralis fecundity. PeVL1-treated plants had far fewer M. ruralis than the control. Exogenous SA and MJ depletes M. ruralis mean fecundity to 50% [36,45]. The lowest M. ruralis fecundity was observed at lower temperatures (e.g., 23 °C), while the highest fecundity was observed at (27 °C), attributed to a declining proportion of metabolic activities [46]. The maximum larval development time was observed even at a lower temperature (23 °C), indicating that a one-degree temperature increase had an effect on the insect’s life cycle [47].
After being exposed to PeVL1, O. sativa became more resistant to M. ruralis than before. A systemic defense response is triggered by beneficial bacteria. This response is controlled by a signaling system that links the plant hormones SA, JA, and ethylene, and it is controlled by the bacteria ET [48]. Some data suggests that the SA, JA, and ET pathways work together to change how the plant responds to different pathogens [48]. These plant defense mechanisms play defensive role against major agricultural pests [28,48]. To activate PR genes via interactions with TGA transcription factors, authors found that PeVL1 increased JA, SA, and ET-responsive genes levels [49]. The data show that PeVL1-mediated systemic defensive responses in O. sativa are influenced by relative expression levels. Discoveries from the present work also support former research by Chaerle et al. [50]; secondary metabolite accumulation can help plants fight infection by generating mechanical barriers to pathogen growth, which stimulates phenolic metabolism and lignin synthesis. In addition, phenolic compounds such as scopoletin and phenolic acids can strengthen the cell wall, making it more resistant to bacteria and fungi. This crosslinking of phenyl propanoidesters with ferulic acid results in auto-fluorescent lignin-like polymers, such as hydroxycinnamic acids and their derivatives [51]. Systemic defenses are activated when an M. ruralis feeds on plants [52]. These findings suggest that our work helps us better understand how PeVL1 works in O. sativa for the management of M. ruralis [53].
5. Conclusions
PeVL1 made O. sativa more resistant to M. ruralis, which made the second and third generations of M. ruralis less fertile and more likely to have M. ruralis. Mechanical defenses played a small role in the resistance characteristics. The surface structure of O. sativa leaves changed after PeVL1 was added to the plant. SA, JA, and ET, which are thought to be involved in systemic defense responses in O. sativa when PeVL1 is used, showed small increases in relative expression levels. PeVL1 also had a big impact on M. ruralis lifespan in the lab, but additional investigation is needed to make it more effective in the field. In our research, we found that PeVL1 may possibly be cast-off as a “vaccine” to protect plants of O. sativa against the insect pest, M. ruralis.
Author Contributions
K.J. deliberated and executed the experimentations, composed the paper, and analyzed the results. H.J. and Y.W. helped manage data curation. Y.W. oversaw the entire experiment and helped revise the paper. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by the Guizhou Provincial Department of Education (202001). International Cooperation Basic Project of Guizhou Science and Technology Department; Talent Project of Guizhou Science and Technology Cooperation Platform ([2017]5788-5; [2019]5641; [2020]5001); Program of Introducing Talents of Discipline to Universities in China (111 Program; D20023) ([2018]5806).
