Estimating the Impact of Consecutive Blood Meals on Vector Competence of Aedes albopictus for Chikungunya Virus
Abstract
1. Introduction
2. Material and Methods
2.1. Mosquitoes Collection
2.2. Virus
2.3. Mosquito Infection
2.4. Mosquito Dissection and Saliva Collection
2.5. Quantification of Virus Infection, Dissemination, and Transmission
2.6. Data Analysis
3. Results
3.1. Virus
3.2. Mosquito Infection
3.3. Virus Amplification, Group Treatment, and Temperature Conditions
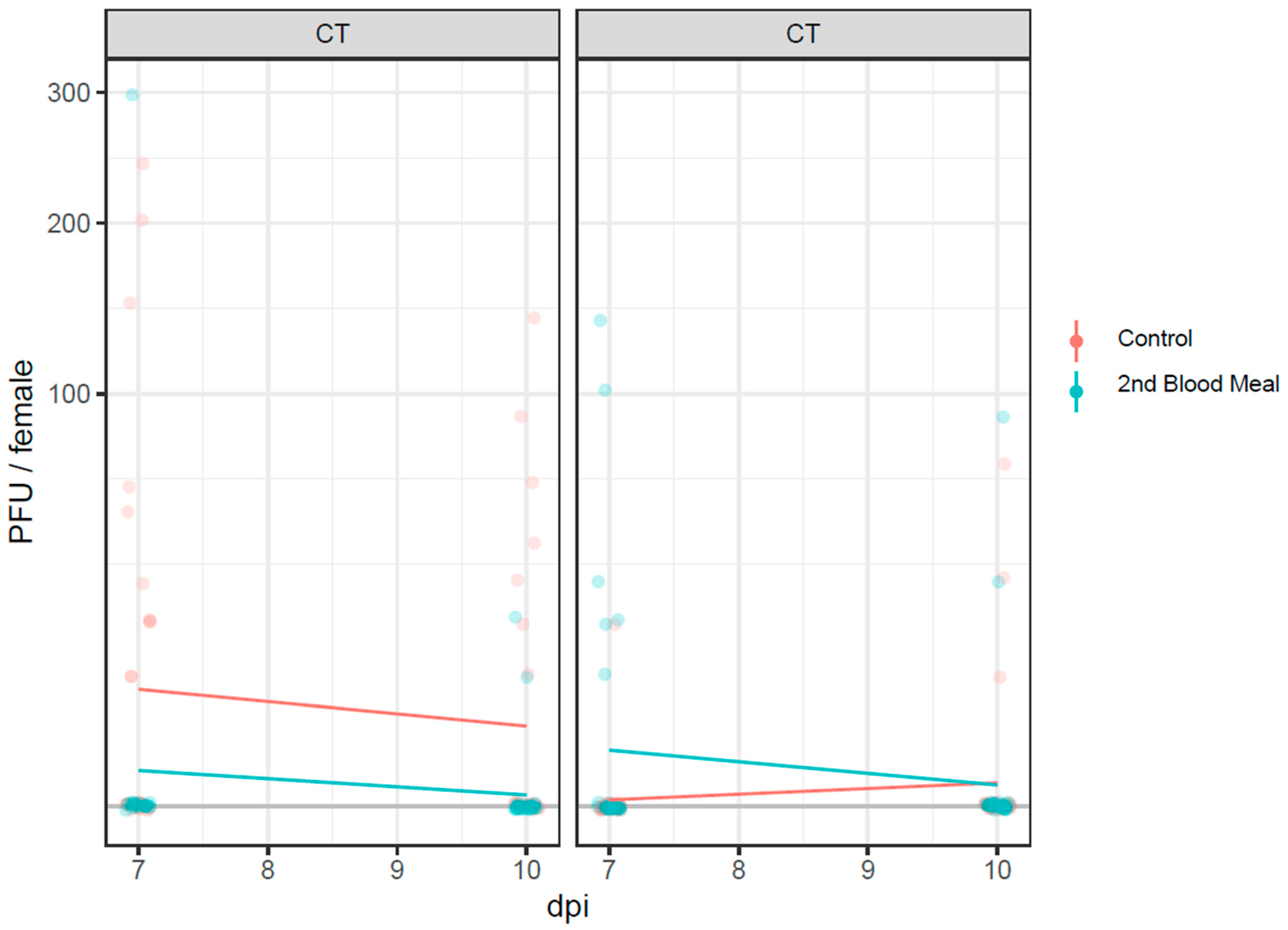
3.4. Infectious Virus Particles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angelini, R.; Finarelli, A.C.; Angelini, P.; Po, C.; Petropulacos, K.; Macini, P.; Fiorentini, C.; Fortuna, C.; Venturi, G.; Romi, R.; et al. An outbreak of chikungunya fever in the province of Ravenna, Italy. Euro Surveill 2007, 12, E070901–E070906. [Google Scholar] [CrossRef] [PubMed]
- Venturi, G.; Di Luca, M.; Fortuna, C.; Remoli, M.E.; Riccardo, F.; Severini, F.; Toma, L.; Del Manso, M.; Benedetti, E.; Caporali, M.G.; et al. Detection of a chikungunya outbreak in Central Italy, August to September 2017. Euro Surveill 2017, 22, 17-00646. [Google Scholar] [CrossRef] [PubMed]
- Cochet, A.; Calba, C.; Jourdain, F.; Grard, G.; Durand, G.A.; Guinard, A.; Investigation, t.; Noel, H.; Paty, M.C.; Franke, F. Autochthonous dengue in mainland France, 2022: Geographical extension and incidence increase. Euro Surveill 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Giron, S.; Franke, F.; Decoppet, A.; Cadiou, B.; Travaglini, T.; Thirion, L.; Durand, G.; Jeannin, C.; L’Ambert, G.; Grard, G.; et al. Vector-borne transmission of Zika virus in Europe, southern France, August 2019. Euro Surveill 2019, 24. [Google Scholar] [CrossRef] [PubMed]
- Flacio, E.; Engeler, L.; Tonolla, M.; Luthy, P.; Patocchi, N. Strategies of a thirteen year surveillance programme on Aedes albopictus (Stegomyia albopicta) in southern Switzerland. Parasit Vectors 2015, 8, 208. [Google Scholar] [CrossRef]
- Flacio, E.; Engeler, L.; Tonolla, M.; Muller, P. Spread and establishment of Aedes albopictus in southern Switzerland between 2003 and 2014: An analysis of oviposition data and weather conditions. Parasit Vectors 2016, 9, 304. [Google Scholar] [CrossRef]
- Ravasi, D.; Parrondo Monton, D.; Guidi, V.; Flacio, E. Evaluation of the public health risk for autochthonous transmission of mosquito-borne viruses in southern Switzerland. Med. Vet. Entomol. 2020, 34, 244–250. [Google Scholar] [CrossRef]
