Abstract
African Swine Fever is a lethal hemorrhagic disease caused by a DNA virus that affects domestic and wild pigs, causing serious economic losses in the swine industry. African Swine Fever virus (ASFV) is maintained in a sylvatic cycle that includes wildlife and Ornithodoros tick species. A huge investigation about ASFV structure and its infection process in pigs has been carried out in recent years, and although these studies have increased our knowledge about its pathogenesis, there are still many unclear aspects about which immune responses protect swine hosts against the disease caused by this virus. The mechanisms of ASFV infection in ticks are even less well understood. This infection is long term and persistent, with relatively high levels of virus replication in different tick tissues. According to specific infected tissues, the Ornithodoros tick species that are ASFV-competent vectors show transstadial, transovarial and/or venereal transmissions. This review is focused on the main process taking place at the virus–vector interface, summarizing the latest findings about the molecular and cellular aspects of ASFV infection in ticks, which could constitute the basis for developing novel strategies to interrupt the arthropod transmission cycle.
Keywords:
ASF; ASFV; Ornithodoros tick species; pigs; ASFV transmission; tick–host interface; ASFV infection 1. Introduction
African Swine Fever (ASF) is a highly contagious viral hemorrhagic disease that affects domestic and wild pigs, whose acute form can reach 100% mortality [1]. This disease is endemic to sub-Saharan Africa and was first described in Kenya in 1921 [2]. It is caused by a virus belonging to the Asfarviridae family (ASFV), which is classified into 24 genotypes and 8 serotypes, with different levels of virulence present only in Africa [3]. Transcontinental spreads of ASFV have included only genotypes I and II [1]. Currently, ASFV persists in Eastern European countries and Asia, with consequent devastating economic and productive losses to the swine industry [4,5,6,7,8]. To date, there are no available effective vaccines or treatments against ASFV due to the complex viral genome and its sophisticated ability to regulate the host immune response [9]. For this reason, timely and rapid diagnosis is essential to control the transmission of the disease; however, these measures have only limited success, and ASF has become a panzootic [10].
The ASFV icosahedral particle has a multilayered structure with a 170–194 kb long linear dsDNA genome encoding more than 150 open reading frames [11,12,13]. It is the only known DNA virus transmitted by arthropods [14]. In Eastern and Southern Africa, the virus circulates in a sylvatic cycle, which includes wildlife reservoirs as warthogs (Phacochoerus africanus) that are infected by soft ticks of the genus Ornithodoros living in their warrens [15]. The common warthog can remain viremic for long time; however, it does not develop clinical disease because its viremia is usually low [16]. Therefore, ASFV transmission among these animals and domestic pigs is unlikely [17]. Other wild suids could be also involved in this sylvatic cycle, but they are considered to have a less significant role in ASFV dissemination to domestic pigs [14]. In this context, the tick–pig cycle is the most relevant for the ASFV outbreaks in domestic pigs. On the contrary, the contact of domestic pigs with infected wild boars is considered key within the ASFV domestic cycle in the Russian Federation, trans-Caucasian countries and Eurasia [17,18,19,20,21]. Wild boars (Sus scrofa) are native species in Europe, Asia and Northern Africa, and they are susceptible to ASFV infection, showing the same clinical symptoms and mortality rates as domestic pigs (Figure 1). In addition to all these dissemination ways, ASFV spreads over long distances as a result of viral survival in pork products stored even at negative temperatures [22].
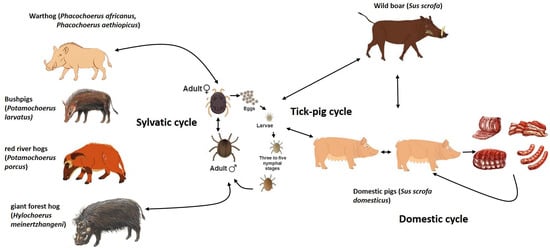
Figure 1.
The life cycle of ASFV involving the tick and swine hosts.
Despite Ornithodoros sp. tick participation in the ASF epidemiology having been suspected since the first description of the disease [2], it was not discovered until 1960, during outbreaks in Spain in the 1960s, with O. erraticus as an actor in the ASFV’s sylvatic cycle in this region [23]. In Africa, it has been demonstrated that O. moubata complex soft ticks are able to maintain an ASFV infection for years and transmit it through sexual, transstadial and/or transovarial ways [24]. Nonetheless, vector competence for ASFV remains an investigation topic [25]. Not all Argasidae can transmit this virus, as only eight taxa from Ornithodoros ticks have demonstrated their ASFV biological vector abilities [26]. Results of these studies have demonstrated that the ability to maintain and transmit this virus is closely linked to the virus strain and the tick species [27]. To date, there is evidence that the ASFV is not able to replicate or be transmitted by hard ticks [28,29,30]. There are other studies where the capacity for ASFV transmission by different arthropods, including other blood-sucking insects, has been investigated and whose results have shown low probability for them to have an important role in long-term virus preservation and successful infection of susceptible pigs [31,32,33]. All these studies suggest a high specificity in the ASFV–tick interaction; however, viral and tick factors governing this interaction remain very limited even today. The scope of this review is, therefore, to synthesize current knowledge on the molecular mechanisms that underpin the complex interactions between ASFV and its tick vectors, highlighting gaps in knowledge and potential targets for intervention. To do this, PubMed (https://www.ncbi.nlm.nih.gov) searches with the terms “ASFV reviews” and “tick immunity”, as well as combinations of “ASFV” and “ticks”, “ASFV” and “pig” and “ASFV” and “tick saliva”, were performed on 15 August 2025 and on 17 October 2025 without date or language limitations. Duplicates were removed using EndNote 20 tools [34].
2. The Tick Vector: More than Just a Syringe
2.1. Biology of Relevant Ticks: Focus on Ornithodoros spp.
Despite the huge diversity of arthropod and arboviruses, the virus–vector combinations are very specific, suggesting precise molecular mechanisms involved in the viral infection and transmission by vectors, which are results from the long-term co-evolution of pathogens and vectors [35]. In the case of ticks, restrictions in vector competencies suggest main differences between tick families in their interactions with viruses [36]. To date, only eight Ornithodoros taxa in the family Argasidae have been described as biological vectors and reservoir hosts for ASFV [25]. Obviously, the specific biology of these species is relevant for the ASFV infection outcome.
Argasid (or soft) ticks lack a dorsal scutum in the adult and nymph stages; however the characteristics of their cuticle reduce water evaporation, allowing them to survive at high temperatures and under relatively dry conditions in tropical and subtropical zones and in arid areas of Central Asia and Africa, where ASFV is endemic and the highest genetic diversity of this virus is maintained in a sylvatic cycle that, up to now, includes only Ornithodoros tick species [15,37,38,39]. These species have an endophilous, nidicolous lifestyle living in a microhabitat in the burrows and caves of vertebrate animals that guarantee the required specific optimal conditions for their development [39]. Nymphs and adults of Ornithodoros species have short blood meals that can last from 15 to 60 min, which makes it unlikely to find them on their hosts. During this blood feeding, biological and mechanical ASFV transmission can happen. In addition, the high tegument distension and elimination of water excess and ions by coxal glands that occur during this feeding process lead to ASFV excretion in the tick vector’s coxal fluid, increasing tick-to-tick transmission [40].
Unlike ixodid ticks, soft ticks such as Ornithodoros species have between 2 and 8 nymph instars that require, just like larvae, at least one blood meal for molting. Their adults are long lived, with a maximum lifespan as great as 25 years for some species, and can feed up to 10 times when hosts are available [39]. Throughout their life cycle, these tick species are likely exposed to ASFV at multiple times depending on host availability and environmental conditions. In infected ticks, the virus can be isolated after many years post-infection [27]. In Madagascar, the presence of ASFV was detected in O. porcinus ticks in domestic pig premises unoccupied for at least 4 years [41]. Another study reported a longevity of O. erraticus ticks of 15–20 years and ASFV transmission after 5 years without a blood meal [42]. Additionally, female soft Ornithodoros ticks are iteroparous: they can lay eggs from two to five small clutches during their lifetime without previous copulation because they retain sperms within endospermatophores as an adaptation to host scarcity and/or climatic variability [43]. In addition, soft ticks, including Ornithodoros species, are very resistant to starvation, entering a quiescent phase or diapause for two to eight months during the winter or dry season until hosts become available [39]. All these characteristics of Ornithodoros spp. contribute to maintaining ASFV circulation in nature and explain why ASF eradication by pig vaccination is a challenging goal in areas where the sylvatic cycle is present.
Despite the vectoral competence of described Ornithodoros species to replicate ASFV, there are differences in viral titer and persistence according to the studied combination of soft tick species and virus strain [27]. Regardless of the fact that many of these results are incomparable due to bias of the different experimental designs used to obtain them, it has become clear that although a tick species can act as a reservoir of ASFV, infection with different viral genotypes could cause minimal cytopathological effects or even increase mortality and variations in the transmission efficiency [27,35,44]. For example, high mortality among O. moubata populations infected with some strains of genotype I (VIC T90/1 and Liv13/33) or genotype II (Georgia2007/1) has been observed. In addition, the Liv13/33 strain was vertically transmitted by this tick species but not the Georgia 2007/1 strain [27,36]. There is also evidence of ASFV clearance from infected O. moubata porcinus tick colonies that are fed with a virus-free blood. This fact could be explicated by the high mortality rates of infected ticks over the uninfected ones [45]. A study comparing fecundity in ASFV-infected and uninfected O. moubata porcinus and O. erraticus ticks showed that mortality rates were considerably higher among the infected ticks [45,46]. The high mortality of ASFV-infected ticks was also observed in O. coriaceus [31,47] and O. moubata (Murray, 1877) ticks [48]. On the other hand, O. coriaceus was able to transmit to pigs the Tengani/62 strain but not the Uganda/61 strain, while O. porcinus transmitted both strains [45]. In general, combinations between ASFV isolates not derived from ticks or Ornithodoros species not native from the place where the virus was isolated show increased tick mortality, suggesting that virus–tick adaptation could likely be necessary for successful ASFV persistence and transmission [18]. In this sense, molecular studies have suggested that the integration of ASFV genetic material in the genomes of soft ticks of the O. moubata complex occurred at least 1.47 million years ago and such long-term co-evolution could be responsible for the current high viral diversity present in Africa [16,49,50,51].
