Abstract
Ferrets are highly susceptible to a wide range of mycobacteria, mainly M. bovis, M. avium, and M. triplex. Therefore, ferrets pose a risk of transmission of mycobacteriosis, especially zoonotically relevant tuberculosis. The aim of this study was to describe the findings of M. xenopi mycobacteriosis in a pet ferret and emphasize its zoonotic potential. A pet ferret had a history of weight loss, apathy, hyporexia, and hair loss. Abdominal ultrasound revealed splenomegaly with two solid masses and cystic lesions of the liver. Fine-needle aspiration cytology revealed numerous acid-fast bacilli in epithelioid cells, thus leading to the suspicion of mycobacterial infection. Because of its poor general condition, the ferret was euthanized. Necropsy examination revealed generalized granulomatous lymphadenitis, pneumonia, myocarditis, splenitis, and hepatitis. Histologically, in all organs, there were multifocal to coalescing areas of inflammatory infiltration composed of epithelioid macrophages, a low number of lymphocytes, and plasma cells, without necrosis nor multinucleated giant cells. Ziehl–Neelsen staining detected the presence of numerous (multibacillary) acid-fast bacteria, which were PCR-typed as M. xenopi. This is the first study showing the antimicrobial susceptibility testing of M. xenopi in veterinary medicine, describing the resistance to doxycycline. Overall, our results could facilitate further diagnosis and provide guidelines for the treatment protocols for such infections.
1. Introduction
The genus Mycobacterium is divided into members of the Mycobacterium tuberculosis complex (MTBC) and non-tuberculous mycobacteria (NTM). Ferrets are considered highly susceptible to a wide range of mycobacteria, with the most commonly reported cases of infection involving species Mycobacterium bovis, M. avium, and M. triplex [1]. Other reported mycobacterial infection isolates in ferrets include M. genavense, M. microti, M. celatum, M. abscessus, M. fortuitum, M. florentinum, M. interjectum, M. septicum, M. peregrinum, and M. xenopi. [2,3,4,5,6]. Infections caused by M. bovis in some ferrets were described as systemic disease, while in others, only one or more lymph nodes were affected. However, infections caused by M. avium complex are mostly described as granulomatous enteritis and pneumonia [2]. So far, mycobacteriosis has been regularly described and is widespread in feral ferrets in New Zealand, while in other countries, only sporadic occurrences have been described [7]. These infections in ferrets most often occur through ingestion, although infection through inhalation is also possible [8]. The disease most often causes granulomatous formations in the digestive tract and associated lymph nodes, but the spleen, liver, and lungs can also be affected, depending on the route of infection entry [8,9,10,11]. The mycobacteria were isolated from the oral cavity and bronchoalveolar lavage but also from urine, mammary tissue, and faeces of infected animals, indicating the sources of mycobacterial excretion [6].
2. Materials and Methods
2.1. In Vivo Clinical Examination
We conducted a clinical study of mycobacteriosis in a male ferret, with further information provided later in the text. After arriving at the clinic, the animal underwent haematological and biochemical tests and an ultrasound examination. A cytological examination of suspicious tissues was also performed, and the obtained material was stained with the May–Grünwald–Giemsa (MGG) and Ziehl–Neelsen (ZN) stains. Amplification of the DNA sequence containing the gene coding for the 65 kDa antigen common in all mycobacteria was performed using conventional PCR to detect members of the genus Mycobacterium in punctate [12,13].
2.2. Post-Mortem Examination
After humane euthanasia, necropsy and histopathological examination were performed. Representative samples of lung, spleen, liver, and mesenteric and tracheobronchial lymph nodes were submitted for bacteriological examination and inoculated onto standard growth medium (Löwenstein–Jensen medium supplemented with pyruvate, Löwenstein–Jensen medium supplemented with glycerine, and Stonebrink medium) specially used for the culture of Mycobacterium species, followed by incubation at 37 °C [14]. Grown colonies were stained using ZN to confirm the presence of acid-fast bacilli. Furthermore, amplification of the DNA sequence containing the gene coding 65 kDa antigen was used to identify the grown colonies as members of the genus Mycobacterium. Isolated mycobacteria were tested with GenoType® Mycobacterium CM (Hain Lifescience, Nehren, Germany) for further identification [12,13,15].
