Adaptive Laboratory Evolution of Microorganisms: Methodology and Application for Bioproduction
Abstract
1. Introduction
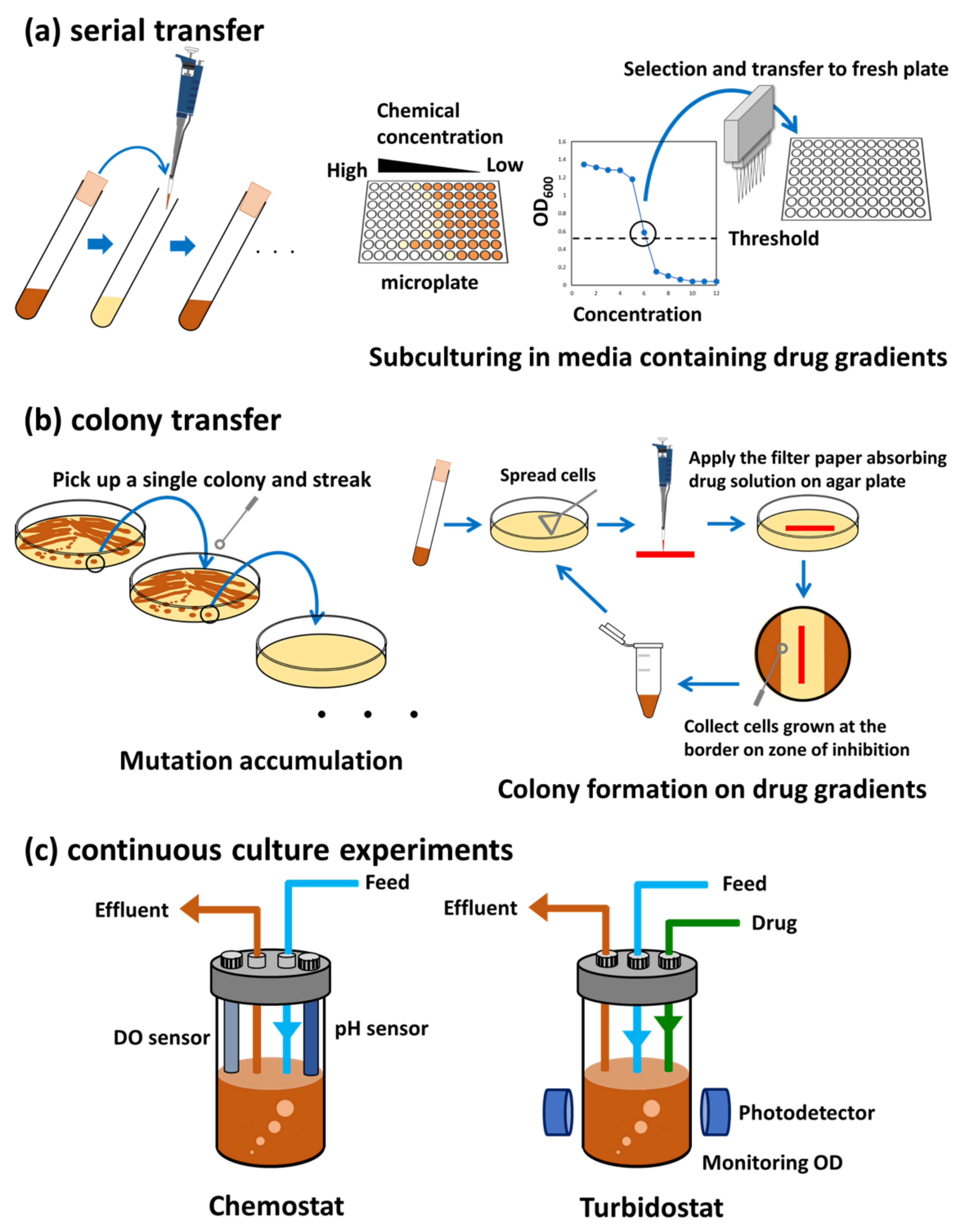
2. ALE Methods
2.1. Serial Transfer
2.2. Colony Transfer
2.3. Continuous Culture Experiments
| ALE Method | Advantage | Disadvantage | Example of Applications |
|---|---|---|---|
| Serial transfer | Easy to automate and conduct high-throughput experiments | Not applicable to cells that aggregate or form biofilm in liquid culture. Growth is inherently discontinuous and control of the growth conditions is often limited and temporal. | E. coli long-term evolution experiment (LTEE) [3]. Co-cultures of obligatory mutualistic communities [10,11,12]. Resistance to chemicals [15,16,17] |
| Colony transfer | Introducing a single-cell bottleneck, applicable to aggregate cells in liquid media, and visualization of evolutionary dynamics using soft agar | Usually low-throughput and limitation to automation. Growth is inherently discontinuous and control of the growth conditions is difficult. | Mutation accumulation [19]. Anti-tuberculosis drug resistance of aggregating Mycobacterium [20]. Increase in antibiotics production by ALE of co-culture using Streptomyces and MRSA [21]. Visualization of antibiotic resistance using MAGA-plate [22]. |
| Continuous culture experiments | It can control constant growth rates, population densities, nutrient supply, and environmental conditions | Limitation of multiple replicates in parallel owing to the costs of operation. Cells may adapt to bioreactors by the formation of biofilm to prevent washout | Morbidostat for antibiotic resistance [24]. ALE of E. coli strain that can synthesize sugars from CO2 [23]. Parallel turbidostat-based ALE of 78 S. cerevisiae populations by eVOLVER [25]. |
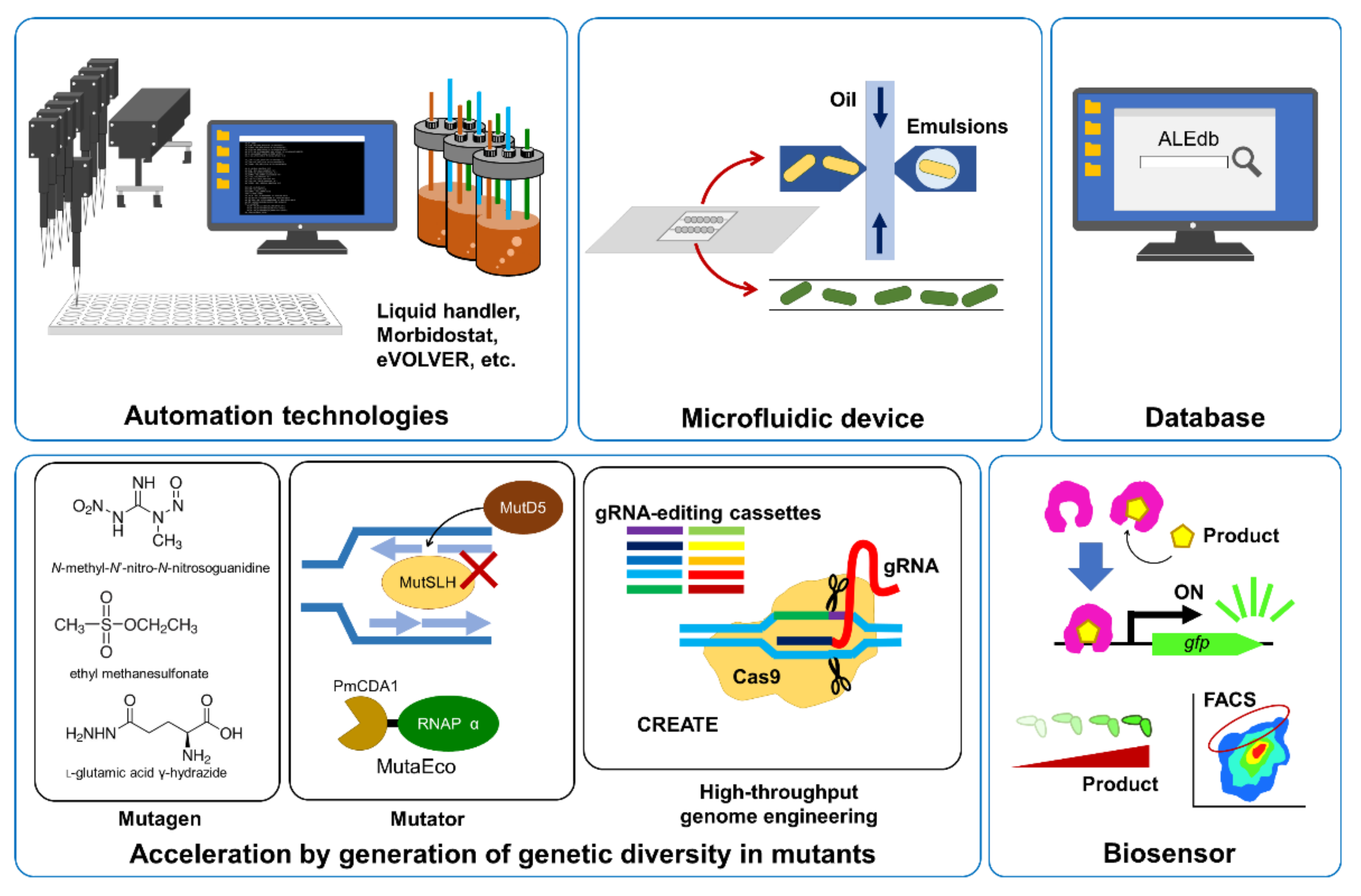
3. Automation Technologies for ALE
4. Acceleration of ALE
5. Microfluidic Devices and Database for ALE
6. Application of ALE to Improvement of Carbon Source Utilization in Microorganisms
6.1. Improvement of Xylose Utilization by ALE
6.2. Improvement of Glycerol Assimilation in S. cerevisiae with ALE
6.3. Creation of the Synthetic Methylotrophic E. coli, Which Can Grow on Methanol as the Sole Carbon Source with the Aid of ALE
6.4. Improvement of Growth of Synthetic Autotrophic E. coli on CO2 by ALE
6.5. Improvement of CO Utilization by Acetogenic Bacterium with ALE
7. Acquisition of Stress Tolerance to Microorganisms with ALE
7.1. ALE for Conferring Stress Tolerance to Methylotroph with ALE
7.2. Stress-Tolerant Strains of Non-Conventional Yeasts Obtained with ALE
7.3. Adaptation of Anaerobic Bacteria to Oxygen or Carbon Monoxide
7.4. High Light Tolerant Cyanobacteria Obtained with ALE
8. Improvement of Production of Target Compounds by Microorganisms with ALE Integrated with Other Methodology
8.1. Application of Co-Culture with ALE for Bioproduction by Microorganisms
8.2. Application of Biosensor Cells with ALE to Improved Bioproduction Using Microbial Cells
9. Conclusions and Future Prospective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sandberg, T.E.; Salazar, M.J.; Weng, L.L.; Palsson, B.O.; Feist, A.M. The emergence of adaptive laboratory evolution as an efficient tool for biological discovery and industrial biotechnology. Metab. Eng. 2019, 56, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Novick, A.; Szilard, L. Experiments with the chemostat on spontaneous mutations of bacteria. Proc. Natl. Acad. Sci. USA 1950, 36, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Lenski, R.E.; Rose, M.R.; Simpson, S.C.; Tadler, S.C. Long-term experimental evolution in Escherichia coli. I. Adaptation and divergence during 2,000 generations. Am. Nat. 1991, 138, 1315–1341. [Google Scholar] [CrossRef]
- Lenski, R.E.; Travisano, M. Dynamics of adaptation and diversification: A 10,000-generation experiment with bacterial populations. Proc. Natl. Acad. Sci. USA 1994, 91, 6808–6814. [Google Scholar] [CrossRef] [PubMed]
- Blount, Z.D.; Borland, C.Z.; Lenski, R.E. Historical contingency and the evolution of a key innovation in an experimental population of Escherichia coli. Proc. Natl. Acad. Sci. USA 2008, 105, 7899–7906. [Google Scholar] [CrossRef] [PubMed]
- Grant, N.A.; Abdel Magid, A.; Franklin, J.; Dufour, Y.; Lenski, R.E. Changes in cell size and shape during 50,000 generations of experimental evolution with Escherichia coli. J. Bacteriol. 2021, 203, e00469-20. [Google Scholar] [CrossRef]