2.2. The Tick Midgut: The First Battlefield
Following the ingestion of ASFV-infected blood by ticks, their competence as vectors is defined by the successful replication and subsequent dissemination of the virus from the midgut to other tick tissues. Effective transmission will require the virus to infect specific target tissues, such as the salivary glands for horizontal transmission via saliva or the reproductive organs for vertical (transovarial) or sexual transmission [27,51]. Studies on O. moubata s.l. ticks have shown efficient ASFV replication in the midgut epithelium, circulating hemocytes, salivary glands and coxal glands [26]. This efficient ASFV multiplication points toward overcoming several barriers within the arthropod, such as the midgut peritrophic membrane, tick immunity and the resistance offered by the tick microbiota to pathogen invasions [52,53,54]. The gut represents the critical entry point that determines the success of the virus’ survival, replication and transmission. The midgut responsible for blood digestion, is additionally a key organ in the tick’s immune response against pathogens [35,36,40]. This tissue constitutes a microenvironment where viruses also come into direct contact with the resident microbial community, which can prevent or enhance the viral infection by direct interaction and/or modulation of the vector’s immune system components [55,56]. In this context, there is a study that has shown divergent midgut microbiome profiles (in composition, diversity and assembly) between O. erraticus and O. moubata s.l. tick species [57]. On the other hand, another study demonstrated ASFV sexual and transstadial transmission but no transovarial transmission in O. erraticus compared to O. moubata s.l. ticks, where all these transmissions have been demonstrated [46]. If demonstrated differences in microbiota between these tick species could underlie their distinct interactions with ASFV and drive the variations in their vector competence is an issue that remains to be investigated. An example of the impact of microbiota manipulation on the regulation of immune response and on ASFV infection vulnerability was demonstrated by the transplantation of African warthog (P. africanus) fecal microbiota to domestic pigs (S. scrofa), which conferred partial protection of these highly susceptible pigs against infection of attenuated ASFV strains [58]. Remarkably, most of the bacteria specifically found in transplanted pigs just before ASFV challenge have been associated with anti-inflammatory states and the production of AMPs in humans [59,60,61,62,63]. Therefore, microbiome transplantation from ASFV-resistant ticks to vector-competent Ornithodoros ticks could be a very interesting approach to evaluate the microbiota’s role in ASFV infection in ticks.
An experimental infection of O. moubata porcinus ticks with the Uganda or KWH/12 ASFV virus strain showed primary viral establishment, the highest titers and the longest persistence in the midgut [64]. Direct immunofluorescence studies of O. turicata ticks fed on an ASFV-infected animal revealed wide infection of the midgut epithelium after 2 or 3 days, while virus detection in the hemolymph and the salivary glands was possible only 20 days after feeding [31,40]. The results of another ultrastructural study using O. porcinus ticks infected with the Chiredzi/83/1 ASFV isolate showed that viral replication started in phagocytic digestive cells of the midgut epithelium, and 2–3 weeks later, virus infection was generalized to other tick tissues [40]. All these studies have demonstrated that efficient ASFV replication in the tick midgut is critical for virus infection generalization [65]. It means that once the virus has crossed midgut barriers, managing its replication in the cells of the midgut epithelium, it would be able disseminate to the salivary glands and reproductive organs, establishing a long-term, persistent and non-pathogenic infection in ticks.
3. Molecular Journey of ASFV in Ticks: A Stepwise Deconstruction
3.1. Viral Entry into Tick Cells
As mentioned before, in nature, ASFV circulates between individuals of different species, including domestic pigs, wild boars, warthogs and soft ticks; therefore, the virus has evolved to infect different cell types in each host. In swine hosts, ASFV has a restricted cellular tropism, targeting mainly macrophages and monocytes for replication [66], but also infects specific lineages of reticular cells in the spleen, lymph node, lung, kidney and liver [67]. In the same way, this virus is able to replicate and persist in different tick tissues, which demonstrates its adaptability for efficient spreading [26,65]. This selective pressure during host adaptation across evolution, geographical expansion and laboratory adaptation has determined the ASFV diversity for which deletions, insertions, inversions and duplications in its genome have been demonstrated [9].
The proteins encoded by the large double-stranded DNA genome of ASFV are classified into structural and nonstructural proteins, according to their roles in the viral particle. Structural proteins like pp220, pp62, p72, p54, p30 and CD2v are major components of viral particles and are crucial in virus assembly and in the interactions between the virus and its host cells [68,69]. They are codified by a conserved central region of the ASFV genome [70]. This central region is flanked by variable regions in both extremes that contain at least five multigene families (MGFs) coding nonstructural proteins that participate in cell tropism, viral replication, gene expression regulation, immune evasion and disease pathogenesis. These MGFs confer plasticity to the ASFV genome, allowing for strain diversification and the adaptations mentioned above [69]. In fact, ASFV avirulent strains frequently have significant deletions of MGF regions [9]. For example, Portugal NH/P68 and OURT88/3 attenuated ASFV strains show extensive deletions in the MGFs compared to the virulent Lisbon 60 (L60) strain [71]. In the same way, ASFV strains adapted to cell culture demonstrated characteristic MGF alterations [72,73,74]. These MGFs are also highly variable in ASFV strains circulating in wild boar and domestic pig populations and in different geographical regions [75,76,77]. Therefore, these genetic divergences allow for differentiation between geographically distinct ASFV strains and for tracking their spreading [78].
Deletions of MGF360 (3HL, 3IL and 3LL) regions impaired viral capacity to establish generalized infections in Ornithodoros vector ticks, preventing efficient viral transmission, which demonstrates their critical role in ASFV replication and persistence in tick tissues [26,67]. These genes, together with the MGF 530 genes, are also significantly involved in promoting survival and virulence in infected swine macrophages [79,80]. All this evidence points out that MGF360 genes as important factors for determining the ASFV host range. However, given the lack of homology of the products of these genes with other known proteins, it has been difficult to speculate on their roles in virus–cell interactions and how they modulate tick cell infection [67].
In ticks, erythrocyte digestion takes place inside microvilli of midgut digestive cells by phagolysosome formation [26,40]. In this regard, ASFV adhesion to erythrocytes could be crucial to enhance virus uptake and tick midgut cell infection [40]. The CD2v is a transmembrane and glycosylated protein of ASFV that has been described as essential in the viral hemadsorption (HAD) to red blood cells and as an enhancer of ASFV replication in O. erraticus ticks [81]. Therefore, adherence of viral particles to red blood cells could constitute a widespread mechanism enabling increased viral uptake and replication in arthropod vectors (Figure 2). Thus, the gene encoding this viral protein could be under strong positive selection in the sylvatic transmission cycle.
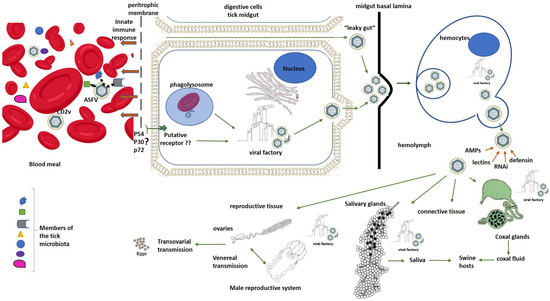
Figure 2.
Schematic ASFV “molecular journey” inside ticks. Black arrows represent interactions between ASFV and microorganisms that are part of the tick microbiota inside the gut when infected blood is ingested by ticks. Brown arrows represent effects of the innate immune response mediators of ticks on ASFV. Green arrows represent hypothetical pathways for ASFV entry and dissemination inside ticks. The symbol ? means that the involvement of these viral structural proteins in a specific mechanism for ASFV entry to tick digestive cells remains to be demonstrated. In the same way, ?? means that the putative specific receptor for ASFV entry to tick digestive cells remains to be identified.
Nevertheless, many viral infections are initiated when the virus binds to a specific receptor in the host cells that triggers a complex cascade of intracellular signaling for virus internalization. The presence of such specific receptors on the luminal surface of the tick gut has not been demonstrated for ASFV entry and infection of tick digestive cells as an alternative mechanism for the processes of phagocytosis and pinocytosis described above. However, it could be supported by the fact that non-HAD mutant ASFV isolates can also enter and replicate in ticks [81]. The presence of this receptor could also be partly supported by the high specificity of ASFV replication in ticks only demonstrated in Ornithodoros species. The ASFV structural proteins, p54 and p30, have been demonstrated as key molecules in viral entry to host mammalian cells [82,83]. In the same way, the propeller-like top structure of p72 extended to outside of the virus has been likely implicated in the receptor-binding area on the pig cell surface [12]. Further studies will be necessary in order to elucidate if these proteins could be also involved in ASFV interaction with the putative receptors on tick cells. Other authors have also speculated a “leaky gut” phenomenon, in which the ASFV virus could pass directly into the hemocoel from the gut lumen through lesions or pores formed between the gut cells during the first phase of digestion without entering the gut cells [84,85]. However, this last theory contradicts some studies that have demonstrated that virus replication in the midgut epithelium is required for the generalization of viral infection in Ornithodoros ticks [27,65]. In this regard, other evidence reinforcing the essential role of the high viral replication in the midgut cells for ASFV dissemination and successful transmission by ticks is the speculation by some authors that it could be responsible for weakening the gut cell monolayer, making it susceptible to rupture, which is supported by the observations of gut rupture as a frequent cause of death in some ASFV-infected ticks [31,47,48]. A critical review of a study that suggested this “leaky gut” phenomenon for ASFV infection shows an experimental design in which ticks were artificially engorged on ASFV-infected blood in the presence of antibiotics and antifungals that could modify the integrity of the tick midgut, favoring the ASFV’s bypassing of midgut replication [27,84]. In any case, gaps in our understanding of ASFV infection and the dynamics of its dissemination in Ornithodoros ticks remain to be unraveled.
After midgut infection, the virus escapes from this organ to infect other tick organs using a not-well-described mechanism [36,86]. In soft ticks, the midgut basal lamina is a homogeneous and continuous layer that has neither been biochemically nor cytochemically characterized [26,40,65]. Possibly, ASFV spreading may involve virus movements across the midgut basal lamina into the hemocoel by the action of other molecules such as matrix metalloproteinases or through mechanical forces [26,40]. The delay in the generalization of ASFV infection in ticks may be explained by inefficiency in the translocation across this basal lamina and/or the need for high viral titers close to the basal lamina, which probably requires extensive virus replication and time [40].
