The Cellular and Humoral Immune Response to SARS-CoV-2 Messenger RNA Vaccines Is Significantly Better in Liver Transplant Patients Compared with Kidney Transplant Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
2.3. ELISpot Assay
2.4. Detection of SARS-CoV-2 Specific Antibodies
2.5. Statistics
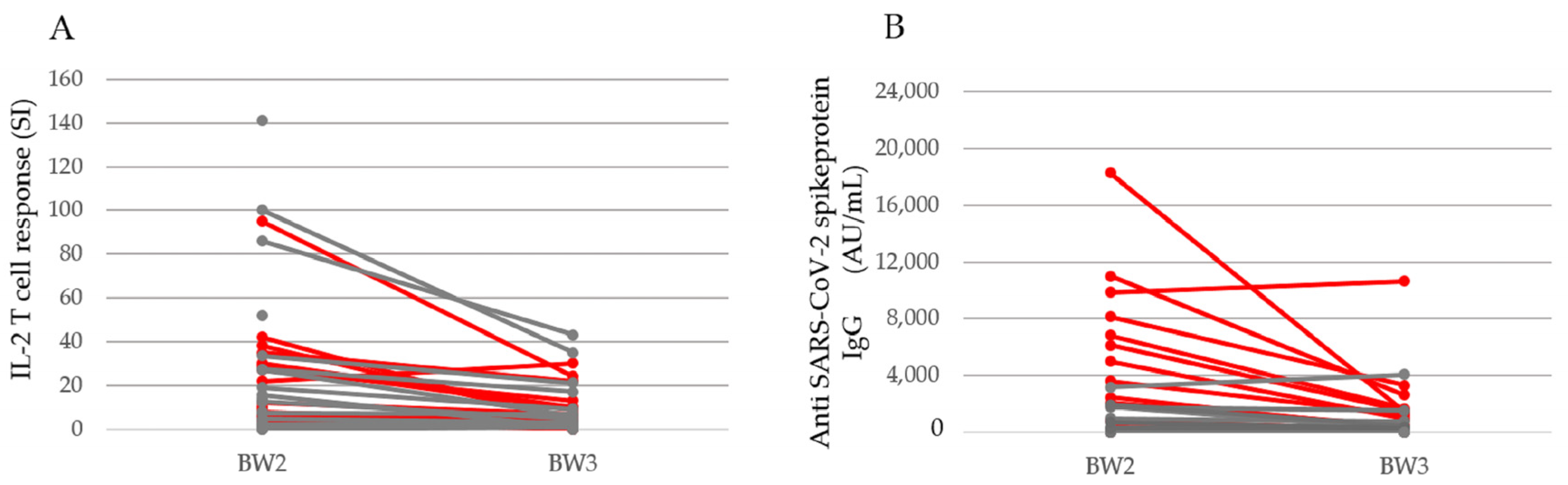
3. Results
3.1. Patients’ Characteristics
3.2. SARS-CoV-2 Antigen-Specific Cellular Immune Response after Vaccination
3.3. Humoral Immune Response after Vaccination with BNT162b2
3.4. Immunosuppression and Immune Response
3.5. SARS-CoV-2 Specific Immune Response One Year after the First Vaccine Dose
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclosure
References
- Cornberg, M.; Buti, M.; Eberhardt, C.S.; Grossi, P.A.; Shouval, D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J. Hepatol. 2021, 74, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Grupper, A.; Katchman, H. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus: Not alarming, but should be taken gravely. Am. J. Transplant. 2021, 21, 2909. [Google Scholar] [CrossRef] [PubMed]
- Benotmane, I.; Gautier-Vargas, G.; Cognard, N.; Olagne, J.; Heibel, F.; Braun-Parvez, L.; Martzloff, J.; Perrin, P.; Moulin, B.; Fafi-Kremer, S.; et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021, 99, 1498–1500. [Google Scholar] [CrossRef] [PubMed]
- Rabinowich, L.; Grupper, A.; Baruch, R.; Ben-Yehoyada, M.; Halperin, T.; Turner, D.; Katchman, E.; Levi, S.; Houri, I.; Lubezky, N.; et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J. Hepatol. 2021, 75, 435–438. [Google Scholar] [CrossRef]
- Schramm, R.; Costard-Jackle, A.; Rivinius, R.; Fischer, B.; Muller, B.; Boeken, U.; Haneya, A.; Provaznik, Z.; Knabbe, C.; Gummert, J. Poor humoral and T-cell response to two-dose SARS-CoV-2 messenger RNA vaccine BNT162b2 in cardiothoracic transplant recipients. Clin. Res. Cardiol. 2021, 110, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, J.; Siepmann, T.; Lindner, T.; Karger, C.; Schwobel, J.; Anders, L.; Faulhaber-Walter, R.; Schewe, J.; Martin, H.; Schirutschke, H.; et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: A prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg. Health Eur. 2021, 9, 100178. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA 2021, 325, 2204–2206. [Google Scholar] [CrossRef]
- Boedecker, S.C.; Klimpke, P.; Kraus, D.; Runkel, S.; Galle, P.R.; Koch, M.; Weinmann-Menke, J. COVID-19-Importance for Patients on the Waiting List and after Kidney Transplantation-A Single Center Evaluation in 2020–2021. Pathogens 2021, 10, 429. [Google Scholar] [CrossRef]
- Guarino, M.; Cossiga, V.; Esposito, I.; Furno, A.; Morisco, F. Effectiveness of SARS-CoV-2 vaccination in liver transplanted patients: The debate is open! J. Hepatol. 2022, 76, 237–239. [Google Scholar] [CrossRef]
