Clinical and Microbiological Risk Factors for 30-Day Mortality of Bloodstream Infections Caused by OXA-48-Producing Klebsiella pneumoniae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Features of Nosocomial BSI Caused by OXA-48-Producing K. pneumoniae
2.2. Microbiological Features
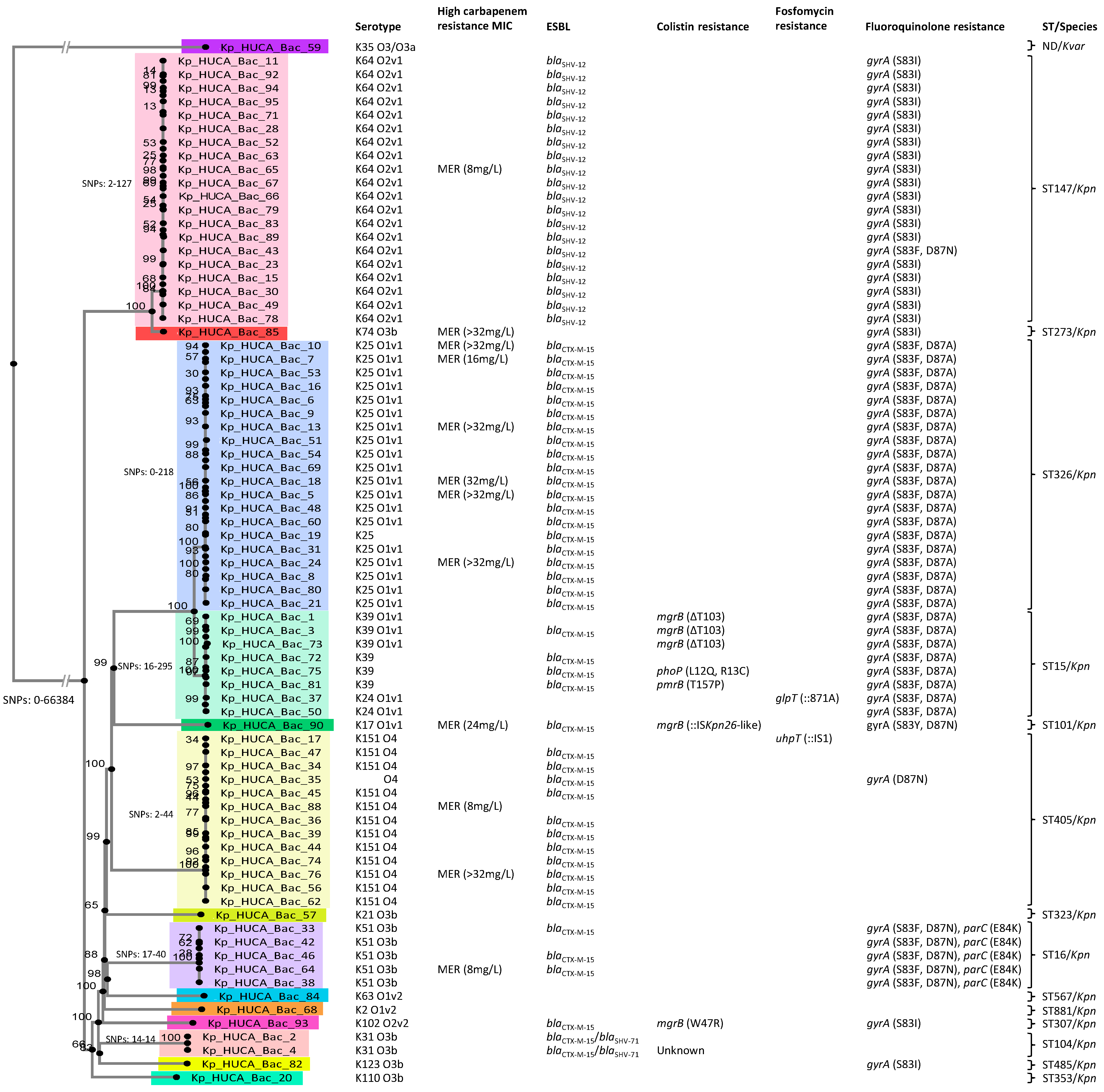
2.3. Phylogenetic Analysis
2.4. Statistical Analysis
3. Results
3.1. Phylogenetic Relationships of the Isolates, In Silico Serotyping, and Resistance Properties
3.2. Patient Characteristics and Therapy
3.3. Risk Factors for 30-Day Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pitout, J.D.; Nordmann, P.; Poirel, L. Carbapenemase-Producing Klebsiella pneumoniae, a Key Pathogen Set for Global Nosocomial Dominance. Antimicrob. Agents Chemother. 2015, 59, 5873–5884. [Google Scholar] [CrossRef]
- Martin, R.M.; Bachman, M.A. Colonization, Infection, and the Accessory Genome of Klebsiella pneumoniae. Front. Cell. Infect. Microbiol. 2018, 8, 4. [Google Scholar] [CrossRef]
- Tamma, P.D.; Han, J.H.; Rock, C.; Harris, A.D.; Lautenbach, E.; Hsu, A.J.; Avdic, E.; Cosgrove, S.E.; Group, A.R.L. Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum β-lactamase bacteremia. Clin. Infect. Dis. 2015, 60, 1319–1325. [Google Scholar] [CrossRef]
- van Duin, D.; Doi, Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 2017, 8, 460–469. [Google Scholar] [CrossRef]
- Rodríguez-Baño, J.; Gutiérrez-Gutiérrez, B.; Machuca, I.; Pascual, A. Treatment of Infections Caused by Extended-Spectrum-Beta-Lactamase-, AmpC-, and Carbapenemase-Producing Enterobacteriaceae. Clin. Microbiol. Rev. 2018, 31. [Google Scholar] [CrossRef]
- Evrard, B.; Balestrino, D.; Dosgilbert, A.; Bouya-Gachancard, J.L.; Charbonnel, N.; Forestier, C.; Tridon, A. Roles of capsule and lipopolysaccharide O antigen in interactions of human monocyte-derived dendritic cells and Klebsiella pneumoniae. Infect. Immun. 2010, 78, 210–219. [Google Scholar] [CrossRef]