The obtained isolate was subjected to antimicrobial susceptibility testing via broth microdilution method using the Sensititre ™ Myco SLOMYCO AST Plate commercial kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the Clinical and Laboratory Standards Institute recommendations [16,17]. Thirteen antibiotics most often used in human medicine were tested: amikacin (AMI), ciprofloxacin (CIP), clarithromycin (CLA), doxycycline (DOX), ethambutol (EMB), ethionamide (ETH), isoniazid (INH), linezolid (LZD), moxifloxacin (MXF), rifabutin (RFB), rifampin (RIF), streptomycin (STR), and trimethoprim-sulfamethoxazole (SXT). Cation-adjusted Mueller–Hinton broth (Thermo Fisher Scientific) supplemented with 5% Middlebrook Oleic Albumin Dextrose Catalase Growth Supplement (Sigma-Aldrich, St. Louis, MO, USA) was used for the preparation of bacterial suspension. Then microplate was incubated at 36 ± 1 °C for 14 days, followed by the classification of isolates as susceptible, intermediate susceptible, or resistant for all tested antibiotics except for EMB, ETH, and STR (no interpretation criteria have been established so far) [17].
3. Case Description
3.1. Patient
A 4-year-old hormonally castrated male ferret with a 3-month history of progressive weight loss, apathy, hyporexia, and hair loss was presented. The animal had been kept together with a female ferret for four years in the owner’s house (in a cage with temporary access to free range in the house) and was fed with commercial feed. The second female ferret had a diagnosis of lymphoma and was humanely euthanized due to poor health conditions and poor prognosis. Both ferrets were obtained from the same breeder from the ferret farm as cubs and were regularly vaccinated against distemper. Besides the ferrets, a 3-year-old female mixed-breed dog was present in the household.
3.2. Diagnostics
The ferret was depressed, hypothermic (rectal temperature of 36.2 °C), had a body condition score of 2 out of 5, and bilateral serous epiphora. A complete blood count revealed elevated values of white blood cells (WBCs) (17.8 109/L, RI 2.5–5.5 109/L), lymphocytes (LYM) (4.5 109/L, RI 0.3–1.3 109/L), granulocytes (GRA) (12 109/L, RI 0.4–2.0 109/L), monocytes (MON) (1.3 109/L, RI 0.0–0.2 109/L), and red cell distribution width (RDW) (17.10%, RI 14–17%), while decreased values of haemoglobin (HGB) (147 g/L, RI 150–180 g/L), mean corpuscular haemoglobin (MCH) (13.7 pg, RI 15–20 pg), and mean corpuscular haemoglobin concentration (MCHC) (290 g/L, RI 300–340 g/L) [18,19]. Other biochemical parameters showed elevated values of gamma-glutamyl transferase (GGT) (0.55 ukat/L, RI 0.00–0.03 ukat/L), cholesterol (CHOL) (8.70 mmol/L, RI 1.29–5.96 mmol/L), lipase (LIPA) (1.17 ukat/L, RI 0.00–0.53 ukat/L), and blood urea nitrogen (BUN) (15.30 mmol/L, RI 5.40–13.20 mmol/L), while decreased values of creatinine (CREA) (27.00 umol/L, RI 62.00–186.00 umol/L) and amylase (AMY) (1.53 ukat/L, RI 8.43–23.34 ukat/L). Palpation revealed enlarged popliteal lymph nodes. Abdominal ultrasound examination showed two hyperechoic round masses of 1.56 × 1.31 cm and 1.47 × 1.5 cm in the pancreaticoduodenal area (lymphadenomegaly) (Figure 1), cystic lesions of the liver, and splenomegaly. Fine-needle aspiration of the pancreaticoduodenal enlarged lymph nodes was performed, and smears were stained with the routine MGG stain. Cytologic examination revealed numerous epithelioid macrophages admixed with small lymphocytes, containing intracytoplasmic negative-staining rods measuring 2 × 0.5 μm. Staining with the ZN stain showed numerous acid-fast bacilli within the macrophages. Members of the genus Mycobacterium were identified in a punctate specimen by amplifying the 65 kDA antigen-specific DNA sequence. The animal was treated with amoxicillin-clavulanate (22 mg/kg every 12 h for 10 days) and, after the Mycobacterium determination, with enrofloxacin (10 mg/kg/day). The ferret became very weak and ill and had hypothermia and pale mucus membranes. He lost his appetite and rejected force-feeding. The ferret was humanely euthanized due to the poor prognosis and progressive clinical deterioration despite therapy.