Once in the hemocoel, the viral particles are picked up by the hemocytes, where they are efficiently replicated and propagated to other tick organs [26]. ASFV virions budding from the plasma membrane of infected hemocytes were observed [40]. ASFV presence was also demonstrated in hemocytes of infected O. coriaceus ticks by electron microscopy and immunofluorescence studies [87]. Secondary sites of virus replication in ticks included connective tissue, coxal glands, salivary glands and reproductive tissue [40]. To infect the salivary glands and be transmitted through saliva, the virus must cross a thin basal lamina that surrounds them [26,40]. ASFV particles have been detected at very high concentrations in the salivary secretions of Ornithodoros ticks, supporting this route as the main ASFV transmission path during tick feeding on hosts [26,40,64,88]. In addition, the fluids from the coxal organ can also contain a high concentration of the virus that can be delivered to the host during feeding [40,65,88]. In O. porcinus porcinus ticks infected with the ASFV Chiredzi/83/1 isolate, the virus replicated in the cells of both the filtration membrane and the collecting tubule within the coxal gland. Numerous virions were observed budding into the lumen of the filtration membrane [40].
Finally, ASFV can also infect reproductive organs in female and male ticks, which allows for transovarial and sexual transmission depending on the viral isolate and Ornithodoros species combination [26,46]. For example, O. moubata porcinus female ticks transmitted the virus to their offspring and also infected males and uninfected females [24,64]. However, O. erraticus female ticks infected with a strain isolated from Portugal transmitted the virus to naive pigs but did not display ASFV transovarial transmission or venereal transmission [24,46]. These results suggest the presence of a specific barrier in both the male and female reproductive tracts of this tick species for ASFV infection [46].
3.2. Viral Replication and Assembly in the Tick Environment
ASFV is a cytoplasmic virus whose DNA replication and morphogenesis take place in viral factories, localized near to the host cell nucleus and the microtubule organizing center [89]. In this localization, ASFV has no access to the transcription machinery within the nucleus of host cells, and therefore, its genome encodes proteins for its own transcription machinery [90]. Probably, this transcriptional independence of ASFV allows for its wide diversity of evolutionarily distant hosts. In these factories, the viral capsid assembles progressively until the formation of the mature viral particles [91,92,93]. It has been recognized that aggresomal pathways could be used by ASFV to concentrate viral proteins and facilitate replication and assembly in infected swine macrophages [89]. It is possible that this same pathway could be used in the replication and assembly of this virus in tick cells due to the fact that similar viral factories have been observed in midgut digestive cells and hemocytes of ASFV-infected O. porcinus ticks [26,65].
In tick cells, viral factories are observed in a uniform cytoplasmic region bordered by filaments in which there are virions at different maturation stages [26]. Frequently, abundant mitochondria are around these viral factories, found subjacent to the plasma membrane. This localization could contribute to the intracellular transportation of viral particles and their budding from the plasma membrane of host cells, giving rise to extracellular virions with an additional external membrane [12,26,40,65]. It has been demonstrated that both intracellular and extracellular ASFV virions are infectious, which indicates that the external membrane is not strictly necessary for infectivity [94]. Additionally, in tick cells, membranes from an unknown source associated closely with mature viral particles have been observed that probably could provide protection during long periods between feedings of the long-lived ticks [26].
On another hand, ASFV was also detected in salivary glands, being first observed in the connective tissue. Later, virus factories and mature virions were also present in the granular cells, with virions accumulating in secretory granules. In this context, virus replication increased 10,000-fold between 21- and 112-days post-infection [40]. Abundant budding and large numbers of mature virions with extensive viral factories are also observed in the filtration membrane and in the tubular portions of the coxal organ in infected ticks [26].
3.3. Step 3: Evasion of Tick Antiviral Defenses
When a pathogen comes into contact with ticks, the arthropod can offer resistance to infection, eradicating this pathogen, or tolerate it, with minimal impact on its health and fitness. This outcome will depend of many factors, such as the immune response required for combating the specific pathogen and its energetic cost compared to the pathology induced by such a pathogen along with co-evolution, the specific microbiota and environmental conditions [95]. In ticks, immunity is based only on an innate immune system, which comprises cellular and humoral responses. The cellular component involves hemocytes circulating in the hemolymph that fills the whole tick body and surrounds all cells. These hemocytes are homologous to the mammalian white blood cells in terms of their immune function, which includes phagocytosis, nodule formation and the encapsulation of invaders [96,97]. The humoral component includes humoral encapsulation, hemagglutination and antimicrobial peptides (AMPs). Similar to other eukaryotes, ticks encode signal transducers involved in signaling pathways that regulate the innate immune response, such as toll, immunodeficiency (IMD) and Janus kinase and also transcription activators of the JAK-STAT pathway [98].
Humoral encapsulation is elicited by the prophenoloxidase activating system, which has been described only in the soft tick O. moubata [99]. Tick lectins function in hemagglutination to enhance phagocytosis by tick hemocytes [53,97]. Studies about pathogen uptake by phagocytic hemocyte populations have suggested that in competent vectors, the pathogens utilize hemocytes for surviving and disseminating inside ticks [96]. Lysozymes, together with other AMPs, are involved in killing invaders in several tick species [97]. Additionally, cystatins, classic cysteine protease inhibitors, have recently been shown to also function in innate immune responses of ticks [97]. It has also been demonstrated that hemoglobin fragments have antimicrobial activity in the midgut of ticks. The combination of all these immune responses in each tick species against a specific pathogen determines the vectoral capacity for such pathogen of the tick species in question [96]. Understanding the complex interaction of cellular and humoral reactions that occurs when ASFV gets the first lines of tick defense in the integument and gut may help to answer questions about why some Ornithodoros tick species are vector competent for this virus and other ticks are not.
Agglutinins/lectins with molecular sizes from 30 to 85 kDa have been described from the hemolymph, hemocytes, gut and salivary glands of several soft tick species [53]. The lectin named Dorin M with high hemagglutinating activity was identified in the hemocytes and plasma of O. moubata ticks as a molecule for recognizing non-self in innate immunity and could also be involved in mechanisms of pathogen transmission; however, its role in the ASFV infection of this tick species is still unknown [100,101]. On the other hand, a lysozyme purified from the gut of O. moubata was significantly upregulated after blood meal [102,103]. However, it is unclear if this lysozyme only has a digestive function or if it is also involved in an immune response against ingested pathogens. Lysozymes were described to be active only in the gut of soft ticks but not in the gut of hard ticks [103]. However, they are upregulated in the hemolymph and hemocytes in hard ticks, which remains to be demonstrated in soft ticks [104]. Further studies are needed to elucidate the different action mechanisms of lysozymes in soft and hard ticks and how this may impact its different capabilities as ASFV vectors. Cystatins, for their part, have been found in hard ticks, but until now, there are no reports about them in soft ticks [97,105,106].
Finally, AMPs like defensins and their upregulation in the midgut and other tick tissues have been reported in O. moubata as part of a strong antibacterial response; however, their role in antiviral response remains to be more deeply studied [107,108]. A relatively recent work discovered a defensin-like peptide named OPTX-1 from Ornithodoros papillipes that inhibited the ASFV protease involved in structural protein processing, which is essential for its viral assembly and replication [109,110,111]. The analogs of OPTX-1 isolated from hard ticks were much more potent inhibitors of this protease, which could explain why ASFV is not able to replicate or be transmitted by hard ticks [28,29,30]. In another study on O. turicata, a putative vector for ASFV, four defensin genes were identified with different expression patterns between organs and physiological stages [112]. Some of them were homologous to defensins without antiviral activity against ASFV as described in O. moubata, which is one of the main vectors for ASFV transmission [109]. Additional studies are needed in order to validate if this is a real mechanism of ASFV vector competence of Ornithodoros sp. (Figure 3)
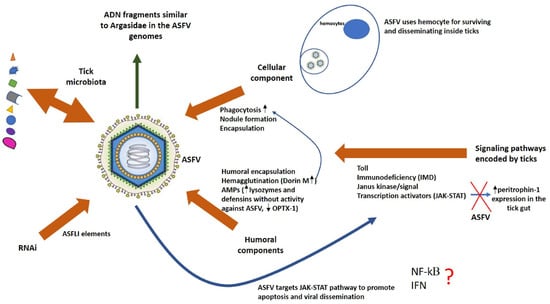
Figure 3.
Schematic representation of ASFV interactions with the innate immune system of competent vector ticks. Brown arrows represent different effectors of the tick innate immune system that could influence the ASFV infection outcome. The wider blue arrow represents the putative effects of ASFV on the signaling pathways that regulate the tick innate immune responses. The thin blue arrows represent the effects of some mediators of the tick immune response over others. The green arrow represents putative mechanisms developed by ASFV to evade and suppress immune responses in their hosts. The symbol ? means that the ASFV interference with NF-kB role in the tick immune response and also if tick IFN response is associated with viral resistance in arthropods are issues that remain to be elucidated.
A proteomic study on O. moubata tick cells without or with ASFV infection, conducted to understand better the role of tick proteins in the interaction with ASFV, found 788 that were differentially expressed [113]. Among these, three of the top five upregulated proteins (ficolin, serine protease precursor and lysozyme precursor) have been reported to contribute to tick innate immunity against pathogens, and the implication of endocytosis in ASFV infection was highlighted by the notable downregulation of nine proteins of this pathway [100]. However, further assessments of this valuable information must be carried in order to validate the role of these proteins in ASFV replication and transmission in this arthropod vector.
RNA interference (RNAi) has been also described as the main antiviral mechanism in ticks [114]. It has been mainly studied in hard ticks, and it is speculated that it could interfere directly with the viral infection or regulate the production of AMPs [115]. However, there are reports in which the RNAi pathway maintains arboviral infection and vector competence for transmission [116]. Genomic studies on O. moubata and O. porcinus soft ticks identified sequences over 20 kb coming from the ASFV genome, which were named African Swine Fever virus-like integrated (ASFLI) elements. These germline integration events could be explained by ASFV infection of reproductive tissues of both tick species. However, the nucleotide sequence of the mapped reads in each Ornithodoros tick species had a different identity percentage with respect to the sequences of the different ASFV genotypes [50,117]. In addition, transcriptomic studies identified small RNAs corresponding to the ASFLI elements identified in each tick species [50]. All data about experimental infection of Ornithodoros tick species by specific ASFV genotypes and the ASFLI element-specific small RNAs taken together suggest that ASFLI elements could be involved in an RNAi-based mechanism of defense against ASFV infection in Ornithodoros ticks, which, in conjunction with other components of the tick immune system, could determine which ASFV strain can infect a specific Ornithodoros tick species [50].
