Monitoring Multiple Sexually Transmitted Pathogens Through Wastewater Surveillance
Abstract
1. Introduction
2. Materials and Methodology
2.1. Sample Collection and Site Selection
2.2. Sample Concentration and DNA/RNA Extraction
2.3. Microbial Quantification by dPCR
2.4. Data Analysis
2.5. Clinical Data
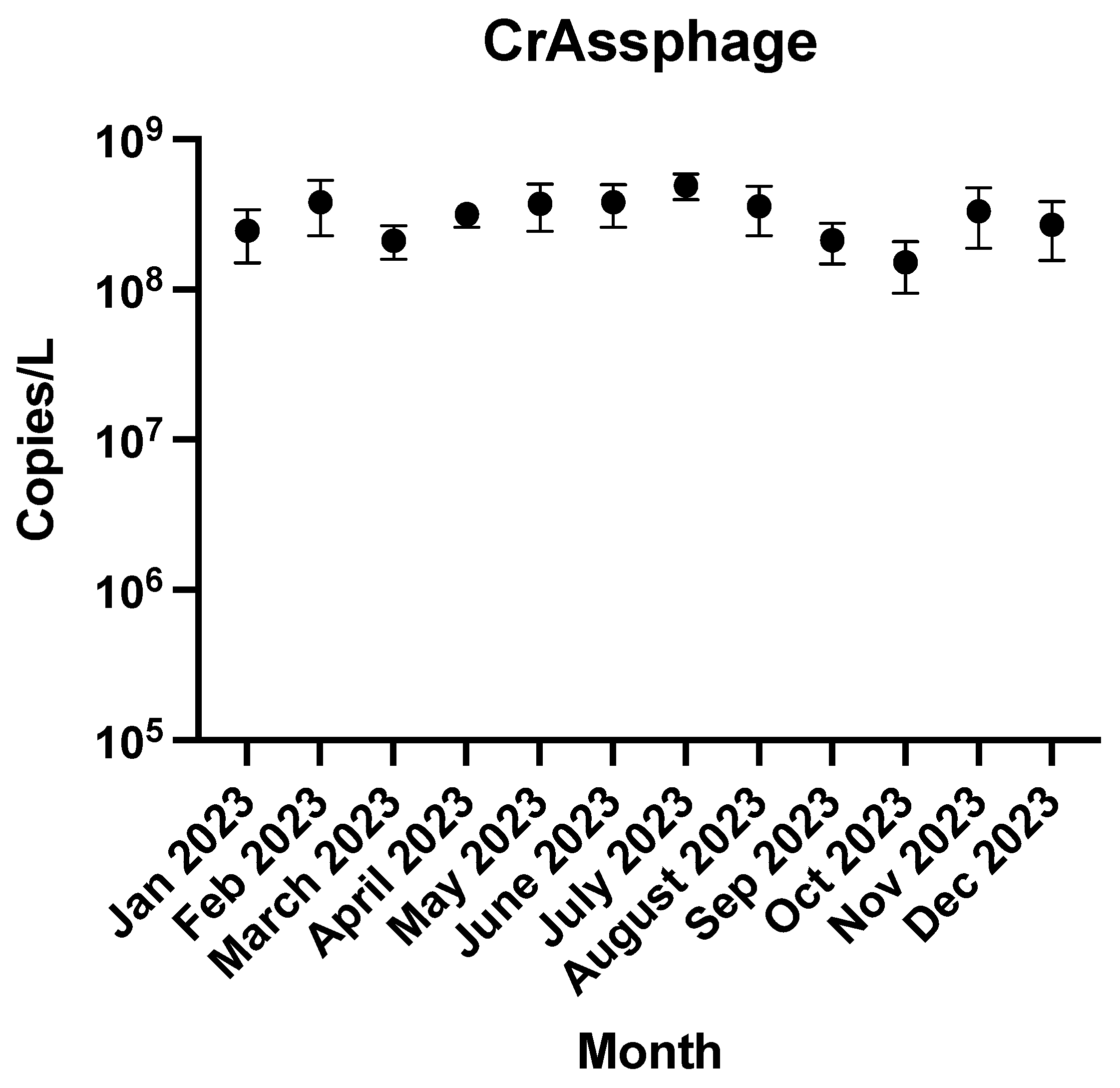
