Abstract
Clade 2.3.4.4 H5Nx influenza viruses have further diversified into several subclades. Sub-clade 2.3.4.4b H5N1 viruses have been widely circulating in wild birds and detected in Europe, Africa, Asia, and North America since October 2020. In this study, we report the first detection of highly pathogenic avian influenza H5N1 clade 2.3.4.4b viruses in wild birds and domestic ducks from live bird markets in Egypt. Phylogenetic analysis revealed that the Egyptian H5N1 virus retained the genomic composition of Eurasian strains. Mutations in the viral proteins associated with zoonotic potential and pathogenicity were detected in Egyptian isolates. Egypt is considered a hot spot for the evolution of the influenza virus, so active surveillance of avian influenza viruses in Egypt is warranted.
1. Introduction
Infectious diseases and pandemics in humans are often caused by pathogens transmitted from non-human animal reservoirs. Influenza A viruses (IAVs) spread among a variety of different hosts and cross species barriers to create new viral strains. Waterfowl serves as the primary reservoir and can perpetuate many avian influenza virus (AIV) subtypes via asymptomatic shedding, which plays an important role in the reassortment and transmission of influenza subtypes to domestic poultry [1]. In 1996, a highly pathogenic (HP) AIV (H5N1) of Goose/Guangdong/1/96 (Gs/GD) lineage emerged in Chinese poultry and has been able to cross the species barrier and infect humans, which eventually spread to Europe, Africa, and the North American continent via migratory birds [2,3,4]. Due to the accumulation of genetic mutations and reassortment with multiple influenza subtypes, Gs/Gd lineage viruses evolved into nine clades and multiple subclades.
The phylogenetic clade 2.3.4.4 of H5Nx viruses has caused extensive outbreaks across the globe and has further evolved into eight subclades (2.3.4.4a–2.3.4.4h) [5]. In 2020/2021, clade 2.3.4.4b H5N1 viruses have spread to many countries in Europe, Africa, Asia, and America [6], and several infections have been reported in wild or captive mammals as well as in humans.
Highly pathogenic AIV (H5N1) was initially introduced into Egypt in 2005 and became endemic in poultry in 2008. Since then, many outbreaks have been reported in domestic poultry farms. Multiple clades of H5N1 Gs/Gd lineage viruses (Clades 2.2, 2.2.1, 2.2.1.1, 2.2.1.1a, and 2.2.1.2) were identified [7]. The 2.3.4.4b H5N8 virus was first detected in Egypt in wild birds in 2016 [8]. Since then, several cases of H5N8 were recorded among domestic poultry in live bird markets, backyard flocks, and commercial farms in several governorates in Egypt. Although all Egyptian H5N8 isolates belong to the clade 2.3.4.4, several independent introductions of the virus have been detected [9]. The 2.3.4.4b H5N8 replaced the clade 2.2.1 H5N1 viruses that subsequently disappeared. H9N2 AIVs were widespread in poultry globally and endemic in poultry in many Middle Eastern countries including Egypt, where H9N2 G1-like lineages were introduced in 2010 [10,11,12]. Extensive surveillance of the H9N2 virus has indicated that the virus was endemic in Egyptian domestic poultry in different geographical regions across the country and reassortant H9N2 viruses were detected [13]. Co-circulation of H5Nx and H9N2 viruses increases the probability of genetic reassortment which might enhance the zoonotic potential.
To monitor the influenza viruses with pandemic potential at the human–animal interface, in this study, we identify the genetic and antigenic characteristics of HPAI H5N1 viruses that were introduced into Egypt through active surveillance of AIVs in live bird markets (LBMs) and migratory wild birds.
2. Materials and Methods
2.1. Sample Collection
Active surveillance of avian influenza viruses has been conducted in Egypt through collaborative efforts of the Center of Scientific Excellence for Influenza viruses, National Research Centre, Egypt, and Center of Excellence for Influenza Research and Surveillance at St. Jude (Memphis, TN, USA) since 2009. In April 2021, we sampled poultry and wild birds sold in LBMs in Egypt. We collected cloacal and oropharyngeal swab samples, which were kept chilled in virus transport medium until they reached the laboratory.