- Lütgens, M.; Gottschalk, G. Why a co-substrate is required for anaerobic growth of Escherichia coli on citrate. Microbiology 1980, 119, 63–70. [Google Scholar] [CrossRef]
- Pos, K.M.; Dimroth, P.; Bott, M. The Escherichia coli citrate carrier CitT: A member of a novel eubacterial transporter family related to the 2-oxoglutarate/malate translocator from spinach chloroplasts. J. Bacteriol. 1998, 180, 4160–4165. [Google Scholar] [CrossRef]
- Good, B.H.; McDonald, M.J.; Barrick, J.E.; Lenski, R.E.; Desai, M.M. The dynamics of molecular evolution over 60,000 generations. Nature 2017, 551, 45–50. [Google Scholar] [CrossRef]
- Konstantinidis, D.; Pereira, F.; Geissen, E.M.; Grkovska, K.; Kafkia, E.; Jouhten, P.; Kim, Y.; Devendran, S.; Zimmermann, M.; Patil, K.R. Adaptive laboratory evolution of microbial co-cultures for improved metabolite secretion. Mol. Syst. Biol. 2021, 17, e10189. [Google Scholar] [CrossRef]
- Zhang, X.; Reed, J.L. Adaptive evolution of synthetic cooperating communities improves growth performance. PLoS ONE 2014, 9, e108297. [Google Scholar] [CrossRef] [PubMed]
- Harcombe, W.R.; Chacon, J.M.; Adamowicz, E.M.; Chubiz, L.M.; Marx, C.J. Evolution of bidirectional costly mutualism from byproduct consumption. Proc. Natl. Acad. Sci. USA 2018, 115, 12000–12004. [Google Scholar] [CrossRef] [PubMed]
- Friedman, L.; Alder, J.D.; Silverman, J.A. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob. Agents Chemother. 2006, 50, 2137–2145. [Google Scholar] [CrossRef]
- Furusawa, C.; Horinouchi, T.; Maeda, T. Toward prediction and control of antibiotic-resistance evolution. Curr. Opin. Biotechnol. 2018, 54, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Imamovic, L.; Ellabaan, M.M.H.; Dantas Machado, A.M.; Citterio, L.; Wulff, T.; Molin, S.; Krogh Johansen, H.; Sommer, M.O.A. Drug-driven phenotypic convergence supports rational treatment strategies of chronic infections. Cell 2018, 172, 121–134.e14. [Google Scholar] [CrossRef] [PubMed]
- Lázár, V.; Nagy, I.; Spohn, R.; Csörgő, B.; Györkei, A.; Nyerges, A.; Horváth, B.; Vörös, A.; Busa-Fekete, R.; Hrtyan, M.; et al. Genome-wide analysis captures the determinants of the antibiotic cross-resistance interaction network. Nat. Commun. 2014, 5, 4352. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Iwasawa, J.; Kotani, H.; Sakata, N.; Kawada, M.; Horinouchi, T.; Sakai, A.; Tanabe, K.; Furusawa, C. High-throughput laboratory evolution reveals evolutionary constraints in Escherichia coli. Nat. Commun. 2020, 11, 5970. [Google Scholar] [CrossRef]
- Suzuki, S.; Horinouchi, T.; Furusawa, C. Prediction of antibiotic resistance by gene expression profiles. Nat. Commun. 2014, 5, 5792. [Google Scholar] [CrossRef]
- Lee, H.; Popodi, E.; Tang, H.; Foster, P.L. Rate and molecular spectrum of spontaneous mutations in the bacterium Escherichia coli as determined by whole-genome sequencing. Proc. Natl. Acad. Sci. USA 2012, 109, E2774–E2783. [Google Scholar] [CrossRef]
- Maeda, T.; Kawada, M.; Sakata, N.; Kotani, H.; Furusawa, C. Laboratory evolution of Mycobacterium on agar plates for analysis of resistance acquisition and drug sensitivity profiles. Sci. Rep. 2021, 11, 15136. [Google Scholar] [CrossRef]
- Charusanti, P.; Fong, N.L.; Nagarajan, H.; Pereira, A.R.; Li, H.J.; Abate, E.A.; Su, Y.; Gerwick, W.H.; Palsson, B.O. Exploiting adaptive laboratory evolution of Streptomyces clavuligerus for antibiotic discovery and overproduction. PLoS ONE 2012, 7, e33727. [Google Scholar] [CrossRef] [PubMed]
- Baym, M.; Lieberman, T.D.; Kelsic, E.D.; Chait, R.; Gross, R.; Yelin, I.; Kishony, R. Spatiotemporal microbial evolution on antibiotic landscapes. Science 2016, 353, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Antonovsky, N.; Gleizer, S.; Noor, E.; Zohar, Y.; Herz, E.; Barenholz, U.; Zelcbuch, L.; Amram, S.; Wides, A.; Tepper, N.; et al. Sugar synthesis from CO2 in Escherichia coli. Cell 2016, 166, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Toprak, E.; Veres, A.; Yildiz, S.; Pedraza, J.M.; Chait, R.; Paulsson, J.; Kishony, R. Building a morbidostat: An automated continuous-culture device for studying bacterial drug resistance under dynamically sustained drug inhibition. Nat. Protoc. 2013, 8, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.G.; Mancuso, C.P.; Kiriakov, S.; Bashor, C.J.; Khalil, A.S. Precise, automated control of conditions for high-throughput growth of yeast and bacteria with eVOLVER. Nat. Biotechnol. 2018, 36, 614–623. [Google Scholar] [CrossRef]