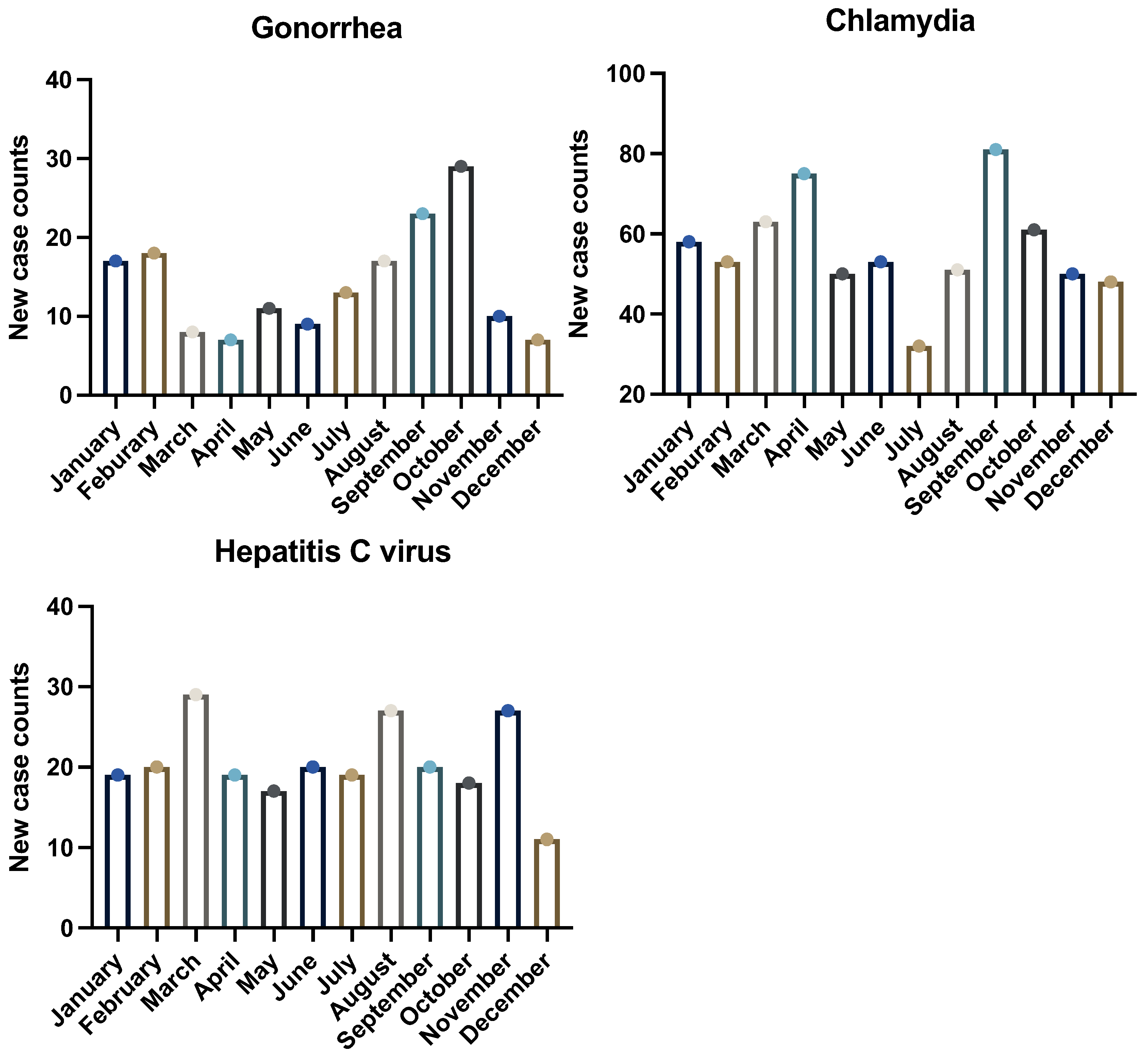
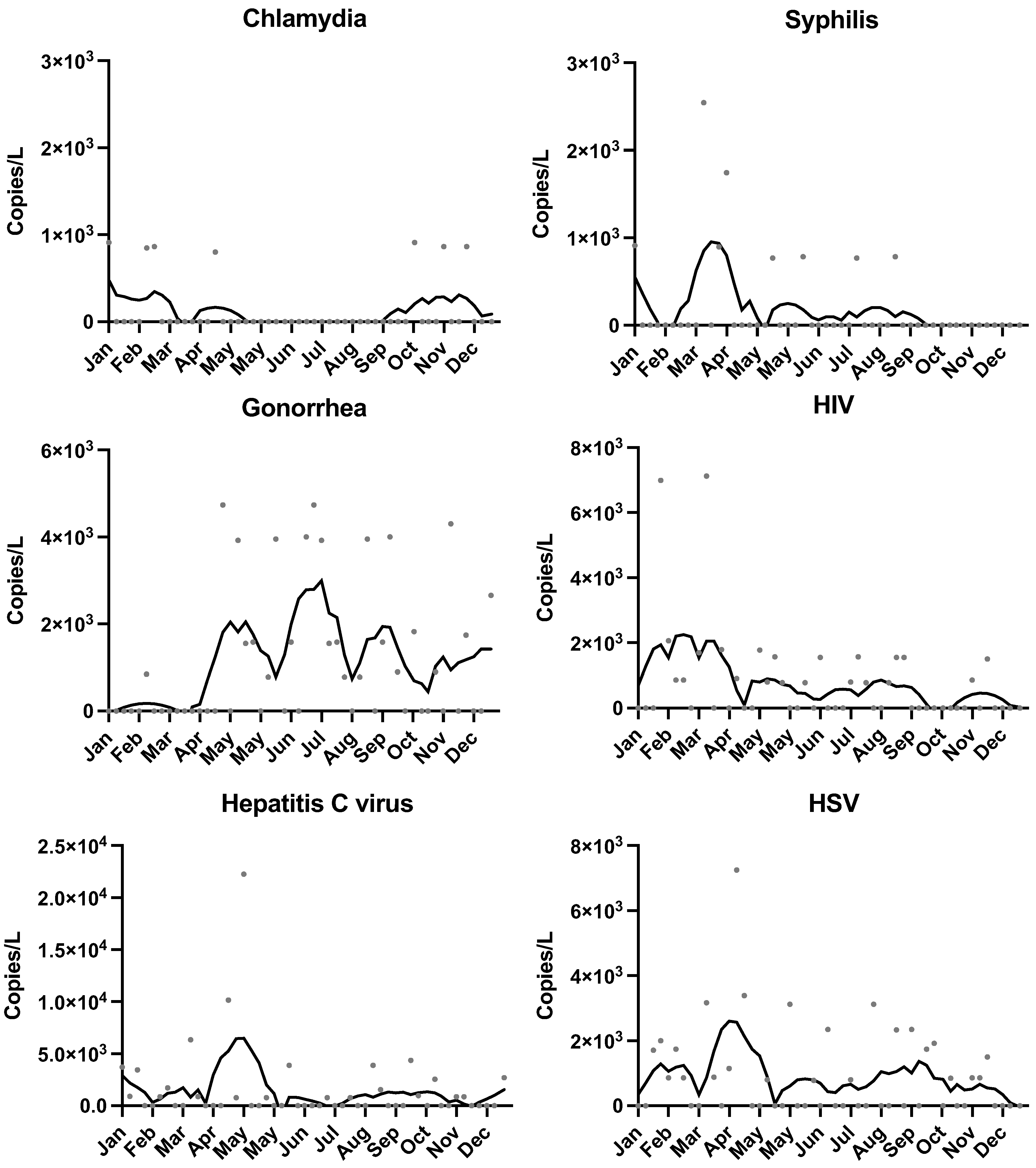
3. Results and Discussion
3.1. Rates of Detection
3.2. Temporal Trends
3.3. Comparison with Clinical Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Sexually transmitted infections (STIs) [Internet]. Geneva: World Health Organization; 2025 May 29. Available online: https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis) (accessed on 29 May 2025).
- Centers for Disease Control and Prevention. Sexually Transmitted Infections Surveillance 2022 [Internet]. Atlanta (GA): CDC; 2022. Available online: https://www.cdc.gov/sti-statistics/media/pdfs/2024/11/2022-STI-Surveillance-Report-PDF.pdf (accessed on 29 May 2025).
