Abstract
The presence and transfer of plasmids from commensal bacteria to more pathogenic bacteria may contribute to the dissemination of antimicrobial resistance. However, the prevalence of plasmids from commensal bacteria, such as the enterococci, in food animals remains largely unknown. In this study, the diversity and prevalence of plasmid families from multidrug-resistant (MDR; resistance to three or more antimicrobials) enterococci from poultry carcasses were determined. Plasmid-positive MDR enterococci were also tested for the ability to transfer plasmids to other enterococci using conjugation. MDR Enterococcus faecalis (n = 98) and Enterococcus faecium (n = 696) that were isolated from poultry carcass rinsates between 2004 and 2011 were tested for the presence of 21 plasmid replicon (rep) families using multiplex PCR. Approximately 48% of E. faecalis (47/98) and 16% of E. faecium (110/696) were positive for at least one rep-family. Fourteen rep-families were detected overall, and ten rep-families were shared between E. faecalis and E. faecium. The rep7 and rep17 families were unique to E. faecalis, while the rep5 and rep8 families were unique to E. faecium. The rep9 family was predominant in both E. faecalis and E. faecium for all the years tested. The greatest number of rep-families detected was in 2005 (n = 10), and the least was in 2009 (n = 1). Eight rep-families were transferred from E. faecalis donors to the E. faecalis JH2-2 recipient using conjugation. Results from this study showed that E. faecalis and E. faecium from poultry carcasses contain numerous and diverse rep-families that are capable of conjugal transfer.
1. Introduction
The Enterococcus spp. are Gram-positive bacteria that are commonly found in the intestinal flora of humans and animals [1,2,3]. They are also important opportunistic pathogens and are among the leading causes of healthcare-associated pathogens in the United States [4,5,6]. Enterococcus faecalis and Enterococcus faecium are the two clinically important species as they account for most enterococcal infections, which cause a variety of infections, including urinary tract infections, soft tissue infections, bacteremia, and endocarditis in humans [2]. Some factors that have made the enterococci a successful nosocomial pathogen are their intrinsic resistance to many antimicrobial agents and their ability to acquire resistance via horizontal gene transfer [2]. The widespread resistance among enterococci has also made them one of the sentinel organisms used to track antimicrobial resistance trends by the National Antimicrobial Resistance Monitoring System (NARMS), a partnership between the Centers for Disease Control and Prevention (CDC), the U.S. Food and Drug Administration (FDA), and the U.S. Department of Agriculture (USDA) [7]. From 1996 to 2011, the surveillance of bacterial isolates of animal origin was conducted by the USDA Agricultural Research Service (USDA-ARS), and from 2011 to the present, by the USDA Food Safety and Inspection Service (FSIS). Certain enteric bacteria are monitored by NARMS to track trends in antimicrobial resistance in humans, retail meats, and food animals in the United States, and the enterococci are included to monitor antimicrobial resistance trends in Gram-positive bacteria.
Antimicrobials administered to food animals for therapeutic or prophylactic purposes have resulted in the development of antimicrobial resistance in enterococci. While most enterococci are commensals and do not cause illnesses in food animals, enterococci commonly carry plasmids encoding antimicrobial resistance, and the transfer of such plasmids from enterococci to more pathogenic bacteria is cause for concern. Studies have shown that enterococci can disseminate their antimicrobial resistance genes to other bacteria, such as Staphylococcus aureus, through horizontal gene transfer [8,9]. Thus, enterococci in food animals serve as a reservoir of antimicrobial resistance, with the potential to transfer antimicrobial resistance to human pathogenic bacteria and spread to the human population through the consumption of animal products or through human–animal contact. Hence, it is important to study the prevalence of plasmids, especially those associated with antimicrobial resistance, in enterococci in food animals and their potential transfer to other bacteria.
In 2010, a system for the classification of plasmids for Gram-positive bacteria was determined by Jensen et al. [10]. The system was established by the comparison of sequences of replication-initiating genes (rep) as well as rep-like genes from Gram-positive plasmids available from GenBank; 21 replicon families (20 rep-families and a unique sequence) were determined. In this study, the distribution and prevalence of plasmid families from multidrug-resistant (MDR; resistance to three or more antimicrobials) E. faecalis and E. faecium from poultry, collected as part of NARMS, were determined using multiplex PCR developed from the analysis. Isolates representative of each plasmid prototype from detected rep-families were selected and used to determine if the plasmids could transfer to other enterococci using bacterial conjugation. Results from this study will provide information on rep-family prevalence, diversity, and mobility in MDR E. faecalis and E. faecium from poultry.