- Cortés, G.; Borrell, N.; de Astorza, B.; Gómez, C.; Sauleda, J.; Albertí, S. Molecular analysis of the contribution of the capsular polysaccharide and the lipopolysaccharide O side chain to the virulence of Klebsiella pneumoniae in a murine model of pneumonia. Infect. Immun. 2002, 70, 2583–2590. [Google Scholar] [CrossRef]
- Liu, P.; Li, X.; Luo, M.; Xu, X.; Su, K.; Chen, S.; Qing, Y.; Li, Y.; Qiu, J. Risk Factors for Carbapenem-Resistant Klebsiella pneumoniae Infection: A Meta-Analysis. Microb. Drug Resist. 2018, 24, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gutiérrez, B.; Salamanca, E.; de Cueto, M.; Hsueh, P.R.; Viale, P.; Paño-Pardo, J.R.; Venditti, M.; Tumbarello, M.; Daikos, G.; Cantón, R.; et al. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): A retrospective cohort study. Lancet Infect. Dis. 2017, 17, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Wu, S.; Hao, M.; Zhu, J.; Ding, B.; Yang, Y.; Xu, X.; Wang, M.; Yang, F.; Hu, F. The Colonization of Carbapenem-Resistant Klebsiella pneumoniae: Epidemiology, Resistance Mechanisms, and Risk Factors in Patients Admitted to Intensive Care Units in China. J. Infect. Dis. 2020, 221, S206–S214. [Google Scholar] [CrossRef] [PubMed]
- Vardakas, K.Z.; Matthaiou, D.K.; Falagas, M.E.; Antypa, E.; Koteli, A.; Antoniadou, E. Characteristics, risk factors and outcomes of carbapenem-resistant Klebsiella pneumoniae infections in the intensive care unit. J. Infect. 2015, 70, 592–599. [Google Scholar] [CrossRef]
- Lumbreras-Iglesias, P.; Rodicio, M.R.; Valledor, P.; Suárez-Zarracina, T.; Fernández, J. High-Level Carbapenem Resistance among OXA-48-Producing Klebsiella pneumoniae with Functional OmpK36 Alterations: Maintenance of Ceftazidime/Avibactam Susceptibility. Antibiotics 2021, 10, 1174. [Google Scholar] [CrossRef]
- Rodríguez-Lucas, C.; Rodicio, M.R.; Rosete, Y.; Fernández, J. Prospective evaluation of an easy and reliable work flow for the screening of OXA-48-producing Klebsiella pneumoniae in endemic settings. J. Hosp. Infect. 2020, 105, 659–662. [Google Scholar] [CrossRef]
- CGE. Center For Genomic Epidemiology. Available online: http://www.genomicepidemiology.org (accessed on 17 January 2020).