2.2. Sample Screening and Virus Isolation
Samples were thoroughly vortexed prior to viral nucleic acid extraction from 200 μL of viral transport media using either the automated MagNA Pure 96 platform, KingFisher Flex instrument (Thermo Fisher Scientific, Rocklin, CA, USA) or the QIAamp Viral RNA Mini kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. Nucleic acid extracts were screened by real-time RT-PCR (rRT-PCR) for the presence of the AIVs (universal M-gene) [14], and samples were classified as positive with a Ct value ≤ 36. All positive samples were individually injected into the allantoic cavity of 10-day-old specific pathogen-free embryonated hens’ eggs, incubated for 48 h post-injection at 37 °C, and then chilled at 4 °C for 4 h or overnight. Allantoic fluids were then collected and analyzed by the hemagglutination assay (HA) using 0.5% chicken red blood cells (RBCs). Hemagglutination assays (HA) of the allantoic fluids from the inoculated eggs were performed to screen for IAV according to the World Health Organization (WHO) and the World Organization for Animal Health (OIE) protocols. The positive samples were aliquoted and stored at −80 °C.
2.3. Sequencing and Sequence Analysis
Viral RNA extracted from allantoic fluid was subjected to reverse transcription to synthesize the first cDNA strand using a SuperScript IV first-strand synthesis kit (Invitrogen, Waltham, MA, USA) and the Uni12 influenza primer. Then, Phusion high-fidelity DNA polymerase (New England Biolabs, Ipswich, MA, USA) and Uni12/13 primers were used for multiplex PCR of all eight gene segments, and PCR products were purified. Sequencing library preparation was performed by using Illumina’s Nextera XT DNA Sample Preparation Kit according to the manufacturer’s protocol. Amplicons were sequenced on Illumina’s MiSeq platform (Illumina, San Diego, CA, USA) by using the paired-end approach. The eight full segments of each H5N8 virus were assembled using CLC Genomics Workbench, version 21 (CLC Bio, Qiagen, Hilden, Germany).
For sequence and phylogenetic analyses, genome sequences of H5Nx were aligned by MAFFT v4.787 [15], and the maximum likelihood (ML) trees were built from each segment alignment by FastTree v2.1.11 with GTR+Gamma model [16]. Temporal phylogeny was constructed by BEAST v1.10.4 under SRD06 substitution model [17,18], uncorrelated lognormal relaxed clock model, and Gaussian Markov random field (GMRF) Bayesian Skyride tree prior [19]. Two independent MCMC chains were run for 100 million iterations and sampled every 10,000 generations. Convergence was examined by Tracer v1.7.2 [20], requiring effective sample size (ESS) of over 200. The maximum clade credibility (MCC) tree was summarized by TreeAnnotator included in the BEAST package.
2.4. Antigenic Analysis
Haemagglutination inhibition (HI) assays were used to antigenically characterize the isolated viruses. The H5N1 AIVs were tested by using post-infection ferret antiserum raised against F.2015-7-A/duck/England/36254/2014 (H5N8), F.2017-13-A/chicken/Kumamoto/1-7/14 (H5N8), F.2016-16- A/gyrfalcon/Washington/410886/2014 (H5N8), and F.2015-48-A/Sichuan/26221/2014 (H5N6) of clade 2.3.4.4 viruses which were produced in Center of Excellence for Influenza Research and Surveillance at St. Jude (Memphis, TN, USA). A panel of post-infection ferret antisera was treated with receptor-destroying enzyme II and heat-inactivated at 56 °C for 30 min and diluted to a final concentration of 1:10 in PBS and 0.5% chicken erythrocytes. The HI test was performed according to the WHO protocols [21].
