Abstract
Chironomidae (chironomid) are one of the dominant families in freshwater ecosystems, and they plays an important role in the food web. They have been used as an indicator for water quality assessment, as they are resistant to diverse environmental pollutants. In this study, we identified the microbiomes of two chironomid species to see if there are any endogenous bacterial groups which could contribute to the host survival. The studied species are Glyptotendipes tokunagai, a model species cultivated in a laboratory-controlled environment, and Chironomus flaviplumus captured in a field stream in Yeosu, Korea. DNAs were extracted from the whole body of the individual species, and the 16S rRNA gene was amplified. The amplified products were sequenced using an Illumina MiSeq platform. The microbiomes of G. tokunagai were homogeneous, having 20% of the core amplicon sequence variants overlapping between replicates sampled from different water tanks. In contrast, none of the core amplicon sequence variants overlapped in C. flaviplumus. In both chironomid groups, potential symbionts were identified. Dysgonomonas, which can degrade complex carbon sources, was found in more than half of the total microbiomes of G. tokunagai. Tyzzerella and Dechloromonas, which have been suggested to detoxify environmental pollutants, were identified in the microbiome of C. flaviplumus. This study can help elucidate the life strategies of chironomids in polluted or organic-rich environments.
1. Introduction
Chironomidae (chironomids) are one of the most dominant and widely distributed macroinvertebrates found in freshwater ecosystems. Chironomids undergo complete metamorphosis involving four stages: (1) eggs, (2) larva, (3) pupa, and (4) adult [1]. They spend most of their lifetime in water until they become adults and fly up into the atmosphere. Chironomid larvae are one of the key intermediate members of the food web. They feed on microbes such as algae and are eaten by diverse animal species, including fishes, amphibians, and larger insects.
Chironomid larvae have long been used as water quality indicators of contaminated environments because they can survive in organic-rich and oxygen-deficient environments [2,3]. They can also survive in polluted environments with high concentrations of heavy metals, pesticides, and herbicides [4,5]. In these environments, they apply distinctive survival strategies, such as having hemoglobin that allows them to store oxygen efficiently [6,7]. Furthermore, microbes living inside chironomid eggs and larvae likely help chironomids survive in polluted environments. Using 454 pyrosequencing, Senderovich and Halpern (2013) [8] identified bacterial communities inhabiting chironomids and detected bacterial species that can detoxify metals. Through Koch-like postulates, they showed that the survival rate of disinfected larvae is lower than that of the control groups in the presence of toxic lead. Sela et al. (2021) [5] studied the endogenous microbiota of wild and cultivated (captured in wild and cultivated for 8 months) Chironomus ramosus larvae in India and examined wild C. ramosus microbiome through metagenomic analysis. They found several genes related to the degradation of toxic compounds, detoxification of metals, and resistance to antibiotics and UV radiation.
Although approximately 10,000 chironomid species exist [9], studies on their microbiome are lacking possibly because, unlike mosquitoes, chironomids are nonbiting midges that do not harm humans. Considering the pivotal role of chironomids in freshwater ecosystems and the potential contribution of endogenous microbes in their survival, it is important to study the microbiomes of various chironomid species.
In this study, we aimed to identify the microbiome of Glyptotendipes tokunagai larvae cultivated for several generations under laboratory conditions and the microbiome of wild Chironomus flaviplumus captured in Yeon Deung stream, an urban stream in Korea [8]. G. tokunagai and C. flaviplumus are two important chironomid species, but their microbiomes have not been studied, to the best of our knowledge. Glyptotendipes tokunagai is often used as a model species in ecotoxicology studies, as it can be easily cultivated under laboratory conditions [10,11]. Chironomus flaviplumus is commonly found in highly polluted sites [12], and it has gained attention from the public since their presence in a tap water purification plant in Korea in 2020 [13]. We further aimed to infer the functional profile of microbial communities through PICRUST2 [14] and discuss how microbial communities and their functions could contribute to host survival.
