Fungi-Based Bioproducts: A Review in the Context of One Health
Abstract
1. Introduction
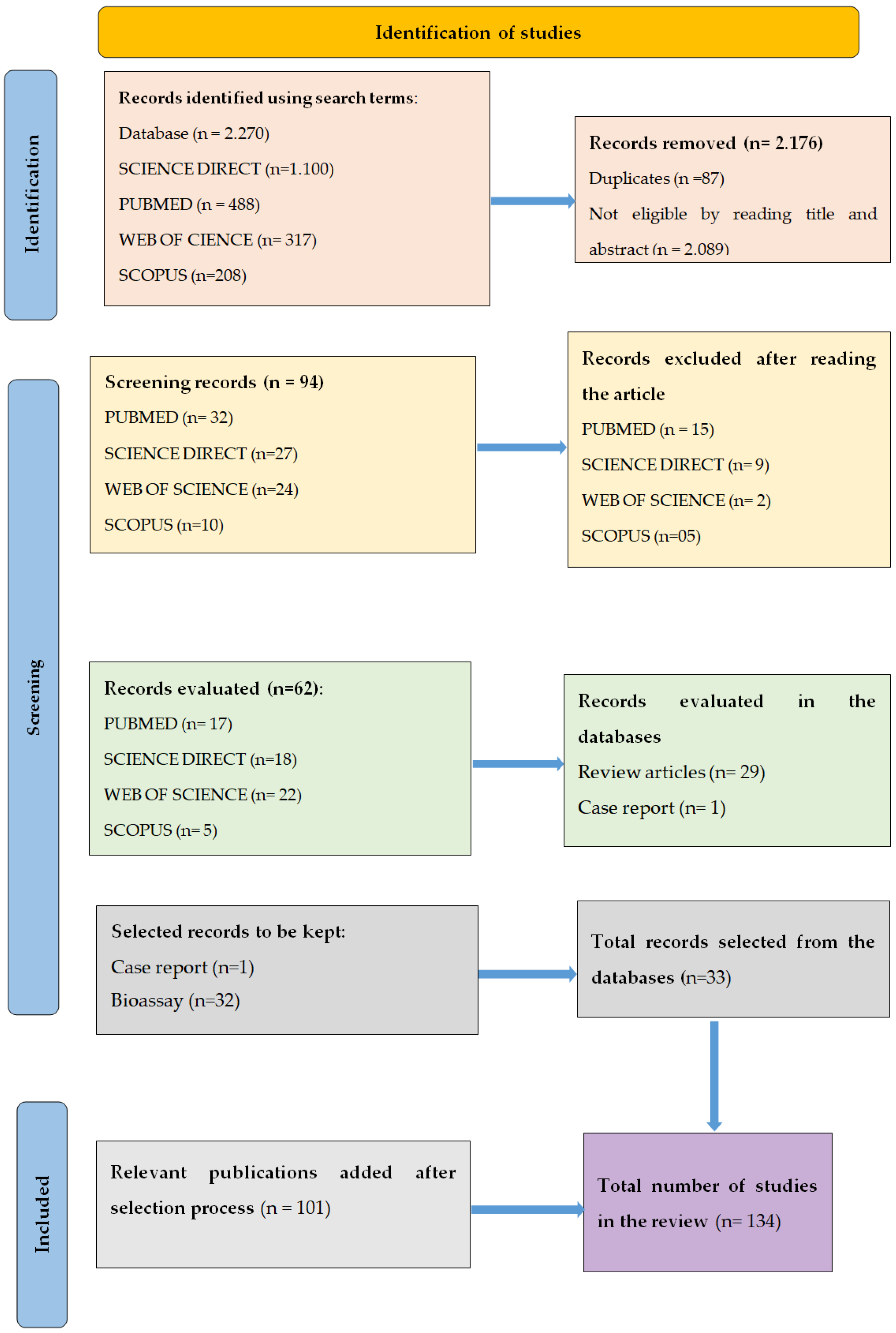
2. Methods
3. Results
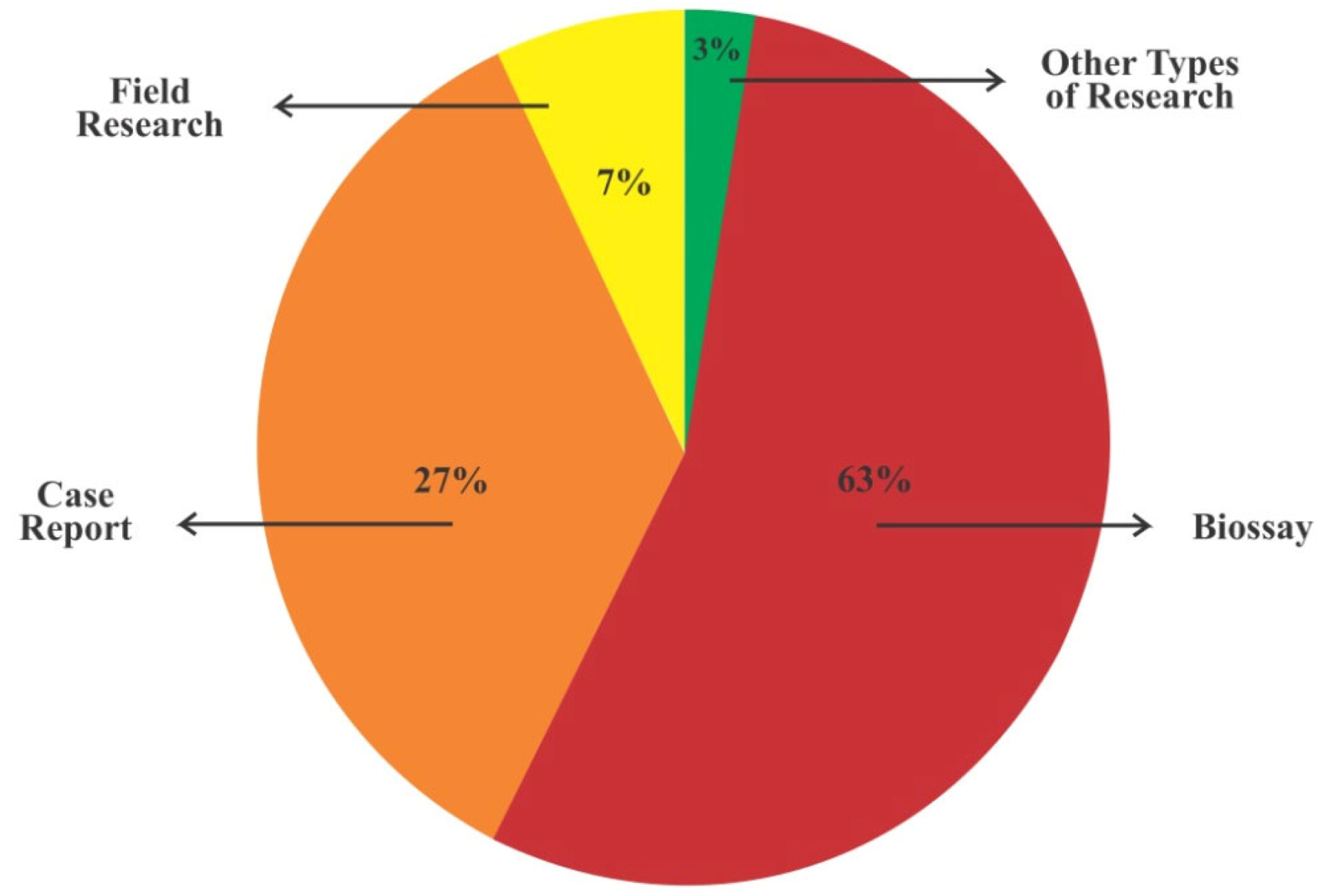
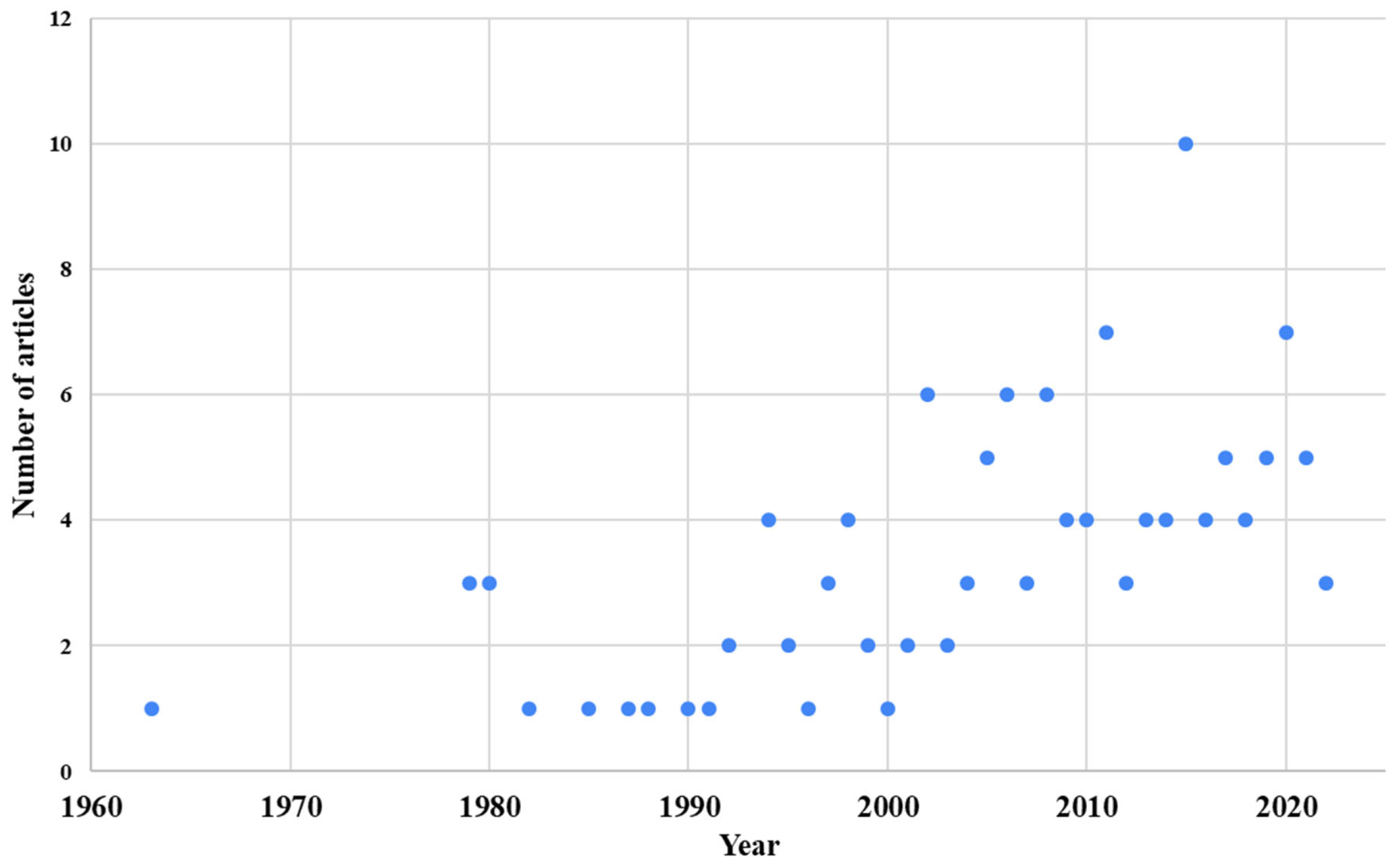
3.1. Types of Study, Location, Fungal Species, Year of Publication, and Most Frequent Journals
3.2. Studies on Vertebrate Animals
3.3. Studies on Invertebrate Animals
3.4. Experimental Studies on Various Organisms
3.5. Other Types of Studies
4. Discussion
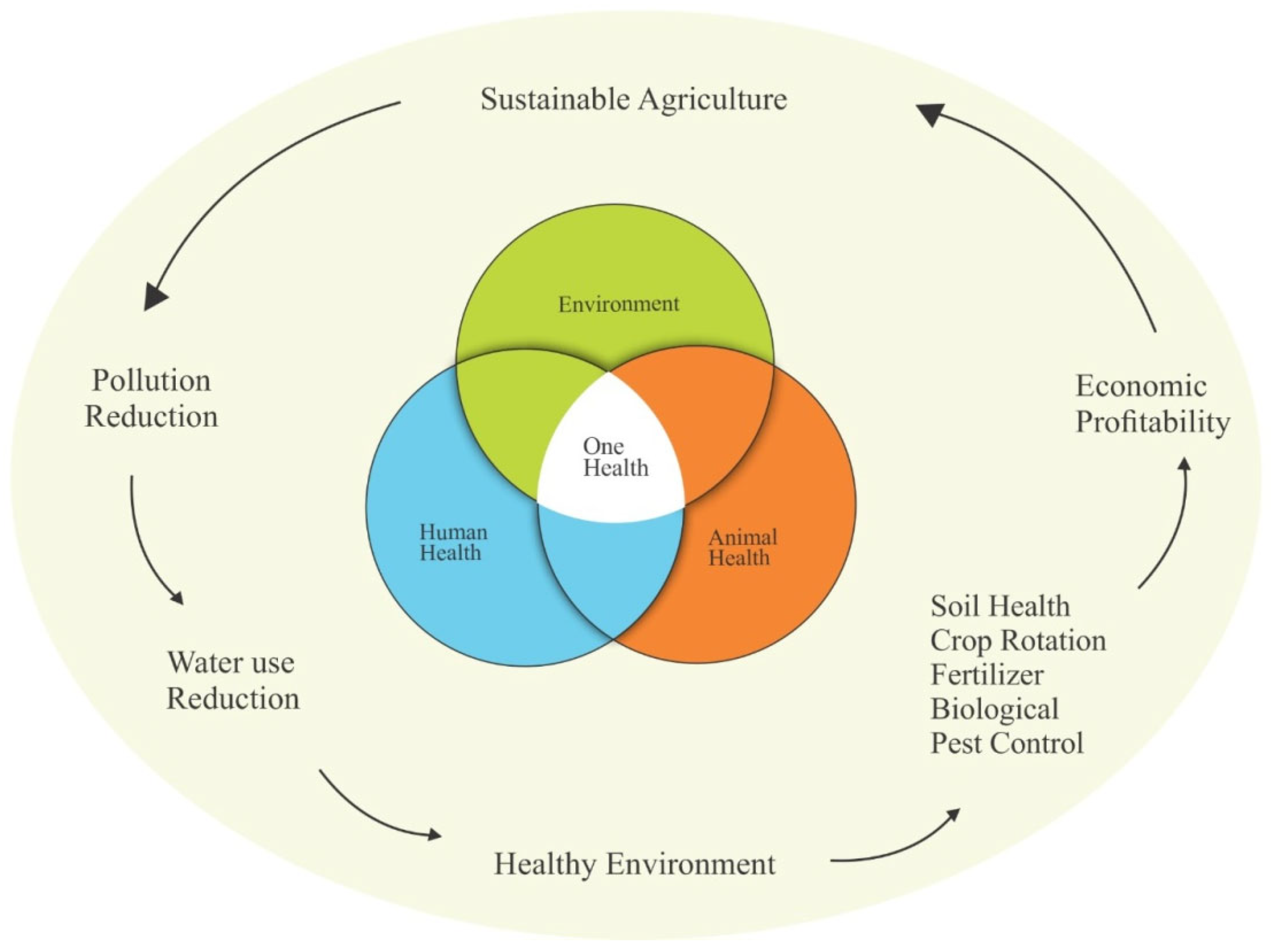
5. Effects on One Health
5.1. Human Health
5.2. Animal Health
5.2.1. Vertebrate Animals
5.2.2. Invertebrate Animals
5.3. Environment
6. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saath, K.C.O.; Fachinello, A.L. Crescimento da demanda mundial de alimentos e restrições do fator terra no Brasil. RESR 2018, 56, 195–212. [Google Scholar] [CrossRef]
- Frota, M.T.B.A.; Siqueira, C.E. Agrotóxicos: Os venenos ocultos na nossa mesa. Cad. Saúde Pública 2021, 37, 00004321. [Google Scholar] [CrossRef] [PubMed]
- Gazzoni, D.L. Como alimentar 10 bilhões de cidadãos na década de 2050? Ciência Cult. 2017, 69, 3–38. [Google Scholar] [CrossRef][Green Version]
- Abreu, L.P.S.; Berbert, P.A.; Teodoro, C.E.S.; Martinazzo, A.P. Alternativa sustentável de uso da bacillus amyloliquefaciens no biocontrole de fungos fitopatogênicos: Uma revisão. Rev. Ciências Ambient. 2022, 16, 1–15. [Google Scholar] [CrossRef]
- Silva, E.D.; Santos, E.E.S.; Santos, A.; Oliveira, F.J.V. Controle biológico de patógenos pós-colheita em videira. Sci. Electron. Arch. 2023, 16, 67–74. [Google Scholar] [CrossRef]
- Fontes, E.M.G.; Pires, C.S.S.; Sujii, E.R. Estratégias de uso e histórico. In Controle Biológico de Pragas da Agricultura; Fontes, E.M.G., Valadares-Inglis, M.C., Eds.; Embrapa: Brasília, Brazil, 2020; Available online: https://www.embrapa.br/en/busca-de-publicacoes/-/publicacao/1121825/controle-biologico-de-pragas-da-agricultura (accessed on 1 April 2023).
- Parra, J.R.P. Controle Biológico. Revista Cultivar. 2015. Available online: https://revistacultivar.com.br/artigos/controle-biologico (accessed on 24 December 2023).
- Filho, E.B.; Ciociola, A.I. Parasitoides ou Predadores? Vantagens e desvantagens. In Controle Biológico no Brasil: Parasitóides e Predadores; Parra, J.R.P., Ed.; Editora Manole Ltd.: São Paulo, Brazil, 2002. [Google Scholar]