- Baraitser, P.; Alexander, S.; Sheringham, J. Chlamydia trachomatis screening in young women. Curr. Opin. Obstet. Gynecol. 2011, 23, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, B. Stigma as a barrier to treatment of sexually transmitted infection in the American deep south: Issues of race, gender and poverty. Soc. Sci. Med. 2003, 57, 2435–2445. [Google Scholar] [CrossRef]
- Balfe, M.; Brugha, R.; O’Connell, E.; McGee, H.; O’Donovan, D.; Vaughan, D. Why don’t young women go for Chlamydia testing? A qualitative study employing Goffman’s stigma framework. Heal. Risk Soc. 2010, 12, 131–148. [Google Scholar] [CrossRef]
- Gitter, A.; Oghuan, J.; Godbole, A.R.; Chavarria, C.A.; Monserrat, C.; Hu, T.; Wang, Y.; Maresso, A.W.; Hanson, B.M.; Mena, K.D.; et al. Not a waste: Wastewater surveillance to enhance public health. Front. Chem. Eng. 2023, 4, 1112876. [Google Scholar] [CrossRef]
- Cunningham, S.D.; Kerrigan, D.L.; Jennings, J.M.; Ellen, J.M. Relationships Between Perceived STD-Related Stigma, STD-Related Shame and STD Screening Among a Household Sample of Adolescents. Perspect. Sex. Reprod. Health 2009, 41, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, K.A.T.M.; Bos, A.E.R.; Hoebe, C.J.P.A.; Kok, G.; Vluggen, S.; Crutzen, R.; Dukers-Muijrers, N.H.T.M. Chlamydia trachomatis testing among young people: What is the role of stigma? BMC Public Health 2015, 15, 651. [Google Scholar] [CrossRef]
- Sentis, A.; Prats-Uribe, A.; López-Corbeto, E.; Montoro-Fernandez, M.; Nomah, D.K.; de Olalla, P.G.; Mercuriali, L.; Borrell, N.; Guadalupe-Fernández, V.; Reyes-Urueña, J.; et al. The impact of the COVID-19 pandemic on Sexually Transmitted Infections surveillance data: Incidence drop or artefact? BMC Public Health 2021, 21, 1637. [Google Scholar] [CrossRef] [PubMed]
- Philo, S.E.; Keim, E.K.; Swanstrom, R.; Ong, A.Q.; Burnor, E.A.; Kossik, A.L.; Harrison, J.C.; Demeke, B.A.; Zhou, N.A.; Beck, N.K.; et al. A comparison of SARS-CoV-2 wastewater concentration methods for environmental surveillance. Sci. Total. Environ. 2021, 760, 144215. [Google Scholar] [CrossRef]
- Zhao, L.; Guzman, H.P.; Xagoraraki, I. Tracking Chlamydia and Syphilis in the Detroit Metro Area by Molecular Analysis of Environmental Samples. Environ. Sci. Technol. 2024, 58, 17606–17616. [Google Scholar] [CrossRef]
- Hart, O.E.; Halden, R.U. Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: Feasibility, economy, opportunities and challenges. Sci. Total Environ. 2020, 730, 138875. [Google Scholar] [CrossRef]
- Medema, G.; Heijnen, L.; Elsinga, G.; Italiaander, R.; Brouwer, A. Presence of SARS-Coronavirus-2 RNA in Sewage and Correlation with Reported COVID-19 Prevalence in the Early Stage of the Epidemic in The Netherlands. Environ. Sci. Technol. Lett. 2020, 7, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Angel, N.; Edson, J.; Bibby, K.; Bivins, A.; O’Brien, J.W.; Choi, P.M.; Kitajima, M.; Simpson, S.L.; Li, J.; et al. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: A proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020, 728, 138764. [Google Scholar] [CrossRef]
- Choi, P.M.; Tscharke, B.J.; Donner, E.; O’Brien, J.W.; Grant, S.C.; Kaserzon, S.L.; Mackie, R.; O’Malley, E.; Crosbie, N.D.; Thomas, K.V.; et al. Wastewater-based epidemiology biomarkers: Past, present and future. TrAC Trends Anal. Chem. 2018, 105, 453–469. [Google Scholar] [CrossRef]
- Mao, K.; Zhang, H.; Pan, Y.; Yang, Z. Biosensors for wastewater-based epidemiology for monitoring public health. Water Res. 2021, 191, 116787. [Google Scholar] [CrossRef] [PubMed]
- Peccia, J.; Zulli, A.; Brackney, D.E.; Grubaugh, N.D.; Kaplan, E.H.; Casanovas-Massana, A.; Ko, A.I.; Malik, A.A.; Wang, D.; Wang, M.; et al. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020, 38, 1164–1167. [Google Scholar] [CrossRef]