- Lukačišinová, M.; Fernando, B.; Bollenbach, T. Highly parallel lab evolution reveals that epistasis can curb the evolution of antibiotic resistance. Nat. Commun. 2020, 11, 3105. [Google Scholar] [CrossRef]
- Horinouchi, T.; Minamoto, T.; Suzuki, S.; Shimizu, H.; Furusawa, C. Development of an automated culture system for laboratory evolution. J. Lab. Autom. 2014, 19, 478–482. [Google Scholar] [CrossRef]
- Takahashi, C.N.; Miller, A.W.; Ekness, F.; Dunham, M.J.; Klavins, E. A low cost, customizable turbidostat for use in synthetic circuit characterization. ACS Synth. Biol. 2015, 4, 32–38. [Google Scholar] [CrossRef]
- Selifonova, O.; Valle, F.; Schellenberger, V. Rapid evolution of novel traits in microorganisms. Appl. Environ. Microbiol. 2001, 67, 3645–3649. [Google Scholar] [CrossRef]
- Tan, Z.L.; Zheng, X.; Wu, Y.; Jian, X.; Xing, X.; Zhang, C. In vivo continuous evolution of metabolic pathways for chemical production. Microb. Cell Fact. 2019, 18, 82. [Google Scholar] [CrossRef]
- Mobini-Dehkordi, M.; Nahvi, I.; Zarkesh-Esfahani, H.; Ghaedi, K.; Tavassoli, M.; Akada, R. Isolation of a novel mutant strain of Saccharomyces cerevisiae by an ethyl methane sulfonate-induced mutagenesis approach as a high producer of bioethanol. J. Biosci. Bioeng. 2008, 105, 403–408. [Google Scholar] [CrossRef]
- Ohnishi, J.; Mizoguchi, H.; Takeno, S.; Ikeda, M. Characterization of mutations induced by N-methyl-N'-nitro-N-nitrosoguanidine in an industrial Corynebacterium glutamicum strain. Mutat. Res. 2008, 649, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Ohnishi, J.; Hayashi, M.; Mitsuhashi, S. A genome-based approach to create a minimally mutated Corynebacterium glutamicum strain for efficient L-lysine production. J. Ind. Microbiol. Biotechnol. 2006, 33, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Shibai, A.; Yokoi, N.; Tarusawa, Y.; Kawada, M.; Kotani, H.; Furusawa, C. Mutational property of newly identified mutagen L-glutamic acid γ-hydrazide in Escherichia coli. Mutat. Res. 2021, 823, 111759. [Google Scholar] [CrossRef]
- Scheuermann, R.H.; Echols, H. A separate editing exonuclease for DNA replication: The ε subunit of Escherichia coli DNA polymerase III holoenzyme. Proc. Natl. Acad. Sci. USA 1984, 81, 7747–7751. [Google Scholar] [CrossRef]
- Schaaper, R.M.; Radman, M. The extreme mutator effect of Escherichia coli mutD5 results from saturation of mismatch repair by excessive DNA replication errors. EMBO J. 1989, 8, 3511–3516. [Google Scholar] [CrossRef] [PubMed]
- Sniegowski, P.D.; Gerrish, P.J.; Lenski, R.E. Evolution of high mutation rates in experimental populations of E. coli. Nature 1997, 387, 703–705. [Google Scholar] [CrossRef] [PubMed]
- Cox, E.C.; Horner, D.L. Structure and coding properties of a dominant Escherichia coli mutator gene, mutD. Proc. Natl. Acad. Sci. USA 1983, 80, 2295–2299. [Google Scholar] [CrossRef]
- Abe, H.; Fujita, Y.; Takaoka, Y.; Kurita, E.; Yano, S.; Tanaka, N.; Nakayama, K. Ethanol-tolerant Saccharomyces cerevisiae strains isolated under selective conditions by over-expression of a proofreading-deficient DNA polymerase δ. J. Biosci. Bioeng. 2009, 108, 199–204. [Google Scholar] [CrossRef]
- Pontrelli, S.; Fricke, R.C.B.; Teoh, S.T.; Lavina, W.A.; Putri, S.P.; Fitz-Gibbon, S.; Chung, M.; Pellegrini, M.; Fukusaki, E.; Liao, J.C. Metabolic repair through emergence of new pathways in Escherichia coli. Nat. Chem. Biol. 2018, 14, 1005–1009. [Google Scholar] [CrossRef]
- Eom, G.E.; Lee, H.; Kim, S. Development of a genome-targeting mutator for the adaptive evolution of microbial cells. Nucleic Acids Res. 2022, 50, e38. [Google Scholar] [CrossRef] [PubMed]
- Garst, A.D.; Bassalo, M.C.; Pines, G.; Lynch, S.A.; Halweg-Edwards, A.L.; Liu, R.; Liang, L.; Wang, Z.; Zeitoun, R.; Alexander, W.G.; et al. Genome-wide mapping of mutations at single-nucleotide resolution for protein, metabolic and genome engineering. Nat. Biotechnol. 2017, 35, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Kong, S.; Luo, S.; Chen, C.; Cui, Z.; Sun, X.; Chen, T.; Wang, Z. Improving furfural tolerance of Escherichia coli by integrating adaptive laboratory evolution with CRISPR-enabled trackable genome engineering (CREATE). ACS Sustain. Chem. Eng. 2022, 10, 2318–2330. [Google Scholar] [CrossRef]