In parallel, the viruses have also developed mechanisms to evade and suppress immune responses in their hosts. A large number of ADN fragments similar to hosts including both Argasidae and suids were found in the ASFV genomes [118,119]. Notably, these fragments were found more frequently in non-coding regions of the viral genome containing promoter sequences, aiding immune evasion and adaptation to the host [118,120]. All viral sequences that resemble those of the host suggest that ASFV may disrupt host cell processes by mimicking their proteins in order to support viral survival, facilitating replication and transmission [121]. Studies comparing genomes of ASFV strains of genotypes I and II identified an exclusive gene named X64R in some genotype I strains that is speculated to be involved in the specificity of the tick–virus combinations [27].
In pigs, it has been demonstrated that ASFV targets cGAS-STING, NF-κB, TGF-β, ubiquitination and apoptosis signaling pathways for promoting viral replication [68,122]. ASFV MGF-encoded proteins suppress interferon signaling, NF-κB and JAK/STAT pathways in susceptible swine hosts [9,122]. Due to the fact that ASFV also adapted to replicate in some soft Ornithodoros ticks and this infection can persist over years, it is also probable that proteins encoded by the viral genome could enhance virus replication and evade the arthropod defense system [81]. There are examples in which arbovirus activated the host JAK-STAT pathway to promote apoptosis, benefiting viral infection and dissemination in the arthropod vector, when, in general, apoptosis is considered to be an efficient defense against viruses in mammals [123,124,125]. This JAK/STAT pathway was demonstrated to be functional in ticks and also associated with AMP expression; however, its signaling mechanism is unclear because the receptor triggering this pathway has not been identified yet [53]. Interestingly, it was reported that I. scapularis ticks use a vertebrate host-derived cytokine to stimulate their own JAK/STAT immune pathway [126], and the STAT knockdown in this tick species decreased peritrophin-1 expression, affecting gut epithelium integrity [55]. In addition, a kappa B (kB)-binding region has been identified in the promotor region of certain insect AMP genes, and it is known that ASFV can regulate the inflammatory response in pigs by regulating NF-kB [53,68].
Several studies have suggested that the transmembrane and glycosylated CD2v protein of ASFV, which is required for hemadsorption, also plays an important role in immune escape and viral pathogenesis by regulating the JAK2-STAT3 pathway and inhibiting apoptosis to facilitate virus replication in pig cells [127,128]. Remarkably, there are significant morphological and functional similarities between pig macrophages and tick phagocytic digestive cells. As mentioned previously, ASFV MGF360 and MGF530 genes suppress interferon (IFN) response genes in primary swine macrophage cultures [129]. Although IFN response had not been associated with viral resistance in arthropods, these ASFV genes may be involved—directly or indirectly—in the survival of infected tick cells by blocking an interferon-like signaling pathway (Figure 3).
3.4. Step 4: Transmission to the Host
Salivary ASFV excretion during feeding has been described as the main route for transmission of this virus from ticks to swine hosts [64,88]. In salivary secretions of Ornithodoros ticks, abundant ASFV particles have been detected. In the same way, crystal arrays of condensate ASFV particles have been observed in granule-forming cells. This evidence supports the fact that ASFV could be delivered at very high concentrations into the host during feeding [26,40]. However, a study showed that ASFV transmission was successful when 30 infected ticks simultaneously bit a pig, whereas transmission failed when multiple tick challenges were carried out using 15 infected ticks each time, which demonstrated that viral loads and the quantitative presence of bioactive salivary gland molecules could play an essential role in the competence of Ornithodoros ticks as ASFV vectors and are additional factors to take into consideration for explaining transmission success [27].
It is known that tick saliva is a pharmacopoeia of biologically active molecules locally regulating host processes such as vasodilation, wound healing, platelet aggregation, blood coagulation, innate immune responses, the complement system and acquired immune responses for facilitating ticks with obtaining a blood meal from their vertebrate hosts [130,131]. Obviously, these interactions at the tick–host interface could also facilitate pathogen infection and proliferation in the hosts; however, there is little evidence about direct interactions between tick-borne viruses with the molecular constituents of tick saliva [36]. Results of a study in which pigs were co-inoculated with ASFV and salivary gland extracts from O. porcinus ticks showed enhanced macrophage recruitment at the inoculation site compared to pigs inoculated with the virus alone, which could promote viral infection, taking into account that macrophages are the main target cells in swine hosts [132]. Additional studies about the effect of saliva containing ASFV on the modulation of the immune cells and the expression levels for cytokines in different host tissues are necessary in order to improve our understanding about how the active components of saliva can facilitate viral infection, bearing in mind our previous knowledge about ASFV pathogenesis in pigs [68].
In general, the basic components of tick saliva are water, ions, non-peptide molecules, tick peptides, tick proteins, host proteins and exosomes. Studies about the salivary transcriptome and proteomics of the soft Ornithodoros tick species have found lipocalins, Kunitz, cystatin, basic tail, hebraein, defensin, TIL domain, metalloprotease, 5′-nucleotidase/apyrase and phospholipase families of proteins and have also identified protein families uniquely found in the Argasidae family, such as the adrenomedullin/CGRP peptides, 7DB, 7 kDa and the RGD-containing single-Kunitz proteins. Additionally, three other unique protein families common only to the Ornithodoros genus were discovered [133,134]. Moreover, it is known that O. erraticus tick bites cause greater inflammation and injuries in host tissues than those caused by ticks from the O. moubata–O. porcinus complex [135]. The role of all these proteins and specially that of those exclusive ones remains to be elucidated, in line with the ASFV vector ability of Ornithodoros tick species. Some studies have also suggested an important role of tick heat shock proteins (HSPs) and organic anion-transporting polypeptides in arthropod blood feeding and in interactions with the pathogens [136]. The knockdown of HSP70 expression in a tick cell line resulted in increased Langat virus replication [137]. It could be an interesting issue to address in order to know if reduced expression of HSPs is also associated with enhanced ASFV replication in tick cells.
In summary, all knowledge about the biology of tick feeding and molecular interactions at the tick–host interface is very important for understanding the mechanisms involved in successful pathogen transmission and will eventually allow us to develop vaccines to block it. Notably, despite the functions of multiple molecules in tick saliva being identified and their contributions to tick feeding success and host immune evasion being described, currently, around 80% of identified saliva proteins do not have a known function, and fewer than 5% of those proteins that have been functionally annotated have the putative function verified [131]. It is also probable that tick saliva contains a number of unknown proteins that have not yet been identified. This scenario is even more complex because there are studies that have shown that tick saliva is dynamic, and its protein expression profile changes according to host responses and when harboring viruses [138].
4. Comparative Mechanisms: ASFV in Ticks vs. Swine
Knowledge about the molecular mechanisms of ASFV infection in ticks remains scarce and poorly understood. Although the ASFV strategies to successfully infect vertebrate cells are better known, their extrapolation cannot be fully assumed in its persistent infection of tick cells, and further investigation is necessary to elucidate specific interactions of this virus with the molecular mechanisms of tick defenses. The Table 1 shows a comparison between the ASFV mechanisms in tick vs. pig hosts

Table 1.
Mechanisms of ASFV in its tick vector versus its swine host.
5. Implications and Applications
Knowledge about the biology of Ornithodoros tick species that are competent for ASFV transmission and the molecular interactions at the tick–host interface during viral transmission is very important for developing disruptive strategies for its transmission cycle and blocking ASFV spread. Anti-tick vaccines targeting important components of tick saliva enhancing ASFV infection, crucial viral attachment molecules or transmission-blocking molecules could constitute interesting approaches to address these strategies and prevent this tick-borne disease [145].
In the same way, the combination of genomic, transcriptome/proteomic and metabolomic data can reveal genes and proteins from both the vector and the virus with important roles in persistent ASFV infection in ticks, which could also guide us in the design of attenuated ASFV strains as vaccine candidates to prevent lethal disease in pigs [146,147]. Functional genomic tools such as mutagenesis, RNAi and CRISPR could facilitate the identification and validation of these essential molecules in tick signaling pathways, as well as the use of modified ASFV strains to validate the specific factors identified as essential for its replication in tick vectors [148,149].
6. Conclusions and Future Perspectives
In summary, the road to fully understanding the underlying mechanisms involved in the vectoral capacity of Ornithodoros tick species for ASFV transmission is still long. Although in recent years a great amount of relevant knowledge about the tick–virus interface and the potential mechanisms implied in tick infection by ASFV and their transmission to vertebrate hosts has been gained, there is still limited understanding about the exact potential tick cell receptors for ASFV, the full repertoire of viral genes essential for tick infection, detailed tick immune responses to ASFV and mechanisms of transovarial transmission, among others. In this scenario, future research should be aimed at clarifying ASFV–tick interactions.