- Hovi, T.; Shulman, L.M.; VAN DER Avoort, H.; Deshpande, J.; Roivainen, M.; DE Gourville, E.M. Role of environmental poliovirus surveillance in global polio eradication and beyond. Epidemiology Infect. 2011, 140, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, R.S.; Munk, P.; Njage, P.; Van Bunnik, B.; McNally, L.; Lukjancenko, O.; Röder, T.; Nieuwenhuijse, D.; Pedersen, S.K.; Kjeldgaard, J.; et al. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage. Nat. Commun. 2019, 10, 1124. [Google Scholar] [CrossRef]
- Chin Quee, J.E. Using Wastewater-Based Epidemiology to Study Chlamydia Occurrence on a College Campus; University of Central Florida: Orlando, FL, USA, 2023. [Google Scholar]
- Gonzalez, R.; Curtis, K.; Bivins, A.; Bibby, K.; Weir, M.H.; Yetka, K.; Thompson, H.; Keeling, D.; Mitchell, J.; Gonzalez, D. COVID-19 surveillance in Southeastern Virginia using wastewater-based epidemiology. Water Res. 2020, 186, 116296. [Google Scholar] [CrossRef]
- Wu, Z.; Greaves, J.; Arp, L.; Stone, D.; Bibby, K. Comparative fate of CrAssphage with culturable and molecular fecal pollution indicators during activated sludge wastewater treatment. Environ. Int. 2020, 136, 105452. [Google Scholar] [CrossRef]
- Chia, C.T.; Bender, A.T.; Lillis, L.; Sullivan, B.P.; Martin, C.D.; Burke, W.; Landis, C.; Boyle, D.S.; Posner, J.D. Rapid detection of hepatitis C virus using recombinase polymerase amplification. PLoS ONE 2022, 17, e0276582. [Google Scholar] [CrossRef]
- Wolfe, M.K.; Varkila, M.R.J.; Zulli, A.; Parsonnet, J.; Boehm, A.B. Detection and quantification of human immunodeficiency virus-1 (HIV-1) total nucleic acids in wastewater settled solids from two California communities. Appl. Environ. Microbiol. 2024, 90, e0147724. [Google Scholar] [CrossRef]
- Aitlhaj-Mhand, R.; Qasmaoui, A.; Bellaji, B.; Remz, C.; Charof, R.; El Jaoudi, R.; Abdelmoumen, H.; Hançali, A.; Oumzil, H. Promoting molecular diagnostic equity: Assessing in-house real-time PCR for Neisseria gonorrhoeae in anal samples from MSM recruited in an outpatient setting in Morocco. Infez. Med. 2024, 32, 352–362. [Google Scholar] [CrossRef]
- Feng, S.; Roguet, A.; McClary-Gutierrez, J.S.; Newton, R.J.; Kloczko, N.; Meiman, J.G.; McLellan, S.L. Evaluation of Sampling, Analysis, and Normalization Methods for SARS-CoV-2 Concentrations in Wastewater to Assess COVID-19 Burdens in Wisconsin Communities. ACS ES&T Water 2021, 1, 1955–1965. [Google Scholar] [CrossRef]
- McCall, C.; Wu, H.; O’brien, E.; Xagoraraki, I. Assessment of enteric viruses during a hepatitis outbreak in Detroit MI using wastewater surveillance and metagenomic analysis. J. Appl. Microbiol. 2021, 131, 1539–1554. [Google Scholar] [CrossRef]
- Wilhelm, A.; Schoth, J.; Meinert-Berning, C.; Bastian, D.; Blum, H.; Elsinga, G.; Graf, A.; Heijnen, L.; Ho, J.; Kluge, M.; et al. Interlaboratory comparison using inactivated SARS-CoV-2 variants as a feasible tool for quality control in COVID-19 wastewater monitoring. Sci. Total. Environ. 2023, 903, 166540. [Google Scholar] [CrossRef]
- Anderson-Coughlin, B.L.; Craighead, S.; Kelly, A.; Gartley, S.; Vanore, A.; Johnson, G.; Jiang, C.; Haymaker, J.; White, C.; Foust, D.; et al. Enteric Viruses and Pepper Mild Mottle Virus Show Significant Correlation in Select Mid-Atlantic Agricultural Waters. Appl. Environ. Microbiol. 2021, 87, e0021121. [Google Scholar] [CrossRef]
- Azzellino, A.; Pellegrinelli, L.; Pedrini, R.; Turolla, A.; Bertasi, B.; Binda, S.; Castiglioni, S.; Cocuzza, C.E.; Ferrari, F.; Franzetti, A.; et al. Evaluating Interlaboratory Variability in Wastewater-Based COVID-19 Surveillance. Microorganisms 2025, 13, 526. [Google Scholar] [CrossRef]
- He, Z.; Dunne, D. Refining the scope of Journal of Hazardous Materials. J. Hazard. Mater. 2022, 432, 128717. [Google Scholar] [CrossRef]
- Banadaki, M.D.; Torabi, S.; Rockward, A.; Strike, W.D.; Noble, A.; Keck, J.W.; Berry, S.M. Simple SARS-CoV-2 concentration methods for wastewater surveillance in low resource settings. Sci. Total. Environ. 2024, 912, 168782. [Google Scholar] [CrossRef]
- Health, I.D.o. STI Morbidity Dashboard. 2025. Available online: https://www.in.gov/health/hiv-std-viral-hepatitis/sexually-transmitted-disease-prevention-program/stds-dashboard/ (accessed on 15 February 2025).
