Author Contributions
Conceptualization, M.T., S.S., Y.S., M.Y., and H.S.; methodology, M.T., S.S., Y.S., K.M., M.Y., I.R., and H.S.; software, M.T., S.S., I.R., and H.S.; validation, M.T., S.S., Y.S, and H.S.; investigation, M.T., S.S., Y.S., K.M., M.Y., I.R., and H.S.; data curation, M.T., S.S., Y.S., and I.R.; writing—original draft preparation, M.T., S.S., and Y.S.; writing—review and editing, M.Y., I.R., and H.S.; visualization, M.T., S.S., Y.S., M.Y., and H.S.; supervision, M.Y. and H.S. All authors have read and agreed to the published version of the manuscript.
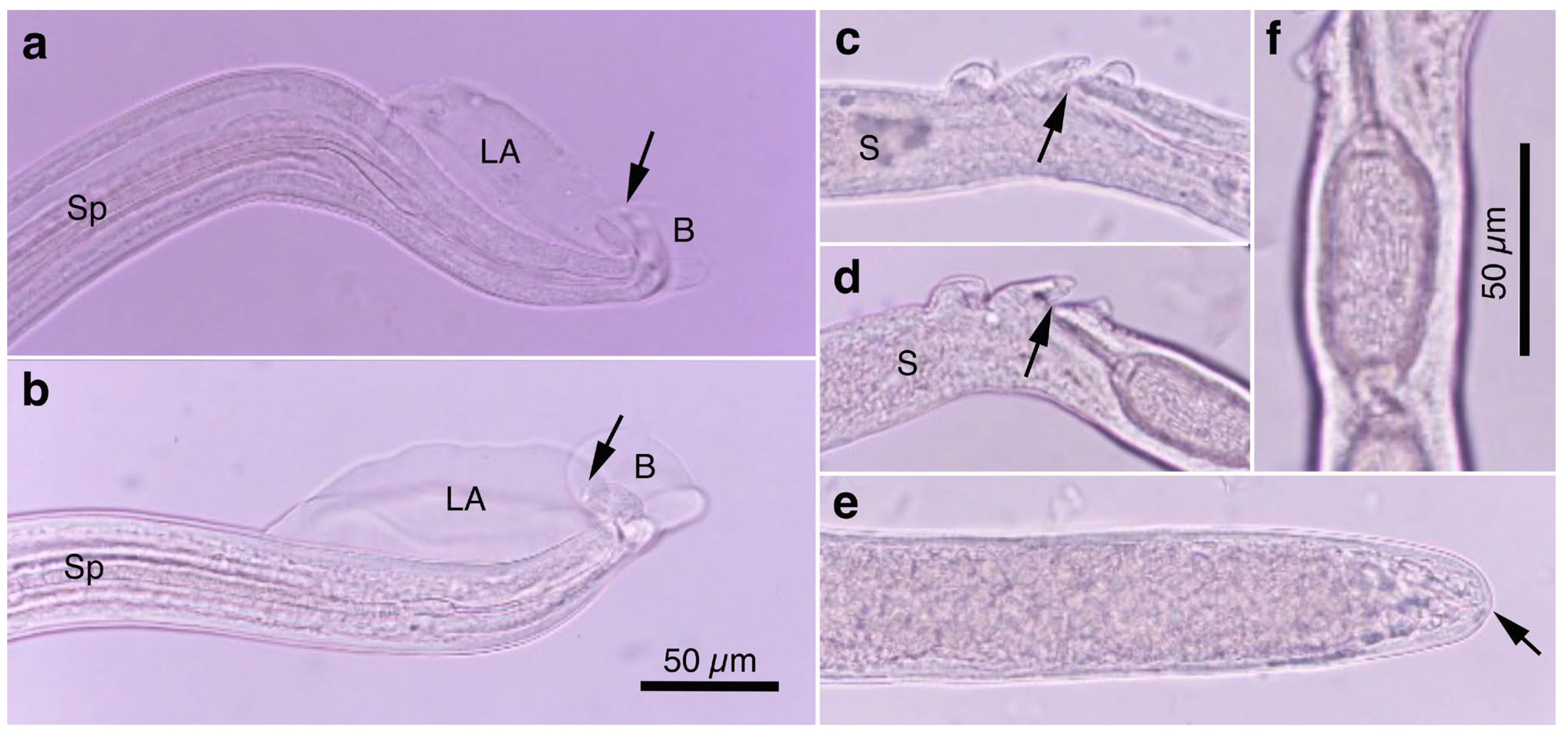
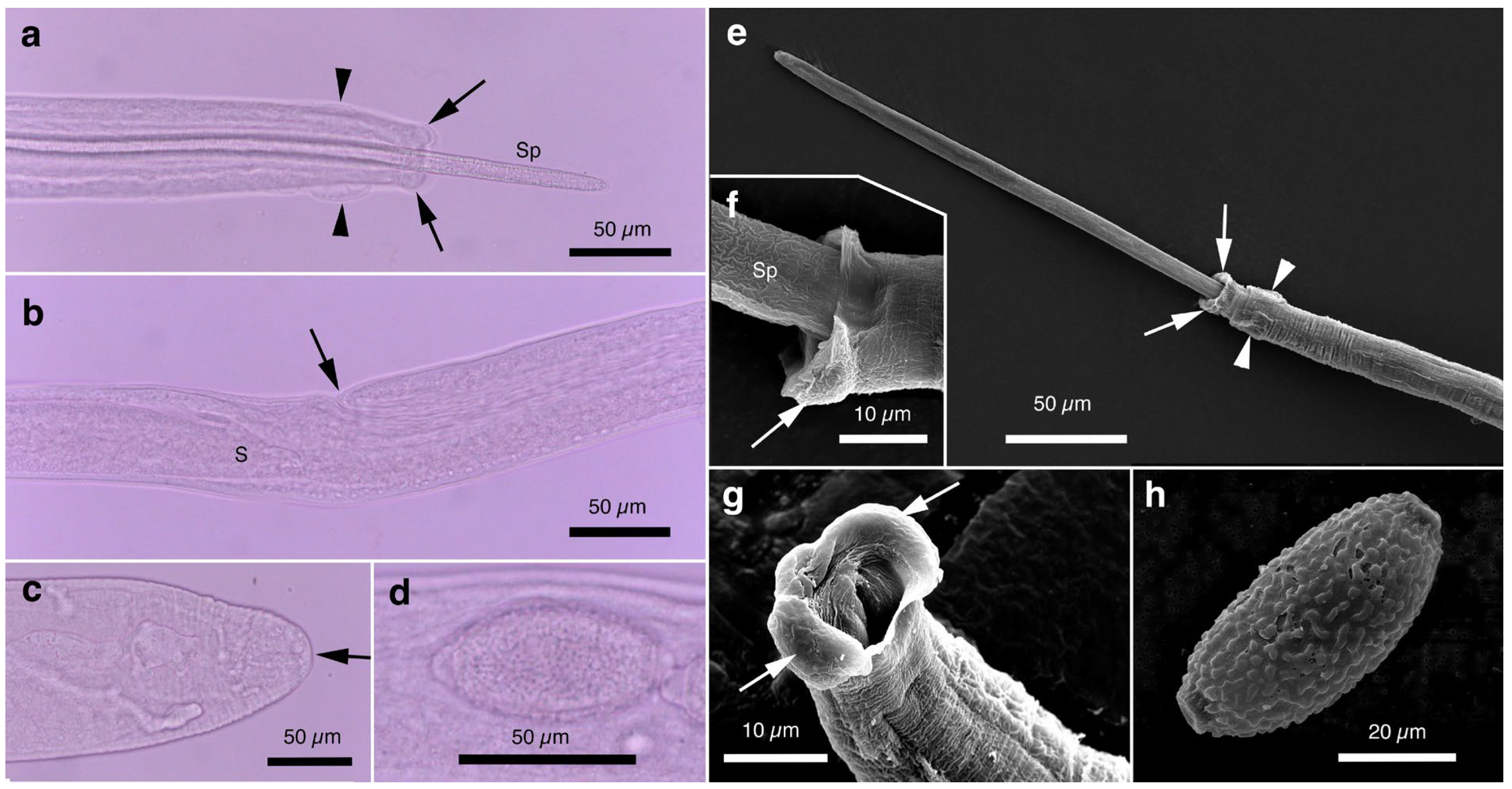
Figure 1.
Morphology of Aonchotheca putorii. (a) Caudal end of morphotype “Type A” male worm with lateral alae (LA) and membranous bursa (B) supported with elongated digitiform projections (arrow). Spicule (Sp). (b) Caudal end of morphotype “Type B” male worm with LA and membranous B supported with stumpy digitiform projections (arrow). (c,d) Vulval appendages of female worms at three regions (anterior and posterior swellings of the vulva, and a vulval plate from the anterior edge of the vulval opening (arrows). End of stichosome (S). (e) Posterior end of a female worm with a terminal anus (arrow). (f) Intrauterine egg with striated eggshell surface texture. Photographs (a–e) at the same magnification and a scale bar is shown in (b).
Figure 1.
Morphology of Aonchotheca putorii. (a) Caudal end of morphotype “Type A” male worm with lateral alae (LA) and membranous bursa (B) supported with elongated digitiform projections (arrow). Spicule (Sp). (b) Caudal end of morphotype “Type B” male worm with LA and membranous B supported with stumpy digitiform projections (arrow). (c,d) Vulval appendages of female worms at three regions (anterior and posterior swellings of the vulva, and a vulval plate from the anterior edge of the vulval opening (arrows). End of stichosome (S). (e) Posterior end of a female worm with a terminal anus (arrow). (f) Intrauterine egg with striated eggshell surface texture. Photographs (a–e) at the same magnification and a scale bar is shown in (b).
