Abstract
Previous studies have shown that Acanthamoeba spp. may invade the eyes by migrating along the optic nerve to the eyes from the brain. This study aimed to confirm the presence of inflammation in the eyes of mice with disseminated acanthamoebiasis by examining prostaglandin E2 (PGE2) and thromboxane B2 (TXB2) concentrations in the eyes of immunocompetent and immunocompromised mice intranasally inoculated with Acanthamoeba spp. The PGE2 concentration was statistically significantly lower in the immunocompromised amoebae-infected mice on 8 dpi compared with the noninfected group of animals, and it was higher in the eyes of immunosuppressed amoebae-infected mice on 16 dpi than in the control group of animals. There was a statistically significant lower TXB2 concentration in the eyes of immunocompetent infected mice compared with the noninfected group on 8 dpi. However, on 24 dpi, we noted statistically significant higher TXB2 levels in the immunocompetent infected mice than in the control group. In immunocompromised mice, there was a lower TXB2 level on 8 dpi than in control mice. This study confirmed the existence of an inflammatory process in the eyes of immunocompetent and immunocompromised mice infected with Acanthamoeba spp. without damaged corneas.
1. Introduction
Particularly dangerous to the health and life of hosts are microorganisms that can have both free-living and parasitic lifestyles. Such species include amoebae from the Acanthamoeba genus. These cosmopolitan organisms occur in water, soil, and air as trophozoites and cysts that are resistant to a wide range of chemical and physical agents [1]. Acanthamoeba spp. cause Acanthamoeba keratitis (AK), granulomatous amebic encephalitis (GAE), and disseminated acanthamoebiasis [2]. To date, more than 3000 cases of AK have been described in the scientific literature [3]. The pathogenesis of AK is well-known [4] in contrast to the mechanisms of Acanthamoeba spp. invasion of the eyes due to disseminated acanthamoebiasis. Acanthamoeba spp. migrate with the blood to distant organs [2], but to the eyes, they may migrate from the brain along the optic nerve [5,6]. Pathomorphological changes are not extensive; however, previously, there were some differences at the cellular and molecular levels in groups of animals experimentally infected with Acanthamoeba spp. compared with control groups. Increased expression of Toll-like receptors (TLR2 and TLR4) [6] and dysregulation of a nonenzymatic antioxidant and antioxidant enzymes [7] in the group of Acanthamoeba spp.-infected animals compared with noninfected hosts were reported. Under the condition of increased oxidative stress, the expression and activation of cyclooxygenases (COXs) are altered [8]. Cyclooxygenase isoenzymes (COX-1 and -2) biosynthesize a conversion reaction in which the substrate is arachidonic acid and the products are prostaglandins (PGs). COXs play an important role in eye health and disease. The prostaglandins are composed of five different classes, PGD2, PGE2, PGF2, PGI2, and thromboxane A (TXA2) [8,9]. Prostaglandin E2 is a metabolite of COX-2. However, in the eye, PGE2 formation may be attributed to both COX-1 and COX-2 [10]. PGE2 is one of the most studied PGs. It is responsible for the generation of fever as well as pain and neurotransmitter modulation. PGE2 has been studied for its role in the eye, particularly in the lowering of intraocular pressure (IOP) [11]. Thromboxane A2 (TXA2) is the prostanoid product of COX-1 [12]. TXA2 contains an unstable ether linkage that is rapidly hydrolyzed to form the biologically inert TXB2. Increased levels of TX are observed in inflammatory diseases. TXB2 may lower IOP. Moreover, TXA2 does not have a direct role in glaucoma, but the physical interaction of TXA2 with a gene product implicated in open-angle glaucoma pathology was previously shown [13].
PGs play a role in parasitic diseases, such as amoebiasis caused by Entamoeba histolytica, leishmaniasis, Chagas disease, malaria, and pulmonary acanthamoebiasis [14,15,16,17,18]. The aim of this study was to confirm the existence of inflammation in the eyes of mice with disseminated acanthamoebiasis. The purpose of this study was achieved by the examination of concentrations of cyclooxygenase products, prostaglandin E2 (PGE2) and thromboxane B2 (TXB2), in the eyes of immunocompetent and immunocompromised mice intranasally inoculated with Acanthamoeba spp. To date, no studies have addressed the implications of the role of COXs’ metabolites in Acanthamoeba spp. eye infections.
2. Results
2.1. Concentration of PGE2
Comparing PGE2 concentrations in the eyes of immunocompromised Acanthamoeba spp.-infected mice on different days after infection, differences occurred and were statistically significantly different (H = 14.10, p < 0.001). There was a decrease in the PGE2 concentration at 8 dpi, an increase at 16 dpi, and a re-decrease at 24 dpi. At 8 dpi, the PGE2 concentration was statistically significantly lower in the immunocompromised parasite-infected mice than in the control group of animals (U = 10, p < 0.01; Figure 1). However, at 16 dpi, a higher PGE2 level was observed in the eyes of immunosuppressed infected hosts than in noninfected animals (U = 23, p = 0.01; Figure 1).