Author Contributions
A.R.-M.: Bibliography review, writing—original draft preparation, writing—review and editing and visualization; T.L.G.: Bibliography review and writing—original draft preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sánchez-Vizcaíno, J.M.; Laddomada, A.; Arias, M.L. African swine fever virus. In Diseases of Swine; Zimmerman, J.J., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 443–452. [Google Scholar]
- Montgomery, R.E. On a form of swine fever occurring in British East Africa (Kenya Colony). J. Comp. Pathol. Ther. 1921, 34, 159–191. [Google Scholar] [CrossRef]
- Malogolovkin, A.; Burmakina, G.; Titov, I.; Sereda, A.; Gogin, A.; Baryshnikova, E.; Kolbasov, D. Comparative analysis of African swine fever virus genotypes and serogroups. Emerg. Infect. Dis. 2015, 21, 312–315. [Google Scholar] [CrossRef]
- Lu, G.; Pan, J.; Zhang, G. African swine fever virus in Asia: Its rapid spread and potential threat to unaffected countries. J. Infect. 2020, 80, 350–371. [Google Scholar] [CrossRef]
- Shurson, G.C.; Palowski, A.; van de Ligt, J.L.; Schroeder, D.C.; Balestreri, C.; Urriola, P.E.; Sampedro, F. New perspectives for evaluating relative risks of African swine fever virus contamination in global feed ingredient supply chains. Transbound. Emerg. Dis. 2022, 69, 31–56. [Google Scholar] [CrossRef]
- Lee, K.; Vu, T.T.H.; Yeom, M.; Nguyen, V.D.; Than, T.T.; Nguyen, V.T.; Jeong, D.G.; Ambagala, A.; Le, V.P.; Song, D. Molecular characterization of emerging recombinant African swine fever virus of genotype I and II in Vietnam 2023. Emerg. Microbes Infect. 2024, 13, 2404156. [Google Scholar] [CrossRef]
- Igolkin, A.; Mazloum, A.; Zinyakov, N.; Chernyshev, R.; Schalkwyk, A.V.; Shotin, A.; Lavrentiev, I.; Gruzdev, K.; Chvala, I. Detection of the first recombinant African swine fever virus (genotypes I and II) in domestic pigs in Russia. Mol. Biol. Rep. 2024, 51, 1011. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Sun, E.; Huang, L.; Ding, L.; Zhu, Y.; Zhang, J.; Bu, Z.; Shen, D.; Zhang, X.; Zhang, Z.; et al. Highly lethal genotype I and II recombinant African swine fever viruses detected in pigs. Nat. Commun. 2023, 14, 3096. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.L.; Luo, R.; Lan, J.; Lu, Z.; Qiu, H.-J.; Wang, T.; Sun, Y. The multigene family genes-encoded proteins of African Swine Fever virus: Roles in evolution, cell tropism, immune evasion, and pathogenesis. Viruses 2025, 17, 865. [Google Scholar] [CrossRef]
- Rossi, G.; Leitch, E.C.M.; Graham, J.; Biccheri, R.; Iscaro, C.; Torresi, C.; Lycett, S.J.; Feliziani, F.; Giammarioli, M. A phylogenetic contribution to understanding the panzootic spread of African swine fever: From the global to the local scale. Virus Evol. 2025, veaf103. [Google Scholar] [CrossRef]
- Salas, M.L.; Andrés, G. African swine fever virus morphogenesis. Virus Res. 2013, 173, 29–41. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, D.; Wang, J.; Zhang, Y.; Wang, M.; Gao, Y.; Li, F.; Wang, J.; Bu, Z.; Rao, Z.; et al. Architecture of African swine fever virus and implications for viral assembly. Science 2019, 366, 640–644. [Google Scholar] [CrossRef]
- Andrés, G.; Charro, D.; Matamoros, T.; Dillard, R.S.; Abrescia, N.G. The cryo-EM structure of African swine fever virus unravels a unique architecture comprising two icosahedral protein capsids and two lipoprotein membranes. J. Biol. Chem. 2020, 295, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ruedas-Torres, I.; Thi to Nga, B.; Salguero, F.J. Pathogenicity and virulence of African swine fever virus. Virulence 2024, 15, 2375550. [Google Scholar] [CrossRef]
- Penrith, M.L.; Duarte Bastos, A.; Etter, E.M.C.; Beltrán-Alcrudo, D. Epidemiology of African swine fever in Africa today: Sylvatic cycle versus socio-economic imperatives. Transbound. Emerg. Dis. 2019, 66, 672–686. [Google Scholar] [CrossRef]
- Salguero, F.J. Comparative Pathology and Pathogenesis of African Swine Fever Infection in Swine. Front. Vet. Sci. 2020, 7, 282. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, N.N.; Madden, D.W.; Wilson, W.C.; Trujillo, J.D.; Richt, J.A. African swine fever virus: An emerging DNA arbovirus. Front. Vet. Sci. 2020, 7, 215. [Google Scholar]
- Sánchez-Vizcaíno, J.M.; Mur, L.; Martínez-López, B. African swine fever: An epidemiological update. Transbound. Emerg. Dis. 2012, 59, 27–35. [Google Scholar] [CrossRef]
- Guinat, C.; Gogin, A.; Blome, S.; Keil, G.; Pollin, R.; Pfeiffer, D.U.; Dixon, L. Transmission routes of African swine fever virus to domestic pigs: Current knowledge and future research directions. Vet. Rec. 2016, 178, 262–267. [Google Scholar] [CrossRef]
- Gabriel, C.; Blome, S.; Malogolovkin, A.; Parilov, S.; Kolbasov, D.; Teifke, J.P.; Beer, M. Characterization of African swine fever virus Caucasus isolate in European wild boars. Emerg. Infect. Dis. 2011, 17, 2342. [Google Scholar] [CrossRef] [PubMed]
- Pietschmann, J.; Guinat, C.; Beer, M.; Pronin, V.; Tauscher, K.; Petrov, A.; Keil, G.; Blome, S. Course and transmission characteristics of oral low-dose infection of domestic pigs and European wild boar with a Caucasian African swine fever virus isolate. Arch. Virol. 2015, 160, 1657–1667. [Google Scholar] [CrossRef]
- Chenais, E.; Depner, K.; Guberti, V.; Dietze, K.; Viltrop, A.; Ståhl, K. Epidemiological considerations on African swine fever in Europe 2014–2018. Porcine Health Manag. 2019, 5, 6. [Google Scholar]
- Sanchez-Botija, C. African swine fever. New developments. Rev. Sci. Tech. 1982, 4, 1065–1094. [Google Scholar]
- Plowright, W.; Perry, C.T.; Peirce, M.A. Transovarial infection with African swine fever virus in the argasid tick, Ornithodoros moubata porcinus, Walton. Res. Vet. Sci. 1970, 11, 582–584. [Google Scholar] [CrossRef]
- Lv, T.; Xie, X.; Song, N.; Zhang, S.; Ding, Y.; Liu, K.; Diao, L.; Chen, X.; Jiang, S.; Li, T.; et al. Expounding the role of tick in Africa swine fever virus transmission and seeking effective prevention measures: A review. Front. Immunol. 2022, 13, 1093599. [Google Scholar] [CrossRef]
- Burrage, T.G. African swine fever virus infection in Ornithodoros ticks. Virus Res. 2013, 173, 131–139. [Google Scholar] [CrossRef]
- Pereira De Oliveira, R.; Hutet, E.; Lancelot, R.; Paboeuf, F.; Duhayon, M.; Boinas, F.; Pérez de León, A.A.; Filatov, S.; Le Potier, M.-F.; Vial, L. Differential vector competence of Ornithodoros soft ticks for African swine fever virus: What if it involves more than just crossing organic barriers in ticks? Parasit. Vectors 2020, 13, 618. [Google Scholar] [CrossRef]
- Bonnet, S.I.; Bouhsira, E.; De Regge, N.; Fite, J.; Etoré, F.; Garigliany, M.M.; Jori, F.; Lempereur, L.; Le Potier, M.F.; Quillery, E.; et al. Putative role of arthropod vectors in African Swine Fever virus transmission in relation to their bio-ecological properties. Viruses 2020, 12, 778. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); Boklund, A.E.; Ståhl, K.; Miranda Chueca, M.Á.; Podgórski, T.; Vergne, T.; Cortiñas Abrahantes, J.; Cattaneo, E.; Dhollander, S.; Papanikolaou, A.; et al. Risk and protective factors for ASF in domestic pigs and wild boar in the EU, and mitigation measures for managing the disease in wild boar. EFSA J. 2024, 22, e9095. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Ferreira, H.C.; Tudela Zúquete, S.; Wijnveld, M.; Weesendorp, E.; Jongejan, F.; Stegeman, A.; Loeffen, W.L. No evidence of African swine fever virus replication in hard ticks. Ticks Tick. Borne Dis. 2014, 5, 582–589. [Google Scholar] [CrossRef]
- Hess, W.R.; Endris, R.G.; Haslett, T.M.; Monahan, M.J.; McCoy, J.P. Potential arthropod vectors of African swine fever virus in North America and the Caribbean basin. Vet. Parasitol. 1987, 26, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Hong, S.K.; Lee, I.; Choi, D.S.; Lee, J.H.; Lee, E.; Wee, S.H. Arthropods as potential vectors of African swine fever virus outbreaks in pig farms in the Republic of Korea. Vet. Med. Sci. 2021, 7, 1841–1844. [Google Scholar] [PubMed]
- Herm, R.; Kirik, H.; Vilem, A.; Zani, L.; Forth, J.H.; Müller, A.; Michelitsch, A.; Wernike, K.; Werner, D.; Tummeleht, L.; et al. No evidence for African swine fever virus DNA in haematophagous arthropods collected at wild boar baiting sites in Estonia. Transbound. Emerg. Dis. 2021, 68, 2696–2702. [Google Scholar] [CrossRef] [PubMed]
- Gotschall, T. EndNote 20 desktop version. J. Med. Libr. Assoc. 2021, 109, 520. [Google Scholar] [CrossRef]
- Viglietta, M.; Bellone, R.; Blisnick, A.A.; Failloux, A.B. Vector Specificity of Arbovirus Transmission. Front. Microbiol. 2021, 12, 773211. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, P.A. Molecular characterization of tick-virus interactions. Front. Biosci. 2009, 14, 2466–2483. [Google Scholar] [CrossRef]
- Penrith, M.L.; Vosloo, W.; Jori, F.; Bastos, A.D. African swine fever virus eradication in Africa. Virus Res. 2013, 173, 228–246. [Google Scholar] [CrossRef]
- Dixon, L.K.; Stahl, K.; Jori, F.; Vial, L.; Pfeiffer, D.U. African swine fever epidemiology and control. Annu. Rev. Anim. Biosci. 2020, 8, 221–246. [Google Scholar] [CrossRef]