- Follador, R.; Heinz, E.; Wyres, K.L.; Ellington, M.J.; Kowarik, M.; Holt, K.E.; Thomson, N.R. The diversity of Klebsiella pneumoniae surface polysaccharides. Microb. Genom. 2016, 2, e000073. [Google Scholar] [CrossRef]
- Simoons-Smit, A.M.; Verweij-van Vught, A.M.; MacLaren, D.M. The role of K antigens as virulence factors in Klebsiella. J. Med. Microbiol. 1986, 21, 133–137. [Google Scholar] [CrossRef]
- Shon, A.S.; Bajwa, R.P.; Russo, T.A. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: A new and dangerous breed. Virulence 2013, 4, 107–118. [Google Scholar] [CrossRef]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.; Clamp, M.; Barton, G.J. Jalview Version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef]
- Choi, Y.; Chan, A.P. PROVEAN web server: A tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics 2015, 31, 2745–2747. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Desai, S.; Passet, V.; Gajjar, D.; Brisse, S. Genomic evolution of the globally disseminated multidrug-resistant Klebsiella pneumoniae clonal group 147. Microb. Genom. 2022, 8, 000737. [Google Scholar] [CrossRef] [PubMed]
- Baraniak, A.; Izdebski, R.; Fiett, J.; Sadowy, E.; Adler, A.; Kazma, M.; Salomon, J.; Lawrence, C.; Rossini, A.; Salvia, A.; et al. Comparative population analysis of Klebsiella pneumoniae strains with extended-spectrum β-lactamases colonizing patients in rehabilitation centers in four countries. Antimicrob. Agents Chemother. 2013, 57, 1992–1997. [Google Scholar] [CrossRef]
- Sánchez-López, J.; García-Caballero, A.; Navarro-San Francisco, C.; Quereda, C.; Ruiz-Garbajosa, P.; Navas, E.; Dronda, F.; Morosini, M.I.; Cantón, R.; Diez-Aguilar, M. Hypermucoviscous Klebsiella pneumoniae: A challenge in community acquired infection. IDCases 2019, 17, e00547. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Fu, Y.; Guo, L.; Xu, Y.; Zhang, W.; Gu, J.; Xu, J.; Chen, X.; Zhao, Y.; Ma, J.; Liu, X.; et al. Alteration of GyrA amino acid required for ciprofloxacin resistance in Klebsiella pneumoniae isolates in China. Antimicrob. Agents Chemother. 2008, 52, 2980–2983. [Google Scholar] [CrossRef]
- Agyepong, N.; Govinden, U.; Owusu-Ofori, A.; Amoako, D.G.; Allam, M.; Janice, J.; Pedersen, T.; Sundsfjord, A.; Essack, S. Genomic characterization of multidrug-resistant ESBL-producing Klebsiella pneumoniae isolated from a Ghanaian teaching hospital. Int. J. Infect. Dis. 2019, 85, 117–123. [Google Scholar] [CrossRef]
- Osei Sekyere, J.; Amoako, D.G. Genomic and phenotypic characterisation of fluoroquinolone resistance mechanisms in Enterobacteriaceae in Durban, South Africa. PLoS ONE 2017, 12, e0178888. [Google Scholar] [CrossRef]
- Fernández, J.; Poirel, L.; Rodicio, M.R.; Nordmann, P. Concomitant and multiclonal dissemination of OXA-48-producing Klebsiella pneumoniae in a Spanish hospital. J. Antimicrob. Chemother. 2016, 71, 1734–1736. [Google Scholar] [CrossRef]