2.5. Nucleotide Sequence Accession Numbers
The nucleotide sequences of the H5N1 AIVs described in this study were deposited in the GenBank database with the accession numbers shown in Table S1.
3. Results and Discussion
Through surveillance, we isolated H5N1 viruses from one wild pintail duck and three domestic Pekin ducks, A/pintail/Egypt/RA19853OP/2021 in late 2021 and A/duck/Egypt/BA20360C/2022, A/duck/Egypt/BA20360OP/2022, and A/duck/Egypt/BA20361OP/2022 isolates in early 2022. The analysis of the complete HA gene segment showed that the HPAI H5N1 viruses belonged to phylogenetic clade 2.3.4.4b. The nucleotide sequence identities across all eight segments of the four viruses were 99.5–100%. As a representative virus, A/pintail/Egypt/RA19853OP/2021 (H5N1) had a high nucleotide identity (99–100%) to the HPAI A(H5N1) viruses of clade 2.3.4.4 from Europe and the Middle East (Table 1). These isolates were identified as HPAI viruses that harbored multiple basic amino acids (PLRERRRKR/G) within the cleavage site of the HA gene, which is characteristic of high pathogenicity in chickens.

Table 1.
Comparison of nucleotide sequence identities of the eight influenza A virus (IAV) gene sequences for the virus isolated in this study (A/pintail/Egypt/RA19853OP/2021 (H5N1)) and nearest virus homologs.
We combined genome sequences generated in this study with all sequences of H5Nx viruses available in GenBank and the GISAID database (11). Phylogenetic analysis confirmed that the Egyptian A(H5N1) isolates are of clade 2.3.4.4b and clustered with the recent HPAIV A(H5N1) isolates from Europe, Africa, and the Middle East (Figure 1). The clade 2.3.4.4b HA genes of H5 viruses have evolved from a sub-linage under clade 2.3.4.4 which includes several subtypes of H5N1, H5N6, and H5N8 viruses. Our isolates detected in this study clustered with the HA genes of H5N1 viruses contemporarily detected in Europe and the Middle East.
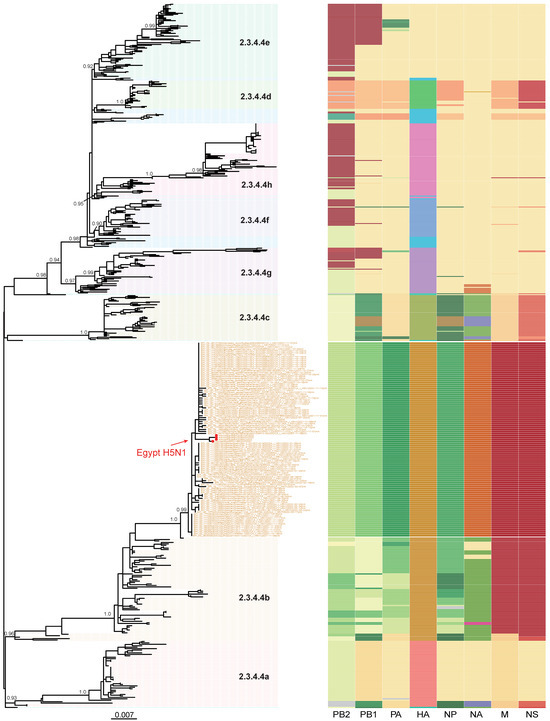
Figure 1.
Phylogenetic tree of H5N1 viruses sequenced in this study, in addition to other publicly available H5Nx clade 2.3.4.4 from GenBank and GISAID. Red dots represent the H5N1 viruses sequenced in this study. Topological support values (SH-like support) of selected nodes are displayed. To the right, a schematic representation of viral clustering of each gene segment (from left to right: PB2, PB1, polymerase acidic, haemagglutinin, nucleoprotein, neuraminidase, matrix, and non-structural) is shown. Segment colors indicate origin of the segment. Within each cluster, a unified color pattern indicates homogeneity and a different color pattern indicates reassortment.