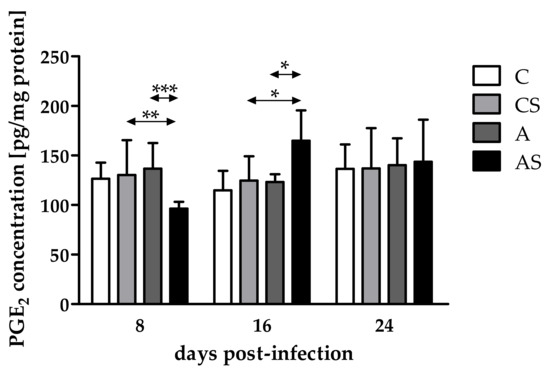
Figure 1.
Concentrations of prostaglandin E2 (PGE2) in the eyes of mice at 8, 16, 24 days post Acanthamoeba spp. infection (dpi) related to immunological status of the host. Data represent mean ± standard deviation for six independent experiments (C, immunocompetent control, noninfected mice; CS, immunosuppressed control, noninfected mice; A, immunocompetent mice infected with Acanthamoeba spp.; AS, immunosuppressed mice infected with Acanthamoeba spp.; * statistical difference at p < 0.05; ** statistical difference at p < 0.01; *** statistical difference at p < 0.001).
Taking into account host immune status, the PGE2 concentration was lower in the eyes of immunosuppressed infected mice than in immunocompetent infected mice at 8 dpi (U = 2, p < 0,001; Figure 1), and it was higher in the eyes of immunocompromised mice than in immunocompetent hosts at 16 dpi (U = 4, p = 0.02; Figure 1). At 24 dpi, the concentration of PGE2 was at a similar level in the eyes of immunosuppressed and immunocompetent infected hosts.
2.2. Concentration of TXB2
The concentration of TXB2 in the eyes of immunocompetent Acanthamoeba spp.-infected mice decreased at 8 dpi and increased at 16 and 24 dpi (H = 12.16, p < 0.01). We noted a statistically significant lower TXB2 concentration in the eyes of immunocompetent infected mice than in the noninfected group at 8 dpi (U = 13, p = 0.02; Figure 2). Additionally, we found statistically significant higher TXB2 levels in the immunocompetent infected mice than in the control group at 24 dpi (U = 0, p < 0.001; Figure 2).
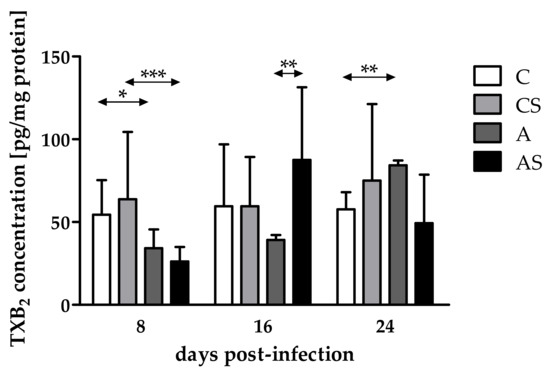
Figure 2.
Concentrations of thromboxane B2 (TXB2) in the eyes of mice at 8, 16, 24 days post Acanthamoeba spp. infection (dpi) related to immunological status of the host. Data represent mean ± standard deviation for six independent experiments (C, immunocompetent control, noninfected mice; CS, immunosuppressed control, noninfected mice; A, immunocompetent mice infected with Acanthamoeba spp.; AS, immunosuppressed mice infected with Acanthamoeba spp.; * statistical difference at p < 0.05; ** statistical difference at p < 0.01; *** statistical difference at p < 0.001).
Comparing TXB2 concentrations in the eyes of immunocompromised mice on different days after infection, differences occurred and were statistically significant (H = 12.38, p < 0.01). The concentration of TXB2 decreased at 8 dpi, increased at 16 dpi, and re-decreased at 24 dpi. Moreover, we found a statistically significant lower TXB2 level in the immunosuppressed infected mice than in the uninfected group at 8 dpi (U = 9, p < 0.001; Figure 2).
Taking into account host immune status, TXB2 concentrations were at the same level in the eyes of immunosuppressed and immunocompetent infected mice at 8 dpi. At 16 dpi, TXB2 levels increased in the immunosuppressed mice compared with the immunocompetent animals, while at 24 dpi, TXB2 levels decreased in the immunosuppressed hosts. The difference was statistically significant only at 16 dpi (U = 0, p < 0.01; Figure 2).
