Abstract
Regulated cell death (RCD) is central to the development, integrity, and functionality of multicellular organisms. In the last decade, evidence has accumulated that RCD is a universal phenomenon in all life domains. Cyanobacteria are of specific interest due to their importance in aquatic and terrestrial habitats and their role as primary producers in global nutrient cycling. Current knowledge on cyanobacterial RCD is based mainly on biochemical and morphological observations, often by methods directly transferred from vertebrate research and with limited understanding of the molecular genetic basis. However, the metabolism of different cyanobacteria groups relies on photosynthesis and nitrogen fixation, whereas mitochondria are the central executioner of cell death in vertebrates. Moreover, cyanobacteria chosen as biological models in RCD studies are mainly colonial or filamentous multicellular organisms. On the other hand, unicellular cyanobacteria have regulated programs of cellular survival (RCS) such as chlorosis and post-chlorosis resuscitation. The co-existence of different genetically regulated programs in cyanobacterial populations may have been a top engine in life diversification. Development of cyanobacteria-specific methods for identification and characterization of RCD and wider use of single-cell analysis combined with intelligent image-based cell sorting and metagenomics would shed more light on the underlying molecular mechanisms and help us to address the complex colonial interactions during these events. In this review, we focus on the functional implications of RCD in cyanobacterial communities.
1. Introduction
Genetically regulated cell death (RCD) was first identified in metazoans and later found in prokaryotes and different eukaryotes [1,2,3]. The multiple forms of RCD execution seem to involve a series of stereotypical morphological and biochemical hallmarks such as initiation of proteolytic cascade and DNA degradation. Apoptosis-like RCD in metazoans is characterized by lipid rearrangement of the plasma membrane and a decrease in cellular volume and changes in ultrastructure, genome fragmentation, and at the final stages, disassembly of the cell into membrane-enclosed vesicles. Research on RCD has made spectacular progress in eukaryotes, including unicellular organisms [4,5,6,7,8]. In the last decade, emerging evidence suggests that RCD-linked genes and activities are also possibly involved in bacterial life cycle regulation and environmental stress adaptations [1,9,10,11], stress-related protein complexes in archaea [12,13,14], and cyanobacteria [15,16,17]. Many hypotheses have been proposed as to why RCD evolved in unicellular prokaryotes despite its obvious disadvantage to individual fitness. The possible evolutionary advantages (i.e., at the population level) can be related to (a) better response to stress and starvation, (b) immunity against pathogens, and (c) development of transiently multicellular organisms [3]. RCD in prokaryotes is triggered by environmental stresses (nutrient deprivation, oxidative stress, high salinity, high light levels, heavy metals pollution) and biotic factors (infection with pathogens, allelopathy) and may lead to autocatalytic cell suicide and, eventually, cell demolition. It is related to massive cyanobacterial cell death and lysis in nature [18]. Importantly, (1) cell death in cyanobacteria is regulated and is not just an accidental event because of harsh environmental conditions or stress and (2) in addition to morphological criteria for RCD identification and characterization, there is increasing molecular evidence about the RCD-like sequence of events in cyanobacteria.
However, a stress response in different cyanobacteria species also relies on regulated cellular survival (RCS) programs such as chlorosis and resuscitation, leading to temporary dormancy in the unicellular non-diazotrophic cyanobacteria [19] and to the maturation of akinetes in heterocyst-forming multicellular filamentous cyanobacteria [20]. Cyanobacteria are one of the most morphologically diverse prokaryotic phyla where multicellularity has evolved [21]. Cyanobacteria represent a group of oxygenic phototrophic bacteria with Gram-negative characteristics, several members of which are colonial (Microcystis) and/or exhibit multicellularity, with up to six distinct cell types in filamentous cyanobacteria [22,23]. Taxonomic research traditionally organizes the cyanobacteria into five subsections based on their morphological traits [24]. Unicellular forms belong to subsection I (Chroococcales), which undergo binary fission, and subsection II (Pleurococcales), which reproduce through multiple fissions in three planes. Evolutionary sequencing-based studies suggested that within the cyanobacterial phylum, reversions and switches from filamentous multi- to unicellularity occurred, affecting a marine planktonic SynPro clade and Gloeocapsopsis clade that has been traditionally classified in subsection II [25,26]. The transition to multicellularity is a major evolutionary transition, and multicellular life forms have evolved independently many times, suggesting multiple advantages associated with this transition [27]. Nitrogen fixation was found to be the prime driver of multicellularity in cyanobacteria [23], and a trade-off between nitrogen fixation and photosynthesis led to the compartmentalization of these two functions through multiple evolutionary solutions [23].
RCD in cyanobacteria is a long-studied phenomenon mainly in multicellular cyanobacteria, in colonial organisms (Microcystis sp.), and in cyanobacteria species that were considered to be unicellular forms but probably at a certain evolutionary point reversed from multicellularity to unicellularity ([28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64], Table 1, Figure 1). Some representatives of section I of cyanobacteria form enclosed sheath cellular aggregates, and some section II representatives form multicellular aggregates enclosed by an additional fibrous layer [23,65].

Table 1.
Characterization of RCD in cyanobacteria.
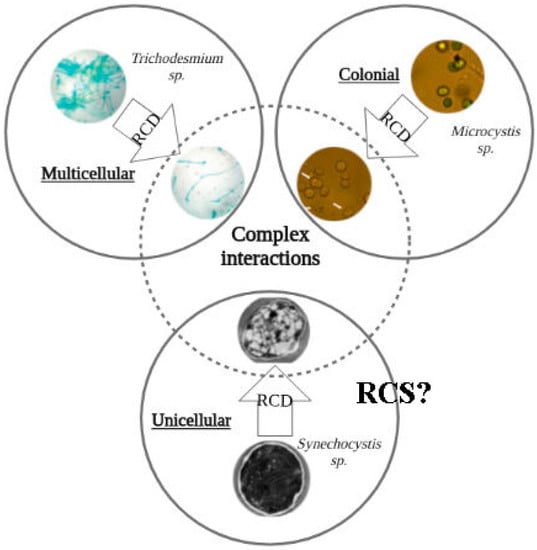
Figure 1.
Regulated cell death (RCD) models in cyanobacteria. Regulated cell death (RCD) has extensively been studied in a few biological models. It includes multicellular (filamentous) cyanobacteria, such as Trichodesmium spp. (top left). RCD was also documented in colonial strains and in some unicellular organisms, which was recently suggested could reverse multicellularity (Microcystis spp.—top right, Synechocystis spp.—bottom).
All this brings us to a question: if practically all studies of RCD in cyanobacteria were performed on multicellular and colonial (at least temporary multicellular) species—what happens with unicellular cyanobacteria in response to the environmental stress? Does their stress response initiate the regulated programs of survival (RCS) following resuscitation or regulated cell death? Opposite to multicellular cyanobacteria RCD research, practically all studies on chlorosis and RCS were conducted on two non-diazotrophic unicellular model species (Synechocystis spp. and Synechococcus elongatus) [19]. For unicellular non-diazotrophic cyanobacteria, exit to reversible dormancy and quiescence by a regulated survival program may be a key to rescue, though co-existence of cells executing RCD and RCS and heterogeneity of cyanobacterial populations along the quiescence–RCD response will provide an advantage at the population level.
Limitations of existing methods to study RCD in cyanobacteria are currently actively discussed (ref. [66]). Methods for studying RCD were developed to investigate vertebrate cells where metabolic changes during apoptosis rely on mitochondria as stress sensors and central executioners of cell death [67,68,69,70]. Therefore, the direct transfer of existing vertebrate methodology to cyanobacteria organisms that have no mitochondria and are using different sources of energy—photosynthesis and nitrogen fixation—may lead to the false findings introduced by analogical reasoning [71].
In this study, we review functional applications of RCD in cyanobacteria in relation to abiotic and biotic (cyanophages and other pathogens) factors.
2. Functional Importance of RCD in Cyanobacteria
There is direct experimental evidence for RCD-associated benefits for higher-group-level prokaryotes, such as survival under phage infection. During infection with a pathogen, a prokaryotic strain capable of RCD outperforms non-RCD strain, indicating surplus at the population level [72]. One of the first routes to multicellularity was to aggregate temporarily in order to resist unfavorable conditions [73]. The colonial cyanobacteria such as Microcystis spp., at least transiently, are part of multicellular systems, and some cyanobacteria, such as diazotrophic filamentous spp. are functionally differentiated (akinetes, heterocytes, vegetative cells) [74]. A bacterial community can induce RCD in a part of population to favor the colony survival in response to the external stress stimulus: phage and fungal infections, nutrient deprivation, radiation exposure and oxidative stress [75]. However, the cell specialization and RCD co-evolved together with other pathways of survival—the ability to develop metabolic rewiring and reorganization of cellular machinery leading to cell dormancy and reversible quiescence [76]. Remodeling of photosynthetic apparatus and cellular metabolic machinery reorganization accompany chlorosis and cellular survival and resuscitation after long-term chlorosis [77].
2.1. Starvation
One of the explanations for bacterial RCD appearance during evolution is a mechanism for functional interaction between unicellular organisms, which further increases their fitness via mutual dependence [78]. Under stress conditions, as a result of nutrient starvation, dying cyanobacteria release nutrients essential for the survival of the remaining population (“nutrient acquisition”) [79,80,81]. In 2004, a study reported an autocatalytic RCD in Trichodesmium spp. in response to nutrient starvation [16]. It happens, for example, in colonial morphospecies when nutrients for the entire colony are limited, and the cell death of some cells may increase the chances for colony survival—similar to results obtained with colonial yeasts [82,83]. Bar-Zeev and colleagues [39] demonstrated the release of transparent polysaccharides during RCD in Trichodesmium algal blooms and the consequent downward flux of nutrients, supplying the bloom participants with carbon and nitrogen. RCD, in this case, facilitates the nutrient cycling in the microbial loop—how a unicellular organism dies was shown to affect the fate of other microorganisms [2]. In particular, the exchange of nutrients released upon the lysis of a photosynthetic organism led to the recycling of these dissolved organic materials (DOMs).
Cyanobacteria evolved under periodic changes between periods of nutrient sufficiency, allowing fast growth, and the following periods of nitrogen depletion when the cells needed to cope with starvation until nutrients were replenished. However, the story of nitrogen starvation shaping cyanobacteria functionality and evolution is rather complicated, and we will briefly discuss just a few aspects of this complex story related to regulated cell survival (RCS) (we recommend an excellent specific review for detailed discussion on the mechanisms of nitrogen starvation and chlorosis [19]). Two major metabolic processes in cyanobacteria are the processes of photosynthesis and N2 fixation. They are regulated to increase cellular fitness and ecological competitiveness by distinct daily cycles such as circadian rhythms [84,85]. Oscillation changes in photosynthesis and nitrogen fixation were found initially in several diazotrophic cyanobacteria strains [86,87], and later Huang and co-authors [88] discovered the classical circadian rhythm of nitrogen fixation in Synechococcus sp. RF-1. Cyanobacteria are the only prokaryotes known to possess a circadian clock [84].