- Zoheir, A.E.; Spath, G.P.; Niemeyer, C.M.; Rabe, K.S. Microfluidic evolution-on-a-chip reveals new mutations that cause antibiotic resistance. Small 2021, 17, e2007166. [Google Scholar] [CrossRef] [PubMed]
- Köster, S.; Angilé, F.E.; Duan, H.; Agresti, J.J.; Wintner, A.; Schmitz, C.; Rowat, A.C.; Merten, C.A.; Pisignano, D.; Griffiths, A.D.; et al. Drop-based microfluidic devices for encapsulation of single cells. Lab Chip 2008, 8, 1110–1115. [Google Scholar] [CrossRef]
- Bachmann, H.; Fischlechner, M.; Rabbers, I.; Barfa, N.; Branco dos Santos, F.; Molenaar, D.; Teusink, B. Availability of public goods shapes the evolution of competing metabolic strategies. Proc. Natl. Acad. Sci. USA 2013, 110, 14302–14307. [Google Scholar] [CrossRef]
- Yuan, H.; Zhou, Y.; Lin, Y.; Tu, R.; Guo, Y.; Zhang, Y.; Wang, Q. Microfluidic screening and genomic mutation identification for enhancing cellulase production in Pichia pastoris. Biotechnol. Biofuels Bioprod. 2022, 15, 50. [Google Scholar] [CrossRef]
- Phaneuf, P.V.; Gosting, D.; Palsson, B.O.; Feist, A.M. ALEdb 1.0: A database of mutations from adaptive laboratory evolution experimentation. Nucleic Acids Res. 2019, 47, D1164–D1171. [Google Scholar] [CrossRef]
- Dev, C.; Jilani, S.B.; Yazdani, S.S. Adaptation on xylose improves glucose-xylose co-utilization and ethanol production in a carbon catabolite repression (CCR) compromised ethanologenic strain. Microb. Cell Fact. 2022, 21, 154. [Google Scholar] [CrossRef]
- Kim, J.; Tremaine, M.; Grass, J.A.; Purdy, H.M.; Landick, R.; Kiley, P.J.; Reed, J.L. Systems metabolic engineering of Escherichia coli improves coconversion of lignocellulose-derived sugars. Biotechnol. J. 2019, 14, e1800441. [Google Scholar] [CrossRef]
- Sarkar, P.; Mukherjee, M.; Goswami, G.; Das, D. Adaptive laboratory evolution induced novel mutations in Zymomonas mobilis ATCC ZW658: A potential platform for co-utilization of glucose and xylose. J. Ind. Microbiol. Biotechnol. 2020, 47, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Wang, J.; Yang, Y.; Yang, Q.; Li, R.; Hu, M.; He, Q.; Du, J.; Wang, X.; Li, M.; et al. Development and characterization of efficient xylose utilization strains of Zymomonas mobilis. Biotechnol. Biofuels 2021, 14, 231. [Google Scholar] [CrossRef] [PubMed]
- Millán, C.; Peña, C.; Flores, C.; Espín, G.; Galindo, E.; Castillo, T. Improving glucose and xylose assimilation in Azotobacter vinelandii by adaptive laboratory evolution. World J. Microbiol. Biotechnol. 2020, 36, 46. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, S.; Klein, M.; Carrillo, M.; McInnes, J.; Nguyen, H.T.T.; Nevoigt, E. Re-evaluation of glycerol utilization in Saccharomyces cerevisiae: Characterization of an isolate that grows on glycerol without supporting supplements. Biotechnol. Biofuels 2013, 6, 157. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Carrillo, M.; Xiberras, J.; Islam, Z.U.; Swinnen, S.; Nevoigt, E. Towards the exploitation of glycerol's high reducing power in Saccharomyces cerevisiae-based bioprocesses. Metab. Eng. 2016, 38, 464–472. [Google Scholar] [CrossRef]
- Strucko, T.; Zirngibl, K.; Pereira, F.; Kafkia, E.; Mohamed, E.T.; Rettel, M.; Stein, F.; Feist, A.M.; Jouhten, P.; Patil, K.R.; et al. Laboratory evolution reveals regulatory and metabolic trade-offs of glycerol utilization in Saccharomyces cerevisiae. Metab. Eng. 2018, 47, 73–82. [Google Scholar] [CrossRef]
- Kawai, K.; Kanesaki, Y.; Yoshikawa, H.; Hirasawa, T. Identification of metabolic engineering targets for improving glycerol assimilation ability of Saccharomyces cerevisiae based on adaptive laboratory evolution and transcriptome analysis. J. Biosci. Bioeng. 2019, 128, 162–169. [Google Scholar] [CrossRef]
- Yuzawa, T.; Shirai, T.; Orishimo, R.; Kawai, K.; Kondo, A.; Hirasawa, T. 13C-metabolic flux analysis in glycerol-assimilating strains of Saccharomyces cerevisiae. J. Gen. Appl. Microbiol. 2021, 67, 142–149. [Google Scholar] [CrossRef]
- Fan, L.; Wang, Y.; Qian, J.; Gao, N.; Zhang, Z.; Ni, X.; Sun, L.; Yuan, Q.; Zheng, P.; Sun, J. Transcriptome analysis reveals the roles of nitrogen metabolism and sedoheptulose bisphosphatase pathway in methanol-dependent growth of Corynebacterium glutamicum. Microb. Biotechnol. 2021, 14, 1797–1808. [Google Scholar] [CrossRef]