The time to the most recent common ancestor (tMRCA) was calculated to explain the emergence of the H5N1 viruses. Taking the intersection of the 95% highest posterior density (HPD) intervals of the tMRCA (Figure 2) suggests that the viruses from Europe, Africa, and the Middle East share a common ancestor of unknown origin that emerged around July 2020 (95%HPD: April 2020–October 2020). We did not find an amino acid deletion at position 133 in the HA protein (H3 numbering) in all our isolates, a feature common with clade 2.3.4.4 isolated from humans (Table 2), and associated with the alteration of the H5 HA receptor binding pocket [22]. The analysis of the NA gene of H5N1 viruses revealed that none of these viruses displayed oseltamivir resistance markers E119, H275, R293, and N295 (N1 numbering) (Table 2). Deletions were also present in both neuraminidase (NA) (an 11-aa deletion in the stalk region) and nonstructural protein 1 (NS1) (deletion from residues 80–84; Table 2), which are associated with high pathogenicity in avian hosts [23]. These analyses suggest that the newly detected H5N1 viruses in Egypt may be able to infect and cause disease in mammals.
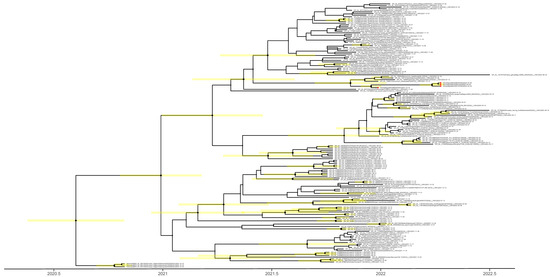
Figure 2.
Time to the most recent common ancestor of H5N1 viruses sequenced in this study; maximum clade credibility temporal phylogeny of the hemagglutinin (HA) gene. The H5N1 viruses from Egypt are represented by red dots. Posterior clade probabilities are indicated by the sizes of the internal node circles. Shaded bars represent the 95% highest probability distribution for the age of each node with posterior clade probability > 0.3.

Table 2.
Assessment of molecular amino acid markers for zoonotic potential of the influenza A(H5N1) virus detected in Egypt.
The receptor binding sites in the viral HA gene of the four viruses possess the conservative amino acid residues (including 190E, 220R, 225G, 226Q, and 228G; H3 numbering), which indicated that these viruses would preferentially bind to the α-2,3-sialic acid linkage, the avian-like receptors.
The antigenic properties of H5N1 viruses were also assessed using ferret antisera against the World Health Organization’s candidate clade 2.3.4.4c H5N8 and clade 2.3.4.4a H5N6 vaccine viruses including A/gyrfalcon/Washington/41088-6/2014 (H5N8), A/duck/England/36254/2014 (H5N8), A/chicken/Japanese Kumamoto/1-7/2014 (H5N8), and A/Sichuan/26221/2014 (H5N6) (Table 3). The presence of E and D at positions 627 and 701 in polymerase basic (PB) 2 in viruses sequenced in this study also confirms a typical characteristic of avian influenza viruses (Table 2). PB2 amino acid substitutions L89V, E249G, G309D, and T339M enhance the replication and increased virulence of the H5N1 virus in mice [26,44], and the substitution L89V, G309D, and T339K were found in all isolates of our Egypt H5N1 viruses. PB1-F1 has been shown to contribute to viral pathogenicity, as well as to enhance inflammation, cytotoxicity, and viral polymerase activity [42,45]. All Egypt H5N1 isolates in this study expressed PB1-F2 of 90 aa and had the N66S mutation, which increases virulence, replication efficiency, and antiviral response in mice.

Table 3.
Antigenic analysis of H5N1 influenza A viruses from Egypt by hemagglutination inhibition assay.