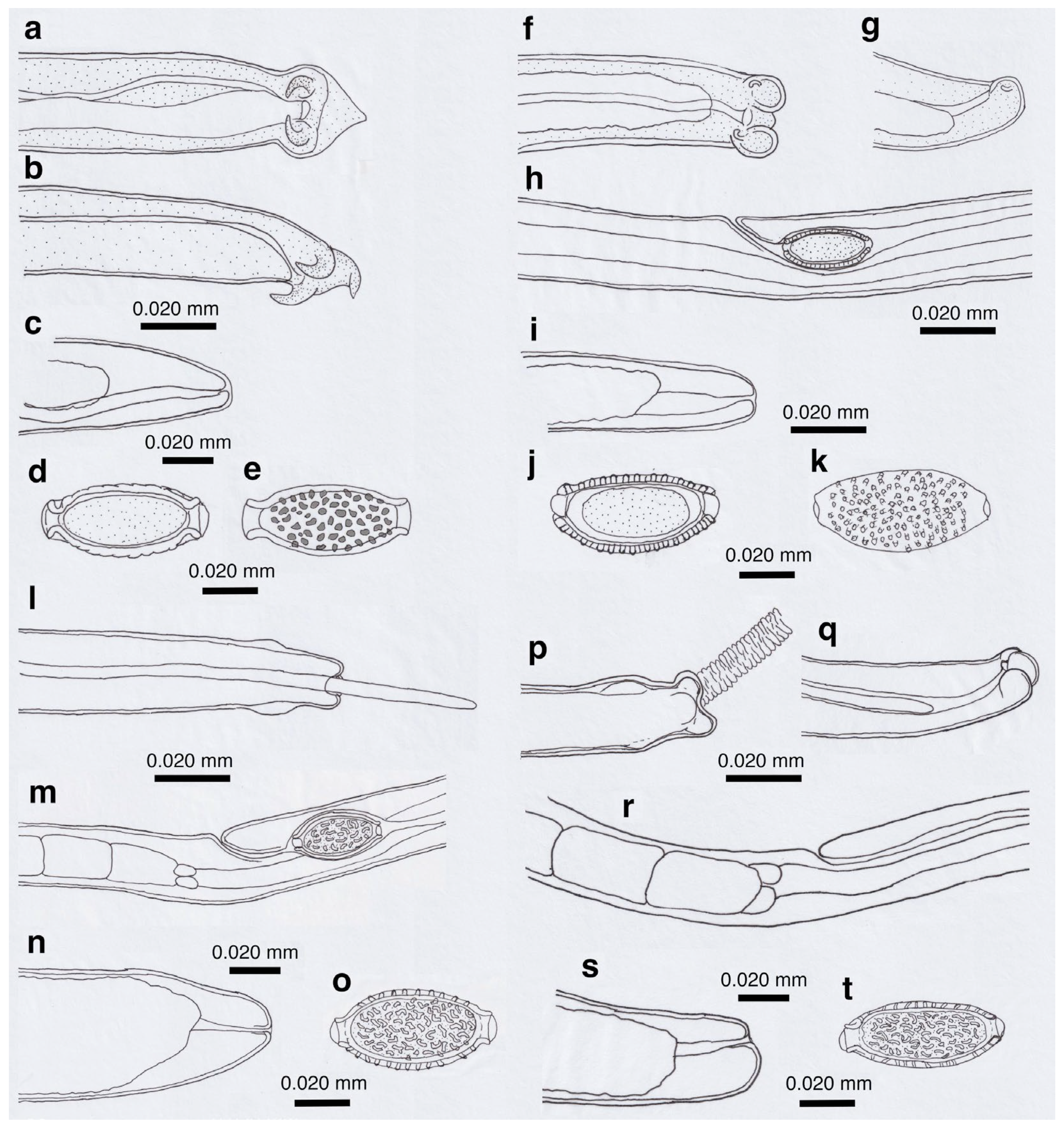
Figure 2.
Line drawings of Aonchotheca putorii (a–d) and A. suzukii n. sp. (e–g). (a) Caudal end of morphotype “Type A” male worm. (b) Caudal end of morphotype “Type B” male worm. (c) Vulval appendages of a female worm at three regions (anterior and posterior swellings of the vulva, and a vulval plate from the anterior edge of the vulval opening), and intrauterine egg with striated eggshell surface texture. (d) Posterior end of a female worm with a terminal anus. (e) Caudal end of A. suzukii n. sp. male worm. (f) Vulval appendages of A. suzukii n. sp. female worm at two regions (small subcuticular elevation anterior to the vulval opening and cuticular ballooned swelling around it), and an intrauterine egg with striated eggshell surface texture. (g) Posterior end of A. suzukii n. sp. female worm with a subterminal anus.
Figure 2.
Line drawings of Aonchotheca putorii (a–d) and A. suzukii n. sp. (e–g). (a) Caudal end of morphotype “Type A” male worm. (b) Caudal end of morphotype “Type B” male worm. (c) Vulval appendages of a female worm at three regions (anterior and posterior swellings of the vulva, and a vulval plate from the anterior edge of the vulval opening), and intrauterine egg with striated eggshell surface texture. (d) Posterior end of a female worm with a terminal anus. (e) Caudal end of A. suzukii n. sp. male worm. (f) Vulval appendages of A. suzukii n. sp. female worm at two regions (small subcuticular elevation anterior to the vulval opening and cuticular ballooned swelling around it), and an intrauterine egg with striated eggshell surface texture. (g) Posterior end of A. suzukii n. sp. female worm with a subterminal anus.
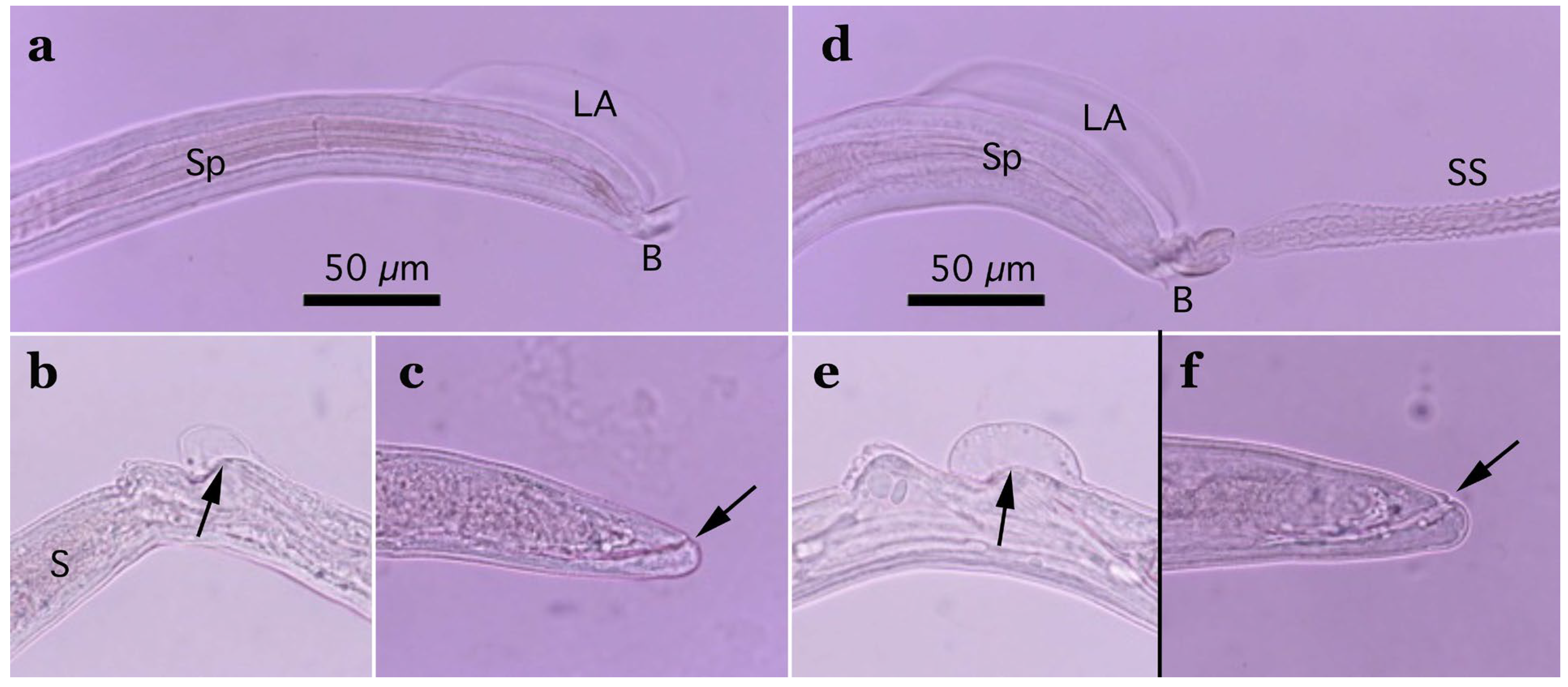
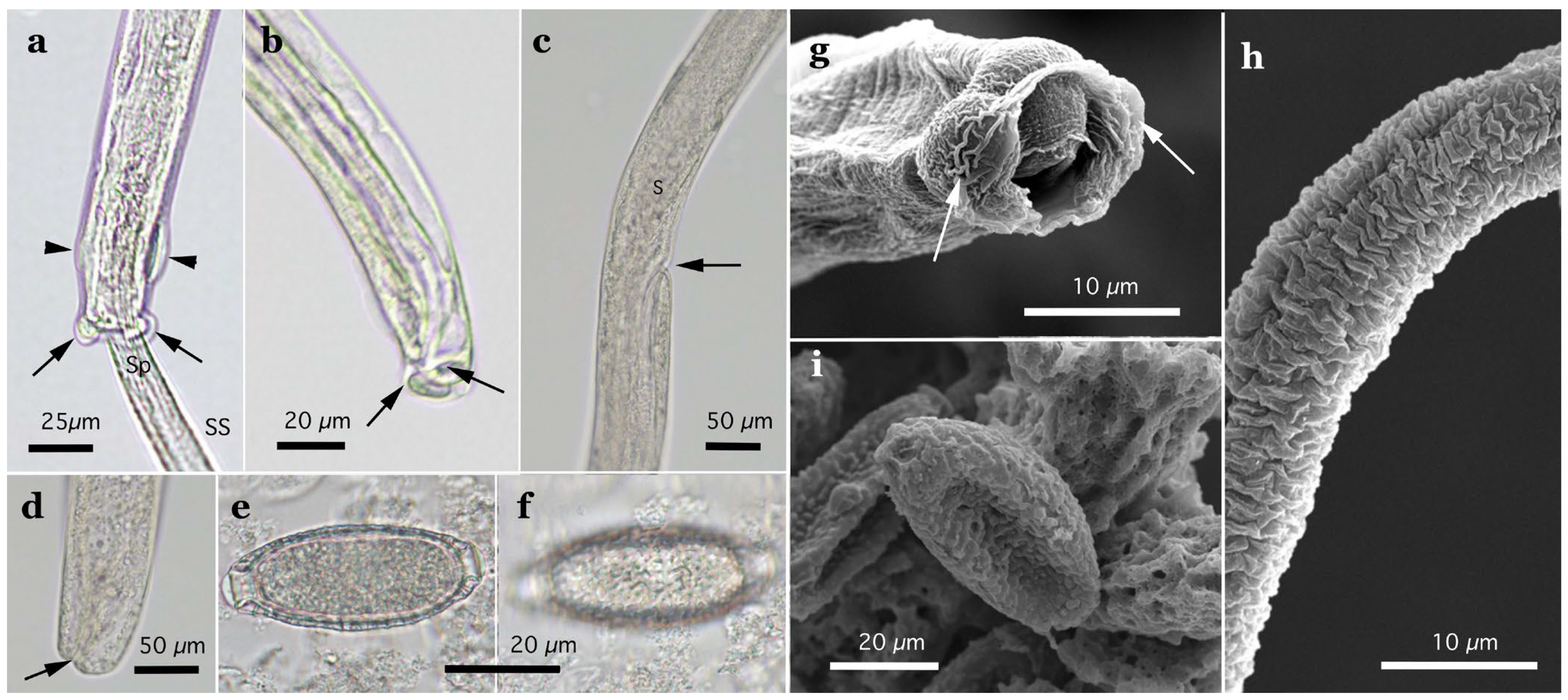
Figure 3.
Morphology of Aonchotheca suzukii n. sp. ((a–c), specimens from feral alien raccoons, (d–f), specimens from wild boars). (a,d) Caudal end of male worm with lateral alae (LA) and membranous bursa (B) supported with short digitiform projections. Spicule (Sp), and extruded spicular sheath (SS) showing transverse striations. (b,e) Vulval appendages of female worm; small subcuticular elevation at the anterior to the vulval opening (arrow) and cuticular ballooned swelling around it. End of stichosome (S). (c,f) Posterior end of female worm with subterminal anus (arrow). Photographs (a–f) at the same magnification, and scale bar is shown in (a,d).