2. Materials and Methods
2.1. Bacterial Strains, Isolation, and Identification
The enterococci in this study were collected from healthy poultry carcass rinsates between 2004 and 2011 by the USDA-ARS as a part of the animal arm of NARMS, as previously described [11]. All enterococci were from poultry carcass rinsates collected from poultry processing facilities located in different geographical regions of the U.S. Excel was used to randomly select 100 multidrug-resistant (MDR; defined as resistance to three or more antimicrobials in this study) enterococci (either E. faecalis or E. faecium) from each study year. Briefly, 1 mL of rinsates were inoculated into 9 mL of BBL Enterococcosel broth (Becton Dickinson, Sparks, MD, USA) and incubated at 37 °C overnight. Positive cultures were then streaked onto BBL Enterococcosel agar and incubated at 37 °C overnight. A single colony of presumptive enterococcal isolates was sub-cultured onto slants of brain heart infusion agar (BHIA) (Becton Dickinson) for storage, followed by sub-culturing twice onto blood agar (trypticase soy agar containing 5% defibrinated sheep blood) for the isolation of pure colonies. Isolates were identified to species using multiplex PCR, as previously described [11].
2.2. Antimicrobial Susceptibility Testing
Minimum inhibitory concentrations (MIC; μg/mL) of enterococci were determined by broth microdilution according to the manufacturer’s directions using the SensititreTM semi-automated antimicrobial susceptibility system (Trek Diagnostic Systems, Inc., Cleveland, OH, USA) and the SensititreTM Gram-Positive Custom Plate CVM2AGPF or CMV3AGPF (chloramphenicol, ciprofloxacin, daptomycin, erythromycin, flavomycin, gentamicin, kanamycin, lincomycin, linezolid, nitrofurantoin, penicillin, streptomycin, Synercid (Quinupristin/Dalfopristin), tetracycline, tigecycline, tylosin, and vancomycin). Results were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) guidelines, when defined [12,13], and NARMS (https://www.fda.gov/media/108180/download, accessed on 13 June 2022). No CLSI interpretive criteria were defined for kanamycin, lincomycin, and tylosin, and only susceptible breakpoints were established for tigecycline (≤0.25 μg/mL). E. faecalis ATCC 29212, E. faecalis ATCC 51299, S. aureus ATCC 29213, and Escherichia coli ATCC 25922 were used as quality controls for the determination of MIC.
2.3. Replicon Typing
Replicon families were determined, as previously described [10]. Six multiplex PCR and one additional PCR (targeting pUB101) were used to detect 21 defined Gram-positive replicon families. A template for the rep-family PCR was prepared by suspending a single bacterial colony in 100 μL of sterile deionized water; 5 μL of template was used in each amplification reaction.
2.4. Bacterial Matings
The transfer of plasmids from different replicon families was determined by filter mating, as previously described [14]. The donor strains were randomly selected based on the plasmid type present and were resistant to either tetracycline or erythromycin. The recipient strain used in the matings was E. faecalis JH2-2 (rifampicin, fusidic acid), and transconjugants were selected using media containing either tetracycline (16 μg/mL) or erythromycin (8 μg/mL) in combination with fusidic acid (25 μg/mL). Rep-family multiplex PCR was performed on transconjugants to confirm the presence of specific plasmid replicon types.
2.5. Statistical Analysis of Tetracycline Resistance and rep-Family
The ratio of tetracycline-susceptible and resistant enterococci that contain plasmid replicons, as determined by rep-family typing, was compared using 95% confidence intervals calculated usingExcel.