4. Conclusions
Several introductions of clade 2.3.4.4b viruses have been seen in Egypt. Those introductions are typically through wild migratory birds but eventually spill over to poultry. Some live bird markets in Northern Egypt sell both poultry and trapped wild birds for human consumption. Such an interface provides ample opportunity for cross-species virus spill-over. The viruses we detected were from such markets where the initial virus was detected in a migratory bird and then in domestic poultry. No human cases of clade 2.3.4.4b H5Nx infections were reported in Egypt but the mutations detected in analyzed viruses suggest that human infections can occur. A vigilant surveillance system at the human-wild bird-poultry interface is necessary.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens12010036/s1, Table S1: GenBank Accession Number.
Author Contributions
Conceptualization, R.E.-S., R.J.W., G.K. and M.A.A..; methodology and investigation, R.E.-S., Y.M., S.H.M., Y.S., A.E.T., M.G., M.N.K. and M.E.S.; software, R.E.-S. and Y.S.; writing—original draft preparation, R.E.-S. and A.K.; writing—review and editing, R.E.-S., A.K., T.T.Y.L., G.K. and M.A.A.; supervision, R.E.-S., G.K. and M.A.A.; project administration, P.P.M. and G.K.; funding acquisition, T.T.Y.L., R.J.W., G.K. and M.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, US Department of Health and Human Services (under contract HHSN272201400006C), and the National Natural Science Foundation of China’s Excellent Young Scientists Fund (Hong Kong and Macau) (31922087) and the InnoHK funding from Innovation and Technology Commission of HKSAR.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef] [PubMed]
- Claes, F.; Morzaria, S.P.; Donis, R.O. Emergence and dissemination of clade 2.3.4.4 H5Nx influenza viruses–how is the Asian HPAI H5 lineage maintained. Curr. Opin. Virol. 2016, 16, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Torchetti, M.K.; Winker, K.; Ip, H.S.; Song, C.S.; Swayne, D.E. Intercontinental Spread of Asian-Origin H5N8 to North America through Beringia by Migratory Birds. J. Virol. 2015, 89, 6521–6524. [Google Scholar] [CrossRef] [PubMed]
- El-Shesheny, R.; Barman, S.; Feeroz, M.M.; Hasan, M.K.; Jones-Engel, L.; Franks, J.; Turner, J.; Seiler, P.; Walker, D.; Friedman, K.; et al. Genesis of Influenza A(H5N8) Viruses. Emerg. Infect. Dis. 2017, 23, 1368–1371. [Google Scholar] [CrossRef]
- Lee, D.H.; Bertran, K.; Kwon, J.H.; Swayne, D.E. Evolution, global spread, and pathogenicity of highly pathogenic avian influenza H5Nx clade 2.3.4.4. J. Vet. Sci. 2017, 18, 269–280. [Google Scholar] [CrossRef]
- Cui, P.; Shi, J.; Wang, C.; Zhang, Y.; Xing, X.; Kong, H.; Yan, C.; Zeng, X.; Liu, L.; Tian, G.; et al. Global dissemination of H5N1 influenza viruses bearing the clade 2.3.4.4b HA gene and biologic analysis of the ones detected in China. Emerg. Microbes Infect. 2022, 11, 1693–1704. [Google Scholar] [CrossRef]
