Modulation of TvRAD51 Recombinase in Trichomonas vaginalis by Zinc and Cadmium as a Potential Mechanism for Genotoxic Stress Response
Abstract
1. Introduction
2. Materials and Methods
2.1. In Silico TvRAD51 Analysis
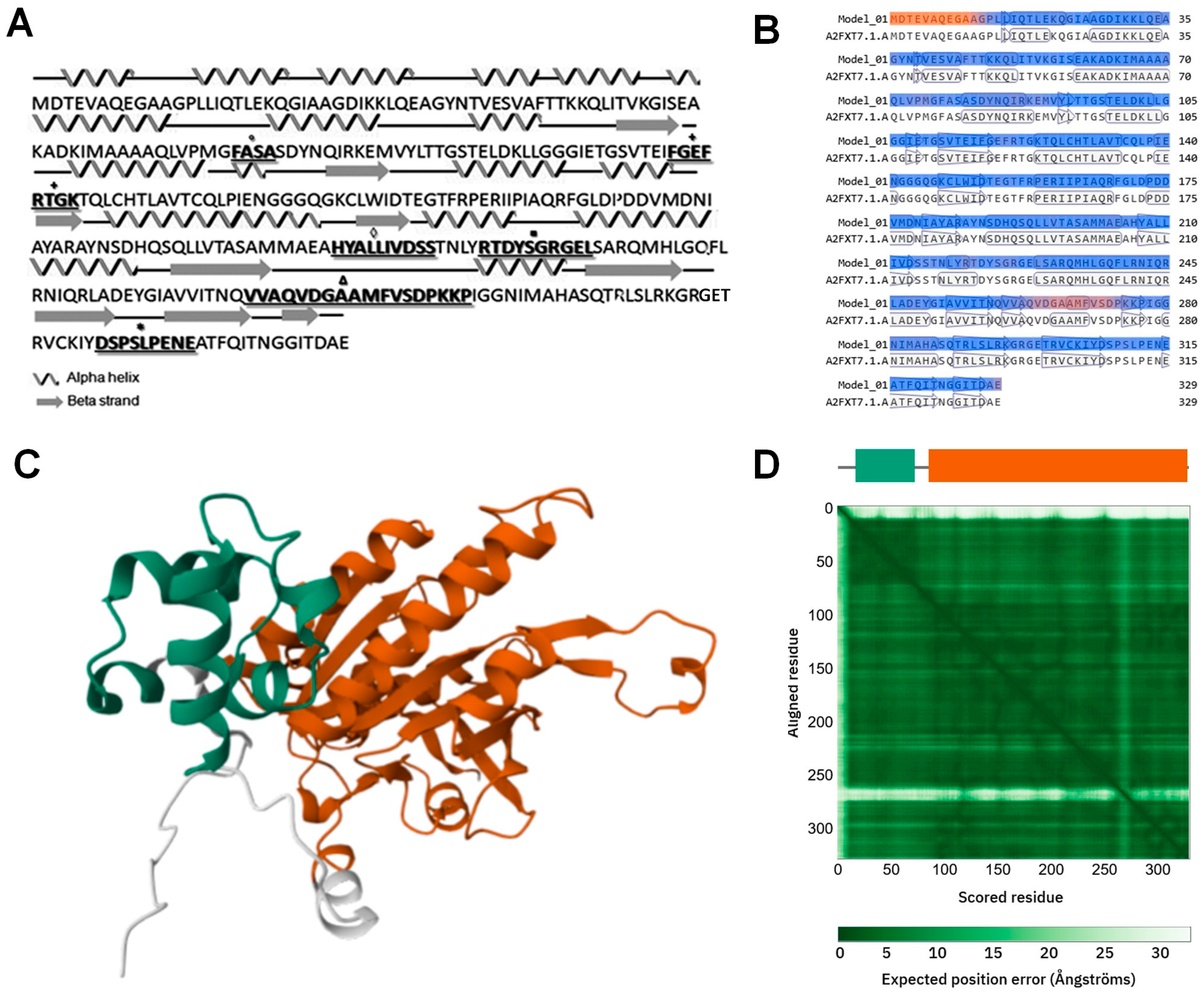
2.2. Sequence, Phylogenetic, and Tertiary Structure Prediction of TvRAD51
2.3. Prediction of Interaction Analysis
2.4. Parasites Culture, UV Irradiation, and Cations Treatments
2.5. RNA Isolation and End-Point PCR