3. Results
3.1. Presence of rep-Families among E. faecalis and E. faecium
Of the 794 MDR enterococcal isolates selected for testing, 48% of E. faecalis (47/98) and 16% of E. faecium (110/696) were positive for at least one rep-family. Six E. faecium that were originally selected were excluded in the final analysis as the isolates were duplicates. Overall, the host range for the 21 rep-families tested was wide as 10 of the 21 were present in both E. faecalis and E. faecium (Table 1). Overall, 14 rep-families were detected, indicating the presence of 14 different plasmids among the isolates. Although 12 rep-families were found among E. faecalis and E. faecium, the host range was different. Two rep-families (rep7, plasmid prototype pUSA02 and rep17, plasmid prototype pRUM) were unique to E. faecalis isolates and not detected in E. faecium. Likewise, rep5 (pSAS/pN315) and rep8 (pAM373) were found in E. faecium, but not in E. faecalis. When examined by species, E. faecalis and E. faecium were each negative for nine rep-families; seven of those rep-families (rep4 (pMBB1), rep10 (pIM13), rep10b (pSK6), rep12 (pBMB67), rep13 (pC194), rep15 (pUSA03), and rep16 (pSAS)) were not found in any of the isolates tested (Table 1).

Table 1.
Presence and prevalence of replicon families and plasmid prototypes among Enterococcus faecalis and E. faecium from 2004–2011.
3.2. Resistance Profiles among rep-Positive Enterococci
Antimicrobial resistance phenotypic patterns were largely variable among the isolates, with few dominant patterns observed for all the species and years examined (Table 2, Table 3, Table 4 and Table 5 and Table S1). The most common MDR pattern among the isolates was GenKanLinTet and LinNitTet (n = 5 for both), with GenKanLinTet only detected in E. faecalis, while LinNitTet was found in both E. faecalis and E. faecium. Due to the high number of different MDR patterns, no distinct pattern was associated with specific plasmid replicons. However, high numbers of plasmid-positive MDR E. faecalis (83%; 39/47) and E. faecium (76%; 84/110) were resistant to tetracycline. After 2005, isolates were not tested against bacitracin as this antibiotic was removed from the susceptibility plate due to widespread resistance in the enterococci. The lack of testing for bacitracin resistance in the subsequent years did not appear to affect the dominant MDR patterns.

Table 2.
Distribution of plasmid replicons and families among multidrug resistant Enterococcus faecalis and Enterococcus faecium from 2004.

Table 3.
Distribution of plasmid replicons and families among multidrug resistant Enterococcus faecalis and Enterococcus faecium from 2005.

Table 4.
Distribution of plasmid replicons and families among multidrug resistant Enterococcus faecalis and Enterococcus faecium from 2006–2007.

Table 5.
Distribution of plasmid replicons and families among multidrug resistant Enterococcus faecalis and Enterococcus faecium from 2008–2011.
3.3. Distribution of rep-Families over Time
The rep9 family (n = 77), followed by rep3 (n = 45) and rep11 (n = 39), were the most prevalent rep-families in both E. faecalis and E. faecium for all the years tested (Table 2, Table 3, Table 4 and Table 5). Abundance of rep-families varied over the years, with the highest number of different rep-families detected in 2005 (n = 10) (Figure 1). The number of rep-families declined from the earlier years (2004–2007) to 2009, in which only one rep-family (rep9) was detected. The number of rep-families detected began to increase again in 2010 and 2011, in which two (rep11 and rep14) and four (rep6, rep9, rep11, rep19) different rep-families, respectively, were found (Figure 1).
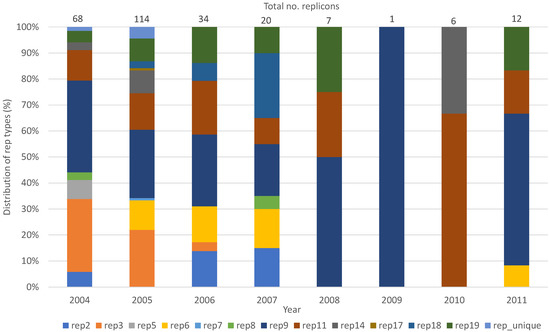
Figure 1.
Distribution of rep-families detected in poultry enterococci (E. faecalis and E. faecium) from 2004 to 2011.
3.4. Transfer of rep-Families
Twenty enterococci (10 each for E. faecalis and E. faecium) containing different plasmid replicons were selected as donors in conjugation studies. Tetracycline was used as the counter-selectable marker, except for one E. faecalis donor for which erythromycin was used. Conjugations using E. faecium as the donor resulted in either no transconjugants, suggesting that the transfer was unsuccessful, or in colonies that were negative for the tested plasmid replicons (data not shown). Eight rep-families (rep3, rep6, rep7, rep9, rep17, rep18, rep19, and repUnique) were transferred from the E. faecalis donors to the E. faecalis JH2-2 recipient in the matings (Table 6). Two E. faecalis donors containing rep1 and rep14 families were characteristic of the E. faecium matings in that colonies were present, but no replicon transfer was detected (Table 6). No transfer of rep-family prototypes 1 (pIP501), 2 (pRE25), 8 (pAM373), 11 (pEF1071), 14 (pRI), or 16 (pSAS) was detected.