- Sonenshine, D.E. Ecology of nidicolous ticks. In Biology of Ticks; N.Y. Oxford University Press: New York, NY, USA, 1993; p. 465. [Google Scholar]
- Kleiboeker, S.B.; Burrage, T.G.; Scoles, G.A.; Fish, D.; Rock, D.L. African swine fever virus infection in the argasid host, Ornithodoros porcinus porcinus. J. Virol. 1998, 72, 1711–1724. [Google Scholar] [CrossRef]
- Ravaomanana, J.; Michaud, V.; Jori, F.; Andriatsimahavandy, A.; Roger, F.; Albina, E.; Vial, L. First detection of African Swine Fever Virus in Ornithodoros porcinus in Madagascar and new insights into tick distribution and taxonomy. Parasit. Vectors 2010, 3, 115. [Google Scholar]
- Boinas, F.S.; Wilson, A.J.; Hutchings, G.H.; Martins, C.; Dixon, L.J. The persistence of African swine fever virus in field-infected Ornithodoros erraticus during the ASF endemic period in Portugal. PLoS ONE 2011, 6, e20383. [Google Scholar] [CrossRef]
- Vial, L. Biological and ecological characteristics of soft ticks (Ixodida: Argasidae) and their impact for predicting tick and associated disease distribution. Parasite 2009, 16, 191–202. [Google Scholar] [CrossRef]
- Bernard, J.; Vial, L.; Madeira, S.; Otte, J.; Boinas, F.; Le Potier, M.F.; Jourdan-Pineau, H. Experimental design impacts the vector competence of Ornithodoros ticks for African swine fever virus: A meta-analysis of published evaluations. Peer Community J. 2025, 5, e58. [Google Scholar] [CrossRef]
- Hess, W.R.; Endris, R.G.; Lousa, A.; Caiado, J.M. Clearance of African swine fever virus from infected tick (Acari) colonies. J. Med. Entomol. 1989, 26, 314–317. [Google Scholar] [CrossRef]
- Endris, R.G.; Hess, W.R. Attempted transovarial and venereal transmission of African swine fever virus by the Iberian soft tick Ornithodoros (Pavlovskyella) macrocanus (Acari: Ixodoidea: Argasidae). J. Med. Entomol. 1994, 31, 373–381. [Google Scholar]
- Groocock, C.M.; Hess, W.R.; Gladney, W.J. Experimental transmission of African Swine Fever virus by Omithodoros coriaceus an Argasid tick indigenous to the United States. Am. J. Vet. Res. 1980, 41, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Rennie, L.; Wilkinson, P.J.; Mellor, P.S.; Mur, L.; Boadella, M.; Martínez-López, B.; Gallardo, C.; Gortazar, C.; Sánchez-Vizcaíno, J.M.; Kayaga, E.B.; et al. Effects of infection of the tick Ornithodoros moubata with African swine fever virus. Med. Vet. Entomol. 2000, 14, 355–360. [Google Scholar]
- Quembo, C.J.; Jori, F.; Vosloo, W.; Heath, L. Genetic characterization of African swine fever virus isolates from soft ticks at the wildlife/domestic interface in Mozambique and identification of a novel genotype. Transbound. Emerg. Dis. 2018, 65, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Forth, J.H.; Forth, L.F.; Lycett, S.; Bell-Sakyi, L.; Keil, G.M.; Blome, S.; Calvignac-Spencer, S.; Wissgott, A.; Krause, J.; Höper, D.; et al. Identification of African swine fever virus-like elements in the soft tick genome provides insights into the virus evolution. BMC Biol. 2020, 18, 136. [Google Scholar] [CrossRef] [PubMed]
- Jori, F.; Bastos, A.; Boinas, F.; Van Heerden, J.; Heath, L.; Jourdan-Pineau, H.; Martinez-Lopez, B.; Pereira de Oliveira, R.; Pollet, T.; Quembo, C.; et al. An Updated Review of Ornithodoros Ticks as Reservoirs of African Swine Fever in Sub-Saharan Africa and Madagascar. Pathogens 2023, 12, 469. [Google Scholar] [CrossRef]
- Wang, J.; Gao, L.; Aksoy, S. Microbiota in disease-transmitting vectors. Nat. Rev. Microbiol. 2023, 21, 604–618. [Google Scholar] [CrossRef]
- Fogaça, A.C.; Sousa, G.; Pavanelo, D.B.; Esteves, E.; Martins, L.A.; Urbanová, V.; Kopáček, P.; Daffre, S. Tick immune system: What is known, the interconnections, the gaps, and the challenges. Front. Immunol. 2021, 12, 628054. [Google Scholar] [CrossRef]
- Kitsou, C.; Foor, S.D.; Dutta, S.; Bista, S.; Pal, U. Tick gut barriers impacting tick–microbe interactions and pathogen persistence. Mol. Microbiol. 2021, 116, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, S.; Rajeevan, N.; Liu, L.; Zhao, Y.O.; Heisig, J.; Pan, J.; Eppler-Epstein, R.; DePonte, K.; Fish, D.; Fikrig, E. Gut microbiota of the tick vector Ixodes scapularis modulate colonization of the Lyme disease spirochete. Cell Host Microbe 2014, 15, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Pavanelo, D.B.; Piloto-Sardiñas, E.; Maitre, A.; Abuin-Denis, L.; Kopáček, P.; Cabezas-Cruz, A.; Fogaça, A.C. Arthropod microbiota: Shaping pathogen establishment and enabling control. Front. Arachn. Sci. 2023, 2, 1297733. [Google Scholar] [CrossRef]
- Piloto-Sardiñas, E.; Cano-Argüelles, A.L.; Maitre, A.; Wu-Chuang, A.; Mateos-Hernández, L.; Corduneanu, A.; Obregón, D.; Oleaga, A.; Pérez-Sánchez, R.; Cabezas-Cruz, A. Comparison of salivary gland and midgut microbiome in the soft ticks Ornithodoros erraticus and Ornithodoros moubata. Front. Microbiol. 2023, 14, 1173609. [Google Scholar] [CrossRef]
- Zhang, J.; Rodríguez, F.; Navas, M.J.; Costa-Hurtado, M.; Almagro, V.; Bosch-Camós, L.; López, E.; Cuadrado, R.; Accensi, F.; Pina-Pedrero, S.; et al. Fecal microbiota transplantation from warthog to pig confirms the influence of the gut microbiota on African swine fever susceptibility. Sci. Rep. 2020, 10, 17605. [Google Scholar] [CrossRef]
- Martín, R.; Miquel, S.; Benevides, L.; Bridonneau, C.; Robert, V.; Hudault, S.; Chain, F.; Berteau, O.; Azevedo, V.; Chatel, J.M. Functional characterization of novel Faecalibacterium prausnitzii strains isolated from healthy volunteers: A step forward in the use of F. prausnitzii as a next-generation probiotic. Front. Microbiol. 2017, 8, 1226. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; Deroos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Rolhion, N.; Chassaing, B. When pathogenic bacteria meet the intestinal microbiota. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150504. [Google Scholar] [CrossRef]
- Ubeda, C.; Bucci, V.; Caballero, S.; Djukovic, A.; Toussaint, N.C.; Equinda, M.; Lipuma, L.; Ling, L.; Gobourne, A.; No, D. Intestinal microbiota containing Barnesiella species cures vancomycin-resistant Enterococcus faecium colonization. Infect. Immun. 2013, 81, 965–973. [Google Scholar] [CrossRef]
- Greig, A. The localization of African swine fever virus in the tick Ornithodoros moubata porcinus. Arch. Gesamte Virusforsch. 1972, 39, 240–247. [Google Scholar] [CrossRef]
- Kleiboeker, S.B.; Scoles, G.A.; Burrage, T.G.; Sur, J. African swine fever virus replication in the midgut epithelium is required for infection of Ornithodoros ticks. J. Virol. 1999, 73, 8587–8598. [Google Scholar] [CrossRef]
- Dixon, L.; Islam, M.; Nash, R.; Reis, A. African swine fever virus evasion of host defenses. Virus Res. 2019, 266, 25–33. [Google Scholar] [CrossRef]
- Burrage, T.G.; Lu, Z.; Neilan, J.G.; Rock, D.L.; Zsak, L. African swine fever virus multigene family 360 genes affect virus replication and generalization of infection in Ornithodoros porcinus ticks. J. Virol. 2004, 78, 2445–2453. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, W.; Yang, W.; Zhang, J.; Li, D.; Zheng, H. Structure of African Swine Fever Virus and associated molecular mechanisms underlying infection and immunosuppression: A Review. Front. Immunol. 2021, 12, 715582. [Google Scholar] [CrossRef]
- Jia, N.; Ou, Y.; Pejsak, Z.; Zhang, Y.; Zhang, J. Roles of African Swine Fever Virus Structural Proteins in Viral Infection. J. Vet. Res. 2017, 61, 135–143. [Google Scholar] [CrossRef]
- Dixon, L.K.; Chapman, D.A.; Netherton, C.L.; Upton, C.; Hakobyan, S.; Bayramyan, N.; Karalyan, Z.; Izmailyan, R.; Avetisyan, A.; Poghosyan, A.; et al. African swine fever virus replication and genomics. Virus Res. 2013, 173, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qi, C.; Ge, S.; Li, J.; Hu, Y.; Qian, Y. Genetic variation and evolution of attenuated African swine fever virus strain isolated in the field: A review. Virus Res. 2022, 319, 198874. [Google Scholar]
- Wang, T.; Wang, L.; Han, Y.; Pan, L.; Yang, J.; Sun, M.; Zhou, P.; Sun, Y.; Bi, Y.; Qiu, H.J.; et al. Adaptation of African swine fever virus to HEK293T cells. Transbound. Emerg. Dis. 2021, 68, 2853–2866. [Google Scholar] [CrossRef] [PubMed]
- Thaweerattanasinp, T.; Kaewborisuth, C.; Viriyakitkosol, R.; Saenboonrueng, J.; Wanitchang, A.; Tanwattana, N.; Sonthirod, C.; Sangsrakru, D.; Pootakham, W.; Tangphatsornruang, S.; et al. Adaptation of African swine fever virus to MA-104 cells: Implications of unique genetic variations. Vet. Microbiol. 2024, 291, 110016. [Google Scholar] [CrossRef]
- Borca, M.V.; Rai, A.; Ramirez-Medina, E.; Silva, E.; Velazquez-Salinas, L.; Vuono, E.; Pruitt, S.; Espinoza, N.; Gladue, D.P. A cell culture-adapted vaccine virus against the current African swine fever virus pandemic strain. J. Virol. 2021, 95, e0012321. [Google Scholar] [CrossRef] [PubMed]
- Farlow, J.; Donduashvili, M.; Kokhreidze, M.; Kotorashvili, A.; Vepkhvadze, N.G.; Kotaria, N.; Gulbani, A. Intra-epidemic genome variation in highly pathogenic African swine fever virus from the country of Georgia. Virol. J. 2018, 15, 190. [Google Scholar] [CrossRef]