- El-Shesheny, R.; Kandeil, A.; Mostafa, A.; Ali, M.A.; Webby, R.J. H5 Influenza Viruses in Egypt. Cold Spring Harb. Perspect. Med. 2021, 11, a038745. [Google Scholar] [CrossRef]
- Kandeil, A.; Kayed, A.; Moatasim, Y.; Webby, R.J.; McKenzie, P.P.; Kayali, G.; Ali, M.A. Genetic characterization of highly pathogenic avian influenza A H5N8 viruses isolated from wild birds in Egypt. J. Gen. Virol. 2017, 98, 1573–1586. [Google Scholar] [CrossRef]
- Moatasim, Y.; Kandeil, A.; Aboulhoda, B.E.; El-Shesheny, R.; Alkhazindar, M.; AbdElSalam, E.T.; Kutkat, O.; Kamel, M.N.; El Taweel, A.N.; Mostafa, A.; et al. Comparative Virological and Pathogenic Characteristics of Avian Influenza H5N8 Viruses Detected in Wild Birds and Domestic Poultry in Egypt during the Winter of 2016/2017. Viruses 2019, 11, 990. [Google Scholar] [CrossRef]
- Kandeil, A.; El-Shesheny, R.; Maatouq, A.M.; Moatasim, Y.; Shehata, M.M.; Bagato, O.; Rubrum, A.; Shanmuganatham, K.; Webby, R.J.; Ali, M.A.; et al. Genetic and antigenic evolution of H9N2 avian influenza viruses circulating in Egypt between 2011 and 2013. Arch. Virol. 2014, 159, 2861–2876. [Google Scholar] [CrossRef]
- Arafa, A.S.; Hagag, N.; Erfan, A.; Mady, W.; El-Husseiny, M.; Adel, A.; Nasef, S. Complete genome characterization of avian influenza virus subtype H9N2 from a commercial quail flock in Egypt. Virus Genes 2012, 45, 283–294. [Google Scholar] [CrossRef] [PubMed]
- El-Zoghby, E.F.; Arafa, A.S.; Hassan, M.K.; Aly, M.M.; Selim, A.; Kilany, W.H.; Selim, U.; Nasef, S.; Aggor, M.G.; Abdelwhab, E.M.; et al. Isolation of H9N2 avian influenza virus from bobwhite quail (Colinus virginianus) in Egypt. Arch. Virol. 2012, 157, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- El Sayes, M.; Kandeil, A.; Moatasim, Y.; El Taweel, A.; Rubrum, A.; Kutkat, O.; Kamel, M.N.; Badra, R.; Barakat, A.B.; McKenzie, P.P.; et al. Insights into Genetic Characteristics and Virological Features of Endemic Avian Influenza A (H9N2) Viruses in Egypt from 2017–2021. Viruses 2022, 14, 1484. [Google Scholar] [CrossRef] [PubMed]
- CDC REF# I-007-05; CDC Realtime RT-PCR (rRTPCR) Protocol for Detection and Characterization of Influenza (Version 2007). Centers for Disease Control and Prevention: Atlanta, GA, USA, 2007.
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Suchard, M.A.; Lemey, P.; Baele, G.; Ayres, D.L.; Drummond, A.J.; Rambaut, A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018, 4, vey016. [Google Scholar] [CrossRef]
- Shapiro, B.; Rambaut, A.; Drummond, A.J. Choosing appropriate substitution models for the phylogenetic analysis of protein-coding sequences. Mol. Biol. Evol. 2006, 23, 7–9. [Google Scholar] [CrossRef]
- Minin, V.N.; Bloomquist, E.W.; Suchard, M.A. Smooth skyride through a rough skyline: Bayesian coalescent-based inference of population dynamics. Mol. Biol. Evol. 2008, 25, 1459–1471. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- WHO. WHO Manual on Animal Influenza Diagnosis and Surveillance. Available online: http://whqlibdoc.who.int/hq/2002/WHO_CDS_CSR_NCS_2002.5.pdf (accessed on 12 December 2011).