- Mariconti, M.; Obadia, T.; Mousson, L.; Malacrida, A.; Gasperi, G.; Failloux, A.B.; Yen, P.S. Estimating the risk of arbovirus transmission in Southern Europe using vector competence data. Sci. Rep. 2019, 9, 17852. [Google Scholar] [CrossRef]
- Stephenson, C.J.; Coatsworth, H.; Kang, S.; Lednicky, J.A.; Dinglasan, R.R. Transmission potential of floridian Aedes aegypti mosquitoes for dengue virus serotype 4: Implications for estimating local dengue risk. mSphere 2021, 6, e0027121. [Google Scholar] [CrossRef]
- Vogels, C.B.; Goertz, G.P.; Pijlman, G.P.; Koenraadt, C.J. Vector competence of European mosquitoes for West Nile virus. Emerg. Microbes Infect. 2017, 6, e96. [Google Scholar] [CrossRef]
- Alto, B.W.; Wiggins, K.; Eastmond, B.; Ortiz, S.; Zirbel, K.; Lounibos, L.P. Diurnal temperature range and chikungunya virus infection in invasive mosquito vectors. J. Med. Entomol. 2018, 55, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Farjana, T.; Tuno, N. Multiple blood feeding and host-seeking behavior in Aedes aegypti and Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2013, 50, 838–846. [Google Scholar] [CrossRef]
- Armstrong, P.M.; Ehrlich, H.Y.; Magalhaes, T.; Miller, M.R.; Conway, P.J.; Bransfield, A.; Misencik, M.J.; Gloria-Soria, A.; Warren, J.L.; Andreadis, T.G.; et al. Successive blood meals enhance virus dissemination within mosquitoes and increase transmission potential. Nat. Microbiol. 2020, 5, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Vazeille, M.; Moutailler, S.; Coudrier, D.; Rousseaux, C.; Khun, H.; Huerre, M.; Thiria, J.; Dehecq, J.S.; Fontenille, D.; Schuffenecker, I.; et al. Two chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PLoS ONE 2007, 2, e1168. [Google Scholar] [CrossRef]
- Balestrino, F.; Puggioli, A.; Gilles, J.R.; Bellini, R. Validation of a new larval rearing unit for Aedes albopictus (Diptera: Culicidae) mass rearing. PLoS ONE 2014, 9, e91914. [Google Scholar] [CrossRef]
- Glavinic, U.; Varga, J.; Paslaru, A.I.; Hauri, J.; Torgerson, P.; Schaffner, F.; Veronesi, E. Assessing the role of two populations of Aedes japonicus japonicus for Zika virus transmission under a constant and a fluctuating temperature regime. Parasit Vectors 2020, 13, 479. [Google Scholar] [CrossRef]