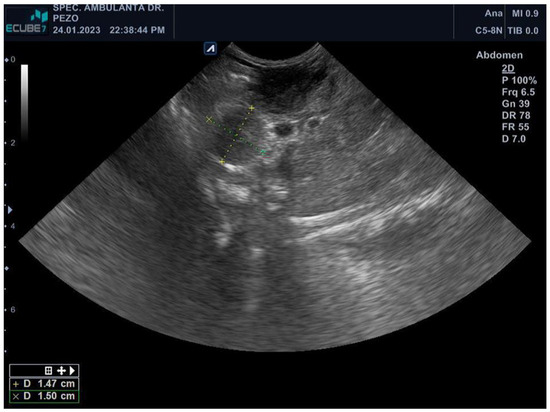
Figure 1.
Abdominal ultrasound (E-Cube 7, Alpinion, Microconvex–array ultrasound transducer C5-8N) revealing two hyperechoic round masses measuring 1.56 × 1.31 cm and 1.47 × 1.5 cm in the pancreaticoduodenal area.
3.3. Necropsy Findings
At post-mortem examination, all subcutaneous lymph nodes were moderately enlarged. The mesenteric and pancreaticoduodenal lymph nodes were markedly enlarged, whereas the mediastinal lymph node was moderately enlarged (Figure 2 and Figure 3). The lungs were voluminous, edematous, full of blood, and diffusely dark red color (edema and congestion, likely due to the euthanasia method), with prominent lobules. In cross-section, light yellow to pink areas were observed around the bronchus and bronchioles. The liver was slightly enlarged, with a pink to light brown color and numerous irregular multifocal to coalescing gray to yellow areas extending through the liver parenchyma. The spleen was markedly enlarged, dark red to dark brown in color, with a smooth capsule and multifocal light-yellow areas in the parenchyma measuring 0.3–0.5 cm in diameter. On the cut section, there was prominent white pulp hyperplasia. The gastric and small intestinal mucosa were moderately thickened with multifocal ulcerations (Figure 4).
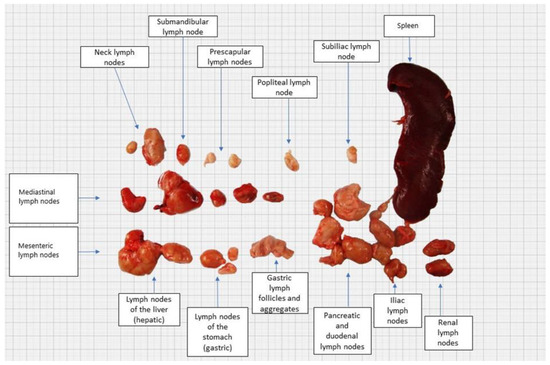
Figure 2.
Gross appearance of enlarged lymph nodes and spleen.
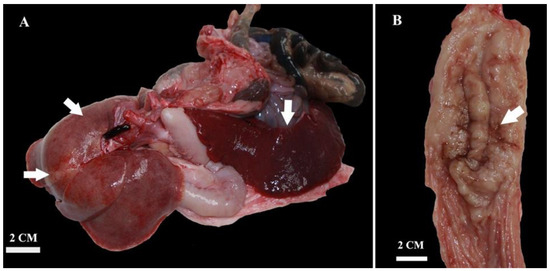
Figure 3.