- Alves, S.B.; Lopes, R.B.; Vielra, A.S.; Tamai, M.A. Fungos entomopatogênicos usados no controle de pragas na América Latina. ln Controle Microbiano de Pragas na América Latina: Avanços e Desafios; FEALQ: Piracicaba, Brazil, 2008; pp. 69–110. [Google Scholar]
- Mascarin, G.M.; Quintela, E.D. Técnica de Produção do Fungo Entomopatogênico Metarhizium Anisopliae Para Uso em Controle Biológico; Embrapa Arroz e Feijão: Santo Antônio de Goiás, Brazil, 2013. [Google Scholar]
- Alves, R.T.; Faria, R. Pequeno Manual Sobre Fungos Entomopatogênicos; Embrapa Cerrados: Planaltina, Brazil, 2010. [Google Scholar]
- Oliveira Filho, E.C.; Faria, M.R.; Castro, M.L.M.P. Regulamentação de Produtos Biológicos Para o Controle de Pragas Agrícolas; Embrapa Recursos Genéticos e Biotecnologia: Brasília, Brazil, 2004. [Google Scholar]
- Oliveira-Filho, E.C.; Monnerat, R.G. Fundamentos Para a Regulação de Semioquímicos, Inimigos Naturais e Agentes Microbiológicos de Controle de Pragas; Embrapa Cerrados: Planaltina, Brazil, 2006. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). National Center for Emerging and Zoonotic Infectious Diseases (NCEZID). Outubro. 2023. Available online: https://www.cdc.gov/onehealth/index.html (accessed on 18 November 2023).
- Carneiro, L.A.; Pettan-Brewer, C. One Health: Conceito, História e Questões Relacionadas Revisão e Reflexão Liliane. In Pesquisa em Saúde & Ambiente na Amazônia: Perspectivas Para Sustentabilidade Humana e Ambiental na Região; Miranda, A.M.M., Ed.; Científica Digital: Guarujá, Brazil, 2021; Available online: https://downloads.editoracientifica.com.br/books/978-65-89826-36-1.pdf (accessed on 1 May 2022).
- Sundh, I.; Del Giudice, T.; Cembalo, L. Reaping the Benefits of Microorganisms in Cropping Systems: Is the Regulatory Policy Adequate? Microorganisms 2021, 9, 1437. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and explanation. American College of Physicians. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Sampaio, R.F.; Mancini, M.C. Estudos de revisão sistemática: Um guia para síntese criteriosa da evidência científica. Rev. Bras. Fisioter. 2007, 11, 83–89. [Google Scholar] [CrossRef]
- Brasil. Ministério da Saúde. Secretaria de Ciência, Tecnologia e Insumos Estratégicos. Departamento de Ciência e Tecnologia. Diretrizes metodológicas: Elaboração de revisão sistemática e metanálise de ensaios clínicos randomizados; Ministério da Saúde: Brasília, Brazil, 2012. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/diretrizes_metodologicas_elaboracao_sistematica.pdf (accessed on 5 May 2025).