2. Materials and Methods
2.1. Sample Collection
Glyptotendipes tokunagai (GT) was cultivated in a laboratory in accordance with the OECD test 233 [15]. GT was reared in an incubator maintained under the following conditions: 20 ± 1 °C, 70% humidity, light–dark cycle of 16:8 h, and light intensity of approximately 500 lx. M4 medium was used as breeding water, and aeration was continuously supplied. The larvae were fed with Tetra-Min (Tetra-Werke, Melle, Germany) at 0.5 mg per larva per day. GT individuals at the 4th larval stage (three replicates, two individuals per replicate) were collected for this study. Each GT replicate was taken from different water tanks. Wild Chironomus flaviplumus (CH) at the 4th larval stage (three replicates, two individuals per replicate) were captured from the Yeon Deung (YD) stream located in Yeosu, South Korea (34°44′ N, 127°43′ E), in February 2022. Developmental stage of chironomids was determined based on body length and body color. The body length of the fourth instar larvae selected for this study was in the range of 5.2–11.2 mm, and the body of the individuals showed clear red color. All of the 12 individuals (2 for each replicate, 3 replicates for GT and 3 replicates for CF) were taken on the same day. The surrounding water samples were collected with three replicates (1 L per each) and filtered through filters with a pore size of 0.22 µm to determine the microbiome of the environment of wild C. flaviplumus and to identify possible contamination from skin microbiome.
2.2. Assessment of Environmental Factors in the Studied Area
Sampling was conducted at one sampling site in the YD stream. Water samples (1 L) were collected thrice. Several water environmental factors were measured to assess water quality. Water temperature (°C), dissolved oxygen (DO, mg/L), specific conductance (SPC, µS/cm), pH, and salinity (PSU) were determined on site by using a portable YSI Professional Plus (Yellow Springs, OH, USA). For nutrients, 1 L samples were filtered onto precombusted 0.7 µm glass fiber filters (GFF) fused to a Nalgene polysulfone reusable bottle top filter. For filtered GFF, chlorophyll a (Chl-a), NO2, NO3−, NH4+, PO43−, total nitrogen (TN), and total phosphorous (TP) were analyzed in duplicate via a standard spectrophotometric method [16]. All parameters were measured using a GENSYSTM visible spectrophotometer (Thermo Scientific, Waltham, MA, USA). Trophic state indices (TSI) for TP and Chl-a were calculated following the method developed by Carson [17].
2.3. DNA Extraction and High-Throughput Sequencing
Total DNAs were extracted from the whole body of GT and CF individuals using a DNeasy blood and tissue kit (Qiagen, Hilden, Germany). Before DNA extraction, individuals were washed several times with 1× PBS to prevent cross-contamination from the field and mechanically homogenized using sterile surgical blades. The total DNA from each of the filtered samples was extracted using the same kit in accordance with a modified protocol [18]. Total DNA was sent to Chunlab Inc. (Seoul, Korea) for 16S rRNA gene amplicon sequencing. The first PCR was carried out under the following conditions: 95 °C for 3 min followed by 25 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s. The final extension was conducted at 72 °C for 5 min. Sequencing was performed on an Illumina MiSeq platform (2 × 250 paired ends).
2.4. Bioinformatics
Sequences were processed in accordance with the “Moving Pictures” tutorial of Qiime2 software [19] (https://docs.qiime2.org/2022.2/tutorials/moving-pictures/, accessed on 1 March 2022). Sequence quality control was performed using a DADA2 plugin with a minimum length cut-off of 220 bp [20]. Sequences were classified using the Silva database v. 138 [21], and the sequences annotated as “Eukaryote”, “Chloroplast”, “Mitochondria”, and “Archaea” were removed for further processing. PICRUST2 v. 2.4.2 was applied to predict the functional profile of the microbial communities [14]. Before PICRUST2 was used, the number of sequences in each sample was normalized with 3793 reads per sample. The raw fastq files have been deposited at the Sequence Read Archive (SRA) under project ID PRJNA880421.