The circadian clock in Synechococcus elongatus comprises the core oscillator proteins KaiA, B, and C [84]. Under light–dark (LD) conditions S. elongatus employs photosynthesis and carbon fixation, and stores fixed carbon as glycogen [89]. In the dark period, glycogen is rapidly degraded via the oxidative pentose phosphate pathway. Regulator of phycobilisome (PBS) association (RpaA) was first identified as a protein that influences the ratio of light energy transfer from PBS to photosystems PSI vs. PSII [90]. RpaA forms a two-component pair with SasA histidine kinase, which interacts directly with central circadian core components (KaiC). The RpaB forms a two-component pair with the histidine kinase Hik33 and participates in the responses to environmental stress such as oxidative stress, high salinity, and high light [91]. Most of the insight into the functionality of this two-component system, as we already mentioned, was obtained using only two model cyanobacteria species—Synechocystis 6803 and Synechococcus elongatus PCC 7942. Typical cyanobacteria possess multiple two-component systems, mainly comprising a membrane-bound histidine kinase (HK) and its cognate cytoplasmic response regulator. Their quantity is widely varied—from just 11 putative genes encoding two-component systems in the genome of Prochlorococcus MED4, up to 146 HK and 168 genome regulators (RR) in filamentous Nostoc punctiforme [92,93]. Recently, the possibility of direct thiol regulation of some cyanobacterial RRs (Rre1, RpaA, and RpaB) by the redox state of the photosynthetic electron transport chain through ferredoxin or thioredoxin was suggested [94]. Moreover, the mutants of S. elongatus defective for the rpaA accumulate excessive reactive oxygen species (ROS) during the day and are inviable after several hours in the dark, likely because NADPH is required to detoxify ROS, and enzymes of the NADPH pathway are direct targets of RpaA [95].
The need to modulate the photosynthetic apparatus between alternative metabolic conditions is responsible for developing of the genetically regulated process of chlorosis associated with the response to nitrogenstarvation (rev. [19]). Chlorosis is characterized by the degradation of photosynthetic pigments and is accompanied by a color change from blue green to yellow (hence the name) [96]. The chlorosis (bleaching of algal cultures in response to nitrogen starvation) was already described in 1910 [97]. Phycobilisomes may account for up to 60% of the cellular protein in the cyanobacterial cell and serve as a major cellular reserve in nutrient starvation conditions [98]. Upon nitrogen depletion, phycobilisomes—giant protein–pigment complexes anchored to the thylakoid membranes—rapidly degrade following a regulated genetic program [99,100,101]. Degradation of PBS supplies nutrients for cellular metabolism during nutrient deprivation conditions, prevents photosynthetic apparatus from undergoing photoinhibition and production of harmful ROS [102], and plays an important role in cell survival [103]. Following long-term nitrogen deprivation, chlorophyll (Chl) level decreases dramatically to <1% of the original [104]. Using Synechocystis PCC 6803 sp. as a model, Murton and co-authors [103] demonstrated heterogeneity of pigment distribution in the single cells that can be explained by the self-shading effect [105] and revealed clustering of cells with high PC and low Chl–PSII in the concerted population response to nitrogen starvation. A first comprehensive analysis of a genetically regulated program for resuscitation from chlorosis was published by Klotz and co-authors [106] using Synechocystis as a model. When long-term chlorotic Synechocystis cells are supplied with nitrogen, they regain photosynthetic activity after about 24 h, and cell division resumes after three days. The transcriptomic and proteomic analysis revealed that during this lag-phase period, cyanobacterial cells are rebuilding the complex photosynthetic machinery [106,107]. In the different resuscitation phases, cyanobacterial cells go through different metabolic phases—from heterotrophic-like metabolism based on glycogen consumption and oxygen-dependent respiration to a mixotrophic metabolism when glycogen assimilation co-occurs with oxygenic photosynthesis, and finally—to the resumption of classical photoautotrophic metabolism [77]. Photosynthetic apparatus remodeling and cellular metabolic machinery reorganization accompany chlorosis and resuscitation after long-term chlorosis. Importantly, it is a genetically regulated program of cellular survival (regulated cellular survival or RCS). During the first steps of the resuscitation of unicellular cyanobacteria, an almost instantaneous (in minutes) increase in the ATP level is observed [77].
2.2. Differentiation and RCD in Cyanobacteria
Complex multicellularity evolved app. 2.5 billion years ago, and since then has been lost and regained several times in cyanobacteria [18,108,109]. In particular, filamentous cyanobacteria may undergo cellular differentiation into at least five specialized cell types: photosynthetic vegetative (photosynthetic) cells, nitrogen-fixating heterocytes (=heterocysts), spore-like cells—akinetes [74hormogonia cells, and necridia. Moreover, vegetative cells can divide and generate other cell types, but heterocytes cannot revert back and thus represent terminally differentiated cells [110]. Apart from nutritional function, heterocytes also regulate the placement and positioning of akinetes [111]. Hormogonia represent another cyanobacterial cell type characterized as short, motile filaments [74], the primary function of which is the colonization of new environments more suitable for growth [112]. The release of hormogonium from the parental trichome happens after the formation of released dead cells, called necridia [112,113,114,115].
Necridia transform and adapt themselves for their final function, the attachment of hormogonia to the colonized surface. This mechanism can act in conjunction with other adhesion mechanisms. The first stage in necridia differentiation is characterized by a peak in fluorescence that accompanies the loss of photosystem II activity, which is well described in heterocytes [116]. The appearance of these highly fluorescent cells allows the fragmented filaments to escape unfavorable environmental conditions. Depending on the mechanical strain, cell bleaching is followed by the breaking of the parental filament. The nonrandom position of the high-fluorescence cell within the filament, the abrupt appearance of the elevated chlorophyll a fluorescence, persisting photochemical activity, and the equally abrupt decomposition of the photosynthetic apparatus indicate that cell death is strongly controlled rather than being an accidental decay process induced by unfavorable environmental conditions [112]. The alternative strategy was developed by the cyanobacteria Plectonema boryanum (=Leptolyngbya boryana), where it switches back and forth between nitrogen and carbon fixation, hence separating processes not in space but in time, i.e., achieving temporal differentiation [117].
Cell death of filamentous cyanobacteria of different genera in exposure to stress leads to fragmentation of filaments and the subsequent autolysis of cells. The extrusion of cellular components and loss of viability that is observed in Anabaena variabilis during heterocyst differentiation can also be considered as evidence for the role of RCD in cell propagation and dispersal [78]. Environmental stress triggers akinete formation and induces reactive oxygen species (ROS) accumulation in the cyanobacterium Aphanizomenon ovalisporum (=Chrysosporum ovalisporum) [118]. However, the differentiating Aphanizomenon cells prevent the oxidative burst by degradation of the antioxidative machinery and phycobilisome antenna [118,119].
Differentiation of vegetative cells to dormant forms (akinetes) in cyanobacteria (Nostocales and Stigonematales orders) has been studied since 1856 [120]. Significant progress in analyzing mechanisms that lead to the akinete formation has been achieved recently [76,77]. Only heterocyst-forming filamentous multicellular cyanobacteria form akinetes, and their differentiation and maturation have been studied in detail using two model organisms: the terrestrial Nostoc punctiforme ATCC 29133 and the freshwater Anabaena variabilis (=Trichormus variabilis) ATCC 29413. The environmental stresses, such as nutrient starvation, temperature changes, and light availability, trigger akinete germination [20,121]. This process begins with a reorganization of the cellular material, followed by elongation and cell division that occur inside the akinete envelope [122]. Despite the last decade's developments, precise molecular and cellular mechanisms involved in the germination of akinetes remain unclear.
2.3. Infection-Triggered Response in Cyanobacteria Is Activated Coordinately with Multiple Defense Functions
Cyanobacteria co-exist under natural conditions with other organisms such as cyanophages, heterotrophic bacteria, protists, and algicidal microfungi [123]. Besides grazers, the parasites participate in top-down control of phytoplankton [124,125] with the coexistence of several parasites with the same target host. Thus, Manage and co-authors [126] demonstrated the coexistence of the algicidal bacteria Alcaligenes denitrificans and cyanophages-like particles, which simultaneously participated in the regulation of Microcystis aeruginosa blooms. Cyanobacteria colonial forms may have even more associated organisms, including bacteria, microalgae (diatoms, chrysophytes, and dinoflagellates), and metazoans (hydroids, juvenile copepods, and decapods) [127]. The colonial structural organization and dissolved nitrogen and carbon organic substances provided by colonies encourage the association with heterotrophic organisms.
Could RCD evolve in colonial and unicellular cyanobacteria as an antipathogen strategy? Given a high abundance of viruses in diverse ecosystems [128,129,130,131] and a co-evolution of viruses with cellular life forms since the earliest stages of life, some forms of RCD might have evolved from antivirus defense machinery.
2.3.1. Cyanophages and RCD in Cyanobacteria
Thus, RCD is thought to be a defense mechanism upon the initiation of which cyanobacterial cells can resist phage infection [132]. Viruses are the most abundant biological entities in the ocean, and through horizontal gene transfer and lysis of specific hosts, cyanophages influence bacterial community diversity and composition [133,134]. The genetic diversity of viruses may substantially exceed the diversity of cellular-like organisms [135]. Natural blooms often terminate abruptly, and cyanophages may affect bloom demise significantly [16]. By lysing cyanobacteria, cyanophages may cause the collapse of cyanobacterial blooms and potentially serve as biological agents to control the cyanobacteria-induced HABs [136].
The filamentous freshwater cyanobacteria and well-studied marine cyanobacteria significantly differ in the size of their genomes (from 3.88 Mb Cylindrospermopsis spp. to 9.06 Mb Nostoc spp. for freshwater vs. 1.6–2.7 Mb for some sequenced Prochlorococcus genomes [137] and 2.1–3.7 Mb for sequenced marine Synechococcus spp. genomes [138]) and cellular size. Chytrids prefer large hosts to small ones [139,140,141,142]. The difference in the cell sizes in freshwater and marine counterparts make cyanophages, and perhaps, heterotrophic bacteria but not fungi (chytrids), major regulators of ocean cyanobacterial blooms.
The current understanding of viral metagenomics and culturing experiments in model systems is that phages hijack host cell metabolism and increase host fitness during frequent periods of nutrient limitation [143]. The virally encoded host-like proteins could have a role in restoring protein synthesis in a starved cell to allow for virus replication and delay RCD. So far, RCD is thought to be an antiviral defense mechanism, upon the initiation of which cyanobacterial cells can withstand phage infection [132].
Distinct transcriptional states may co-exist during the active viral infection of cyanobacteria: (1) cells resistant to lysis that keep persisting viruses (“persisters”) and could potentially serve as an inoculum for next blooms; (2) metabolically active infected cells responsible for viral release followed by lysis, and (3) a population of cells dying without releasing any viral particles (Figure 2). Similar observations were made by Tucker and Pollard [144] on how a Microcystis aeruginosa population recovered from a cyanophage infection in about three weeks, possibly due to a developed resistance. So, it is reasonable to assume that cyanophage infection could ultimately result in diverse cell fates in the bacterial host. Because late-phase blooms are characterized by low [P] and [N] levels, phage infection could be activated, and cyanophage would actively utilize host cellular machinery to maintain and expand the phage population [16]. At the same time, the release of organic material during lysis would contribute to the decomposition of organic matter by heterotrophic microbes [145]. Fuchsman and co-authors [136] have proposed a similar mechanism for Prochlorococcus, and Bar-Zeev et al. [39] for a nitrogen-fixing cyanobacterium, Trichodesmium. In the latter study, recently fixed nitrogen was sinking into the sediments with the cell for further decomposition by heterotrophic bacteria.