2.6. Western Blot Assays
2.7. Immunolocalization of T. vaginalis TvRAD51 in Zinc (Zn2+) and Cadmium (Cd2+)
2.8. Statistical Analysis
3. Results
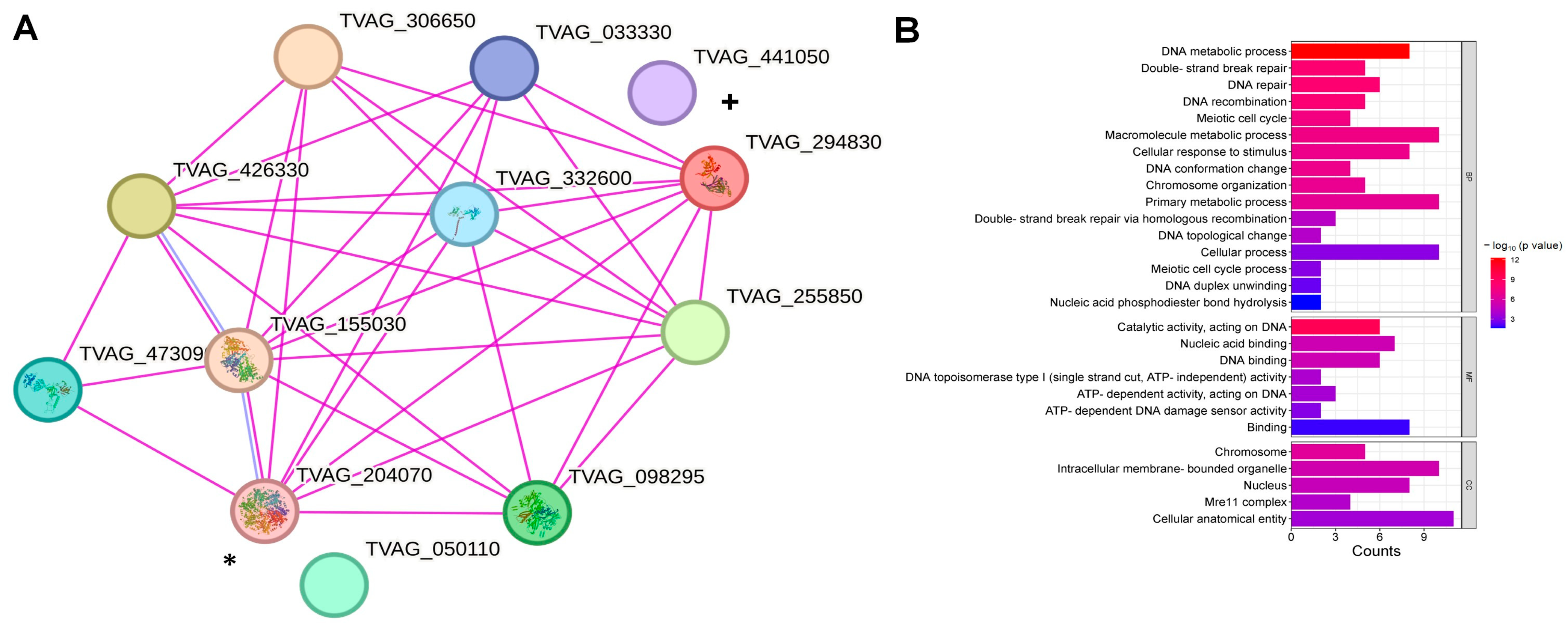
3.1. In Silico Analysis of tvrad51 Nucleotide and TvRAD51 Amino Acid Sequences and Identification of Potential Relative Genes Involved in HR Machinery in T. vaginalis
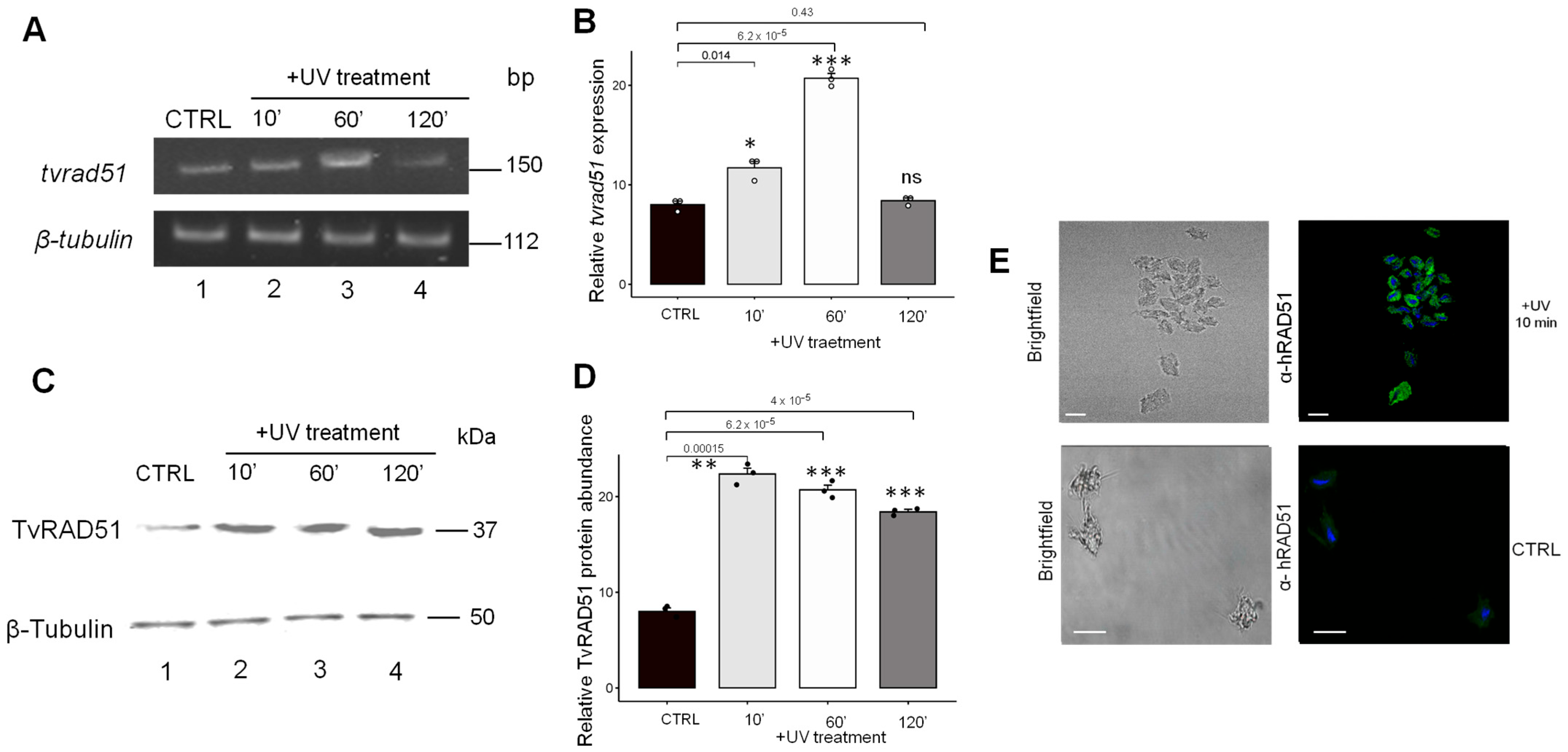
3.2. Expression of TvRAD51 in Trichomonas vaginalis in Response to UV Insult