3. Discussion
In our previous studies, we demonstrated that Acanthamoeba spp. can invade the eyes of a host without a damaged cornea. Amoebae may migrate from the brain to the eyes along the optic nerve in hosts with disseminated acanthamoebiasis [6]. In the scientific literature, intraocular colonization secondary to disseminated acanthamoebiasis was reported only once in humans in post mortem examinations [5], but the reproduction of this finding in an animal model [6] warns that the situation could occur with more frequency. Moreover, among symptoms occurring during GAE, eye distention and photophobia with blurred vision are sometimes described [19,20,21]. We speculate that in these patients with GAE and vision problems, Acanthamoeba spp. invaded the eyes. However, we know neither what circumstances have to occur to make amoebae migrate through the optic nerve nor how fast they invade the eyes. In live patients, we found no case reports concerning eye invasions by amoebae in GAE or disseminated acanthamoebiasis. Most probably, it is associated with high mortality in patients with GAE, and that diagnostic is often confirmed post mortem [22].
Symptoms occurring with Acanthamoeba spp. infection are associated with an ongoing inflammatory process. The proteins involved in this process are PGs, products of the reaction carried out by cyclooxygenases [23,24]. Under normal physiological conditions, PGs have crucial homeostatic functions, including maintaining IOP. However, prostaglandins are also involved in some pathological conditions, including ocular inflammation [25]. PGs increase vasodilation, facilitate leukocyte migration, and damage the blood–ocular barrier [26]. Thromboxanes are important mediators of inflammation. However, data concerning the role of TXB2 in the eyes and the influence of TXB2 on the pathogenesis of various eye diseases is limited. TXB2 may be involved in inflammation in glaucoma and dry eye [13,27]. The upregulation of TXB2 was demonstrated in response to a cornea infection with Pseudomonas aeruginosa [28]. Thromboxane may be involved in host–parasite interactions. In the eyes of mice with disseminated acanthamoebiasis, changes in TXB2 levels were observed in immunocompetent hosts on 8 and 24 dpi and in immunocompromised mice at the beginning of infection.
PGE2 is important in the inflammatory process [29]. There is experimental evidence indicating the involvement of PGE2 in the pathogenesis of various eye diseases, including diabetic retinopathy and glaucoma [11,23]. Through studying diabetic retinopathy in animal models, it has been reported that retinal cells constantly increase COXs and PGE2 enzyme levels [30,31]. Schoenberger et al. [23] found higher PGE2 levels in diabetic human eyes. Lekhanont et al. [32] reported a positive correlation between the PGE2 concentration in tears and the symptoms of dry eye. Considering parasitic diseases, it was suggested that PGE2 is involved in the pathogenesis of ocular toxoplasmosis [33]. PGE2 may modulate a host’s immune system in terms of the inhibition of nitrogen oxide (NO) production, immunosuppression, and the inhibition of interferon (IFN) and apoptotic pathways. Additionally, PGE2 may play a role in infections by the induction of autoimmune disorder and disruption of signaling via TLRs [34]. In this study, we also noted the role of PGE2 in the pathomechanism and pathophysiology of Acanthamoeba spp. invasion in the eyes of immunosuppressed mice. Degraaf et al. [35] reported that PGE2 reduces TLR4 expression, which agrees with our research. In immunosuppressed mice, an increased level of TLR4 expression was not observed [6].
The increased production of PGE2 in pathological conditions is mostly regulated by the induction of the COX-2 gene [36]. In human keratinocytes, increased intracellular ROS as a result of mechanical injury stimulates PGE2 production via the activation of COX-2 [37]. In pulmonary acanthamoebiasis, differences in COX-1 and COX-2 expressions were only observed between immunocompetent infected and noninfected mice. Interestingly, there were no differences in PGE2 and TXB2 concentrations between amoebae-infected and noninfected mice regardless of the immunological status of the animals [18]. Despite antioxidant dysregulation in both immunocompetent and immunosuppressed eyes of mice infected with Acanthamoeba spp. [7], in our study, we noted differences in TXB2 in immunocompetent and immunosuppressed mice, while differences in PGE2 concentration were observed only in immunosuppressed mice. We suggest that in the immunocompetent mice, there was a blockage of PGE2 production. Strong et al. [38] reported that suppressing PGE2 production leads to a decrease in proinflammatory cytokines levels and provides a survival advantage to the host.