- Veronesi, E.; Paslaru, A.; Silaghi, C.; Tobler, K.; Glavinic, U.; Torgerson, P.; Mathis, A. Experimental evaluation of infection, dissemination, and transmission rates for two West Nile virus strains in European Aedes japonicus under a fluctuating temperature regime. Parasitol. Res. 2018, 117, 1925–1932. [Google Scholar] [CrossRef]
- Balestrino, F.; Bouyer, J.; Vreysen, M.J.B.; Veronesi, E. Impact of irradiation on vector competence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) for dengue and chikungunya viruses. Front. Bioeng. Biotechnol. 2022, 10, 876400. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Fortuna, C.; Toma, L.; Remoli, M.E.; Amendola, A.; Severini, F.; Boccolini, D.; Romi, R.; Venturi, G.; Rezza, G.; Di Luca, M. Vector competence of Aedes albopictus for the Indian Ocean lineage (IOL) chikungunya viruses of the 2007 and 2017 outbreaks in Italy: A comparison between strains with and without the E1:A226V mutation. Euro Surveill 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Balaraman, V.; Kantor, A.M.; Lin, J.; Grant, D.G.; Held, N.L.; Franz, A.W.E. Chikungunya virus dissemination from the midgut of Aedes aegypti is associated with temporal basal lamina degradation during bloodmeal digestion. PLoS Negl. Trop. Dis. 2017, 11, e0005976. [Google Scholar] [CrossRef] [PubMed]
- Klowden, M.J. The endogenous regulation of mosquito reproductive behavior. Experientia 1990, 46, 660–670. [Google Scholar] [CrossRef]
- Klowden, M.J.; Lea, A.O. Blood meal size as a factor affecting continued host-seeking by Aedes aegypti (L.). Am. J. Trop. Med. Hyg. 1978, 27, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Briegel, H.; Lea, A.O. Relationship between protein and proteolytic activity in the midgut of mosquitoes. J. Insect Physiol. 1975, 21, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Mordecai, E.A.; Caldwell, J.M.; Grossman, M.K.; Lippi, C.A.; Johnson, L.R.; Neira, M.; Rohr, J.R.; Ryan, S.J.; Savage, V.; Shocket, M.S.; et al. Thermal biology of mosquito-borne disease. Ecol. Lett. 2019, 22, 1690–1708. [Google Scholar] [CrossRef]
- Delatte, H.; Gimonneau, G.; Triboire, A.; Fontenille, D. Influence of temperature on immature development, survival, longevity, fecundity, and gonotrophic cycles of Aedes albopictus, vector of chikungunya and dengue in the Indian Ocean. J. Med. Entomol. 2009, 46, 33–41. [Google Scholar] [CrossRef]
- Verhulst, N.O.; Brendle, A.; Blanckenhorn, W.U.; Mathis, A. Thermal preferences of subtropical Aedes aegypti and temperate Ae. japonicus mosquitoes. J. Therm. Biol. 2020, 91, 102637. [Google Scholar] [CrossRef] [PubMed]