2.5. Statistical Analysis
Rarefaction curves were generated using the “rarecurve” function in R package “vegan” [22] with a step size of five. A principal coordinate analysis (PCoA) plot was generated with R “vegan” package based on the Bray–Curtis dissimilarity between samples calculated with square-root-transformed amplicon sequence variant (ASV) abundance data. Venn diagrams of ASVs were generated for each chironomid species using R “ampvis2” package [23]. The ASVs over 10% relative abundance were considered as core ASVs.
3. Results
3.1. Environmental Conditions of the Studied Sites
The environmental conditions of the studied area are summarized in Table 1. The trophic state index (TSI) was 50.8 when calculated based on the average chlorophyl-a concentration and 75.5 when calculated based on the average total phosphorous concentration, representing an eutrophic condition.

Table 1.
Environmental conditions of the studied area.
3.2. Microbial Community Composition
The rarefaction curves for the chironomid samples were saturated at the subsampling point (3793 reads), although the curves were unsaturated for the filter samples (Figure 1). The PCoA plot showed a clear distinction between the microbiomes of wild and cultivated chironomids (Figure 2). The microbiome of wild C. flaviplumus was also separated from the microbial communities of the surrounding water environment (Figure 2). In terms of diversity, the Shannon diversity (α-diversity) and the within-group Bray–Curtis dissimilarity (β-diversity) were the lowest in the cultivated G. tokunagai microbiome (Figure 3). About 20% of the core ASVs overlapped between G. tokunagai replicates, whereas in the replicates of C. flaviplumus none of the core ASVs overlapped (Figure 4).
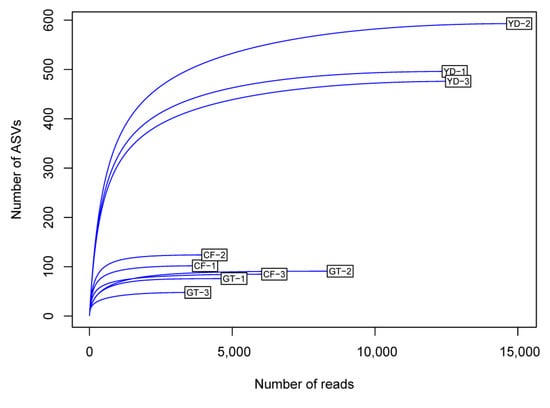
Figure 1.
Rarefaction curves for the chironomid samples (GT and CF) and the filter samples (YD). GT: G. to-kunagai; CF: C. flaviplumus; YD: Yeon Deung water.
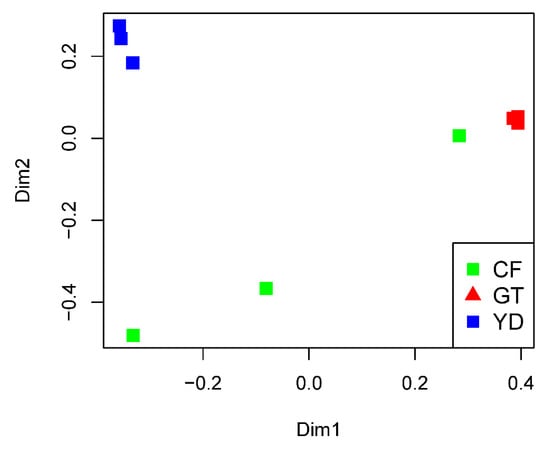
Figure 2.
PCoA plot showing Bray–Curtis dissimilarities between the studied samples. GT: G. tokunagai; CF: C. flaviplumus; YD: Yeon Deung water.