- Chen, L.; Han, X.; Li, Y.; Li, M. Assessment of Mortality-Related Risk Factors and Effective Antimicrobial Regimens for Treatment of Bloodstream Infections Caused by Carbapenem-Resistant Enterobacterales. Antimicrob. Agents Chemother. 2021, 65, e0069821. [Google Scholar] [CrossRef]
- Ara-Montojo, M.F.; Escosa-García, L.; Alguacil-Guillén, M.; Seara, N.; Zozaya, C.; Plaza, D.; Schuffelmann-Gutiérrez, C.; de la Vega, Á.; Fernández-Camblor, C.; Ramos-Boluda, E.; et al. Predictors of mortality and clinical characteristics among carbapenem-resistant or carbapenemase-producing Enterobacteriaceae bloodstream infections in Spanish children. J. Antimicrob. Chemother. 2021, 76, 220–225. [Google Scholar] [CrossRef]
- Viale, P.; Giannella, M.; Lewis, R.; Trecarichi, E.M.; Petrosillo, N.; Tumbarello, M. Predictors of mortality in multidrug-resistant Klebsiella pneumoniae bloodstream infections. Expert Rev. Anti-Infect. Ther. 2013, 11, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Anton-Vazquez, V.; Evans, T.J.; Fernando, S.; Somasunderam, D.; David, K.; Melzer, M.; Hawkins, L.; Morris-Jones, S.; Arias, M.; Drazho, B.; et al. Clinical, microbiological characteristics and predictors of mortality in patients with carbapenemase-producing Enterobacterales bloodstream infections: A multicentre study. Infect. Prev. Pract. 2023, 5, 100298. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.S.; Tong, Y.S.; Lee, M.T.; Lin, H.Y.; Lu, M.C. Risk Factors of 30-Day All-Cause Mortality in Patients with Carbapenem-Resistant Klebsiella pneumoniae Bloodstream Infection. J. Pers. Med. 2021, 11, 616. [Google Scholar] [CrossRef] [PubMed]
- Corbella, L.; Fernández-Ruiz, M.; Ruiz-Ruigómez, M.; Rodríguez-Goncer, I.; Silva, J.T.; Hernández-Jiménez, P.; López-Medrano, F.; Lizasoain, M.; Villa, J.; Carretero, O.; et al. Prognostic factors of OXA-48 carbapenemase-producing Klebsiella pneumoniae infection in a tertiary-care Spanish hospital: A retrospective single-center cohort study. Int. J. Infect. Dis. 2022, 119, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Rojo, V.; Vázquez, P.; Reyes, S.; Puente Fuertes, L.; Cervero, M. Risk factors and clinical evolution of carbapenemase-producing Klebsiella pneumoniae infections in a university hospital in Spain. Case-control study. Rev. Esp. Quimioter. 2018, 31, 427–434. [Google Scholar] [PubMed]
- Zhou, C.; Sun, L.; Li, H.; Huang, L.; Liu, X. Risk Factors and Mortality of Elderly Patients with Hospital-Acquired Pneumonia of Carbapenem-Resistant Klebsiella pneumoniae Infection. Infect. Drug Resist. 2023, 16, 6767–6779. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, J.; Hu, H.; Zhang, S.; Wei, J.; Yang, Q.; Qu, T. Clinical Characteristics and Risk Factors for Bloodstream Infection Due to Carbapenem-Resistant Klebsiella pneumoniae in Patients with Hematologic Malignancies. Infect. Drug Resist. 2020, 13, 3233–3242. [Google Scholar] [CrossRef]