Figure 3.
Morphology of Aonchotheca suzukii n. sp. ((a–c), specimens from feral alien raccoons, (d–f), specimens from wild boars). (a,d) Caudal end of male worm with lateral alae (LA) and membranous bursa (B) supported with short digitiform projections. Spicule (Sp), and extruded spicular sheath (SS) showing transverse striations. (b,e) Vulval appendages of female worm; small subcuticular elevation at the anterior to the vulval opening (arrow) and cuticular ballooned swelling around it. End of stichosome (S). (c,f) Posterior end of female worm with subterminal anus (arrow). Photographs (a–f) at the same magnification, and scale bar is shown in (a,d).
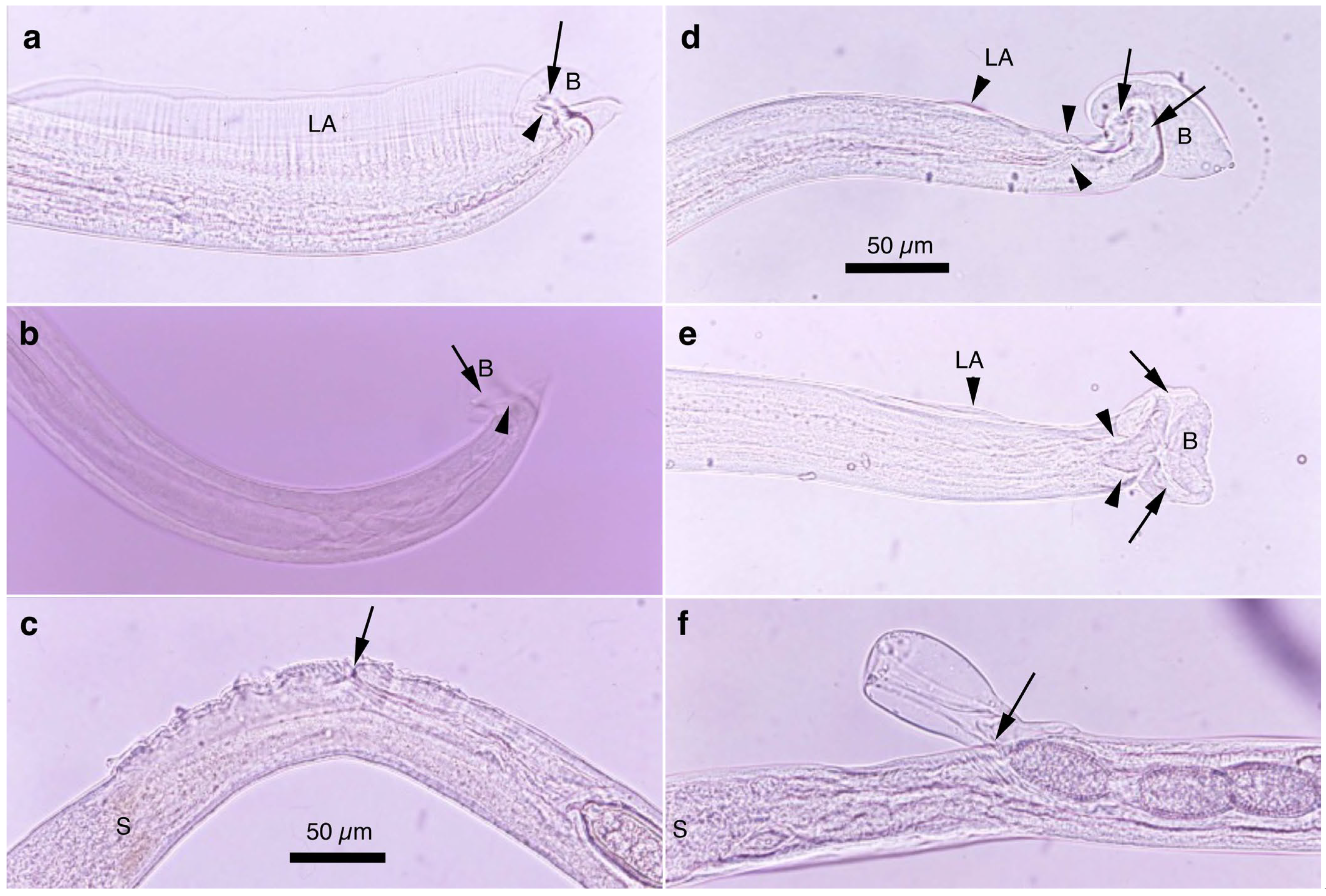
Figure 4.
Morphology of Aonchotheca suis n. comb. (a–c) and A. riukiuensis (d–f) from the stomach of wild boars. (a) Caudal end of male worm with conspicuous lateral alae (LA) and membranous bursa (B) supported with two pairs of ventrolateral projections, simple digitiform ones (arrowheads) and hammer-shaped ones (arrow). (b) Caudal end of male worm lacking lateral alae but with membranous bursa (B) supported with two pairs of ventrolateral projections, simple digitiform ones (arrowhead) and hammer-shaped ones (arrow). (c) Evidently and finely roughened cuticular surface around the vulval opening (arrow). End of stichosome (S). (d,e) Caudal end of male worms with rudimentary lateral alae (LA) and membranous bursa (B) supported with two pairs of ventrolateral projections, simple digitiform ones (arrowhead) and thick hammer-shaped ones (arrow). (f) Bell-shaped vulval appendage at the vulval opening (arrow). End of stichosome (S). Photographs (a–c) at the same magnification, and scale is shown in (c). Similarly, photographs (d–f) at the same magnification, and scale bar is shown in (d).
Figure 4.
Morphology of Aonchotheca suis n. comb. (a–c) and A. riukiuensis (d–f) from the stomach of wild boars. (a) Caudal end of male worm with conspicuous lateral alae (LA) and membranous bursa (B) supported with two pairs of ventrolateral projections, simple digitiform ones (arrowheads) and hammer-shaped ones (arrow). (b) Caudal end of male worm lacking lateral alae but with membranous bursa (B) supported with two pairs of ventrolateral projections, simple digitiform ones (arrowhead) and hammer-shaped ones (arrow). (c) Evidently and finely roughened cuticular surface around the vulval opening (arrow). End of stichosome (S). (d,e) Caudal end of male worms with rudimentary lateral alae (LA) and membranous bursa (B) supported with two pairs of ventrolateral projections, simple digitiform ones (arrowhead) and thick hammer-shaped ones (arrow). (f) Bell-shaped vulval appendage at the vulval opening (arrow). End of stichosome (S). Photographs (a–c) at the same magnification, and scale is shown in (c). Similarly, photographs (d–f) at the same magnification, and scale bar is shown in (d).
![Pathogens 14 00455 g004 Pathogens 14 00455 g004]()
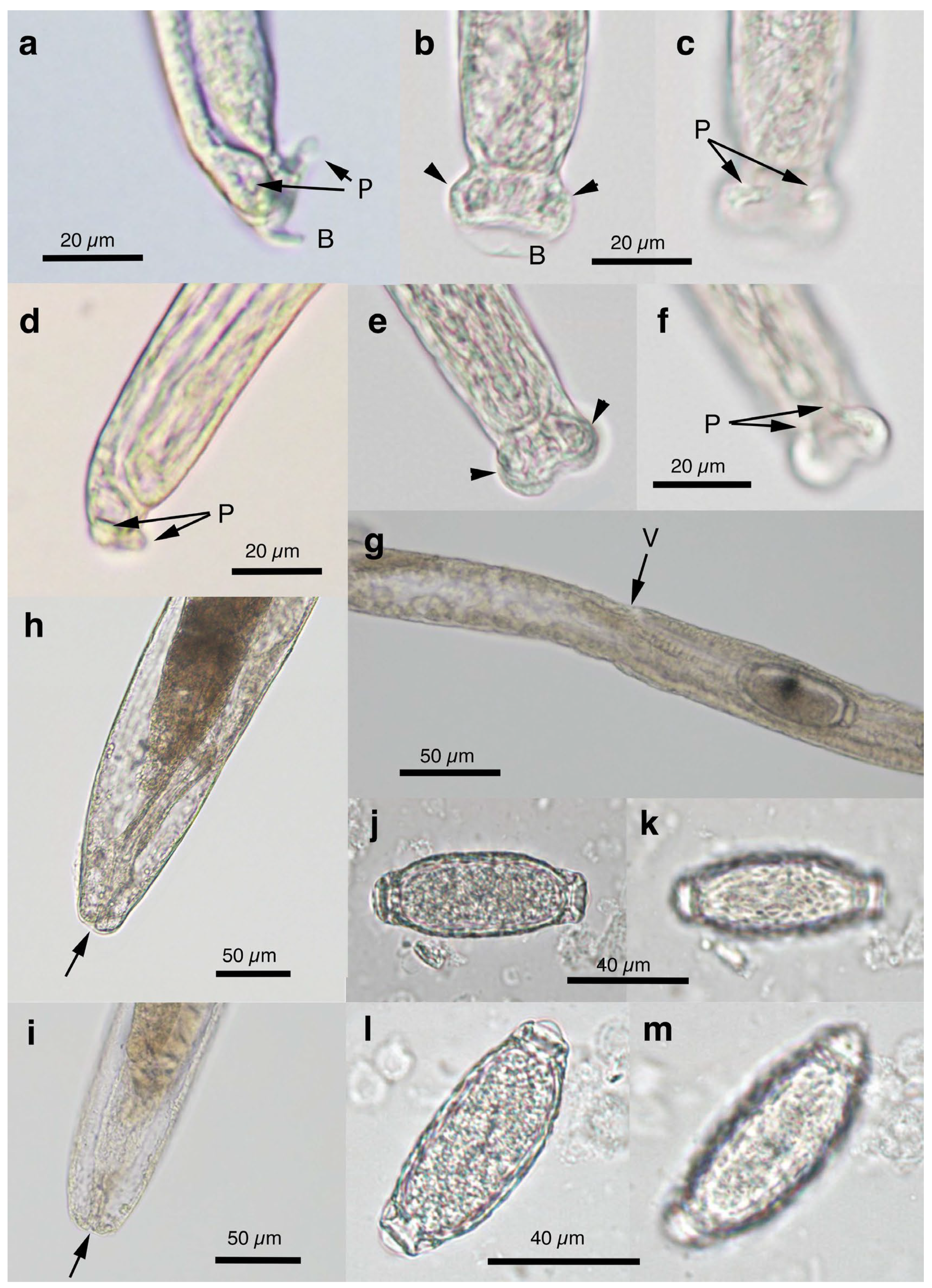
Figure 5.
Morphology of Pearsonema neoplica n. sp. (a–c) Caudal end of male worm with caudal triangular membranous bursa (B) connected to dorsolateral lobes (arrowheads) and supported with digitiform projections (P). (d–f) Caudal end of male worm lacking caudal triangular membranous bursa. Dorsolateral lobes (arrowheads) and digitiform projections (P). (g) Vulva (V) without appendages of female worm. (h,i) Posterior end of female worms with terminal anus (arrow). (j–m) Bioperculated barrel-shaped eggs, with reticulated eggshell surface texture. Sagittal section (j,l), and reticulated eggshell surface (k,m). Scale bar is shown in each photograph.