- Hennig, G.; Haupka, C.; Brito, L.F.; Ruckert, C.; Cahoreau, E.; Heux, S.; Wendisch, V.F. Methanol-essential growth of Corynebacterium glutamicum: Adaptive laboratory evolution overcomes limitation due to methanethiol assimilation pathway. Int. J. Mol. Sci. 2020, 21, 3617. [Google Scholar] [CrossRef]
- Tuyishime, P.; Wang, Y.; Fan, L.; Zhang, Q.; Li, Q.; Zheng, P.; Sun, J.; Ma, Y. Engineering Corynebacterium glutamicum for methanol-dependent growth and glutamate production. Metab. Eng. 2018, 49, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Har, J.R.G.; Agee, A.; Bennett, R.K.; Papoutsakis, E.T.; Antoniewicz, M.R. Adaptive laboratory evolution of methylotrophic Escherichia coli enables synthesis of all amino acids from methanol-derived carbon. Appl. Microbiol. Biotechnol. 2021, 105, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.Y.; Jung, H.W.; Tsuei, C.Y.; Liao, J.C. Converting Escherichia coli to a synthetic methylotroph growing solely on methanol. Cell 2020, 182, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Gleizer, S.; Ben-Nissan, R.; Bar-On, Y.M.; Antonovsky, N.; Noor, E.; Zohar, Y.; Jona, G.; Krieger, E.; Shamshoum, M.; Bar-Even, A.; et al. Conversion of Escherichia coli to generate all biomass carbon from CO2. Cell 2019, 179, 1255–1263.e12. [Google Scholar] [CrossRef]
- Ragsdale, S.W.; Pierce, E. Acetogenesis and the Wood-Ljungdahl pathway of CO2 fixation. Biochim. Biophys. Acta 2008, 1784, 1873–1898. [Google Scholar] [CrossRef]
- Kang, S.; Song, Y.; Jin, S.; Shin, J.; Bae, J.; Kim, D.R.; Lee, J.K.; Kim, S.C.; Cho, S.; Cho, B.K. Adaptive laboratory evolution of Eubacterium limosum ATCC 8486 on carbon monoxide. Front. Microbiol. 2020, 11, 402. [Google Scholar] [CrossRef]
- Jin, S.; Kang, S.; Bae, J.; Lee, H.; Cho, B.-K. Development of CO gas conversion system using high CO tolerance biocatalyst. Chem. Eng. J. 2022, 449, 137678. [Google Scholar] [CrossRef]
- Nguyen-Vo, T.P.; Liang, Y.; Sankaranarayanan, M.; Seol, E.; Chun, A.Y.; Ashok, S.; Chauhan, A.S.; Kim, J.R.; Park, S. Development of 3-hydroxypropionic-acid-tolerant strain of Escherichia coli W and role of minor global regulator yieP. Metab. Eng. 2019, 53, 48–58. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, L.; Tuyishime, P.; Liu, J.; Zhang, K.; Gao, N.; Zhang, Z.; Ni, X.; Feng, J.; Yuan, Q.; et al. Adaptive laboratory evolution enhances methanol tolerance and conversion in engineered Corynebacterium glutamicum. Commun. Biol. 2020, 3, 217. [Google Scholar] [CrossRef]
- Caspeta, L.; Coronel, J.; Montes de Oca, A.; Abarca, E.; González, L.; Martínez, A. Engineering high-gravity fermentations for ethanol production at elevated temperature with Saccharomyces cerevisiae. Biotechnol. Bioeng. 2019, 116, 2587–2597. [Google Scholar] [CrossRef]
- Salas-Navarrete, P.C.; de Oca Miranda, A.I.M.; Martínez, A.; Caspeta, L. Evolutionary and reverse engineering to increase Saccharomyces cerevisiae tolerance to acetic acid, acidic pH, and high temperature. Appl. Microbiol. Biotechnol. 2022, 106, 383–399. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.Y.; Wang, S.S.; Guan, C.G.; Liang, W.F.; Xue, Z.L.; Zhang, C.; Xing, X.H. Breeding of methanol-tolerant Methylobacterium extorquens AM1 by atmospheric and room temperature plasma mutagenesis combined with adaptive laboratory evolution. Biotechnol. J. 2018, 13, e1700679. [Google Scholar] [CrossRef]
- Belkhelfa, S.; Roche, D.; Dubois, I.; Berger, A.; Delmas, V.A.; Cattolico, L.; Perret, A.; Labadie, K.; Perdereau, A.C.; Darii, E.; et al. Continuous culture adaptation of Methylobacterium extorquens AM1 and TK 0001 to very high methanol concentrations. Front. Microbiol. 2019, 10, 1313. [Google Scholar] [CrossRef] [PubMed]
- Catrileo, D.; Acuna-Fontecilla, A.; Godoy, L. Adaptive laboratory evolution of native Torulaspora delbrueckii YCPUC10 with enhanced ethanol resistance and evaluation in co-inoculated fermentation. Front. Microbiol. 2020, 11, 595023. [Google Scholar] [CrossRef] [PubMed]
- Phommachan, K.; Keo-oudone, C.; Nurcholis, M.; Vongvilaisak, N.; Chanhming, M.; Savanhnaly, V.; Bounphanmy, S.; Matsutani, M.; Kosaka, T.; Limtong, S.; et al. Adaptive laboratory evolution for multistress tolerance, including fermentability at high glucose concentrations in thermotolerant Candida tropicalis. Energies 2022, 15, 561. [Google Scholar] [CrossRef]