PGE2 is expressed in the human cornea, iris, trabecular meshwork, ciliary body, conjunctiva, and retina [11]. The retinal pigment epithelium is capable of inducing the differentiation of T cells into regulatory T cells through the expression of prostaglandin E2. The RPE induces the production of PGE2, thereby increasing granulocyte maturation and the inflammatory process [38]. Wang et al. [39] found retinal structural abnormalities, retinal edema, and neovascularization in the eyes of rats with diabetic retinopathy after PGE2 treatment compared with rats with diabetic retinopathy not treated with PGE2. In our study, we found a higher level of PGE2 in immunosuppressed mice infected with the parasite on 16 dpi compared with the noninfected group. In this infected group of mice, we also found an increased thickness in the outer nuclear layer of the retina, vacuolization inside the outer plexiform layer, and invagination of the Bowman’s membrane into the substantia propria. However, an increased thickness in the layers of the retina was observed in immunocompetent infected mice at 16 and 24 dpi as well as in immunosuppressed infected mice at 24 dpi [7], in which increased PGE2 levels were not found.
Yamanishi et al. [24] found decreased production of PGE2 in the tears of patients with severe conjunctivitis after dexamethasone administration. Fuller et al. [40] reported that dexamethasone inhibits the production of thromboxane B2. Dexamethasone and methylprednisolone are immunosuppressive drugs known as corticosteroids. In our study, we observed decreased PGE2 levels in the immunocompromised infected mice compared with the immunocompetent infected hosts on 8 dpi. On 16 dpi, PGE2 and TXB2 levels were higher in the AS group than in the A group.
Ocular pathology in disseminated acanthamoebiasis may be affected by a Th17-dependent immune response. It was suggested that Toxoplasma gondii induce an IL-17 increase, which exacerbates pathology and inflammation [41]. This cytokine may allow antigens, antibodies, and activated immune cells to cross the blood–retinal barrier, leading to increased inflammation and subsequent tissue damage [33,42]. In sharp contrast, Suryawanshi et al. [43] reported that IL-17 plays a significant role in host protection against Acanthamoeba spp. invasion. Łanocha-Arendarczyk et al. [44] showed that in disseminated acanthamoebiasis in immunocompetent hosts, parasites induce Th1, Th2, and Th17 responses. Meanwhile, in immunocompromised hosts, Acanthamoeba spp. induces strong immunity mediated by Th1 cells without Th17 involvement. IL-17 activates the TXB2 pathway [45], while prostaglandin E2 is essential for the production of IL-17, the Th17 effector cytokine [46]. However, Dejani et al. [47] reported that Th17 cell differentiation during intestinal Citrobacter rodentium infection is inhibited by PGE2. In our study, we did not examine IL-17 levels in the eyes of mice infected with amoebae, but it should be done in a future study to confirm our suggestions.
4. Materials and Methods
4.1. Animal Model
Approval was obtained from the ethics committees in Szczecin (Resolution No. 29/2015, 22 June 2015) and Poznań (Resolution No. 64/2016, 9 September 2016) for the study. The Acanthamoeba strain was classified as a T16 genotype, and it was isolated from a patient who suffered from acute myeloid leukemia (AML).
Ninety-six male mice of strain BALB/c, aged 6–10 weeks, with an average weight of 23 g, were used for the study. The animals were obtained from the Center of Experimental Medicine, Medical University of Bialystok. They were properly examined by a veterinarian who issued certificates of their health status.
Animals were divided into four groups on the basis of their immune status and parasite infection (Figure 3).
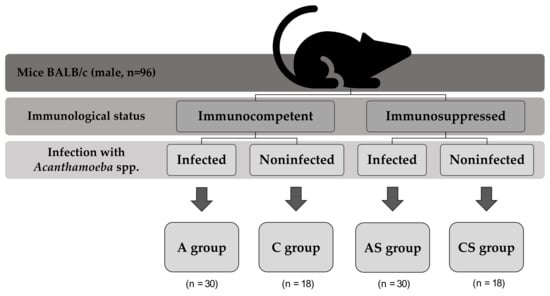
Figure 3.
Division of mice in the experiment.
For four days prior to inoculation of mice with Acanthamoeba spp., AS and CS animals were administered 0.22 mg (10 mg/kg body weight, b.w.) of methylprednisolone dissolved in 0.1 mL of 0.1% saline to induce immunosuppression. Infections of A and AS groups were performed by intranasal inoculation with 3 µL of a suspension containing 10–20,000 amoebae in accordance with the animal model used by Górnik and Kuźna-Grygiel [48]. C and CS groups received 3 µL of saline (0.9% NaCl).
Animals were monitored daily for clinical signs of Acanthamoeba spp. infection. We observed higher activity, changes in fur aspect, and ataxia in some mice. All observations of each mouse in each group were presented in previous papers [18,49].