- Rashidi-Alavijeh, J.; Frey, A.; Passenberg, M.; Korth, J.; Zmudzinski, J.; Anastasiou, O.E.; Saner, F.H.; Jahn, M.; Lange, C.M.; Willuweit, K. Humoral Response to SARS-CoV-2 Vaccination in Liver Transplant Recipients-A Single-Center Experience. Vaccines 2021, 9, 738. [Google Scholar] [CrossRef]
- Ruether, D.F.; Schaub, G.M.; Duengelhoef, P.M.; Haag, F.; Brehm, T.T.; Fathi, A.; Wehmeyer, M.; Jahnke-Triankowski, J.; Mayer, L.; Hoffmann, A.; et al. SARS-CoV2-specific Humoral and T-cell Immune Response After Second Vaccination in Liver Cirrhosis and Transplant Patients. Clin. Gastroenterol. Hepatol. 2022, 20, 162–172.e9. [Google Scholar] [CrossRef] [PubMed]
- Marion, O.; Del Bello, A.; Abravanel, F.; Couat, C.; Faguer, S.; Esposito, L.; Hebral, A.L.; Izopet, J.; Kamar, N. Safety and Immunogenicity of Anti-SARS-CoV-2 Messenger RNA Vaccines in Recipients of Solid Organ Transplants. Ann. Intern. Med. 2021, 174, 1336–1338. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, A.; Todesco, E.; Drouin, S.; Hazan, F.; Marot, S.; Thabut, D.; Varnous, S.; Soulie, C.; Barrou, B.; Marcelin, A.G.; et al. Poor Antibody Response After Two Doses of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine in Transplant Recipients. Clin. Infect. Dis. 2022, 74, 1093–1096. [Google Scholar] [CrossRef] [PubMed]
- Strauss, A.T.; Hallett, A.M.; Boyarsky, B.J.; Ou, M.T.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Hamilton, J.P.A.; Garonzik-Wang, J.M.; et al. Antibody Response to Severe Acute Respiratory Syndrome-Coronavirus-2 Messenger RNA Vaccines in Liver Transplant Recipients. Liver Transpl. 2021, 27, 1852–1856. [Google Scholar] [CrossRef] [PubMed]
- Nazaruk, P.; Monticolo, M.; Jedrzejczak, A.M.; Krata, N.; Moszczuk, B.; Sanko-Resmer, J.; Pilecki, T.; Urbanowicz, A.; Florczak, M.; Paczek, L.; et al. Unexpectedly High Efficacy of SARS-CoV-2 BNT162b2 Vaccine in Liver versus Kidney Transplant Recipients-Is It Related to Immunosuppression Only? Vaccines 2021, 9, 1454. [Google Scholar] [CrossRef]
- Amanna, I.J.; Slifka, M.K. Contributions of humoral and cellular immunity to vaccine-induced protection in humans. Virology 2011, 411, 206–215. [Google Scholar] [CrossRef]
- DiPiazza, A.T.; Graham, B.S.; Ruckwardt, T.J. T cell immunity to SARS-CoV-2 following natural infection and vaccination. Biochem. Biophys. Res. Commun. 2021, 538, 211–217. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef]
- Geers, D.; Shamier, M.C.; Bogers, S.; den Hartog, G.; Gommers, L.; Nieuwkoop, N.N.; Schmitz, K.S.; Rijsbergen, L.C.; van Osch, J.A.T.; Dijkhuizen, E.; et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci. Immunol. 2021, 6, eabj1750. [Google Scholar] [CrossRef]
- Miele, M.; Busa, R.; Russelli, G.; Sorrentino, M.C.; Di Bella, M.; Timoneri, F.; Mularoni, A.; Panarello, G.; Vitulo, P.; Conaldi, P.G.; et al. Impaired anti-SARS-CoV-2 humoral and cellular immune response induced by Pfizer-BioNTech BNT162b2 mRNA vaccine in solid organ transplanted patients. Am. J. Transplant. 2021, 21, 2919–2921. [Google Scholar] [CrossRef]