- Mazur-Panasiuk, N.; Walczak, M.; Juszkiewicz, M.; Wozniakowski, G. The spillover of African swine fever in western Poland revealed its estimated origin on the basis of O174L, K145R, MGF 505-5R and IGR I73R/I329L genomic sequences. Viruses 2020, 12, 1094. [Google Scholar] [CrossRef]
- Mazloum, A.; van Schalkwyk, A.; Shotin, A.; Igolkin, A.; Shevchenko, I.; Gruzdev, K.N.; Vlasova, N. Comparative analysis of full genome sequences of African swine fever virus isolates taken from wild boars in Russia in 2019. Pathogens 2021, 10, 521. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Zhu, Z.; Wang, S.; Tu, S.; Zhang, Y.; Zou, Y.; Liu, Y.; Liu, C.; Ren, W.; et al. Tracing the Origin of Genotype II African Swine Fever Virus in China by Genomic Epidemiology Analysis. Transbound. Emerg. Dis. 2023, 2023, 4820809. [Google Scholar] [CrossRef]
- Neilan, J.G.; Zsak, L.; Lu, Z.; Kutish, G.F.; Afonso, C.L.; Rock, D.L. Novel swine virulence determinant in the left variable region of the African swine fever virus genome. J. Virol. 2002, 76, 3095–3104. [Google Scholar] [CrossRef] [PubMed]
- Zsak, L.; Sur, J.H.; Burrage, T.G.; Neilan, J.G.; Rock, D.L. African Swine Fever Virus (Asfv) Multigene families 360 and 530 genes promote infected macrophage survival. Sci. World J. 2001, 1, 97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rowlands, R.J.; Duarte, M.M.; Boinas, F.; Hutchings, G.; Dixon, L.K.; Liu, W.; Yang, L.; Di, C.; Sun, J.; Liu, P.; et al. The CD2v protein enhances African swine fever virus replication in the tick vector, Ornithodoros erraticus. Virology 2009, 393, 319–328. [Google Scholar] [CrossRef]
- Rodriguez, F.; Ley, V.; Gómez-Puertas, P.; García, R.; Rodriguez, J.F.; Escribano, J.M.; Suh, T.Y.; Park, J.H.; Park, C.R.; Kim, J.E.; et al. The structural protein p54 is essential for African swine fever virus viability. Virus Res. 1996, 40, 161–167. [Google Scholar] [CrossRef]
- Sánchez, E.G.; Quintas, A.; Nogal, M.; Castelló, A.; Revilla, Y.; Zhu, G.; Ren, J.; Li, D.; Ru, Y.; Qin, X.; et al. African swine fever virus controls the host transcription and cellular machinery of protein synthesis. Virus Res. 2013, 173, 58–75. [Google Scholar] [CrossRef]
- Sonenshine, D.E. Biology of Ticks; Oxford University Press: New York, NY, USA, 1993; p. 465. [Google Scholar]
- Diaz, A.V.; Netherton, C.L.; Dixon, L.K.; Wilson, A.J. African swine fever virus strain Georgia 2007/1 in Ornithodoros erraticus ticks. Emerg. Infect Dis. 2012, 18, 1026–1028. [Google Scholar] [CrossRef]
- Labuda, M.; Nuttall, P.A. Tick-borne viruses. Parasitology 2004, 129, S221–S245. [Google Scholar] [CrossRef] [PubMed]
- Endris, R.G.; Hess, W.R. Experimental transmission of African swine fever virus by the soft tick Ornithodoros (Pavlovskyella) marocanus (Acari: Ixodoidea: Argasidae). J. Med. Entomol. 1992, 29, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Plowright, W.; Perry, C.T.; Peirce, M.A.; Parker, J. Experimental infection of the argasid tick, Ornithodoros moubata porcinus, with African swine fever virus. Arch. Gesamte Virusforsch. 1970, 31, 33–50. [Google Scholar] [CrossRef]
- Heath, C.M.; Windsor, M.; Wileman, T. Aggresomes resemble sites specialized for virus assembly. J. Cell Biol. 2001, 153, 449–455. [Google Scholar] [CrossRef]
- Cackett, G.; Sýkora, M.; Werner, F. Transcriptome view of a killer: African swine fever virus. Biochem. Soc. Trans. 2020, 48, 1569–1581. [Google Scholar] [CrossRef]
- Suarez, C.; Andres, G.; Kolovou, A.; Hoppe, S.; Salas, M.L.; Walther, P.; Krijnse Locker, J. A frican swine fever virus assembles a single membrane derived from rupture of the endoplasmic reticulum. Cell. Microbiol. 2015, 17, 1683–1698. [Google Scholar] [PubMed]
- Suárez, C.; Gutiérrez-Berzal, J.; Andrés, G.; Salas, M.L.; Rodríguez, J.M. African swine fever virus protein p17 is essential for the progression of viral membrane precursors toward icosahedral intermediates. J. Virol. 2010, 84, 7484–7499. [Google Scholar] [CrossRef]
- Epifano, C.; Krijnse-Locker, J.; Salas, M.L.; Rodríguez, J.M.; Salas, J. The African swine fever virus nonstructural protein pB602L is required for formation of the icosahedral capsid of the virus particle. J. Virol. 2006, 80, 12260–12270. [Google Scholar] [CrossRef]
- Andrés, G.; García-Escudero, R.; Viñuela, E.; Salas, M.L.; Rodrίguez, J.M. African swine fever virus structural protein pE120R is essential for virus transport from assembly sites to plasma membrane but not for infectivity. J. Virol. 2001, 75, 6758–6768. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.K.; Tate, A.T.; Schneider, D.S.; Levashina, E.A.; Kagan, J.C.; Pal, U.; Fikrig, E.; Pedra, J.H. Vector immunity and evolutionary ecology: The harmonious dissonance. Trends Immunol. 2018, 39, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, A. Understanding the tick cellular immunity to pathogen infection. In Biological, Environmental, and Earth Sciences; University of Southern Mississippi: Hattiesburg, MS, USA, 2024. [Google Scholar]
- Taylor, D. Innate Immunity in Ticks: A review. J. Acarol. Soc. Jpn. 2006, 15, 109–127. [Google Scholar] [CrossRef][Green Version]
- Sonenshine, D.E.; Macaluso, K.R. Microbial invasion vs. tick immune regulation. Front. Cell. Infect. Microbiol. 2017, 7, 390. [Google Scholar] [CrossRef]
- Kadota, K.; Satoh, E.; Ochiai, M.; Inoue, N.; Tsuji, N.; Igarashi, I.; Nagasawa, H.; Mikami, T.; Claveria, F.G.; Fujisaki, K. Existence of phenol oxidase in the argasid tick Ornithodoros moubata. Parasitol. Res. 2002, 88, 781–784. [Google Scholar] [CrossRef]
- Rego, R.O.; Hajdušek, O.; Kovář, V.; Kopáček, P.; Grubhoffer, L.; Hypša, V. Molecular cloning and comparative analysis of fibrinogen-related proteins from the soft tick Ornithodoros moubata and the hard tick Ixodes ricinus. Insect Biochem. Mol. Biol. 2005, 35, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Kovář, V.; Kopáček, P.; Grubhoffer, L. Isolation and characterization of Dorin M, a lectin from plasma of the soft tick Ornithodoros moubata. Insect Biochem. Mol. Biol. 2000, 30, 195–205. [Google Scholar] [CrossRef]
- Kopáček, P.; Vogt, R.; Jindrák, L.; Weise, C.; Šafařík, I. Purification and characterization of the lysozyme from the gut of the soft tick Ornithodoros moubata. Insect Biochem. Mol. Biol. 1999, 29, 989–997. [Google Scholar] [CrossRef]
- Grunclová, L.; Fouquier, H.; Hypša, V.; Kopáček, P. Lysozyme from the gut of the soft tick Ornithodoros moubata: The sequence, phylogeny and post-feeding regulation. Dev. Comp. Immunol. 2003, 27, 651–660. [Google Scholar] [CrossRef]
- Kopáček, P.; Hajdušek, O.; Burešová, V.; Daffre, S. Tick innate immunity. Invertebrate Immunity. In Invertebrate Immunity; Advances in Experimental Medicine and Biology; Söderhäll, K., Ed.; Springer: Boston, MA, USA, 2010; pp. 137–162. [Google Scholar]
- Zhou, J.; Ueda, M.; Umemiya, R.; Battsetseg, B.; Boldbaatar, D.; Xuan, X.; Fujisaki, K. A secreted cystatin from the tick Haemaphysalis longicornis and its distinct expression patterns in relation to innate immunity. Insect Biochem. Mol. Biol. 2006, 36, 527–535. [Google Scholar] [CrossRef]
- Lima, C.A.; Sasaki, S.D.; Tanaka, A.S. Bmcystatin, a cysteine proteinase inhibitor characterized from the tick Boophilus microplus. Biochem. Biophys. Res. Commun. 2006, 347, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Van Der Goes, V.N.Y. Purification and partial amino acid sequence of antibacterial peptides from the hemolymph of the soft tick, Ornithodoros moubata (Acari: Argasidae). In Proceedings of the 3rd International Conference on Ticks and Tick-borne Pathogens: Into the 21st Century, Bratislava, Slovakia, 2000; Institute of Zoology, Slovak Academy of Sciences: Bratislava, Slovakia, 2010. [Google Scholar]
- Nakajima, Y.; Van Der Goes van Naters-Yasui, A.; Taylor, D.; Yamakawa, M. Antibacterial peptide defensin is involved in midgut immunity of the soft tick, Ornithodoros moubata. Insect Mol. Biol. 2002, 11, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ji, M.; Yuan, B.; Luo, A.; Jiang, Z.; Zhu, T.; Liu, Y.; Kamau, P.M.; Jin, L.; Lai, R. Peptide OPTX-1 From Ornithodoros papillipes tick inhibits the pS273R protease of African Swine Fever Virus. Front. Microbiol. 2021, 12, 778309. [Google Scholar] [CrossRef]
- Li, G.; Liu, X.; Yang, M.; Zhang, G.; Wang, Z.; Guo, K.; Gao, Y.; Jiao, P.; Sun, J.; Chen, C. Crystal structure of African swine fever virus pS273R protease and implications for inhibitor design. J. Virol. 2020, 94, e02125-19. [Google Scholar] [CrossRef] [PubMed]
- Alejo, A.; Andrés, G.; Salas, M.L. African swine fever virus proteinase is essential for core maturation and infectivity. J. Virol. 2003, 77, 5571–5577. [Google Scholar] [CrossRef]
- Armstrong, B.A.; Kneubehl, A.R.; Mitchell, R.D., III; Krishnavajhala, A.; Teel, P.D.; Pérez de León, A.A.; Lopez, J.E. Differential expression of putative Ornithodoros turicata defensins mediated by tick feeding. Front. Cell. Infect. Microbiol. 2020, 10, 152. [Google Scholar]