- Hillary, L.S.; Farkas, K.; Maher, K.H.; Lucaci, A.; Thorpe, J.; Distaso, M.A.; Gaze, W.H.; Paterson, S.; Burke, T.; Connor, T.R.; et al. Monitoring SARS-CoV-2 in municipal wastewater to evaluate the success of lockdown measures for controlling COVID-19 in the UK. Water Res. 2021, 200, 117214. [Google Scholar] [CrossRef]
- Nam, J.-Y.; Jwa, E.; Eom, H.; Kim, H.; Hwang, K.; Jeong, N. Enhanced energy recovery using a cascaded reverse electrodialysis stack for salinity gradient power generation. Water Res. 2021, 200, 117255. [Google Scholar] [CrossRef]
- Manns, M.P.; But, M.; Gane, E.; Pawlotsky, J.-M.; Razavi, H.; Terrault, N.; Younossi, Z. Hepatitis C virus infection. Nat. Rev. Dis. Primers 2017, 3, 17006. [Google Scholar] [CrossRef]
- Beld, M.; Sentjens, R.; Rebers, S.; Weel, J.; Dillen, P.W.-V.; Sol, C.; Boom, R. Detection and Quantitation of Hepatitis C Virus RNA in Feces of Chronically Infected Individuals. J. Clin. Microbiol. 2000, 38, 3442–3444. [Google Scholar] [CrossRef]
- Reyne, M.I.; Allen, D.M.; Levickas, A.; Allingham, P.; Lock, J.; Fitzgerald, A.; McSparron, C.; Nejad, B.F.; McKinley, J.; Lee, A.; et al. Detection of human adenovirus F41 in wastewater and its relationship to clinical cases of acute hepatitis of unknown aetiology. Sci. Total. Environ. 2022, 857, 159579. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; O’Brien, J.; Sivakumar, M.; Jiang, G. Back-estimation of norovirus infections through wastewater-based epidemiology: A systematic review and parameter sensitivity. Water Res. 2022, 219, 118610. [Google Scholar] [CrossRef]
- Meyer-Weitz, A.; Reddy, P.; Borne, H.V.D.; Kok, G.; Pietersen, J. Health care seeking behaviour of patients with sexually transmitted diseases: Determinants of delay behaviour. Patient Educ. Couns. 2000, 41, 263–274. [Google Scholar] [CrossRef]
- Rotchford, K.; Strum, A.W.; Wilkinson, D. Effect of coinfection with STDs and of STD treatment on HIV shedding in genital-tract secretions: Systematic review and data synthesis. Sex. Transm. Dis. 2000, 27, 243–248. [Google Scholar] [CrossRef]
- Betancourt, W.Q.; Schmitz, B.W.; Innes, G.K.; Prasek, S.M.; Brown, K.M.P.; Stark, E.R.; Foster, A.R.; Sprissler, R.S.; Harris, D.T.; Sherchan, S.P.; et al. COVID-19 containment on a college campus via wastewater-based epidemiology, targeted clinical testing and an intervention. Sci. Total. Environ. 2021, 779, 146408. [Google Scholar] [CrossRef]
- Kilaru, P.; Hill, D.; Anderson, K.; Collins, M.B.; Green, H.; Kmush, B.L.; Larsen, D.A. Wastewater Surveillance for Infectious Disease: A Systematic Review. Am. J. Epidemiology 2022, 192, 305–322. [Google Scholar] [CrossRef]
- Bivins, A.; Kaya, D.; Ahmed, W.; Brown, J.; Butler, C.; Greaves, J.; Leal, R.; Maas, K.; Rao, G.; Sherchan, S.; et al. Passive sampling to scale wastewater surveillance of infectious disease: Lessons learned from COVID-19. Sci. Total. Environ. 2022, 835, 155347. [Google Scholar] [CrossRef]