Data Availability Statement
The required data set is already available in manuscript file; other data sets generated during the study are available upon request from corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Silipo, A.; Erbs, G.; Shinya, T.; Maxwell Dow, J.M.; Parrilli, M.; Lanzetta, R.; Shibuya, N.; Newman, M.A.; Molinaro, A. Glycoconjugates as elicitors or suppressors of plant innate immunity. Glycobiology 2009, 20, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Berkowitz, G.A. The grateful dead: Calcium and cell death in plant innate immunity. Cell. Microbiol. 2007, 9, 2571–2585. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Brugger, A.; Lamotte, O.; Vandelle, E.; Bourque, S.; Lecourieux, D.; Poinssot, B.; Wendehenne, D.; Pugin, A. Early signaling events induced by elicitors of plant defenses. Mol. Plant-Microbe Interact. 2006, 19, 711–724. [Google Scholar] [CrossRef]
- Nürnberger, T.; Brunner, F. Innate immunity in plants and animals: Emerging parallels between the recognition of general elicitors and pathogen-associated molecular patterns. Curr. Opin. Plant Biol. 2002, 5, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Thomma, B.P.H.J.; Nu, T.; Joosten, M.H.A.J. Of PAMPs and Effectors: The Blurred PTI-ETI Dichotomy. Plant Cell 2011, 23, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Yano, A.; Suzuki, K.; Uchimiya, H.; Shinshi, H. Induction of hypersensitive cell death by a fungal protein in cultures of tobacco cells. Mol. Plant-Microbe Interact. 1998, 11, 115–123. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Chen, J.L.; Cheng, D.F.; Sun, J.R.; Liu, Y.; Tian, Z. Biochemical and molecular characterizations of Sitobion avenae-induced wheat defense responses. Crop Prot. 2009, 28, 435–442. [Google Scholar] [CrossRef]
- Ellis, J.G.; Rafiqi, M.; Gan, P.; Chakrabarti, A.; Dodds, P.N. Recent progress in discovery and functional analysis of effector proteins of fungal and oomycete plant pathogens. Curr. Opin. Plant Biol. 2009, 12, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Bent, A.F.; Mackey, D. Elicitors, effectors, and R genes: The new paradigm and a lifetime supply of questions. Annu. Rev. Phytopathol. 2008, 45, 399–436. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Montesano, M.; Brader, G.; Palva, E.T. Pathogen derived elicitors: Searching for receptors in plants. Mol. Plant Pathol. 2003, 4, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Hael-Conrad, V.; Perato, S.M.; Arias, M.E.; Martínez-Zamora, M.G.; Di Peto, P.D.L.Á.; Martos, G.G.; Castagnaro, A.P.; Díaz-Ricci, J.C.; Chalfoun, N.R. The elicitor protein AsES induces a systemic acquired resistance response accompanied by systemic microbursts and micro-hypersensitive responses in Fragaria ananassa. Mol. Plant-Microbe Interact. 2018, 31, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Javed, K.; Javed, H.; Qiu, D. PeBL1 of Brevibacillus laterosporus a new biocontrol tool for wheat aphid management (Sitobion avenae) in triticum aestivum. Int. J. Trop. Insect Sci. 2021. [Google Scholar] [CrossRef]
- Javed, K.; Javed, H.; Qiu, D. Biocontrol Potential of Purified Elicitor Protein PeBL1 Extracted from Brevibacillus laterosporus Strain A60 and Its Capacity in the Induction of Defense Process against Cucumber Aphid (Myzus persicae ) in Cucumber (Cucumis sativus). Biology 2020, 9, 179. [Google Scholar] [CrossRef]
- Javed, K.; Javed, H.; Mukhtar, T.; Qiu, D. Pathogenicity of some entomopathogenic fungal strains to green peach aphid, Myzus persicae Sulzer (Homoptera: Aphididae). Egypt. J. Biol. Pest Control 2019, 29, 92. [Google Scholar] [CrossRef]
- Javed, K.; Javed, H.; Mukhtar, T.; Qiu, D. Efficacy of Beauveria bassiana and Verticillium lecanii for the management of whitefly and aphid. Pak. J. Agric. Sci. 2019, 56, 669–674. [Google Scholar] [CrossRef]
- Javed, K.; Talha, H.; Ayesha, H.; Wang, Y.; Javed, H. PeaT1 and PeBC1 Microbial Protein Elicitors Enhanced Resistance against Myzus persicae Sulzer in Chili. Microorganisms 2021, 9, 2197. [Google Scholar] [CrossRef]