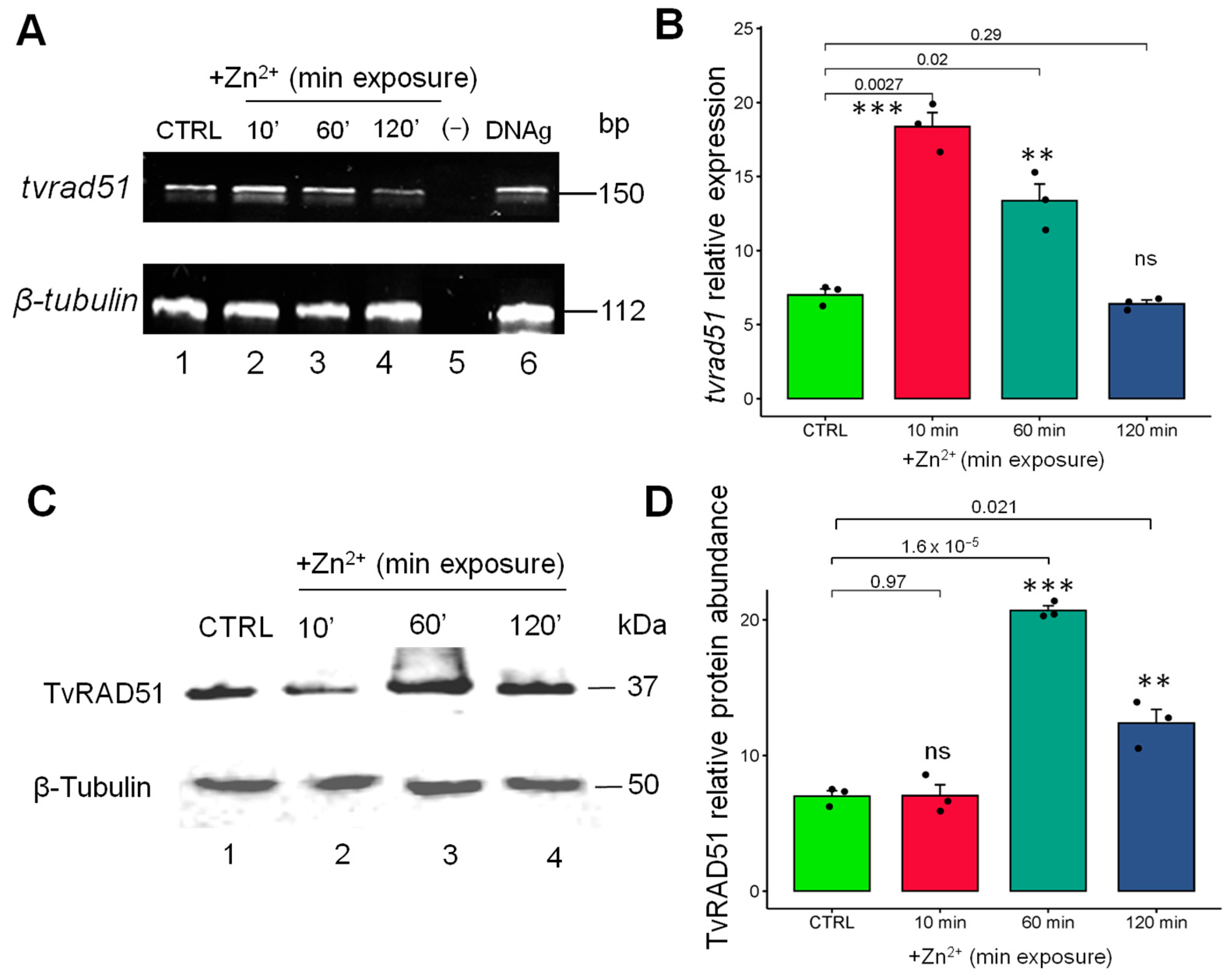
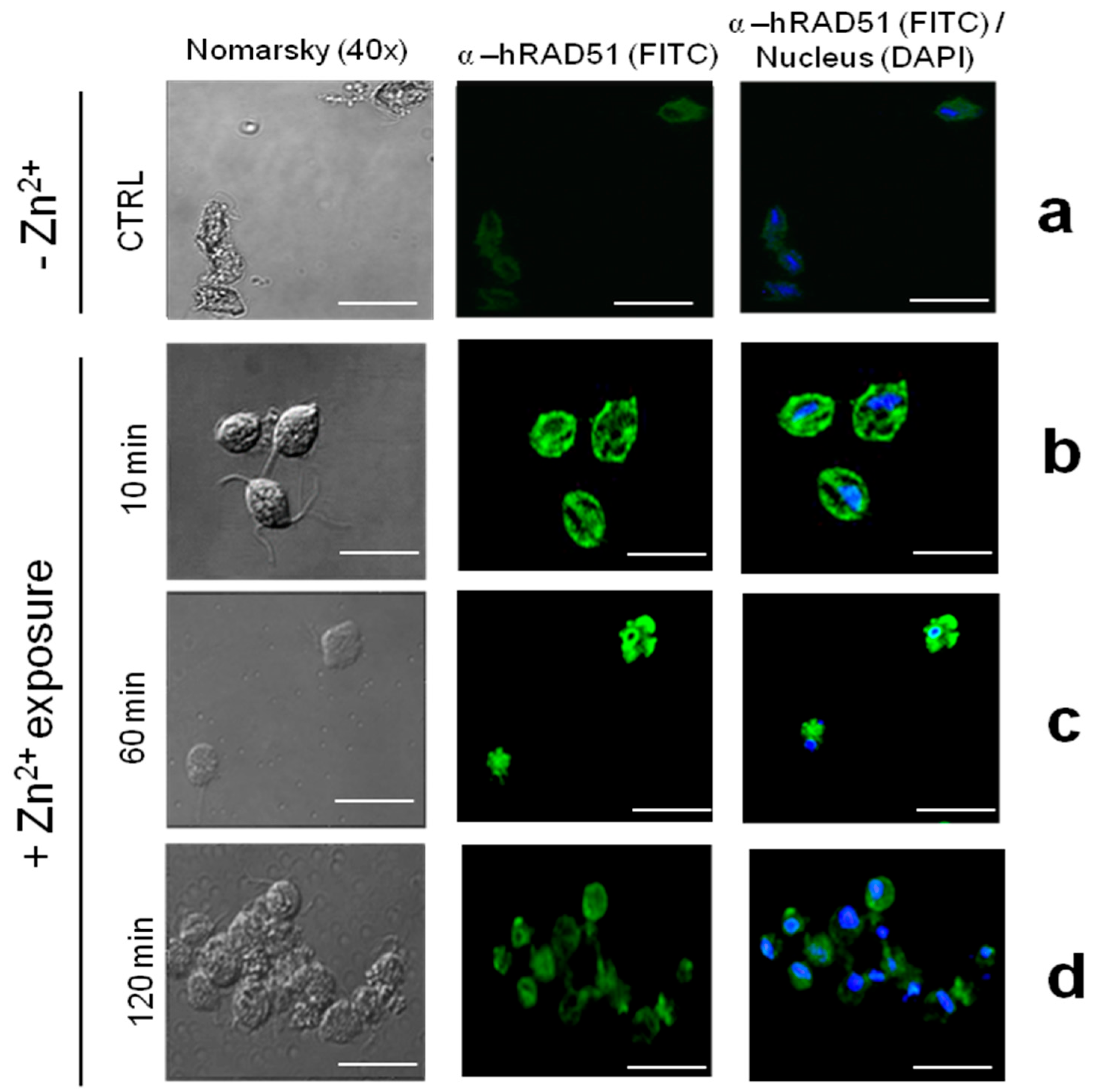
3.3. The Expression, Protein Abundance, and Localization of the TvRAD51 Protein Is Modulated by Zinc Exposure in T. vaginalis
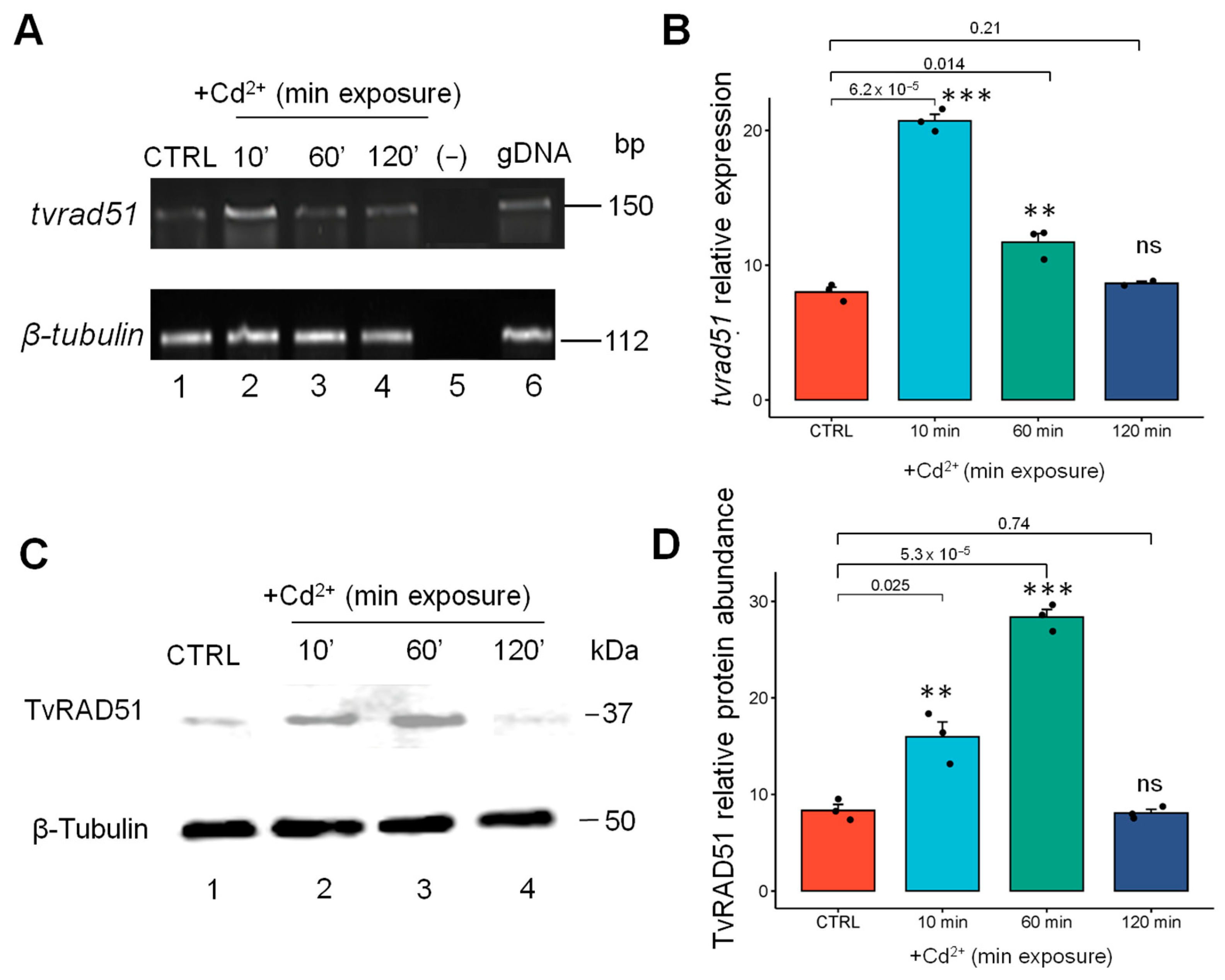
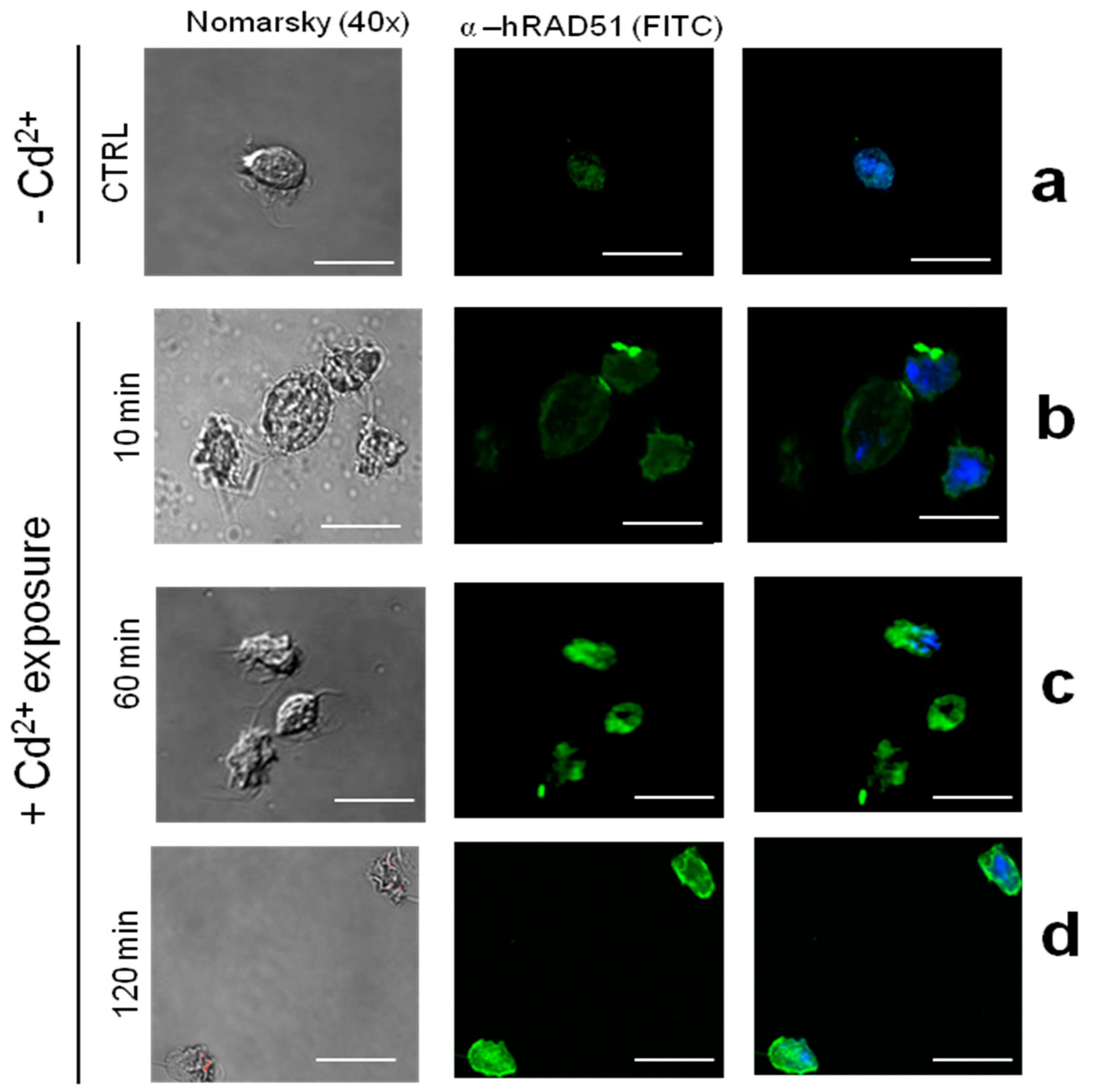
3.4. The Expression and Abundance of TvRAD51 Is Also Modulated by Cadmium in T. vaginalis but at an Early Time Point Compared to Zinc
4. Discussion
4.1. HR in Protozoa and Their Impact
4.2. T. vaginalis and TvRAD51 Network
4.3. TvRAD51 Gene and Protein Expression Under Genotoxic Conditions
4.4. Differential Responses to Zn2+ and Cd2+
4.5. Subcellular Localization of TvRAD51 and Implications for DNA Repair
4.6. Potential Biological and Clinical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HR | Homologous recombination |
| UV | Ultraviolet light |
| DSB | Double-strand break |
| Zn2+ | Zinc cation |
| Cd2+ | Cadmium cation |
| CTRL | Control condition |
References
- Van Gerwen, O.T.; Opsteen, S.A.; Graves, K.J.; Muzny, C.A. Trichomoniasis. Infect. Dis. Clin. N. Am. 2023, 37, 245–265. [Google Scholar] [CrossRef]
- World Health Organization. Sexually Transmitted Infections (STIs). World Health Organization. Available online: https://www.who.int/es/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis) (accessed on 10 December 2024).