Figure 5.
Morphology of Pearsonema neoplica n. sp. (a–c) Caudal end of male worm with caudal triangular membranous bursa (B) connected to dorsolateral lobes (arrowheads) and supported with digitiform projections (P). (d–f) Caudal end of male worm lacking caudal triangular membranous bursa. Dorsolateral lobes (arrowheads) and digitiform projections (P). (g) Vulva (V) without appendages of female worm. (h,i) Posterior end of female worms with terminal anus (arrow). (j–m) Bioperculated barrel-shaped eggs, with reticulated eggshell surface texture. Sagittal section (j,l), and reticulated eggshell surface (k,m). Scale bar is shown in each photograph.
Figure 6.
Scanning electron microscopic view of Pearsonema neoplica n. sp. (a,b) Caudal end of male worm with caudal triangular membranous bursa (B) connected to dorsolateral lobes (arrowheads) and supported with digitiform projections (arrows). Extruded spicular sheath (SS) from cloaca. (c,d) Caudal end of male worm lacking caudal triangular membranous bursa. Dorsolateral lobes (arrowheads) and digitiform projections (arrows). Extruded spicular sheath (SS) from cloaca. (e,f) Bioperculated barrel-shaped eggs, with reticulated eggshell surface texture. Scale bar is shown in each photograph.
Figure 6.
Scanning electron microscopic view of Pearsonema neoplica n. sp. (a,b) Caudal end of male worm with caudal triangular membranous bursa (B) connected to dorsolateral lobes (arrowheads) and supported with digitiform projections (arrows). Extruded spicular sheath (SS) from cloaca. (c,d) Caudal end of male worm lacking caudal triangular membranous bursa. Dorsolateral lobes (arrowheads) and digitiform projections (arrows). Extruded spicular sheath (SS) from cloaca. (e,f) Bioperculated barrel-shaped eggs, with reticulated eggshell surface texture. Scale bar is shown in each photograph.
Figure 7.
Line drawings of Pearsonema neoplica n. sp. (a–e), P. feliscati (f–k), P iharai n. sp. (l–o), and P. toriii n. sp. (p,q). Caudal end of male worms (a,b,f,g,l,p,q), vulval region of female worms (h,m,r), posterior end of female worms (c,i,n,s), sagittal section of eggs (d,j), eggshell surface (e,k), and jointed view of sagittal egg section and eggshell surface (o,t).
Figure 7.
Line drawings of Pearsonema neoplica n. sp. (a–e), P. feliscati (f–k), P iharai n. sp. (l–o), and P. toriii n. sp. (p,q). Caudal end of male worms (a,b,f,g,l,p,q), vulval region of female worms (h,m,r), posterior end of female worms (c,i,n,s), sagittal section of eggs (d,j), eggshell surface (e,k), and jointed view of sagittal egg section and eggshell surface (o,t).
Figure 8.
Morphology of Pearsonema feliscati. (a,b) Ventral and lateral views of caudal end of male worms, lacking apparent membranous bursa. Dorsolateral lobes (arrowheads) and a nodular projection (arrow). (c) Vulva (arrow) without appendages of female worm. (d–g) Bioperculated barrel-shaped egg, with punctuated eggshell surface texture, collected from urine of a feral alien raccoon (d,e), or a domestic cat (f,g). (h) Scanning electron microscopic view of male caudal end with dorsolateral lobes (arrowheads) in ventral view. (i) Scanning electron microscopic view of P. feliscati egg surface. Scale bar is shown in each photograph.
Figure 8.
Morphology of Pearsonema feliscati. (a,b) Ventral and lateral views of caudal end of male worms, lacking apparent membranous bursa. Dorsolateral lobes (arrowheads) and a nodular projection (arrow). (c) Vulva (arrow) without appendages of female worm. (d–g) Bioperculated barrel-shaped egg, with punctuated eggshell surface texture, collected from urine of a feral alien raccoon (d,e), or a domestic cat (f,g). (h) Scanning electron microscopic view of male caudal end with dorsolateral lobes (arrowheads) in ventral view. (i) Scanning electron microscopic view of P. feliscati egg surface. Scale bar is shown in each photograph.
Figure 9.
Morphology of Pearsonema iharai n. sp. (a) Ventral view of caudal end of male worm, lacking apparent membranous bursa. Circular cuticular swelling (arrowheads) before caudal end with dorsolateral lobes (arrows). Protruded spicule (Sp). (b) Vulva (arrow) without appendages of female worm. (c) Caudal end of female worm with a terminal anus (arrow). (d) Intrauterine bioperculated lemon-shaped egg, with vermiculated eggshell surface texture. (e–g) Scanning electron microscopic view of male caudal end with dorsolateral lobes (arrows) in ventral view. Circular cuticular swelling (arrowheads) prior to the caudal end. (h) Scanning electron microscopic view of P. iharai n. sp. egg with vermiculated eggshell surface. Scale bar is shown in each photograph.
Figure 9.
Morphology of Pearsonema iharai n. sp. (a) Ventral view of caudal end of male worm, lacking apparent membranous bursa. Circular cuticular swelling (arrowheads) before caudal end with dorsolateral lobes (arrows). Protruded spicule (Sp). (b) Vulva (arrow) without appendages of female worm. (c) Caudal end of female worm with a terminal anus (arrow). (d) Intrauterine bioperculated lemon-shaped egg, with vermiculated eggshell surface texture. (e–g) Scanning electron microscopic view of male caudal end with dorsolateral lobes (arrows) in ventral view. Circular cuticular swelling (arrowheads) prior to the caudal end. (h) Scanning electron microscopic view of P. iharai n. sp. egg with vermiculated eggshell surface. Scale bar is shown in each photograph.
Figure 10.
Morphology of Pearsonema toriii n. sp. (a) Dorsal view of caudal end of male worm, lacking apparent membranous bursa. Lateral cuticular swelling (arrowheads) before caudal end with conspicuous spheroid dorsolateral lobes (arrows). Protruded spicular sheath (SS) and spicule (Sp). (b) Lateral view of caudal end of male worm. Dorsolateral lobes (arrows). (c) Vulva (arrow) without appendages of female worm. End of stichosome (S). (d) Caudal end of female worm with subterminal anus (arrow). (e,f) Bioperculated barrel-shaped egg, with vermiculated eggshell surface texture. (g) Scanning electron microscopic view of male caudal end with spheroid dorsolateral lobes (arrows). (h) Scanning electron microscopic view of transversely wrinkled spicular sheath. (i) Scanning electron microscopic view of P. toriii n. sp. egg with vermiculated eggshell surface. Scale bar is shown in each photograph.
Figure 10.
Morphology of Pearsonema toriii n. sp. (a) Dorsal view of caudal end of male worm, lacking apparent membranous bursa. Lateral cuticular swelling (arrowheads) before caudal end with conspicuous spheroid dorsolateral lobes (arrows). Protruded spicular sheath (SS) and spicule (Sp). (b) Lateral view of caudal end of male worm. Dorsolateral lobes (arrows). (c) Vulva (arrow) without appendages of female worm. End of stichosome (S). (d) Caudal end of female worm with subterminal anus (arrow). (e,f) Bioperculated barrel-shaped egg, with vermiculated eggshell surface texture. (g) Scanning electron microscopic view of male caudal end with spheroid dorsolateral lobes (arrows). (h) Scanning electron microscopic view of transversely wrinkled spicular sheath. (i) Scanning electron microscopic view of P. toriii n. sp. egg with vermiculated eggshell surface. Scale bar is shown in each photograph.
Figure 11.
Morphology of Echinocoleus yokoyamae n. sp. (a–c) specimens from badgers; (d–f) specimens from wild boars. (a,d) Ventral view of caudal end of male worm, terminated in two lobes, extending to sickle-shaped thick projections (arrows on right side), which support two-lobed membranous pseudobursa (PB). In addition, a pair of digitiform ventrolateral projections at precloacal level (arrows on left side) border anterior edge of membranous bursal lobes. (b,e) Vulva (arrow) with cylindrical vulvar appendage. (c,f) Caudal end of female worm with terminal anus (arrow). All photograph at the same magnification, and scale is shown in (c,f).
Figure 11.
Morphology of Echinocoleus yokoyamae n. sp. (a–c) specimens from badgers; (d–f) specimens from wild boars. (a,d) Ventral view of caudal end of male worm, terminated in two lobes, extending to sickle-shaped thick projections (arrows on right side), which support two-lobed membranous pseudobursa (PB). In addition, a pair of digitiform ventrolateral projections at precloacal level (arrows on left side) border anterior edge of membranous bursal lobes. (b,e) Vulva (arrow) with cylindrical vulvar appendage. (c,f) Caudal end of female worm with terminal anus (arrow). All photograph at the same magnification, and scale is shown in (c,f).
Figure 12.
Line drawings of Echinocoleus yokoyamae n. sp. (a–c), and Eucoleus kaneshiroi n. sp. (d–f). (a,d) Ventral view of caudal end of male worms. (b,e) Vulval region of female worms, and intrauterine eggs. (c,f) Caudal end of female worm with terminal anus.
Figure 12.
Line drawings of Echinocoleus yokoyamae n. sp. (a–c), and Eucoleus kaneshiroi n. sp. (d–f). (a,d) Ventral view of caudal end of male worms. (b,e) Vulval region of female worms, and intrauterine eggs. (c,f) Caudal end of female worm with terminal anus.
Figure 13.
Morphology of Eucoleus kaneshiroi n. sp. (a) Ventral view of caudal end of male worm, terminated in two small lobes (arrows), supporting clear semilunar-shape membranous pseudobursa (PB). A pair of papillae, close to each other, on each caudal lobe. (b) Vulva (arrow) without vulvar appendage. (c) Caudal end of female worm with terminal anus (arrow). (d) Intrauterine bioperculated lemon-shaped egg, with minutely pitted eggshell surface. Photographs (a–c) at the same magnification, and scale is shown in (c).
Figure 13.
Morphology of Eucoleus kaneshiroi n. sp. (a) Ventral view of caudal end of male worm, terminated in two small lobes (arrows), supporting clear semilunar-shape membranous pseudobursa (PB). A pair of papillae, close to each other, on each caudal lobe. (b) Vulva (arrow) without vulvar appendage. (c) Caudal end of female worm with terminal anus (arrow). (d) Intrauterine bioperculated lemon-shaped egg, with minutely pitted eggshell surface. Photographs (a–c) at the same magnification, and scale is shown in (c).