Animals were injected intraperitoneally (i.p.) with sodium pentobarbital (2 mL/kg b.w.) and were sacrificed on days 8, 16, and 24 post Acanthamoeba spp. infection (dpi). Eyes were collected from mice using sterilized equipment. Confirmation of Acanthamoeba spp. infection was determined by amoeba re-isolation from the organs, which consisted of placing the eyes and optic nerve on a plate with non-nutrient agar and bacteria and then incubating the plate for 10 days at 41 °C [6]. Eyes for biochemical analysis were fixed in liquid nitrogen and stored at −80 °C.
4.2. Homogenization
Frozen eyes were placed individually in a metal homogenizer cooled in liquid nitrogen and then fragmented thoroughly by hitting the metal mandrel, which was also cooled in liquid nitrogen, several times with a hammer. The crushed and frozen eye fragments were transferred with a liquid-nitrogen-cooled spoon into Eppendorf test tubes. The homogenizer and hammer were thoroughly cleaned with ethanol after each use. The prepared samples were stored at −80 °C.
4.3. Total Protein Concentration
To each sample, 200 µL of buffer (150 mM NaCl, 50 mM Tris-HCl, and 0.5% Triton X-100) was added. Then, they were vortexed and incubated in an ice cartridge for 15 min. The samples were then centrifuged (14,000 rpm for 20 min at 4 °C), and the supernatant was gently extracted. Total protein concentration in the supernatant was examined by the bicinchoninic acid method using a commercial test (MicroBCA Protein Assay Kit, Thermo Scientific, Pierce Biotechnology, Waltham, MA, USA). The absorbance of the samples was then measured spectrophotometrically using a Biochrom EZ Read 2000 plate reader at 562 nm. The essence of this method is the reduction reaction of Cu2+ to Cu+ ions in an alkaline medium and the formation of a colored BCA complex with Cu+ ions, which shows an absorbance maximum at 562 nm. The absorbance value is directly proportional to the total protein concentration.
4.4. PGE2 and TXB2 Concentrations
PGE2 and TXB2 were extracted from eye sample homogenates using Bakerbond columns (Witko Group, Łódź, Poland). The concentration of PGE2 was measured using the Prostaglandin E2 EIA Kit according to the manufacturer’s procedure (Cayman, Ann Arbor, MI, USA). TXB2 concentration was measured using the Thromboxane B2 EIA Kit assay according to the manufacturer’s procedure (Cayman, Ann Arbor, MI, USA). Results were read using a microplate reader (EZ Read 2000, Biochrom Ltd., Cambridge, UK) at 420 nm. The concentrations of PGE2 and TXB2 were expressed in pg/mg protein.
4.5. Statistical Analysis
Statistical analysis was performed using Microsoft Excel 2016, StatSoft Statistica 12.0, and GraphPad 5.0. We calculated the arithmetic mean (AM) and standard deviation of the arithmetic mean (SD). Because the data did not follow a normal distribution, nonparametric tests were used for statistical analysis. The Mann–Whitney U test (U) was used to compare differences between two study groups (Acanthamoeba spp. infection and immune status), and the Kruskal–Wallis test (H) was used to compare differences among three study groups (days after infection). Significant statistical differences were assumed when p < 0.05.
5. Conclusions
The pathomechanism and pathophysiology of disseminated acanthamoebiasis accompanied by an infection of amoebae to the eyes, is still not fully understood. In the present study, we confirmed the inflammatory process in the eyes of immunocompetent and immunocompromised mice infected with Acanthamoeba spp. without damaged cornea. In immunocompetent infected mice, the main mediator of inflammation is TXB2, while in immunosuppressed infected mice, both TXB2 and PGE2 are mediators of inflammation.
Author Contributions
Conceptualization, K.K. and P.K.; methodology, K.K. and P.K.; software, K.K., D.K. and P.K.; validation, K.K. and P.K.; formal analysis, K.K. and D.K.; investigation, K.K.; resources, K.K.; data curation, K.K.; writing—original draft preparation, K.K.; writing—review and editing, D.I.K.-B. and N.Ł.-A.; visualization, D.K.; supervision, D.I.K.-B. and N.Ł.-A.; project administration, N.Ł.-A.; funding acquisition, D.I.K.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Local Ethics Committee for Scientific Experiments on Animals in Szczecin (no. 29/2015 of 22 June 2015) and Poznań (no. 64/2016 of 9 September 2016).
Informed Consent Statement
Not applicable.