- Van Gerwen, O.T.; Muzny, C.A. Recent Advances in the Epidemiology, Diagnosis, and Management of Trichomonas vaginalis Infection. F1000Research 2019, 8, F1000 Faculty Rev-1666. [Google Scholar] [CrossRef] [PubMed]
- Vickram, S.; Rohini, K.; Srinivasan, S.; Nancy Veenakumari, D.; Archana, K.; Anbarasu, K.; Jeyanthi, P.; Thanigaivel, S.; Gulothungan, G.; Rajendiran, N.; et al. Role of Zinc (Zn) in Human Reproduction: A Journey from Initial Spermatogenesis to Childbirth. Int. J. Mol. Sci. 2021, 22, 2188. [Google Scholar] [CrossRef]
- Quintas-Granados, L.I.; Villalpando, J.L.; Vázquez-Carrillo, L.I.; Arroyo, R.; Mendoza-Hernández, G.; Alvarez-Sánchez, M.E. TvMP50 is an Immunogenic Metalloproteinase During Male Trichomoniasis. Mol. Cell. Proteom. 2013, 12, 1953–1964. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martín, K.G.; Alvarez-Sánchez, M.E.; Arana-Argáez, V.E.; Alvarez-Sánchez, L.C.; Lara-Riegos, J.C.; Torres-Romero, J.C. Genome-wide Identification, In Silico Characterization and Expression Analysis of ZIP-like Genes from Trichomonas vaginalis in Response to Zinc and Iron. Biometals 2017, 30, 663–675. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, L.; Li, J.; Wang, H.; Yang, H. The Roles of TG-Interacting Factor in Cadmium Exposure-Promoted Invasion and Migration of Lung Cancer Cells. Toxicol. In Vitro 2019, 61, 104630. [Google Scholar] [CrossRef]
- Li, X.; Heyer, W.D. Homologous Recombination in DNA Repair and DNA Damage Tolerance. Cell Res. 2008, 18, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Skipper, A.; Sims, J.N.; Yedjou, C.G.; Tchounwou, P.B. Cadmium Chloride Induces DNA Damage and Apoptosis of Human Liver Carcinoma Cells via Oxidative Stress. Int. J. Environ. Res. Public Health 2016, 13, 88. [Google Scholar] [CrossRef]
- Kondo, T.; Kanai, M.; Matsubara, J.; Yamaguchi, D.; Ura, T.; Kou, T.; Itani, T.; Nomura, M.; Funakoshi, T.; Yokoyama, A.; et al. Association Between Homologous Recombination Gene Variants and Efficacy of Oxaliplatin-Based Chemotherapy in Advanced Pancreatic Cancer: Prospective Multicenter Observational Study. Med. Oncol. 2023, 40, 144. [Google Scholar] [CrossRef]
- del Socorro Charcas-Lopez, M.; Garcia-Morales, L.; Pezet-Valdez, M.; Lopez-Camarillo, C.; Zamorano-Carrillo, A.; Marchat, L.A. Expression of EhRAD54, EhRAD51, and EhBLM Proteins During DNA Repair by Homologous Recombination in Entamoeba histolytica. Parasite 2014, 21, 7. [Google Scholar] [CrossRef]
- Genois, M.M.; Mukherjee, A.; Ubeda, J.M.; Buisson, R.; Paquet, E.; Roy, G.; Plourde, M.; Coulombe, Y.; Ouellette, M.; Masson, J.Y. Interactions Between BRCA2 and RAD51 for Promoting Homologous Recombination in Leishmania infantum. Nucleic Acids Res. 2012, 40, 6570–6584. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, M.K.; Norris, D.E.; Kumar, N. Molecular Players of Homologous Recombination in Protozoan Parasites: Implications for Generating Antigenic Variation. Infect. Genet. Evol. 2004, 4, 91–98. [Google Scholar] [CrossRef]
- Roy, N.; Bhattacharyya, S.; Chakrabarty, S.; Laskar, S.; Babu, S.M.; Bhattacharyya, M.K. Dominant Negative Mutant of Plasmodium Rad51 Causes Reduced Parasite Burden in Host by Abrogating DNA Double-Strand Break Repair. Mol. Microbiol. 2014, 94, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Flores-Vega, J.J.; Puente-Rivera, J.; Sosa-Mondragón, S.I.; Camacho-Nuez, M.; Alvarez-Sánchez, M.E. RAD51 Recombinase and Its Paralogs: Orchestrating Homologous Recombination and Unforeseen Functions in Protozoan Parasites. Exp. Parasitol. 2024, 267, 108847. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein-Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Diamond, L.S. The Establishment of Various Trichomonads of Animals and Man in Axenic Cultures. J. Parasitol. 1957, 43, 488–490. [Google Scholar] [CrossRef]
- Vazquez Carrillo, L.I.; Quintas Granados, L.I.; Arroyo, R.; Mendoza Hernández, G.; González Robles, A.; Carvajal Gamez, B.I.; Alvarez Sánchez, M.E. The Effect of Zn2+ on Prostatic Cell Cytotoxicity Caused by Trichomonas vaginalis. J. Integr. OMICS 2011, 1, 198–210. [Google Scholar]
- Netzahualcoyotzi, B.A.; Puente-Rivera, J.; Arreola, R.; Romero, J.C.T.; Benitez, M.M.; Carmona, R.L.; Reyes, J.A.M.; de Jesús Olivares Trejo, J.; Sánchez, M.E.A. Cadmium-Dependent Expression of a New Metallothionein Identified in Trichomonas vaginalis. Biometals 2019, 32, 887–899. [Google Scholar] [CrossRef]
- López-Casamichana, M.; Orozco, E.; Marchat, L.A.; López-Camarillo, C. Transcriptional Profile of the Homologous Recombination Machinery and Characterization of the EhRAD51 Recombinase in Response to DNA Damage in Entamoeba histolytica. BMC Mol. Biol. 2008, 9, 35. [Google Scholar] [CrossRef]
- Madico, G.; Quinn, T.C.; Rompalo, A.; McKee, K.T., Jr.; Gaydos, C.A. Diagnosis of Trichomonas vaginalis Infection by PCR Using Vaginal Swab Samples. J. Clin. Microbiol. 1998, 36, 3205–3210. [Google Scholar] [CrossRef]
- Puente-Rivera, J.; Villalpando, J.L.; Villalobos-Osnaya, A.; Vázquez-Carrillo, L.I.; León-Ávila, G.; Ponce-Regalado, M.D.; López-Camarillo, C.; Elizalde-Contreras, J.M.; Ruiz-May, E.; Arroyo, R.; et al. The 50kDa Metalloproteinase TvMP50 is a Zinc-Mediated Trichomonas vaginalis Virulence Factor. Mol. Biochem. Parasitol. 2017, 217, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Liston, D.R.; Johnson, P.J. Gene Transcription in Trichomonas vaginalis. Parasitol. Today 1998, 14, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Johnson, P. Gene Expression in the Unicellular Eukaryote Trichomonas vaginalis. Res. Microbiol. 2011, 162, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, N.; Hernández, R.; López-Griego, L.; López-Villaseñor, I. Separable Putative Polyadenylation and Cleavage Motifs in Trichomonas vaginalis mRNAs. Gene 2002, 289, 81–86. [Google Scholar] [CrossRef]
- Carlton, J.M.; Hirt, R.P.; Silva, J.C.; Delcher, A.L.; Schatz, M.; Zhao, Q.; Wortman, J.R.; Bidwell, S.L.; Alsmark, U.C.; Besteiro, S.; et al. Draft Genome Sequence of the Sexually Transmitted Pathogen Trichomonas vaginalis. Science 2007, 315, 207–212. [Google Scholar] [CrossRef]
- Machida, S.; Takaku, M.; Ikura, M.; Sun, J.; Suzuki, H.; Kobayashi, W.; Kinomura, A.; Osakabe, A.; Tachiwana, H.; Horikoshi, Y.; et al. Nap1 Stimulates Homologous Recombination by RAD51 and RAD54 in Higher-Ordered Chromatin Containing Histone H1. Sci. Rep. 2014, 4, 4863. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Adolph, M.; Tsai, M.S.; Gallagher, K.; Cortez, D.; Chazin, W.J. Structure of RADX and Mechanism for Regulation of RAD51 Nucleofilaments. bioRxiv 2023, 121, e2316491121. [Google Scholar] [CrossRef]
- Stacey, R.G.; Skinnider, M.A.; Chik, J.H.L.; Foster, L.J. Context-Specific Interactions in Literature-Curated Protein Interaction Databases. BMC Genom. 2018, 19, 758. [Google Scholar] [CrossRef]
- Choi, E.H.; Yoon, S.; Koh, Y.E.; Seo, Y.J.; Kim, K.P. Maintenance of Genome Integrity and Active Homologous Recombination in Embryonic Stem Cells. Exp. Mol. Med. 2020, 52, 1220–1229. [Google Scholar] [CrossRef]
- Ham, R.E. Investigating Rad51 and Dmc1 in Entamoeba histolytica: Homologous Recombinases with Drug Target Potential. Master’s Thesis, Clemson University, Clemson, SC, USA, 2020. Available online: https://tigerprints.clemson.edu/all_theses/3392 (accessed on 15 May 2025).