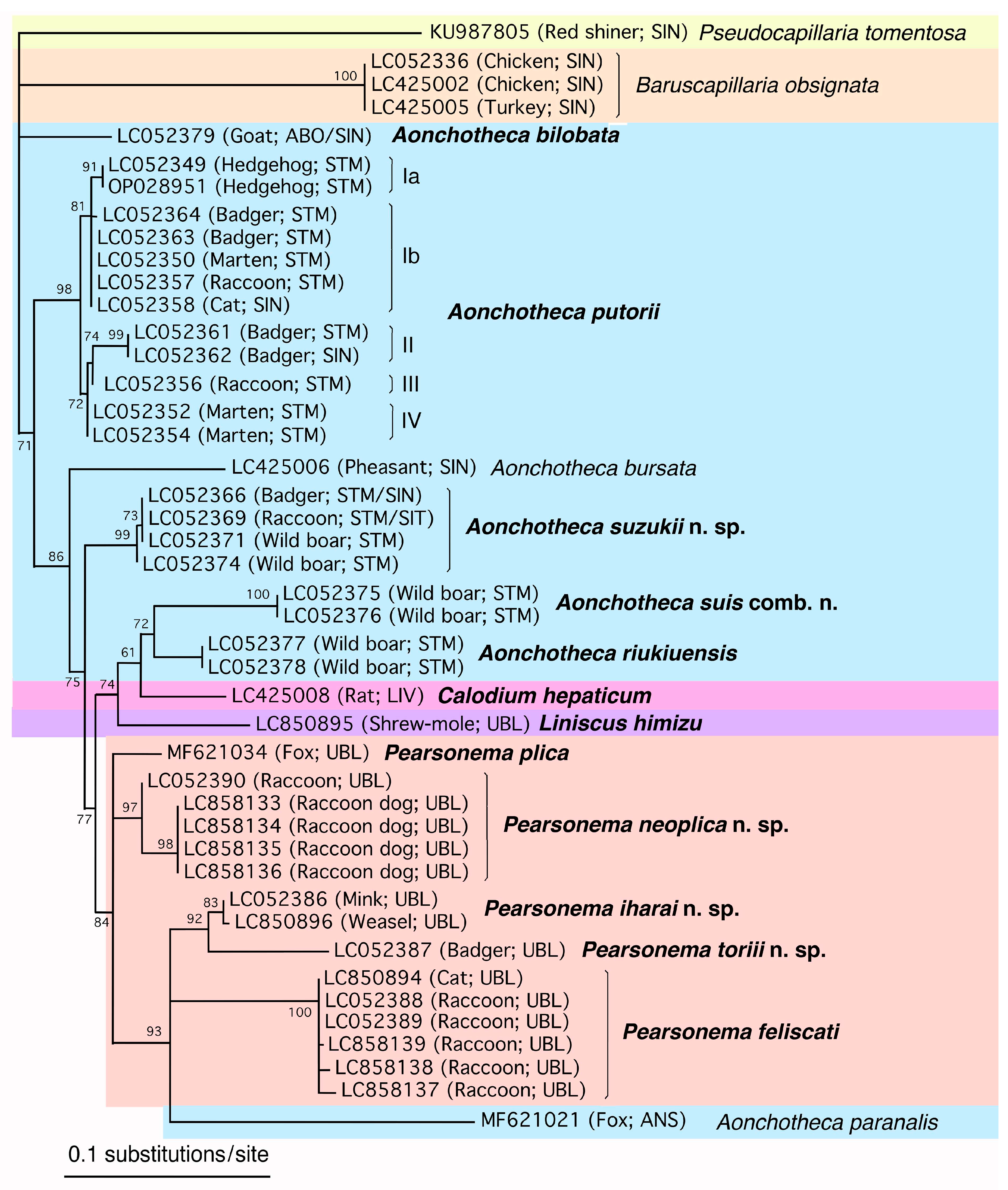
Figure 14.
ML phylogenetic tree based on the SSU rDNA sequence. The GenBank accession number is followed by the host and isolation organ in parentheses and the species name. ABO, abomasum; CEM, cecum; ESP, esophagus; LIN, large intestine; LIV, liver; RES, respiratory system; SIN, small intestine; STM, stomach; TRA, trachea; and UBL, urinary bladder. Capillariid species reported in this study are indicated in bold letters. Sequences of isolates belonging to the same genus are placed on a single background color: Aonchotheca, light blue; Baruscapillaria, light orange; Calodium, pink; Capillaria, light violet; Echinocoleus, light gree; Eucoleus, emerald green, Liniscus, violet; Pearsonema, orange; and Pseudocapillaria, light yellow.
Figure 14.
ML phylogenetic tree based on the SSU rDNA sequence. The GenBank accession number is followed by the host and isolation organ in parentheses and the species name. ABO, abomasum; CEM, cecum; ESP, esophagus; LIN, large intestine; LIV, liver; RES, respiratory system; SIN, small intestine; STM, stomach; TRA, trachea; and UBL, urinary bladder. Capillariid species reported in this study are indicated in bold letters. Sequences of isolates belonging to the same genus are placed on a single background color: Aonchotheca, light blue; Baruscapillaria, light orange; Calodium, pink; Capillaria, light violet; Echinocoleus, light gree; Eucoleus, emerald green, Liniscus, violet; Pearsonema, orange; and Pseudocapillaria, light yellow.
Figure 15.
ML phylogenetic tree based on the SSU rDNA sequence, focusing on a clade of
Aonchotheca/
Pearsonema/
Calodium/
Liniscus. The labeling of each sequence, organ abbreviation, and species name are expressed as explained in the legend for
Figure 14.
Figure 15.
ML phylogenetic tree based on the SSU rDNA sequence, focusing on a clade of
Aonchotheca/
Pearsonema/
Calodium/
Liniscus. The labeling of each sequence, organ abbreviation, and species name are expressed as explained in the legend for
Figure 14.
Figure 16.
Locations of interspecific and intraspecific nucleotide changes (substitutions and indels) of 88 isolates (classified into 28 species of 9 genera) plotted on a putative SSU rDNA secondary structure of A. putorii (DDBJ/EMBL/GenBank accession no. LC052349). Eighty-eight sequences (isolates) are classified as Aonchotheca (34 sequences of 7 species), Baruscapillaria (5 sequences of B. obsignata), Capillaria (15 sequences of 5 species), Echinocoleus (2 sequences of E. yokoyamae n. sp.), Eucoleus (13 sequences of 8 species), Pseudocapillaria (one sequence of P._tomentosa), Calodium (one sequence of C. hepaticum), Pearsonema (16 sequence of 5 species), and Liniscus (one sequence of L. himizu). Base sites of intergeneric and interspecific nucleotide variation are expressed in red letters on a yellow background color, and those of intraspecific nucleotide variation are with blue letters. Bases expressed in black letters are highly conserved nucleotides with all capillariid isolates analyzed here.
Figure 16.
Locations of interspecific and intraspecific nucleotide changes (substitutions and indels) of 88 isolates (classified into 28 species of 9 genera) plotted on a putative SSU rDNA secondary structure of A. putorii (DDBJ/EMBL/GenBank accession no. LC052349). Eighty-eight sequences (isolates) are classified as Aonchotheca (34 sequences of 7 species), Baruscapillaria (5 sequences of B. obsignata), Capillaria (15 sequences of 5 species), Echinocoleus (2 sequences of E. yokoyamae n. sp.), Eucoleus (13 sequences of 8 species), Pseudocapillaria (one sequence of P._tomentosa), Calodium (one sequence of C. hepaticum), Pearsonema (16 sequence of 5 species), and Liniscus (one sequence of L. himizu). Base sites of intergeneric and interspecific nucleotide variation are expressed in red letters on a yellow background color, and those of intraspecific nucleotide variation are with blue letters. Bases expressed in black letters are highly conserved nucleotides with all capillariid isolates analyzed here.
![Pathogens 14 00455 g016 Pathogens 14 00455 g016]()
Table 1.
Measurements of Aonchotheca putorii (types A and B) from different mammalian species.
Table 1.
Measurements of Aonchotheca putorii (types A and B) from different mammalian species.
| Worm type | Type A | Type B | –a | – | – | – | – |
| Host species | Martes melampus; Mustela sibirica; Felis silvastris catus; Erinaceus amurensis | Martes melampus; Mustela sibirica; Procyon lotor | Lutra lutra; Mustela spp.; Neogale vison (syn. Neovison vison); Martes foina | Neogale vison (syn. Neovison vison) | Neogale vison (syn. Neovison vison) | Erinaceus europaeus | Procyon lotor |
| Location | stomach, small intestine | stomach, small intestine | esophagus, stomach, small intestine | stomach | small intestine | stomach | stomach |
| Locality | Japan (Aomori, Akita, Shizuoka, Wakayama, Kochi) | Japan (Aomori, Akita, Kochi, Saga, Nagasaki) | Europe, North America | Canada (Ontario) | Canada (Ontario) | Canada (Ontario) | Canada (Ontario) |
| Reference | Present study | Present study | [2] | [4] | [4] | [4] | [4] |
| Male worms | (n = 44) | (n = 14) | – | (n = 10) | (n = 10) | (n = 10) | (n = 10) |
| Worm length | 4.47–7.56 (6.10) | 4.47–6.74 (5.52) | 5.4–8.0 | 5.6–8.0 (6.8) | 6.9–9.6 (8.4) | 5.1–6.9 (5.9) | 4.5–7.1 (5.8) |
| Max. worm width | 0.037–0.070 (0.047) | 0.042–0.055 (0.046) | 0.058–0.068 | 0.043–0.053 (0.046) | 0.048–0.060 (0.053) | 0.038–0.048 (0.040) | 0.035–0.048 (0.041) |
| Esophagus length | 2.25–3.78 (3.01) | 2.17–3.01 (2.63) | 2.41–2.55 | 2.6–3.5 (3.1) | 3.2–3.9 (3.5) | 2.2–3.5 (2.8) | 2.3–3.4 (2.9) |
| P/A proportion c | 0.80–1.29 (1.00) | 0.91–1.41 (0.96) | 1.25–2.17 | – | – | – | – |
| Spicule length | 0.301–0.482 (0.363) | 0.203–0.247 (0.216) | 0.149–0.168 | 0.241–0.368 (0.323) | 0.240–0.357 (0.288) | 0.243–0.351 (0.309) | 0.194–0.351 (0.242) |
| Female worms | (n = 70) b | – | (n = 10) | (n = 10) | (n = 10) | (n = 10) |
| Worm length | 5.32–12.16 (8.54) | 9.4–15.0 | 7.1–11.8 (9.7) | 9.6–14.1 (12.1) | 8.9–12.4 (10.9) | 5.4–11.1 (9.1) |
| Max. worm width | 0.043–0.112 (0.067) | 0.068–0.088 | 0.053–0.066 (0.058) | 0.065–0.075 (0.072) | 0.049–0.069 (0.056) | 0.043–0.065 (0.058) |
| Esophagus length | 2.19–4.33 (3.27) | 3.02–3.59 | 2.6–3.9 (3.4) | 3.1–4.2 (3.9) | 3.3–4.1 (3.7) | 2.7–4.3 (3.7) |
| P/A proportion c | 1.17–1.95 (1.60) | 2.10–3.17 | – | – | – | – |
| Vulva from the esophageal end | 0.041–0.142 (0.081) | – | – | – | – | – |
| Egg length | 0.053–0.070 (0062) | 0.064–0.072 | 0.044–0.066 (0.061) | 0.059–0.068 (0.064) | 0.055–0.065 (0.059) | 0.058–0.066 (0.063) |
| Egg width | 0.022–0.036 (0028) | 0.028–0.032 | 0.021–0.026 (0.024) | 0.025–0.028 (0.026) | 0.021–0.025 (0.024) | 0.020–0.026 (0.024) |
Table 2.