- Silva, M.R.; Hayashi, C.R.M.; Hayashi, M.C.P.I. Análise bibliométrica e cientométrica: Desafios para especialistas que atuam no campo. Rev. Ci. Inf. Doc. 2011, 2, 110–129. [Google Scholar] [CrossRef]
- Barbieri, R.T.; Croce, J.; Gandra, R.F.; Gagete, E.; Paula, C.R.; Gambale, W. Allergenic extracts from Metarhizium anisopliae: Obtainment and characterization. J. Investig. Allergol. Clin. Immunol. 2005, 15, 131–139. [Google Scholar] [PubMed]
- Westwood, G.S.; Huang, S.W.; Keyhani, N.O. Allergens of the entomopathogenic fungus Beauveria bassiana. Clin. Mol. Allergy 2005, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Westwood, G.S.; Huang, S.W.; Keyhani, N.O. Molecular and immunological characterization of allergens from the entomopathogenic fungus Beauveria bassiana. Clin. Mol. Allergy 2006, 4, 12. [Google Scholar] [CrossRef]
- Celik, M.; Aksoy, H.; Yilmaz, S. Evaluation of beauvericin genotoxicity with the chromosomal aberrations, sister-chromatid exchanges and micronucleus assays. Ecotoxicol. Environ. Saf. 2010, 73, 1553–1557. [Google Scholar] [CrossRef]
- Sachs, S.W.; Baum, J.; Mies, C. Beauveria bassiana keratitis. Br. J. Ophthalmol. 1985, 69, 548–550. [Google Scholar] [CrossRef] [PubMed]
- García, M.C.; Arboleda, M.L.; Barraquer, F.; Grose, E. Fungal keratitis caused by Metarhizium anisopliae var. anisopliae. J. Med. Vet. Mycol. 1997, 35, 361–363. [Google Scholar] [CrossRef]
- Furukawa, H.; Kusne, S.; Sutton, D.A.; Manez, R.; Carrau, R.; Nichols, L.; Abu-Elmagd, K.; Skedros, D.; Todo, S.; Rinaldi, M.G. Acute invasive sinusitis due to Trichoderma longibrachiatum in a liver and small bowel transplant recipient. Clin. Infect. Dis. 1998, 26, 487–489. [Google Scholar] [CrossRef]
- Burgner, D.; Eagles, G.; Burgess, M.; Procopis, P.; Rogers, M.; Muir, D.; Pritchard, R.; Hocking, A.; Priest, M. Disseminated invasive infection due to Metarrhizium anisopliae in an immunocompromised child. J. Clin. Microbiol. 1998, 36, 1146–1150. [Google Scholar] [CrossRef]
- Revankar, S.G.; Sutton, D.A.; Sanche, S.E.; Rao, J.; Zervos, M.; Dashti, F.; Rinaldi, M.G. Metarrhizium anisopliae as a cause of sinusitis in immunocompetent hosts. J. Clin. Microbiol. 1999, 37, 195–198. [Google Scholar] [CrossRef]
- Kisla, T.A.; Cu-Unjieng, A.; Sigler, L.; Sugar, J. Medical management of Beauveria bassiana keratitis. Cornea 2000, 19, 405–406. [Google Scholar] [CrossRef]
- Jani, B.R.; Rinaldi, M.G.; Reinhart, W.J. An unusual case of fungal keratitis: Metarrhizium anisopliae. Cornea 2001, 20, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Henke, M.O.; De Hoog, G.S.; Gross, U.; Zimmermann, G.; Kraemer, D.; Weig, M. Human deep tissue infection with an entomopathogenic Beauveria species. J. Clin. Microbiol. 2002, 40, 2698–2702. [Google Scholar] [CrossRef] [PubMed]
- Tucker, D.L.; Beresford, C.H.; Sigler, L.; Rogers, K. Disseminated Beauveria bassiana infection in a patient with acute lymphoblastic leukemia. J. Clin. Microbiol. 2004, 42, 5412–5414. [Google Scholar] [CrossRef]
- Doekes, G.; Larsen, P.; Sigsgaard, T.; Baelum, J. IgE sensitization to bacterial and fungal biopesticides in a cohort of Danish greenhouse workers: The BIOGART study. Am. J. Ind. Med. 2004, 46, 404–407. [Google Scholar] [CrossRef]
- Gürcan, S.; Tuğrul, H.M.; Yörük, Y.; Ozer, B.; Tatman-Otkun, M.; Otkun, M. First case report of empyema caused by Beauveria bassiana. Mycoses 2006, 49, 246–248. [Google Scholar] [CrossRef]
- Tu, E.Y.; Park, A.J. Recalcitrant Beauveria bassiana keratitis: Confocal microscopy findings and treatment with posaconazole (Noxafil). Cornea 2007, 26, 1008–1010. [Google Scholar] [CrossRef]
- Amiel, H.; Chohan, A.B.; Snibson, G.R.; Vajpayee, R. Atypical fungal sclerokeratitis. Cornea 2008, 27, 382–383. [Google Scholar] [CrossRef]
- Sonoyama, H.; Araki-Sasaki, K.; Kazama, S.; Kawasaki, T.; Ideta, H.; Sunada, A.; Asari, S.; Inoue, Y.; Hayashi, K. The characteristics of keratomycosis by Beauveria bassiana and its successful treatment with antimycotic agents. Clin. Ophthalmol. 2008, 2, 675–678. [Google Scholar]
- Marsh, R.A.; Lucky, A.W.; Walsh, T.J.; Pacheco, M.C.; Rinaldi, M.G.; Mailler-Savage, E.; Puel, A.; Casanova, J.L.; Bleesing, J.J.; Filippi, M.D.; et al. Cutaneous infection with Metarhizium anisopliae in a patient with hypohidrotic ectodermal dysplasia and immune deficiency. Pediatr. Infect. Dis. J. 2008, 27, 283–284. [Google Scholar] [CrossRef]
- Oh, J.Y.; Lee, M.J.; Wee, W.R.; Léo, J.W. A case of necrotizing sclerokeratitis and endophthalmitis caused by Beauveria bassiana. Jpn. J. Ophthalmol. 2009, 53, 551–553. [Google Scholar] [CrossRef]
- Pariseau, B.; Nehls, S.; Ogawa, G.S.; Sutton, D.A.; Wickes, B.L.; Romanelli, A.M. Beauveria keratitis and biopesticides: Case histories and a random amplification of polymorphic DNA comparison. Cornea. 2010, 29, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Motley, W.W.; Melson, A.T.; Mortensen, J.E. Pediatric Metarrhizium anisopliae keratitis. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2011, 15, 101–103. [Google Scholar] [CrossRef]
- Figueira, L.; Pinheiro, D.; Moreira, R.; Pinto, E.; Simões, J.; Camisa, E.; Torrão, L.; Palmares, J.; Falcão-Reis, F. Beauveria bassiana keratitis in bullous keratopathy: Antifungal sensitivity testing and management. Eur. J. Ophthalmol. 2012, 22, 814–818. [Google Scholar] [CrossRef]
- Molnár-Gábor, E.; Dóczi, I.; Hatvani, L.; Vágvölgyi, C.; Kredics, L. Isolated sinusitis sphenoidalis caused by Trichoderma longibrachiatum in an immunocompetent patient with headache. J. Med. Microbiol. 2013, 62, 1249–1252. [Google Scholar] [CrossRef]
- Ligozzi, M.; Maccacaro, L.; Passilongo, M.; Pedrotti, E.; Marchini, G.; Koncan, R.; Cornaglia, G.; Centonze, A.R.; Lo Cascio, G. A case of Beauveria bassiana keratitis confirmed by internal transcribed spacer and LSU rDNA D1-D2 sequencing. New Microbes New Infect. 2014, 2, 84–87. [Google Scholar] [CrossRef]
- Mitani, A.; Shiraishi, A.; Miyamoto, H.; Sunada, A.; Ueda, A.; Asari, S.; Zheng, X.; Yamamoto, Y.; Hara, Y.; Ohashi, Y. Fungal keratitis caused by Beauveria bassiana: Drug and temperature sensitivity profiles: A case report. BMC Res. Notes. 2014, 7, 677. [Google Scholar] [CrossRef]
- Dorin, J.; Debourgogne, A.; Zaïdi, M.; Bazard, M.C.; Machouart, M. First unusual case of keratitis in Europe due to the rare fungus Metarhizium anisopliae. Int. J. Med. Microbiol. 2015, 305, 408–412. [Google Scholar] [CrossRef]
- Ogawa, A.; Matsumoto, Y.; Yaguchi, T.; Shimmura, S.; Tsubota, K. Successful treatment of Beauveria bassiana fungal keratitis with topical voriconazole. J. Infect. Chemother. 2016, 22, 257–260. [Google Scholar] [CrossRef]
- Eguchi, H.; Toibana, T.; Hotta, F.; Miyamoto, T.; Mitamura, Y.; Yaguchi, T. Severe fungal sclerokeratitis caused by Metarhizium anisopliae: A case report and literature review. Mycoses 2015, 58, 88–92. [Google Scholar] [CrossRef]
- Lara Oya, A.; Medialdea Hurtado, M.E.; Rojo Martín, M.D.; Aguilera Pérez, A.; Alastruey-Izquierdo, A.; Miranda Casas, C.; Rubio Prats, M.; Medialdea Marcos, S.; Navarro Marí, J.M. Fungal Keratitis Due to Beauveria bassiana in a Contact Lenses Wearer and Review of Published Reports. Mycopathologia. 2016, 181, 745–752. [Google Scholar] [CrossRef]
- Showail, M.J.; Kus, J.V.; Tsui, G.K.; Chew, H.F. Fungal keratitis caused by Metarhizium anisopliae complex. Med. Mycol. Case Rep. 2017, 17, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Derhy, D.; Sauer, A.; Sabou, M.; Letsch, J.; Candolfi, E.; Letscher-Bru, V.; Bourcier, T. Surgical treatment of Metarhizium anisopliae sclerokeratitis and endophthalmitis. Indian J. Ophthalmol. 2017, 65, 523–526. [Google Scholar] [CrossRef]
- Gunn, D.J.; Tavassoli, S.; Darcy, K. Treatment of Metarhizium fungal keratitis in the United Kingdom. Eye 2018, 32, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Ducange, P.; Verdina, T.; Stiro, F.; Grottola, A.; Orlando, G.; Delvecchio, G.; Mastropasqua, R. Beauveria bassiana keratitis: Management of an atypical clinical presentation. Med. Mycol. Case Rep. 2021, 33, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Georg, L.K.; Williamson, W.M.; Tilden, E.B.; Getty, R.E. Mycotic pulmonary disease of captive giant tortoises due to Beauvaria bassiana and Paecilomyces fumoso-roseus. Med. Mycol. 1963, 2, 80–86. [Google Scholar] [CrossRef]
- Fromtling, R.A.; Jensen, J.M.; Robinson, B.E.; Bulmer, G.S. Fatal mycotic pulmonary disease of captive American alligators. Vet. Pathol. 1979, 16, 428–431. [Google Scholar]
- González Cabo, J.F.; Espejo Serrano, J.; Bárcena Asensio, M.C. Mycotic pulmonary disease by Beauveria bassiana in a captive tortoise. Mycoses 1995, 3, 167–169. [Google Scholar] [CrossRef]
- Muir, D.; Martin, P.; Kendall, K.; Malik, R. Invasive hyphomycotic rhinitis in a cat due to Metarhizium anisopliae. Med. Mycol. 1998, 36, 51–54. [Google Scholar] [CrossRef]
- Hall, N.H.; Conley, K.; Berry, C.; Farina, L.; Sigler, L.; Wellehan, J.F., Jr.; Roehrl, M.H.; Heard, D. Computed tomography of granulomatous pneumonia with oxalosis in an American alligator (Alligator mississippiensis) associated with Metarhizium anisopliae var anisopliae. J. Zoo. Wildl. Med. 2011, 42, 700–708. [Google Scholar] [CrossRef]
- Hartmann, G.C.; Wasti, S.S.; Hendrickson, D.L. Murine Safety of Two Species of Entomogenous Fungi, Cordyceps militaris (FRIES) LINK and Paecilomyces fumoso-roseus (WIZE) BROWN and SMITH. Appl. Entomol. Zool. 1979, 14, 217–220. [Google Scholar] [CrossRef]
- Wasti, S.; Hartmann, G.; Rousseau, A. Gypsy moth mycoses by two species of entomogenous fungi and an assessment of their avian toxicity. Parasitology 1980, 80, 419–424. [Google Scholar] [CrossRef]
- Donovan Peluso, M.; Wasti, S.S.; Hartmann, G.C. Safety of entomogenous fungi to vertebrate hosts. Appl. Entomol. Zool. 1980, 15, 498–499. [Google Scholar] [CrossRef]
- Mccoy, C.W.; Heimpel, A.M. Safety of the Potential Mycoacaricide, Hirsutella thompsonii, to Vertebrates. Environ. Entomol. 1980, 9, 47–49. [Google Scholar] [CrossRef]