Table 6.
Transfer of plasmid replicons and replicon families.
3.5. Frequency of rep-Families in Tetracycline-Resistant Enterococci
The frequency of the presence or absence of rep-families in tetracycline-susceptible and resistant enterococci was determined for the years examined (Figure 2). It was only in 2005 when the frequency of tetracycline resistance and the presence of at least one plasmid replicon (tet+/plasmid+) were greater than tetracycline-resistant isolates without a plasmid (tet+/plasmid−) (Figure 2). For all other years, the opposite was observed as the frequency of tetracycline-resistant isolates without a plasmid replicon was higher than all other combinations (tet+/plasmid+, tet−/plasmid+ and tet−/plasmid−).
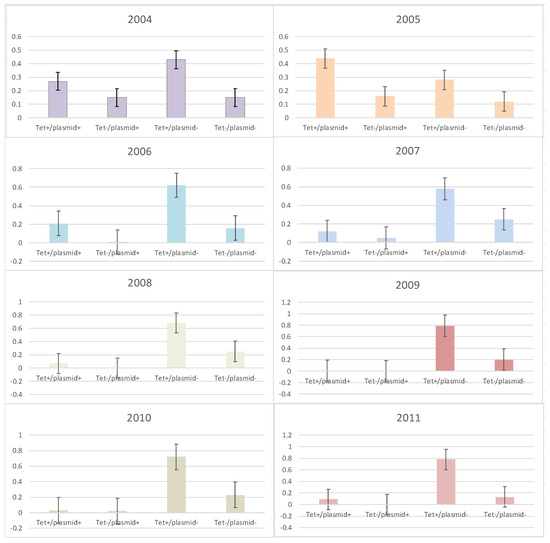
Figure 2.
Frequency of tetracycline-susceptible and resistant enterococci with and without plasmid replicons from 2004 to 2011. Error bars reflect 95% confidence intervals.
4. Discussion
Studies characterizing the antimicrobial resistance and plasmid content of bacterial strains of clinical origin are well-represented in the literature; however, knowledge of plasmid distribution for strains isolated from non-clinical sources such as food animals is largely unavailable. The use of antimicrobials in the food supply, including food animal production, coupled with the potential for transfer of antimicrobial-resistant bacteria into the human population, supports the need for additional data from non-clinical sources. As commensal bacteria such as enterococci contain plasmids that can harbor multiple resistances and are often mobilizable, the present study aimed to analyze the plasmid replicon content of E. faecalis and E. faecium collected from poultry during an eight-year period, in which the USDA-ARS participated as a part of NARMS. The analysis utilized a PCR-based plasmid replicon typing system designed to define plasmid replicon families from Gram-positive bacteria, including enterococci [10].
Of the replicon families examined in this study, ten of the rep-families were found in both E. faecalis and E. faecium, indicating a broad range of distribution. This observation was different from that previously described as only four rep-families were shared between E. faecalis and E. faecium based on information from PubMed and GenBank [10]. Limited information on the distribution of rep-families in enterococci can be attributed to the low number of strains examined, the limited sources of the strains (i.e., clinical sources), and specific strain characteristics such as certain antimicrobial resistance phenotypes or genotypes [15]. Although the present study specifically examined E. faecalis and E. faecium from poultry, a large collection was analyzed, and the different origins of the isolates allowed for a comparison to the clinical as well as non-clinical sources. Furthermore, biasing due to antimicrobial resistance to one specific antimicrobial was minimized by targeting MDR isolates, which allowed for a greater number of isolates to be included as well as a higher probability of isolates containing at least one plasmid replicon family.
About 20% of the total enterococcal isolates were positive for at least one rep-family. Previous studies on the plasmid classification of antimicrobial-resistant Enterococcus have shown that a considerable portion of Enterococcus from animal and environment sources (approximately 30%) did not harbor any plasmids from the 21 rep-families, while Enterococcus from human sources were mostly positive for rep genes [10,16,17,18,19,20]. This suggests that plasmids present in non-human enterococcal isolates are different from those found in enterococcal isolates of human origin and may be comprised of those not included in this classification system. It could also indicate that less antibiotic pressure in non-clinical settings may have resulted in the loss of plasmids.