- Jakobs, R.; Schweiger, R.; Müller, C. Aphid infestation leads to plant part-specific changes in phloem sap chemistry, which may indicate niche construction. New Phytol. 2019, 221, 503–514. [Google Scholar] [CrossRef]
- Nouri-Ganbalani, G.; Borzoui, E.; Shahnavazi, M.; Nouri, A. Induction of resistance against Plutella xylostella (L.) (Lep.: Plutellidae) by jasmonic acid and mealy cabbage aphid feeding in Brassica napus L. Front. Physiol. 2018, 9, 859. [Google Scholar] [CrossRef]
- Salzman, R.A.; Brady, J.A.; Finlayson, S.A.; Buchanan, C.D.; Summer, E.J.; Sun, F.; Klein, P.E.; Klein, R.R.; Pratt, L.H.; Cordonnier-Pratt, M.M.; et al. Transcriptional profiling of sorghum induced by methyl jasmonate, salicylic acid, and aminocyclopropane carboxylic acid reveals cooperative regulation and novel gene responses. Plant Physiol. 2005, 138, 352–368. [Google Scholar] [CrossRef]
- Lazebnik, J.; Frago, E.; Dicke, M.; van Loon, J.J.A. Phytohormone Mediation of Interactions Between Herbivores and Plant Pathogens. J. Chem. Ecol. 2014, 40, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.G.; Agrawal, A.A. Asymmetry of plant-mediated interactions between specialist aphids and caterpillars on two milkweeds. Funct. Ecol. 2014, 28, 1404–1412. [Google Scholar] [CrossRef]
- Sandoya, G.V.; de Oliveira Buanafina, M.M. Differential responses of Brachypodium distachyon genotypes to insect and fungal pathogens. Physiol. Mol. Plant Pathol. 2014, 85, 53–64. [Google Scholar] [CrossRef]
- Nisar, M.S. Comparative effect of termiticides and plant extracts on mortality and tunnel formation of Odontotermes obesus. Pur. and Appl. Biol. 2020, 9, 1903–1910. [Google Scholar] [CrossRef]
- Kabaluk, J.T.; Ericsson, J.D. Metarhizium anisopliae seed treatment increases yield of field corn when applied for wireworm control. Agr. Jour. 2007, 99, 1377–1381. [Google Scholar] [CrossRef]
- Thaler, J.S.; Agrawal, A.A.; Rayko, H. Salicylate-mediated interactions between pathogens and herbivores. Ecology 2010, 91, 1075–1082. [Google Scholar] [CrossRef]
- Liao, X.; O’Brien, T.R.; Fang, W.; St. Leger, R.J. The plant beneficial effects of Metarhizium species correlate with their association with roots. App. Micro. Biotech. 2014, 98, 7089–7096. [Google Scholar] [CrossRef]
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012, 17, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, H.; Jia, Q.; Wu, X.; Guo, X.; Zhang, S.; Song, F.; Dong, H. The internal glycine-rich motif and cysteine suppress several effects of the HpaGXooc protein in plants. Phytopathology 2006, 96, 1052–1059. [Google Scholar] [CrossRef]
- Mishra, A.K.; Sharma, K.; Misra, R.S. Elicitor recognition, signal transduction and induced resistance in plants. J. Plan. Int. 2012, 7, 95–120. [Google Scholar] [CrossRef]
- Nam, T.D.; Abdulle, Y.A.; Javed, K.; Sokea, T.; Qiu, D. A Novel Protein Elicitor PevL1, from Verticillium lecanii 2, Induces Systemic Resistance against Bean Aphid (Megoura Japonica Matsumura) in Phaseolus Vulgaris L. Int. J. Plan. Ani. Env. Sci. 2020, 10, 81–94. [Google Scholar]
- Li, Y.H.; Wei, F.; Dong, X.Y.; Peng, J.H.; Liu, S.Y.; Chen, H. Simultaneous analysis of multiple endogenous plant hormones in leaf tissue of oilseed rape by solid-phase extraction coupled with high-performance liquid chromatography-electrospray ionisation tandem mass spectrometry. Phyto. Anal. 2011, 22, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Jarošová, J.; Kundu, J.K. Validation of reference genes as internal control for studying viral infections in cereals by quantitative real-time RT-PCR. BMC. Pla. Biol. 2010, 10. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Javed, K.; Qiu, D. Protein Elicitor PeBL1 of Brevibacillus laterosporus Enhances Resistance Against Myzus persicae in Tomato. Pathogens 2020, 9, 57. [Google Scholar] [CrossRef]
- Boughton, A.J.; Hoover, K.; Felton, G.W. Impact of chemical elicitor applications on greenhouse tomato plants and population growth of the green peach aphid, Myzus persicae. Entomol. Exp. Appl. 2006, 120, 175–188. [Google Scholar] [CrossRef]