- Shadduck, J.A.; Roberts, D.W.; Lause, S. Mammalian Safety Tests of Metarhizium anisopliae: Preliminary results. Environ. Entomol. 1982, 11, 189–192. [Google Scholar] [CrossRef]
- Siegel, J.P.; Shadduck, J.A. Safety of the entomopathogenic fungus Lagenidium giganteum (Oomycetes: Lagenidiales) to mammals. J. Econ. Entomol. 1987, 80, 994–997. [Google Scholar] [CrossRef]
- Genthner, F.J.; Middaugh, D.P. Effects of Beauveria bassiana on embryos of the inland silverside fish (Menidia beryllina). Appl. Environ. Microbiol. 1992, 58, 2840–2845. [Google Scholar] [CrossRef]
- Johnson, D.L.; Smits, J.E.; Jaronski, S.T.; Weaver, D.K. Assessment of health and growth of ring-necked pheasants following consumption of infected insects or conidia of entomopathogenic fungi, Metarhizium anisopliae var. acridum and Beauveria bassiana, from Madagascar and North America. J. Toxicol. Environ. Health 2002, 65, 2145–2162. [Google Scholar] [CrossRef]
- Milner, R.J.; Lim, R.P.; Hunter, D.M. Risks to the aquatic ecosystem from the application of Metarhizium anisopliae for locust control in Australia. Pest Manag. Sci. 2002, 58, 718–723. [Google Scholar] [CrossRef]
- Peveling, R.; Demba, S.A. Toxicity and pathogenicity of Metarhizium anisopliae var. acridum (Deuteromycotina, Hyphomycetes) and fipronil to the fringe-toed lizard Acanthodactylus dumerili (Squamata: Lacertidae). Environ. Toxicol. Chem. 2003, 22, 1437–1447. [Google Scholar] [CrossRef]
- Mier, T.; Olivares-Redonda, G.; Navarro-Barranco, H.; Pérez-Mejía, A.; Lorenzana, M.; Pérez-Torres, A.; Toriello, C. Acute oral intragastric pathogenicity and toxicity in mice of Paecilomyces fumosoroseus isolated from whiteflies. Antonie Van. Leeuwenhoek 2005, 88, 103–111. [Google Scholar] [CrossRef]
- Instanes, C.; Ward, M.D.; Hetland, G. The fungal biopesticide Metarhizium anisopliae has an adjuvant effect on the allergic response to ovalbumin in mice. Toxicol. Lett. 2006, 161, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.D.; Chung, Y.J.; Copeland, L.B.; Doerfler, D.L. Allergic Responses Induced by a Fungal Biopesticide Metarhizium anisopliae and House Dust Mite Are Compared in a Mouse Model. J. Toxicol. 2011, 2011, 360805. [Google Scholar] [CrossRef] [PubMed]
- Alves-Filho, E.R.; Maioli, T.U.; Faria, A.M.; Noronha, F.S.; Silva, N.M.; Costa, M.G.; Santos, J.L. The biocontrol fungus Trichoderma stromaticum downregulates respiratory burst and nitric oxide in phagocytes and IFN-gamma and IL-10. J. Toxicol. Environ. Health 2011, 74, 943–958. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Liu, Y.; Liu, C.; Meng, Q.; Song, J.; Wang, D.; Lu, J.; Teng, L.; Zhou, Y.; Teng, L. Acute and subchronic toxicity studies on safety assessment of Paecilomyces tenuipes N45 extracts. Comb. Chem. High. Throughput Screen. 2015, 18, 809–818. [Google Scholar] [CrossRef]
- Brunner-Mendoza, C.; Navarro-Barranco, H.; León-Mancilla, B.; Pérez-Torres, A.; Toriello, C. Biosafety of an entomopathogenic fungus Isaria fumosorosea in an acute dermal test in rabbits. Cutan. Ocul. Toxicol. 2016, 36, 12–18. [Google Scholar] [CrossRef]
- Schmidt, V.; Klasen, L.; Schneider, J.; Hübel, J.; Pees, M. Characterization of Metarhizium viride Mycosis in Veiled Chameleons (Chamaeleo calyptratus), Panther Chameleons (Furcifer pardalis), and Inland Bearded Dragons (Pogona vitticeps). J. Clin. Microbiol. 2017, 55, 832–843. [Google Scholar] [CrossRef]
- Fu, H.I.; Hsu, J.H.; Li, T.J.; Yeh, S.H.; Chen, C.C. Safety assessment of HEA-enriched Cordyceps cicadae mycelia on the central nervous system (CNS), cardiovascular system, and respiratory system in ICR male mice. Food Sci. Nutr. 2020, 9, 4905–4915. [Google Scholar] [CrossRef]
- Sayed Ali, S.; El-Saadany, H.M.; Kotb, G.A.M.; Elshaer, N.; Melebary, S.J.; Soliman, S.M.; AGh Farag, A. Biosafety evaluation of two Beauveria bassiana products on female albino rats using acute oral test. Saudi J. Biol. Sci. 2022, 29, 103293. [Google Scholar] [CrossRef]
- Abrar, A.; Abbas, M.; Mehmood, S.; Ghani, N.; Fatima, A.; Shahzadi, R. Scanning electron microscopy for identification of local strain of Aspergillus parasiticus and its larvicidal efficacy against Aedes Aegypti and non-target toxicity testing on fingerlings of Hypophthalmichthys Molitrix. Microsc. Res. Tech. 2022, 85, 3187–3192. [Google Scholar] [CrossRef]
- Pell, J.K.; Vandenberg, J.D. Interactions Among the Aphid Diuraphis noxia, the Entomopathogenic Fungus Paecilomyces fumosoroseus and the Coccinellid Hippodamia convergens. Biocontrol Sci. Technol. 2002, 12, 217–224. [Google Scholar] [CrossRef]
- Wu, S.; Xie, H.; Li, M.; Xu, X.; Lei, Z. Highly virulent Beauveria bassiana strains against the two-spotted spider mite, Tetranychus urticae, show no pathogenicity against five phytoseiid mite species. Exp. Appl. Acarol. 2016, 70, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Karthi, S.; Senthil-Nathan, S.; Kalaivani, K.; Vasantha-Srinivasan, P.; Chellappandian, M.; Thanigaivel, A.; Ponsankar, A.; Sivanesh, H.; Stanley-Raja, V.; Chanthini, K.M.; et al. Comparative efficacy of two mycotoxins against Spodoptera litura Fab. And their non-target activity against Eudrilus eugeniae Kinb. Ecotoxicol. Environ. Saf. 2019, 183, 109474. [Google Scholar] [CrossRef] [PubMed]
- Vasantha-Srinivasan, P.; Karthi, S.; Chellappandian, M.; Ponsankar, A.; Thanigaivel, A.; Senthil-Nathan, S.; Chandramohan, D.; Ganesan, R. Aspergillus flavus (Link) toxins reduces the fitness of dengue vector Aedes aegypti (Linn.) and their non-target toxicity against aquatic predator. Microb. Pathog. 2019, 128, 281–287. [Google Scholar] [CrossRef]
- Mei, L.; Chen, M.; Shang, Y.; Tang, G.; Tao, Y.; Zeng, L.; Huang, B.; Li, Z.; Zhan, S.; Wang, C. Population genomics and evolution of a fungal pathogen after releasing exotic strains to control insect pests for 20 years. ISME J. 2020, 14, 1422–1434. [Google Scholar] [CrossRef]
- Cantwell, G.E.; Lehnert, T. Lack of effect of certain microbial insecticides on the honeybee. J. Invertebr. Pathol. 1979, 33, 381–382. [Google Scholar] [CrossRef]
- Vandenbergi, J.D. Safety of Four Entomopathogens for Caged Adult Honey Bees (Hymenoptera: Apidae). J. Econ. Entomol. 1990, 83, 755–759. [Google Scholar] [CrossRef]
- Nuutinen, V.; Tyni-juslin, J.; Vänninen, I.; Vainio, A. The effects of four entomopathogenic fungi and an entomoparasitic nematode on the hatching of earthworm (Aporrectodea caliginosa) cocoons in laboratory. J. Invertebr. Pathol. 1991, 58, 147–149. [Google Scholar] [CrossRef]
- Lipa, J.J. Safety of Nosema meligethi I. and R. (Microsporida) to Apis mellifera L. and Coccinella septempunctata L. J. Invertebr. Pathol. 1992, 60, 310–311. [Google Scholar] [CrossRef]
- Butt, T.M.; Ibrahim, L.; Ball, B.V.; Clark, S.J. Pathogenicity of the entomogenous fungi Metarhizium anisopliae and Beauveria bassiana against crucifer pests and the honey bee. Biocontrol Sci. Technol. 1994, 4, 207–214. [Google Scholar] [CrossRef]
- Ball, B.V.; Pye, B.J.; Carreck, N.L.; Moore, D.; Bateman, R.P. Laboratory testing of amycopesticide on non-target organisms: The effects of an oil formulation of Metarhizium flavoviride applied to Apis mellifera. Biocontrol Sci. Technol. 1994, 4, 289–296. [Google Scholar] [CrossRef]
- Genthner, F.J.; Foss, S.S.; Fisher, W.S. Testing of the insect pest control fungus Beauveria bassiana in grass shrimp Pslaemonetes pugic. Dis. Aquat. Org. 1994, 20, 49–57. [Google Scholar] [CrossRef]
- Rath, A.C.; Worledge, D.; Koen, T.B.; Rowe, B.A. Long-term Field Efficacy of the Entomogenous Fungus Metarhizium anisopliae against the Subterranean Scarab, Adoryphorus couloni. Biocontrol Sci. Technol. 1995, 5, 439–452. [Google Scholar] [CrossRef]
- Alves, S.B.; Marchini, L.C.; Pereira, R.M.; Baumgratz, L.L. Effects of some insect pathogens on the Africanized honey bee, Apis mellifera L. (Hym., Apidae). J. Appl. Entomol. 1996, 120, 559–564. [Google Scholar] [CrossRef]
- Genthner, F.J.; Foss, S.S.; Glas, P.S. Virulence of Metarhizium anisopliae to Embryos of the Grass Shrimp Palaemonetes pugio. J. Invertebr. Pathol. 1997, 69, 157–164. [Google Scholar] [CrossRef]
- Sitch, J.C.; Jackson, C.W. Pre-penetration events affecting host specificity of Verticillium lecanii. Mycol. Res. 1997, 101, 535–541. [Google Scholar] [CrossRef]
- Danfa, A.; Van Der Valk, H.C.H.G. Laboratory Testing of Metarhizium spp. and Beauveria bassiana on Sahelian Non-target Arthropods. Biocontrol Sci. Technol. 1999, 9, 187–198. [Google Scholar] [CrossRef]
- Wang, Y.; Crocker, R.; Wilson, L.T.; Smart, G.; Wei, X.; Nailon, W.T.; Cobb, P.P. Effect of Nematode and Fungal Treatments on Nontarget Turfgrass-Inhabiting Arthropod and Nematode Populations. Environ. Entomol. 2001, 30, 196–203. [Google Scholar] [CrossRef]
- Ivie, M.A.; Pollock, D.A.; Gustafson, D.L.; Rasolomandimby, J.; Ivie, L.L.; Swearingen, W.D. Field-Based Evaluation of Biopesticide Impacts on Native Biodiversity: Malagasy coleoptera and anti-locust entomopathogenic fungi. J. Econ. Entomol. 2002, 95, 651–660. [Google Scholar] [CrossRef]
- Dromph, K.M.; Vestergaard, S. Pathogenicity and attractiveness of entomopathogenic hyphomycete fungi to collembolans. Appl. Soil Ecol. 2002, 21, 197–210. [Google Scholar] [CrossRef]
- Arthurs, S.; Thomas, M.B.; Langewald, J. Field observations of the effects of fenitrothion and Metarhizium anisopliae var. acridum on non-target ground dwelling arthropods in the Sahel. Biol. Control 2003, 26, 333–340. [Google Scholar] [CrossRef]
- Wang, C.; Fan, M.; Li, Z.; Butt, T.M. Molecular monitoring and evaluation of the application of the insect-pathogenic fungus Beauveria bassiana in southeast China. J. Appl. Microbiol. 2004, 96, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Traugott, M.; Weissteiner, S.; Strasser, H. Effects of the entomopathogenic fungus Beauveria brongniartii on the non-target predator Poecilus versicolor (Coleoptera: Carabidae). Biol. Control 2005, 33, 107–112. [Google Scholar] [CrossRef]