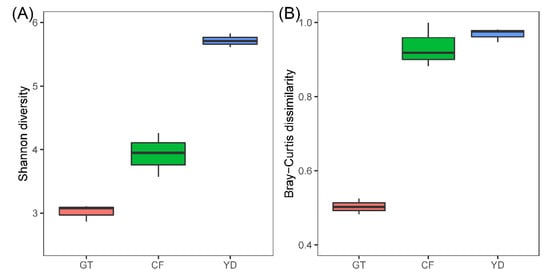
Figure 3.
Shannon diversity (α-diversity) (A) and the within-group Bray–Curtis dissimilarity (β-diversity) (B) of the studied samples. GT (red): G. tokunagai; CF (green): C. flaviplumus; YD (blue): Yeon Deung water.
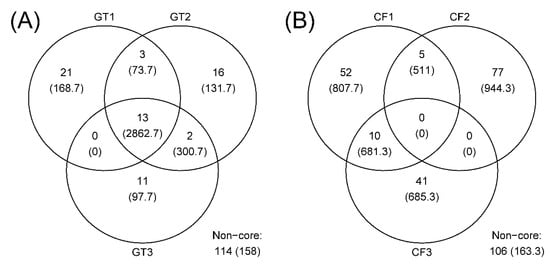
Figure 4.
Venn diagrams showing the number of ASVs and their average abundance (the number of reads) in each chironomid species. (A) GT: G. tokunagai; (B) CF: C. flaviplumus.
The three most abundant phyla accounting for more than 85% in all the studied samples were Proteobacteria, Bacteroidota, and Firmicutes (Figure 5). At the genus level, Dysgonomonas was found in more than half of all G. tokunagai samples and in one of the wild C. flaviplumus samples (Figure 6). In two of the wild C. flaviplumus samples, Sphaerotilus and Dechloromonas were dominant, accounting for 20% and 5% each of the total abundance. Tyzzerella and Flavobacterium were found to be abundant in all three C. flaviplumus samples. The average relative abundance of Dysgonomonas, Sphaerotilus, and Tyzzerella was less than 1% in Yeon Deung samples. In contrast, Flavobacterium was found to be abundant (average relative abundance of 6%) in Yeon Deung samples as well.
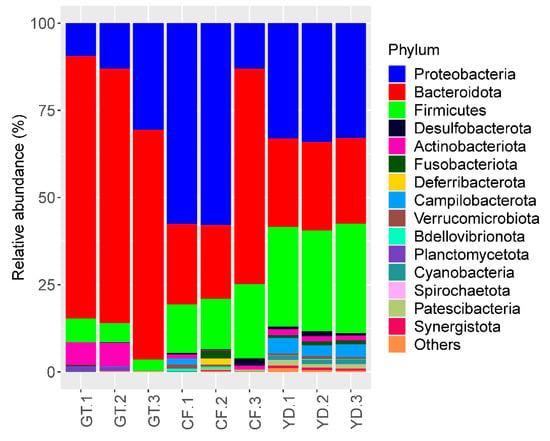
Figure 5.
Relative abundance of the 10 most abundant phyla in the studied samples. GT: G. tokunagai; CF: C. flaviplumus; YD: Yeon Deung water.
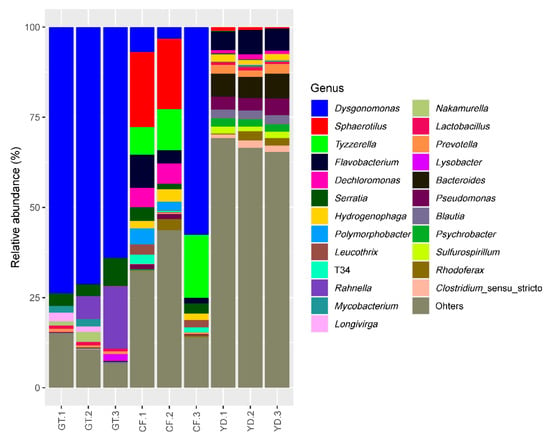
Figure 6.