Comparison of nucleotide variations in the SSU rDNA sequences of Aonchotheca putorii from different mammalian species in Japan.
Table 2.
Comparison of nucleotide variations in the SSU rDNA sequences of Aonchotheca putorii from different mammalian species in Japan.
| Genotype | Morphotype | Host | Locality | GenBank Accession No. | Relative Position of Nucleotide Where Nucleotide Changes Occur a |
| 108 | 167 | 171 | 172 | 174 | 210 | 212 | 213 | 214 | 218 | 220 | 224 | 225 | 226 | 229 | —229/230— | 230 | 234 | 859 | 1506 | 1545 | 1546 | 1547 | 1556 | 1701 | 1709 | 1712 | 1719 | 1736 | 1737 |
| Ia | A | Erinaceus amurensis | Shizuoka | LC052349 | T | G | C | G | T | C | C | A | T | T | G | C | T | C | T | – | – | – | – | – | – | G | A | G | A | A | A | T | C | C | C | T | T | T | T |
| | n.d. b | Erinaceus europaeus c | Beijing, China | OP028951 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ |
| Ib | A | Martes m. melampus | Kochi | LC052350 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | C | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | T | ▪ | ▪ | ▪ | ▪ | ▪ |
| | A | Meles anakuma | Wakayama | LC052363 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | C | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | T | ▪ | ▪ | ▪ | ▪ | ▪ |
| | n.d. | Felis silvestris catus | Wakayama | LC052358–LC052360 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | C | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | T | ▪ | ▪ | ▪ | ▪ | ▪ |
| | n.d. | Meles anakuma | Kochi | LC052364 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | C | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | T | T | ▪ | ▪ | ▪ | ▪ | ▪ |
| | n.d. | Procyon lotor | Saga | LC052356 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | C | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | T | ▪ | ▪ | ▪ | ▪ | ▪ |
| | n.d. | Procyon lotor | Nagasaki | LC052357 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | C | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | T | ▪ | ▪ | ▪ | ▪ | ▪ |
| II | n.d. | Meles anakuma | Shiga | LC052361 | A | ▪ | T | T | C | T | T | G | C | C | ▪ | G | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | G | ▪ | ▪ | ▪ | ▪ | T | T | C | C | G | – |
| | n.d. | Meles anakuma | Shiga | LC052362 | A | ▪ | T | T | C | T | T | G | C | C | ▪ | G | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | G | ▪ | ▪ | ▪ | ▪ | T | T | C | C | G | – |
| III | B | Procyon lotor | Saga | LC052356 | ▪ | T | T | ▪ | ▪ | T | G | ▪ | ▪ | C | A | ▪ | G | T | G | ▪ | ▪ | ▪ | ▪ | ▪ | G | T | T | C | ▪ | ▪ | ▪ | ▪ | ▪ | T | T | ▪ | C | G | – |
| | AB | Meles anakuma | Wakayama | LC052365 | ▪ | T | T | ▪ | ▪ | T | G | ▪ | ▪ | C | A | ▪ | G | T | G | ▪ | ▪ | ▪ | ▪ | ▪ | G | T | T | C | ▪ | T | T | A | ▪ | | | | | | |
| IV | B | Martes m. melampus | Kochi | LC052352 | ▪ | ▪ | T | ▪ | ▪ | T | T | ▪ | ▪ | C | A | ▪ | ▪ | T | ▪ | T | G | C | G | G | G | T | T | C | ▪ | ▪ | ▪ | ▪ | ▪ | T | C | ▪ | C | G | – |
| | n.d. | Martes m. melampus | Kyoto | LC052353 | ▪ | ▪ | T | ▪ | ▪ | T | T | ▪ | ▪ | C | A | ▪ | ▪ | T | ▪ | T | G | C | G | G | G | T | T | C | ▪ | ▪ | ▪ | ▪ | ▪ | T | C | ▪ | C | G | – |
| | B | Martes m. melampus | Wakayama | LC052354 | ▪ | ▪ | T | ▪ | ▪ | T | T | ▪ | ▪ | C | A | ▪ | ▪ | T | ▪ | T | G | C | G | G | G | T | T | C | ▪ | ▪ | ▪ | ▪ | ▪ | T | C | ▪ | C | G | – |
| | B | Procyon lotor | Saga | LC052355 | ▪ | ▪ | T | ▪ | ▪ | T | T | ▪ | ▪ | C | A | ▪ | ▪ | T | ▪ | T | G | C | G | G | G | T | T | C | ▪ | ▪ | ▪ | ▪ | ▪ | T | C | ▪ | C | G | – |
Table 3.
Pairwise comparison of nucleotide variations in the SSU rDNA sequences of different genotypes of Aonchotheca putorii from wild mammalian hosts in Japan a.
Table 3.
Pairwise comparison of nucleotide variations in the SSU rDNA sequences of different genotypes of Aonchotheca putorii from wild mammalian hosts in Japan a.
| Genotype | GenBank Accession No. | Aonchotheca putorii Genotype |
| Type Ia | Type Ib | Type II | Type IIIa | Type IIIb | Type IV |
| Type Ia | LC052349 (1813 bp) | | 2 | 16 (1) | 14 (2) | 14 (1) | 12 (7) |
| Type Ib | LC052350 (1813 bp) | 99.89 | | 14 (1) | 12 (2) | 13 (1) | 10 (7) |
| Type II | LC052361 (1812 bp) | 99.12 | 99.23 | | 16 (1) | 18 (1) | 12 (6) |
| Type IIIa | LC052356 (1813 bp) | 99.23 | 99.34 | 99.12 | | 3 | 4 (5) |
| Type IIIb | LC052365 (1590 bp) | 99.12 | 99.18 | 98.87 | 99.81 | | 6 (5) |
| Type IV | LC052352 (1818 bp) | 99.34 | 99.45 | 99.34 | 99.79 | 99.62 | |
Table 4.
Measurements of Aonchotheca suzukii n. sp. from different mammalian species.
Table 4.
Measurements of Aonchotheca suzukii n. sp. from different mammalian species.
| | Aonchotheca suzukii n. sp. | Aonchotheca putorii |
| Morphotype A | Morphotype B |
| Host species | Procyon lotor | Nyctereutes procyonoides | Meles anakuma | Sus scrofa
leucomystax | Martes melampus; Mustela sibirica; Felis silvastris catus; Erinaceus amurensis | Martes melampus; Mustela sibirica; Procyon lotor |
| Locality | Japan (Saga, Nagasaki) | Japan (Gunma, Wakayama) | Japan (Kyoto, Wakayama, Saga) | Japan (Hyogo, Wakayama) | Japan (Aomori, Akita, Shizuoka, Wakayama, Kochi) | Japan (Aomori, Akita, Kochi, Saga, Nagasakai) |
| Male worms | (n = 4) | (n = 2) | (n = 2) | (n = 2) | (n = 44) | (n = 14) |
| Worm length | 5.78–6.66 (6.13) | 6.11–6.22 | 4.52–4.60 | 6.90–7.59 | 4.47–7.56 (6.10) | 4.47–6.74 (5.52) |
| Max. worm width | 0.042–0.050 (0.045) | 0.040–0.042 | 0.032–0.034 | 0.050–0.061 | 0.037–0.070 (0.047) | 0.042–0.055 (0.046) |
| Esophagus length | 2.74–3.12 (2.93) | 2.85–2.90 | 2.30–2.52 | 3.21–3.70 | 2.25–3.78 (3.01) | 2.17–3.01 (2.63) |
| P/A proportion a | 1.05–1.13 (1.09) | 1.10–1.18 | 0.83–0.97 | 1.05–1.15 | 0.80–1.29 (1.003) | 0.91–1.41 (0.955) |
| Spicule length | 0.370–0.455 (0.410) | 0.364–0.381 | 0.240–0.357 (0.288) | 0.390–0.460 | 0.301–0.482 (0.363) | 0.203–0.247 (0.216) |
| Female worms | (n = 5) | (n = 5) | (n = 17) | (n = 6) | | (n = 70) b |
| Worm length | 6.82–9.23 (8.26) | 8.49–10.16 (9.23) | 5.56–7.45 (6.24) | 8.96–9.73 (9.22) | | 5.32–12.16 (8.54) |
| Max. worm width | 0.049–0.057 (0.052) | 0.0503–0.065 (0.056) | 0.039–0.054 (0.047) | 0.053–0.061 (0.056) | | 0.043–0.112 (0.067) |
| Esophagus length | 2.44–3.29 (2.99) | 2.82–3.07 (2.94) | 2.16–2.79 (2.41) | 2.66–3.12 (2.90) | | 2.19–4.33 (3.27) |
| P/A proportion a | 1.67–1.86 (1.77) | 1.92–2.37 (2.14) | 1.41–1.74 (1.59) | 1.92–2.39 (2.18) | | 1.17–1.95 (1.60) |
| Vulva from the esophageal end | 0.068–0.186 (0.135) | 0.060–0.121 (0.087) | 0.041–0.082 (0060) | 0.082–0.132 (0.110) | | 0.041–0.142 (0.081) |
| Egg length | 0.058–0.064 (0.060) | 0.056–0.063 (0.060) | 0.053–0.064 (0.058) | 0.054–0.066 (0.059) | | 0.053–0.070 (0.062) |
| Egg width | 0.023–0.029 (0.026) | 0.024–0.028 (0.026) | 0.021–0.030 (0.025) | 0.024–0.027 (0.025) | | 0.022–0.036 (0.028) |
Table 5.
Prevalence of capillariid worms from the urinary bladder of wild mammals in Japan.
Table 5.
Prevalence of capillariid worms from the urinary bladder of wild mammals in Japan.
| Host | Locality | Date | No. of Animals Examined | No. of Positive Animals with Parasites | Parasite Species |
| Procyon lotor | Hyogo Pref. | Aug. 2009–Jul. 2010 | 154 | 9 (5.8%) a | ![Pathogens 14 00455 i001 Pathogens 14 00455 i001]() | Pearsonema neoplica n. sp.
Pearsonema feliscati |
| | Wakayama Pref. | Feb. 2015–Jul. 2017 | 477 | 26 (5.5%) a |
| Nyctereutes procyonoides viverrinus | Wayakama Pref. | Feb. 2015–Jan. 2016 | 32 | 14 (43.8%) | Pearsonema neoplica n. sp. |
| Neogale vison | Fukushima Pref. | Aug. 2010–Oct. 2010 | 76 | 31 (40.8%) | Pearsonema iharai n. sp. |
| Meles anakuma | Wakayama Pref. | Mar. 2010–Oct. 2010 | 29 | 13 (44.8%) | Pearsonema toriii n. sp. |
Table 6.
Measurements of Pearsonema spp. from the urinary bladder of Carnivora mammals (expressed in mm).
Table 6.