It is interesting to note that the number of enterococcal isolates positive for rep genes decreased over time, as did the diversity of rep-families. About half of the isolates tested were identified to harbor plasmids in 2004 and 2005, while the number of the plasmid-positive isolates decreased to just one isolate in 2009. This was unexpected as the selective pressure of antibiotics would enhance the acquisition and exchange of resistance genes through various mechanisms, including horizontal gene transfer via plasmids. In the absence of antibiotics, plasmids encoding antimicrobial resistance tend to be lost as plasmid maintenance is a burden to the bacterial cell [21]. However, it is unlikely that selective conditions were absent in the poultry farms during the years the samples were taken, although changes in the class of antibiotics used cannot be ruled out. Antibiotics were still being used in food animals until their use in farm animals for growth promotion purposes was banned in the U.S. in 2017 [22,23]. The reason for the decrease in the number of rep-families in enterococcal isolates may be due to the presence of plasmids that do not belong to the 21 rep-families or due to unknown conditions that led to an instability and the subsequent loss of plasmids.
The predominant rep-family detected in the strains was rep9, represented by the pCF10 prototype, one of the pheromone-responsive plasmids in enterococci. As pheromone-responsive plasmids have been found almost exclusively in E. faecalis [10,24], the prevalence of this plasmid in this species was not unexpected. However, the predominance of rep9 in E. faecium isolates was surprising and could be due to the insufficient study of a wide variety of enterococci from poultry sources. The small sampling of rep-families from poultry E. faecalis and E. faecium previously reported rep0, rep2, and rep9 in E. faecalis, with a variety of rep-families (rep2, rep3, rep4, rep5, rep6, rep7, rep14, rep17) found in E. faecium [10,25]. Because of the nature of poultry production, such as the number of birds and environmental conditions pre-processing, it is not unreasonable that the opportunity for the transfer of plasmid rep-families is far greater in food animal production than clinical medicine, which may also account for the different rep-families in enterococci from poultry.
Both E. faecalis and E. faecium contained rep-families that were restricted to one or the other species in this study. Rep7 and rep17 were found exclusively in E. faecalis, while rep5 and rep8 were only found in E. faecium. Prototype pUSA02 (rep7) is characterized as a broad-host range plasmid that has been detected primarily in clinical E. faecalis [15,26,27], while prototype pRUM (rep17) is a conjugative, MDR plasmid originally isolated from a clinical E. faecium [28]. Surprisingly, none of the E. faecium in the present study were positive for rep17, which is a major deviation from results of some previous studies [10,29]. Both rep5 and rep8 were found only in E. faecium in this study. Both rep-families are described as having a narrow-host range, with rep5 predominating in S. aureus, while rep8 is another rep-family containing pheromone-responsive plasmids primarily found in E. faecalis [10]. Rep5 has previously been identified in E. faecium from chicken [25] and in a recent study of S. aureus from retail poultry from the U.S. [30], suggesting the genetic exchange of plasmid replicons among enterococci and staphylococci in poultry.
As most of the MDR isolates in this study included resistance to tetracycline, this antibiotic phenotype was used to determine the frequency of plasmid replicons associated with tetracycline resistance and was employed as a marker in conjugation studies. Interestingly, only for year 2005 was tetracycline resistance associated with the presence of a plasmid replicon. Although the specific rep-families associated with tetracycline resistance were not identified, 2005 was also the year with the highest number of different rep-families detected. The transfer of tetracycline resistance and rep17 using conjugation indicated a possible linkage between the antibiotic and rep type in a previous study [10]. It is also possible that some or more plasmid replicons for that year harbored tetracycline resistance genes. A definitive reason for the association of tetracycline resistance with the rep-family in this study was not determined.