- Afayibo, D.J.A.; Yang, J.; Hao, R.; Zhang, Z.; Sun, H.; Luo, J.; Ren, Q.; Tadele, B.A.; Guan, G.; Niu, Q. Protein profile and protein interaction network analysis of Ornithodoros moubata during African swine fever virus infection. Res. Sq. 2025, preprint. [Google Scholar] [CrossRef]
- Kazimírová, M.; Thangamani, S.; Bartíková, P.; Hermance, M.; Holíková, V.; Štibrániová, I.; Nuttall, P.A. Tick-borne viruses and biological processes at the tick-host-virus interface. Front. Cell. Infect. Microbiol. 2017, 7, 339. [Google Scholar] [CrossRef]
- Talactac, M.R.; Hernandez, E.P.; Hatta, T.; Yoshii, K.; Kusakisako, K.; Tsuji, N.; Tanaka, T. The antiviral immunity of ticks against transmitted viral pathogens. Dev. Comp. Immunol. 2021, 119, 104012. [Google Scholar] [CrossRef]
- Lan, H.H.; Chen, H.Y.; Liu, Y.Y.; Jiang, C.Y.; Mao, Q.Z.; Jia, D.S.; Chen, Q.; Wei, T. Small interfering RNA pathway modulates initial viral infection in midgut epithelium of insect after ingestion of virus. J. Virol. 2015, 90, 917–929. [Google Scholar] [CrossRef]
- Forth, J.H.; Forth, L.F.; Blome, S.; Höper, D.; Beer, M. African swine fever whole-genome sequencing-Quantity wanted but quality needed. PLoS Pathog. 2020, 16, e1008779. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, N.; Sun, Q.; Long, X.; Wang, T.; Qiu, H.J. Host-similar fragments in the African swine fever virus genome: Distribution, functions, and evolution. Vet. Res. 2025, 56, 108. [Google Scholar] [CrossRef]
- Wang, Y.; Chi, C.; Zhang, J.; Zhang, K.; Deng, D.; Zheng, W.; Chen, N.; Meurens, F.; Zhu, J. Systematic analysis of the codon usage patterns of African swine fever virus genome coding sequences reveals its host adaptation phenotype. Microb. Genom. 2024, 10, 001186. [Google Scholar] [CrossRef] [PubMed]
- Bohn-Wippert, K.; Tevonian, E.N.; Megaridis, M.R.; Dar, R.D. Similarity in viral and host promoters couple viral reactivation with host cell migration. Nat. Commun. 2017, 8, 15006. [Google Scholar] [CrossRef][Green Version]
- Kaján, G.L.; Doszpoly, A.; Tarján, Z.L.; Vidovszky, M.Z.; Papp, T. Virus-host coevolution with a focus on animal and human DNA viruses. J. Mol. Evol. 2020, 88, 41–56. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, X.X.; Jiang, S.W.; Gao, X.T.; Huang, S.Y.; Liang, Y.; Jia, H.; Zhu, H.F.; Han, S.; Oh, D.; et al. MGF360-12L of ASFV-SY18 is an immune-evasion protein that inhibits host type I IFN, NF-κB, and JAK/STAT pathways. Pol. J. Vet. Sci. 2023, 26, 119–130. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.X.; Mao, Q.Z.; Zhuo, J.C.; Huang, H.J.; Lu, J.B.; Zhang, C.X.; Li, J.M.; Chen, J.P.; Lu, G. The JAK-STAT pathway promotes persistent viral infection by activating apoptosis in insect vectors. PLoS Pathog. 2023, 19, e1011266. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, K.; Olson, B.J.; Huang, N.; Unis, D.; Clem, R.J. Rapid selection against arbovirus-induced apoptosis during infection of a mosquito vector. Proc. Nat. Acad. Sci. USA 2015, 112, E1152–61. [Google Scholar] [CrossRef]
- Clarke, P.; Tyler, K.L. Apoptosis in animal models of virus-induced disease. Nat. Rev. Microbiol. 2009, 7, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.A.; Navasa, N.; Yang, X.; Wilder, C.N.; Buyuktanir, O.; Marques, A.; Anguita, J.; Pal, U. Cross-species interferon signaling boosts microbicidal activity within the tick vector. Cell Host Microbe 2016, 20, 91–98. [Google Scholar] [CrossRef]
- Gao, Q.; Yang, Y.; Luo, Y.; Chen, X.; Gong, T.; Wu, D.; Feng, Y.; Zheng, X.; Wang, H.; Zhang, G.; et al. African Swine Fever virus envelope glycoprotein CD2v interacts with host CSF2RA to regulate the JAK2-STAT3 pathway and inhibit apoptosis to facilitate virus replication. J. Virol. 2023, 97, e0188922. [Google Scholar] [CrossRef]
- Borca, M.V.; Carrillo, C.; Zsak, L.; Laegreid, W.W.; Kutish, G.F.; Neilan, J.G.; Burrage, T.G.; Rock, D.L. Deletion of a CD2-like gene, 8-DR, from African swine fever virus affects viral infection in domestic swine. J. Virol. 1998, 72, 2881–2889. [Google Scholar] [CrossRef]
- Zsak, L.; Lu, Z.; Burrage, T.G.; Neilan, J.G.; Kutish, G.F.; Moore, D.M.; Rock, D.L. African swine fever virus multigene family 360 and 530 genes are novel macrophage host range determinants. J. Virol. 2001, 75, 3066–3076. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, P.A. Wonders of tick saliva. Ticks Tick. Borne Dis. 2019, 10, 470–481. [Google Scholar] [CrossRef]
- Simo, L.; Kazimirova, M.; Richardson, J.; Bonnet, S.I. The essential role of tick salivary glands and saliva in tick feeding and pathogen transmission. Front. Cell Infect. Microbiol. 2017, 7, 281. [Google Scholar] [CrossRef]
- Bernard, J.; Hutet, E.; Paboeuf, F.; Randriamparany, T.; Holzmuller, P.; Lancelot, R.; Rodrigues, V.; Vial, L.; Le Potier, M.F. Effect of O. porcinus tick salivary gland extract on the African swine fever virus infection in domestic pig. PLoS ONE 2016, 11, e0147869. [Google Scholar] [CrossRef] [PubMed]
- Oleaga, A.; Carnero-Morán, A.; Valero, M.L.; Pérez-Sánchez, R. Proteomics informed by transcriptomics for a qualitative and quantitative analysis of the sialoproteome of adult Ornithodoros moubata ticks. Parasit. Vectors 2021, 14, 396. [Google Scholar] [CrossRef]
- Pérez-Sánchez, R.; Carnero-Morán, Á.; Soriano, B.; Llorens, C.; Oleaga, A. RNA-seq analysis and gene expression dynamics in the salivary glands of the argasid tick Ornithodoros erraticus along the trophogonic cycle. Parasit. Vectors 2021, 14, 170. [Google Scholar] [CrossRef]
- Riek, R.F.; Lavoipierre, M.M.J. Reaction of the skin of laboratory animals to the bites of argasid ticks. R. Soc. Trop. Med. 1954, 8–9. [Google Scholar]
- Bernardo, J.M.G.; Serdeña, A.P.R.; Pangga, G.M.V.; Salamat, S.E.A.; Agulto, T.N.; Fernandez-Colorado, C.P. Risk scoring of African swine fever transmission in selected provinces of the Philippines. J. Vet. Sci. 2025, 26, e2. [Google Scholar] [CrossRef]
- Weisheit, S.; Villar, M.; Tykalova, H.; Popara, M.; Loecherbach, J.; Watson, M.; Růžek, D.; Grubhoffer, L.; de la Fuente, J.; Fazakerley, J.K.; et al. Ixodes Scapularis and Ixodes Ricinus tick cell lines respond to infection with tick-borne encephalitis virus: Transcriptomic and proteomic analysis. Parasites Vectors 2015, 8, 599–26. [Google Scholar] [CrossRef]
- Maqbool, M.; Sajid, M.S.; Saqib, M.; Anjum, F.R.; Tayyab, M.H.; Rizwan, H.M.; Rashid, M.I.; Rashid, I.; Iqbal, A.; Siddique, R.M.; et al. Potential mechanisms of transmission of tick-borne viruses at the virus-tick interface. Front. Microbiol. 2022, 13, 846884. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, X.; Cao, Y.; Li, S.; Shao, J.; Sun, S.; Guo, H.; Yin, S. Identification of a new cell-penetrating peptide derived from the african swine fever virus CD2v protein. Drug Deliv. 2021, 28, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Miao, C.; Liu, W.; Zhang, G.; Shao, J.; Chang, H. Structure and function of African swine fever virus proteins: Current understanding. Front. Microbiol. 2023, 14, 1043129. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Xue, Y.; Niu, T.; Li, X.; Cheng, M.; Bao, M.; Zou, B.; Shi, C.; Wang, J.; Yang, W.; et al. African swine fever virus MGF505-7R protein interacted with IRF7and TBK1 to inhibit type I interferon production. Virus Res. 2022, 322, 198931. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, D.; Shi, X.; Shen, C.; Hao, Y.; Zhang, T.; Yang, J.; Yuan, X.; Chen, X.; Zhao, D.; et al. Construction, identification and analysis of the interaction network of African Swine Fever Virus MGF360-9L with host proteins. Viruses 2021, 13, 1804. [Google Scholar] [CrossRef]
- Afe, A.E.; Shen, Z.J.; Guo, X.; Zhou, R.; Li, K. African Swine Fever Virus Interaction with Host Innate Immune Factors. Viruses 2023, 15, 1220. [Google Scholar] [CrossRef]
- Dixon, L.K.; Sánchez-Cordón, P.J.; Galindo, I.; Alonso, C. Investigations of pro- and anti-apoptotic factors affecting African Swine Fever Virus replication and pathogenesis. Viruses 2017, 9, 241. [Google Scholar] [CrossRef]
- Neelakanta, G.; Sultana, H. Tick saliva and salivary glands: What do we know so far on their role in arthropod blood feeding and pathogen transmission. Front. Cell. Infect. Microbiol. 2022, 11, 816547. [Google Scholar] [CrossRef]
- Urbano, A.C.; Ferreira, F. African swine fever control and prevention: An update on vaccine development. Emerg. Microbes Infect. 2022, 11, 2021–2033. [Google Scholar] [CrossRef]
- Zhu, J.J. African Swine Fever Vaccinology: The Biological Challenges from Immunological Perspectives. Viruses 2022, 14, 2021. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Pham, M.N.; Reyes, J.B.; Chana, R.; Yim, W.C.; Heu, C.C.; Kim, D.; Chaverra-Rodriguez, D.; Rasgon, J.L.; Harrell, R.A., II; et al. Cas9-mediated gene editing in the black-legged tick, Ixodes scapularis, by embryo injection and ReMOT Control. iScience 2022, 25, 103781. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, J.; Kocan, K.M.; Almazán, C.; Blouin, E.F. RNA interference for the study and genetic manipulation of ticks. Trends Parasitol. 2007, 23, 427–433. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.