| Group | dpi | IR (%) | DR (%) | TR (%) | DE (%) | TE (%) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CT (%) | CI95 (%) | FT (%) | CI95 (%) | CT (%) | CI95 (%) | FT (%) | CI95 (%) | CT (%) | CI95 (%) | FT (%) | CI95 (%) | CT (%) | CI95 % | FT (%) | CI95 (%) | CT (%) | CI95 (%) | PFU (min-max) | FT (%) | CI95 (%) | PFU (min-max) | ||
| Control | 7 | 26/29 (90) | 74–96 | 21/29 (72) | 52–85 | 19/29 (66) | 47–80 | 11/29 (38) | 23–56 | 11/29 (38) | 23–56 | 1/29 (3) | 1–17 | 19/26 (73) | 54–86 | 11/21 (52) | 32–72 | 11/19 (58) | 36–77 | 10–240 | 1/11 (9) | 2–38 | 0–20 |
| 10 | 21/25 (84) | 65–94 | 29/30 (97) | 83–99 | 19/25 (76) | 57–89 | 26/30 (87) | 70–95 | 7/25 (28) | 14–48 | 3/30 (10) | 3–26 | 19/21 (90) | 71–97 | 26/29 (90) | 75–96 | 7/19 (37) | 19–56 | 10–140 | 3/26 (12) | 4–29 | 10–70 | |
| Second blood meal | 7 | 6/20 (30) | 15–52 | 18/29 (62) | 44–77 | 6/20 (30) | 15–52 | 14/29 (48) | 31–66 | 1/20 (5) | 1–24 | 6/29 (21) | 10–38 | 6/6 (100) | 61–100 | 14/18 (78) | 55–91 | 1/6 (17) | 3–56 | 0–300 | 6/14 (43) | 21–67 | 10–140 |
| 10 | 9/28 (32) | 18–51 | 21/29 (72) | 54–85 | 6/28 (21) | 10–40 | 20/29 (69) | 51–83 | 2/28 (7) | 2–23 | 2/29 (7) | 2–22 | 6/9 (67) | 35–88 | 20/21 (100) | 77–99 | 2/6 (33) | 10–70 | 10–20 | 2/20 (10) | 3–30 | 30–90 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veronesi, E.; Paslaru, A.; Ettlin, J.; Ravasi, D.; Flacio, E.; Tanadini, M.; Guidi, V. Estimating the Impact of Consecutive Blood Meals on Vector Competence of Aedes albopictus for Chikungunya Virus. Pathogens 2023, 12, 849. https://doi.org/10.3390/pathogens12060849
Veronesi E, Paslaru A, Ettlin J, Ravasi D, Flacio E, Tanadini M, Guidi V. Estimating the Impact of Consecutive Blood Meals on Vector Competence of Aedes albopictus for Chikungunya Virus. Pathogens. 2023; 12(6):849. https://doi.org/10.3390/pathogens12060849
Chicago/Turabian StyleVeronesi, Eva, Anca Paslaru, Julia Ettlin, Damiana Ravasi, Eleonora Flacio, Matteo Tanadini, and Valeria Guidi. 2023. "Estimating the Impact of Consecutive Blood Meals on Vector Competence of Aedes albopictus for Chikungunya Virus" Pathogens 12, no. 6: 849. https://doi.org/10.3390/pathogens12060849
APA StyleVeronesi, E., Paslaru, A., Ettlin, J., Ravasi, D., Flacio, E., Tanadini, M., & Guidi, V. (2023). Estimating the Impact of Consecutive Blood Meals on Vector Competence of Aedes albopictus for Chikungunya Virus. Pathogens, 12(6), 849. https://doi.org/10.3390/pathogens12060849