- Bertrand, D.; Hamzaoui, M.; Lemee, V.; Lamulle, J.; Hanoy, M.; Laurent, C.; Lebourg, L.; Etienne, I.; Lemoine, M.; Le Roy, F.; et al. Antibody and T Cell Response to SARS-CoV-2 Messenger RNA BNT162b2 Vaccine in Kidney Transplant Recipients and Hemodialysis Patients. J. Am. Soc. Nephrol. 2021, 32, 2147–2152. [Google Scholar] [CrossRef]
- Cucchiari, D.; Egri, N.; Bodro, M.; Herrera, S.; Del Risco-Zevallos, J.; Casals-Urquiza, J.; Cofan, F.; Moreno, A.; Rovira, J.; Banon-Maneus, E.; et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am. J. Transplant. 2021, 21, 2727–2739. [Google Scholar] [CrossRef] [PubMed]
- Raszeja-Wyszomirska, J.; Janik, M.K.; Wojcicki, M.; Milkiewicz, P. SARSCoV2 vaccination in liver transplant recipients: Factors affecting immune response and refusal to vaccine. Pol. Arch. Intern. Med. 2022, 132, 16274. [Google Scholar] [PubMed]
- Caillard, S.; Chavarot, N.; Bertrand, D.; Kamar, N.; Thaunat, O.; Moal, V.; Masset, C.; Hazzan, M.; Gatault, P.; Sicard, A.; et al. Occurrence of severe COVID-19 in vaccinated transplant patients. Kidney Int. 2021, 100, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Abravanel, F.; Marion, O.; Couat, C.; Izopet, J.; Del Bello, A. Three Doses of an mRNA COVID-19 Vaccine in Solid-Organ Transplant Recipients. N. Engl. J. Med. 2021, 385, 661–662. [Google Scholar] [CrossRef]
- Saiag, E.; Grupper, A.; Avivi, I.; Elkayam, O.; Ram, R.; Herishanu, Y.; Cohen, Y.; Perry, C.; Furer, V.; Katchman, H.; et al. The effect of a third-dose BNT162b2 vaccine on anti-SARS-CoV-2 antibody levels in immunosuppressed patients. Clin. Microbiol. Infect. 2022, 28, 735.e5–735.e8. [Google Scholar] [CrossRef]
- Stumpf, J.; Tonnus, W.; Paliege, A.; Rettig, R.; Steglich, A.; Gembardt, F.; Kessel, F.; Kroger, H.; Arndt, P.; Sradnick, J.; et al. Cellular and Humoral Immune Responses After 3 Doses of BNT162b2 mRNA SARS-CoV-2 Vaccine in Kidney Transplant. Transplantation 2021, 105, e267–e269. [Google Scholar] [CrossRef]
- Hall, V.G.; Ferreira, V.H.; Ku, T.; Ierullo, M.; Majchrzak-Kita, B.; Chaparro, C.; Selzner, N.; Schiff, J.; McDonald, M.; Tomlinson, G.; et al. Randomized Trial of a Third Dose of mRNA-1273 Vaccine in Transplant Recipients. N. Engl. J. Med. 2021, 385, 1244–1246. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef]
- Bertrand, D.; Lemee, V.; Laurent, C.; Lemoine, M.; Hanoy, M.; Le Roy, F.; Nezam, D.; Pruteanu, D.; Lebourg, L.; Grange, S.; et al. Waning antibody response and cellular immunity 6 months after third dose SARS-CoV-2 mRNA BNT162b2 vaccine in kidney transplant recipients. Am. J. Transplant. 2022, 22, 1498–1500. [Google Scholar] [CrossRef]