Measurements of Pearsonema spp. from the urinary bladder of Carnivora mammals (expressed in mm).
| Species | Pearsonema neoplica n. sp. | Pearsonema plica (Rudolphi, 1819) | Pearsonema feliscati (Bellingham, 1845) |
| (with triangular bursa / no vulval appendage) | (without triangular bursa / no vulval appendage)a | (with triangular bursa / cylindrical vulval appendage) | (with triangular bursa / no vulval appendage) | (without triangular bursa / no vulval appendage) |
| Host | Nyctereutes procyonoides viverrinus | Nyctereutes procyonoides viverrinus | Vulpes vulpes | Procyon lotor | Meles meles | Vulpes spp. | Procyon lotor | Felis spp. |
| Locality | Japan (Wakayama) | Japan (Wakayama) | Canada (Ontario) | Europe | Japan (Wakayama) | – |
| Reference | Present study | Present study | [4] | Rukhlyadev (1948) in [3] | Present study | [1] |
| Male worms | (n = 4) | (n = 8) | (n = 15) | (n = 15) | (n = ?) | (n = ?) | (n = 4) | (n = ?) |
| Worm length | 19.29–23.85 (20.65) | 16.76–22.21 (20.60) | 28.6–53.3 (39.9) | 16.7–31.2 (22.6) | 21.06–32.94 | 27.00–37.80 | 20.54–24.23 (22.27) | 25.5 |
| Max. worm width | 0.052–0.061 (0.056) | 0.044–0.057 (0.051) | 0.055–0.070 (0.060) | 0.031–0.048 (0.041) | 0.044–0.060 | 0.052–0.060 | 0.046–0.055 (0.050) | 0.032–0.064 |
| Esophagus length | 4.97–7.39 (6.25) | 5.23–7.47 (6.53) | 7.7–11.7 (9.3) | 3.5–6.8 (5.4) | 6.66–7.92 | 9.00–10.26 | 4.06–7.17 (6.04) | 6.7 |
| P/A proportion b | 1.61–3.80 (2.41) | 1.95–2.39 (2.17) | – (3.29) | – (3.19) | – c | – | 2.32–4.33 (2.85) | 3 |
| Spicule length | 1.55–1.89 (1.78) | 1.47–1.80 (1.61) | 3.4–5.2 (4.5) | 2.3–3.5 (2.9) | 2.80–3.85 | 2.83–3.87 | 2.22–2.46 (2.33) | 2.5 |
| Female worms | (n = 7) | (n = 9) | (n = 15) | (n = 15) | (n = ?) | (n = ?) | (n = 10) | (n = ?) |
| Worm length | 20.45–30.63 (26.34) | 19.96–26.69 (21.80) | 29.4–52.2 (42.7) | 17.6–44.9 (25.9) | 27.9–36.0 | 28.44–29.34 | 13.32–34.70 (22.91) | 28.6–31.9 |
| Max. worm width | 0.070–0.103 (0.082) | 0.067–0.087 (0.079) | 0.089–0.114 (0.100) | 0.060–0.109 (0.070) | 0.081–0.085 | 0.088 | 0.047–0.108 (0.081) | 0.032–0.144 |
| Esophagus length | 6.59–8.31 (7.79) | 7.15–9.84 (8.10) | 6.9–11.9 (9.9) | 4.4–8.4 (6.6) | 8.82–9.90 | 10.08–10.98 | 5.22–13.48 (9.22) | 10.2–10.8 |
| P/A proportion b | 2.06–2.97 (2.39) | 1.48–2.02 (1.73) | – (3.31) | – (2.92) | – | – | 1.21–2.08 (1.52) | 2 |
| Vulva from the esophageal end | 0.052–0.235 (0.107) | 0.058–0.208 (0.128) | – | – | – | – | 0.073–0.525 (0.248) | 0.034–0.544 |
| Egg length | 0.060–0.068 (0.064) | 0.064–0.073 (0.068) | 0.058–0.071 (0.065) | 0.059–0.074 (0.064) | 0.062–0.068 | 0.062–0.065 | 0.057–0.078 (0.063) | 0.051–0.062 |
| Egg width | 0.027–0.030 (0.028) | 0.026–0.029 (0.028) | 0.025–0.031 (0.028) | 0.023–0.028 (0.026) | 0.029–0.031 | 0.026–0.028 | 0.022–0.031 (0.026) | 0.024–0.032 |
Table 7.
Comparison of nucleotide variations in the SSU rDNA sequences of Pearsonema spp. from different mammalian species in Japan.
Table 7.
Comparison of nucleotide variations in the SSU rDNA sequences of Pearsonema spp. from different mammalian species in Japan.
| Parasite a | Host | GenBank Accession No. | Sequence Length (bp) | Relative position of nucleotide where nucleotide changes occur b |
| 52 | 54 | 61 | 104 | 109 | 147 | 148 | 150 | 151 | 153 | 154 | 164 | 166 | 167 | —167/168— | 168 | 169 | 172 | 173 | 174 | 198 | 205 | 206 |
| P. neoplica n. sp. | Raccoon dog | LC858133 | 1831 | C | T | A | G | A | A | C | C | C | C | G | A | T | A | – | – | – | T | G | G | G | T | A | A | G |
| P. neoplica n. sp. | Raccoon dog | LC858134 | 1808 | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| P. neoplica n. sp. | Raccoon dog | LC858135 | 1808 | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| P. neoplica n. sp. | Raccoon dog | LC858136 | 1808 | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| P. neoplica n. sp. | Raccoon | LC052390 | 1808 | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| P. plica | Red foxa | MF621034 | 1743 | • | • | • | • | • | • | • | G | T | • | • | • | C | T | • | • | • | • | • | • | • | • | • | G | • |
| P. iharai n. sp. | Mink | LC052386 | 1810 | • | • | • | • | • | • | • | • | T | • | • | • | G | T | • | • | • | • | T | • | – | • | • | G | • |
| P. iharai n. sp. | Weasel | LC850896 | 1816 | • | • | • | • | • | • | • | • | T | • | • | • | G | T | • | • | • | • | T | • | – | C | • | G | • |
| P. toriii n. sp. | Badger | LC052387 | 1813 | G | • | T | A | G | G | T | • | T | T | • | • | G | C | • | • | • | • | • | • | • | C | G | • | • |
| P. feliscati | Raccoon | LC052388 | 1824 | • | G | • | A | • | • | • | • | • | • | • | G | C | T | T | T | T | • | • | T | • | C | • | G | • |
| P. feliscati | Raccoon | LC052389 | 1827 | • | G | • | A | • | • | • | • | • | • | • | G | C | T | T | T | T | • | • | T | • | C | • | G | • |
| P. feliscati | Raccoon | LC858137 | 1850 | • | G | • | A | • | • | • | • | • | • | • | G | C | T | T | T | T | • | • | T | • | C | • | G | • |
| P. feliscati | Raccoon | LC858138 | 1850 | • | G | • | A | • | • | • | • | • | • | • | G | C | T | T | T | T | • | • | T | • | C | • | G | • |
| P. feliscati | Raccoon | LC858139 | 1850 | • | G | • | A | • | • | • | • | • | • | • | G | C | T | T | T | T | • | • | T | • | C | • | G | • |
| P. feliscati | Cat | LC850894 | 1827 | • | G | • | A | • | • | • | • | • | • | • | G | C | T | T | T | T | • | • | T | • | C | • | G | • |
| Liniscus himizu | Shrew mole | LC850895 | 1798 | • | • | • | A | • | • | • | • | • | • | A | • | • | T | G | • | • | C | T | • | T | • | • | • | A |
| Relative position of nucleotide where nucleotide changes occur b |
| 207 | 209 | 210 | 211 | 212 | 213 | 216 | 217 | 218 | 219 | 220 | 221 | 222 | 223 | 224 | 225 | 226 | —226/227— | 227 | 228 | —228/229— | 229 |
| C | T | A | T | G | G | G | G | C | T | A | C | C | T | T | G | C | – | – | – | – | – | – | – | G | T | – | – | – | – | – | – | G |
| • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| T | • | • | • | • | • | • | • | • | • | • | • | G | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| • | • | G | • | T | • | • | • | T | • | G | • | • | • | G | C | T | • | • | • | • | • | • | A | A | • | G | G | G | C | A | • | A |
| • | • | G | • | T | • | • | • | T | • | G | • | • | • | A | C | T | T | C | T | A | G | T | A | T | • | G | G | G | C | A | • | A |
| • | C | G | • | T | • | • | • | T | • | G | G | • | • | G | C | T | • | • | • | • | • | • | A | A | • | G | G | C | C | A | • | A |
| • | • | G | • | T | • | T | A- | T | • | G | G | T | G | C | C | G | C | T | A | A | G | C | A | • | C | G | G | T | A | G | C | A |
| • | • | G | • | T | • | T | A | T | • | G | G | T | G | C | C | G | C | T | A | A | G | C | A | • | C | G | G | T | A | G | C | A |
| • | • | G | • | T | • | T | A | T | • | G | G | T | G | C | C | G | C | T | A | A | G | C | A | • | C | G | G | T | A | G | C | A |
| • | • | G | • | T | • | T | A | T | • | G | G | T | G | C | C | G | C | T | A | A | G | C | A | • | C | G | G | T | A | G | C | A |
| • | • | G | • | T | • | T | A | T | • | G | G | T | G | C | C | G | C | T | A | A | G | C | A | • | C | G | G | T | A | G | C | A |
| • | • | G | • | T | • | T | A | T | • | G | G | T | G | C | C | G | C | T | A | A | G | C | A | • | C | G | G | T | A | G | C | A |
| • | • | T | A | T | A | • | • | – | – | – | – | – | • | • | C | A | • | • | • | • | • | • | • | A | • | • | • | • | • | • | • | A |
| Relative position of nucleotide where nucleotide changes occur b |
| 230 | 239 | 245 | 266 | 267 | 290 | 305 | 319 | 412 | 493 | 522 | 534 | 614 | 667 | 674 | 685 | 686 | 686/687 | 718 | 719 | 724 | 737 | 738 | 740 | 749 | 753 | 754 | 756 | 757 | 853 | 855 | 860 | 861 | 912 | 1075 |
| G | C | A | A | T | C | G | A | C | T | T | T | C | T | A | T | C | – | G | C | C | T | G | C | T | T | G | G | A | G | G | A | A | T | T |
| • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| • | • | • | T | • | T | • | • | • | • | • | C | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | T | • | C | C |
| – | • | • | A | • | • | • | • | • | • | • | C | • | C | G | • | • | • | • | • | • | • | A | • | C | • | • | • | • | • | • | – | G | • | C |
| – | • | • | A | • | • | • | • | • | • | • | C | • | C | G | • | • | • | • | • | • | • | A | • | C | • | • | • | • | • | • | – | G | • | C |
| A | • | G | A | • | • | • | • | • | • | • | C | • | C | G | • | • | • | • | • | • | • | • | • | C | • | • | • | • | • | • | • | G | • | C |
| • | • | • | • | C | • | • | • | • | C | C | C | • | C | G | C | T | G | • | • | T | • | • | • | • | C | • | • | • | • | • | • | G | • | G |
| • | • | • | • | C | • | • | • | • | C | C | C | • | C | G | C | T | G | • | • | T | • | • | • | • | C | • | • | • | • | • | • | G | • | G |
| • | • | • | • | C | • | • | • | • | C | C | C | • | C | G | C | T | G | • | • | T | • | • | • | • | C | • | • | • | • | • | • | G | • | G |
| • | • | • | • | C | • | • | • | • | C | C | C | • | C | G | C | T | G | • | • | T | • | • | • | • | C | • | • | • | • | • | • | G | • | G |
| • | • | • | • | C | • | • | • | • | C | C | C | • | C | G | C | T | G | • | • | T | • | • | • | • | C | • | • | • | • | • | • | G | • | G |
| • | • | • | • | C | • | • | • | • | C | C | C | • | C | G | C | T | G | • | • | T | • | • | • | • | C | • | • | • | • | • | • | G | • | G |
| A | T | • | • | • | • | A | T | G | • | • | C | T | • | • | • | • | • | T | G | • | C | • | T | • | • | A | C | G | A | C | – | • | • | C |
| Relative position of nucleotide where nucleotide changes occur b |
| 1112 | 1222 | —1373/1374— | 1383 | 1386 | 1410 | 1520 | 1521 | 1524 | 1526 | 1527 | 1562 | 1565 | 1704 | 1715 | 1720 | 1721 | 1723 | 1724 | 1725 | 1726 | 1730 | 1735 | 1747 | 1750 |
| C | C | – | – | – | T | C | G | T | T | A | A | G | G | C | G | G | G | C | C | G | T | T | A | A | C | G |
| • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | C | C | • | • | T | • | • | • | T | G | T | A |
| • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | T | T | • | • | T | • | • | • | T | G | T | A |
| • | • | • | • | • | – | • | • | • | C | • | G | • | • | • | C | C | • | • | • | • | • | • | T | G | T | A |
| • | • | • | • | • | – | • | • | • | C | • | G | • | • | • | C | C | • | • | • | • | • | • | T | G | T | A |
| G | • | • | • | • | – | T | • | C | C | G | G | C | • | • | C | C | • | T | • | C | • | • | T | • | T | A |
| G | • | G | T | C | – | • | • | • | • | • | • | • | • | • | • | • | • | • | • | – | – | – | • | • | • | • |
| G | • | G | T | C | – | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| G | T | G | T | C | – | T | A | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| G | • | G | T | C | – | • | • | • | • | • | • | • | A | G | • | • | • | • | • | • | • | • | • | • | • | • |
| G | • | G | T | C | – | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| G | • | G | T | C | – | • | • | • | • | • | • | • | • | • | • | • | • | • | C | • | • | • | • | • | • | • |
| • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | C | C | A | • | C | • | • | • | T | G | T | A |
Table 8.