Relative abundance of the 10 most abundant genera in the studied samples. GT: G. tokunagai; CF: C. flaviplumus; YD: Yeon Deung stream water.
3.3. Functional Potential of Microbial Communities
Table 2 summarizes the genes related to resistance to antibiotics, toxic metals, and toxic compounds that were identified in our study via PICRUST2 and in C. ramosus larval microbiome in Sela et al.’s study (2021) [5] through metagenome sequencing. These included an arsenic resistance gene (arsH), cobalt–zinc–cadmium resistance genes (e.g., czcA and zitB), copper resistnace genes (e.g., cusR, copR, and pcoD genes), multidrug resistance genes (e.g., mdtK and emrE), and a gene related to phenylacetic acid degradation (paaY).

Table 2.
List of the KEGG gene categories related to resistance to antibiotics, toxic metals, and toxic compounds found in our study and in C. ramosus larval microbiome in Sele et al.’s study (2021). GT: G. tokunagai; CF: C. flaviplumus.
4. Discussion
In this study, we used high-throughput sequencing methods to identify the microbiomes of cultivated G. tokunagai and wild C. flaviplumus. As indicated by the low within-group dissimilarity and highly overlapping core ASVs, the microbiome of G. tokunagai was homogeneous suggesting that environmental conditions were controlled well. The Shannon diversity of G. tokunagai microbiome was low as well, possibly because they have been cultivated for several generations, which could result in simpler microbial forms that only include key microbes.
At the phylum level, Proteobacteria, Bacteroidota, and Firmicutes were dominant in both groups; this finding was consistent with previous results on chironomid microbiome [5,8,24]. At the genus level, Dysgonomonas was observed in more than half of the microbiomes of all three G. tokunagai individuals. Dysgonomonas is often identified as one of the most dominant genera in the microbiome of insects, including chironomids [5,24,25]. Dysgonomonas is an anaerobic bacterial genus that grows on nutrient-rich media and can degrade diverse polysaccharides and complex compounds, such as lignocellulose [26]. In chironomids, they could work as potential symbionts that provide nutrients as simpler forms to their host.
Sphaerotilus, Tyzzerella, and Dechloromonas, were found to be dominant in C. flaviplumus. Sphaerotilus is represented by Sphaerotilus natans, which have often been isolated from nutrient-rich environments such as sewage and activated sludge samples [27,28,29]. Tyzzerella and Dechloromonas are associated with various types of environmental pollutants. The relative abundance of Tyzzerella was higher in the gut microbiome of Achirus lineatus exposed to a water-accommodated fraction of light crude oil [30] and in the gut microbiome of residents with long-term exposure to heavy metals in a mining area [31]. Species belonging to Dechloromonas have a broad range of metabolic capabilities including reduction of perchlorate and oxidization of chlorobenzoate, toluene, and xylene [32,33]. For this reason, they have been suggested as candidate organisms for bioremediation [34]. Possibly, these organisms could aid detoxification of pollutants and provide nutrient sources to their host. Flavobacterium was one of the abundant taxa in the microbiome of wild C. flaviplumus as well, but it was also abundant in the surrounding environment. This could be due to contamination from the surrounding environment or from skin microbiome. The high abundance of this taxa corresponds with previous findings that show prevalence of Flavobacterium in eutrophic lakes [35,36].
Although heavy metal concentration was not measured in this study, a previous study shows a high level of heavy metals in the sediment of Yeon Deung stream, exceeding the limit designated by Korean law, Enforcement decree of the water quality and aquatic ecosystem conservation act [37]. Functional genes inferred by PICRUST2 included gene categories related to resistance to antibiotics, toxic metals (e.g., copper, arsenic, and zinc), and toxic compounds (e.g., quaternary ammonium compound), as found by Sela et al. (2021) [5]. This finding strengthened the hypothesis of Senderovich and Halpern (2013) [8], who described the protective role of chironomid microbiome. The copper resistance gene in G. tokunagai microbiome was more abundant than that in C. flaviplumus microbiome, possibly because of the water pipe composed of copper, although the copper level in the cultivation environment was not tested in this study.