Characterizing the mobility of plasmids is fundamental to understanding the epidemiology of plasmid-encoded antimicrobial resistance. In the present study, conjugation was performed to see if plasmids present in enterococcal isolates were transmissible which would enable determination of the potential transmissibility of the traits encoded on the enterococcal plasmids from animal sources to human sources. The transfer of plasmid replicons using conjugation, which was conducted using an E. faecalis recipient, was seen in the E. faecalis donors but not in E. faecium, which is indicative of the narrow-host range of some of the rep-families, and that intraspecies transfer is preferred over interspecies transfer [31]. Moreover, not all plasmids of the rep-families tested were transferred, which agrees with the previous analysis that revealed how some of the plasmids are non-transmissible [32]. Eight rep-families were successfully transferred from E. faecalis donors to the E. faecalis recipient, and some of these rep-families included rep9-containing pheromone-responsive plasmids such as pAD1 and pPD1—in addition to pCF10 discussed above—and the unique rep, which contains pMG1, a pheromone-independent conjugative plasmid. Pheromone-responsive plasmids, well-described in E. faecalis, transfer at a high frequency using aggregation substances for the clumping of donor and recipient cells; non-pheromone-responding plasmids are also known to transfer efficiently between different Enterococcus species [24,33]. There were four rep-families that were not transferred from E. faecalis donors to the E. faecalis recipient, and these included rep1, which contains pIP501, pAMβ1, and pSM19035, as well as rep2, which contains pRE25. These broad-host range conjugative plasmids do not transfer well in broth suspensions and transfer at a lower frequency on solid surfaces as compared to pheromone-responding plasmids and pMG1-related, pheromone-independent conjugative plasmids that transfer well on solid and in liquid, as reflected in our mating results [24,33].
5. Conclusions
This study clearly demonstrated that MDR E. faecalis and E. faecium from poultry contain multiple and diverse replicons. A shared pool of rep-families was evident, but a portion of rep-families was also unique to one or the other species. Although further testing with an E. faecium recipient is needed to test the host range, some of the replicons transferred to E. faecalis provide a mechanism for the spread of antimicrobial resistance and other genes among the enterococci. As the poultry carcass rinsates used in this study were from poultry processing facilities that provide poultry for human consumption, further studies on the prevalence, distribution, and transfer of the rep-families of enterococci from poultry and on the impact that these plasmid replicons have on human health are warranted.
Supplementary Materials
The following supporting information can be downloaded at:https://www.mdpi.com/article/10.3390/microorganisms10061244/s1, Table S1: Multidrug resistance patterns of Enterococcus faecalis and Enterococcus faecium from poultry.
Author Contributions
Conceptualization, C.R.J.; methodology, S.C., E.A.M., J.B.B., L.M.H., T.A.W. and S.L.H.; validation, S.C., E.A.M., J.B.B., L.M.H., T.A.W., S.L.H. and C.R.J.; formal analysis, S.C., E.A.M., J.B.B., L.M.H., T.A.W., S.L.H. and C.R.J.; investigation, J.G.F. and C.R.J.; resources, J.G.F. and C.R.J.; data curation, S.C., E.A.M., J.B.B., L.M.H., T.A.W. and S.L.H.; writing—original draft preparation, S.C. and C.R.J.; writing—review and editing, S.C., E.A.M., J.B.B., L.M.H., T.A.W., S.L.H., J.G.F. and C.R.J.; visualization, C.R.J.; supervision, J.G.F., J.B.B. and C.R.J.; project administration, J.B.B. and C.R.J.; funding acquisition, J.G.F. and C.R.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the U.S. Department of Agriculture, Agricultural Research Service internal project plan 6040-32000-079-000-D.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research was supported in part by an appointment to the Agricultural Research Service (ARS) Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy (DOE) and the U.S. Department of Agriculture (USDA).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Disclaimer
The mention of trade names or commercial products in this manuscript is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
References
- Martin, J.D.; Mundt, J.O. Enterococci in insects. J. Appl. Microbiol. 1972, 24, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.E. The life and times of the Enterococcus. Clin. Microbiol. Rev. 1990, 3, 46–65. [Google Scholar] [CrossRef] [PubMed]