- Lu, Z.; Imlay, J.A. When anaerobes encounter oxygen: Mechanisms of oxygen toxicity, tolerance and defence. Nat. Rev. Microbiol. 2021, 19, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Schoeffler, M.; Gaudin, A.L.; Ramel, F.; Valette, O.; Denis, Y.; Hania, W.B.; Hirschler-Rea, A.; Dolla, A. Growth of an anaerobic sulfate-reducing bacterium sustained by oxygen respiratory energy conservation after O2-driven experimental evolution. Environ. Microbiol. 2019, 21, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Esquivel-Elizondo, S.; Delgado, A.G.; Krajmalnik-Brown, R. Evolution of microbial communities growing with carbon monoxide, hydrogen, and carbon dioxide. FEMS Microbiol. Ecol. 2017, 93, fix076. [Google Scholar] [CrossRef]
- Dann, M.; Ortiz, E.M.; Thomas, M.; Guljamow, A.; Lehmann, M.; Schaefer, H.; Leister, D. Enhancing photosynthesis at high light levels by adaptive laboratory evolution. Nat. Plants 2021, 7, 681–695. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Ogawa, K.; Toya, Y.; Akimoto, S.; Matsuda, F.; Shimizu, H. Mutations in hik26 and slr1916 lead to high-light stress tolerance in Synechocystis sp. PCC6803. Commun. Biol. 2021, 4, 343. [Google Scholar] [CrossRef]
- Moser, J.W.; Prielhofer, R.; Gerner, S.M.; Graf, A.B.; Wilson, I.B.; Mattanovich, D.; Dragosits, M. Implications of evolutionary engineering for growth and recombinant protein production in methanol-based growth media in the yeast Pichia pastoris. Microb. Cell Fact. 2017, 16, 49. [Google Scholar] [CrossRef] [PubMed]
- Zambanini, T.; Hosseinpour Tehrani, H.; Geiser, E.; Merker, D.; Schleese, S.; Krabbe, J.; Buescher, J.M.; Meurer, G.; Wierckx, N.; Blank, L.M. Efficient itaconic acid production from glycerol with Ustilago vetiveriae TZ1. Biotechnol. Biofuels 2017, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Cubas-Cano, E.; Gonzalez-Fernández, C.; Tomás-Pejó, E. Evolutionary engineering of Lactobacillus pentosus improves lactic acid productivity from xylose-rich media at low pH. Bioresour. Technol. 2019, 288, 121540. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Sathesh-Prabu, C.; Kwak, G.H.; Bang, I.; Jung, H.W.; Kim, D.; Lee, S.K. Enhanced production of nonanedioic acid from nonanoic acid by engineered Escherichia coli. Biotechnol. J. 2022, 17, e2000416. [Google Scholar] [CrossRef] [PubMed]
- Prell, C.; Busche, T.; Ruckert, C.; Nolte, L.; Brandenbusch, C.; Wendisch, V.F. Adaptive laboratory evolution accelerated glutarate production by Corynebacterium glutamicum. Microb. Cell Fact. 2021, 20, 97. [Google Scholar] [CrossRef]
- Kim, K.; Hou, C.Y.; Choe, D.; Kang, M.; Cho, S.; Sung, B.H.; Lee, D.H.; Lee, S.G.; Kang, T.J.; Cho, B.K. Adaptive laboratory evolution of Escherichia coli W enhances gamma-aminobutyric acid production using glycerol as the carbon source. Metab. Eng. 2022, 69, 59–72. [Google Scholar] [CrossRef]
- Tokuyama, K.; Toya, Y.; Horinouchi, T.; Furusawa, C.; Matsuda, F.; Shimizu, H. Application of adaptive laboratory evolution to overcome a flux limitation in an Escherichia coli production strain. Biotechnol. Bioeng. 2018, 115, 1542–1551. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, B.; Fu, J.; Wang, Z.; Chen, T. Adaptive laboratory evolution of Halomonas bluephagenesis enhances acetate tolerance and utilization to produce poly(3-hydroxybutyrate). Molecules 2022, 27, 22. [Google Scholar] [CrossRef]
- Kawai, R.; Toya, Y.; Miyoshi, K.; Murakami, M.; Niide, T.; Horinouchi, T.; Maeda, T.; Shibai, A.; Furusawa, C.; Shimizu, H. Acceleration of target production in co-culture by enhancing intermediate consumption through adaptive laboratory evolution. Biotechnol. Bioeng. 2022, 119, 936–945. [Google Scholar] [CrossRef]
- Kawai, R.; Toya, Y.; Shimizu, H. Metabolic pathway design for growth-associated phenylalanine production using synthetically designed mutualism. Bioprocess Biosyst. Eng. 2022, 45, 1539–1546. [Google Scholar] [CrossRef]
- Mahr, R.; Gatgens, C.; Gatgens, J.; Polen, T.; Kalinowski, J.; Frunzke, J. Biosensor-driven adaptive laboratory evolution of L-valine production in Corynebacterium glutamicum. Metab. Eng. 2015, 32, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Gwon, D.A.; Seok, J.Y.; Jung, G.Y.; Lee, J.W. Biosensor-assisted adaptive laboratory evolution for violacein production. Int. J. Mol. Sci. 2021, 22, 6594. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Shi, F.; Liu, H.; Tan, S.; Li, Y. Programming adaptive laboratory evolution of 4-hydroxyisoleucine production driven by a lysine biosensor in Corynebacterium glutamicum. AMB Express 2021, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.Y.; Han, Y.H.; Yang, J.S.; Yang, J.; Lim, H.G.; Kim, S.G.; Seo, S.W.; Jung, G.Y. Synthetic biosensor accelerates evolution by rewiring carbon metabolism toward a specific metabolite. Cell Rep 2021, 36, 109589. [Google Scholar] [CrossRef]