- Watanabe, Y.; Ibrahim, M.S.; Ellakany, H.F.; Kawashita, N.; Mizuike, R.; Hiramatsu, H.; Sriwilaijaroen, N.; Takagi, T.; Suzuki, Y.; Ikuta, K. Acquisition of human-type receptor binding specificity by new H5N1 influenza virus sublineages during their emergence in birds in Egypt. PLoS Pathog. 2011, 7, e1002068. [Google Scholar] [CrossRef]
- Cui, Y.; Li, Y.; Li, M.; Zhao, L.; Wang, D.; Tian, J.; Bai, X.; Ci, Y.; Wu, S.; Wang, F.; et al. Evolution and extensive reassortment of H5 influenza viruses isolated from wild birds in China over the past decade. Emerg. Microbes Infect. 2020, 9, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, E.K.; London, W.; Murphy, B.R. A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. J. Virol. 1993, 67, 1761–1764. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, Y.; Shinya, K.; Deng, G.; Jiang, Y.; Li, Z.; Guan, Y.; Tian, G.; Li, Y.; Shi, J.; et al. Identification of amino acids in HA and PB2 critical for the transmission of H5N1 avian influenza viruses in a mammalian host. PLoS Pathog. 2009, 5, e1000709. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ishaq, M.; Prudence, M.; Xi, X.; Hu, T.; Liu, Q.; Guo, D. Single mutation at the amino acid position 627 of PB2 that leads to increased virulence of an H5N1 avian influenza virus during adaptation in mice can be compensated by multiple mutations at other sites of PB2. Virus Res. 2009, 144, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Ma, W.; Sun, N.; Huang, L.; Li, Y.; Zeng, Z.; Wen, Y.; Zhang, Z.; Li, H.; Li, Q.; et al. PB2-588 V promotes the mammalian adaptation of H10N8, H7N9 and H9N2 avian influenza viruses. Sci. Rep. 2016, 6, 19474. [Google Scholar] [CrossRef] [PubMed]
- Conenello, G.M.; Zamarin, D.; Perrone, L.A.; Tumpey, T.; Palese, P. A single mutation in the PB1-F2 of H5N1 (HK/97) and 1918 influenza A viruses contributes to increased virulence. PLoS Pathog. 2007, 3, 1414–1421. [Google Scholar] [CrossRef]
- Schmolke, M.; Manicassamy, B.; Pena, L.; Sutton, T.; Hai, R.; Varga, Z.T.; Hale, B.G.; Steel, J.; Perez, D.R.; Garcia-Sastre, A. Differential contribution of PB1-F2 to the virulence of highly pathogenic H5N1 influenza A virus in mammalian and avian species. PLoS Pathog. 2011, 7, e1002186. [Google Scholar] [CrossRef]
- Yamayoshi, S.; Yamada, S.; Fukuyama, S.; Murakami, S.; Zhao, D.; Uraki, R.; Watanabe, T.; Tomita, Y.; Macken, C.; Neumann, G.; et al. Virulence-affecting amino acid changes in the PA protein of H7N9 influenza A viruses. J. Virol. 2014, 88, 3127–3134. [Google Scholar] [CrossRef]
- Hulse-Post, D.J.; Franks, J.; Boyd, K.; Salomon, R.; Hoffmann, E.; Yen, H.L.; Webby, R.J.; Walker, D.; Nguyen, T.D.; Webster, R.G. Molecular changes in the polymerase genes (PA and PB1) associated with high pathogenicity of H5N1 influenza virus in mallard ducks. J. Virol. 2007, 81, 8515–8524. [Google Scholar] [CrossRef]
- Maines, T.R.; Chen, L.M.; Van Hoeven, N.; Tumpey, T.M.; Blixt, O.; Belser, J.A.; Gustin, K.M.; Pearce, M.B.; Pappas, C.; Stevens, J.; et al. Effect of receptor binding domain mutations on receptor binding and transmissibility of avian influenza H5N1 viruses. Virology 2011, 413, 139–147. [Google Scholar] [CrossRef]