Data Availability Statement
Derived data supporting the findings of this study are available from the corresponding author (D.K.-B.) upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Visvesvara, G.S.; Moura, H.; Schuster, F.L. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 2007, 50, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, N.A. Acanthamoeba: Biology and increasing importance in human health. FEMS Microbiol. Rev. 2006, 30, 564–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Król-Turmińska, K.; Olender, A. Human infections caused by free-living amoebae. Ann. Agric. Environ. Med. 2017, 24, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Morales, J.; Khan, N.A.; Walochnik, J. An update on Acanthamoeba keratitis: Diagnosis, pathogenesis and treatment. Parasite 2015, 22, 10. [Google Scholar] [CrossRef] [Green Version]
- Chandra, S.R.; Adwani, S.; Mahadevan, A. Acanthamoeba meningoencephalitis. Ann. Indian Acad. Neurol. 2014, 17, 108–112. [Google Scholar] [CrossRef]
- Kot, K.; Kosik-Bogacka, D.; Łanocha-Arendarczyk, N.; Wojtkowiak-Giera, A.; Kolasa-Wołosiuk, A. Expression of toll-like receptors (TLR2 and TLR4) in the eyes of mice with disseminated acanthamoebiasis. Biomed. Res. Int. 2019, 2019, 1401894. [Google Scholar] [CrossRef] [Green Version]
- Kot, K.; Kosik-Bogacka, D.; Kupnicka, P.; Łanocha-Arendarczyk, N. Antioxidant defense in the eyes of immunocompetent and immunosuppressed mice infected with Acanthamoeba spp. Parasites Vectors 2020, 13, 123. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Cheng, C.K.; Zhang, C.L.; Huang, Y. Interplay between oxidative stress, cyclooxygenases, and prostanoids in cardiovascular diseases. Antioxid. Redox Signal. 2021, 34, 784–799. [Google Scholar] [CrossRef]
- Korbecki, J.; Baranowska-Bosiacka, I.; Gutowska, I.; Chlubek, D. Cyclooxygenase pathways. Acta Biochim. Pol. 2014, 61, 639–649. [Google Scholar] [CrossRef] [Green Version]
- Liclican, E.L.; Nguyen, V.; Sullivan, A.B.; Gronert, K. Selective activation of the prostaglandin E2 circuit in chronic injury-induced pathologic angiogenesis. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6311–6320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doucette, L.P.; Walter, M.A. Prostaglandins in the eye: Function, expression, and roles in glaucoma. Ophthalmic Genet. 2017, 38, 108–116. [Google Scholar] [CrossRef]
- Lucotti, S.; Cerutti, C.; Soyer, M.; Bernabé, A.M.J.; Gomes, A.L.; Allen, P.D.; Smart, S.; Markelc, B.; Watson, K.; Armstrong, P.C.; et al. Aspirin blocks formation of metastatic intravascular niches by inhibiting platelet-derived COX-1/thromboxane A2. J. Clin. Investig. 2019, 129, 1845–1862. [Google Scholar] [CrossRef] [Green Version]
- Cartier, A.; Parent, A.; Labrecque, P.; Laroche, G.; Parent, J.L. WDR36 acts as a scaffold protein tethering a G-protein-coupled receptor, Gαq and phospholipase Cβ in a signalling complex. J. Cell Sci. 2011, 124, 3292–3304. [Google Scholar] [CrossRef]
- Lonardoni, M.V.; Russo, M.; Jancar, S. Essential role of platelet-activating factor in control of Leishmania (Leishmania) amazonensis infection. Infect. Immun. 2000, 68, 6355–6361. [Google Scholar] [CrossRef]
- Lejeune, M.; Moreau, F.; Chadee, K. Prostaglandin E2 produced by Entamoeba histolytica signals via EP4 receptor and alters claudin-4 to increase ion permeability of tight junctions. Am. J. Pathol. 2011, 179, 807–818. [Google Scholar] [CrossRef]
- Abdalla, G.K.; Faria, G.E.; Silva, K.T.; Castro, E.C.; Reis, M.A.; Michelin, M.A. Trypanosoma cruzi: The role of PGE2 in immune response during the acute phase of experimental infection. Exp. Parasitol. 2008, 118, 514–521. [Google Scholar] [CrossRef]
- Anyona, S.B.; Kempaiah, P.; Davenport, G.C.; Vulule, J.M.; Hittner, J.B.; Ong’echa, J.M.; Perkins, D.J. Suppressed circulating bicyclo-PGE2 levels and leukocyte COX-2 transcripts in children co-infected with P. falciparum malaria and HIV-1 or bacteremia. Biochem. Biophys. Res. Commun. 2013, 436, 585–590. [Google Scholar] [CrossRef] [Green Version]