Gross appearance of the liver showing multifocal to coalescing gray to yellow areas and severely enlarged spleen (splenomegaly) (A). Diffuse thickening and multifocal ulcerations of gastric mucosa (B).
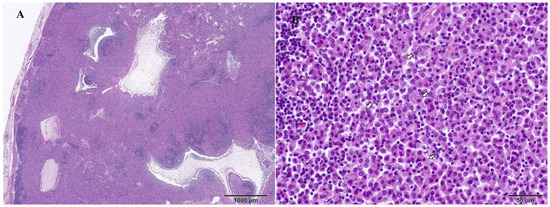
Figure 4.
Mesenteric lymph node, ferret, and H&E stains. Large multifocal to coalescing area of epithelioid macrophage infiltration that disrupts lymph node architectures (A). Higher magnification shows epithelioid macrophages with large, bright cytoplasm that infiltrate the lymph node (B).
3.4. Histopathological Findings
Representative samples from the mesenteric and mediastinal lymph nodes, stomach, liver, spleen, pancreas, intestine, heart, lung, kidney, and brain were fixed in 10% neutral-buffered formalin and routinely processed for histologic examination. Microscopically, samples from all organs revealed the presence of multifocal to coalescing poorly circumscribed areas of granulomatous inflammation containing numerous epithelioid macrophages (Figure 4) filled with myriad intracytoplasmic ZN-positive rod-shaped bacteria (Figure 5). There were no visible areas of necrosis among the granulomatous inflammation or multinucleated cells. Disseminated areas of granulomatous inflammation were observed around blood vessels in the lung, measuring 50 to 150 µm. The spleen also had marked extramedullary hematopoiesis (this is a common, likely incidental finding in ferrets).
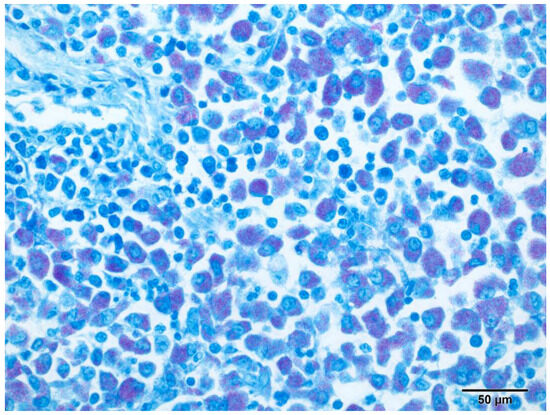
Figure 5.
Pancreaticoduodenal lymph node, ferret. Abundant (multibacillary) slender rod-shaped intracytoplasmic acid-fast M. xenopi within infiltrating macrophages. Ziehl–Neelsen, original magnification 200×.
3.5. Bacterial Examination and Molecular Identification
Grown colonies appeared on Löwenstein–Jensen medium supplemented with pyruvate on the 38th day of incubation, and the presence of acid-fast bacilli was determined using ZN staining. Amplification of the DNA sequence containing the gene coding for the 65 kDa antigen identified the grown colonies as a member of the genus Mycobacterium. The Geno Type® Mycobacterium CM hybridization test (Hain Lifescience, Nehren, Germany) revealed that the isolate belonged to M. xenopi, which was confirmed in all tested tissues.
Regarding antimicrobial susceptibility testing, the M. xenopi isolate was susceptible to all tested antibiotics except for DOX, where it proved to be resistant. For antibiotics EMB, ETH, and STR, we recorded only minimum inhibitory concentration (MIC) values because no interpretation criteria have been established so far (Table 1).

Table 1.
Breakpoints used for M. xenopi drug susceptibility testing and minimum inhibitory concentration (MIC) values of all drugs included in the panel.