- Hook, E.W.; Richey, C.M.; Leone, P.; Bolan, G.; Spalding, C.; Henry, K.; Clarke, P.; Smith, M.; Celum, C.L. Delayed presentation to clinics for sexually transmitted diseases by symptomatic patients—A potential contributor to continuing STD morbidity. Sex. Transm. Dis. 1997, 24, 443–448. [Google Scholar] [CrossRef]
- Kersh, E.N.; Shukla, M.; Raphael, B.H.; Habel, M.; Park, I. At-Home Specimen Self-Collection and Self-Testing for Sexually Transmitted Infection Screening Demand Accelerated by the COVID-19 Pandemic: A Review of Laboratory Implementation Issues. J. Clin. Microbiol. 2021, 59, e0264620. [Google Scholar] [CrossRef] [PubMed]
- Farkas, K.; Cooper, D.M.; McDonald, J.E.; Malham, S.K.; de Rougemont, A.; Jones, D.L. Seasonal and spatial dynamics of enteric viruses in wastewater and in riverine and estuarine receiving waters. Sci. Total Environ. 2018, 634, 1174–1183. [Google Scholar] [CrossRef]
- Huisman, J.S.; Scire, J.; Caduff, L.; Fernandez-Cassi, X.; Ganesanandamoorthy, P.; Kull, A.; Scheidegger, A.; Stachler, E.; Boehm, A.B.; Hughes, B.; et al. Wastewater-Based Estimation of the Effective Reproductive Number of SARS-CoV-2. Environ. Health Perspect. 2022, 130, 57011. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, S.; Ronquillo, N.; Belda-Ferre, P.; Alvarado, D.; Javidi, T.; Longhurst, C.A.; Knight, R. High-Throughput Wastewater SARS-CoV-2 Detection Enables Forecasting of Community Infection Dynamics in San Diego County. mSystems 2021, 6, e00045-21. [Google Scholar] [CrossRef]
- Brisebois, E.; Veillette, M.; Dion-Dupont, V.; Lavoie, J.; Corbeil, J.; Culley, A.; Duchaine, C. Human viral pathogens are pervasive in wastewater treatment center aerosols. J. Environ. Sci. (China). 2018, 67, 45–53. [Google Scholar] [CrossRef] [PubMed]
| Target Gene | No. of Tested Samples | No. of Positive Samples (%) | Mean Concentration ± Standard Deviation (log10 copies/L) |
|---|---|---|---|
| Chlamydia | 51 | 7 (13.7%) | 2.64 ± 0.002 |
| Syphilis | 51 | 8 (15.6%) | 2.76 ± 0.041 |
| Gonorrhea | 51 | 24 (47.1%) | 3.08 ± 0.091 |
| HIV | 51 | 22 (43.1%) | 2.95 ± 0.110 |
| Hep C | 51 | 22 (43.1%) | 3.23 ± 0.300 |
| HSV | 51 | 24 (47.1%) | 3.00 ± 0.089 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshehri, B.; Birch, O.N.; Greaves, J.C. Monitoring Multiple Sexually Transmitted Pathogens Through Wastewater Surveillance. Pathogens 2025, 14, 562. https://doi.org/10.3390/pathogens14060562
Alshehri B, Birch ON, Greaves JC. Monitoring Multiple Sexually Transmitted Pathogens Through Wastewater Surveillance. Pathogens. 2025; 14(6):562. https://doi.org/10.3390/pathogens14060562
Chicago/Turabian StyleAlshehri, Balghsim, Olivia N. Birch, and Justin C. Greaves. 2025. "Monitoring Multiple Sexually Transmitted Pathogens Through Wastewater Surveillance" Pathogens 14, no. 6: 562. https://doi.org/10.3390/pathogens14060562
APA StyleAlshehri, B., Birch, O. N., & Greaves, J. C. (2025). Monitoring Multiple Sexually Transmitted Pathogens Through Wastewater Surveillance. Pathogens, 14(6), 562. https://doi.org/10.3390/pathogens14060562