- Łanocha-Arendarczyk, N.; Baranowska-Bosiacka, I.; Kot, K.; Gutowska, I.; Kolasa-Wołosiuk, A.; Chlubek, D.; Kosik-Bogacka, D. Expression and activity of COX-1 and COX-2 in Acanthamoeba sp.-infected lungs according to the host immunological status. Int. J. Mol. Sci. 2018, 19, 121. [Google Scholar] [CrossRef] [Green Version]
- Fu, N.X.; Song, J.; Huang, X.; Lin, G.H. Granulomatous amoebic encephalitis presenting as a solitary mass lesion. Radiol. Infect. Dis. 2020, 7, 204–207. [Google Scholar] [CrossRef]
- Paudel, A.C.; Patel, N.; Quang, J.; Casella, C.; Sigal, A.; Parajuli, P.; Oladunjove, O.; Oke, I.O.; Khanal, S.; Bhattari, K. Rapidly progressive granulomatous amoebic encephalitis in a diabetic individual. Cureus 2021, 13, e19336. [Google Scholar] [CrossRef]
- Akpek, G.; Uslu, A.; Huebner, T.; Taner, A.; Rapoport, A.P.; Gojo, I.; Akpolat, Y.T.; Loffe, O.; Kleinberg, M.; Baer, M.R. Granulomatous amebic encephalitis: An under-recognized cause of infectious mortality after hematopoietic stem cell transplantation. Transpl. Infect. Dis. 2011, 13, 366–373. [Google Scholar] [CrossRef]
- Kalra, S.K.; Sharma, P.; Shyam, K.; Tejan, N.; Ghoshal, U. Acanthamoeba and its pathogenic role in granulomatous amebic encephalitis. Exp. Parasitol. 2020, 208, 107788. [Google Scholar] [CrossRef]
- Schoenberger, S.D.; Kim, S.J.; Sheng, J.; Rezaei, K.A.; Lalezary, M.; Cherney, E. Increased prostaglandin E2 (PGE2) levels in proliferative diabetic retinopathy, and correlation with VEGF and inflammatory cytokines. Invest. Ophthalmol. Vis. Sci. 2012, 53, 5906–5911. [Google Scholar] [CrossRef] [Green Version]
- Yamanishi, R.; Okada, N.; Shimizu, E.; Fujishima, H. Elevated levels of prostaglandin E2 in the tears of patients with severe allergic conjunctivitis and primary cultured conjunctival cells are suppressed by ketotifen and dexamethasone. BMJ Open Ophthalmol. 2021, 6, e000571. [Google Scholar] [CrossRef]
- Toris, C.B.; Gulati, V. The biology, pathology and therapeutic use of prostaglandins in the eye. Clin. Lipidol. 2011, 6, 577–591. [Google Scholar] [CrossRef]
- Kim, S.J.; Flach, A.J.; Jampol, L.M. Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv. Ophthalmol. 2010, 55, 108–133. [Google Scholar] [CrossRef]
- Ambaw, Y.A.; Chao, C.; Ji, S.; Raida, M.; Torta, F.; Wenk, M.R.; Tong, L. Tear eicosanoids in healthy people and ocular surface disease. Sci. Rep. 2018, 8, 11296. [Google Scholar] [CrossRef]
- Kallinikos, P.; Efron, N. On the etiology of keratocyte loss during contact lens wear. Invest. Ophthalmol. Vis. Sci. 2004, 45, 3011–3020. [Google Scholar] [CrossRef] [Green Version]
- Ricciotti, E.; Fitz-Gerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Johnson, E.I.; Dunlop, M.E.; Larkins, R.G. Increased vasodilatory prostaglandin production in the diabetic rat retinal vasculature. Curr. Eye Res. 1999, 18, 79–82. [Google Scholar] [CrossRef]
- Amrite, A.C.; Ayalasomayajula, S.P.; Cheruvu, N.P.; Kompella, U.B. Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Invest. Ophthalmol. Vis. Sci. 2006, 47, 1149–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lekhanont, K.; Sathianvichitr, K.; Pisitpayat, P.; Anothaisintawee, T.; Soontrapa, K.; Udomsubpayakul, U. Association between the levels of prostaglandin E2 in tears and severity of dry eye. Int. J. Ophthalmol. 2019, 12, 1127–1133. [Google Scholar] [CrossRef]
- Greigert, V.; Bittich-Fahmi, F.; Pfaff, A.W. Pathophysiology of ocular toxoplasmosis: Facts and open questions. PLoS Negl. Trop. Dis. 2020, 14, e0008905. [Google Scholar] [CrossRef] [PubMed]
- Sander, W.J.; O’Neill, H.G.; Pohl, C.H. Prostaglandin E2 As a Modulator of Viral Infections. Front Physiol. 2017, 8, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degraaf, A.J.; Zasłona, Z.; Bourdonnay, E.; Peters-Golden, M. Prostaglandin E2 reduces Toll-like receptor 4 expression in alveolar macrophages by inhibition of translation. Am. J. Respir. Cell Mol. Biol. 2014, 51, 242–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, J.; Yang, X.; Han, X. Several transcription factors regulate COX-2 gene expression in pancreatic beta-cells. Mol. Biol. Rep. 2007, 34, 199–206. [Google Scholar] [CrossRef]