- Al Mazra’awi, M.S.; Shipp, J.L.; Broadbent, A.B.; Kevan, P.G. Dissemination of Beauveria bassiana by Honey Bees (Hymenoptera: Apidae) for Control of Tarnished Plant Bug (Hemiptera: Miridae) on Canola. Environ. Entomol. 2006, 35, 1569–1577. [Google Scholar] [CrossRef]
- Favilla, M.; Macchia, L.; Gallo, A.; Altomare, C. Toxicity assessment of metabolites of fungal biocontrol agents using two different (Artemia salina and Daphnia magna) invertebrate bioassays. Food Chem. Toxicol. 2006, 44, 1922–1931. [Google Scholar] [CrossRef]
- Kapongo, J.P.; Shipp, L.; Kevan, P.; Broadbent, B. Optimal concentration of Beauveria bassiana vectored by bumble bees in relation to pest and bee mortality in greenhouse tomato and sweet pepper. BioControl 2008, 53, 797–812. [Google Scholar] [CrossRef]
- Thungrabeab, M.; Tongma, S. Effect of Entomopathogenic Fungi, Beauveria bassiana (Balsam) and Metarhizium anisopliae (Metsch) on non Target Insect. KMITL Sci. Tech. J. 2007, 7, 8–12. [Google Scholar]
- Mommaerts, V.; Platteau, G.; Boulet, J.; Sterk, G.; Smagghe, G. Trichoderma-based biological control agents are compatible with the pollinator Bombus terrestris: A laboratory study. Biol. Control 2008, 46, 463–466. [Google Scholar] [CrossRef]
- García-Fernández, P.; Santiago-Álvarez, C.; Quesada-Moraga, E. Pathogenicity and thermal biology of mitosporic fungi as potential microbial control agents of Varroa destructor (Acari: Mesostigmata), an ectoparasitic mite of honey bee, Apis melífera (hymenoptera). Apidologie 2008, 39, 662–673. [Google Scholar] [CrossRef]
- Mommaerts, V.; Sterk, G.; Hoffmann, L.; Smagghe, G. A laboratory evaluation to determine the compatibility of microbiological control agents with the pollinator Bombus terrestris. Pest Manag. Sci. 2009, 65, 949–955. [Google Scholar] [CrossRef]
- Rodríguez, M.; Gerding, M.; France, A.; Ceballos, R. Evaluation of Metarhizium anisopliae var. anisopliae Qu-M845 Isolate to Control Varroa destructor (Acari: Varroidae) in Laboratory and Field Trials. Chil. J. Agric. Res. 2009, 69, 541–547. [Google Scholar] [CrossRef]
- Mao, B.Z.; Huang, C.; Yang, G.M.; Chen, Y.Z.; Chen, S.Y. Separation and determination of the bioactivity of oosporein from Chaetomium cupreum. Afr. J. Biotechnol. 2010, 9, 5955–5961. [Google Scholar]
- Soni, J.; Thakur, M. Effect of Biopathogens on Honey Bees. Pest Technol. 2011, 5, 86–90. [Google Scholar]
- Luan, F.; Zhang, S.; Cai, Y.; Sun, Z.; Wang, B.; Huang, B.; Li, Z. Identification of the molecular origin and development of a panzootic caused by Beauveria bassiana in praying mantis populations in eastern China. J. Invertebr. Pathol. 2011, 108, 98–105. [Google Scholar] [CrossRef]
- James, R.R.; Mcguire, M.R.; Leland, J.E. Susceptibility of Adulo Alfalfa Leafcutting Bees1and Honey Bees to a Microbial Control Agent, Beauveria bassiana. Southwest. Entomol. 2012, 37, 13–21. [Google Scholar] [CrossRef]
- Ramanaidu, K.; Cutler, G.C. Different toxic and hormetic responses of Bombus impatiens to Beauveria bassiana, Bacillus subtilis and spirotetramat. Pest Manag. Sci. 2013, 69, 949–954. [Google Scholar] [CrossRef]
- Abdel Rasoul, M.A.; Eid, K.S.A.; Marei, G.I.K. Impacts of multiple applications with biofly (Beauveria bassiana) and spintor® (spinosad) on honey bee (Apis mellifera) larvae. J. Plant Prot. Pathol. 2013, 4, 49–66. [Google Scholar] [CrossRef]
- Luan, F.; Zhang, S.; Wang, B.; Huang, B.; Li, Z. Genetic diversity of the fungal pathogen Metarhizium spp., causing epizootics in Chinese burrower bugs in the Jingting Mountains, eastern China. Mol. Biol. Rep. 2013, 40, 515–523. [Google Scholar] [CrossRef]
- Smagghe, G.; De Meyer, L.; Meeus, I.; Mommaerts, V. Safety and acquisition potential of Metarhizium anisopliae in entomovectoring with bumble bees, Bombus terrestris. J. Econ. Entomol. 2013, 106, 277–282. [Google Scholar] [CrossRef]
- Conceição, P.J.; Neves Cynthia, M.L.; Sodré, G.S.; Carvalho, C.; Souza, A.V.; Ribeiro, G.S.; Pereira, R.C. Susceptibility of Melipona scutellaris Latreille, 1811 (Hymenoptera: Apidae) worker bees to Beauveria bassiana (bals.) vuill. Sociobiology 2014, 61, 184–188. [Google Scholar] [CrossRef]
- Chen, X.; Huang, C.; He, L.; Zhang, S.; Li, Z. Molecular tracing of white muscardine in the silkworm, Bombyx mori (Linn.) II. Silkworm white muscardinas is not caused by artificial release or natural epizootic of Beauveria bassiana in China. J. Invertebr. Pathol. 2015, 125, 16–22. [Google Scholar] [CrossRef]
- Karise, R.; Muljar, R.; Smagghe, G.; Kaart, T.; Kuusik, A.; Dreyersdorff Williams, I.; Mänd, M. Sublethal effects of kaolin and the biopesticides Prestop-Mix and BotaniGard on metabolic rate, water loss and longevity in bumble bees (Bombus terrestris). J. Pest Sci. 2015, 89, 71–178. [Google Scholar] [CrossRef]
- Seiedy, M.; Tork, M.; Deyhim, F. Effect of the entomopathogenic fungus Beauveria bassiana on the predatory mite Amblyseius swirskii (Acari: Phytoseiidae) as a non-target organism. Syst. Appl. Acarol. 2015, 20, 241. [Google Scholar]
- Rännbäck, L.M.; Cotes, B.; Anderson, P.; Rämert, B.; Meyling, N.V. Mortality risk from entomopathogenic fungi affects oviposition behavior in the parasitoid wasp Trybliographa rapae. J. Invertebr. Pathol. 2015, 124, 78–86. [Google Scholar] [CrossRef]
- Bajya, D.R.; Ranjith, M.; Raza, S.K. Evaluation of Beauveria bassiana against chickpea pod borer, Helicoverpa armigera and its safety to natural enemies. Indian J. Agric. Sci. 2015, 85, 378–381. [Google Scholar] [CrossRef]
- Babendreier, D.; Jeannere, P.; Pilz, C.; Toepfer, S. Non-target effects of insecticides, entomopathogenic fungi and nematodes applied against western corn rootworm larvae in maize. J. Appl. Entomol. 2015, 139, 457–467. [Google Scholar] [CrossRef]
- Toledo-Hernández, R.A.; Ruíz-Toledo, J.; Toledo, J.; Sánchez, D. Effect of Three Entomopathogenic Fungi on Three Species of Stingless Bees (Hymenoptera: Apidae) Under Laboratory Conditions. J. Econ. Entomol. 2016, 109, 1015–1019. [Google Scholar] [CrossRef]
- Portilla, M.; Luttrell, R.; Snodgrass, G.; Zhu, Y.C.; Riddick, E. Lethality of the Entomogenous Fungus Beauveria bassiana Strain NI8 on Lygus lineolaris (Hemiptera: Miridae) and its Possible Impact on Beneficial Arthropods. J. Entomol. Sci. 2017, 52, 352–369. [Google Scholar]
- Ríos-Moreno, A.; Garrido-Jurado, I.; Raya-Ortega, M.C.; Quesada-Moraga, E. Quantification of fungal growth and destruxin A during infection of Galleria mellonella larvae by Metarhizium brunneum. J. Invertebr. Pathol. 2017, 149, 29–35. [Google Scholar] [CrossRef]
- Karise, R.; Raimets, R.; Dreyersdorff, G.; Mänd, M. Using respiratory physiology techniques in assessments of pesticide effects. Jul. Kühn Arch. 2018, 462, 61–66. [Google Scholar]
- Bilgo, E.; Lovett, B.; St Leger, R.J.; Sanon, A.; Dabiré, R.K.; Diabaté, A. Native entomopathogenic Metarhizium spp. from Burkina Faso and their virulence against the malaria vector Anopheles coluzzii and non-target insects. Parasit. Vectors 2018, 11, 209. [Google Scholar] [CrossRef]
- Potrich, M.; Silva, R.T.L.; Maia, F.M.C.; Lozanoa, E.R.; Rossi, R.M.; Colomboa, F.C.; Tedescoa, F.G.; Gouvea, A. Effect of entomopathogens on Africanized Apis mellifera L. (Hymenoptera: Apidae). Rev. Bras. Entomol. 2018, 62, 23–28. [Google Scholar] [CrossRef]
- Cappa, F.; Petrocelli, I.; Dani, F.R.; Dapporto, L.; Giovannini, M.; Silva-Castellari, J.; Turillazzi, S.; Cervo, R. Natural biocide disrupts nestmate recognition in honeybees. Sci. Rep. 2019, 9, 3171. [Google Scholar] [CrossRef] [PubMed]
- Akkoc, S.; Karaca, İ.; Karaca, G. Effects of Some Entomopathogen Fungi on Apis mellifera L. and Bombus terrestris L. Süleyman Demirel Üniversitesi Fen Bilimleri Enstitüsü Dergisi. J. Nat. Appl. Sci. 2019, 23, 433–439. [Google Scholar]
- Challa, G.K.; Firake, D.M.; Behere, G.T. Bio-pesticide applications may impair the pollination services and survival of foragers of honey bee, Apis cerana Fabricius in oilseed brassica. Environ. Pollut. 2019, 249, 598–609. [Google Scholar] [CrossRef]
- Peng, G.; Tong, S.M.; Zeng, D.; Xia, Y.; Feng, M.G. Colony leasing protects honey bee populations from a risk of contact with wide-spectrum Beauveria bassiana insecticides applied in the field. Pest Manag. Sci. 2020, 76, 2627–2634. [Google Scholar] [CrossRef]
- Carlesso, D.; Smargiassi, S.; Sassoli, L.; Cappa, F.; Cervo, R.; Baracchi, D. Exposure to a biopesticide interferes with sucrose responsiveness and Lear Ning in honey bees. Sci. Rep. 2020, 10, 19929. [Google Scholar] [CrossRef]
- Colombo, F.C.; Maciel, R.M.A.; Abati, R.; Raulino-domanski, F.; Longhim, S.J.; Costa-Maia, F.M.; Vismara, E.S.; Lozano, E.R.; Potrich, M. Do Beauveria bassiana and Metarhizium anisopliae affect worker survival and the production of Africanized Apis mellifera queens? J. Apic. Res. 2020, 60, 260–269. [Google Scholar] [CrossRef]
- Bordalo, M.D.; Gravato, C.; Beleza, S.; Campos, D.; Lopes, I.; Pestana, J.L.T. Lethal and sublethal toxicity assessment of Bacillus thuringiensis var. israelensis and Beauveria bassiana based bioinsecticides to the aquatic insect Chironomus riparius. Sci. Total Environ. 2020, 698, 134155. [Google Scholar] [CrossRef]
- Omuse, E.R.; Niassy, S.; Wagacha, J.M.; Ong’amo, G.O.; Lattorff, H.M.G.; Kiatoko, N.; Mohamed, S.A.; Subramanian, S.; Afuste, K.S.; Dubois, T. Susceptibility of the Western Honey Bee Apis mellifera and the African Stingless Bee Meliponula ferruginea (Hymenoptera: Apidae) to the Entomopathogenic Fungi Metarhizium anisopliae and Beauveria bassiana. J. Econ. Entomol. 2021, 115, 46–55. [Google Scholar] [CrossRef]
- Almeida, F.C.R.; Magalhães, D.M.; Favaris, A.P.; Rodríguez, J.; Azevedo, K.E.X.; Bento, J.M.S.; Alves, D.A. Side effects of a fungus-based biopesticide on stingless bee guarding behaviour. Chemosphere 2021, 287, 132147. [Google Scholar] [CrossRef]
- Reinbacher, L.; Bacher, S.; Praprotnik, E.; Grabenweger, G. Standard non-target tests for risk assessment of plant protection products are unsuitable for entomopathogenic fungi a proposal for a new protocol. J. Soils Sediments 2021, 21, 2357–2368. [Google Scholar] [CrossRef]