Representative samples from the second female ferret were also subjected to a bacteriological examination, which ended with a negative result.
4. Discussion
Ferrets are particularly susceptible to a wide range of mycobacteria, with the most common ones being M. bovis and M. avium [7], followed by M. genavense, M. celatum, and M. microti [5,6,20]. Our case provides a clinical, ultrasonographic, cytologic, macroscopic, microscopic, and molecular description of M. xenopi in a ferret showing lesions that were more severe in the mesenteric and pancreaticoduodenal lymph nodes, stomach, intestines, spleen, and liver than the lung, suggesting a primary oral route of exposure, as most often diagnosed in ferrets with other mycobacteriosis [1,2,3,6,8,9,11]. However, the previous two publications on M. xenopi infection in ferrets describe more pronounced pathological changes in the lungs, which differs from our case [3,21]. It is also interesting to note that only the male ferret suffered from mycobacteriosis with a rapidly developing clinical picture, while the female was not infected, nor were mycobacteria isolated from her sample, even though she had been diagnosed with lymphoma. The animals were kept strictly indoors, and the source of infection has not been determined. Because of this, many issues from the epidemiological side remain unclear, as well as the fact about possible, so far undiscovered predispositions that led to the mycobacteria infection. The question of individual susceptibility to this infection remains unanswered, which indicates the need for further research and publication of such clinical cases.
In human medicine, M. xenopi is associated with the highest mortality among pulmonary nontuberculous mycobacterial (NTM) infections and is the second most commonly isolated NTM species responsible for pulmonary infection, behind M. avium complex [22]. A predisposing factor is immunosuppression, either local (lung disease) or systemic (lymphoproliferative malignancy, immunosuppressive therapy, or HIV infection) [3,23]. The preferred treatment regimen for M. xenopi infection in humans includes clarithromycin or azithromycin, ethambutol, and rifampin [24]. The isolate in our study did not show resistance to the mentioned antibiotics, but we should be aware that the treatment protocol for such infection is long-term with a very low success rate; in some studies, only 8.8% [25]. M. xenopi is an emerging opportunistic pathogen and should be considered as a potential zoonotic agent [2,3]. Although we have no confirmed disease in the owner of the ferret, we should be aware that the onset of symptoms is insidious, and the infection may progress slowly or increase and decrease over the months or years [26]. The rates of NTM lung diseases, of which M. xenopi is one of the most common causes, increase with human age and chronic diseases as well as menopause in women [27,28]. In this study, the organs of the ferret’s digestive tract were most affected, which indicates their excretion in faeces and the consequent contamination of the living space, thus exposing the owners and others who come into contact since infection occurs mainly through ingestion, inhalation, or direct inoculation.
Owners should be warned of the potential zoonotic risks if an infection with M. xenopi is suspected or detected in their pets. The diagnostic challenge for veterinary practice is the similarity of clinical signs and necropsy findings with lymphoma and systemic coronavirus-associated diseases. In our case, cytology and especially histopathologic evaluation were important tools to confirm the diagnosis of mycobacterial infection and to exclude the most important clinical differential diagnoses. Fine-needle aspiration of a lymph node or granuloma, stained with the ZN stain, also allows rapid, minimally invasive detection of acid-fast bacilli in macrophages and should be considered as a recommended procedure in the diagnostic process. PCR and molecular species typing via 16S rDNA sequencing are essential for a definitive etiologic diagnosis.
In conclusion, our results confirm the first detection of M. xenopi infection in a pet ferret in Croatia and present for the first time antimicrobial susceptibility testing for M. xenopi in veterinary medicine in general. Our study emphasizes the crucial importance of early detection of such infection and provides guidelines for rapid diagnosis. We should realize that this disease is a potential zoonotic threat to the most vulnerable groups and should not be ignored.