- Fava, A.; Donadeu, L.; Sabe, N.; Pernin, V.; Gonzalez-Costello, J.; Llado, L.; Meneghini, M.; Charmetant, X.; Garcia-Romero, E.; Cachero, A.; et al. SARS-CoV-2-specific serological and functional T cell immune responses during acute and early COVID-19 convalescence in solid organ transplant patients. Am. J. Transplant. 2021, 21, 2749–2761. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Immunogenicity of a Single Dose of SARS-CoV-2 Messenger RNA Vaccine in Solid Organ Transplant Recipients. JAMA 2021, 325, 1784–1786. [Google Scholar] [CrossRef]
- Benotmane, I.; Gautier-Vargas, G.; Cognard, N.; Olagne, J.; Heibel, F.; Braun-Parvez, L.; Martzloff, J.; Perrin, P.; Moulin, B.; Fafi-Kremer, S.; et al. Weak anti-SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int. 2021, 99, 1487–1489. [Google Scholar] [CrossRef] [PubMed]
- Korth, J.; Jahn, M.; Dorsch, O.; Anastasiou, O.E.; Sorge-Hadicke, B.; Eisenberger, U.; Gackler, A.; Dittmer, U.; Witzke, O.; Wilde, B.; et al. Impaired Humoral Response in Renal Transplant Recipients to SARS-CoV-2 Vaccination with BNT162b2 (Pfizer-BioNTech). Viruses 2021, 13, 756. [Google Scholar] [CrossRef] [PubMed]
- Rincon-Arevalo, H.; Choi, M.; Stefanski, A.L.; Halleck, F.; Weber, U.; Szelinski, F.; Jahrsdorfer, B.; Schrezenmeier, H.; Ludwig, C.; Sattler, A.; et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci. Immunol. 2021, 6, eabj1031. [Google Scholar] [CrossRef] [PubMed]
- Furian, L.; Russo, F.P.; Zaza, G.; Burra, P.; Hartzell, S.; Bizzaro, D.; Di Bello, M.; Di Bella, C.; Nuzzolese, E.; Agnolon, C.; et al. Differences in Humoral and Cellular Vaccine Responses to SARS-CoV-2 in Kidney and Liver Transplant Recipients. Front. Immunol. 2022, 13, 853682. [Google Scholar] [CrossRef]
- Qin, C.X.; Moore, L.W.; Anjan, S.; Rahamimov, R.; Sifri, C.D.; Ali, N.M.; Morales, M.K.; Tsapepas, D.S.; Basic-Jukic, N.; Miller, R.A.; et al. Risk of Breakthrough SARS-CoV-2 Infections in Adult Transplant Recipients. Transplantation 2021, 105, e265–e266. [Google Scholar] [CrossRef]
- Caillard, S.; Thaunat, O.; Benotmane, I.; Masset, C.; Blancho, G. Antibody Response to a Fourth Messenger RNA COVID-19 Vaccine Dose in Kidney Transplant Recipients: A Case Series. Ann. Intern. Med. 2022, 175, 455–456. [Google Scholar] [CrossRef]
- Kamar, N.; Abravanel, F.; Marion, O.; Romieu-Mourez, R.; Couat, C.; Del Bello, A.; Izopet, J. Assessment of 4 Doses of SARS-CoV-2 Messenger RNA-Based Vaccine in Recipients of a Solid Organ Transplant. JAMA Netw. Open 2021, 4, e2136030. [Google Scholar] [CrossRef]
- Babu, T.M.; Kotton, C.N. Immunizations in Chronic Kidney Disease and Kidney Transplantation. Curr. Treat. Options Infect. Dis. 2021, 13, 47–65. [Google Scholar] [CrossRef]



| LTX (n = 21) | KTX (n = 28) | Control (n = 10) | |
|---|---|---|---|
| Gender (male/female) | 10/11 | 19/9 * | 4/6 |
| Age at first vaccination, years median (IQR 25–75) | 59 (53–67) | 54 (37–61) * | 54 (43–58) |
| Years since transplantation median (IQR 25–75) | 4 (1.5–11) | 3 (1–11) | |
| Immunosuppression regimen, n (%) | |||
| triple therapy | 3 (14.0) | 25 (89.0) | |
| Tac 1, MMF 2, prednisone | 2 | 17 | |