- Hu, Y.P.; Peng, Y.B.; Zhang, Y.F.; Wang, Y.; Yu, W.R.; Yao, M.; Fu, X.J. Reactive Oxygen Species Mediated Prostaglandin E2 Contributes to Acute Response of Epithelial Injury. Oxid. Med. Cell Longev. 2017, 2017, 4123854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strong, V.E.; Mackrell, P.J.; Concannon, E.M.; Naama, H.A.; Schaefer, P.A.; Shaftan, G.W.; Stapleton, P.P.; Daly, J.M. Blocking prostgalndin E2 after trauma attenuates pro-inflammatory cytokines and improves survivial. Shock 2000, 14, 374–379. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Xie, T.; Zhan, P.; Zou, J.; Nie, X.; Shao, J.; Zhuang, M.; Tan, C.; Tan, J.; et al. Prostaglandin E2/EP2 receptor signalling pathway promotes diabetic retinopathy in a rat model of diabetes. Diabetologia 2019, 62, 335–348. [Google Scholar] [CrossRef] [Green Version]
- Fuller, R.W.; Keisey, C.R.; Cole, P.J.; Dollery, C.T.; MacDermot, J. Dexamethasone inhibits the production of thromboxane B2 and leukotriene B4 by human alveolar and peritoneal macrophages in culture. Clin. Sci. 1984, 67, 653–656. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yang, P.; Li, F.; Kijlstra, A. The effects of Th17 cytokines on the inflammatory mediator production and barrier function of ARPE-19 cells. PLoS ONE 2011, 6, e18139. [Google Scholar] [CrossRef] [Green Version]
- Sauer, A.; Pfaff, A.W.; Villard, O.; Creuzot-Garcher, C.; Dalle, F.; Chiquet, C.; Pelloux, H.; Speeg-Schatz, C.; Gaucher, D.; Prevost, G.; et al. Interleukin 17A as an effective target for anti-inflammatory and antiparasitic treatment of toxoplasmic uveitis. J. Infect. Dis. 2012, 206, 1319–1329. [Google Scholar] [CrossRef] [Green Version]
- Suryawanshi, A.; Cao, Z.; Sampson, J.F.; Panjwani, N. IL-17A-mediated protection against Acanthamoeba keratitis. J. Immunol. 2015, 194, 650–663. [Google Scholar] [CrossRef] [Green Version]
- Łanocha-Arendarczyk, N.; Kolasa-Wołosiuk, A.; Wojciechowska-Koszko, I.; Kot, K.; Roszkowska, P.; Krasnodębska-Szponder, B.; Paczkowska, E.; Machaliński, B.; Łuczkowska, K.; Wiszniewska, B.; et al. Changes in the immune system in experimental acanthamoebiasis in immunocompetent and immunosuppressed hosts. Parasites Vectors 2018, 11, 517. [Google Scholar] [CrossRef]
- Östling, J.; van Geest, M.; Schofield, J.P.R.; Jevnikar, Z.; Wilson, S.; Ward, J.; Lutter, R.; Shaw, D.E.; Bakke, P.S.; Caruso, M.; et al. U-BIOPRED Study Group. IL-17-high asthma with features of a psoriasis immunophenotype. J. Allergy Clin. Immunol. 2019, 144, 1198–1213. [Google Scholar] [CrossRef] [Green Version]
- Boniface, K.; Bak-Jensen, K.S.; Li, Y.; Blumenschein, W.M.; McGeachy, M.J.; McClanahan, T.K.; McKenzie, B.S.; Kastelein, R.A.; Cua, D.J.; de Waal Malefyt, R. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J. Exp. Med. 2009, 206, 535–548. [Google Scholar] [CrossRef] [Green Version]
- Dejani, N.N.; Orlando, A.B.; Niño, V.E.; Penteado, L.A.; Verdan, F.F.; Bazzano, J.M.R.; Codo, A.C.; Salina, A.C.G.; Saraiva, A.C.; Avelar, M.R.; et al. Intestinal host defense outcome is dictated by PGE2 production during efferocytosis of infected cells. Proc. Natl. Acad. Sci. USA 2018, 115, E8469–E8478. [Google Scholar] [CrossRef] [Green Version]
- Górnik, K.; Kuźna-Grygiel, W. Histological studies of selected organs of mice experimentally infected with Acanthamoeba spp. Folia Morphol. 2005, 64, 161–167. [Google Scholar]
- Kot, K.; Kosik-Bogacka, D.; Łanocha-Arendarczyk, N.; Ptak, M.; Roszkowska, P.; Kram, A. Histological changes in the kidneys and heart in experimental acanthamoebiasis in immunocompetent and immunosuppressed hosts. Folia Biol. 2021, 4, 167–178. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).