5. Conclusions
In this study, we identified the microbiome of cultivated G. tokunagai, which is often used as a model species, and the microbiome of wild C. flaviplumus. We found that G. tokunagai possessed a homogeneous microbiome where a single genus (Dysgonomonas) dominating, possibly because G. tokunagai was cultivated in a controlled environment. In both chironomid groups, we found bacterial genera which have ability to degrade complex carbon sources possibly aiding host digestion. Tyzzerella and Dechloromonas that could detoxify toxic metals/compounds were more abundant in wild C. flaviplumus than in G. tokunagai, strengthening the hypothesis on the protective role of chironomid microbiome against environmental pollutants. In the future, metatranscriptomics will provide further information to understand the response mechanisms of chironomids against toxic compounds and the symbiotic roles of chironomid microbiome.
Author Contributions
Conceptualization, I.-S.K.; methodology, W.-S.K. and J.-W.P.; formal analysis, H.S.; investigation, H.S., W.-S.K. and J.-W.P.; data curation, H.S.; writing—original draft preparation, H.S.; writing—review and editing, W.-S.K. and I.-S.K.; visualization, H.S.; supervision, I.-S.K.; project administration, I.-S.K.; funding acquisition, I.-S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF), grant numbers [NRF2020R1A2C1013936] and [NRF-2018R1A6A1A03024314]. Hokyung Song was funded by the Basic Science Research Program of NRF [2016R1A6A1A03012862].
Data Availability Statement
FASTQ Illumina sequence data have been deposited at the Sequence Read Archive (SRA) under project ID PRJNA880421.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Armitage, P.D.; Pinder, L.; Cranston, P. The Chironomidae: Biology and Ecology of Non-Biting Midges; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Mezgebu, A.; Lakew, A.; Lemma, B.; Beneberu, G. The potential use of chironomids (Insecta: Diptera) as bioindicators in streams and rivers around Sebeta, Ethiopia. Afr. J. Aquat. Sci. 2019, 44, 369–376. [Google Scholar] [CrossRef]
- Molineri, C.; Tejerina, E.G.; Torrejón, S.E.; Pero, E.J.I.; Hankel, G.E. Indicative value of different taxonomic levels of Chironomidae for assessing the water quality. Ecol. Indic. 2020, 108, 105703. [Google Scholar] [CrossRef]
- Al-Shami, S.A.; Rawi, C.S.M.; HassanAhmad, A.; Nor, S.A.M. Distribution of Chironomidae (Insecta: Diptera) in polluted rivers of the Juru River Basin, Penang, Malaysia. J. Environ. Sci. 2010, 22, 1718–1727. [Google Scholar] [CrossRef]
- Sela, R.; Laviad-Shitrit, S.; Thorat, L.; Nath, B.B.; Halpern, M. Chironomus ramosus Larval Microbiome Composition Provides Evidence for the Presence of Detoxifying Enzymes. Microorganisms 2021, 9, 1571. [Google Scholar] [CrossRef]
- Sun, X.; Liu, W.; Li, R.; Zhao, C.; Pan, L.; Yan, C. A chromosome level genome assembly of Propsilocerus akamusi to understand its response to heavy metal exposure. Mol. Ecol. Resour. 2021, 21, 1996–2012. [Google Scholar] [CrossRef]
- Nath, B.B. Extracellular hemoglobin and environmental stress tolerance in Chironomus larvae. J. Limnol. 2018, 77, 104–112. [Google Scholar] [CrossRef]