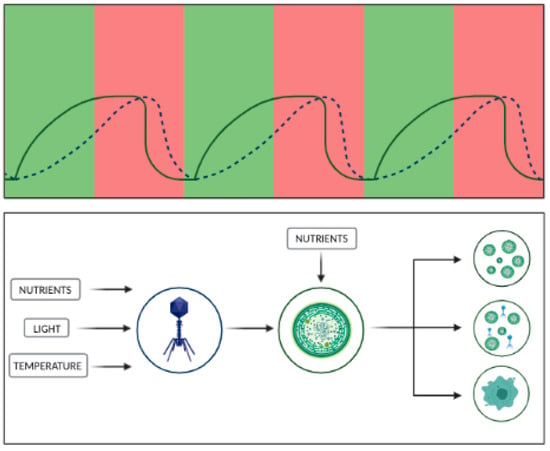
Figure 2.
Cyanophages and nutrient cycling in cyanobacteria. The viral infection of algae can result in multiple cell fates. According to the diagram, part of the population would be metabolically active while still being infected, serving as viral particle releasing machines; the second part of the population would be lysed upon the infection, releasing organic matter; lastly, a part of the population would be resistant to the infection, thus serving as an inoculum for future populations. On top of this, the cycle of cyanobacterial population growth is also closely linked to the level of available nutrients. This relationship (similar to a typical predator–prey curve) can thus be described as follows, high-nutrient phases are characterized by progressive growth of algal populations (solid line), which in turn leads to the progressive growth of viruses (dotted line). Upon reaching a certain growth point, the cyanobacterial population experiences nutrient limitation, as late phases of algal blooms have been demonstrated to have low levels of available P and N. Under such conditions, phages can utilize cellular host machinery in order to maintain the phage population, leading to (1) release of viral particles for maintenance of the phage population, (2) release of organic matter for further decomposition by microbes, and (3) development of a resistant cyanobacterial population for the beginning of a new cycle.
However, it is unclear what the primary factors that stabilize the host–phage relationship are and whether this relationship is cyclic [146]. Light and photosynthetic processes strongly influence the success of phage infection and the number of generated cyanophage particles and their release [147]. Multiple studies have identified photosynthetic “host” genes present in the phage genome, which are hypothesized to be significant during phage infections as they increase the physiological and ecological fitness of infected cyanobacteria [148]. One of these examples, the speD gene—a homolog found in all marine cyanobacteria—is responsible for polyamine biosynthesis. Along with the psbA gene, also present in the marine phage genome [149], if expressed, speD is thought to maintain the activity of the host photosystem II reaction center during phage infection [133,148]. It is hypothesized that phages perturb cyanobacteria metabolism to keep them alive for a more extended period stimulating phage multiplication.
2.3.2. Bacteria
Bloom-forming cyanobacteria are closely associated with heterotrophic bacteria [150,151,152,153,154]. Multiple sampling across continents confirms the phylogenetic and functional similarity of Microcystis-associated bacteria and the existence of complementary pathways between Microcystis and associated heterotrophs [155]. The heterotrophic bacterial community changes when it participates in cyanobacteria blooms. Thus, Shi and co-authors [156] observed that Rhodospirillales, Burkholderiales, and Verrucomicrobiales dominated during the rapid decomposition phase, whereas Sphingomonadales, Rhizobiales, and Xanthomonadales dominated during the slow decomposition phase. Changes in a bacterial community are likely involved in the formation and maintenance of Microcystis colonial morphoforms [157]. These findings are in accord with reports that heterotrophic bacteria secrete extracellular components that affect the metabolism and growth of cyanobacteria [152,158,159]. In nature, and even when recently isolated Microcystis was maintained in the lab culture, colonies contain heterotrophic bacteria [160]. Furthermore, bacteria of the genus Sphingomonas are associated with Microcystis blooms and actively break down toxins [161,162]. Several bacterial strains were found to inhibit or enhance the growth of associated cyanobacteria [163]. Recent studies by Jankowiak and Gobler [164] confirmed that Microcystis colonies harbor bacterial assemblages that may be conserved across geographically distinct blooms and over different stages of blooms [164]. This finding also suggests that Microcystis colonies harbor a selected pool of bacteria with a conserved functional potential that may help bloom persistence even under unfavorable environmental conditions. Parasite strategies, such as bacterial regulation of host RCD, can drive the evolution of host–parasite interactions leading to a possible outcome of cell death, dormancy, or infection. If bacteria delay and/or promote Microcystis colony growth and participate in colony decay, they may participate more actively in inducing cell death in the old colonies.
2.3.3. Fungi
In addition to well-studied viruses, many cyanobacteria species are affected by fungal parasites mainly belonging to Chytridiomycota (chytrids) [165]. These microfungi infect their host cells by zoospores that digest the cytoplasm, eventually form sporangia, and finally produce and release new zoospores [125]. Chytrid parasites of cyanobacteria are ubiquitous, undergo strong variations from year to year, and may burst into epidemics, reaching a prevalence of infection up to 90–98% [142,166]. Chytrids infect different freshwater genera of cyanobacteria, including Anabaena, Aphanizomenon, Cylindrospermopsis, other filamentous taxa, and Microcystis [123,125,167]. Chytrids infecting cyanobacteria are obligate parasites, and many of them have narrow host limits and cannot use alternative food sources [168]. In diatoms (Asterionella formosa), RCD accelerates the death of infected host cells and the chytrid parasite before it completes its life cycle [169]. A fungal infection may also reduce the length of cyanobacterial filaments by so-called “mechanistic fragmentation” [170] and, therefore, may promote bloom decline by enhancing grazing [171] and facilitating host–parasite dispersal [172]. However, it is unclear if the “mechanistic fragmentation” of cyanobacterial filaments happens because of the RCD-like mechanism participating in releasing uninfected parts of the filament.
3. Methods to Study RCD in Cyanobacteria
Early examination of phytoplankton automortality characterized by a loss of membrane integrity and degradation of photosynthetic activity, led scientists to supposition of a genetically based process of RCD as a source of phytoplankton mortality [15]. Current research on cyanobacterial RCD [50,69,72,73] concentrates on several morphological and biochemical features parallel to those found in eukaryotic RCD, including ultrastructure changes and cell membrane rearrangement, and activation of proteolytic enzymes [132,173].
Initially, the hallmarks of RCD in cyanobacteria were meant to be visualized using a standard for mammalian apoptosis reagents and techniques, such as labeling cell membrane with fluorochrome-conjugated annexin V to verify exposure of phosphatidylserine (PS) ([43,72,173], Table 1). During apoptosis in eukaryotes, the annexin V signal parallels effector caspase activation [68]. Positive staining with annexin V was demonstrated in cyanobacterial akinetes, heterocytes, and vegetative cells. However, peptidoglycan, a key component of a prokaryotic cell wall, can be an obstruction in the staining of phosphatidylcholine with annexin V [174]. Also, the shrinking/changing volume of the cell, which is considered an early sign of RCD in the mammalian cell, is limited due to a rigid peptidoglycan wall. In cyanobacteria, the peptidoglycan’s layer status may serve as an important indicator of living/dead status [72]. Cyanobacterial viability was estimated by the ratio of dead and viable cells using fluorescent dyes such as propidium iodide (PI), SYTO 9, and SYTOX Green [175,176] or, alternatively, staining with fluorogenic ester dyes based on the enzymes of living cells; however, these methods are limited in assessing microbial cells [177].
The cellular ultrastructure of cyanobacteria undergoes significant changes under RCD, and due to the bacterial size, scanning electron microscopy (SEM) and transmission electron microscopy (TEM) are the major technologies of choice. A number of studies describing the detailed ultrastructure changes during cell death in cyanobacteria are available [37,40,50,72,73]. Distorted cell walls, surface deformation, and enlarged disintegrated thylakoid membranes were observed in the cells. Moreover, cells undergoing RCD appeared to be smaller in size in comparison with controls [36,37,40,50,72,73].
The discovery of RCD-like events in prokaryotes reveals the possibility that widely divergent organisms possess conserved and previously unrecognized RCD pathways [44]. Caspases (cysteine-dependent aspartyl-specific proteases) are a superfamily of conserved, cysteine-dependent, aspartate-directed effector proteases that play an essential role in regulating PCD in metazoans [178,179,180]. Cyanobacteria lack true caspases, and therefore the practice of studying their caspase-like activities using fluorogenic caspase substrates for metazoans is questionable (rev. [66]).
Most published caspase activity assays in cyanobacteria rely on commercial fluorogenic substrates that produce fluorescence signals upon proteolytic cleavage, indicating the presence of an active caspase [173]. Fluorescence of subsequently cleaved substrate in cyanobacteria in response to H2O2, salinity changes, and allelopathic stimuli was quantified using a microplate reader or conventional flow cytometer [40,45,50,54,55,73], and the activity of cyanobacterial caspase-like enzymes in some cases was reported to be inhibited by pan-caspase inhibitors (such as zVAD-fmk) [62]. However, the caspase-like homologs in cyanobacteria are more likely to have different specificity for the cleavage site (Arg or Lys) than mammalian caspase homologs and, therefore, would require different specific substrates and inhibitors [132]. The pan-caspase inhibitors (including the most commonly used zVAD-fmk) also inhibit serine non-caspase proteases and N-glycanases, inducing autophagy [181,182,183].
Recently, the first attempt to compare meta-caspase activity using specific substrates for cyanobacterial caspase-like enzymes and standard substrates for mammalian caspase evaluation was made [184]. The design of specific substrates for caspase-like enzymes in cyanobacteria, using non-specific substrates (such as fluorescein casein), and monitoring overall proteolytic activities may be required [66]. The interpretation of experiments with commercially available mammalian caspase kits should be made cautiously in the absence of complementary assays.
Targeting intracellular components for degradation is a crucial intracellular process that can counteract the effects of external stress such as invading pathogens [185]. The bioinformatic approach helped to explore the distribution and conservation of autophagy-related genes in yeast, photosynthetic eukaryotes, and possibly of their remote homologs in prokaryotes [186,187,188]. In unicellular cyanobacteria regulated degradation of thylakoids may be part of cell survival and not a cell death program due to the need to modulate the photosynthetic apparatus in response to starvation. The classical TEM approach was important in the initial detailed characterization of RCD and RCS mechanisms in cyanobacteria but had limited capabilities to differentiate between these two genetically regulated mechanisms. Further progress in our understanding of RCD would require the implementation of a statistically sound multi-omics analysis of thousands of single cells in combination with cell sorting and approaches allowing the visualization of phenotypic heterogeneity at a single cell level [189,190,191,192]. Single-cell genomics and single-cell transcriptomics target individual pre-selected cells. However, only genes that are expressed above certain thresholds can be sampled. In the future, we expect these thresholds to be significantly lowered, allowing us to go beyond taxonomic diversity research toward quantitative analysis of gene-regulated processes in the unicellular cyanobacteria, cell cycles, physiology, and ecological interactions. Valuable additional information would come from the imaging of cells before transcriptome profiling or whole-genome sequencing, allowing the association of results with specific cell images unambiguously. This approach was applied for single-cell sequencing of marine protists [193,194]. The collaborative efforts would require facilitating steps for the development of new cyanobacteria model systems beyond the existing few. The genetic manipulation systems similar to those developed by the Environmental Model Systems (EMC) initiative [195], which will enable deeper insights into the cell biology of cyanobacteria and the understanding of genetically regulated mechanisms such as RCD and RCS. The major methodological challenge is the current absence of a link between high-throughput genomic and morphological data. A comparison of parallel approaches demonstrates a low degree of overlap between these two approaches [196]. We need technologies that allow image-based cell sorting with a sequential application of genomic methods [197,198]. Cell sorting technologies require classifying cells in real-time [199], and complex phenotypes may require machine learning algorithms for analysis [200].