- Mallinger, R.E.; Hogg, D.B.; Gratton, C. Methyl Salicylate Attracts Natural Enemies and Reduces Populations of Soybean Aphids (Hemiptera: Aphididae) in Soybean Agroecosystems. J. Econ. Entomol. 2011, 104, 115–124. [Google Scholar] [CrossRef]
- Schaller, F.; Schaller, A.; Stintzi, A. Biosynthesis and metabolism of jasmonates. J. Plant Growth Regul. 2004, 23, 179–199. [Google Scholar] [CrossRef]
- Glas, J.J.; Schimmel, B.C.J.; Alba, J.M.; Escobar-Bravo, R.; Schuurink, R.C.; Kant, M.R. Plant glandular trichomes as targets for breeding or engineering of resistance to herbivores. Int. J. Mol. Sci. 2012, 13, 17077–17103. [Google Scholar] [CrossRef]
- Shenashen, M.; Derbalah, A.; Hamza, A.; Mohamed, A.; El Safty, S. Antifungal activity of fabricated mesoporous alumina nanoparticles against root rot disease of tomato caused by Fusarium oxysporium. Pest Manag. Sci. 2017, 73, 1121–1126. [Google Scholar] [CrossRef]
- Tian, D.; Tooker, J.; Peiffer, M.; Chung, S.H.; Felton, G.W. Role of trichomes in defense against herbivores: Comparison of herbivore response to woolly and hairless trichome mutants in tomato (Solanum lycopersicum). Planta 2012, 236, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sheng, L.; Zhang, H.; Du, X.; An, C.; Xia, X.; Chen, F.; Jiang, J.; Chen, S. CmMYB19 over-expression improves aphid tolerance in Chrysanthemum by promoting lignin synthesis. Int. J. Mol. Sci. 2017, 18, 619. [Google Scholar] [CrossRef]
- dos Santos Tozin, L.R.; Marques, M.O.M.; Rodrigues, T.M. Herbivory by leaf-cutter ants changes the glandular trichomes density and the volatile components in an aromatic plant model. AoB Plants 2017, 9, plx057. [Google Scholar] [CrossRef]
- Boughton, A.J.; Hoover, K.; Felton, G.W. Methyl jasmonate application induces increased densities of glandular trichomes on tomato, Lycopersicon esculentum. J. Chem. Ecol. 2005, 31, 2211–2216. [Google Scholar] [CrossRef]
- Ni, Y.; Wang, J.; Song, C.; Xia, R.-E.; Sun, Z.-Y.; Guo, Y.-J.; Li, J.-N. Effects of SA Induction on Leaf Cuticular Wax and Resistance to Sclerotinia sclerotiorurn in Brassica napus. Acta Agron. Sin. 2013, 39, 110. [Google Scholar] [CrossRef]
- Farmer, E.E.; Johnson, R.R.; Ryan, C.A. Regulation of expression of proteinase inhibitor genes by methyl jasmonate and jasmonic acid. Plant Physiol. 1992, 98, 995–1002. [Google Scholar] [CrossRef]
- Mahmoud, F.; Mahfouz, H. Effects of salicylic acid elicitor against aphids on wheat and detection of infestation using infrared thermal imaging technique in Ismailia, Egypt. Pestic. Fitomed. 2015, 30, 91–97. [Google Scholar] [CrossRef]
- Hammond-Kosack, K.E.; Parker, J.E. Deciphering plant-pathogen communication: Fresh perspectives for molecular resistance breeding. Curr. Opin. Biotechnol. 2003, 14, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Delaney, T.P. Over-expression of TGA5, which encodes a bZIP transcription factor that interacts with NIM1/NPR1, confers SAR-independent resistance in Arabidopsis thaliana to Peronospora parasitica. Plant J. 2002, 32, 151–163. [Google Scholar] [CrossRef]
- Chaerle, L.; Lenk, S.; Hagenbeek, D.; Buschmann, C.; Van Der Straeten, D. Multicolor fluorescence imaging for early detection of the hypersensitive reaction to tobacco mosaic virus. J. Plant Physiol. 2007, 164, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, R.L.; Hammerschmidt, R. Phenolic Compounds And Their Role In Disease Resistance. Annu. Rev. Phytopathol. 1992, 30, 369–389. [Google Scholar] [CrossRef]
- De Vos, M.; Van Oosten, V.R.; Van Poecke, R.M.P.; Van Pelt, J.A.; Pozo, M.J.; Mueller, M.J.; Buchala, A.J.; Métraux, J.P.; Van Loon, L.C.; Dicke, M.; et al. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol. Plant-Microbe Interact. 2005, 18, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.G.; Agrawal, A.A. Specialist versus generalist insect herbivores and plant defense. Trends Plant Sci. 2012, 17, 293–302. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).