- Luo, H.; Hansen, A.S.L.; Yang, L.; Schneider, K.; Kristensen, M.; Christensen, U.; Christensen, H.B.; Du, B.; Ozdemir, E.; Feist, A.M.; et al. Coupling S-adenosylmethionine-dependent methylation to growth: Design and uses. PLoS Biol. 2019, 17, e2007050. [Google Scholar] [CrossRef]
- He, Y.; Yin, H.; Dong, J.; Yu, J.; Zhang, L.; Yan, P.; Wan, X.; Hou, X.; Zhao, Y.; Chen, R.; et al. Reduced sensitivity of lager brewing yeast to premature yeast flocculation via adaptive evolution. Food Microbiol. 2022, 106, 104032. [Google Scholar] [CrossRef]
- Gorter de Vries, A.R.; Voskamp, M.A.; van Aalst, A.C.A.; Kristensen, L.H.; Jansen, L.; van den Broek, M.; Salazar, A.N.; Brouwers, N.; Abeel, T.; Pronk, J.T.; et al. Laboratory evolution of a Saccharomyces cerevisiae x S. eubayanus hybrid under simulated lager-brewing conditions. Front. Genet 2019, 10, 242. [Google Scholar] [CrossRef]
- Gibson, B.; Vidgren, V.; Peddinti, G.; Krogerus, K. Diacetyl control during brewery fermentation via adaptive laboratory engineering of the lager yeast Saccharomyces pastorianus. J. Ind. Microbiol. Biotechnol. 2018, 45, 1103–1112. [Google Scholar] [CrossRef]
| Organism/Strain | ALE Method | Results/Outcome | Reference |
|---|---|---|---|
| E. coli B with deletion of ldhA, frdA and pflB and replacement of pdh promoter with gapA promoter evolved on glucose and xylose, followed by ptsG deletion | Chemostat on glucose and xylose in bioreactor at a dilution rate of 0.01 h–1 for 7 days, followed by passages on agar plates containing xylose for 10 days and in Hungate tube containing liquid medium with stepwise increase in xylose concentration for 41 d | Adapted strain SCD78 simultaneously consumed both 10 g L–1 each of glucose and xylose in 36 h. High ethanol production was achieved. | [49] |
| E. coli MG1655 with deletion of ptsH and ldhA genes and replacement of pflB with Z. mobilis pdc and adhB | Serial transfers of culture on 10 g L–1 glucose for 5 times and on 6 g L–1 glucose and 4 g L–1 xylose for 5 times followed by transfers of cultures on 10 g L–1 glucose plus 5% ethanol | Adapted strain JK32E improved co-utilization of both glucose and xylose and produced high concentration of ethanol by additional introduction of the plasmid carrying Z. mobilis pdc and adhB. | [50] |
| Z. mobilis harboring the genes from E. coli related to xylose metabolism on the genome | Serial transfers of culture with increasing concentration of xylose from 3% (10 transfers), 5% (20 transfers), to 10% (20 transfers), followed by single colony isolations, for over 200 d in total. | The evolved strain AD50 showed increased xylose uptake rate by 1.65 times. Co-utilization of glucose and xylose and high ethanol production was achieved. | [51] |
| Z. mobilis carrying xylose isomerase gene from Reticulitermes speratus and xylB from E. coli | Serial transfers of culture containing 50 g L–1 xylose 38 times over 100 days. | Xylose consumption rate (50–150 g L–1) was increased and ethanol yield was enhanced by 60–140% | [52] |
| Azotobacter vinelandii OP | Serial transfers of culture on 2 g L–1 xylose and 8 g L–1 glucose and finally 8 g L–1 xylose and 2 g L–1 glucose in shake flasks (380 generations). | Specific growth rate, glucose uptake rate and xylose uptake rate in the evolved strain increase 2, 6.5 and 3.6-fold, respectively. | [53] |
| Organism/Strain | Stress | ALE Method | Reference |
|---|---|---|---|
| E. coli | 3-Hydroxypropionic acid | Serial transfers of culture in M9 medium containing 0.5 g L–1 yeast extract, 100 mM glycerol for 5 months. Concentration of 3-hydroxy propionic acid increased stepwise by 50 mM in the range from 200 to 800 mM | [68] |
| S. cerevisiae S288c | Thermal stress | Serial transfers of culture at 39.5 °C for 342 d (1200 generations) | [70] |
| S. cerevisiae and thermotolerant TTY23 strains | Acetic acid, acidic pH | Serial transfers of culture in the medium containing 3 g L–1 acetic acid at pH 3 for 41 d, 4 g L–1 for 57 d, and 12 g L–1 at pH 4 for 102 d (243 d and 816 generations in total) | [71] |
| Target Product | Organism/Strain | ALE Method | Outcome | Reference |
|---|---|---|---|---|