- Ribeiro, K.C.; Monteiro-Leal, L.H.; Benchimol, M. Contributions of the Axostyle and Flagella to Closed Mitosis in the Protists Tritrichomonas foetus and Trichomonas vaginalis. J. Eukaryot. Microbiol. 2000, 47, 481–492. [Google Scholar] [CrossRef]
- Noël, C.J.; Diaz, N.; Sicheritz-Ponten, T.; Safarikova, L.; Tachezy, J.; Tang, P.; Fiori, P.L.; Hirt, R.P. Trichomonas vaginalis Vast BspA-Like Gene Family: Evidence for Functional Diversity from Structural Organisation and Transcriptomics. BMC Genom. 2010, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Bradic, M.; Warring, S.D.; Tooley, G.E.; Scheid, P.; Secor, W.E.; Land, K.M.; Huang, P.J.; Chen, T.W.; Lee, C.C.; Tang, P.; et al. Genetic Indicators of Drug Resistance in the Highly Repetitive Genome of Trichomonas vaginalis. Genome Biol. Evol. 2017, 9, 1658–1672. [Google Scholar] [CrossRef] [PubMed]
- Guttery, D.S.; Ramaprasad, A.; Ferguson, D.J.P.; Zeeshan, M.; Pandey, R.; Brady, D.; Holder, A.A.; Pain, A.; Tewari, R. MRE11 Is Crucial for Malaria Parasite Transmission and Its Absence Affects Expression of Interconnected Networks of Key Genes Essential for Life. Cells 2020, 9, 2590. [Google Scholar] [CrossRef]
- Mehnert, A.K.; Prorocic, M.; Dujeancourt-Henry, A.; Hutchinson, S.; McCulloch, R.; Glover, L. The MRN Complex Promotes DNA Repair by Homologous Recombination and Restrains Antigenic Variation in African Trypanosomes. Nucleic Acids Res. 2021, 49, 1436–1454. [Google Scholar] [CrossRef]
- Girasol, M.J.; Krasilnikova, M.; Marques, C.A.; Damasceno, J.D.; Lapsley, C.; Lemgruber, L.; Burchmore, R.; Beraldi, D.; Carruthers, R.; Briggs, E.M.; et al. RAD51-mediated R-loop formation acts to repair transcription-associated DNA breaks driving antigenic variation in Trypanosoma brucei. Proc. Natl. Acad. Sci. USA 2023, 120, e2309306120. [Google Scholar] [CrossRef]
- Faria, J.; Briggs, E.M.; Black, J.A.; McCulloch, R. Emergence and adaptation of the cellular machinery directing antigenic variation in the African trypanosome. Curr. Opin. Microbiol. 2022, 70, 102209. [Google Scholar] [CrossRef]
- McKean, P.G.; Keen, J.K.; Smith, D.F.; Benson, F.E. Identification and characterisation of a RAD51 gene from Leishmania major. Mol. Biochem. Parasitol. 2001, 115, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Spies, J.; Polasek-Sedlackova, H.; Lukas, J.; Somyajit, K. Homologous Recombination as a Fundamental Genome Surveillance Mechanism during DNA Replication. Genes 2021, 12, 1960. [Google Scholar] [CrossRef]
- Bhowmick, R.; Lerdrup, M.; Gadi, S.A.; Rossetti, G.G.; Singh, M.I.; Liu, Y.; Halazonetis, T.D.; Hickson, I.D. RAD51 Protects Human Cells from Transcription–Replication Conflicts. Mol. Cell 2022, 82, 3366–3381.e9. [Google Scholar] [CrossRef]
- San Filippo, J.; Sung, P.; Klein, H. Mechanism of eukaryotic homologous recombination. Annu. Rev. Biochem. 2008, 77, 229–257. [Google Scholar] [CrossRef]
- Forget, A.L.; Kowalczykowski, S.C. Single-molecule imaging brings Rad51 nucleoprotein filaments into focus. Trends Cell Biol. 2010, 20, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Branca, J.J.V.; Fiorillo, C.; Carrino, D.; Paternostro, F.; Taddei, N.; Gulisano, M.; Pacini, A.; Becatti, M. Cadmium-Induced Oxidative Stress: Focus on the Central Nervous System. Antioxidants 2020, 9, 492. [Google Scholar] [CrossRef] [PubMed]
- Davidova, S.; Milushev, V.; Satchanska, G. The Mechanisms of Cadmium Toxicity in Living Organisms. Toxics 2024, 12, 875. [Google Scholar] [CrossRef] [PubMed]
- Giaginis, C.; Gatzidou, E.; Theocharis, S. DNA Repair Systems as Targets of Cadmium Toxicity. Toxicol. Appl. Pharmacol. 2006, 213, 282–290. [Google Scholar] [CrossRef]
- Zandi, P.; Schnug, E. Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef]
- Wang, X.; Teng, Y.; Ji, C.; Wu, H.; Li, F. Critical Target Identification and Human Health Risk Ranking of Metal Ions Based on Mechanism-Driven Modeling. Chemosphere 2022, 301, 134724. [Google Scholar] [CrossRef]
- Qu, F.; Zheng, W. Cadmium Exposure: Mechanisms and Pathways of Toxicity and Implications for Human Health. Toxics 2024, 12, 388. [Google Scholar] [CrossRef]