When we had already submitted our manuscript, we came upon the interesting recent publication of Li and co-authors (2022) proposing to subdivide RCD cell types in cyanobacteria based on the involvement of caspase homologs [201]. In our opinion, the proteolytic system in the unicellular cyanobacteria involved in the highly specific proteolysis and a multi-step repair of the thylakoid complexes with de novo synthesized copies may go far beyond caspase family homologs [202,203].
4. Conclusions
RCD in cyanobacteria contributes to increased population survival and is an essential part of the ecological process. The multicellularity for many cyanobacterial clades suggests cell-death-related mechanisms of differentiation and protection against infection from viruses, fungi, and other pathogens. In published research, cyanobacteria species used in RCD studies are mainly represented by multicellular filamentous organisms or colonial and unicellular species having reversed from multicellularity in their evolutionary history. On the other side, there are research studies of regulated cellular survival programs in cyanobacteria, mainly using unicellular species (Synechococcus and Synechocystis spp.). The key question to be addressed is—are the data obtained with comparative genomics and supporting execution of regulated cell death in multicellular cyanobacteria applicable to unicellular cyanobacteria? We suggest that the enzymes in mechanisms in unicellular cyanobacteria are very different from those in other cyanobacteria groups. In fact, the execution of regulated cellular survival and not a regulated cellular death can be a desired outcome for these bacteria.
The existing techniques are borrowed from vertebrate RCD research, particularly from mammalian cells equipped with mitochondria—central organelles involved in their process of cell death—and are limited in their capabilities. Further RCD research in cyanobacteria requires the development of proper methods for assessment and wider use of molecular genetics and single-cell analysis in combination with cell sorting based on advanced imaging. This may lead to the discovery of novel RCD molecular pathways and regulatory and inhibitory molecules and contribute to understanding and managing bloom-forming cyanobacteria.
Author Contributions
Authorship conceptualization: N.S.B.; writing, original draft preparation: A.M. and N.S.B.; review and editing: D.M., I.A.V. and N.S.B. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported in part funding from Nazarbayev University FDCGRP #110119FD4513 grants and #SSH2020028 to N.S.B, Ministry of Sciences, Kazakhstan grants MES #4350/GF4 to N.S.B and grants AP08857554 (MES, Kazakhstan) and FDCGRP 240919FD3937 (Nazarbayev University) to I.A.V.
Acknowledgments
The figures were created with a help of the Biorender program.
Conflicts of Interest
The authors declare no competing interests.
References
- Koonin, E.V.; Aravind, L. Origin and evolution of eukaryotic apoptosis: The bacterial connection. Cell Death Differ. 2002, 9, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Bidle, K.D. Programmed cell death in unicellular phytoplankton. Curr. Biol. 2016, 26, R594–R607. [Google Scholar] [CrossRef]
- Durand, P.M.; Ramsey, G. The nature of programmed cell death. Biol. Theory 2019, 14, 30–41. [Google Scholar] [CrossRef]
- Martin, S.J.; Green, D.R. Protease activation during apoptosis: Death by a thousand cuts? Cell 1995, 82, 349–352. [Google Scholar] [CrossRef]
- Raff, M. Cell suicide for beginners. Nature 1998, 396, 119. [Google Scholar] [CrossRef] [PubMed]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef]
- McCall, K. Genetic control of necrosis—Another type of programmed cell death. Curr. Opin. Cell Biol. 2010, 22, 882–888. [Google Scholar] [CrossRef]
- Fuchs, Y.; Steller, H. Live to die another way: Modes of programmed cell death and the signals emanating from dying cells. Nat. Rev. Mol. Cell Biol. 2015, 16, 329–344. [Google Scholar] [CrossRef]
- Yarmolinsky, M.B. Programmed cell death in bacterial populations. Science 1995, 267, 836–837. [Google Scholar] [CrossRef]
- Bidle, K.D.; Falkowski, P.G. Cell death in planktonic, photosynthetic microorganisms. Nat. Rev. Microbiol. 2004, 2, 643–655. [Google Scholar] [CrossRef]
- Zheng, W.; Rasmussen, U.; Zheng, S.; Bao, X.; Chen, B.; Gaon, Y.; Guan, X.; Larsson, J.; Bergman, B. Multiple modes of cell death discovered in a prokaryotic (cyanobacterial) endosymbiont. PLoS ONE 2013, 8, e66147. [Google Scholar] [CrossRef] [PubMed]
- Bidle, K.A.; Haramaty, L.; Baggett, N.; Nannen, J.; Bidle, K.D. Tantalizing evidence for caspase-like protein expression and activity in the cellular stress response of Archaea. Environ. Microbiol. 2010, 12, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Seth-Pasricha, M.; Bidle, K.A.; Bidle, K.D. Specificity of archaeal caspase activity in the extreme halophile Haloferax volcanii. Environ. Microbiol. Rep. 2013, 5, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Seth-Pasricha, M.; Senn, S.; Sanman, L.E.; Bogyo, M.; Nanda, V.; Bidle, K.A.; Bidle, K.D. Catalytic linkage between caspase activity and proteostasis in Archaea. Environ. Microbiol. 2019, 21, 286–298. [Google Scholar] [CrossRef]
- Veldhuis, M.J.; Kraay, G.W.; Timmermans, K.R. Cell death in phytoplankton: Correlation between changes in membrane permeability, photosynthetic activity, pigmentation and growth. Eur. J. Phycol. 2001, 36, 167–177. [Google Scholar] [CrossRef]
- Berman-Frank, I.; Bidle, K.D.; Haramaty, L.; Falkowski, P.G. The demise of the marine cyanobacterium, Trichodesmium spp., via an autocatalyzed cell death pathway. Limnol. Oceanogr. 2004, 49, 997–1005. [Google Scholar] [CrossRef]
- Berman-Frank, I.; Rosenberg, G.; Levitan, O.; Haramaty, L.; Mari, X. Coupling between autocatalytic cell death and transparent exopolymeric particle production in the marine cyanobacterium Trichodesmium. Environ. Microbiol. 2007, 9, 1415–1422. [Google Scholar] [CrossRef]
- Ding, Y.; Gan, N.; Lin, J.; Sedmak, B.; Song, L. Hydrogen peroxide induces apoptotic-like cell death in Microcystis aeruginosa (Chroococcales, Cyanobacteria) in a dose-dependent manner. Phycologia 2012, 51, 567–575. [Google Scholar] [CrossRef]
- Forchhammer, K.; Schwarz, R. Nitrogen chlorosis in unicellular cyanobacteria—A developmental program for surviving nitrogen deprivation. Environ. Microbiol. 2019, 21, 1173–1184. [Google Scholar] [CrossRef]
- Maldener, I.; Summers, M.L.; Sukenik, A. Cellular differentiation in filamentous cyanobacteria. The cell biology of cyanobacteria 2014, 15, 263–291. [Google Scholar]
- Schirrmeister, B.E.; Antonelli, A.; Bagheri, H.C. The origin of multicellularity in cyanobacteria. BMC Evol. Biol. 2011, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Komárek, J.; Johansen, J.R. Filamentous cyanobacteria. In Freshwater Algae of North America; Elsevier: Amsterdam, The Netherlands, 2015; pp. 135–235. [Google Scholar]
- Hammerschmidt, K.; Landan, G.; Domingues Kümmel Tria, F.; Alcorta, J.; Dagan, T. The order of trait emergence in the evolution of cyanobacterial multicellularity. Genome Biol. Evol. 2021, 13, evaa249. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Shih, P.M.; Wu, D.; Latifi, A.; Axen, S.D.; Fewer, D.P.; Talla, E.; Calteau, A.; Cai, F.; Tandeau de Marsac, N.; Rippka, R.; et al. Improving the coverage of the cyanobacterial phylum using diversity-driven genome sequencing. Proc. Natl. Acad. Sci. USA 2013, 110, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Urrejola, C.; von Dassow, P.; van den Engh, G.; Salas, L.; Mullineaux, C.W.; Vicuna, R.; Sanchez-Baracaldo, P. Loss of filamentous multicellularity in cyanobacteria: The extremophile Gloeocapsopsis sp. strain UTEX B3054 retained multicellular features at the genomic and behavioral levels. J. Bacteriol. 2020, 202, e00514–e00519. [Google Scholar] [CrossRef]
- Bonner, J.T. The origins of multicellularity. Integr. Biol. Issues News Rev. Publ. Assoc. Soc. Integr. Comparative Biol. 1998, 1, 27–36. [Google Scholar] [CrossRef]
- Lee, D.Y.; Rhee, G.Y. Kinetics of growth and death in Anabaena flos-aquae (cyanobacteria) under light limitation and supersaturation. J. Phycol. 1999, 35, 700–709. [Google Scholar] [CrossRef]
- Ning, S.B.; Guo, H.L.; Wang, L.; Song, Y.C. Salt stress induces programmed cell death in prokaryotic organism Anabaena. J. Appl. Microbiol. 2002, 93, 915–928. [Google Scholar] [CrossRef]
- Agusti, S.; Alou, E.; Hoyer, M.V.; Frazer, T.K.; Canfield, D.E. Cell death in lake phytoplankton communities. Freshw. Biol. 2006, 51, 1496–1506. [Google Scholar] [CrossRef]
- Ross, C.; Santiago-Vazquez, L.; Paul, V. Toxin release in response to oxidative stress and programmed cell death in the cyanobacterium Microcystis aeruginosa. Aquat. Toxicol. 2006, 78, 66–73. [Google Scholar] [CrossRef]
- Sigee, D.; Selwyn, A.; Gallois, P.; Dean, A. Patterns of cell death in freshwater colonial cyanobacteria during the late summer bloom. Phycologia 2007, 46, 284–292. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Singh, S.P.; Hader, D.P.; Sinha, R.P. Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2’,7’-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochem. Biophys. Res. Commun. 2010, 397, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zhang, X.; Au, D.W.; Mao, X.; Yuan, K. The effects of sub-lethal UV-C irradiation on growth and cell integrity of cyanobacteria and green algae. Chemosphere 2010, 78, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, J.N.; Purdie, D.A. Effect of elevated temperature, darkness, and hydrogen peroxide treatment on oxidative stress and cell death in the bloom-forming toxic cyanobacterium Microcystis aeruginosa. J. Phycol. 2011, 47, 1316–1325. [Google Scholar] [CrossRef]