- Leite, M.O.G.; Alves, D.A.; Lecocq, A.; Malaquias, J.B.; Delalibera, I., Jr.; Jensen, A.B. Laboratory Risk Assessment of Three Entomopathogenic Fungi Used for Pest Control toward Social Bee Pollinators. Microorganisms 2022, 10, 1800. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, J.L.; Dritz, D.A.; Washino, R.K. Nonmammalian safety tests for Lagenidium giganteum (Oömycetes: Lagenidiales). J. Econ. Entomol. 1988, 81, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Nestrud, L.B.; Anderson, R.L. Aquatic safety of Lagenidium giganteum: Effects on freshwater fish and invertebrates. J. Invertebr. Pathol. 1994, 64, 228–233. [Google Scholar] [CrossRef]
- Genthner, F.J.; Chancy, C.A.; Couch, J.A.; Foss, S.S.; Middaugh, D.P.; George, S.E.; Warren, M.A.; Bantle, J.A. Toxicity and Pathogenicity Testing of the Insect Pest Control Fungus Metarhizium anisopliae. Arch. Environ. Contam. Toxicol. 1998, 35, 317–324. [Google Scholar] [CrossRef]
- Skrobek, A.; Boss, D.; Défago, G.; Butt, T.M.; Maurhofer, M. Evaluation of different biological test systems to assess the toxicity of metabolites from fungal biocontrol agents. Toxicol. Lett. 2006, 161, 43–52. [Google Scholar] [CrossRef]
- Tanner, R.A.; Pollard, K.M.; Varia, S.; Evans, H.C.; Ellison, C.A. First release of a fungal classical biocontrol agent against an invasive alien weed in Europe: Biology of the rust, Puccinia komarovii var. glanduliferae. Plant Pathol. 2015, 64, 1130–1139. [Google Scholar] [CrossRef]
- Kaur, M.; Chadha, P.; Kaur, S.; Kaur, A. Aspergillus flavus induced oxidative stress and immunosuppressive activity in Spodoptera litura as well as safety for mammals. BMC Microbiol. 2021, 21, 180. [Google Scholar] [CrossRef]
- Darbro, J.M.; Thomas, M.B. Spore persistence and likelihood of aeroallergenicity of entomopathogenic fungi used for mosquito control. Am. J. Trop. Med. Hyg. 2009, 80, 992–997. [Google Scholar] [CrossRef]
- Hansen, V.M.; Winding, A.; Madsen, A.M. Exposure to bioaerosols during the growth season of tomatoes in an organic greenhouse using Supresivit (Trichoderma harzianum) and Mycostop (Streptomyces griseoviridis). Appl. Environ. Microbiol. 2010, 76, 5874–5881. [Google Scholar] [CrossRef]
- Seger, C.; Erlebach, D.; Stuppner, H.; Griesser, U.J.; Strasser, H. Physicochemical Properties of Oosporein, the Major Secreted Metabolite of the Entomopathogenic Fungus Beauveria brongniartii. Helv. Chim. Acta 2005, 88, 802–810. [Google Scholar] [CrossRef]
- Kouvelis, V.N.; Wang, C.; Skrobek, A.; Pappas, K.M.; Typas, M.A.; Butt, T.M. Assessing the cytotoxic and mutagenic effects of secondary metabolites produced by several fungal biological control agents with the Ames assay and the VITOTOX (®) test. Mutat. Res. 2011, 722, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Moreira, S.M.C.O. Perspectivas para uso de fungos entomopatogênicos no território da Transamazônica e Xingu—Pará—Brasil. Rev. Agron. Bras. 2018, 2, 1–2. [Google Scholar] [CrossRef]
- Faria, M.; Mascarin, G.M.; Souza, D.A.; Lopes, R.B. Controle de Qualidade de Produtos Comerciais à Base de Fungos Para Manejo de Invertebrados (Insetos, Ácaros, Nematoides) em Sistemas Agropecuários; Embrapa Recursos Genéticos e Biotecnologia: Brasília, Brazil, 2022. [Google Scholar]
- Valadares-Inglis, M.C.; Lopes, R.B.; Faria, M.R. Controle de artrópodes-praga com fungos entomopatogênicos. In Controle Biológico de Pragas da Agricultura; Fontes, E.M.G., Valadares-Inglis, M.C., Eds.; Embrapa: Brasília, Brazil, 2020; Available online: https://www.embrapa.br/en/busca-de-publicacoes/-/publicacao/1121825/controle-biologico-de-pragas-da-agricultura (accessed on 16 July 2022).
- Sá, L.A.N.; Capalbo, D.M.F. Regulamentações. Agricultura e Meio ambiente. Embrapa. 2021. Available online: https://www.embrapa.br/en/agencia-de-informacao-tecnologica/tematicas/agricultura-e-meio-ambiente/manejo/avaliacao-de-risco/regulamentacoes (accessed on 13 January 2024).
- De la Cruz Quiroz, R.; Cruz Maldonado, J.; Rostro Alanis, M.; Torres, J.A.; Parra Saldívar, R. Fungi-based biopesticides: Shelf-life preservation technologies used in commercial products. J. Pest Sci. 2019, 92, 1003–1015. [Google Scholar] [CrossRef]
- Islam, W.; Adnan, M.; Shabbir, A.; Naveed, H.; Abubakar, Y.S.; Qasim, M.; Tayyab, M.; Noman, A.; Nisar, M.S.; Khan, K.A. Insect-fungal-interactions: A detailed review on entomopathogenic fungi pathogenicity to combat insect pests. Microb. Pathog. 2021, 159, 105–122. [Google Scholar] [CrossRef]
- Kaushal, K.S.; Ajoy, K.C.; Priyanka, K. Entomopathogenic Fungi. In Omkar. Ecofriendly Pest Management for Food Security; Academic Press: Cambridge, MA, USA, 2016; pp. 475–505. [Google Scholar]
- Tiago, P.V.; Oliveira, N.T.; Lima, E.Á.L.A. Biological insect control using Metarhizium anisopliae: Morphological, molecular, and ecological aspects. Cienc. Rural. 2014, 44, 645–651. [Google Scholar] [CrossRef]
- Kepler, R.M.; Luangsa-Ard, J.J.; Hywel-Jones, N.L.; Quandt, C.A.; Sung, G.H.; Rehner, S.A.; Aime, M.C.; Henkel, T.W.; Sanjuan, T.; Zare, R.; et al. A phylogenetically-based nomenclature for Cordycipitaceae (Hypocreales). IMA Fungus 2017, 8, 335–353. [Google Scholar] [CrossRef]
- Harris-Shultz, K.; Knoll, J.; Punnuri, S.; Niland, E.; Ni, X. Evaluation of strains of Beauveria bassiana and Isaria fumosorosea to control sugarcane aphids on grain sorghum. Agrosyst. Geosci. Environ. 2020, 3, e20047. [Google Scholar] [CrossRef]
- Vivian, R.; Quirino, R.B. Mercado de Agentes de Controle Biológico. In Controle Biológico de Pragas da Agricultura; Fontes, E.M.G., Valadares-Inglis, M.C., Eds.; Embrapa: Brasília, Brazil, 2020; Available online: https://www.embrapa.br/en/busca-de-publicacoes/-/publicacao/1121825/controle-biologico-de-pragas-da-agricultura (accessed on 16 July 2022).
- Bettiol, W.; Medeiros, F.H.V. Como o Brasil se Tornou o Maior Produtor e Consumidor de Produtos de Biocontrole. 17/03/2023. Available online: https://www.embrapa.br/en/busca-de-noticias/-/noticia/79156418/artigo-como-o-brasil-se-tornou-o-maior-produtor-e-consumidor-de-produtos-de-biocontrole (accessed on 18 December 2023).
- Ministério da Agricultura, Pecuária e Abastecimento. Agrofit: System of Phytosanitary Pesticides. Available online: http://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons (accessed on 5 April 2025).
- Ortiz-Urquiza, A.; Luo, Z.; Keyhani, N.O. Improving mycoinsecticides for insect biological control. Appl. Microbiol. Biotechnol. 2015, 99, 1057–1068. [Google Scholar] [CrossRef]
- Oliveira, M.T.; Monteiro, A.C.; Junior, N.S.; Barbosa, J.C.; Mochi, D.A. Sensibilidade de isolados de fungos entomopatogênicos às radiações solar, ultravioleta e à temperatura. Arq. Inst. Biol. 2016, 83, 1–7. [Google Scholar] [CrossRef]
- Centro de Microcirurgia Ocular (CEMO). Entendendo a Ceratite: Causas, Sintomas e Tratamentos. Brasil. 2023. Available online: https://oftalmologiacemo.com.br/2023/07/05/entendendo-a-ceratite/ (accessed on 27 December 2023).
- Murray, P.R.; Rosenthal, K.S.; Pfaller, M.A. Microbiologia médica; Elsevier: Rio de Janeiro, Brazil, 2009. [Google Scholar]
- Ortiz-martinez, Y.; Fajardo-Rivero, J.E.; Mendoza-Herrera, T.; Ruiz, C.; Figueroa-Pineda, C.; Masías, Y.; Moreno-Moreno, D.; Rodríguez-Morales, A.J. Epidemiologia e fatores preditivos de infecções fúngicas invasivas em pacientes com leucemia. Braz. J. Infect. Dis. 2022, 26, 101996. [Google Scholar] [CrossRef]
- Valadares-Inglis, M.C.; Martins, I.; Silva, J.P.; Mello, S.C.M. Seleção in vitro de Linhagens de Trichoderma Para Controle da Podridão-branca Doa Alho e da Cebola; Embrapa Recursos Genéticos e Biotecnologia: Brasília, Brazil, 2018. [Google Scholar]
- Camargo, J.M.M.; Leite, M.S.P.; Zaleski, S.E.M.; Ribeiro, R.D.; Penteado, S.R.C. Impacto do Fungo Entomopatogênico Verticillium lecanii (Zimm.) Viègas Sobre Larvas do Predador Syrphus Phaeostigma Wiedemann (Diptera: Syrphidae); Embrapa Floresta: Colombo, Brazil, 2004. [Google Scholar]
- Castillo, H.; Rojas, R.; Villalta, M. Gliocladium sp., important biocontrol agent with promising applications. Rev. Tecnol. Marcha. 2016, 29, 65–73. [Google Scholar]
- Juariyah, S.; Tondok, E.T.; Sinaga, M.S. Trichoderma dan Gliocladium untuk mengendalikan penyakit busuk akar Fusarium pada bibit kelapa sawit. J. Fitopatol. Indones. 2018, 14, 196. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef]
- Hassine, M.; Aydi-Ben-Abdallah, R.; Jabnoun-Khireddine, H.; Daami-Remadi, M. Soil-borne and compost-borne Penicillium sp. and Gliocladium spp. as potential microbial biocontrol agents for the suppression of anthracnose-induced decay on tomato fruits. Egypt. J. Biol. Pest. Control 2022, 32, 20. [Google Scholar] [CrossRef]
- Isah, U.; Ahmad, M.A. Microorganisms as bioinsecticides; short review. BAJOPAS 2020, 12, 274–279. [Google Scholar] [CrossRef]
- Luccâs, N.; Santos, C.L.A.; Furlanetti, A.C. Abelhas polinizadoras, agricultura sustentável e segurança alimentar no brasil: Refletindo os ODS 2 à luz da ciência pós-normal. Rev. Bras. Meio Ambiente Sustentabilidade 2021, 1, 79–104. [Google Scholar]
- Půža, V.; Tarasco, E. Interactions between entomopathogenic fungi and entomopathogenic nematodes. Microorganisms 2023, 11, 163. [Google Scholar] [CrossRef]
- Abbas, M.S.T. Interactions between entomopathogenic fungi and entomophagous insects. Adv. Entomol. 2020, 8, 130–146. [Google Scholar] [CrossRef]
- Draganova, S.; Donkova, R.; Georgieva, D. Impact of strains of entomopathogenic fungi on some main groups of soil microorganisms. J. Plant Prot. Res. 2008, 48, 83–88. [Google Scholar] [CrossRef]
- Canfora, L.; Tartanus, M.; Manfredini, A.; Tkaczuk, C.; Majchrowska-Safaryan, A.; Malusà, E. The impact of Beauveria species bioinocula on the soil microbial community structure in organic strawberry plantations. Front. Microbiol. 2022, 13, 1073386. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Environmental Health. 2024. Available online: https://www.who.int/health-topics/environmental-health#tab=tab_1 (accessed on 14 January 2024).