Measurements of capillariid species from the urinary bladder of wild Carnivorra mammals in Japan (expressed in mm).
Table 8.
Measurements of capillariid species from the urinary bladder of wild Carnivorra mammals in Japan (expressed in mm).
| Species | Pearsonema iharai n. sp. | Pearsonema toriii n. sp. | Pearsonema neoplica n. sp. | P. feliscati (Bellingham, 1845) | Pearsonema plica (Rudolphi, 1819) |
| Host | Neogale vison | Meles anakuma | Nyctereutes procyonoides viverrinus | Procyon lotor | Meles meles | Vulpes spp. |
| Locality | Japan (Fukushima) | Japan (Wakayama) | Japan (Wakayama) | Japan (Wakayama) | Europe |
| Reference | Present study | Present study | Present study | Present study | Rukhlyadev (1948) in [3] |
| Male worms | (n = 10) | (n = 4) | (n = 6) | (n = 4) | (n = ?) | (n = ?) |
| Worm length | 16.19–26.19 (21.09) | 14.48–18.82 (15.86) | 19.51–21.45 (20.69) | 20.54–24.23 (22.27) | 21.06–32.94 | 27.00–37.80 |
| Max. worm width | 0.054–0.070 (0.065) | 0.035–0.049 (0.043) | 0.051–0.057 (0.053) | 0.046–0.055 (0.050) | 0.044–0.060 | 0.052–0.060 |
| Esophagus length | 4.90–6.49 (5.80) | 3.85–5.46 (4.91) | 5.78–7.19 (6.42) | 4.06–7.17 (6.04) | 6.66–7.92 | 9.00–10.26 |
| P/A proportion a | 1.99–3.12 (2.62) | 1.73–2.76 (2.27) | 1.97–2.39 (2.23) | 2.32–4.33 (2.85) | – b | – |
| Spicule length | 1.60–2.46 (2.18) | 1.95–2.10 (2.03) | 1.52–1.89 (1.67) | 2.22–2.46 (2.33) | 2.80–3.85 | 2.83–3.87 |
| Female worms | (n = 11) | (n = 7) | (n = 10) | (n = 10) | (n = ?) | (n = ?) |
| Worm length | 18.68–29.07 (23.85) | 18.32–26.53 (23.50) | 20.68–30.63 (23.56) | 13.32–34.70 (22.91) | 27.9–36.0 | 28.44–29.34 |
| Max. worm width | 0.104–0.148 (0.118) | 0.080–0.120 (0.103) | 0.070–0.91 (0.080) | 0.047–0.108 (0.081) | 0.081–0.085 | 0.088 |
| Esophagus length | 7.45–9.40 (8.24) | 7.29–9.02 (8.08) | 6.59–8.31 (7.77) | 5.22–13.48 (9.22) | 8.82–9.90 | 10.08–10.98 |
| P/A proportion a | 1.41–2.24 (1.89) | 1.50–2.27 (1.91) | 1.48–2.97 (2.05) | 1.21–2.08 (1.52) | – | – |
| Vulva from the esophageal end | 0.071–0.148 (0.118) | 0.214–0.667 (0.346) | 0.052–0.235 (0.122) | 0.073–0.525 (0.248) | – | – |
| Egg length | 0.064–0.075 (0.069) | 0.058–0.074 (0.067) | 0.060–0.074 (0.067) | 0.057–0.078 (0.063) | 0.062–0.068 | 0.062–0.065 |
| Egg width | 0.026–0.034 (0.030) | 0.027–0.031 (0.029) | 0.025–0.031 (0.028) | 0.022–0.031 (0.026) | 0.029–0.031 | 0.026–0.028 |
Table 9.
Pairwise comparison of nucleotide variations in the SSU rDNA sequences of urinary bladder worms (Pearsonema spp. and Liniscus himizu) a.
Table 9.
Pairwise comparison of nucleotide variations in the SSU rDNA sequences of urinary bladder worms (Pearsonema spp. and Liniscus himizu) a.
| | Species | GenBank Accession No. | P neoplica n. sp. | P. plica | P. iharai n. sp. | P. toriii n.sp. | Pearsonema feliscati | L. himizu |
| | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| 1 | P neoplica n.sp. | LC858133 (1831 bp) | | 0 (0) | 0 (0) | 0 (0) | 7 (0) | 19 (0) | 29 (8) | 30 (14) | 46 (7) | 30 (25) | 30 (25) | 31 (21) | 33 (21) | 30 (25) | 30 (21) | 40 (7) |
| 2 | P neoplica n.sp. | LC858134 (1808 bp) | 100 | | 0 (0) | 0 (0) | 7 (0) | 19 (0) | 29 (8) | 30 (14) | 46 (7) | 30 (25) | 30 (25) | 31 (21) | 33 (21) | 30 (25) | 30 (21) | 40 (7) |
| 3 | P neoplica n.sp. | LC858135 (1808 bp) | 100 | 100 | | 0 (0) | 7 (0) | 19 (0) | 29 (8) | 30 (14) | 46 (7) | 30 (25) | 30 (25) | 31 (21) | 33 (21) | 30 (25) | 30 (21) | 40 (7) |
| 4 | P neoplica n.sp. | LC858136 (1808 bp) | 100 | 100 | 100 | | 7 (0) | 19 (0) | 29 (8) | 30 (14) | 46 (7) | 30 (25) | 30 (25) | 31 (21) | 33 (21) | 30 (25) | 30 (21) | 40 (7) |
| 5 | P neoplica n.sp. | LC052390 (1808 bp) | 99.6 | 99.6 | 99.6 | 99.6 | | 14 (0) | 24 (8) | 24 (14) | 43 (7) | 36 (24) | 36 (24) | 40 (21) | 40 (21) | 36 (24) | 37 (21) | 35 (7) |
| 6 | P. plica | MF621034 (1743 bp) | 98.9 | 98.9 | 98.9 | 98.9 | 99.2 | | 24 (8) | 25 (14) | 50 (7) | 39 (24) | 39 (24) | 42 (21) | 42 (21) | 39 (24) | 40 (21) | 42 (7) |
| 7 | P. iharai n sp. | LC052386 (1810 bp) | 98.4 | 98.4 | 98.4 | 98.4 | 98.7 | 98.7 | | 3 (0) | 25 (3) | 31 (20) | 31 (20) | 33 (17) | 33 (17) | 31 (20) | 31 (17) | 35 (13) |
| 8 | P. iharai n.sp. | LC850896 (1816 bp) | 98.3 | 98.3 | 98.3 | 98.3 | 98.7 | 98.6 | 99.8 | | 31 (7) | 39 (14) | 39 (14) | 41 (11) | 40 (11) | 39 (14) | 39 (11) | 40 (19) |
| 9 | P. toriii n.sp. | LC052387 (1813 bp) | 97.5 | 97.5 | 97.5 | 97.5 | 97.6 | 97.1 | 98.6 | 98.3 | | 47 (17) | 47 (17) | 48 (14) | 52 (13) | 47 (17) | 49 (14) | 51 (14) |
| 10 | P. feliscati | LC052388 (1824 bp) | 98.3 | 98.3 | 98.3 | 98.3 | 98.0 | 97.8 | 98.3 | 97.8 | 97.4 | | 0 (0) | 3 (3) | 2 (3) | 0 (0) | 0 (3) | 50 (29) |
| 11 | P. feliscati | LC052389 (1827 bp) | 98.3 | 98.3 | 98.3 | 98.3 | 98.0 | 97.8 | 98.3 | 97.8 | 97.4 | 100 | | 3 (3) | 2 (3) | 0 (0) | 0 (3) | 50 (29) |
| 12 | P. feliscati | LC858137 (1850 bp) | 98.3 | 98.3 | 98.3 | 98.3 | 97.8 | 97.6 | 98.2 | 97.7 | 97.4 | 99.8 | 99.8 | | 5 (0) | 4 (0) | 3 (0) | 53 (26) |
| 13 | P. feliscati | LC858138 (1850 bp) | 98.2 | 98.2 | 98.2 | 98.2 | 97.8 | 97.6 | 98.2 | 97.8 | 97.1 | 99.9 | 99.9 | 99.7 | | 0 (0) | 0 (3) | 53 (26) |
| 14 | P. feliscati | LC858139 (1850 bp) | 98.3 | 98.3 | 98.3 | 98.3 | 98.0 | 97.8 | 98.3 | 97.8 | 97.4 | 100 | 100 | 99.8 | 100 | | 1 (0) | 50 (29) |
| 15 | P. feliscati | LC850894 (1827 bp) | 98.4 | 98.4 | 98.4 | 98.4 | 98.5 | 97.7 | 98.3 | 97.9 | 97.3 | 100 | 100 | 99.8 | 100 | 99.9 | | 51 (26) |
| 16 | L. himizu | LC850895 (1798 bp) | 97.8 | 97.8 | 97.8 | 97.8 | 98.1 | 97.6 | 98.0 | 97.8 | 97.2 | 97.2 | 97.2 | 97.1 | 97.1 | 97.2 | 97.2 | |