- Chang, D.W.; Hsieh, M.L.; Chen, Y.M.; Lin, T.F.; Chang, J.S. Kinetics of cell lysis for Microcystis aeruginosa and Nitzschia palea in the exposure to beta-cyclocitral. J. Hazard Mater. 2011, 18, 1214–1220. [Google Scholar] [CrossRef]
- Gumbo, J.R.; Cloete, T.E. The mechanism of Microcystis aeruginosa death upon exposure to Bacillus mycoides. Phys. Chem. Earth Parts A/B/C 2011, 36, 881–886. [Google Scholar] [CrossRef]
- Mikula, P.; Zezulka, S.; Jancula, D.; Marsalek, B. Metabolic activity and membrane integrity changes in Microcystis aeruginosa—New findings on hydrogen peroxide toxicity in cyanobacteria. Eur. J. Phycol. 2012, 47, 195–206. [Google Scholar] [CrossRef]
- Bar-Zeev, E.; Avishay, I.; Bidle, K.D.; Berman-Frank, I. Programmed cell death in the marine cyanobacterium Trichodesmium mediates carbon and nitrogen export. ISME J. 2013, 7, 2340–2348. [Google Scholar] [CrossRef]
- He, Y.; Zhou, Q.-H.; Liu, B.-Y.; Cheng, L.; Tian, Y.; Zhang, Y.-Y.; Wu, Z.-B. Programmed cell death in the cyanobacterium Microcystis aeruginosa induced by allelopathic effect of submerged macrophyte Myriophyllum spicatum in co-culture system. J. Appl. Phycol. 2016, 28, 2805–2814. [Google Scholar] [CrossRef]
- Tiwari, A.; Singh, P.; Asthana, R.K. Role of calcium in the mitigation of heat stress in the cyanobacterium Anabaena PCC 7120. J. Plant Physiol. 2016, 199, 67–75. [Google Scholar] [CrossRef]
- Wu, L.; Qiu, Z.; Zhou, Y.; Du, Y.; Liu, C.; Ye, J.; Hu, X. Physiological effects of the herbicide glyphosate on the cyanobacterium Microcystis aeruginosa. Aquat. Toxicol. 2016, 178, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Dashkova, V.; Malashenkov, D.; Poulton, N.; Vorobjev, I.; Barteneva, N.S. Imaging flow cytometry for phytoplankton analysis. Methods 2017, 112, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Chen, Q.; Zhang, D.; Zhang, H.; Lei, X.; Chen, Z.; Li, Y.; Hong, Y.; Ma, X.; Zheng, W.; et al. The algicidal mechanism of prodigiosin from Hahella sp. KA22 against Microcystis aeruginosa. Sci. Rep. 2017, 7, 7750. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Sha, J.; Tian, Y.; Zhang, X.; Liu, B.; Wu, Z. Polyphenolic allelochemical pyrogallic acid induces caspase-3 (like)-dependent programmed cell death in the cyanobacterium Microcystis aeruginosa. Algal Res. 2017, 21, 148–155. [Google Scholar] [CrossRef]
- Srikumar, A.; Krishna, P.S.; Sivaramakrishna, D.; Kopfmann, S.; Hess, W.R.; Swamy, M.J.; Lin-Chao, S.; Prakash, J.S. The Ssl2245-Sll1130 toxin-antitoxin system mediates heat-induced programmed cell death in Synechocystis sp. PCC6803. J. Biol. Chem. 2017, 292, 4222–4234. [Google Scholar] [CrossRef] [PubMed]
- Swapnil, P.; Yadav, A.K.; Srivastav, S.; Sharma, N.K.; Srikrishna, S.; Rai, A.K. Biphasic ROS accumulation and programmed cell death in a cyanobacterium exposed to salinity (NaCl and Na2SO4). Algal Res. 2017, 23, 88–95. [Google Scholar] [CrossRef]
- Ding, Y.; Gan, N.; Liu, J.; Zheng, L.; Li, L.; Song, L. Survival, recovery and microcystin release of Microcystis aeruginosa in cold or dark condition. Chin. J. Oceanol. Limnol. 2017, 35, 313–323. [Google Scholar] [CrossRef]
- Lee, H.W.; Park, B.S.; Joo, J.H.; Patidar, S.K.; Choi, H.J.; Jin, E.; Han, M.S. Cyanobacteria-specific algicidal mechanism of bioinspired naphthoquinone derivative, NQ 2-0. Sci. Rep. 2018, 8, 11595. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zheng, J.; Cao, H.; Wang, X.; Lou, K.; Zhang, X.; Tao, Y. Growth suppression and apoptosis-like cell death in Microcystis aeruginosa by H2O2: A new insight into extracellular and intracellular damage pathways. Chemosphere 2018, 211, 1098–1108. [Google Scholar] [CrossRef]
- Spungin, D.; Belkin, N.; Foster, R.A.; Stenegren, M.; Caputo, A.; Pujo-Pay, M.; Leblond, N.; Dupouy, C.; Bonnet, S.; Berman-Frank, I. Programmed cell death in diazotrophs and the fate of organic matter in the western tropical South Pacific Ocean during the OUTPACE cruise. Biogeosciences 2018, 15, 3893–3908. [Google Scholar] [CrossRef]
- Daniel, E.; Weiss, G.; Murik, O.; Sukenik, A.; Lieman-Hurwitz, J.; Kaplan, A. The response of Microcystis aeruginosa strain MGK to a single or two consecutive H2O2 applications. Environ. Microbiol. Rep. 2019, 11, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Kozik, C.; Young, E.B.; Sandgren, C.D.; Berges, J.A. Cell death in individual freshwater phytoplankton species: Relationships with population dynamics and environmental factors. Eur. J. Phycol. 2019, 54, 369–379. [Google Scholar] [CrossRef]
- Ross, C.; Warhurst, B.C.; Brown, A.; Huff, C.; Ochrietor, J.D. Mesohaline conditions represent the threshold for oxidative stress, cell death and toxin release in the cyanobacterium Microcystis aeruginosa. Aquat. Toxicol. 2019, 206, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Spungin, D.; Bidle, K.D.; Berman-Frank, I. Metacaspase involvement in programmed cell death of the marine cyanobacterium Trichodesmium. Environ. Microbiol. 2019, 21, 667–681. [Google Scholar] [CrossRef]
- Ye, J.; Huang, C.; Qiu, Z.; Wu, L.; Xu, C. The growth, apoptosis and oxidative stress in Microcystis viridis exposed to glyphosate. Bull. Environ. Contam. Toxicol. 2019, 103, 585–589. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, T.; Tao, Y. Optimized inhibition on Microcystis aeruginosa by combined use of UV-C irradiation and hydrogen peroxide. IOP Conf. Ser. Earth Environ. Sci. 2019, 295, 012084. [Google Scholar] [CrossRef]
- Fernández-Juárez, V.; Bennasar-Figueras, A.; Sureda-Gomila, A.; Ramis-Munar, G.; Agawin, N.S. Differential effects of varying concentrations of phosphorus, iron, and nitrogen in N2-fixing cyanobacteria. Front. Microbiol. 2020, 11, 541558. [Google Scholar] [CrossRef]
- Rzymski, P.; Klimaszyk, P.; Jurczak, T.; Poniedzialek, B. Oxidative stress, programmed cell death and microcystin release in Microcystis aeruginosa in response to Daphnia grazers. Front. Microbiol. 2020, 11, 1201. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, S.; Huang, W.; He, L.; Li, J.; Zhou, J.; Zhou, J. Influence of eugenol on algal growth, cell physiology of cyanobacteria Microcystis aeruginosa and its interaction with signaling molecules. Chemosphere 2020, 255, 126935. [Google Scholar] [CrossRef]
- Zheng, T.; Zhou, M.; Yang, L.; Wang, Y.; Wang, Y.; Meng, Y.; Liu, J.; Zuo, Z. Effects of high light and temperature on Microcystis aeruginosa cell growth and beta-cyclocitral emission. Ecotoxicol. Environ. Saf. 2020, 192, 110313. [Google Scholar] [CrossRef]
- Zhou, T.; Cao, H.; Zheng, J.; Teng, F.; Wang, X.; Lou, K.; Zhang, X.; Tao, Y. Suppression of water-bloom cyanobacterium Microcystis aeruginosa by algaecide hydrogen peroxide maximized through programmed cell death. J. Hazard. Mater. 2020, 393, 122394. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, A.; Berdun, F.; Bartoli, C.; Steelheart, C.; Alegre, M.; Bayir, H.; Tyurina, Y.Y.; Kagan, V.E.; Salerno, G.; Pagnussat, G.; et al. C-ferroptosis is an iron-dependent form of regulated cell death in cyanobacteria. J. Cell Biol. 2022, 221, e201911005. [Google Scholar] [CrossRef]
- Giannuzzi, L.; Lombardo, T.; Juarez, I.; Aguilera, A.; Blanco, G. A stochastic characterization of hydrogen peroxide-induced regulated cell death in Microcystis aeruginosa. Front. Microbiol. 2021, 12, 636157. [Google Scholar] [CrossRef] [PubMed]
- Herrero, A.; Stavans, J.; Flores, E. The multicellular nature of filamentous heterocyst-forming cyanobacteria. FEMS Microbiol. Rev. 2016, 40, 831–854. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, A.; Klemenčič, M.; Sueldo, D.J.; Rzymski, P.; Gianuzzi, L.; Martin, M.V. Cell death in cyanobacteria: Current understanding and recommendations for a consensus on its nomenclature. Front. Microbiol. 2021, 12, 416. [Google Scholar] [CrossRef]
- Green, D.R.; Reed, J.C. Apoptosis: Mitochondria and apoptosis. Science 1998, 281, 1309–1312. [Google Scholar] [CrossRef]
- Reed, J.C.; Green, D.R. Remodeling for demolition: Changes in mitochrondrial ultrastructure during apoptosis. Mol. Cell 2002, 9, 1–3. [Google Scholar] [CrossRef]
- Vorobjev, I.A.; Barteneva, N.S. Temporal heterogeneity metrics in apoptosis induced by anticancer drugs. J. Histochem. Cytochem. 2015, 63, 494–510. [Google Scholar] [CrossRef]
- Bock, F.J.; Tait, S.W. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef]
- Glynn, S.M.; Britton, B.K.; Semrud-Clikeman, M.; Muth, K.D. Analogical reasoning and problem solving in science textbooks. In Handbook of Creativity; Springer: Boston, MA, USA, 1989; pp. 383–398. [Google Scholar]
- Pepper, J.W.; Shelton, D.E.; Rashidi, A.; Dur, P.M. Are internal, death-promoting mechanisms ever adaptive? J. Phylogen. Evol. Biol. 2013, 1, 3. [Google Scholar]
- Lyons, N.A.; Kolter, R. On the evolution of bacterial multicellularity. Curr. Opin. Microbiol. 2015, 24, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Flores, E.; Herrero, A. Compartmentalized function through cell differentiation in filamentous cyanobacteria. Nat. Rev. Microbiol. 2010, 8, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Allocati, N.; Masulli, M.; Di Ilio, C.; De Laurenzi, V. Die for the community: An overview of programmed cell death in bacteria. Cell Death Dis. 2015, 6, e1609. [Google Scholar] [CrossRef] [PubMed]