- Balkan, I.I.; Aygün, G.; Aydın, S.; Mutcalı, S.I.; Kara, Z.; Kuşkucu, M.; Midilli, K.; Şemen, V.; Aras, S.; Yemişen, M.; et al. Blood stream infections due to OXA-48-like carbapenemase-producing Enterobacteriaceae: Treatment and survival. Int. J. Infect. Dis. 2014, 26, 51–56. [Google Scholar] [CrossRef]
- Chen, J.; Ma, H.; Huang, X.; Cui, Y.; Peng, W.; Zhu, F.; Ma, S.; Rao, M.; Zhang, P.; Yang, H.; et al. Risk factors and mortality of carbapenem-resistant Klebsiella pneumoniae bloodstream infection in a tertiary-care hospital in China: An eight-year retrospective study. Antimicrob. Resist. Infect. Control 2022, 11, 161. [Google Scholar] [CrossRef]
- Papadimitriou-Olivgeris, M.; Fligou, F.; Bartzavali, C.; Zotou, A.; Spyropoulou, A.; Koutsileou, K.; Vamvakopoulou, S.; Sioulas, N.; Karamouzos, V.; Anastassiou, E.D.; et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infection in critically ill patients: Risk factors and predictors of mortality. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1125–1131. [Google Scholar] [CrossRef]
- Cheng, J.; Zhao, D.; Ma, X.; Li, J. Molecular epidemiology, risk factors, and outcomes of carbapenem-resistant Klebsiella pneumoniae infection in a tertiary hospital in eastern China: For a retrospective study conducted over 4 years. Front. Microbiol. 2023, 14, 1223138. [Google Scholar] [CrossRef]
- Peirano, G.; Chen, L.; Kreiswirth, B.N.; Pitout, J.D.D. Emerging Antimicrobial-Resistant High-Risk Klebsiella pneumoniae Clones ST307 and ST147. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef] [PubMed]
- Turton, J.; Davies, F.; Perry, C.; Payne, Z.; Pike, R. Hybrid Resistance and Virulence Plasmids in “High-Risk” Clones of Klebsiella pneumoniae, Including Those Carrying blaNDM-5. Microorganisms 2019, 7, 326. [Google Scholar] [CrossRef] [PubMed]
- Clegg, S.; Murphy, C.N. Epidemiology and Virulence of Klebsiella pneumoniae. Microbiol. Spectr. 2016, 4, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Cannatelli, A.; D’Andrea, M.M.; Giani, T.; Di Pilato, V.; Arena, F.; Ambretti, S.; Gaibani, P.; Rossolini, G.M. In vivo emergence of colistin resistance in Klebsiella pneumoniae producing KPC-type carbapenemases mediated by insertional inactivation of the PhoQ/PhoP mgrB regulator. Antimicrob. Agents Chemother. 2013, 57, 5521–5526. [Google Scholar] [CrossRef]
- Esposito, E.P.; Cervoni, M.; Bernardo, M.; Crivaro, V.; Cuccurullo, S.; Imperi, F.; Zarrilli, R. Molecular Epidemiology and Virulence Profiles of Colistin-Resistant Klebsiella pneumoniae Blood Isolates from the Hospital Agency “Ospedale dei Colli,” Naples, Italy. Front. Microbiol. 2018, 9, 1463. [Google Scholar] [CrossRef]