Author Contributions
Conceptualization, Ž.M., S.Š. and I.R.; methodology—clinical pathology, J.H.; methodology—ultrasound diagnostics, G.J.K.; methodology—cytopathology, Z.Š.; methodology—pathology and histopathology, Š.N.; methodology—clinical observation, T.K.; methodology—bacteriology, M.Z.-T. and S.Š.; methodology—molecular identification and antimicrobial susceptibility testing, S.D. and I.R.; writing—original draft preparation, Ž.M. and S.Š.; writing—review and editing, I.R. and Š.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding from any funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Humane euthanasia of the animal was performed on the owner's request and was in accordance with Article 11 of the Animal Protection Act, published in Official Gazette No. 102/17 and 32/19.
Informed Consent Statement
Informed Consent for the publication of all data and images has been granted by the owner of the ferret pet.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to different institutional practices of data storage and access but are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Iland, C.N.; Symmers, W.S.; Thomson, A.P. A note on tuberculosis in the ferret (Mustela furo L.). J. Pathol. Bacteriol. 1951, 63, 554–556. [Google Scholar] [PubMed]
- Pollock, C. Mycobacterial infection in the ferret. Vet. Clin. N. Am. Exot. Anim. Pract. 2012, 15, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Davendralingam, N.; Davagnanam, I.; Stidworthy, M.F.; Baldrey, V.; Peters, L.M.; Stapleton, N. Transmission of Mycobacterium xenopi to a pet albino ferret (Mustela putorius furo) from a domestic aquarium. Vet. Rec. 2017, 181, 169. [Google Scholar] [CrossRef]
- Dequeant, B.; Pascal, Q.; Bilbault, H.; Dagher, E.; Boschiroli, M.L.; Cordonnier, N.; Reyes-Gomez, E. Identification of Mycobacterium genavense natural infection in a domestic ferret. J. Vet. Diagn. Investig. 2019, 31, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Valheim, M.; Djonne, B.; Heiene, R.; Caugant, D.A. Disseminated Mycobacterium celatum (Type 3) infection in a domestic ferret (Mustela putorius furo). Vet. Pathol. 2001, 38, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Barth, S.A.; Menge, C.; Hillemann, D.; Lauda, A.; Pfleghaar, S. Tuberculosis in a pet ferret (Mustela putorius furo). Tierärztliche Prax. Ausg. K Kleintiere Heimtiere 2020, 48, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Lugton, I.W.; Wobeser, G.; Morris, R.S.; Caley, P. Epidemiology of Mycobacterium bovis infection in feral ferrets (Mustela furo) in New Zealand: II. Routes of infection and excretion. N. Z. Vet. J. 1997, 45, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Ragg, J.R.; Waldrup, K.A.; Moller, H. The distribution of gross lesions of tuberculosis caused by Mycobacterium bovis in feral ferrets (Mustela furo) from Otago, New Zealand. N. Z. Vet. J. 1995, 43, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Lugton, I.W.; Wobeser, G.; Morris, R.S.; Caley, P. Epidemiology of Mycobacterium bovis infection in feral ferrets (Mustela furo) in New Zealand: I. Pathology and diagnosis. N. Z. Vet. J. 1997, 45, 140–150. [Google Scholar] [CrossRef]
- Mentre, V.; Bulliot, C. A Retrospective Study of 17 Cases of Mycobacteriosis in Domestic Ferrets (Mustela Putorius furo) between 2005 and 2013. J. Exot. Pet. Med. 2015, 24, 340–349. [Google Scholar] [CrossRef]
- Schultheiss, P.C.; Dolginow, S.Z. Granulomatous enteritis caused by Mycobacterium avium in a ferret. J. Am. Vet. Med. Assoc. 1994, 204, 1217–1218. [Google Scholar] [CrossRef]
- Hance, A.J.; Grandchamp, B.; Lévi-Frébault, V.; Lecossier, D.; Rauzier, J.; Bocart, D.; Gicquel, B. Detection and identification of mycobacteria by amplification of mycobacterial DNA. Mol. Microbiol. 1989, 3, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.H.; Sheu, M.M.; Lin, S.R. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis—A case report of corneal ulcer. Kaohsiung J. Med. Sci. 1997, 13, 583–588. [Google Scholar]
- Kent, P.T.; Kubica, G.P. Public Health Mycobacteriology: A Guide for the Level III; U.S. Department of Health and Human Services, Centers for Disease Control: Atlanta, GA, USA, 1985.