- Daignan-Fornier, B.; Laporte, D.; Sagot, I. Quiescence through the prism of evolution. Front. Cell Dev. Biol. 2021, 9, 745069. [Google Scholar] [CrossRef]
- Neumann, N.; Doello, S.; Forchhammer, K. Recovery of unicellular cyanobacteria from nitrogen chlorosis: Model for resuscitation of dormant bacteria. Microbiol. Physiol. 2021, 21, 78–87. [Google Scholar] [CrossRef]
- Durand, P.M.; Barreto Filho, M.M.; Michod, R.E. Cell death in evolutionary transitions in individuality. Yale J. Biol. Med. 2019, 92, 651–662. [Google Scholar]
- Kaiser, D. Building a multicellular organism. Annu. Rev. Genet. 2001, 35, 103–123. [Google Scholar] [CrossRef]
- West, S.A.; Griffin, A.S.; Gardner, A.; Diggle, S.P. Social evolution theory for microorganisms. Nat. Rev. Microbiol. 2006, 4, 597–607. [Google Scholar] [CrossRef]
- Rossetti, V.; Schirrmeister, B.E.; Bernasconi, M.V.; Bagheri, H.C. The evolutionary path to terminal differentiation and division of labor in cyanobacteria. J. Theor. Biol. 2010, 262, 23–34. [Google Scholar] [CrossRef]
- Fabrizio, P.; Pletcher, S.D.; Minois, N.; Vaupel, J.W.; Longo, V.D. Chronological aging-independent replicative life span regulation by Msn2/Msn4 and Sod2 in Saccharomyces cerevisiae. FEBS Lett. 2004, 557, 136–142. [Google Scholar] [CrossRef]
- Herker, E.; Jungwirth, H.; Lehmann, K.A.; Maldener, C.; Frohlich, K.U.; Wissing, S.; Buttner, S.; Fehr, M.; Sigrist, S.; Madeo, F. Chronological aging leads to apoptosis in yeast. J. Cell Biol. 2004, 164, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.E.; Golden, S.S. Circadian rhythms in cyanobacteria. Microbiol. Molecular. Biol. Rev. 2015, 79, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Reimers, A.M.; Knoop, H.; Bockmayr, A.; Steuer, R. Cellular trade-offs and optimal resource allocation during cyanobacterial diurnal growth. Proc. Natl. Acad. Sci. USA 2017, 114, E6457–E6465. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, A.; Kumazawa, S.; Takahashi, A.; Ikemoto, H.; Cao, S.; Arai, T. Strategy by which nitrogen-fixing unicellular cyanobacteria grow photoautotrophically. Nature 1986, 323, 720–722. [Google Scholar] [CrossRef]
- Stal, L.J.; Krumbein, W.E. Temporal separation of nitrogen fixation and photosynthesis in the filamentous, non-heterocystous cyanobacterium Oscillatoria sp. Arch. Microbiol. 1987, 149, 76–80. [Google Scholar] [CrossRef]
- Huang, T.C.; Tu, J.; Chow, T.J.; Chen, T.H. Circadian rhythm of the prokaryote Synechococcus sp. RF-1. Plant Physiol. 1990, 92, 531–533. [Google Scholar] [CrossRef]
- Diamond, S.; Jun, D.; Rubin, B.E.; Golden, S.S. The circadian oscillator in Synechococcus elongatus controls metabolite partitioning during diurnal growth. Proc. Natl. Acad. Sci. USA 2015, 112, E1916–E1925. [Google Scholar] [CrossRef]
- Ashby, M.K.; Mullineaux, C.W. Cyanobacterial ycf 27 gene products regulate energy transfer from phycobilisomes to photosystems I and II. FEMS Microbiol. Lett. 1999, 181, 253–260. [Google Scholar] [CrossRef]
- Riediger, M.; Kadowaki, T.; Nagayama, R.; Georg, J.; Hihara, Y.; Hess, W.R. Biocomputational analyses and experimental validation identify the regulon controlled by the redox-responsive transcription factor RpaB. iScience 2019, 15, 316–331. [Google Scholar] [CrossRef]
- Mary, I.; Vaulot, D. Two-component systems in Prochlorococcus MED4: Genomic analysis and differential expression under stress. FEMS Microbiol. Lett. 2003, 226, 135–144. [Google Scholar] [CrossRef]
- Ashby, M.K.; Houmard, J. Cyanobacterial two-component proteins: Structure, diversity, distribution, and evolution. Microbiol. Mol. Biol. Rev. 2006, 70, 472–509. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.M.; Rowden, S.J.; Cramer, W.A.; Howe, C.J.; Puthiyaveetil, S. Thiol redox switches regulate the oligomeric state of cyanobacterial Rre1, RpaA, and RpaB response regulators. FEBS Lett. 2022, 596, 1533–1543. [Google Scholar] [CrossRef]
- Diamond, S.; Rubin, B.E.; Shultzaberger, R.K.; Chen, Y.; Barber, C.D.; Golden, S.S. Redox crisis underlies conditional light–dark lethality in cyanobacterial mutants that lack the circadian regulator, RpaA. Proc. Natl. Acad. Sci. USA 2017, 114, E580–E589. [Google Scholar] [CrossRef]
- Allen, M.M.; Smith, A.J. Nitrogen chlorosis in blue-green algae. Arch. Mikrobiol. 1969, 69, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Boresch, K. Zur physiologie der blaualgenfarbstoffe. Lotos 1910, 58, 344–345. [Google Scholar]
- Bogorad, L. Phycobiliproteins and complementary chromatic adaptation. Annu. Rev. Plant Physiol. 1975, 26, 369–401. [Google Scholar] [CrossRef]
- Collier, J.L.; Grossman, A. A small polypeptide triggers complete degradation of light-harvesting phycobiliproteins in nutrient-deprived cyanobacteria. EMBO J. 1994, 13, 1039–1047. [Google Scholar] [CrossRef]
- Collier, J.L.; Herbert, S.K.; Fork, D.C.; Grossman, A.R. Changes in the cyanobacterial photosynthetic apparatus during acclimation to macronutrient deprivation. Photosynth. Res. 1994, 42, 173–183. [Google Scholar] [CrossRef]
- Krauspe, V.; Fahrner, M.; Spät, P.; Steglich, C.; Frankenberg-Dinkel, N.; Maček, B.; Schilling, O.; Hess, W.R. Discovery of a small protein factor involved in the coordinated degradation of phycobilisomes in cyanobacteria. Proc. Natl. Acad. Sci. USA 2021, 118, e2012277118. [Google Scholar] [CrossRef]
- Adir, N.; Dines, M.; Klartag, M.; McGregor, A.; Melamed-Frank, M. Assembly and disassembly of phycobilisomes. In Complex Intracellular Structures in Prokaryotes; Microbiology Monographs; Springer: Berlin/Heidelberg, Germany, 2006; Volume 2, pp. 47–77. [Google Scholar]
- Murton, J.; Nagarajan, A.; Nguyen, A.Y.; Liberton, M.; Hancock, H.A.; Pakrasi, H.B.; Timlin, J.A. Population-level coordination of pigment response in individual cyanobacterial cells under altered nitrogen levels. Photosynth. Res. 2017, 134, 165–174. [Google Scholar] [CrossRef]
- Görl, M.; Sauer, J.; Baier, T.; Forchhammer, K. Nitrogen-starvation-induced chlorosis in Synechococcus PCC 7942: Adaptation to long-term survival. Microbiology 1998, 144, 2449–2458. [Google Scholar] [CrossRef] [PubMed]
- Shigesada, N.; Okubo, A. Analysis of the self-shading effect on algal vertical distribution in natural waters. J. Math. Biol. 1981, 12, 311–326. [Google Scholar] [CrossRef]
- Klotz, A.; Georg, J.; Bučinská, L.; Watanabe, S.; Reimann, V.; Januszewski, W.; Sobotka, R.; Jendrossek, D.; Hess, W.R.; Forchhammer, K. Awakening of a dormant cyanobacterium from nitrogen chlorosis reveals a genetically determined program. Curr. Biol. 2016, 26, 2862–2872. [Google Scholar] [CrossRef] [PubMed]
- Spät, P.; Klotz, A.; Rexroth, S.; Maček, B.; Forchhammer, K. Chlorosis as a developmental program in cyanobacteria: The proteomic fundament for survival and awakening. Mol. Cell. Proteom. 2018, 17, 1650–1669. [Google Scholar] [CrossRef] [PubMed]
- Tomitani, A.; Knoll, A.H.; Cavanaugh, C.M.; Ohno, T. The evolutionary diversification of cyanobacteria: Molecular-phylogenetic and paleontological perspectives. Proc. Natl. Acad. Sci. USA 2006, 103, 5442–5447. [Google Scholar] [CrossRef]
- Claessen, D.; Rozen, D.E.; Kuipers, O.P.; Sogaard-Andersen, L.; van Wezel, G.P. Bacterial solutions to multicellularity: A tale of biofilms, filaments and fruiting bodies. Nat. Rev. Microbiol. 2014, 12, 115–124. [Google Scholar] [CrossRef]
- Muro-Pastor, A.M.; Hess, W.R. Heterocyst differentiation: From single mutants to global approaches. Trends Microbiol. 2012, 20, 548–550. [Google Scholar] [CrossRef]
- Singh, P.; Khan, A.; Srivastava, A. Heterocyst and akinete differentiation in cyanobacteria: A view toward cyanobacterial symbiosis. In Advances in Cyanobacterial Biology; Academic Press: Cambridge, MA, USA, 2020; pp. 235–248. [Google Scholar]
- Adamec, F.; Kaftan, D.; Nedbal, L. Stress-induced filament fragmentation of Calothrix elenkinii (cyanobacteria) is facilitated by death of high-fluorescence cells. J. Phycol. 2005, 41, 835–839. [Google Scholar] [CrossRef]
- Kohl, F.G. On the Organization and Physiology of Cyanophyceae and the Mitotic Division of Its Nucleus; G. Fischer: Stuttgart, Germany, 1903. [Google Scholar]
- Lamont, H.C. Sacrificial cell death and trichome breakage in an oscillatoriacean blue-green alga: The role of murein. Arch. Mikrobiol. 1969, 69, 237–259. [Google Scholar] [CrossRef]
- Brown, I.I.; Bryant, D.A.; Casamatta, D.; Thomas-Keprta, K.L.; Sarkisova, S.A.; Shen, G.; Graham, J.E.; Boyd, E.S.; Peters, J.W.; Garrison, D.H.; et al. Polyphasic characterization of a thermotolerant siderophilic filamentous cyanobacterium that produces intracellular iron deposits. Appl. Environ. Microbiol. 2010, 76, 6664–6672. [Google Scholar] [CrossRef]
- Hernández-Mariné, M.; Roldán, M. Adherence of hormogonia to substrata is mediated by polysaccharides produced by necridic cells. Arch. Hydrobiol. Algol. Stud. 2005, 117, 239–249. [Google Scholar] [CrossRef]
- Misra, H.S.; Tuli, R. Differential expression of photosynthesis and nitrogen fixation genes in the cyanobacterium Plectonema boryanum. Plant Physiol. 2000, 122, 731–736. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaplan-Levy, R.N.; Hadas, O.; Sukenik, A. Deciphering the mechanisms against oxidative stress in developing and mature akinetes of the cyanobacterium Aphanizomenon ovalisporum. Microbiology 2015, 161, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- Sukenik, A.; Beardall, J.; Hadas, O. Photosynthetic characterization of developing and mature akinetes of Aphanizomenon ovalisporum (cyanoprokaryota). J. Phycol. 2007, 43, 780–788. [Google Scholar] [CrossRef]