- González, A.; Laporte, D.; Moenne, A. Cadmium Accumulation Involves Synthesis of Glutathione and Phytochelatins, and Activation of CDPK, CaMK, CBLPK, and MAPK Signaling Pathways in Ulva compressa. Front. Plant Sci. 2021, 12, 669096. [Google Scholar] [CrossRef]
- Compadre, A.J.; van Biljon, L.N.; Valentine, M.C.; Llop-Guevara, A.; Graham, E.; Fashemi, B.; Herencia-Ropero, A.; Kotnik, E.N.; Cooper, I.; Harrington, S.P.; et al. RAD51 Foci as a Biomarker Predictive of Platinum Chemotherapy Response in Ovarian Cancer. Clin. Cancer Res. 2023, 29, 2466–2479. [Google Scholar] [CrossRef]
- Krejci, L.; Altmannova, V.; Spirek, M.; Zhao, X. Homologous recombination and its regulation. Nucleic Acids Res. 2012, 40, 5795–5818. [Google Scholar] [CrossRef]
- Antoniuk-Majchrzak, J.; Enkhbaatar, T.; Długajczyk, A.; Kaminska, J.; Skoneczny, M.; Klionsky, D.J.; Skoneczna, A. Stability of Rad51 Recombinase and Persistence of Rad51 DNA Repair Foci Depends on Post-Translational Modifiers, Ubiquitin and SUMO. Biochim. Biophys. Acta Mol. Cell Res. 2023, 1870, 119526. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | ID (TrichDB) | EST ID | EST Length | Condition * |
|---|---|---|---|---|
| TvRAD51 | TVAG_204070 | TvG004A11 | 693 | G2/M trophozoite (TvG2M) |
| TvXRCC3 | TVAG_144570 | LK995170 | 538 | TvC |
| TvC127B06 | 538 | Cold-induced pseudocyst (TvCS) & | ||
| TvBRCA2 | TVAG_473090 | JK970464 | 422 | T. vaginalis log-phase trophozoite library (TvE) |
| TvE051F07 | 422 | Normal unsynchronized culture (TvEST) | ||
| TvRAD54B | TVAG_441050 | LK991215 | 502 | TvC |
| TvC074A09 | 706 | Cold-induced pseudocyst (TvCS) & | ||
| TvE077C08 | 461 | Normal unsynchronized culture (TvEST) | ||
| TvRAD50 | TVAG_332600 | CV204056 | 365 | Non-normalized T1 cDNA library # |
| CV204057 | 452 | Non-normalized T1 cDNA library # | ||
| TvMRE11 | TVAG_098295 | GT110554 | 700 | Normalized cDNA library from T. vaginalis trophozoites grown in vitro (mid-log phase) |
| GT110567 | 700 | Normalized cDNA library from T. vaginalis trophozoites grown in vitro (mid-log phase) |
| Gene ID | Gene | Protein Length (aa) | Uniprot ID | Identity % * |
|---|---|---|---|---|
| TVAG_021820 | rad51pseudogene | 115 | A2DHG2 | 53.91% |
| TVAG_204070 | tvrad51 | 329 | A2FXT7 | 69.91% |
| TVAG_021810 | dmc1 | 153 | A2DHG1 | 79.08% |
| TVAG_155030 | dmc1 | 338 | A2FY08 | 53.15% |
| TVAG_144570 | xrcc3 | 328 | A2G1B8 | 23.68% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puente-Rivera, J.; Flores-Vega, J.J.; Morales-Reyna, M.; Figueroa-Angulo, E.E.; Pérez-Navarro, Y.; Salgado-Aguayo, A.; Carlos-Reyes, Á.; Alvarez-Sánchez, M.E. Modulation of TvRAD51 Recombinase in Trichomonas vaginalis by Zinc and Cadmium as a Potential Mechanism for Genotoxic Stress Response. Pathogens 2025, 14, 565. https://doi.org/10.3390/pathogens14060565
Puente-Rivera J, Flores-Vega JJ, Morales-Reyna M, Figueroa-Angulo EE, Pérez-Navarro Y, Salgado-Aguayo A, Carlos-Reyes Á, Alvarez-Sánchez ME. Modulation of TvRAD51 Recombinase in Trichomonas vaginalis by Zinc and Cadmium as a Potential Mechanism for Genotoxic Stress Response. Pathogens. 2025; 14(6):565. https://doi.org/10.3390/pathogens14060565
Chicago/Turabian StylePuente-Rivera, Jonathan, José Jesús Flores-Vega, Marcos Morales-Reyna, Elisa Elvira Figueroa-Angulo, Yussel Pérez-Navarro, Alfonso Salgado-Aguayo, Ángeles Carlos-Reyes, and Maria Elizbeth Alvarez-Sánchez. 2025. "Modulation of TvRAD51 Recombinase in Trichomonas vaginalis by Zinc and Cadmium as a Potential Mechanism for Genotoxic Stress Response" Pathogens 14, no. 6: 565. https://doi.org/10.3390/pathogens14060565
APA StylePuente-Rivera, J., Flores-Vega, J. J., Morales-Reyna, M., Figueroa-Angulo, E. E., Pérez-Navarro, Y., Salgado-Aguayo, A., Carlos-Reyes, Á., & Alvarez-Sánchez, M. E. (2025). Modulation of TvRAD51 Recombinase in Trichomonas vaginalis by Zinc and Cadmium as a Potential Mechanism for Genotoxic Stress Response. Pathogens, 14(6), 565. https://doi.org/10.3390/pathogens14060565