- Chutinimitkul, S.; van Riel, D.; Munster, V.J.; van den Brand, J.M.; Rimmelzwaan, G.F.; Kuiken, T.; Osterhaus, A.D.; Fouchier, R.A.; de Wit, E. In vitro assessment of attachment pattern and replication efficiency of H5N1 influenza A viruses with altered receptor specificity. J. Virol. 2010, 84, 6825–6833. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Perez, D.R. Amino acid 226 in the hemagglutinin of H9N2 influenza viruses determines cell tropism and replication in human airway epithelial cells. J. Virol. 2007, 81, 5181–5191. [Google Scholar] [CrossRef] [PubMed]
- Auewarakul, P.; Suptawiwat, O.; Kongchanagul, A.; Sangma, C.; Suzuki, Y.; Ungchusak, K.; Louisirirotchanakul, S.; Lerdsamran, H.; Pooruk, P.; Thitithanyanont, A.; et al. An avian influenza H5N1 virus that binds to a human-type receptor. J. Virol. 2007, 81, 9950–9955. [Google Scholar] [CrossRef] [PubMed]
- Ilyushina, N.A.; Seiler, J.P.; Rehg, J.E.; Webster, R.G.; Govorkova, E.A. Effect of neuraminidase inhibitor-resistant mutations on pathogenicity of clade 2.2 A/Turkey/15/06 (H5N1) influenza virus in ferrets. PLoS Pathog. 2010, 6, e1000933. [Google Scholar] [CrossRef] [PubMed]
- Sleeman, K.; Guo, Z.; Barnes, J.; Shaw, M.; Stevens, J.; Gubareva, L.V. R292K substitution and drug susceptibility of influenza A(H7N9) viruses. Emerg. Infect. Dis. 2013, 19, 1521–1524. [Google Scholar] [CrossRef] [PubMed]
- Abed, Y.; Goyette, N.; Boivin, G. Generation and characterization of recombinant influenza A (H1N1) viruses harboring amantadine resistance mutations. Antimicrob Agents Chemother 2005, 49, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Bean, W.J.; Threlkeld, S.C.; Webster, R.G. Biologic potential of amantadine-resistant influenza A virus in an avian model. J. Infect Dis. 1989, 159, 1050–1056. [Google Scholar] [CrossRef]
- Cheung, C.L.; Rayner, J.M.; Smith, G.J.; Wang, P.; Naipospos, T.S.; Zhang, J.; Yuen, K.Y.; Webster, R.G.; Peiris, J.S.; Guan, Y.; et al. Distribution of amantadine-resistant H5N1 avian influenza variants in Asia. J. Infect Dis. 2006, 193, 1626–1629. [Google Scholar] [CrossRef]
- Jiao, P.; Tian, G.; Li, Y.; Deng, G.; Jiang, Y.; Liu, C.; Liu, W.; Bu, Z.; Kawaoka, Y.; Chen, H. A single-amino-acid substitution in the NS1 protein changes the pathogenicity of H5N1 avian influenza viruses in mice. J. Virol. 2008, 82, 1146–1154. [Google Scholar] [CrossRef]
- Seo, S.H.; Hoffmann, E.; Webster, R.G. Lethal H5N1 influenza viruses escape host anti-viral cytokine responses. Nat. Med. 2002, 8, 950–954. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, Y.; Jiao, P.; Wang, A.; Zhao, F.; Tian, G.; Wang, X.; Yu, K.; Bu, Z.; Chen, H. The NS1 gene contributes to the virulence of H5N1 avian influenza viruses. J. Virol. 2006, 80, 11115–11123. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, R.; Yamada, S.; Le, M.Q.; Li, C.; Chen, H.; Qurnianingsih, E.; Nidom, C.A.; Ito, M.; Sakai-Tagawa, Y.; Kawaoka, Y. Identification of PB2 mutations responsible for the efficient replication of H5N1 influenza viruses in human lung epithelial cells. J. Virol. 2015, 89, 3947–3956. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.; Hossain, M.J.; Hickman, D.; Perez, D.R.; Lamb, R.A. A new influenza virus virulence determinant: The NS1 protein four C-terminal residues modulate pathogenicity. Proc. Natl. Acad. Sci. USA 2008, 105, 4381–4386. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).