- Herdman, M. Akinetes: Structure and function. In Cyanobacteria; Fay, P., van Baalen, C., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 227–250. [Google Scholar]
- Carey, C.C.; Ibelings, B.W.; Hoffmann, E.P.; Hamilton, D.P.; Brookes, J.D. Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Res. 2012, 46, 1394–1407. [Google Scholar] [CrossRef]
- Perez, R.; Wörmer, L.; Sass, P.; Maldener, I. A highly asynchronous developmental program triggered during germination of dormant akinetes of filamentous diazotrophic cyanobacteria. FEMS Microbiol. Ecol. 2018, 94, fix131. [Google Scholar] [CrossRef]
- Van Wichelen, J.; Vanormelingen, P.; Codd, G.A.; Vyverman, W. The common bloom-forming cyanobacterium Microcystis is prone to a wide array of microbial antagonists. Harmful Algae 2016, 55, 97–111. [Google Scholar] [CrossRef]
- Gachon, C.M.; Sime-Ngando, T.; Strittmatter, M.; Chambouvet, A.; Kim, G.H. Algal diseases: Spotlight on a black box. Trends Plant. Sci. 2010, 1, 633–640. [Google Scholar] [CrossRef]
- Gerphagnon, M.; Macarthur, D.J.; Latour, D.; Gachon, C.M.; van Ogtrop, F.; Gleason, F.H.; Sime-Ngando, T. Microbial players involved in the decline of filamentous and colonial cyanobacterial blooms with a focus on fungal parasitism. Environ. Microbiol. 2015, 17, 2573–2587. [Google Scholar] [CrossRef]
- Manage, P.M.; Kawabata, Z.I.; Nakano, S.I. Dynamics of cyanophage-like particles and algicidal bacteria causing Microcystis aeruginosa mortality. Limnology 2001, 2, 73–78. [Google Scholar] [CrossRef]
- Sheridan, C.; Steinberg, D.K.; Kling, G. The microbial and metazoan community associated with colonies of Trichodesmium spp.: A quantitative survey. J. Plankton Res. 2002, 24, 913–922. [Google Scholar] [CrossRef]
- Bergh, Ø.; Børsheim, K.Y.; Bratbak, G.; Heldal, M. High abundance of viruses found in aquatic environments. Nature 1989, 340, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Chibani-Chennoufi, S.; Bruttin, A.; Dillmann, M.L.; Brussow, H. Phage-host interaction: An ecological perspective. J. Bacteriol. 2004, 186, 3677–3686. [Google Scholar] [CrossRef] [PubMed]
- Suttle, C.A. Viruses in the sea. Nature 2005, 437, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Suttle, C.A. Marine viruses-major players in the global ecosystem. Nat. Rev. Microbiol. 2007, 5, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Mishra, A.K. The tale of caspase homologues and their evolutionary outlook: Deciphering programmed cell death in cyanobacteria. J. Exp. Bot. 2020, 71, 4639–4657. [Google Scholar] [CrossRef]
- Sullivan, M.B.; Coleman, M.L.; Weigele, P.; Rohwer, F.; Chisholm, S.W. Three Prochlorococcus cyanophage genomes: Signature features and ecological interpretations. PLoS Biol. 2005, 3, e144. [Google Scholar] [CrossRef]
- Breitbart, M. Marine viruses: Truth or dare. Ann. Rev. Mar. Sci. 2012, 4, 425–448. [Google Scholar] [CrossRef]
- Koonin, E.V.; Dolja, V.V. A virocentric perspective on the evolution of life. Curr. Opin. Virol. 2013, 3, 546–557. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microbial. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef]
- Sun, Z.; Blanchard, J.L. Strong genome-wide selection early in the evolution of Prochlorococcus resulted in a reduced genome through the loss of a large number of small effect genes. PLoS ONE 2014, 9, e88837. [Google Scholar] [CrossRef] [PubMed]
- Fucich, D.; Chen, F. Presence of toxin-antitoxin systems in picocyanobacteria and their ecological implications. ISME J. 2020, 14, 2843–2850. [Google Scholar] [CrossRef] [PubMed]
- Canter, H.M.; Lund, J.W.G. The parasitism of planktonic desmids by fungi. Österr. Bot. Z. 1969, 116, 351–377. [Google Scholar] [CrossRef]
- Holfeld, H. Relative abundance, rate of increase, and fungal infections of freshwater phytoplankton. J. Plankton Res. 2000, 22, 987–995. [Google Scholar] [CrossRef]
- Rasconi, S.; Jobard, M.; Jouve, L.; Sime-Ngando, T. Use of calcofluor white for detection, identification, and quantification of planktonic fungal parasites. Appl. Environ. Microbiol. 2009, 75, 2545–2553. [Google Scholar] [CrossRef]
- Rasconi, S.; Niquil, N.; Sime-Ngando, T. Phytoplankton chytridiomycosis: Community structure and infectivity of fungal parasites in aquatic ecosystems. Environ. Microbiol. 2012, 14, 2151–2170. [Google Scholar] [CrossRef]
- Warwick-Dugdale, J.; Buchholz, H.H.; Allen, M.J.; Temperton, B. Host-hijacking and planktonic piracy: How phages command the microbial high seas. Virol. J. 2019, 16, 15. [Google Scholar] [CrossRef]
- Tucker, S.; Pollard, P. Identification of cyanophage Ma-LBP and infection of the cyanobacterium Microcystis aeruginosa from an Australian subtropical lake by the virus. Appl. Environ. Microbiol. 2005, 71, 629–635. [Google Scholar] [CrossRef]
- Fuchsman, C.A.; Palevsky, H.I.; Widner, B.; Duffy, M.; Carlson, M.C.G.; Neibauer, J.A.; Mulholland, M.R.; Keil, R.G.; Devol, A.H.; Rocap, G. Cyanobacteria and cyanophage contributions to carbon and nitrogen cycling in an oligotrophic oxygen-deficient zone. ISME J. 2019, 13, 2714–2726. [Google Scholar] [CrossRef]
- Gons, H.J.; Ebert, J.; Hoogveld, H.L.; van den Hove, L.; Pel, R.; Takkenberg, W.; Woldringh, C.J. Observations on cyanobacterial population collapse in eutrophic lake water. Antonie Leeuwenhoek 2002, 81, 319–326. [Google Scholar] [CrossRef]
- Liu, R.; Liu, Y.; Chen, Y.; Zhan, Y.; Zeng, Q. Cyanobacterial viruses exhibit diurnal rhythms during infection. Proc. Natl. Acad. Sci. USA 2019, 116, 14077–14082. [Google Scholar] [CrossRef] [PubMed]
- Gao, E.B.; Huang, Y.; Ning, D. Metabolic genes within cyanophage genomes: Implications for diversity and evolution. Genes 2016, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Lindell, D.; Jaffe, J.D.; Johnson, Z.I.; Church, G.M.; Chisholm, S.W. Photosynthesis genes in marine viruses yield proteins during host infection. Nature 2005, 438, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Kapustina, L.L. Experimental study of Microcystis-associated and free-living cyanobacteria. Microbiology 2006, 75, 606–610. [Google Scholar] [CrossRef]
- Shi, L.; Cai, Y.; Yang, H.; Xing, P.; Li, P.; Kong, L.; Kong, F. Phylogenetic diversity and specificity of bacteria associated with Microcystis aeruginosa and other cyanobacteria. J. Environ. Sci. 2009, 21, 1581–1590. [Google Scholar] [CrossRef]
- Liu, L.; Yang, J.; Lv, H.; Yu, Z. Synchronous dynamics and correlations between bacteria and phytoplankton in a subtropical drinking water reservoir. FEMS Microbiol. Ecol. 2014, 90, 126–138. [Google Scholar] [CrossRef]
- Osman, O.A.; Beier, S.; Grabherr, M.; Bertilsson, S. Interactions of freshwater cyanobacteria with bacterial antagonists. Appl. Environ. Microbiol. 2017, 83, e02634-16. [Google Scholar] [CrossRef]
- Li, Q.; Lin, F.; Yang, C.; Wang, J.; Lin, Y.; Shen, M.; Park, M.S.; Li, T.; Zhao, J. A large-scale comparative metagenomic study reveals the functional interactions in six bloom-forming Microcystis-epibiont communities. Front. Microbiol. 2018, 9, 746. [Google Scholar] [CrossRef]
- Cook, K.V.; Li, C.; Cai, H.; Krumholz, L.R.; Hambright, K.D.; Paerl, H.W.; Steffen, M.M.; Wilson, A.E.; Burford, M.A.; Grossart, H.-P.; et al. The global Microcystis interactome. Limnol. Oceanogr. 2020, 65, S194–S207. [Google Scholar] [CrossRef]
- Shi, L.; Huang, Y.; Zhang, M.; Yu, Y.; Lu, Y.; Kong, F. Bacterial community dynamics and functional variation during the long-term decomposition of cyanobacterial blooms in-vitro. Sci. Total Environ. 2017, 598, 77–86. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Shen, H.; Xie, P.; Yu, J. Changes in the bacterial community and extracellular compounds associated with the disaggregation of Microcystis colonies. Biochem. Syst. Ecol. 2015, 61, 62–66. [Google Scholar] [CrossRef]
- Brunberg, A.-K. Contribution of bacteria in the mucilage of Microcystis spp. (Cyanobacteria) to benthic and pelagic bacterial production in a hypereutrophic lake. FEMS Microbiol. Ecol. 1999, 29, 13–22. [Google Scholar] [CrossRef]
- Shen, H.; Niu, Y.; Xie, P.; Tao, M.; Yang, X. Morphological and physiological changes in Microcystis aeruginosa as a result of interactions with heterotrophic bacteria. Freshw. Biol. 2011, 56, 1065–1080. [Google Scholar] [CrossRef]
- Xie, M.; Ren, M.; Yang, C.; Yi, H.; Li, Z.; Li, T.; Zhao, J. Metagenomic analysis reveals symbiotic relationship among bacteria in Microcystis-dominated community. Front. Microbiol. 2016, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Park, H.D.; Sasaki, Y.; Maruyama, T.; Yanagisawa, E.; Hiraishi, A.; Kato, K. Degradation of the cyanobacterial hepatotoxin microcystin by a new bacterium isolated from a hypertrophic lake. Environ. Toxicol. 2001, 16, 337–343. [Google Scholar] [CrossRef]
- Dziallas, C.; Grossart, H.P. Temperature and biotic factors influence bacterial communities associated with the cyanobacterium Microcystis sp. Environ. Microbiol. 2011, 13, 1632–1641. [Google Scholar] [CrossRef]
- Berg, K.A.; Lyra, C.; Sivonen, K.; Paulin, L.; Suomalainen, S.; Tuomi, P.; Rapala, J. High diversity of cultivable heterotrophic bacteria in association with cyanobacterial water blooms. ISME J. 2009, 3, 314–325. [Google Scholar] [CrossRef]