- Mundt, J.O. Occurrence of enterococci in animals in a wild environment. Appl. Microbiol. 1963, 11, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Weiner, L.M.; Webb, A.K.; Limbago, B.; Dudeck, M.A.; Patel, J.; Kallen, A.J.; Edwards, J.R.; Sievert, D.M. Antimicrobial-resistant pathogens associated with healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef]
- Magill, S.S.; O’Leary, E.; Janelle, S.J.; Thompson, D.L.; Dumyati, G.; Nadle, J.; Wilson, L.E.; Kainer, M.A.; Lynfield, R.; Greissman, S.; et al. Emerging Infections Program Hospital Prevalence Survey, T. Changes in prevalence of health care-associated infections in U.S. hospitals. N. Engl. J. Med. 2018, 379, 1732–1744. [Google Scholar] [CrossRef]
- Lake, J.G.; Weiner, L.M.; Milstone, A.M.; Saiman, L.; Magill, S.S.; See, I. Pathogen distribution and antimicrobial resistance among pediatric healthcare-associated infections reported to the National Healthcare Safety Network, 2011–2014. Infect. Control Hosp. Epidemiol. 2018, 39, 1–11. [Google Scholar] [CrossRef]
- Karp, B.E.; Tate, H.; Plumblee, J.R.; Dessai, U.; Whichard, J.M.; Thacker, E.L.; Hale, K.R.; Wilson, W.; Friedman, C.R.; Griffin, P.M.; et al. National Antimicrobial Resistance Monitoring System: Two decades of advancing public health through integrated surveillance of antimicrobial resistance. Foodborne Pathog. Dis. 2017, 14, 545–557. [Google Scholar] [CrossRef]
- Weigel, L.M.; Clewell, D.B.; Gill, S.R.; Clark, N.C.; McDougal, L.K.; Flannagan, S.E.; Kolonay, J.F.; Shetty, J.; Killgore, G.E.; Tenover, F.C. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science 2003, 302, 1569–1571. [Google Scholar] [CrossRef]
- Courvalin, P. Transfer of antibiotic resistance genes between gram-positive and gram-negative bacteria. Antimicrob. Agents Chemother. 1994, 38, 1447–1451. [Google Scholar] [CrossRef]
- Jensen, L.B.; Garcia-Migura, L.; Valenzuela, A.J.; Lohr, M.; Hasman, H.; Aarestrup, F.M. A classification system for plasmids from enterococci and other Gram-positive bacteria. J. Microbiol. Methods 2010, 80, 25–43. [Google Scholar] [CrossRef]
- Jackson, C.R.; Fedorka-Cray, P.J.; Barrett, J.B. Use of a genus- and species-specific multiplex PCR for identification of enterococci. J. Clin. Microbiol. 2004, 42, 3558–3565. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-First Informational Supplement, CLSI Document M100-S; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2011. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Supplement M; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2020; Volume M100. [Google Scholar]
- Simjee, S.; Fraise, A.P.; Gill, M.J. Plasmid heterogeneity and identification of a Tn5281-like element in clinical isolates of high-level gentamicin-resistant Enterococcus faecium isolated in the UK. J. Antimicrob. Chemother. 1999, 43, 625–635. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mikalsen, T.; Pedersen, T.; Willems, R.; Coque, T.M.; Werner, G.; Sadowy, E.; van Schaik, W.; Jensen, L.B.; Sundsfjord, A.; Hegstad, K. Investigating the mobilome in clinically important lineages of Enterococcus faecium and Enterococcus faecalis. BMC Genom. 2015, 16, 282. [Google Scholar]
- Alzahrani, O.M.; Fayez, M.; Alswat, A.S.; Alkafafy, M.; Mahmoud, S.F.; Al-Marri, T.; Almuslem, A.; Ashfaq, H.; Yusuf, S. Antimicrobial resistance, biofilm formation, and virulence genes in Enterococcus species from small backyard chicken flocks. Antibiotics 2022, 11, 380. [Google Scholar] [CrossRef] [PubMed]
- Dabul, A.N.G.; Avaca-Crusca, J.S.; Navais, R.B.; Merlo, T.P.; Van Tyne, D.; Gilmore, M.S.; Camargo, I. Molecular basis for the emergence of a new hospital endemic tigecycline-resistant Enterococcus faecalis ST103 lineage. Infect. Genet. Evol. 2019, 67, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Barrett, J.B.; Frye, J.G.; Jackson, C.R. Antimicrobial resistance gene detection and plasmid typing among multidrug resistant enterococci isolated from freshwater environment. Microorganisms 2020, 8, 1338. [Google Scholar] [CrossRef] [PubMed]
- Wardal, E.; Gawryszewska, I.; Hryniewicz, W.; Sadowy, E. Abundance and diversity of plasmid-associated genes among clinical isolates of Enterococcus faecalis. Plasmid 2013, 70, 329–342. [Google Scholar] [CrossRef]
- Wardal, E.; Markowska, K.; Zabicka, D.; Wroblewska, M.; Giemza, M.; Mik, E.; Polowniak-Pracka, H.; Wozniak, A.; Hryniewicz, W.; Sadowy, E. Molecular analysis of VanA outbreak of Enterococcus faecium in two Warsaw hospitals: The importance of mobile genetic elements. Biomed. Res. Int. 2014, 2014, 575367. [Google Scholar] [CrossRef] [PubMed]
- San Millan, A.; MacLean, R.C. Fitness costs of plasmids: A limit to plasmid transmission. Microb. Transm. 2017, 5, 65–79. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration, CFVM. Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals; FDA: Rockville, MD, USA, 2015. Available online: https://www.fda.gov/media/102160/download (accessed on 22 March 2022).