| CyA 3, MMF, prednisone | 0 | 1 | |
| Tac, mTORinhibitor, prednisone | 1 | 6 | |
| mTORinhibitor, MMF, prednisone | 0 | 1 | |
| dual therapy | 9 (43.0) | 3 (11.0) | |
| Tac, MMF | 3 | 1 | |
| Tac, mTOR inhibitor | 4 | 0 | |
| CyA, MMF | 1 | 0 | |
| CyA, prednisone | 0 | 1 | |
| mTOR inhibitor, MMF | 1 | 0 | |
| mTOR inhibitor, prednisone | 0 | 1 | |
| mono therapy | 9 (43.0) | 0 (0.0) | |
| Tac | 7 | 0 | |
| CyA | 2 | 0 |
| BW1 | BW2 | BW3 | ||
|---|---|---|---|---|
| LTX | Neither T-cell nor IgG response, n (%) | 6/11 (55%) | 0/20 (0%) | 2/18 (11%) |
| T-cell response only, n (%) | 2/11 (18%) | 5/20 (25%) | 1/18 (6%) | |
| IgG response only, n (%) | 3/11 (27%) | 3/20 (15%) | 7/18 (39%) | |
| IgG and T-cell response, n (%) | 0/11 (0%) | 12/20 (60%) | 8/18 (44%) | |
| KTX | Neither T-cell nor IgG response, n (%) | 8/9 (89%) | 8/25 (32%) | 6/16 (37%) |
| T-cell response only, n (%) | 1/9 (11%) | 6/25 (24%) | 2/16 (13%) | |
| IgG response only, n (%) | 0/9 (0%) | 3/25 (12%) | 3/16 (19%) | |
| IgG and T-cell response, n (%) | 0/9 (0%) | 8/25 (32%) | 5/16 (31%) | |
| HV | Neither T-cell nor IgG response, n (%) | 0/5 (0%) | 0/5 (0%) | 0/10 (0%) |
| T-cell response only, n (%) | 0/5 (0%) | 0/5 (0%) | 0/5 (0%) | |
| IgG response only, n (%) | 0/5 (0%) | 0/5 (0%) | 2/10 (20%) | |
| IgG and T-cell response, n (%) | 5/5 (100%) | 5/5 (100%) | 8/10 (80%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lautem, A.; Boedecker-Lips, S.C.; Schneider, E.; Runkel, S.; Feist, C.; Lang, H.; Weinmann-Menke, J.; Koch, M. The Cellular and Humoral Immune Response to SARS-CoV-2 Messenger RNA Vaccines Is Significantly Better in Liver Transplant Patients Compared with Kidney Transplant Patients. Pathogens 2023, 12, 910. https://doi.org/10.3390/pathogens12070910
Lautem A, Boedecker-Lips SC, Schneider E, Runkel S, Feist C, Lang H, Weinmann-Menke J, Koch M. The Cellular and Humoral Immune Response to SARS-CoV-2 Messenger RNA Vaccines Is Significantly Better in Liver Transplant Patients Compared with Kidney Transplant Patients. Pathogens. 2023; 12(7):910. https://doi.org/10.3390/pathogens12070910
Chicago/Turabian StyleLautem, Anja, Simone Cosima Boedecker-Lips, Elisa Schneider, Stefan Runkel, Christina Feist, Hauke Lang, Julia Weinmann-Menke, and Martina Koch. 2023. "The Cellular and Humoral Immune Response to SARS-CoV-2 Messenger RNA Vaccines Is Significantly Better in Liver Transplant Patients Compared with Kidney Transplant Patients" Pathogens 12, no. 7: 910. https://doi.org/10.3390/pathogens12070910
APA StyleLautem, A., Boedecker-Lips, S. C., Schneider, E., Runkel, S., Feist, C., Lang, H., Weinmann-Menke, J., & Koch, M. (2023). The Cellular and Humoral Immune Response to SARS-CoV-2 Messenger RNA Vaccines Is Significantly Better in Liver Transplant Patients Compared with Kidney Transplant Patients. Pathogens, 12(7), 910. https://doi.org/10.3390/pathogens12070910