- Ringuet, H.; Akoua-Koffi, C.; Honore, S.; Varnerot, A.; Vincent, V.; Berche, P.; Gaillard, J.L.; Pierre-Audigier, C. hsp65 sequencing for identification of rapidly growing mycobacteria. J. Clin. Microbiol. 1999, 37, 852–857. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Susceptibility Testing of Mycobacteria, Nocardia spp., and Other Aerobic Actinomycetes (M24), 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; pp. 37–44. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Susceptibility Testing of Mycobacteria, Nocardia spp., and Other Aerobic Actinomycetes (M62), 1st ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; pp. 4–11. [Google Scholar]
- Hein, J.; Spreyer, F.; Sauter-Louis, C.; Hartmann, K. Reference ranges for laboratory parameters in ferrets. Vet. Rec. 2012, 171, 218. [Google Scholar] [CrossRef] [PubMed]
- Reference Ranges for Serum Biochemical Values in Ferrets. Merck & Co., Inc., Rahway, NJ, USA. Available online: https://www.msdvetmanual.com/multimedia/table/reference-ranges-for-serum-biochemical-values-in-ferrets (accessed on 6 September 2023).
- Lucas, J.; Lucas, A.; Furber, H.; James, G.; Hughes, M.S.; Martin, P.; Chen, S.C.; Mitchell, D.H.; Love, D.N.; Malik, R. Mycobacterium genavense infection in two aged ferrets with conjunctival lesions. Aust. Vet. J. 2000, 78, 685–689. [Google Scholar] [CrossRef]
- Gupta, A.; McBride, A.M.; Holder, K.A.; Heggem, B.; Royal, A.B.; Wakamatsu, N. Pathology in Practice. J. Am. Vet. Med. Assoc. 2012, 240, 1427–1429. [Google Scholar] [CrossRef]
- Zaheen, A.; Hirama, T.; Mehrabi, M.; Brode, S.K.; Marras, T.K. Clinical outcomes in Mycobacterium xenopi versus Mycobacterium avium complex pulmonary disease: A retrospective matched cohort study. Respir. Med. 2020, 167, 105967. [Google Scholar] [CrossRef]
- Jiva, T.M.; Jacoby, H.M.; Weymouth, L.A.; Kaminski, D.A.; Portmore, A.C. Mycobacterium xenopi: Innocent bystander or emerging pathogen? Clin. Infect. Dis. 1997, 24, 226–232. [Google Scholar] [CrossRef]
- Brown-Elliott, B.A.; Nash, K.A.; Wallace, R.J., Jr. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin. Microbiol. Rev. 2012, 25, 545–582. [Google Scholar] [CrossRef]
- Andréjak, C.; Lescure, F.X.; Pukenyte, E.; Douadi, Y.; Yazdanpanah, Y.; Laurans, G.; Schmit, J.L.; Jounieaux, V.; Xenopi Group. Mycobacterium xenopi pulmonary infections: A multicentric retrospective study of 136 cases in north-east France. Thorax 2009, 64, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Salmon, J.H.; Direz, G.; Ziza, J.M.; Desplaces, N.; Brochot, P.; Eschard, J.P. Discitis and sacroiliitis diagnosed 15years after iatrogenic Mycobacterium xenopi inoculation. Jt. Bone Spine 2012, 79, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Adjemian, J.; Olivier, K.N.; Seitz, A.E.; Holland, S.M.; Prevots, D.R. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am. J. Respir. Crit. Care Med. 2012, 185, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Weathered, C.; Wei, N.; Pienaar, E. Reduced macrophage killing of M. avium drives infection risk in post-menopausal patients. Tuberculosis 2023, 139, 102304. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).