- Senderovich, Y.; Halpern, M. The protective role of endogenous bacterial communities in chironomid egg masses and larvae. ISME J. 2013, 7, 2147–2158. [Google Scholar] [CrossRef]
- Cranston, P. Morphology. In The Chironomidae. The Biology and Ecology of Non-Biting Midges; Armitage, P., Cranston, P.S., Pinder, L.C.V., Eds.; Chapman and Hall: London, UK, 1995. [Google Scholar]
- Baek, M.J.; Yoon, T.J.; Bae, Y.J. Development of Glyptotendipes tokunagai (Diptera: Chironomidae) Under Different Temperature Conditions. Environ. Entomol. 2012, 41, 950–958. [Google Scholar] [CrossRef]
- Baek, M.J.; Yoon, T.J.; Kang, H.J.; Bae, Y.J. Biological and Genetic Characteristics of Glyptotendipes tokunagai (Diptera: Chironomidae) on the Basis of Successive Rearing of Forty-Two Generations Over Seven Years Under Laboratory Conditions. Environ. Entomol. 2014, 43, 1406–1418. [Google Scholar] [CrossRef]
- Park, K.; Kim, W.-S.; Park, J.-W.; Kwak, I.-S. Complete mitochondrial genome of Chironomus flaviplumus (Diptera: Chironomidae) collected in Korea. Mitochondrial DNA Part B 2021, 6, 2843–2844. [Google Scholar] [CrossRef]
- Kwak, I.-S.; Park, J.-W.; Kim, W.-S.; Park, K. Morphological and genetic species identification in the Chironomus larvae (Diptera: Chironomidae) found in domestic tap water purification plants. Korean J. Ecol. Environ. 2020, 53, 286–294. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 233: Sediment-Water Chironomid Life-Cycle Toxicity Test Using Spiked Water or Spiked Sediment; Organisation for Economic Co-Operation and Development Publishing: Paris, France, 2010. [Google Scholar]
- Hamilton, E. A Manual of Chemical & Biological Methods for Seawater Analysis; Parsons, T.R., Maita, Y., Lalli, C.M., Eds.; Pergamon Press: Oxford, UK, 1984; p. 184. ISBN 0-08-030287-4. [Google Scholar]
- Carlson, R.E. A trophic state index for lakes1. Limnol. Oceanogr. 1977, 22, 361–369. [Google Scholar] [CrossRef]
- Djurhuus, A.; Closek, C.J.; Kelly, R.P.; Pitz, K.J.; Michisaki, R.P.; Starks, H.A.; Walz, K.R.; Andruszkiewicz, E.A.; Olesin, E.; Hubbard, K.; et al. Environmental DNA reveals seasonal shifts and potential interactions in a marine community. Nat. Commun. 2020, 11, 254. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Package ‘Vegan’. Community Ecology Package, version 2; R Core Team: Vienna, Austria, 2013; Volume 2, pp. 1–295. [Google Scholar]
- Andersen, K.S.; Kirkegaard, R.H.; Karst, S.M.; Albertsen, M. ampvis2: An R package to analyse and visualise 16S rRNA amplicon data. bioRxiv 2018, 299537. [Google Scholar] [CrossRef]
- Chernitsyna, S.M.; Khalzov, I.A.; Sitnikova, T.Y.; Naumova, T.V.; Khabuev, A.V.; Zemskaya, T.I. Microbial Communities Associated with Bentic Invertebrates of Lake Baikal. Curr. Microbiol. 2021, 78, 3020–3031. [Google Scholar] [CrossRef]
- Laviad-Shitrit, S.; Sharaby, Y.; Sela, R.; Thorat, L.; Nath, B.B.; Halpern, M. Copper and chromium exposure affect chironomid larval microbiota composition. Sci. Total Environ. 2021, 771, 145330. [Google Scholar] [CrossRef]