- Racine, E.; Nordmann, P.; Pantel, L.; Sarciaux, M.; Serri, M.; Houard, J.; Villain-Guillot, P.; Demords, A.; Vingsbo Lundberg, C.; Gualtieri, M. In Vitro and In Vivo Characterization of NOSO-502, a Novel Inhibitor of Bacterial Translation. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Baron, S.; Rolain, J. Epidemiology of Acquired Colistin-Resistant Enterobacteriaceae in Marseille, from February 2014 to September 2016. In Proceedings of the ECCMID, Vienna, Austria, 22–25 April 2017. [Google Scholar]
- Baron, S.A.; Cassir, N.; Hamel, M.; Hadjadj, L.; Saidani, N.; Dubourg, G.; Rolain, J.M. Risk factors for acquisition of colistin-resistant Klebsiella pneumoniae and expansion of a colistin-resistant ST307 epidemic clone in hospitals in Marseille, France, 2014 to 2017. Eurosurveillance 2021, 26, 2000022. [Google Scholar] [CrossRef]
- Bernardini, A.; Cuesta, T.; Tomás, A.; Bengoechea, J.A.; Martínez, J.L.; Sánchez, M.B. The intrinsic resistome of Klebsiella pneumoniae. Int. J. Antimicrob. Agents 2019, 53, 29–33. [Google Scholar] [CrossRef]
- Karageorgopoulos, D.E.; Wang, R.; Yu, X.H.; Falagas, M.E. Fosfomycin: Evaluation of the published evidence on the emergence of antimicrobial resistance in Gram-negative pathogens. J. Antimicrob. Chemother. 2012, 67, 255–268. [Google Scholar] [CrossRef]
- Falagas, M.E.; Vouloumanou, E.K.; Samonis, G.; Vardakas, K.Z. Fosfomycin. Clin. Microbiol. Rev. 2016, 29, 321–347. [Google Scholar] [CrossRef]
- Ortiz-Padilla, M.; Portillo-Calderón, I.; de Gregorio-Iaria, B.; Blázquez, J.; Rodríguez-Baño, J.; Pascual, A.; Rodríguez-Martínez, J.M.; Docobo-Pérez, F. Interplay among Different Fosfomycin Resistance Mechanisms in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2021, 65. [Google Scholar] [CrossRef] [PubMed]
| Dead (n = 24) | Survival (n = 52) | Univariate Analysis | Multivariate Analysis 1 | |||
|---|---|---|---|---|---|---|
| OR (CI 95%) | p | OR (CI 95%) | p | |||
| Clinical features | ||||||
| Age 2 | 1.5 (1.0–2.2) | 0.042 | 1.5 (0.8–2.8) | 0.197 | ||
| Gender n (%) | ||||||
| Female | 2 (8.3) | 21 (40.4) | 0.1 (0.0–0.6) | 0.009 | 0.1 (0.0–0.7) | 0.026 |
| Male | 22 (91.7) | 31 (56.4) | 1 | 1 | ||
| Hospital ward n (%) | ||||||
| ICU | 8 (33.3) | 9 (17.3) | 2 (0.4–9.1) | 0.370 | - | |
| Medical | 12 (50.0) | 34 (65.4) | 0.8 (0.2–3.1) | 0.738 | - | |
| Surgical | 4 (16.7) | 9 (17.3) | 1 | - | ||
| Charlson 3 n (%) | 1.2 (1.0–1.5) | 0.063 | 1.2 (0.9–1.7) | 0.238 | ||
| Hematological malignancy | 9 (37.5) | 9 (17.3) | 2.9 (1.0–8.6) | 0.059 | 10.5 (1.8–61.6) | 0.009 |
| Source of infection 4 n (%) | ||||||
| Catheter-related infection | 2 (8.3) | 2 (3.9) | 4.5 (0.5–42.2) | 0.188 | - | |
| Intra-abdominal infection | 1 (4.2) | 10 (19.2) | 0.5 (0.0–4.6) | 0.501 | - | |
| Lower respiratory system infection | 7 (29.2) | 5 (9.6) | 6.3 (1.3–30.5) | 0.022 | 9.7 (1.3–71.5) | 0.025 |