- World Health Organization (WHO). One Health. 2024. Available online: https://www.who.int/health-topics/one-health#tab=tab_1 (accessed on 14 January 2024).
- Weihs, M.; Mertens, F. Os desafios da geração do conhecimento em saúde ambiental: Uma perspectiva ecossistêmica. Ciência Saúde Coletiva 2013, 18, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Vivekanandhan, P.; Swathy, K.; Shivakumar, M.S. Identification of insecticidal molecule aucubin from Metarhizium anisopliae ethyl acetate crude extract against disease mosquito vector. Int. J. Trop. Insect Sci. 2022, 42, 3303–3318. [Google Scholar] [CrossRef]
- Santos, M.O. Atualizações Sobre o Diagnóstico Laboratorial das Infecções Fúngicas, 1st ed.; SBCSaúde: Goiânia, Brazil, 2019. [Google Scholar]
- Brasil. Classificação de risco dos agentes biológicos, 3rd ed.; Ministério da Saúde, Secretaria de Ciência, Tecnologia e Insumos Estratégicos, Departamento do Complexo Industrial e Inovação em Saúde, Ed.; Ministério da Saúde: Brasília, Brazil, 2017. [Google Scholar]
- Lim, L.; Hj Awg Besar, A.S.; Kean, S.; Iswari, D.; Sikaisone, P.; Jafar, A.; Cayabyab, B.; Jia Yih Wong Winotai, A.; Phong, B.; Khoa, D.; et al. ASEAN Guidelines on the Regulation, Use, and Trade of Biological Control Agents (BCA); Implementing Biological Control Agents in the ASEAN Region: Guidelines for Policy Makers and Practitioners; ASEAN Sustainable Agrifood Systems: Bangkok, Thailand, 2014. [Google Scholar]



| Search Terms |
|---|
| 1. “biological control” AND “safety” |
| 2. “biological control” AND “safety” AND “entomopathogenic fungi” |
| 3. “biological control” AND “safety” AND “nematophagous fungi” |
| 4. “entomopathogenic fungi” AND “infection” |
| 5. “entomopathogenic fungi” AND “biocontrol” |
| 6. “entomopathogenic fungi” AND “biosafety” |
| 7. “entomopathogenic fungi” AND “infection” AND “safety” |
| 8. “entomopathogenic fungi” AND “microbiology” OR “fungi” OR “safety” |
| 9. “entomopathogenic fungi” AND “safety” |
| 10. “entomopathogenic fungi” OR “biocontrol” AND “safety” |
| 11. “nematophagous fungi” AND “biocontrol” |
| 12. “nematophagous fungi”AND “safety” |
| 13. “nematophagus fungi” AND “biological control” AND “safety” |
| 14. “nematophagus fungi” AND “biological control” |
| 15. “nematophagus fungi” AND “infection” AND “safety” |
| 16. “safety” AND “biocontrol” |
| 17. “safety” AND “biocontrol” AND “entomopathogenic fungi” |
| 18. “safety” AND “biocontrol” AND “nematophagous fungi” |
| Fungus | Targets | Infection/ Effects | Assessed Endpoint | References |
|---|---|---|---|---|
| Beauveria bassiana | man | Fungal keratitis | Pathogenicity, infectivity | [26] |
| Metarhizium anisopliae | man | Fungal keratitis | Pathogenicity, infectivity | [27] |
| Trichoderma longibrachiatum | woman | Invasive sinusitis | Pathogenicity | [28] |
| Metarhizium anisopliae | child | Skin lesions | Pathogenicity | [29] |
| Metarhizium anisopliae | man and woman | Invasive sinusitis | Pathogenicity | [30] |
| Beauveria bassiana | woman | Fungal keratitis | Pathogenicity, infectivity | [31] |
| Metarhizium anisopliae | woman | Fungal keratitis | Pathogenicity, infectivity | [32] |
| Beauveria bassiana ou Beauveria brongniartii | woman | Systemic fungal infection | Pathogenicity, infectivity | [33] |
| Beauveria bassiana | woman | Skin lesions, respiratory symptoms, and pleural effusion | Pathogenicity | [34] |
| Akanthomyces lecani | 216 women and 113 men | High prevalence of sensitization | Allergenicity | [35] |
| Metarhizium anisopliae | allergic patients | Positivity to the allergenicity test with atopic individuals from the sugar cane area (29%), atopic individuals from urban areas (95%) | Allergenicity | [22] |
| Beauveria bassiana | patients with hypersensitivity to fungi | Allergenic potential | Allergenicity | [23] |
| Beauveria bassiana | man | Empyema | Pathogenicity | [36] |
| Beauveria bassiana | serum from patients with fungal allergies | Four alleged allergens identified | Allergenicity | [24] |
| Beauveria bassiana | woman | Fungal keratitis | Pathogenicity, infectivity | [37] |
| Metarhizium anisopliae | woman | Sclerokeratitis | Pathogenicity | [38] |
| Beauveria bassiana | woman | Fungal keratitis | Pathogenicity, infectivity | [39] |
| Metarhizium anisopliae | child | Skin lesions | Pathogenicity | [40] |
| Beauveria bassiana | woman | Necrotizing scleritis and endophthalmitis | Pathogenicity | [41] |
| Beauveria bassiana | woman | Fungal keratitis | Pathogenicity, infectivity | [42] |
| Beauveria bassiana | human lymphocytes | Beauvericin (BEA) is genotoxic to human lymphocytes in vitro | Toxicity | [25] |
| Metarhizium anisopliae | child | Fungal keratitis | Pathogenicity, infectivity | [43] |
| Beauveria bassiana | woman | Bullous keratopathy | Pathogenicity | [44] |
| Trichoderma longibrachiatum | woman | Rhinosinusitis | Pathogenicity | [45] |
| Beauveria bassiana | woman | Fungal keratitis | Pathogenicity, infectivity | [46] |
| Beauveria bassiana | man | Fungal keratitis | Pathogenicity, infectivity | [47] |
| Metarhizium anisopliae | homem | Fungal keratitis | Pathogenicity, infectivity | [48] |
| Beauveria bassiana | woman | Fungal keratitis | Pathogenicity, infectivity | [49] |
| Metarhizium anisopliae | man | Sclerokeratitis | Pathogenicity | [50] |
| Beauveria bassiana | woman | Fungal keratitis | Pathogenicity, infectivity | [51] |
| Metarhizium anisopliae | woman | Fungal keratitis | Pathogenicity, infectivity | [52] |
| Metarhizium anisopliae | woman | Sclerokeratitis and endophthalmitis | Pathogenicity | [53] |
| Metarhizium anisopliae | woman | Fungal keratitis | Pathogenicity, infectivity | [54] |
| Beauveria bassiana | man | Fungal keratitis | Pathogenicity, infectivity | [55] |
| Fungi | Vertebrate Animals | Effects | References |
|---|---|---|---|
| Beauveria bassiana Cordyceps fumosorosea (Paecilomyces fumosoroseus) | Turtles (Testudo elephantopus, Testudo gigantea elephantina, and Terrapene carolina) | Pulmonary infection | [56] |
| Beauveria bassiana | American alligator (Alligator mississippiensis) | Pulmonary lesions | [57] |
| Cordyceps fumosorosea (Paecilomyces fumosoroseus) | murine | No effects | [61] |
| Metarhizium anisopliae Cordyceps fumosorosea (Paecilomyces fumosoroseus) | Japanese quails (Coturnix coturnix japonica) | Loss of body weight | [62] |
| Beauveria bassiana Metarhizium anisopliae Metarhizium rileyi (Nomuraea rileyi) Cordyceps farinosa (Paecilomyces farinosus) Cordyceps fumosorosea (Paecilomyces fumosoroseus) Neoconidiobolus thromboides (Entomophthora virulenta) | Birds, rats, and frogs | Temporary changes in the stomach and intestines | [63] |
| Hirsutella thompsonii | Rats, rabbits, and guinea pigs | No effects | [64] |
| Metarhizium anisopliae | Rats | Tissue damage | [65] |
| Lagenidium giganteum | Mice, rats, and rabbits | The fungus can persist in mammalian tissues | [66] |
| Beauveria bassiana | Fish embryos (Menidia beryllina) | Embryo rupture and death were observed | [67] |
| Beauveria bassiana | Turtle (Trachemys scripta) | Pulmonary infection | [58] |
| Metarhizium anisopliae | Cat | Invasive rhinitis with spread of infection to the nasal bones and subcutaneous tissues | [59] |
| Metarhizium anisopliae var. acridum Beauveria bassiana | Ring-necked pheasants (Phasianus colchicus) | No effects | [68] |
| Metarhizium anisopliae | Fishes (Melanotaenia duboulayi, Ulmerophlebia spp., and Ceriodaphnia dubia) | 100% mortality of Ceriodaphnia dubia in 48 h | [69] |
| Metarhizium anisopliae | Lizard (Acanthodactylus dumerili) | Death of 2 lizards and liver changes | [70] |
| Cordyceps fumosorosea (Paecilomyces fumosoroseus) | Mice | No permanent damage to liver tissue | [71] |
| Metarhizium anisopliae | BALB/c Mice | Increased allergic response, weight and number of popliteal lymph node cells | [72] |
| Metarhizium anisopliae | BALB/c Mice | Potential for allergy induction | [73] |
| Metarhizium anisopliae | American alligator (Alligator mississippiensis) | Fungal pneumonia, anorexia, and abnormal buoyancy | [60] |
| Trichoderma stromaticum | BALB/c Mice | Immunosuppressive effect | [74] |
| Cordyceps tenuipes (Paecilomyces tenuipes) | Mice and rats | No toxicological effects | [75] |
| Cordyceps fumosorosea (Isaria fumosorosea) | Rabbits | No toxic effects | [76] |
| Metarhizium viride | Veiled chameleons (Chamaeleo calyptratus), panther chameleons (Furcifer pardalis), and lizards (Pogona vitticeps) | Fungal glossitis, stomatitis, pharyngitis, or visceral mycosis | [77] |
| Isaria cicadae (Cordyceps cicadae) | Mice | No effects on the CNS or cardiovascular and respiratory systems | [78] |
| Beauveria bassiana | Albino rats | Effect on liver function and size | [79] |
| Aspergillus parasiticus | Fish (Hypophthalmichthys molitrix) | Cracked eyes (64%), bleeding in the fins (33%), and infection in the scales (30%) | [80] |
| Fungi | Invertebrate Animals | Effects | References |
|---|---|---|---|
| Hirsutella thompsonii | Apis mellifera | No effects | [86] |
| Entomophaga maimaiga Beauveria bassiana | Apis mellifera | E. naimaga did not affect the longevity of the bees. B. bassiana reduced longevity and caused mycosis in bees at higher concentrations | [87] |
| Beauveria bassiana Metarhizium anisopliae Cordyceps fumosorosea (Paecilomyces fumosoroseus) Paecilomyces farinosus | Earthworm cocoons (Aporrectodes caliginosa) | The fungi did not reduce the hatching rate of the cocoons | [88] |
| Nosema meligethi I. | Apis mellifera | Apis mellifera showed no sign of infection after 30 days | [89] |
| Metarhizium anisopliae Beauveria bassiana | Apis mellifera | Almost all the bees died at the higher concentration | [90] |
| Metarhizium flavoviride | Apis mellifera | A field dose formulated in oil killed 11% of the bees and a similar dose formulated in water killed 8% | [91] |
| Beauveria bassiana | Shrimp (Palaemonetes pugio) | Lethal infections when conidiospores were injected | [92] |
| Metarhizium anisopliae | Beetles, grasshoppers, and butterflies | There were no differences in the number of late-stage larvae of the non-target species | [93] |
| Metarhizium rileyi (Nomuraea rileyi) Beauveria bassiana Metarhizium anisopliae | Apis mellifera | B. bassiana and M. anisopliae caused greater mortality in the bees’ liquid diet | [94] |
| Metarhizium anisopliae | Shrimp (Palaemonetes pugio) | Dead embryos and larvae with fungal growth and delayed embryo hatching | [95] |
| Akanthomyces lecani | 20 non-target invertebrates | There was no evidence of infection in non-target species | [96] |
| Metarhizium anisopliae Beauveria bassiana | Beetles (Pimelia senegalensis; Trachyderma hispida) | No infection was observed | [97] |