- Bridges, C.M.; Gage, D.J. Development and application of aerobic, chemically defined media for Dysgonomonas. Anaerobe 2021, 67, 102302. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chua, H.; Lo, W.-h.; Lawford, H.; Yu, P.H.-F. Sphaerotilus natans isolated from activated sludge and its production of poly (3-hydroxybutyrate-co-3-hydroxyvalerate). In Biotechnology for Fuels and Chemicals; Springer: Champs, Switzerland, 2002; pp. 1061–1073. [Google Scholar]
- Ferreira, R.; Amado, R.; Padrão, J.; Ferreira, V.; Dias, N.M.; Melo, L.D.R.; Santos, S.B.; Nicolau, A. The first sequenced Sphaerotilus natans bacteriophage– characterization and potential to control its filamentous bacterium host. FEMS Microbiol. Ecol. 2021, 97, fiab029. [Google Scholar] [CrossRef] [PubMed]
- Pellegrin, V.; Juretschko, S.; Wagner, M.; Cottenceau, G. Morphological and biochemical properties of a Sphaerotilus sp. Isolated from paper mill slimes. Appl. Env. Microbiol. 1999, 65, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Cerqueda-García, D.; Améndola-Pimenta, M.; Zamora-Briseño, J.A.; González-Penagos, C.E.; Árcega-Cabrera, F.; Ceja-Moreno, V.; Rodríguez-Canul, R. Effects of chronic exposure to water accommodated fraction (WAF) of light crude oil on gut microbiota composition of the lined sole (Achirus lineatus). Mar. Environ. Res. 2020, 161, 105116. [Google Scholar] [CrossRef]
- Shao, M.; Zhu, Y. Long-term metal exposure changes gut microbiota of residents surrounding a mining and smelting area. Sci. Rep. 2020, 10, 4453. [Google Scholar] [CrossRef]
- Salinero, K.K.; Keller, K.; Feil, W.S.; Feil, H.; Trong, S.; Di Bartolo, G.; Lapidus, A. Metabolic analysis of the soil microbe Dechloromonas aromatica str. RCB: Indications of a surprisingly complex life-style and cryptic anaerobic pathways for aromatic degradation. BMC Genom. 2009, 10, 351. [Google Scholar] [CrossRef]
- Zhang, S.; Amanze, C.; Sun, C.; Zou, K.; Fu, S.; Deng, Y.; Liu, X.; Liang, Y. Evolutionary, genomic, and biogeographic characterization of two novel xenobiotics-degrading strains affiliated with Dechloromonas. Heliyon 2021, 7, e07181. [Google Scholar] [CrossRef]
- Husain, R.; Vikram, N.; Pandey, S.; Yadav, G.; Bose, S.K.; Ahamad, A.; Shamim, M.; Kumar, K.G.; Khan, N.A.; Zuhaib, M.; et al. Microorganisms: An eco-friendly tools for the waste management and environmental safety. In Biotechnological Innovations for Environmental Bioremediation; Arora, S., Kumar, A., Ogita, S., Yau, Y.Y., Eds.; Springer: Singapore, 2022; pp. 949–981. [Google Scholar]
- Eiler, A.; Bertilsson, S. Flavobacteria blooms in four eutrophic lakes: Linking population dynamics of freshwater bacterioplankton to resource availability. Appl. Environ. Microbiol. 2007, 73, 3511–3518. [Google Scholar] [CrossRef]
- Shao, K.; Yao, X.; Wu, Z.; Jiang, X.; Hu, Y.; Tang, X.; Xu, Q.; Gao, G. The bacterial community composition and its environmental drivers in the rivers around eutrophic Chaohu Lake, China. BMC Microbiol. 2021, 21, 179. [Google Scholar] [CrossRef]
- Kim, W.-S.; Kim, R.; Park, K.; Chamilani, N.; Kwak, I.-S. The molecular biomarker genes expressions of rearing species Chironomus riparious and field species Chironomus plumosus exposure to heavy metals. Korean J. Ecol. Environ. 2015, 48, 86–94. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).