| Recombinant protein | Pichia pastoris | Serial transfer of culture on methanol media for 250 generations. | One evolved clone using an alcohol oxidase (AOX) promoter for recombinant protein production showed 2.5- and 1.8-fold increased production in batch and fed-batch cultivations, respectively. | [81] |
| Itaconic acid | Ustilago vetiveriae | Serial transfer of culture on glycerol medium for 62 d. | Growth was not improved, while glycerol uptake was improved. Production of itaconic acid was improved, reaching 4.4 g L–1. Itaconic acid production by the evolved clone reached 35 g L–1 from 196 g L–1 glycerol with medium optimization. | [82] |
| Lactic acid | Lactobacillus pentosus | Serial transfers of anaerobic culture with increasing xylose concentration 100 times (850 generations). | Xylose consumption and lactic acid production increased by 1.5–2-fold compared to the parental strain on 20 g L–1 xylose. | [83] |
| Nonanedioic acid | E. coli fadR mutant | Serial transfers of culture on 3 g L–1 nonanoic acid 30 times (90 d). | The evolved strain with fadE deletion to prevent nonanoic acid degradation produced nonanedioic acid by 12.8-fold compared to the wild-type strain. | [84] |
| Glutaric acid | C. glutamicum recombinant strain for glutaric acid production applied to repeated cultivation | Serial culture transfers in glucose medium 8 times. | Growth was improved from 0.10 h–1 to 0.17 h–1. The volumetric productivity of the strain after the 7th transfer (0.18 g L–1 h–1) was two-fold higher than that of the starting strain. | [85] |
| γ-Aminobutyric acid | E. coli W engineered for γ-aminobutyric acid production from glycerol | Serial transfers of cultures in medium containing 0.2% glycerol for 1300 generations. | The specific growth rate was improved by 40%. Glycerol utilization was also improved. γ-aminobutyric acid production was increased by 2.6-fold. | [86] |
| Succinic acid | E. coli engineered for succinic acid production from glycerol | Serial transfers of culture on glycerol for about 100 generations. | Succinic acid yield from glycerol (C-mol/C-mol) was increased from 0.08 to 0.45. | [87] |
| Poly(3-hydroxybutyrate) | Halomonas bluephagenesis | Serial transfers of culture 71 times with increasing acetic acid concentration (20–120 g L–1). | Cell growth, acetic acid consumption, and tolerance to acetic acid were improved. Poly(3-hydroxybutyrate) production from acetic acid was improved as well. | [88] |
| Organism | Target Product | Biosensor | Outcome | Reference |
|---|---|---|---|---|
| C. glutamicum | L-Valine | L-Valine biosensor consists of lrp, the intergenic region between lrp and brnF, and a transcriptional fusion of brnF with eyfp. | Successfully evolved to a 25% increase in L-valine titers and a 3–4-fold reduction in by-product formation. | [91] |
| E. coli | Violacein | L-Tryptophan biosensor consists of tnaC fused with tetA-sgfp. | Successfully evolved to a 2.7-fold higher violacein production compared to the parent strain. | [92] |
| C. glutamicum | 4-Hydroxyisoleucine | L-Lysine biosensor based on the transcriptional regulator LysG and EYFP. | Successfully evolved to a 28.4 % increase in 4-hydroxy isoleucine titers. | [93] |
| E. coli | 3-Hydroxypropionic acid | 3-Hydroxypropionic acid biosensor consists of two modules: the transcription factor C4-lysR sensing 3-hydroxy propionic acid, and the responsive module based on tetA expression conferring tetracycline resistance. | Archived production of 55 g L–1 3-hydroxy propionic acid which is the highest yield reported to date. | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirasawa, T.; Maeda, T. Adaptive Laboratory Evolution of Microorganisms: Methodology and Application for Bioproduction. Microorganisms 2023, 11, 92. https://doi.org/10.3390/microorganisms11010092
Hirasawa T, Maeda T. Adaptive Laboratory Evolution of Microorganisms: Methodology and Application for Bioproduction. Microorganisms. 2023; 11(1):92. https://doi.org/10.3390/microorganisms11010092
Chicago/Turabian StyleHirasawa, Takashi, and Tomoya Maeda. 2023. "Adaptive Laboratory Evolution of Microorganisms: Methodology and Application for Bioproduction" Microorganisms 11, no. 1: 92. https://doi.org/10.3390/microorganisms11010092
APA StyleHirasawa, T., & Maeda, T. (2023). Adaptive Laboratory Evolution of Microorganisms: Methodology and Application for Bioproduction. Microorganisms, 11(1), 92. https://doi.org/10.3390/microorganisms11010092