| Beauveria bassiana | Oribatida (scarab mites) | Reduction in the abundance of Oribatida | [98] |
| Metarhizium flavoviride Beauveria bassiana | Beetle (Malagasy Coleoptera) | B. bassiana had no impact on the number of species. M. flavoviride had a significant impact on non-target beetles | [99] |
| Cordyceps fumosorosea (Paecilomyces fumosoroseus) | Aphid (Diuraphis noxia) ladybug (Hippodamia convergens) | Infection of predators (aphids and ladybugs) | [81] |
| Beauveria bassiana Beauveria brongniartii Metarhizium anisopliae | Three species of collembola (Folsomia fimetaria; Hypogastrura assimilis; Proisotoma minuta) | Low virulence for non-target springtails | [100] |
| Metarhizium anisopliae | Epigean arthropods | No evidence of infection of native non-target arthropods in field conditions | [101] |
| Beauveria bassiana | Insect cadaver samples (non-target hosts) | Released strains of B. bassiana can be isolated from non-target insects | [102] |
| Beauveria brongniartii | Poecilus versicolor | No negative effects were observed | [103] |
| Beauveria bassiana | Apis mellifera | No adverse effects on bees | [104] |
| Trichoderma viride Trichoderma spp. Clonostachys rosea f. catenulata (Gliocladium catenulatum) Trichoderma virens (Gliocladium virens) Metarhizium anisopliae Beauveria brongniartii Stagonospora spp. | Crustaceans (Artemia salina and Daphia magna) | Both invertebrates were very sensitive to all the metabolites examined | [105] |
| Beauveria bassiana | Bombus impatiens | The average concentration was considered ideal with the least impact on bees | [106] |
| Beauveria bassiana Metarhizium anisopliae | Natural enemies (Coccinella septempunctata L.; Chrysoperla carnea; Dicyphus tamaninii) and beneficial insect (Heteromurus nitidus) | B. bassiana and M. anisopliae are relatively safe for non-target insects | [107] |
| Trichoderma harzianum Trichoderma polysporum | Bombus terrestris | The biofungicides did not cause any bee mortality | [108] |
| Beauveria bassiana | Apis mellifera | Did not affect the health of the bee colony | [109] |
| Beauveria bassiana Metarhizium anisopliae Akanthomyces lecani Hirsutella kirchneri Hirsutella nodulosa Hirsutella sp. | Apis mellifera | Adult bees and pupae were not susceptible to the fungal isolates tested | [110] |
| Trichoderma harzianum Beauveria bassiana | Bombus terrestris | B. bassiana caused 92% mortality after 11 weeks of exposure; T. harzianum caused no negative effect | [111] |
| Metarhizium anisopliae | Apis mellifera | Higher bee mortality with conidial spraying | [112] |
| Arcopilus cupreus (Chaetomium cupreum) | Artemia salina | Toxicity caused by the secondary metabolite oosporein | [113] |
| Beauveria bassiana Metarhizium anisopliae Akanthomyces lecani | Apis mellifera | Higher mortality of bees treated (feeding) with V. lecanni and B. bassiana | [114] |
| Beauveria bassiana | Mantis (Tenodera sinensis; Statilia maculate) | Epizootic in mantis populations in a vast region of China (panzootia) in 2009 | [115] |
| Beauveria bassiana | Megachile rotundata Apis mellifera | Susceptibility (mortality) of bees dependent on fungal concentration | [116] |
| Beauveria bassiana | Bombus impatiens | Oral or topical application of B. bassiana had no effect on bees | [117] |
| Beauveria bassiana | Apis mellifera | Toxic to worker larvae | [118] |
| Metarhizium anisopliae Metarhizium robertsii | Bed bugs (Schiodtella formosana) | Causative agents of epizootic green muscardine disease in populations of S. formosana | [119] |
| Metarhizium anisopliae | Bombus terrestris | Dry exposure to M. anisopliae spores caused mortality | [120] |
| Beauveria bassiana | Melipona scutellaris | Highly virulent (bee mortality at lower dose) | [121] |
| Beauveria bassiana | Silkworm (Bombyx mori), Pine caterpillar (Dendrolimus punctuatus) | The fungal infection did not originate from the fungal insecticide | [122] |
| Clonostachys rosea f. catenulata (Gliocladium catenulatum) Beauveria bassiana | Bombus terrestris | Cuticular water loss and reduced survival | [123] |
| Beauveria bassiana | Amblyseius swirskii (predatory mite) | A. swirskii was susceptible to B. bassiana when the conidia were applied directly | [124] |
| Metarhizium brunneum Beauveria bassiana | Trybliographa rapae | Low risk to T. rapae populations (parasitoid) | [125] |
| Beauveria bassiana | Natural enemies: spiders, coccinellids, and lacewings | No effects on natural enemies | [126] |
| Metarhizium anisopliae | 1944 non-target arthropod species | Mortality of small numbers of non-target insects living in the soil | [127] |
| Beauveria bassiana Metarhizium anisopliae Isaria fumosorosea | Stingless bees (Tetragonisca angustias Latreille, Scaptotrigona mexicana Guérin-Meneville, and Melipona beecheii Bennett) | Moderate to high impact on bee mortality | [128] |
| Beauveria bassiana | Phytoseiid mites (Neoseiulus cucumeris; N. californicus; Phytoseiulus persimilis; N. womersleyi; Amblyseius swirskii) | No effect on predatory mites | [82] |
| Beauveria bassiana | Apis mellifera, Chrysoperla rufilabris;Orius insidiosus; Hippodamia convergens; Harmonia axyridis;Coleomegilla maculata and Aranea | Low lethality at low doses | [129] |
| Metarhizium brunneum | Galleria mellonella | Mortality of larvae and cadavers with low fungal growth | [130] |
| Clonostachys rosea f. catenulata (Gliocladium catenulatum) Beauveria bassiana Metarhizium brunneum | Apis mellifera Bombus terrestris | Effects on the longevity of the two bee species | [131] |
| Metarhizium spp. | American cockroaches (Periplaneta americana), bees (Apis mellifera adansonii) | There was no significant increase in mortality and no mycosis was observed on the cadavers | [132] |
| Beauveria bassiana Metarhizium anisopliae | Apis mellifera | Reduced worker survival | [133] |
| Beauveria bassiana | Foraging bees | Changes to the nest recognition system | [134] |
| Beauveria bassiana Akanthomyces lecani Metarhizium anisopliae | Apis mellifera Bombus terrestris | Slight effect on bee movement and death of some individuals | [135] |
| Aspergillus flavus | Crustacean (Alpheus bouvieri); mosquito (Toxorhynchites splenden) | 80% mortality of both species | [84] |
| Beauveria bassiana Metarhizium rileyi (Nomuraea rileyi) | Apis cerana | B. bassiana was slightly to moderately toxic to bees. Metarhizium rileyi (Nomuraea rileyi) was harmless to bees | [136] |
| Beauveria bassiana Metarhizium anisopliae | Eudrilus eugeniae | Little impact on earthworm mortality | [83] |
| Beauveria bassiana | Apis cerana | No effect on bees. Temperature inside the hive at 35 °C abolished conidia germination | [137] |
| Beauveria bassiana | Apis mellifera | Effect on behavior and cognition (inconsistent response to sucrose) | [138] |
| Beauveria bassiana Metarhizium anisopliae | Africanized Apis mellifera queens | Reduced worker survival | [138] |
| Beauveria bassiana | Africanized Apis mellifera | Increase in weight and time of bee emergence | [139] |
| Beauveria bassiana | Aquatic insects (Chironomu riparius) | Lethal and sublethal effects and on larval growth | [140] |
| Beauveria bassiana | Non-target insects | Released strains can infect non-target insects | [85] |
| Metarhizium anisopliae Beauveria bassiana | Apis meelifera Melipoluna ferruginea | Reduced survival of Apis mellifera | [141] |
| Beauveria bassiana | Tetragonisca angustula | Change in behavior | [142] |
| Metarhizium brunneum | Predatory mite Gaeolaelaps (Hypoaspis) aculeifer | No negative impact | [143] |
| Beauveria bassiana Metarhizium anisopliae Cordyceps fumosorosea (Paecilomyces fumosoroseus) | Bees (Tetragonisca angustula; Scaptotrigona depilis; Apis melífera Bombus terrestris) | Reduced bee survival | [144] |
| Fungus | Organisms | Effects | References |
|---|---|---|---|
| Lagenidium giganteum | Apis mellifera; microcustaceans (decapods, copepods, ostracods); quail (Colinus virginianus L.); ducks, fish, insects, plants, and algae | Two species of insects were infected by exposure to the fungus | [145] |
| Lagenidium giganteum | Cladocerans (Ceriodaphnia dubia, Daphnia pulex, D. magna) and fish (Pimephales promelas) | L. giganteum can affect some non-target aquatic species | [146] |
| Metarhizium anisopliae | Embryos of grass shrimp (Palaemonetes pugio), kingfishers (Menidia beryllina), and frogs (Xenopus laevis) | Eye abnormalities in shrimp and frog embryos. Frog embryos with moderate to severe cranial, facial, and intestinal malformations. Significant mortality in grass shrimp and kingfish embryos | [147] |
| Metarhizium anisopliae | Bacterium (Pseudomonas syringae), protozoan (Tetrahymena pyriformis), arthropod (Daphnia magna), human cell line and an insect cell line (Spodoptera frugiperda) | Toxicity to insect cells. Secondary metabolites without risk to humans or the environment | [148] |
| Puccinia komarovii var. glanduliferae | 74 plant species tested | An ornamental species was susceptible (Impatiens balsamina) | [149] |
| Aspergillus flavus | Spodoptera litura and rats | No toxicity to mammals | [150] |
| Fungus | Test | Toxicity | Reference |
|---|---|---|---|
| Metarhizium anisopliae Beauveria bassiana | Viability of airborne conidia | No risk to humans | [151] |
| Trichoderma harzianum | Bioaerosol analysis | T. harzianum was detected in the air only on the day of the treatment | [152] |
| Beauveria brongniartii | Physico-chemical analysis | It is unlikely that the metabolite oosporein can be absorbed into organisms and pass through the biological membrane | [153] |
| Beauveria bassiana Metarhizium anisopliae Beauveria brongniartii Albifimbria verrucaria (Gliocladium fimbriatum) | Ames Test and VITOTOX | No genotoxicity of secondary metabolites was observed: oosporein, gliotoxin, and destruxins B, D, and E | [154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sousa, T.K.; Silva, A.T.d.; Soares, F.E.d.F. Fungi-Based Bioproducts: A Review in the Context of One Health. Pathogens 2025, 14, 463. https://doi.org/10.3390/pathogens14050463
de Sousa TK, Silva ATd, Soares FEdF. Fungi-Based Bioproducts: A Review in the Context of One Health. Pathogens. 2025; 14(5):463. https://doi.org/10.3390/pathogens14050463
Chicago/Turabian Stylede Sousa, Thais Kato, Adriane Toledo da Silva, and Filippe Elias de Freitas Soares. 2025. "Fungi-Based Bioproducts: A Review in the Context of One Health" Pathogens 14, no. 5: 463. https://doi.org/10.3390/pathogens14050463
APA Stylede Sousa, T. K., Silva, A. T. d., & Soares, F. E. d. F. (2025). Fungi-Based Bioproducts: A Review in the Context of One Health. Pathogens, 14(5), 463. https://doi.org/10.3390/pathogens14050463