- Jankowiak, J.G.; Gobler, C.J. The composition and function of microbiomes within Microcystis colonies are significantly different than native bacterial assemblages in two North American lakes. Front. Microbiol. 2020, 11, 1016. [Google Scholar] [CrossRef]
- Agha, R.; Saebelfeld, M.; Manthey, C.; Rohrlack, T.; Wolinska, J. Chytrid parasitism facilitates trophic transfer between bloom-forming cyanobacteria and zooplankton (Daphnia). Sci. Rep. 2016, 6, 35039. [Google Scholar] [CrossRef]
- Sen, B. Fungal parasitism of planktonic algae in Shearwater. VI. Parasitic occurrence of a new chytrid species on the blue-green alga Microcystis aeruginosa Kuetz emand. Elenkin. Arch. Hydrobiol. Suppl. 1988, 79, 177–184. [Google Scholar]
- Kagami, M.; de Bruin, A.; Ibelings, B.W.; van Donk, E. Parasitic chytrids: Their effects on phytoplankton communities and food-web dynamics. Hydrobiologia 2007, 578, 113–129. [Google Scholar] [CrossRef]
- Sparrow, F. Aquatic Phycomycetes; University of Michigan Press: Ann Arbor, MI, USA, 1960. [Google Scholar]
- Canter, H.M.; Jaworski, G. The occurrence of a hypersensitive reaction in the planktonic diatom Asterionella formosa Hassall parasitized by the chytrid Rhizophydium planktonicum Canter emend., in culture. New Phytol. 1979, 82, 187–206. [Google Scholar] [CrossRef]
- Gerphagnon, M.; Latour, D.; Colombet, J.; Sime-Ngando, T. Fungal parasitism: Life cycle, dynamics and impact on cyanobacterial blooms. PLoS ONE 2013, 8, e60894. [Google Scholar] [CrossRef] [PubMed]
- Bouvy, M.; Pagano, M.; Troussellier, M. Effects of a cyanobacterial bloom (Cylindrospermopsis raciborskii) on bacteria and zooplankton communities in Ingazeira reservoir (northeast Brazil). Aquat. Microb. Ecol. 2001, 25, 215–227. [Google Scholar] [CrossRef]
- Pollard, P.C.; Young, L.M. Lake viruses lyse cyanobacteria, Cylindrospermopsis raciborskii, enhances filamentous-host dispersal in Australia. Acta Oecol. 2010, 36, 114–119. [Google Scholar] [CrossRef]
- Hu, C.; Rzymski, P. Programmed cell death-like and accompanying release of microcystin in freshwater bloom-forming cyanobacterium Microcystis: From identification to ecological relevance. Toxins 2019, 11, 706. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, Y.; Li, Z.; Pan, J.; Wu, H.; Qiu, G.; Feng, C.; Wei, C. Immobilization of phosphatidylserine by ethanol and lysozyme on the cell surface for evaluation of apoptosis-like decay in activated-sludge bacteria. Appl. Environ. Microbiol. 2020, 86, e00345-20. [Google Scholar] [CrossRef]
- Tashyreva, D.; Elster, J.; Billi, D. A novel staining protocol for multiparameter assessment of cell heterogeneity in Phormidium populations (cyanobacteria) employing fluorescent dyes. PLoS ONE 2013, 8, e55283. [Google Scholar] [CrossRef][Green Version]
- Johnson, T.J.; Hildreth, M.B.; Gu, L.; Zhou, R.; Gibbons, W.R. Testing a dual-fluorescence assay to monitor the viability of filamentous cyanobacteria. J. Microbiol. Methods 2015, 113, 57–64. [Google Scholar] [CrossRef]
- Ziglio, G.; Andreottola, G.; Barbesti, S.; Boschetti, G.; Bruni, L.; Foladori, P.; Villa, R. Assessment of activated sludge viability with flow cytometry. Water Res. 2002, 36, 460–468. [Google Scholar] [CrossRef]
- Thornberry, N.A.; Lazebnik, Y. Caspases: Enemies within. Science 1998, 281, 1312–1316. [Google Scholar] [CrossRef]
- Uren, A.G.; O’Rourke, K.; Aravind, L.A.; Pisabarro, M.T.; Seshagiri, S.; Koonin, E.V.; Dixit, V.M. Identification of paracaspases and metacaspases: Two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol. Cell 2000, 6, 961–967. [Google Scholar] [CrossRef]
- Klemenčič, M.; Novinec, M.; Dolinar, M. Orthocaspases are proteolytically active prokaryotic caspase homologues: The case of Microcystis aeruginosa. Mol. Microbiol. 2015, 98, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Rozman-Pungercar, J.; Kopitar-Jerala, N.; Bogyo, M.; Turk, D.; Vasiljeva, O.; Stefe, I.; Vandenabeele, P.; Bromme, D.; Puizdar, V.; Fonovic, M.; et al. Inhibition of papain-like cysteine proteases and legumain by caspase-specific inhibitors: When reaction mechanism is more important than specificity. Cell Death Differ. 2003, 10, 881–888. [Google Scholar] [CrossRef]
- Vandenabeele, P.; Vanden Berghe, T.; Festjens, N. Caspase inhibitors promote alternative cell death pathways. Sci. STKE 2006, 2006, pe44. [Google Scholar] [CrossRef]
- Needs, S.H.; Bootman, M.D.; Grotzke, J.E.; Kramer, H.B.; Allman, S.A. Off-target inhibition of NGLY1 by the polycaspase inhibitor Z-VAD-fmk induces cellular autophagy. FEBS J. 2022, 289, 3115–3131. [Google Scholar] [CrossRef] [PubMed]
- Spungin, D.; Berman-Frank, I. Assessment of metacaspase activity in phytoplankton. Bio-protocol 2019, 9, e3341. [Google Scholar] [CrossRef] [PubMed]
- Ke, P.Y. Autophagy and antiviral defense. IUBMB Life 2022, 74, 317–338. [Google Scholar] [CrossRef]
- Shemi, A.; Ben-Dor, S.; Vardi, A. Elucidating the composition and conservation of the autophagy pathway in photosynthetic eukaryotes. Autophagy 2015, 11, 701–715. [Google Scholar] [CrossRef]
- Farré, J.C.; Subramani, S. Mechanistic insights into selective autophagy pathways: Lessons from yeast. Nat. Rev. Mol. Cell Biol. 2016, 17, 537–552. [Google Scholar] [CrossRef]
- Zhang, S.; Hama, Y.; Mizushima, N. The evolution of autophagy proteins–diversification in eukaryotes and potential ancestors in prokaryotes. J. Cell Sci. 2021, 134, jcs233742. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, P.M.; Woodhouse, J.N.; Makower, A.K.; Yeung, A.C.; Ongley, S.E.; Micallef, M.L.; Moffitt, M.C.; Neilan, B.A. Advances in genomics, transcriptomics and proteomics of toxin-producing cyanobacteria. Environ. Microbiol. Rep. 2016, 8, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.R.; Tan, S.I.; Hsiang, C.C.; Sung, P.K.; Ng, I.S. Challenges and opportunity of recent genome editing and multi-omics in cyanobacteria and microalgae for biorefinery. Bioresour. Technol. 2019, 291, 121932. [Google Scholar] [CrossRef] [PubMed]
- Barteneva, N.S.; Vorobjev, I.A. Heterogeneity of metazoan cells and beyond: To integrative analysis of cellular populations at single-cell level. In Cellular Heterogeneity; Springer: New York, NY, USA, 2018; pp. 3–23. [Google Scholar]
- Pathania, R.; Srivastava, A.; Srivastava, S.; Shukla, P. Metabolic systems biology and multi-omics of cyanobacteria: Perspectives and future directions. Bioresour. Technol. 2022, 343, 126007. [Google Scholar] [CrossRef] [PubMed]
- Strassert, J.F.; Karnkowska, A.; Hehenberger, E.; Del Campo, J.; Kolisko, M.; Okamoto, N.; Burki, F.; Janouškovec, J.; Poirier, C.; Leonard, G.; et al. Single cell genomics of uncultured marine alveolates shows paraphyly of basal dinoflagellates. ISME J. 2018, 12, 304–308. [Google Scholar] [CrossRef]
- Gawryluk, R.M.; Del Campo, J.; Okamoto, N.; Strassert, J.F.; Lukeš, J.; Richards, T.A.; Worden, A.Z.; Santoro, A.E.; Keeling, P.J. Morphological identification and single-cell genomics of marine diplonemids. Curr. Biol. 2016, 26, 3053–3059. [Google Scholar] [CrossRef]
- Faktorová, D.; Nisbet, R.E.R.; Fernández Robledo, J.A.; Casacuberta, E.; Sudek, L.; Allen, A.E.; Ares, M.; Aresté, C.; Balestreri, C.; Barbrook, A.C.; et al. Genetic tool development in marine protists: Emerging model organisms for experimental cell biology. Nat. Methods 2020, 17, 481–494. [Google Scholar] [CrossRef]
- Malashenkov, D.V.; Dashkova, V.; Zhakupova, K.; Vorobjev, I.A.; Barteneva, N.S. Comparative analysis of freshwater phytoplankton communities in two lakes of Burabay National Park using morphological and molecular approaches. Sci. Rep. 2021, 11, 16130. [Google Scholar] [CrossRef]
- Nitta, N.; Sugimura, T.; Isozaki, A.; Mikami, H.; Hiraki, K.; Sakuma, S.; Iino, T.; Arai, F.; Endo, T.; Fujiwaki, Y.; et al. Intelligent image-activated cell sorting. Cell 2018, 175, 266–276. [Google Scholar] [CrossRef]
- Schraivogel, D.; Kuhn, T.M.; Rauscher, B.; Rodríguez-Martínez, M.; Paulsen, M.; Owsley, K.; Middlebrook, A.; Tischer, C.; Ramasz, B.; Ordoñez-Rueda, D.; et al. High-speed fluorescence image–enabled cell sorting. Science 2022, 375, 315–320. [Google Scholar] [CrossRef]
- Barteneva, N.S.; Ketman, K.; Fasler-Kan, E.; Potashnikova, D.; Vorobjev, I.A. Cell sorting in cancer research—Diminishing degree of cell heterogeneity. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2013, 1836, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Isozaki, A.; Herbig, M.; Hayashi, M.; Hiramatsu, K.; Yamazaki, S.; Kondo, N.; Ohnuki, S.; Ohya, Y.; Nitta, N.; et al. Intelligent sort-timing prediction for image-activated cell sorting. Cytom. Part A 2022. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, L.; Li, J. Regulated cell death in cyanobacteria: Evidences, classification, and significances. In Cyanobacterial Physiology; Academic Press: Cambridge, MA, USA, 2022; pp. 69–82. [Google Scholar]
- Kanervo, E.; Spetea, C.; Nishiyama, Y.; Murata, N.; Andersson, B.; Aro, E.M. Dissecting a cyanobacterial proteolytic system: Efficiency in inducing degradation of the D1 protein of photosystem II in cyanobacteria and plants. Biochim. Biophys. Acta (BBA)-Bioenerg. 2003, 1607, 131–140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bouchnak, I.; van Wijk, K.J. Structure, function, and substrates of Clp AAA+ protease systems in cyanobacteria, plastids, and apicoplasts: A comparative analysis. J. Biol. Chem. 2021, 296, 100338. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).