| Skin and soft tissue infection | 1 (4.2) | 0 | 1 | - | ||
| Surgical site infection | 1 (4.2) | 4 (7.7) | 1.1 (0.1–13) | 0.925 | - | |
| Unknown | 8 (33.3) | 13 (25.0) | 2.8 (0.7–11.2) | 0.153 | - | |
| Urinary system infection | 4 (16.7) | 18 (34.7) | 1 | - | ||
| Therapy features | ||||||
| Therapy included carbapenem 5 | 14 (58.3) | 29 (55.8) | 1.1 (0.4–3.0) | 0.834 | - | |
| Combined therapy 5 | 16 (66.7) | 34 (65.4) | 1.1 (0.4–3.0) | 0.913 | - | |
| Inadequate therapy 6 | 3 (12.5) | 1 (1.9) | 7.3 (0.7–74.1) | 0.093 | 75.8 (2.1–2745.6) | 0.018 |
| Microbiological features | ||||||
| High carbapenem resistance | 2 (8.3) | 10 (19.2) | 0.4 (0.1–1.9) | 0.239 | ||
| Clone | ||||||
| ST147 | 10 (41.7) | 10 (19.2) | 6.7 (1.5–29.8) | 0.013 | 4.5 (1.0–20.0) | 0.045 |
| ST326 | 7 (29.2) | 13 (25) | 3.6 (0.8–16.4) | 0.100 | - | |
| ST405 | 4 (16.7) | 9 (17.3) | 3 (0.5–16.1) | 0.208 | - | |
| Other 7 | 3 (12.5) | 21 (38.5) | 1 | - | ||
| K-antigen | ||||||
| K151 | 4 (16.7) | 7 (13.5) | 3.8 (0.7–21.4) | 0.129 | - | |
| K25 | 7 (29.2) | 14 (26.9) | 3.3 (0.7–15.2) | 0.119 | - | |
| K64 | 10 (41.7) | 10 (19.2) | 6.7 (1.5–29.8) | 0.013 | 1 | |
| Other 8 | 3 (12.5) | 21 (40.4) | 1 | - | ||
| O-antigen | ||||||
| O1 | 6 (27.3) | 22 (44.0) | 0.5 (0.1–2.4) | 0.429 | - | |
| O2 | 10 (45.5) | 11 (22.0) | 1.8 (0.4–7.9) | 0.427 | - | |
| O3 | 2 (9.1) | 9 (18.0) | 0.4 (0.1–3.1) | 0.414 | - | |
| O4 | 4 (16.0) | 8 (18.2) | 1 | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lumbreras-Iglesias, P.; Rodrigo-Arrazola, E.; López-Amor, L.; Fernández-Suárez, J.; Rodicio, M.R.; Fernández, J. Clinical and Microbiological Risk Factors for 30-Day Mortality of Bloodstream Infections Caused by OXA-48-Producing Klebsiella pneumoniae. Pathogens 2024, 13, 11. https://doi.org/10.3390/pathogens13010011
Lumbreras-Iglesias P, Rodrigo-Arrazola E, López-Amor L, Fernández-Suárez J, Rodicio MR, Fernández J. Clinical and Microbiological Risk Factors for 30-Day Mortality of Bloodstream Infections Caused by OXA-48-Producing Klebsiella pneumoniae. Pathogens. 2024; 13(1):11. https://doi.org/10.3390/pathogens13010011
Chicago/Turabian StyleLumbreras-Iglesias, Pilar, Edurne Rodrigo-Arrazola, Lucía López-Amor, Jonathan Fernández-Suárez, María Rosario Rodicio, and Javier Fernández. 2024. "Clinical and Microbiological Risk Factors for 30-Day Mortality of Bloodstream Infections Caused by OXA-48-Producing Klebsiella pneumoniae" Pathogens 13, no. 1: 11. https://doi.org/10.3390/pathogens13010011
APA StyleLumbreras-Iglesias, P., Rodrigo-Arrazola, E., López-Amor, L., Fernández-Suárez, J., Rodicio, M. R., & Fernández, J. (2024). Clinical and Microbiological Risk Factors for 30-Day Mortality of Bloodstream Infections Caused by OXA-48-Producing Klebsiella pneumoniae. Pathogens, 13(1), 11. https://doi.org/10.3390/pathogens13010011