- U.S. Food and Drug Administration, CFVM. Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals; FDA: Rockville, MD, USA, 2017. Available online: https://www.fda.gov/media/119332/download (accessed on 22 March 2022).
- Clewell, D.B.; Weaver, K.E.; Dunny, G.M.; Coque, T.M.; Francia, M.V.; Hayes, F. Extrachromosomal and mobile elements in enterococci: Transmission, maintenance, and epidemisology. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Gilmore, M.S., Clewell, D.B., Ike, Y., Shankar, N., Eds.; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Torres, C.; Alonso, C.A.; Ruiz-Ripa, L.; Leon-Sampedro, R.; Del Campo, R.; Coque, T.M. Antimicrobial resistance in Enterococcus spp. of animal origin. Microbiol. Spectr. 2018, 6, 4. [Google Scholar] [CrossRef]
- Song, X.; Sun, J.; Mikalsen, T.; Roberts, A.P.; Sundsfjord, A. Characterisation of the plasmidome within Enterococcus faecalis isolated from marginal periodontitis patients in Norway. PLoS ONE 2013, 8, e62248. [Google Scholar] [CrossRef] [PubMed]
- Hegstad, K.; Mikalsen, T.; Coque, T.M.; Werner, G.; Sundsfjord, A. Mobile genetic elements and their contribution to the emergence of antimicrobial resistant Enterococcus faecalis and Enterococcus faecium. Clin. Microbiol. Infect. 2010, 16, 541–554. [Google Scholar] [CrossRef]
- Rosvoll, T.C.; Lindstad, B.L.; Lunde, T.M.; Hegstad, K.; Aasnaes, B.; Hammerum, A.M.; Lester, C.H.; Simonsen, G.S.; Sundsfjord, A.; Pedersen, T. Increased high-level gentamicin resistance in invasive Enterococcus faecium is associated with aac(6′)Ie-aph(2″)Ia-encoding transferable megaplasmids hosted by major hospital-adapted lineages. FEMS Immunol. Med. Microbiol. 2012, 66, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Rosvoll, T.C.; Pedersen, T.; Sletvold, H.; Johnsen, P.J.; Sollid, J.E.; Simonsen, G.S.; Jensen, L.B.; Nielsen, K.M.; Sundsfjord, A. PCR-based plasmid typing in Enterococcus faecium strains reveals widely distributed pRE25-, pRUM-, pIP501- and pHTbeta-related replicons associated with glycopeptide resistance and stabilizing toxin-antitoxin systems. FEMS Immunol. Med. Microbiol. 2010, 58, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Neyaz, L.; Rajagopal, N.; Wells, H.; Fakhr, M.K. Molecular characterization of Staphylococcus aureus plasmids associated with strains isolated from various retail meats. Front. Microbiol. 2020, 11, 223. [Google Scholar] [CrossRef]
- Garcia-Migura, L.; Sanchez-Valenzuela, A.J.; Jensen, L.B. Presence of glycopeptide-encoding plasmids in enterococcal isolates from food and humans in Denmark. Foodborne Pathog. Dis. 2011, 8, 1191–1197. [Google Scholar] [CrossRef]
- Smillie, C.; Garcillan-Barcia, M.P.; Francia, M.V.; Rocha, E.P.; de la Cruz, F. Mobility of plasmids. Microbiol. Mol. Biol Rev. 2010, 74, 434–452. [Google Scholar] [CrossRef]
- Palmer, K.L.; Kos, V.N.; Gilmore, M.S. Horizontal gene transfer and the genomics of enterococcal antibiotic resistance. Curr. Opin. Microbiol. 2010, 13, 632–639. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).